Abstract
Edible coatings can extend the shelf-life of many foods, controlling moisture and solute migration, gas exchange and oxidative reaction rates. Besides, edible coatings can be used as carriers of bioactive compounds to improve the quality of food products such as antioxidants, antimicrobials, flavors and probiotics. These approaches can be useful to extend shelf-life as well as provide a functional product. When edible coatings are used as a matrix holding bioactive compounds remarkable benefits arise; off odors and flavors can be masked, bioactive compounds are protected from the environment, and controlled release is allowed. In this sense, the present review will be focused on analyzing the potential use of encapsulation with edible coatings to incorporate bioactive compounds, solving the disadvantages of direct application.
Keywords: Edible coatings, Matrix, Bioactive compounds, Encapsulation, Functional food
Introduction
An edible coating is defined as a thin layer of edible material applied to the surface of a food, which provides a barrier against migration of moisture, oxygen, carbon dioxide, aromas, lipids, and other solutes (Kester and Fennema 1986; Biquet and Labuza 1988; Cuq et al. 1995). Furthermore, their use can have more innovative applications; edible coatings can be utilized as encapsulating matrices of bioactive compounds to improve the quality of food products, allowing controlled release; strategy by which bioactive compounds are made available at a desired site and time at a specific rate (Pothakamury and Barbosa-Cánovas 1995). This application is an interesting tool not only to extend shelf-life and reduce the risk of pathogen growth on food surfaces, but also to provide a functional product with health benefits to the consumer.
Bioactive compounds are non nutritional constituents that typically occur in small quantities in foods. Generally, these compounds are found in millions of species of plants, animals, marine organisms and microorganisms, and can be obtained by extraction and biotechnological methods (Kris-Etherton et al. 2002). Extracted bioactive compounds can be incorporated to produce new functional foods, enhancing its shelf life, nutritional quality and increasing consumer acceptance of these commodities. Among the most used bioactive compounds are antioxidants, antimicrobials, probiotics and flavors, in addition to nutraceutical substances (Ayala-Zavala et al. 2011; Muranyi 2013).
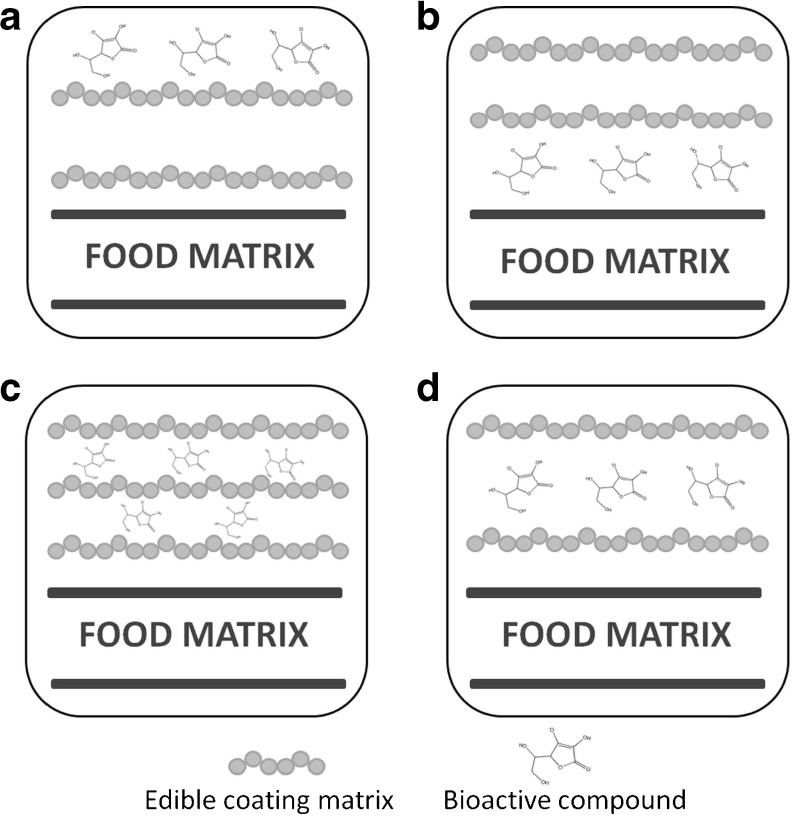
The incorporation of bioactive compounds into food products provides many advantages in food preservation and contributes to the development of functional foods. However, bioactive compounds may have certain disadvantages such as off flavors and an early loss of functionality (Ayala-Zavala et al. 2008; Silva-Weiss et al. 2013). In this sense, encapsulation with edible coatings is a promising technique that can solve the disadvantages of the use of bioactive compounds as food additives. Different encapsulation strategies may be used, depending on the nature and purpose of the bioactive compound (Fig. 1). For example, (a) incorporation on the external surface of the film, (b) at the interface between the film and the food, (c) among multilayered edible coatings and (d) dispersed among different section of the film. These systems are more efficient than a direct application on the food surface, because edible coatings delay the migration of the agents away from the surface, helping to maintain a high concentration of bioactive compounds where it is needed.
Fig. 1.
Possible incorporation systems of bioactive compounds into edible coatings: a bioactive compounds incorporated on the external surface of the film in contact with the environment, b at the interface between the film and the food, c among multilayered edible coating, d dispersed among different sections of the film
Therefore, the aim of this review is to describe the potential use of edible coatings as stabilizing matrices holding bioactive compounds, counteracting the disadvantages of direct application and enhancing the functional properties of edible coatings through the incorporation of bioactive compounds.
Bioactive compounds as food additives: advantages and disadvantages
Bioactive compounds are extra nutritional constituents that typically occur in small quantities in foods (Kris-Etherton et al. 2002). Include groups of compounds such as carotenoids, flavonoids, phenolic acids, glucosinolates, dietary fiber, phytosterols, monoterpenes and very active molecules like ascorbic acid (Ayala-Zavala et al. 2011). Currently, their use and consumption have been gained increasing interest among consumers and researchers because studies have been associated with a positive effect on human health.
In recent years, the use of bioactive compounds has been oriented to pharmaceutical, medical and food applications. In the food area, these compounds are used to create functional foods, with enhanced shelf life and nutritional quality, and with increased consumer acceptance (Tajkarimi et al. 2010). In addition, these compounds have functional properties such as antioxidants (phenolics), antimicrobials (essential oils), probiotics and flavors. Although bioactive compounds offer many advantages in the production of functional foods; these compounds may impart off flavors, be prone to rapid degradation and interact with other components in the food matrix, leading to loss in quality of the functional food products.
Antioxidants
Antioxidants are molecules which offer protection against the harmful effect of free radicals and other reactive oxygen species, offering human health and technological food benefits. Potential sources of natural antioxidants have been found in several materials and/or tissues such as vegetables, fruits, leaves, oilseeds, cereal crops and herbs. As food additives, antioxidants can protect against detrimental change of oxidizable nutrients and consequently can extend the shelf-life of foods. These agents include groups of compounds such as ascorbic acid, carotenoids, flavonoids and other phenolic compounds. However, the use of these compounds involves certain disadvantages such as vulnerability to high temperature and light, high volatility, limited solubility and unpleasant flavor; these characteristics result in a loss of functionality, limiting their application (Ayala-Zavala et al. 2008; Fang and Bhandari 2010).
Some flavonoids (isoflavones) and phenols have limited solubility into lipophilic systems (Viskupicova et al. 2010). In addition, many polyphenols (quercetin, myricetin, taxifolin, procyanidines, salicin, thymol, and eugenol), terpenes and carotenoids are astringent and present an unpleasant flavor. Another problem that some antioxidants present, such as ferulic acid, is that these are easily volatilized, thus cannot inhibit oxidation at high temperatures for long periods of time (Nyström et al. 2007). Furthermore, various vitamins (C, E, K) and some phytochemicals (phenols, flavonoids) are sensitive to UV-B and UV-C light, limiting their use since most foods on shelf life are exposed to such conditions (Durand et al. 2010). In this sense, some technologies of encapsulation can effectively alleviate these deficiencies.
Antimicrobials
In the nature there are a large number of different antimicrobial compounds that play an important role in the defense against all kinds of living microorganisms (Rauha et al. 2000). In this context, incorporation of antimicrobials as food additives provides a way to enhance the safety and shelf life of foods. Among the natural antimicrobial agents more commonly used in foods are enzymes like lactoperoxidase and lysozyme; polysaccharides such as chitosan; bacteriocins and more recently, herbs, spices and essential oils including terpenes, alcohols, acetones, phenols, acids, aldehydes and esters (Tajkarimi et al. 2010). Essential oils have been one of the most studied and used compounds as antimicrobials, because they have been known for centuries, as generally recognized as safe (GRAS) and have been found to be effective against a wide range of bacteria (Fisher and Phillips 2008).
