Abstract
Physicochemical properties of composite flours made of wheat from low to high protein contents and canna or konjac at different levels of substitution (0, 25, 50, 75, and 100 %) were prepared and analyzed. Compared to that of wheat flour alone, increasing levels of canna inclusions significantly increased the amount of resistant starch (RS) but decreased the protein content of composite flours. This substitution did not alter the total starch (TS), amylase and amylopectin contents of these mixtures. Changes in physicochemical properties were also observed in wheat-konjac composite flours. Increasing amounts of konjac flour decreased the TS, amylase, amylopectin, and protein content of the mixtures, but increased the amount of RS. Substitution of wheat flour with 75 % of canna or konjac flours in HPWC (High Protein Wheat-Canna), HPWK (High Protein Wheat-Konjac), and LPWK (Low Protein Wheat-Konjac) increased the swelling power of these mixtures at 80 and 90 °C. In general, substitution of wheat flour with up to 50 % of canna or konjac flours did not cause any other observable decline. In addition, the substitution of wheat flour with canna or konjac flours increased the gelatinization temperature of all composite flours.
Keywords: Composite flour, Physicochemical properties, HPWC, HPWK, LPWC, LPWK
Introduction
Indonesia has many tropical root and tuber crops that can be used as alternative sources of carbohydrate. These crops include taro (Colocasia esculenta), yam (Dioscorea alata), sweet potato (Ipomoea batatas), canna (Canna edulis), konjac (Amorphophallus campanulatus), arrowroot (Marantha arundinaceae), and cassava (Manihot uttilisima) (Deshaliman 2003). Flours and starches isolated from roots and tubers of these plants usually have higher viscosity compared to that of cereal flours, which allows them to be used as thickening or gelling agents in some food products. In addition, these isolated materials have higher paste clarity, which is important for certain food applications. Compared to cereal flours and starches, these materials also have less starchy flavors (Radley 1976; Moorthy 2002). Moreover, these materials also offer health benefits especially in preventing obesity, constipation, cardiovascular disease, diabetes and colon cancer due to the high amount of fiber content (Chen et al. 2003). The absence of gluten within these materials may help provide nutrition for those who have celiac disease (CD) (Hung and Morita 2005).
Except for cassava and sweet potato, many of these starchy materials have not been utilized optimally due to the lack of knowledge of suitable processing techniques and product development (Deshaliman 2003; Nasution 2003). Canna and konjac are among these underutilized roots and tubers even though they have been consumed as a source of carbohydrate by local people in rural areas. Flour and starch isolated from canna and konjac rhizomes have unique characteristics that may be important for use in some food applications (Thitipraphunkul et al. 2003; Hung and Morita 2005). Canna starch has a large particle size, high amylose and phosphate content, as well as a high paste viscosity and clarity (Piyachomkwan et al. 2002; Santacruz et al. 2003; Thitipraphunkul et al. 2003; Srichuwong et al. 2005; Puncha-arnon et al. 2008). Due to its high paste clarity, canna starch has been used for making transparent noodles. In addition, the high amount of resistant starch of canna flour may be beneficial in preventing cardiovascular and other related diseases (Srichuwong et al. 2005). Konjac flour has a high viscosity, which may allow the application of this flour to be used for thickening, as a gelling agent and for water binding in food products (Nishinari et al. 1992).
Despite the availability of starchy materials derived from roots and tubers as mentioned above, rice and wheat are still the main sources of carbohydrates in Indonesia. The need for wheat in Indonesia is relatively high and nearly all of it is fulfilled by import. Cultivation of wheat in this country has been hampered by the incompatibility between the tropical climate and the growth requirements of this plant (Nasution 2003). Based on the end products, most imported wheat is used for the production of wet noodles (30 %), bread (25 %), and instant noodles (20 %). The rest is used in the biscuit industry (15 %), households (5 %), and fried foods (5 %). With the periodic increase in wheat prices accompanied by the changes in consumption patterns and people’s preference toward wheat flour-food products, fulfillment of domestic need of wheat by import is a large financial burden. To reduce the dependency on wheat, partially substituting wheat flour with other flours and starches derived from underutilized roots and tubers, which are widely available in Indonesia, may provide a solution to this need.