Essential oils are natural antimicrobials derived from vegetable extracts. It has been suggested that their effect depend on their hydrophobicity, which enables them partition in the lipid of the microbial cell membrane and mitochondria, disturbing the pathogen cell structures (Ayala-Zavala et al. 2009). In spite of the advantages of using essential oils as antimicrobials, their high volatility and reactivity, and the unpleasant aroma can limit their potential use (Del Toro-Sánchez et al. 2010). In addition, a common problem in the food industry is the incompatibility of the additive and the food. Essential oils have a hydrophobic character and their addition to high water content foods can cause phase separation (Ayala-Zavala et al. 2008). Therefore, in recent years studies have been focused on looking for techniques to reduce these problems.
Probiotics
Probiotics are microbial cell preparations or components of microbial cells that have a beneficial effect on the health and well-being of the host (Salminen et al. 1999). This definition implies that probiotics do not necessarily need to be viable. It has been speculated that some of the mechanism behind the probiotic health effects may not be dependent on the viability of the cells and, therefore, it is also possible that non-viable probiotics could have some health benefits. Even the mechanism of action is not clear; it appears to be associated with adhesion. Probiotic adhesion to host tissues is thought to facilitate the host-microbial interactions such as the effects of microbes on the immune system of the host. Therefore, adhesion may be a key determinant for probiotic efficacy. Some reports suggest that viable and non-viable probiotics are equally adherent to intestinal mucus (Lahtinen 2012).
The most commonly used microorganisms are bifidobacteria, lactic acid bacteria and certain yeast. Among the beneficial effects of probiotics are prevention and shortening of diarrhea and respiratory tract infections, reducing the risk of necrotizing enterocolitis in premature infants, alleviation of symptoms of lactose intolerance, treatment of food allergy, and binding of toxins and pathogens from ingested foods or from the gastrointestinal tract (Salminen and Von Wright 2011). In addition, some probiotics can be used as antimicrobial compounds, against food pathogens (Sharafi et al. 2013; Souza et al. 2013). Several mechanisms have been proposed by which probiotics act, these include competing for nutrients and epithelial attachment site; modifying the environment making it unfavorable for pathogens; and producing antimicrobial peptides (Chichlowski et al. 2007). In addition, foods as carriers of probiotics help to buffer the probiotic through the gastrointestinal tract, regulate their colonization and contain other functional ingredients, which may interact with probiotics to modify their functionality and efficacy. For this reason, the incorporation of probiotics to food products has been increasing, to assurance safety and health products.
Some works have studied the effect of the incorporation of probiotics in food, and have found some protective qualities. Franz et al. (2003) showed that Enterococcus faecium, which is used in meat products as a probiotic, have anti-listerial properties. Pidcock et al. (2002) demonstrated that L. acidophilus and bifidobacteria have inhibitory effects against L. monocytogenes and E. coli in Hungarian salami. Similarly, Lactobacillus reuteri Protectis, which is used as a probiotic in dairy products and fermented sausages, inhibits Helicobacter pylori (Muthukumarasamy and Holley 2006). However, to guarantee the survival of these microorganisms in food and gastrointestinal tract is questionable, due to their poor stability (Ranadheera et al. 2010). The growth and survival of probiotics during gastrointestinal transit is affected by the physical and chemical properties of food carriers. Low pH foods such as fruit juices, salads and condiments present a problem for probiotic survival, as well as foods prepared or stored at high temperatures (Rodgers 2007). Thus, selection of suitable food systems to deliver probiotics is a key factor that should be considered in developing functional probiotic foods (Desobry and Debeaufort 2012).
Flavors
Natural flavorings substances are small molecules responsible for flavor that are obtained by appropriate physical, enzymatic or microbiological processes from animal or vegetal material. Since flavor is a key contributor for the acceptance of foods by the consumer, the incorporation of flavoring substances in foods plays an important role in consumer satisfaction and influences further consumption. Among the most important compounds used as flavors are the essential oils; since there is a wide diversity of compounds, which are approved by the United States Food and Drug Administration (FDA). The aromatic compounds of essential oils are mainly constituted of short hydrocarbon chains, complemented with oxygen, nitrogen, and sulfur atoms attached at various points of the chain (Ayala-Zavala et al. 2009). Several essential oils from diverse sources such as peppermint, oregano, coriander, cinnamon, among others, have been used as flavorings in foods.
However, the incorporation of flavors into foods is a difficult task, because, even in small amounts, aroma compounds are usually delicate, volatile and expensive. In this context, the incorporation of natural food additives within edible coatings could be a beneficial technique to increase the effectiveness of flavoring preventing degradation or loss of these functional compounds.
Edible coatings as a structural matrix
An edible coating is a thin layer of material that can be consumed and provides a barrier to moisture, oxygen and solute movement for the food (Falguera et al. 2011). Nevertheless, their use can have more innovative uses beyond their current applications. Edible coatings can be utilized as encapsulating matrices to many bioactive compounds that improve the quality of food products (Krochta and Nisperos-Carriedo 1994; Baldwin et al. 2012; Dhall 2013). These approaches can be useful to extend shelf-life and reduce the risk of pathogen growth on food surfaces as well as provide a functional product with health benefits to the consumer. In addition, the encapsulation of bioactive compounds into edible coatings is a way to protect these additives against severe environmental factors (Oliveira et al. 2012).
According on the properties of the compound to be encapsulated and the purpose of encapsulation, the structural material of edible coatings are based on proteins (such as zein, soy, collagen and gelatin), polysaccharides (such as cellulose derivates, starch, alginate and chitosan) and lipids (such as glycerol esters and waxes), which may be used alone or in combination. The functionality of these materials may vary, as each component offers different properties to the composite matrix. Indeed, the mechanical and barrier properties of these films not only depend on the compounds used in the polymer matrix, but also on their compatibility and on cohesion forces, including covalent bonds, ionic bonds, and H-bonding, between coating-forming polymer molecules (Janjarasskul and Krochta 2010). However, cohesive strength depends upon material structure and chemistry and by physical treatment (Janjarasskul and Krochta 2010). Chemical treatments include the use of plasticizers and emulsifiers. Plasticizers are typically small-molecular weight hydrophilic agents added to improve coatings properties by situating themselves in their polymeric network and competing form chain-to-chain H-bonding along the polymer chains. Commonly used plasticizers are glycerol, sorbitol polyethylene glycols, fatty acids, surfactants and phospholipids (Krochta and Nisperos-Carriedo 1994). Indeed, emulsifiers are surface active compounds, with both polar and non-polar character, capable of modifying interfacial energy at the interface of immiscible systems, such a water-lipid interface or a water-air-surface. Some common emulsifiers are acetylated monoglyceride, lecithin, glycerol monopalmitate, glycerol monoesterate, polysorbate 60, among others (Janjarasskul and Krochta 2010). Nevertheless, the use of physical treatments, such as heat and irradiation, can also increase the cohesive strength of the coatings by the formation of cross-inks (Pascall and Lin 2013).
Among polysaccharides used for edible coatings include cellulose, starch derivates, pectins, gums, seaweed extracts and chitosan (Bourtoom 2008; Baldwin et al. 2012; Espitia et al. 2013). However, these compounds are generally very hydrophilic resulting in poor water vapor and gas barrier properties in moist conditions. It has been reported that at low RH <25 % conditions most of the polysaccharides have good barrier characteristics (Baldwin et al. 2012). As a result of the large number of hydroxyl groups and other hydrophilic moieties present in their structure, H-bonds play significant roles in film formation and characteristics. In polysaccharides, the hydroxyl is the only reactive group. Generally, polysaccharides coatings are formed by disrupting interactions among long-chain polymer segments during the co-acervation process and forming new intermolecular hydrophilic and H-bonding upon evaporation of the solvent to create a coating matrix (Janjarasskul and Krochta 2010). Co-acervation is an encapsulation method based on the separation of a macromolecular solution into two immiscible liquid phases; a dense coacervation phase, which is relatively concentrated into two macromolecules, and a dilute equilibrium phase (De Kruif et al. 2004).
In the other hand, proteins are great materials for the formation of edible coatings, as they show excellent mechanical and optical properties and they are good barriers against the transport of O2 and CO2 (Janjarasskul and Krochta 2010). However, the barrier and mechanical properties are compromised by moisture owing to their inherent hydrophilic nature; as moisture increase, the plasticization induces loss barrier performances. Proteins present a large variety of possible interactions and chemical reactions (Hernández Izquierdo and Krochta 2008); they may participle in chemical reactions through covalent (peptide and disulfide) linkages and non-covalent interactions (ionic hydrogen and van der Waals bonding). In addition, hydrophobic interactions occur between non-polar groups of amino acids chains (Guerrero et al. 2010). Interlinkages between proteins participating in the formation of a film can lead to improve film properties.
Lipid compounds utilized as edible coatings consist of acetylated monoglycerides, natural wax, and surfactants. The most effective lipid substances are paraffin wax and beeswax (Debeaufort and Voilley 2009). The primarily function of a lipid coating is to block transport of moisture due to their relative low polarity. In contrast, the hydrophobic characteristic of lipid forms thicker and more brittle films; they must be associated with film forming agents such as proteins or cellulose derivates. Nevertheless, lipid-based films are often supported on a polymer structure matrix, usually polysaccharide, to provide mechanical strength.
Encapsulation systems
Edible coatings can be utilized as encapsulating matrices to many bioactive compounds. The encapsulation process is based on the embedding effect of an edible coating, which creates a microenvironment in the structural matrix able to control the interactions between the internal part and the external one (Ravichandran et al. 2012). In this context, there are different types of encapsulation systems, such as spray drying, spray chilling, co-extrusion and liposomes. These techniques enclose liquid droplets or small particles of a sensitive substance within a continuous edible coating. Therefore, the encapsulation technique most used in edible coating is the spray drying (Skurtys et al. 2010). This technique requires emulsifying the substance into the encapsulating agent. This is important for flavor applications, in particular considering the fact that most flavors are made up of components of various chemistries (polarity, hydrophobic to hydrophilic ratios), thus limiting their stability when dispersed or suspended in different solvents. Spray-chilling is based on the injection of cold air into the vessel to enable solidification of the gel particle, rather than on hot air which dries the droplet into a fine powder particle. The liquid droplet thus solidifies and entraps the bioactive compounds. In the extrusion technology the goal is to entrap the bioactive compounds in a dense, impermeable gas (Champagne and Fustier 2007). In other hand, liposomes are microscopic spherical particles consisting of one or more lipid bilayers that can encapsulate or bind a variety of molecules (Fang and Bhandari 2010). Consequently, all these properties make them attractive for use as food delivery vehicles. In spray drying, the bioactive compound is trapped within porous membranes of hollow spheres. However, the choice of method used for encapsulation depends on the properties of the bioactive compound, the edible coating materials and the requirements of the target food application.
Edible coatings as a stabilizing matrix for bioactive compounds
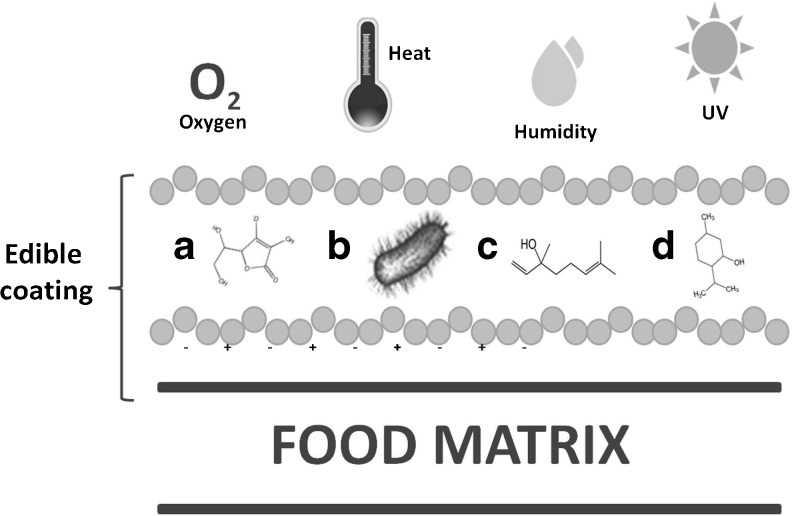
Considering some of the disadvantages of bioactive compounds, edible coatings can be visualized as encapsulating matrices able to offer protection against the environment (temperature and light), improving solubility and controlling compound release (Fig. 2).
Fig. 2.
Edible coating can visualized as encapsulating matrix able to offers protection to a antioxidants, b probiotics, c antimicrobials, and d flavor compounds, from adverse conditions such as UV light, heat, humidity and gases
High temperature protection
Edible coatings can offer the necessary protection of bioactive compounds against high temperature. Most of food additives have high temperature sensitivity; however, these conditions are necessary in a large number of food processes in food industry. Therefore, during encapsulation of bioactive compounds (antioxidants, antimicrobials, probiotics and flavors) these are introduced into a matrix or wall system (edible coating) to prevent their loss; by creating a solid barrier between the additive and environmental conditions, so it is protected. Kayaci and Uyar (2012) produced functional capsules matrix, containing vanillin, having prolonged shelf-life and high temperature stability facilitated by cyclodextrin inclusion complexation. Milanovic et al. (2010) showed that the release of ethyl vanillin from wax matrix occurs under heating proceeds at different temperatures; vanillin evaporation occurs close to 200 °C, while matrix degradation starts at 250 °C. The release of catechins from wax capsules at pH 5 was observed after heating the solution at temperature above 80 °C. Moreover, Partanen et al. (2002) reported the benefits of cyclodextrin encapsulation of extracted caraway fruit oil with maltodextrin and starch derivate. The inclusion complex seemed to protect volatile substances more efficiently during storage, whereas microcapsules with modified starches as wall material were more heat tolerant. The release and thermal stability were assessed on dry samples. During rapid heating, the cyclodextrin microcapsules protected the volatile substances from evaporation up to 100 °C. The protection properties of the maltodextrin microcapsules seemed to depend on the encapsulated molecules (160 °C for limonene and 120 °C for carvone). This indicates that the capsule contributes for the stabilization of bioactive compounds and prevents from degradation, and is useful for being incorporated into beverages. In this context, the edible coatings can protect the additives during the duration in such critical conditions (Ayala-Zavala et al. 2008).
UV light protection
Some bioactive compounds such as vitamins and antioxidants are adversely affected by UV light. However, the edible coatings as a matrix containing these additives may create a barrier to avoid chemical degradation by UV light and oxygen. It has been reported that exposure of essential oil of cinnamon leaf to UV light has a negative effect on the content of active compounds. However, saccharide matrix like as cyclodextrin can prevent deterioration keeping active compounds even in the most severe conditions of exposure to UV light (Ayala-Zavala et al. 2008). Ferreira et al. (2007) studied nanoparticles containing catechins encapsulated in a carbohydrate matrix by homogenization and spray-drying. Durand et al. (2010) reported the efficiency of encapsulation of ethyl-hexyl methoxycinnamate (EMC) in a solid lipid coating in to reduce its photo-instability under UV light exposure. The suspensions of nanoparticles contained 70 % encapsulated EMC (relative to the lipid mass). However, free EMC lost 30 % of its efficiency after 2 h irradiation, whereas the three lipid coating formulations showed a lower loss of absorbency (between 10 and 21 %).
Solubility enhancement
A common problem in the food industry is the incompatibility of the solubility of additive and food. This causes problems such as the occurrence of lumps, precipitates and phase separation among them. An important alternative to improve the solubility of additives is the encapsulation with edible coating matrices. For this reason, encapsulation in cyclodextrins mixtures of these components can effectively increase the solubility of the additive in the food matrix. Another alternative is to change the solubility of lipophobic additives to be incorporated in the matrix of fat foods (Kolanowski et al. 2004; Sereno et al. 2009). Liposomes are an alternative that allows increasing the fat solubility of the compound in the food matrix, making possible to include water-soluble vitamins in fatty foods, without causing phase separation between components.
Controlled release
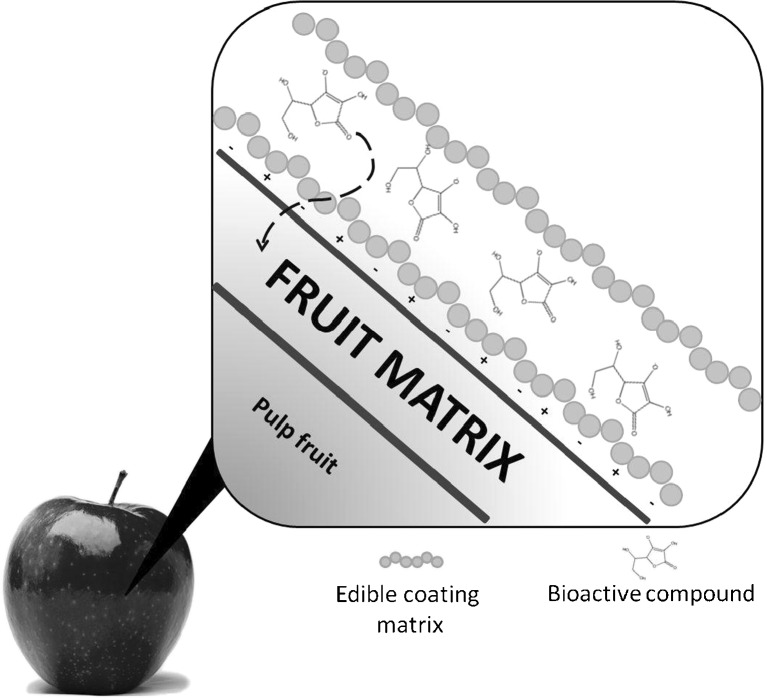
One of the most attractive advantages of the use of edible coatings is the ability to control the release of the bioactive compounds by simple parameters such as humidity, temperature, changes in pH and mechanical rupture of the matrix, among others. The mode of action of an additive may be required at different times during processing, storage or ingestion of food. Therefore, controlling the release of the additive at a given point is crucial to improve quality, ensure food safety or functionality of the edible coating or coated food (Fig. 3) (Ayala Zavala et al. 2008). There are different mechanisms of release such as melting, diffusion, degradation, or particle fracture (Madene et al. 2006).