The objective of this study was to determine the level of substitution of both canna and konjac flours for wheat flour, which would display physicochemical properties suitable for use in food applications in place of wheat flour alone. Therefore, the physicochemical properties of these substituted mixtures were examined and compared to unsubstituted wheat flour. Information obtained in this study may be useful in helping to lessen the dependency on wheat flour as the main source of carbohydrate in Indonesia. Such an outcome may be used to broaden the application of canna and konjac flours in the food industry with particular emphasis on biscuit and noodle production.
Materials and methods
Materials
Matured roots of canna (Canna edulis var Ganyong merah) and konjac (Amorphophallus campanulatus var Mutiara) were collected from Tulung Agung (East Java, Indonesia). Samples were prepared by drying thick chips of these roots in an oven (30 °C; 40 h). Subsequently, these dried chips were milled and sifted (300-μm sieve) to produce flours. Wheat flours with different protein contents i.e.: 11.1 % (high protein wheat flour or HPW) and 8.7 % (low protein wheat flour or LPW) were provided by Allied Mills (Ballarat, Victoria, Australia). Composite materials were prepared by mixing each of the wheat flours (HPW or LPW) with 0, 25, 50, 75, and 100 % of canna or konjac flours (on weight basis).
Proximate analysis of composite flours
Moisture and protein contents of composite flours were determined using AACC (2000) standard method #44-15A and #46-12, respectively. Amylose content was determined using the method proposed by Hoover and Ratnayake (2005). To avoid the interference of lipids on amylose determination, lipids within the samples were firstly removed with 75 % n-propanol for 7 h in a Soxhlet extractor. In this study, amylose and amylopectin content were expressed relative to total starch content. Therefore, amylopectin content was calculated by the formula: % total starch content - % amylose content. Total starch assay Kit (Megazyme, Ireland) was used to measure the amount of total starch, while a Megazyme resistant starch assay kit (Megazyme, Ireland) was used to measure resistant and non-resistant starch contents. The digestibility was determined based on the ratio of non-resistant starch to total amount of resistant and non-resistant starch (Liu et al. 2006).
Swelling power
Swelling power was determined by heating the composite flour suspensions (0.5 %) in a water bath (60, 70, 80 and 90 °C; 30 min) with constant stirring to avoid sedimentation. Pellets were obtained after centrifugation (Sorvall) at 1,000-x g for 15 min at 20 °C. The pellets were weighed and then oven dried until they reached a constant weight. Swelling power (g/g) was expressed as the sedimented fraction, which was weighed and its mass related to that of dry starch to give the swelling power (g/g) (Santacruz et al. 2003).
Color
Color measurements of samples were carried out using a Minolta chromameter (CR-300, Minolta Corporation, Ramsey, NJ). Each sample was scanned at different locations to determine L*, a*, and b* values. The L* value indicates the lightness with 0–100 representing dark to light; a* value presents the degree of red-green color with a higher positive a* value indicating more red. The b* values, on the other hand, indicate the degree of yellow-blue color with a higher positive b* value being more yellow (Shandu et al. 2007).
Pasting properties
A starch cell (Physica SmartStarch analyzer-Anton Paar) attached to a CR/CS rheometer (Physica MCR 33011, Anton Paar, GmbH, Germany) was used to determine the pasting properties of composite flour suspensions (7 % w/w). Samples were equilibrated at 50 °C for 1 min, then heated from 50 to 95 °C at 6 °C/min, held at 95 °C for 5 min, cooled to 50 °C at 6 °C/min, and held at 50 °C for 2 min. The speed was 960 rpm for the first 10 s, then 160 rpm for the reminder of the experiment (Jayakody et al. 2007).