Fig. 3.
Bioactive compounds can be transported from the edible coating to the food or fruit skin by diffusion release. The diffusion release is controlled by the solubility of a bioactive compound in the structural matrix and the permeability of the bioactive compound through the matrix
Melting mechanism involves the melting of the structural matrix to release the bioactive compounds. This is readily accomplished in the food industry as there are numerous materials that can be melted and approved for food use (lipids or waxes) (Charve and Reineccius 2009). The diffusion release is controlled by the solubility of a bioactive compound in the structural matrix (this establishes a concentration in the matrix which drives division) and the permeability of the bioactive compound through the matrix. Two distinct mechanisms of diffusion may apply. One is molecular or static diffusion, which is caused by the random movement of the molecules in the stagnant fluid (De Roos 2000). Other is the eddy or convective diffusion, which transports elements of the fluid from one location to another, carrying with them the dissolved solute. The release of an active compound from a matrix-type delivery system may be controlled by diffusion, erosion or a combination of both. Homogenous and heterogeneous erosion are both detectable. Heterogeneous erosion occurs when degradation is confined to a thin layer at the surface of the delivery system, whereas homogenous erosion is a result of degradation occurring at a uniform rate throughout the polymer matrix (Pothakamury and Barbosa-Cánovas 1995).
The effect of relative humidity on the release of volatile compounds has been observed. At low humidity there is a slow release of host, keeping intact the structure of carbohydrate matrices as cyclodextrins (Ayala-Zavala et al. 2008). In matrices of maltodextrin/gum arabic capsules of ethyl butyrate produced by spray drying, the absorption of water at high RH destroyed the structure of the capsule causing the release of ethyl butyrate. The release rate and oxidation of D-limonene contained in starch matrices was associated with high levels of relative humidity and structural changes of the matrix encapsulating (Soottitantawat et al. 2004). In addition, Fabra et al. (2012) reported the release of n-hexanal and D-limonene from iota-carrageenan edible film (with and without lipid) in water. The release of both aroma compounds was studied at 25 and 37 °C. D-limonene was released quickly at higher temperatures. However, no effect of the temperature was observed on the release of n-hexanal. Thus, this work gives an idea of the release of aroma compounds encapsulated in iota-carrageenan films in aqueous media. On the other hand, in l-menthol added in gum arabic and modified starch, there was an increase in the rate of release by increasing the levels of relative humidity (Soottitantawat et al. 2004; Del Toro-Sánchez et al. 2010).
Food quality improvement by the use of bioactive compounds encapsulated with edible coatings
While an edible coating served as carrier of bioactive compound, it will provide an excellent vehicle to enhance foods quality and create functional products. In this sense, the incorporation of bioactive compounds such as antioxidants, antimicrobials, probiotics and flavors into edible coatings has been recently studied by various authors (Table 1).
Table 1.
Quality improvement of food coated with edible materials incorporated with bioactive compounds
| Bioactive compound | Encapsulating material | Quality improvement of food | Reference |
|---|---|---|---|
| Antioxidants | |||
| N-acetylcysteine and glutathione | Alginate based (2 % w/w) pectin based (2 % w/w) gellan based (0.5 % w/w) | Reduce microbial growth and preventing fresh-cut pears for browning | Oms-Oliu et al. (2008) |
| Essential oils | Chitosan | Protecting minimally processed squash from oxidation | Ponce et al. (2008) |
| Vitamin C and tea polyphenols | Alginate based | Retarded chemical spoilage, water loss and increased the overall sensory quality of fish | Song et al. (2010) |
| Vitamin E | Xanthan gum | Enhancing nutritional quality of peeled baby carrots. Vitamin E content increase from 0 to 67 % | Mei et al. (2002) |
| Ellagic acid | Candelilla wax | Reduce significantly the damage caused by C. gloesporioides and improve quality and shelf life of avocado | Saucedo-Pompa et al. (2009) |
| Ascorbic and citric acid | Comercial film coatings | Reducing browning and deterioration of fresh –cut mangoes | Gonzalez‐Aguilar et al. (2008) |
| Ascorbic acid and citric acid | Alginate | Highest antioxidant activity and preservation of the color of fresh-cut mangoes cubes | Robles-Sánchez et al. (2013) |
| Antimicrobials | |||
| Cinnamon, palmarosa and lemongrass oils | Alginate based | Improve shelf life of fresh-cut melon from microbiological (up to 9.6 days) and physicochemical (>14 days) | Raybaudi-Massilia et al. (2008) |
| Lemongrass, oregano oil and vanillin | Apple pure alginate | All coatings significantly inhibited growth of psychrophilic aerobes, yeasts and molds | Rojas-Graü et al. (2007) |
| Thyme essential oil | β-Cyclodextrin capsule | Reducing growth of Alternaria alternata | Del Toro-Sánchez et al. (2010) |
| Green tea extract | Tapioca starch/decolorized hsian-tsao leaf gum | Pronounced antimicrobial activity on Gram positive bacteria | Chiu and Lai (2010) |
| Oregano oil | Sorbitol-plasticized whey protein | Reduced specific growth rate of total flora and pseudomonads and inhibited completely the lactic acid bacteria growth in fresh beef | Zinoviadou et al. (2009) |
| Cinnamon leaf and garlic oil | β-Cyclodextrin capsule | Inhibiting growth of Alternaria alternata | Ayala-Zavala et al. (2008) |
| Probiotics | |||
| Bifidobacterium lactis Bb-12 | Alginate (0.5 w/w) and gellan film | Carrying viable probiotics on fresh-cut fruit | Tapia et al. (2007) |
| Lactobacillus acidophilus | Alginate | Protecting bacteria against low temperature (5 °C) during 8-day storage period | Moayednia et al. (2009). |
| Lactobacillus acidophilus | Sodium alginate | Protected probiotic cells against injuries in the freezing stage as well as, during frozen storage of yogurt ice cream | Ahmadi et al. (2012) |
| Lactobacillus rhamnosus GG | Alginate micro beads (10–40 μm) | Excellent survival in orange juice and potential reducing acidification | Sohail et al. (2012) |
| Lactobacillus acidophilus | Alginate | Increased the survival of probiotic in milk (stored 4 C for 50 days) or in acidic water (pH 2) | Shinde et al. (2013) |
| Flavors | |||
| Pandan leaf extract | 30 % sorbitol-plasticized rice starch | Flavor holding after 6 months of storage | Laohaknjit and Kerdchoechuen (2007) |
| Peppermint | Modified starch | Flavor holding and controlled release at different rates | Baranauskien et al. (2007) |
| Thymol and geraniol | β-Cyclodextrin capsule | Protecting against oxidation, remaining intact in temperatures at which monoterpenes were oxidized | Mourtzinos et al. (2008) |
| Limonene and β-unsaturated aldehydes | Gum acacia and modified starch | Improved retention during drying and limited flavor losses during storage after 28 days at 40 °C | Charve and Reineccius (2009) |
| n-hexanal and D-limonene | Iota-carrageenan matrix (with and without lipid) | Quickly releasing aroma compounds at higher temperatures (25 and 37 °C) | Fabra et al. (2012) |
| Linoleic acid and isoleucine | Alginate-calcium matrix | Increasing aroma production in apple wedges | Olivas et al. (2012) |
Antioxidants
Oms-Oliu et al. (2008) reported that the incorporation of N-acetylcysteine and glutathione into coating formulations was effective in preventing fresh-cut pears from browning for 2 weeks without affecting firmness of fruit wedges. Ponce et al. (2008) observed an improved of the antioxidant protection of the minimally processed squash using chitosan enriched with essential oils. Song et al. (2010) observed a retarded of fish spoilage using alginate-based edible coating containing vitamin C and tea polyphenols for shelf life extension. Saucedo-Pompa et al. (2009) reported that the use of ellagic acid into candelilla wax matrix has an important effect to improve the quality and shelf life of avocado. Gonzalez‐Aguilar et al. (2008) studied different treatments to inhibit browning, decay and to extend shelf life of “Keitt”, “Kent” and “Ataulfo” fresh-cut mango. Combinations of calcium chloride (CaCl2), antioxidants (ascorbic acid, citric acid) and two commercial film coatings resulted in a reduction of browning and deterioration of fresh-cut mangoes stored at 5 °C, especially for the “Ataulfo” cultivar. Robles-Sánchez et al. (2013) used alginate-based coating as carrier of ascorbic and citric acid which enhanced nutritional quality and preserved the color of fresh-cut mangoes and increased the antioxidant potential of cubes.
Antimicrobials
Raybaudi-Massilia et al. (2008) reported the effectiveness of palmarosa oil (0.3 %) into the coating to preserve fresh-cut melon. Rojas-Graü et al. (2007) investigated the effect of lemongrass, oregano oil and vanillin incorporated in apple puree-alginate edible coatings, oregano oil showed effectiveness against E. coli O157:H7. Del Toro-Sánchez et al. (2010) reported the encapsulation of thyme essential oil in a β-cyclodextrin (β-CD) to be used as a trigger to release antimicrobial thyme oil volatiles, reducing microbial growth and the sensory effect of the treatment caused by the free thyme oil. Chiu and Lai (2010) reported the efficacy of antimicrobial edible coatings based on a tapioca starch/decolorized hsian-tsao leaf gum matrix with various green tea extracts to preserve various types of salads. Zinoviadou et al. (2009) reported the effectiveness of oregano oil containing whey protein coatings to increase the shelf life of fresh beef. Ayala-Zavala et al. (2008) studied microencapsulation of cinnamon leaf (CLO) and garlic oil (GO) with β-cyclodextrin (β-CD) as good antimicrobials. Microcapsules displayed good antifungal activity against Alternaria alternata. Therefore, CLO and GO microcapsules can have important applications in the food industry as stable natural antimicrobial compound systems. Azarakhsh et al. (2014) reported that an alginate-based edible coating formulation incorporated with 0.3 % (w/v) lemongrass has potential to extend the shelf-life and maintain quality of fresh-cut pineapple, decreasing significantly (p < 0.05) the yeast and mould counts.
Probiotics
Tapia et al. (2007) developed the first edible films for probiotic coatings on fresh-cut apple and papaya, observing that both fruits were successfully coated with alginate or gellan film-forming solutions containing viable bifidobacteria. In fact, values higher than >10−6 cfu/g Bifidobacterium lactis Bb-12 were maintained for 10 days during refrigerated storage of both papaya and apple pieces, demonstrating the feasibility of these polysaccharide coatings to carry and support viable probiotics on fresh-cut fruit. Another study related to probiotics and coating is the immobilization of Lactobacillus acidophilus in the alginate coating surface by using two different concentrations of sodium alginate solutions (2 and 3 % v/w). Micrographs along with the microbiological analysis of two samples revealed the higher load of probiotic bacteria in fruits with higher concentration of sodium alginate in their coating formulations over 8-day study period at 5 °C. The viable count of entrapped L. acidophilus in fruit coating materials did not change significantly throughout the storage period at 5 °C. So it can be said that immobilization of L. acidophilus in alginate matrix of strawberry coating effectively protected bacteria against the low temperature storage (Moayednia et al. 2009).
Recently Nasrin Moayednia et al. (2010) immobilized Lactobacillus acidophilus and Bifidobacterium lactis in calcium alginate and coated strawberries by using 2 % (w/v) concentration of sodium alginate solution. The maximum reduction of the viability of Lactobacillus acidophilus in the final day of storage was 0.31 log. The viable count of entrapped bacteria in fruit coating materials did not change significantly throughout the storage period at 5 °C. Results indicated that this probiotic immobilization technique more effectively protected the viability of Lactobacillus acidophilus than of Bifidobacterium lactis against effects of storage environment. López de Lacey et al. (2012) incorporated probiotic bacteria Lactobacillus acidophilus and Bifidobacterium bifidum into gelatin edible coatings applied to fish to assess its effect during chilled storage. B. bifidum remained viable during the storage and H2S-producing microorganisms were reduced in 2 log cycles. In a further experiment, fish coated with gelatin films incorporated with bifidobacteria was treated with a high pressure (200 MPa/10 min/20 °C). At the end of the storage, a reduction of total viable counts (<2 log cycles) were obtained. Moreover, the combined treatments drastically reduced the content of volatile bases and lowered the pH by more than 1 unit. Therefore, the application of gelatin edible packaging incorporated with bacteria can be promising for fish preservation, especially when combined with other technologies such as a high-pressure. Ahmadi et al. (2012) produced yogurt-ice cream via incorporation of microencapsulated Lactobacillus acidophilus (Ia-5) into alginate microbeads. The viable counts of free probiotics decreased from 9.55 to ~7.3 log cfu/g after 60 days of frozen storage while that of encapsulated cells merely decreased less than 1 log cycle. Furthermore, Amine et al. (2014) evaluated the encapsulation in alginate beads on the viability of Bifidobacterium longum 15708 in terms of cheddar cheese manufacturing and storage as well as to a simulated gastro-intestinal environment. Cheddar cheese containing encapsulated B. longum in alginate showed a good survival with 2 log CFU/mL reduction after 21 days. The immobilized bacteria were also more resistant than free cells to simulated gastric and intestinal environments by a factor of 30. Encapsulation matrix protected the probiotic cells against injuries in the freezing stage as well as, during frozen storage. These studies represent promising advances in the search for new applications of edible films and coatings as carriers of diverse probiotics, and open new possibilities for the development of novel food with probiotic products.
Flavors
Encapsulation of flavors by the use of edible coatings represents an effective method for adding flavors to foods, which allows control flavor loss, and controlled release. Laohaknjit and Kerdchoechuen (2007) coated non-aromatic milled rice with 30 % sorbitol-plasticized rice starch, containing 25 % natural pandan leaf extract (Pandanus amaryllifolius Roxb.). This extract is responsible for the jasmine aroma of aromatic rice. The rice starch coating containing natural pandan extract produced rice with aroma compounds similar to that of aromatic rice. Baranauskien et al. (2007) reported the flavor retention of the essential oil of peppermint (Mentha piperita L.) in different commercial modified food starch. Flavor compounds were released at different rates from each of the encapsulated products.
Mourtzinos et al. (2008) reported the incorporation of thymol and geraniol in β-cyclodextrin and modified starch. Both matrices showed protection against oxidation of thymol and geraniol, remaining intact in temperatures at which free monoterpenes were oxidized. Charve and Reineccius (2009) reported the potential use of gum acacia and modified starch for the encapsulation of limonene and β-unsaturated aldehydes ((E)-2-hexenal, (E)-cinnamaldehyde, citral). Fabra et al. (2012) analyzed the release of n-hexanal and D-limonene from edible coatings. Aroma compounds were released easily in water medium in which D-limonene was released quickly at higher temperatures (37 °C). Therefore, these coating technologies represent a promising approach for improving food aroma or flavor.
Another way to add bioactive compounds in order to increase flavor of coated foods, is by the application of flavor precursors that, once in contact with the food, may react with food components to produce flavoring compounds (Olivas et al. 2007). Alginate-calcium coatings were used as a holding matrix for linoleic acid and isoleusine on apple wedges, and an increase on the concentration of aroma compounds was observed. This is possibly due to the metabolization of linoleic acid and isoleusine by the fruit producing compounds such as hexanal. trans-2-hexenal, 2-methyl-1-butanol, 2-methyl-butyl acetate (Olivas et al. 2012).
Other bioactive compounds
There are other bioactive compounds that could be encapsulated into edible coatings to improve the functionality and enhance the nutritional value of foods; particularly minerals, vitamins, phytosterols, lutein, fatty acids and lycopene. The edible coating promotes the delivery of vitamins and minerals to foods mainly by preventing their interaction with other food components; for example, iron bioavailability is commonly affected by interactions with food ingredients (Lynch 1997). Additionally, iron catalyzes the oxidative degradation of fatty acids and vitamins. Calcium lactate encapsulation in lecithin liposomes it was possible to fortify soymilk with levels of calcium equivalent to those found in cow’s milk (Hirotsuka et al. 1984). Moreover, omega-3 and omega-6 fatty acids have been encapsulated in edible coatings for food fortification with considerable human health benefits. Efraim et al. (2011) reported that different types of phytosterols added to chocolate improved the shelf-life and prevent the oxidation. Furthermore, several studies reported the encapsulation of lutein, a natural pigment widely found in fruits, vegetables, flowers and some algae, that play a beneficial role in the prevention of cardiovascular disease, stroke, lung cancer and breast cancer (Ribaya-Mercado and Blumberg 2004; Riccioni 2009). Lutein is unstable against light and heat, and has a low-water solubility, poor absorption and low bioavailability (Granado-Lorencio et al. 2010). Therefore, it is necessary to protect it from light degradation and improve its aqueous solubility; edible coatings could be a good strategy. In this context, is widely know that other types of bioactives may also be encapsulated into edible coatings for obtaining desired characters and add functionality to the foods.