Gel firmness
Following rheological testing, the paste was poured into a plastic container. A few drops of vegetable oil were added onto the surface of the paste to prevent evaporation. The paste was then kept overnight at room temperature. The firmness of gels was tested by force in compression with the TA.XT 2 Texture Analyzer using a 0.5-cm diameter cylinder probe. Texture profile analysis was carried out with a test speed of 1.00 mm/sec. The test strain was set at 50 %. Three determinations were made per sample.
Thermal properties
The thermal properties including the onset (To), peak (Tp) and conclusion (Tc) temperatures, and gelatinization enthalpy (∆H) of the composite flours were determined using a differential scanning calorimetry (DSC-7, Perkin Elmer, Norwalk, CT, USA). Deionized water (11 μl) was added to 3 mg of sample in an aluminum pan (BO160932). The sample was kept overnight at room temperature before analysis. It was heated from 20 to 100 °C with 10 °C/min scan rate. An empty aluminum pan was used as a reference (Ratnayake et al. 2001).
Statistical analysis
A randomized block design was applied in the design of all experiments, with tubers and replications (block) as the main effects. This block structure was repeated at least three times with at least 2 sub samplings. Results were analyzed using a General Linier Model (SAS systems, 1996) with a level of significance preset at p < 0.05.
Results and discussion
Chemical properties
Chemical composition of wheat/canna and wheat/konjac composite flours is presented in Table 1. Notably, the levels of total starch, amylose and amylopectin in the composite flours did not alter substantially with the different percentage substitution using canna flour. In contrast, the percentage of protein decreased significantly (p < 0.05) with increasing levels of canna flour. Wheat flour with a protein content ranging from 8.4 to 10 % is suitable for biscuit sponge making, while flour with lower protein content 7.0–8.5 % is good to make sweet biscuits (Radley 1976). Therefore, based on their protein content, substitution of HPW flour with 50 % of canna flour or replacement of LPW with 25 % of canna flour can produce composite flours that are suitable for sweet biscuit production. Furthermore, composite flour that is suitable for sponge biscuit making can be obtained by substituting HPW flour with canna flour at 75:25 ratio or by using the LPW flour alone. As for biscuit making, different types of noodles have differing requirements for protein content (Fu 2008; Shiau and Yeh 2001). White salted noodles (WSN) are generally made from wheat flour having 8–11 % protein content while yellow alkaline noodles (YAN) are made from flour with protein content between 9–13 %. Instant noodle requires wheat flour with a protein content ranging from 8.5–12.5 %, while udon requires flour with 9–9.5 % protein content. Therefore, based on protein content, substitution of HPW flour with 25 % of canna flour may be used to replace HPW flour in WSN, YAN, instant noodle, and udon making. However, all of the wheat/canna composite flours in this study would not be suitable for Chinese wet noodles production as these require high protein content (11.0–12.5 %) (Charles et al. 2007).
Table 1.