Conclusions
The potential of edible coatings has been well recognized by many research groups as an alternative to conventional packaging and to enhance food protection. However, among the main advantages of using edible films and coatings is that several bioactive compounds, such antioxidants, antimicrobials, flavors and probiotics, can be incorporated into the polymer matrix and consumed with the food, enhancing safety or even better nutritional and sensory attributes. Indeed, the incorporation of bioactive compounds into edible coatings can be an alternative to protect them from the environment or the surrounding food, while allowing controlled released. Thus, is a solving to the disadvantages of direct application of these compounds. However, when bioactive ingredients are added to edible coatings, mechanical, sensory and even functional properties can be dramatically affected. Therefore, studies on this subject are required in order to develop new coating applications with improved functionality and high sensory performance.
References
- Ahmadi A, Milani E, Madadlou A, Mortazavi SA, Mokarram RR, Salarbashi D (2012) Synbiotic yogurt-ice cream produced via incorporation of microencapsulated lactobacillus acidophilus (la-5) and fructooligosaccharide. J Food Sci Technol:1–7. [DOI] [PMC free article] [PubMed]
- Amine KM, Champagne CP, Raymond Y, St-Gelais D, Britten M, Fustier P, Salmieri S, Lacroix M. Survival of microencapsulated Bifidobacterium longum in Cheddar cheese during production and storage. Food Control. 2014;37:193–199. doi: 10.1016/j.foodcont.2013.09.030. [DOI] [Google Scholar]
- Ayala Zavala J, Del Toro Sánchez L, Álvarez Parrilla E, González Aguilar G. High relative humidity in package of fresh cut fruits and vegetables: advantage or disadvantage considering microbiological problems and antimicrobial delivering systems? J Food Sci. 2008;73(4):R41–R47. doi: 10.1111/j.1750-3841.2008.00705.x. [DOI] [PubMed] [Google Scholar]
- Ayala-Zavala J, Soto-Valdez H, González-León A, Álvarez-Parrilla E, Martín-Belloso O, González-Aguilar G. Microencapsulation of cinnamon leaf (Cinnamomum zeylanicum) and garlic (Allium sativum) oils in -cyclodextrin. J Incl Phenom Macro. 2008;60(3):359–368. doi: 10.1007/s10847-007-9385-1. [DOI] [Google Scholar]
- Ayala-Zavala JF, González-Aguilar GA, Del-Toro-Sánchez L. Enhancing safety and aroma appealing of fresh-cut fruits and vegetables using the antimicrobial and aromatic power of essential oils. J Food Sci. 2009;74(7):R84–R91. doi: 10.1111/j.1750-3841.2009.01294.x. [DOI] [PubMed] [Google Scholar]
- Ayala-Zavala JF, Vega-Vega V, Rosas-Domínguez C, Palafox-Carlos H, Villa-Rodríguez JA, Siddiqui MW, Dávila-Avina JE, González-Aguilar GA. Agro-industrial potential of exotic fruit byproducts as a source of food additives. Food Res Int. 2011;44(7):1866–1874. doi: 10.1016/j.foodres.2011.02.021. [DOI] [Google Scholar]
- Azarakhsh N, Osman A, Ghazali HM, Tan CP, Mohd Adzahan N. Lemongrass essential oil incorporated into alginate-based edible coating for shelf-life extension and quality retention of fresh-cut pineapple. Postharvest Biol Technol. 2014;88:1–7. doi: 10.1016/j.postharvbio.2013.09.004. [DOI] [Google Scholar]
- Baldwin EA, Hagenmaier RD, Bai J. Edible coatings and films to improve food quality. Boca Raton: CRC Press Llc; 2012. [Google Scholar]
- Baranauskien R, Bylait E, Žukauskait J, Venskutonis RP. Flavor retention of peppermint (Mentha piperita L.) essential oil spray-dried in modified starches during encapsulation and storage. J Agr Food Chem. 2007;55(8):3027–3036. doi: 10.1021/jf062508c. [DOI] [PubMed] [Google Scholar]
- Biquet B, Labuza T. Evaluation of the moisture permeability characteristics of chocolate films as an edible moisture barrier. J Food Sci. 1988;53(4):989–998. doi: 10.1111/j.1365-2621.1988.tb13516.x. [DOI] [Google Scholar]
- Bourtoom T. Edible films and coatings: characteristics and properties. Int Food Res J. 2008;15(3):237–248. [Google Scholar]
- Champagne CP, Fustier P. Microencapsulation for the improved delivery of bioactive compounds into foods. Curr Opin Biotechnol. 2007;18(2):184–190. doi: 10.1016/j.copbio.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Charve J, Reineccius GA. Encapsulation performance of proteins and traditional materials for spray dried flavors. J Agr Food Chem. 2009;57(6):2486–2492. doi: 10.1021/jf803365t. [DOI] [PubMed] [Google Scholar]
- Chichlowski M, Croom J, McBride B, Havenstein G, Koci M. Metabolic and physiological impact of probiotics or direct-fed-microbials on poultry: a brief review of current knowledge. Int J Poult Sci. 2007;6(10):694–704. doi: 10.3923/ijps.2007.694.704. [DOI] [Google Scholar]
- Chiu P, Lai L. Antimicrobial activities of tapioca starch/decolorized hsian-tsao leaf gum coatings containing green tea extracts in fruit-based salads, romaine hearts and pork slices. Int J Food Microbiol. 2010;139(1–2):23–30. doi: 10.1016/j.ijfoodmicro.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Cuq B, Gontard N, Guilbert S (1995) Edible films and coatings as active layers. In: Active food packaging. Springer, pp 111–142
- De Kruif CG, Weinbreck F, de Vries R. Complex coacervation of proteins and anionic polysaccharides. Curr Opin Colloid Interface Sci. 2004;9(5):340–349. doi: 10.1016/j.cocis.2004.09.006. [DOI] [Google Scholar]
- De Roos KB (2000) Physicochemical models of flavor release from foods. In: ACS symposium series, ACS Publications, pp 126–141
- Debeaufort F, Voilley A. Edible films and coatings for food applications. New York: Springer; 2009. [Google Scholar]
- Del Toro-Sánchez C, Ayala-Zavala J, Machi L, Santacruz H, Villegas-Ochoa M, Álvarez-Parrilla E, González-Aguilar G. Controlled release of antifungal volatiles of thyme essential oil from -cyclodextrin capsules. J Incl Phenom Macro. 2010;67(3):431–441. doi: 10.1007/s10847-009-9726-3. [DOI] [Google Scholar]
- Desobry S, Debeaufort F (2012) 11 Encapsulation of flavors, nutraceuticals, and antibacterials. In: Bai J (ed) Edible Coatings and Films to Improve Food Quality. p 333
- Dhall R. Advances in edible coatings for fresh fruits and vegetables: a review. Crit Rev Food Sci. 2013;53(5):435–450. doi: 10.1080/10408398.2010.541568. [DOI] [PubMed] [Google Scholar]
- Durand L, Habran N, Henschel V, Amighi K. Encapsulation of ethylhexyl methoxycinnamate, a light-sensitive UV filter, in lipid nanoparticles. J Microencapsul. 2010;27(8):714–725. doi: 10.3109/02652048.2010.513455. [DOI] [PubMed] [Google Scholar]
- Efraim P, Marson GC, Jardim DC, Garcia AO, Yotsuynagi K. Influence of phytosterols addition in the rheology and sensory attributes of dark chocolate. Proc Food Sci. 2011;1:1633–1637. doi: 10.1016/j.profoo.2011.09.241. [DOI] [Google Scholar]
- Espitia PJP, Du W-X, Avena-Bustillos RJ, NdFF S, McHugh T. Edible films from pectin: physical-mechanical and antimicrobial properties-a review. Food Hydrocoll. 2013;35:287–296. doi: 10.1016/j.foodhyd.2013.06.005. [DOI] [Google Scholar]
- Fabra MJ, Chambin O, Voilley A, Gay JP, Debeaufort F. Influence of temperature and NaCl on the release in aqueous liquid media of aroma compounds encapsulated in edible films. J Food Eng. 2012;108(1):30–36. doi: 10.1016/j.jfoodeng.2011.07.035. [DOI] [Google Scholar]