Chemical composition of wheat/canna and wheat/konjac composite flours
| Wheat/canna and wheat/konjac ratio | Protein (%) | Total Starch (%) | Amylose (%) | Amylopectin (%) | Moisture (%) |
|---|---|---|---|---|---|
| Wheat/canna | |||||
| HPWC | |||||
| 100:0 | 11.1 ± 0.8a | 77.2 ± 5.8a | 21.9 ± 1.5a | 55.3 ± 6.3a | 11.7 ± 0.9b |
| 75:25 | 9.4 ± 0.6b | 79.2 ± 4.2a | 22.9 ± 1.4a | 56.2 ± 4.8a | 11.4 ± 0.1d |
| 50:50 | 7.6 ± 0.0d | 81.3 ± 2.7a | 24.1 ± 1.4a | 57.3 ± 3.5a | 11.1 ± 0.1e |
| 25:75 | 5.9 ± 0.1g | 81.1 ± 1.5a | 25.3 ± 1.5a | 58.0 ± 2.4a | 13.0 ± 0.1f |
| 0:100 | 4.2 ± 0.0i | 81.0 ± 1.6a | 23.0 ± 1.7a | 57.9 ± 1.7a | 15.3 ± 0.1g |
| LPWC | |||||
| 100:0 | 8.7 ± 0.8c | 79.1 ± 5.8 a | 24.0 ± 1.9a | 54.9 ± 5.8a | 12.2 ± 0.1a |
| 75:25 | 7.6 ± 0.0e | 81.0 ± 1.4a | 22.7 ± 1.7a | 58.3 ± 1.8a | 11.4 ± 0.1c |
| 50:50 | 6.4 ± 0.0f | 82.5 ± 1.5a | 23.9 ± 1.6a | 58.6 ± 1.5a | 11.1 ± 0.1e |
| 25:75 | 5.3 ± 0.0h | 82.3 ± 1.5a | 23.4 ± 1.5a | 58.9 ± 1.5a | 12.5 ± 0.1f |
| 0:100 | 4.2 ± 0.0i | 81.0 ± 1.6a | 23.0 ± 1.7a | 57.9 ± 1.7a | 15.3 ± 0.1 g |
| Wheat/konjac | |||||
| HPWK | |||||
| 100:0 | 11.1 ± 0.8a | 77.2 ± 5.8a | 21.9 ± 1.5a | 55.3 ± 6.3a | 11.7 ± 0.9b |
| 75:25 | 9.9 ± 0.1b | 75.4 ± 4.6cd | 21.3 ± 1.4abc | 53.7 ± 5.3abc | 11.8 ± 0.2d |
| 50:50 | 8.6 ± 0.2c | 73.0 ± 3.4ef | 20.8 ± 1.5cd | 52.7 ± 4.5cd | 12.1 ± 0.4f |
| 25:75 | 7.5 ± 0.2e | 69.6 ± 2.3g | 20.6 ± 1.6de | 44.7 ± 3.7de | 10.6 ± 0.7g |
| 0:100 | 6.2 ± 0.3g | 62.3 ± 1.3h | 19.9 ± 1.9e | 38.3 ± 3.2e | 9.5 ± 0.9h |
| LPWK | |||||
| 100:0 | 8.7 ± 0.8c | 79.1 ± 5.8 a | 24.0 ± 1.9a | 54.9 ± 5.8a | 12.2 ± 0.1a |
| 75:25 | 8.08 ± 0.4d | 77.5 ± 1.1bc | 21.9 ± 1.6bcd | 55.5 ± 1.9abc | 11.7 ± 0.2c |
| 50:50 | 7.47 ± 0.2e | 75.7 ± 1.0de | 21.6 ± 1.5abc | 53.7 ± 2.1bcd | 12.2 ± 0.4e |
| 25:75 | 6.85 ± 0.2f | 71.0 ± 1.1fg | 21.1 ± 1.6d | 46.2 ± 2.5de | 10.7 ± 0.7g |
| 0:100 | 6.23 ± 0.3g | 62.3 ± 1.3h | 19.9 ± 1.9e | 38.3 ± 3.2e | 9.5 ± 0.9h |
All data reported on dry basis and represent the mean of four replicates ± standard deviation. Means followed by a different superscript in each column are significantly different (p < 0.05). HPWC: High protein wheat/canna composite flour; LPWC: Low protein wheat/canna composite flour, HPWK: High protein wheat/konjac composite flour; LPWK: Low protein wheat/konjac composite flour
The total starch of HPWK and LPWK composite flours decreased significantly (p < 0.05) with increasing amounts of konjac flour in the mixture. The percentages of amylose and amylopectin tended to decrease gradually with the increasing amount of konjac flour in the mixture, even though the reduction was not significant (p > 0.05). The increasing amount of konjac flour from 0 to 100 % decreased the protein content in all of the wheat/konjac composite flours. Based on the protein requirements for biscuit production, flour that would be suitable for sweet biscuit making can be obtained by substituting HPW flour with 50 and 75 % of konjac flour or by replacing LPW flour with 25 or 50 % of konjac flour in the mixture. The use of LPW flour in sponge biscuit production can be replaced by substituting HPW flour with 25 % or 50 % of konjac flour. In terms of noodle production, the use of HPW and LPW flours in WSN making can be replaced by substituting HPW flour with up to 50 % of konjac flour or by substitution of LPW flour with up to 25 % of konjac flour. Substitution of HPW flour with 25 % of konjac flour might be used to replace the HPW flour in YAN production. Substituting HPW flour with up to 50 % of konjac flour can produce flours suitable for instant noodle. This composite flour then can replace LPW flour for instant noodle production. Udon noodles can be made from HPWK composite flour that contains 25 % konjac flour in the mixture.