- Falguera V, Quintero JP, Jimenez A, Munoz JA, Ibarz A. Edible films and coatings: structures, active functions and trends in their use. Trends Food Sci Technol. 2011;22(6):292–303. doi: 10.1016/j.tifs.2011.02.004. [DOI] [Google Scholar]
- Fang Z, Bhandari B. Encapsulation of polyphenols-a review. Trends Food Sci Technol. 2010;21(10):510–523. doi: 10.1016/j.tifs.2010.08.003. [DOI] [Google Scholar]
- Ferreira I, Rocha S, Coelho M. Encapsulation of antioxidants by spray-drying. Chem Eng Trans. 2007;11(2):713–717. [Google Scholar]
- Fisher K, Phillips C. Potential antimicrobial uses of essential oils in food: is citrus the answer? Trends Food Sci Technol. 2008;19(3):156–164. doi: 10.1016/j.tifs.2007.11.006. [DOI] [Google Scholar]
- Franz C, Stiles M, Schleifer K, Holzapfel W. Enterococci in foods–a conundrum for food safety. Int J Food Microbiol. 2003;88(2–3):105–122. doi: 10.1016/S0168-1605(03)00174-0. [DOI] [PubMed] [Google Scholar]
- González Aguilar GA, Celis J, Sotelo Mundo RR, De La Rosa LA, Rodrigo García J, Álvarez Parrilla E. Physiological and biochemical changes of different fresh cut mango cultivars stored at 5 °C. Int J Food Sci Technol. 2008;43(1):91–101. doi: 10.1111/j.1365-2621.2006.01394.x. [DOI] [Google Scholar]
- Granado-Lorencio F, Herrero-Barbudo C, Olmedilla-Alonso B, Blanco-Navarro I, Pérez-Sacristán B. Lutein bioavailability from lutein ester-fortified fermented milk: in vivo and in vitro study. J Nutr Biochem. 2010;21(2):133–139. doi: 10.1016/j.jnutbio.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Guerrero P, Retegi A, Gabilondo N, De la Caba K. Mechanical and thermal properties of soy protein films processed by casting and compression. J Food Eng. 2010;100(1):145–151. doi: 10.1016/j.jfoodeng.2010.03.039. [DOI] [Google Scholar]
- Hernández Izquierdo V, Krochta J. Thermoplastic processing of proteins for film formation—a review. J Food Sci. 2008;73(2):30–39. doi: 10.1111/j.1750-3841.2007.00636.x. [DOI] [PubMed] [Google Scholar]
- Hirotsuka M, Taniguchi H, Narita H, Kito M. Calcium fortification of soy milk with calcium lecithin liposome system. J Food Sci. 1984;49(4):1111–1112. doi: 10.1111/j.1365-2621.1984.tb10405.x. [DOI] [Google Scholar]
- Janjarasskul T, Krochta J. Edible packaging materials. Annu Rev Food Sci Technol. 2010;1:415–448. doi: 10.1146/annurev.food.080708.100836. [DOI] [PubMed] [Google Scholar]
- Kayaci F, Uyar T. Encapsulation of vanillin/cyclodextrin inclusion complex in electrospun polyvinyl alcohol (PVA) nanowebs: prolonged shelf-life and high temperature stability of vanillin. Food Chem. 2012;133(3):641–649. doi: 10.1016/j.foodchem.2012.01.040. [DOI] [Google Scholar]
- Kester J, Fennema O. Edible films and coatings: a review. Food Technol. 1986;40:47–59. [Google Scholar]
- Kolanowski W, Laufenberg G, Kunz B. Fish oil stabilisation by microencapsulation with modified cellulose. Int J Food Sci Nutr. 2004;55(4):333–343. doi: 10.1080/09637480410001725157. [DOI] [PubMed] [Google Scholar]
- Kris-Etherton PM, Hecker KD, Bonanome A, Coval SM, Binkoski AE, Hilpert KF, Griel AE, Etherton TD. Bioactive compounds in foods: their role in the prevention of cardiovascular disease and cancer. Am J Med. 2002;113(9):71–88. doi: 10.1016/S0002-9343(01)00995-0. [DOI] [PubMed] [Google Scholar]
- Krochta JM, Nisperos-Carriedo MO. Edible coatings and films to improve food quality. Boca Raton: CRC Press Llc; 1994. [Google Scholar]
- Lahtinen SJ. Probiotic viability–does it matter? Microb Ecol Health Dis. 2012;23:10–14. doi: 10.3402/mehd.v23i0.18567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laohakunjit N, Kerdchoechuen O. Aroma enrichment and the change during storage of non-aromatic milled rice coated with extracted natural flavor. Food Chem. 2007;101(1):339–344. doi: 10.1016/j.foodchem.2005.12.055. [DOI] [Google Scholar]
- López de Lacey A, López-Caballero M, Gómez-Guillén M, Montero P. Functionality of Lactobacillus acidophilus and Bifidobacterium bifidum incorporated to edible coatings and films. Innov Food Sci Emerg Technol. 2012;16:277–282. doi: 10.1016/j.ifset.2012.07.001. [DOI] [Google Scholar]
- Lynch SR. Interaction of iron with other nutrients. Nutr Rev. 1997;55(4):102–110. doi: 10.1111/j.1753-4887.1997.tb06461.x. [DOI] [PubMed] [Google Scholar]
- Madene A, Jacquot M, Scher J, Desobry S. Flavour encapsulation and controlled release–a review. Int J Food Sci Technol. 2006;41(1):1–21. doi: 10.1111/j.1365-2621.2005.00980.x. [DOI] [Google Scholar]
- Mei Y, Zhao Y, Yang J, Furr H. Using edible coating to enhance nutritional and sensory qualities of baby carrots. J Food Sci. 2002;67(5):1964–1968. doi: 10.1111/j.1365-2621.2002.tb08753.x. [DOI] [Google Scholar]
- Milanovic J, Manojlovic V, Levic S, Rajic N, Nedovic V, Bugarski B. Microencapsulation of flavors in carnauba wax. Sensor. 2010;10(1):901–912. doi: 10.3390/s100100901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moayednia N, Ehsani MR, Emamdjomeh Z, Asadi MM, Mizani M, Mazaheri AF. The Effect of sodium alginate concentrations on viability of immobilized lactobacillus acidophilus in fruit alginate coating during refrigerator storage. Aust J Basic Appl Sci. 2009;3(4):3213–3226. [Google Scholar]
- Moayednia N, Ehsani MR, Emamdjomeh Z, Asadi MM, Mizani M, Mazaheri AF. Effect of refrigeration on viability of immobilized probiotic bacteria in alginate coat of strawberry. World Appl Sci J. 2010;10(4):472–476. [Google Scholar]
- Mourtzinos I, Kalogeropoulos N, Papadakis S, Konstantinou K, Karathanos V. Encapsulation of nutraceutical monoterpenes in cyclodextrin and modified starch. J Food Sci. 2008;73(1):89–94. doi: 10.1111/j.1750-3841.2007.00609.x. [DOI] [PubMed] [Google Scholar]
- Muranyi P. Functional edible coatings for fresh food products. J Food Process Technol. 2013;4:e114. [Google Scholar]
- Muthukumarasamy P, Holley R. Microbiological and sensory quality of dry fermented sausages containing alginate-microencapsulated Lactobacillus reuteri. Int J Food Microbiol. 2006;111(2):164–169. doi: 10.1016/j.ijfoodmicro.2006.04.036. [DOI] [PubMed] [Google Scholar]
- Nyström L, Achrenius T, Lampi A, Moreau R, Piironen V. A comparison of the antioxidant properties of steryl ferulates with tocopherol at high temperatures. Food Chem. 2007;101(3):947–954. doi: 10.1016/j.foodchem.2006.02.046. [DOI] [Google Scholar]
- Olivas G, Mattinson D, Barbosa-Cánovas G. Alginate coatings for preservation of minimally processed ‘Gala’ apples. Postharvest Biol Tecnol. 2007;45(1):89–96. doi: 10.1016/j.postharvbio.2006.11.018. [DOI] [Google Scholar]
- Olivas G, Maya I, Espino-Díaz J, Molina-Corral J, Olivas-Dorantes C, Sepulveda D (2012) Metabolization of linoleic acid and isoleucine for aroma production in fresh-cut ‘Golden Delicious’ apples using alginate coatings as the holding matrix. Paper presented at the Institute of Food Technologists IFT Annual Meeting, Las Vegas, NV, E.U
- Oliveira DM, Kwiatkowski A, Rosa CILF, Clemente E (2012) Refrigeration and edible coatings in blackberry (Rubus spp.) conservation. J Food Sci Technol:1–7. [DOI] [PMC free article] [PubMed]
- Oms-Oliu G, Soliva-Fortuny R, Martín-Belloso O. Edible coatings with antibrowning agents to maintain sensory quality and antioxidant properties of fresh-cut pears. Postharvest Biol Technol. 2008;50(1):87–94. doi: 10.1016/j.postharvbio.2008.03.005. [DOI] [Google Scholar]
- Partanen R, Ahro M, Hakala M, Kallio H, Forssell P. Microencapsulation of caraway extract in ß-cyclodextrin and modified starches. Eur Food Res Technol. 2002;214(3):242–247. doi: 10.1007/s00217-001-0446-1. [DOI] [Google Scholar]
- Pascall MA, Lin S-J. The application of edible polymeric films and coatings in the food industry. J Food Process Technol. 2013;4(2):e116. [Google Scholar]
- Pidcock K, Heard G, Henriksson A. Application of nontraditional meat starter cultures in production of hungarian salami. Int J Food Microbiol. 2002;76(1–2):75–81. doi: 10.1016/S0168-1605(02)00002-8. [DOI] [PubMed] [Google Scholar]
- Ponce A, Roura S, del Valle C, Moreira M. Antimicrobial and antioxidant activities of edible coatings enriched with natural plant extracts: in vitro and in vivo studies. Postharvest Biol Technol. 2008;49(2):294–300. doi: 10.1016/j.postharvbio.2008.02.013. [DOI] [Google Scholar]
- Pothakamury UR, Barbosa-Cánovas GV. Fundamental aspects of controlled release in foods. Trends Food Sci Technol. 1995;6(12):397–406. doi: 10.1016/S0924-2244(00)89218-3. [DOI] [Google Scholar]
- Ranadheera R, Baines S, Adams M. Importance of food in probiotic efficacy. Food Res Int. 2010;43(1):1–7. doi: 10.1016/j.foodres.2009.09.009. [DOI] [Google Scholar]
- Rauha JP, Remes S, Heinonen M, Hopia A, Kähkönen M, Kujala T, Pihlaja K, Vuorela H, Vuorela P. Antimicrobial effects of Finnish plant extracts containing flavonoids and other phenolic compounds. Int J Food Microbiol. 2000;56(1):3–12. doi: 10.1016/S0168-1605(00)00218-X. [DOI] [PubMed] [Google Scholar]
- Ravichandran K, Palaniraj R, Saw NMMT, Gabr AMM, Ahmed AR, Knorr D, Smetanska I (2012) Effects of different encapsulation agents and drying process on stability of betalains extract. J Food Sci Technol:1–6. [DOI] [PMC free article] [PubMed]
- Raybaudi-Massilia RM, Mosqueda-Melgar J, Martín-Belloso O. Edible alginate-based coating as carrier of antimicrobials to improve shelf-life and safety of fresh-cut melon. Int J Food Microbiol. 2008;121(3):313–327. doi: 10.1016/j.ijfoodmicro.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Ribaya-Mercado JD, Blumberg JB. Lutein and zeaxanthin and their potential roles in disease prevention. J Am Coll Nutr. 2004;23(sup6):567S–587S. doi: 10.1080/07315724.2004.10719427. [DOI] [PubMed] [Google Scholar]
- Riccioni G. Carotenoids and cardiovascular disease. Curr Atheroscler Rep. 2009;11(6):434–439. doi: 10.1007/s11883-009-0065-z. [DOI] [PubMed] [Google Scholar]
- Robles-Sánchez RM, Rojas-Graü MA, Odriozola-Serrano I, González-Aguilar G, Martin-Belloso O. Influence of alginate-based edible coating as carrier of antibrowning agents on bioactive compounds and antioxidant activity in fresh-cut Kent mangoes. LWT-Food Sci Technol. 2013;50(1):240–246. doi: 10.1016/j.lwt.2012.05.021. [DOI] [Google Scholar]
- Rodgers S. Incorporation of probiotic cultures in foodservice products: an exploratory study. J Food Serv. 2007;18(3):108–118. doi: 10.1111/j.1745-4506.2007.00055.x. [DOI] [Google Scholar]
- Rojas-Graü M, Raybaudi-Massilia R, Soliva-Fortuny R, Avena-Bustillos R, McHugh T, Martín-Belloso O. Apple puree-alginate edible coating as carrier of antimicrobial agents to prolong shelf-life of fresh-cut apples. Postharvest Biol Technol. 2007;45(2):254–264. doi: 10.1016/j.postharvbio.2007.01.017. [DOI] [Google Scholar]
- Salminen S, Von Wright A. Lactic acid bacteria: microbiological and functional aspects. Boca Raton: CRC Press; 2011. [Google Scholar]
- Salminen S, Ouwehand A, Benno Y, Lee Y. Probiotics: how should they be defined? Trends Food Sci Technol. 1999;10(3):107–110. doi: 10.1016/S0924-2244(99)00027-8. [DOI] [Google Scholar]
- Saucedo-Pompa S, Rojas-Molina R, Aguilera-Carbó AF, Saenz-Galindo A, Garza HL, Jasso-Cantú D, Aguilar CN. Edible film based on candelilla wax to improve the shelf life and quality of avocado. Food Res Int. 2009;42(4):511–515. doi: 10.1016/j.foodres.2009.02.017. [DOI] [Google Scholar]
- Sereno N, Hill S, Taylor A, Mitchell J, Davies S. Aroma permeability of hydroxypropyl maize starch films. J Agr Food Chem. 2009;57(3):985–990. doi: 10.1021/jf8036775. [DOI] [PubMed] [Google Scholar]
- Sharafi H, Alidost L, Lababpour A, Zahiri HS, Abbasi H, Vali H, Noghabi KA (2013) Antibacterial activity of probiotic lactobacillus plantarum HK01: effect of divalent metal cations and food additives on production efficiency of antibacterial compounds. Probiotics Antimicrob Proteins:1–10. [DOI] [PubMed]
- Shinde T, Sun-Waterhouse D, Brooks J (2013) Co-extrusion encapsulation of probiotic lactobacillus acidophilus alone or together with apple skin polyphenols: an aqueous and value-added delivery system using alginate. Food Bioprocess Technol:1–16.
- Silva-Weiss A, Ihl M, Sobral P, Gómez-Guillén M, Bifani V. Natural additives in bioactive edible films and coatings: functionality and applications in foods. Food Eng Rev. 2013;5(4):200–216. doi: 10.1007/s12393-013-9072-5. [DOI] [Google Scholar]
- Skurtys O, Acevedo C, Pedreschi F, Enrione J, Osorio F, Aguilera J. Food hydrocolloid edible films and coatings. Hauppauge: Nova Science Pub Inc; 2010. [Google Scholar]
- Sohail A, Turner MS, Prabawati EK, Coombes AG, Bhandari B. Evaluation of Lactobacillus rhamnosus GG and Lactobacillus acidophilus NCFM encapsulated using a novel impinging aerosol method in fruit food products. Int J Food Microbiol. 2012;157(2):162–166. doi: 10.1016/j.ijfoodmicro.2012.04.025. [DOI] [PubMed] [Google Scholar]
- Song Y, Liu L, Shen H, You J, Luo Y. Effect of sodium alginate-based edible coating containing different antioxidants on quality and shelf life of refrigerated bream (Megalobrama amblycephala) Food Control. 2010;22(3–4):608–615. [Google Scholar]
- Soottitantawat A, Yoshii H, Furuta T, Ohgawara M, Forssell P, Partanen R, Poutanen K, Linko P. Effect of water activity on the release characteristics and oxidative stability of d-limonene encapsulated by spray drying. J Agric Food Chem. 2004;52(5):1269–1276. doi: 10.1021/jf035226a. [DOI] [PubMed] [Google Scholar]
- Souza T, Silva A, Drews J, Gomes D, Vinderola C, Nicoli J. In vitro evaluation of Bifidobacterium strains of human origin for potential use in probiotic functional foods. Benefic Microbes. 2013;4(2):179–186. doi: 10.3920/BM2012.0052. [DOI] [PubMed] [Google Scholar]
- Tajkarimi MM, Ibrahim SA, Cliver DO. Antimicrobial herb and spice compounds in food. Food Control. 2010;21(9):1199–1218. doi: 10.1016/j.foodcont.2010.02.003. [DOI] [Google Scholar]
- Tapia M, Rojas Graü M, Rodríguez F, Ramírez J, Carmona A, Martin Belloso O. Alginate and gellan based edible films for probiotic coatings on fresh cut fruits. J Food Sci. 2007;72(4):190–196. doi: 10.1111/j.1750-3841.2007.00318.x. [DOI] [PubMed] [Google Scholar]
- Viskupicova J, Danihelova M, Ondrejovic M, Liptaj T, Sturdik E. Lipophilic rutin derivatives for antioxidant protection of oil-based foods. Food Chem. 2010;123(1):45–50. doi: 10.1016/j.foodchem.2010.03.125. [DOI] [Google Scholar]
- Zinoviadou KG, Koutsoumanis KP, Biliaderis CG. Physico-chemical properties of whey protein isolate films containing oregano oil and their antimicrobial action against spoilage flora of fresh beef. Meat Sci. 2009;82(3):338–345. doi: 10.1016/j.meatsci.2009.02.004. [DOI] [PubMed] [Google Scholar]