Table 2 presents the results for the levels of resistant starch (RS), non-resistant starch (NRS) and digestibility of HPWC, LPWC, HPWK and LPWK composite flours. Increasing amount of canna flour in these composite flours increased the percentage of RS significantly (p < 0.05). As a consequence, the percentage of NRS and digestibility of these mixtures were reduced. Similar increase of RS and decrease of NRS and digestibility were also observed in HPWK and LPWK composite flours. Thus, substitution of wheat flour with canna or konjac flours may offer health benefits due to the low digestibility of the composite flour which has been shown to be important in the prevention of obesity, diabetes and other related diseases (Chen et al. 2003; Hung and Morita 2005). In terms of snack food production, the increase in RS in these mixtures could be used to improve the texture of final products. Flours that contain high amount of RS could produce snack foods with a more expanded, light, and crunchy texture (Taggart 2004).
Table 2.
Resistant and non-resistant starch content and digestibility of wheat/canna and wheat/konjac composite flours
| Wheat/canna and wheat/konjac ratio | Wheat/canna composite flours | Wheat/conjac composite flours | ||||
|---|---|---|---|---|---|---|
| Resistant starch (%) | Non resistant starch (%) | Digestibility (%) | Resistant starch (%) | Non resistant starch (%) | Digestibility (%) | |
| HPW | ||||||
| 100:0 | 1.3 ± 0.2e | 87.1 ± 4.3b | 98.5 ± 0.2a | 1.3 ± 0.2e | 87.1 ± 4.3a | 98.5 ± 0.2a |
| 75:25 | 15.1 ± 0.8d | 72.4 ± 3.3c | 82.2 ± 0.5b | 13.9 ± 0.5d | 72.7 ± 3.1b | 82.9 ± 0.7b |
| 50:50 | 28.8 ± 1.7c | 57.6 ± 2.4d | 65.9 ± 1. 1c | 26.5 ± 0.9c | 58.4 ± 2.2c | 67.5 ± 1.4c |
| 25:75 | 42.6 ± 2.7b | 42.8 ± 1.5e | 49.6 ± 1.6d | 39.1 ± 1.3b | 43.9 ± 1.8d | 51.9 ± 2.0d |
| 0:100 | 56.4 ± 3.6a | 28.1 ± 1.0f | 33.3 ± 2.2e | 51.7 ± 1.7a | 29.6 ± 2.4 d | 36.4 ± 2.7e |
| LPW | ||||||
| 100:0 | 1.3 ± 0.2e | 92.7 ± 1.5a | 98.3 ± 0.1a | 1.6 ± 0.1e | 92.7 ± 1.5a | 98.3 ± 0.1a |
| 75:25 | 15.3 ± 0.9d | 76.5 ± 0.9c | 82.1 ± 0.5b | 14.1 ± 0.5d | 76.9 ± 1.7b | 82.8 ± 0.7b |
| 50:50 | 29.0 ± 1.8c | 60.4 ± 0.3d | 65.8 ± 1.1c | 26.7 ± 0.9c | 61.1 ± 1.9c | 67.3 ± 1.4c |
| 25:75 | 42.7 ± 2.7b | 44.3 ± 0.4e | 49.6 ± 1.6d | 39.2 ± 1.3b | 45.4 ± 2.2d | 51.8 ± 2.0d |
| 0:100 | 56.4 ± 3.6a | 28.1 ± 1.0f | 33.3 ± 2.2e | 51.7 ± 1.7a | 29.6 ± 2.4e | 36.4 ± 2.7e |
All data reported on dry basis and represent the mean of four replicates ± standard deviation. Means followed by a different superscript in each column are significantly different (p < 0.05). HPW: High protein wheat composite flour; LPW: Low protein wheat composite flour.
Swelling power
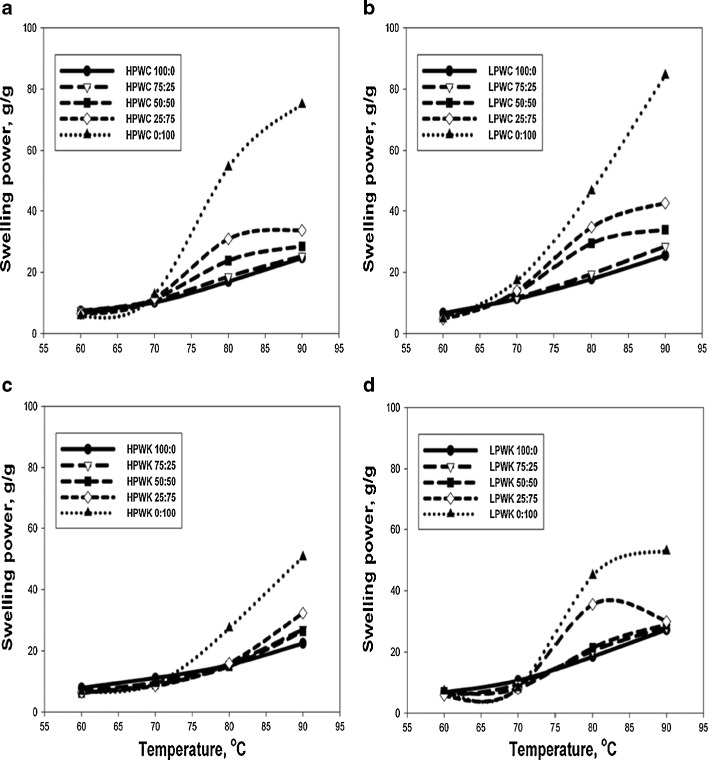
Swelling power of HPWC and LPWC composite flours is summarized in Fig. 1a and Fig. 1b, respectively. The starch granules within these flours started to swell at ~60 °C. The swelling power increased slightly with increasing temperature to 70 °C. A significant (p < 0.05) increase of swelling power was observed with increasing temperature from 70 to 90 °C. The swelling power of HPWC composite flours that contained 25 or 50 % canna flour showed no significant (p > 0.05) difference to that of the wheat flour alone at all tested temperatures. HPW flour containing 75 % canna flour had a higher swelling power compared to that of HPW flour alone at 80 and 90 °C. A similar effect on swelling behavior was also observed in LPWC composite flours. For LPWC composite flours, substitution of LPW flour with an even lower amount of canna flour (50 %) increased the swelling power of the composite flours. The increase in swelling power would be beneficial for food applications that require a high swelling power. For example udon and Korean dried salted noodles, which require soft, smooth, and elastic textural properties (Fu 2008).
Fig. 1.
Swelling power of composite flours created by mixing high protein wheat (HPW) or low protein wheat flour (LPW) with canna (C) and with konjac (K) flour in different proportions. High protein wheat/canna (HPWC), low protein wheat/canna (LPWC), high protein wheat/konjac (HPWK), low protein wheat/konjac (LPWK) flours and their proportions are presented as figure A, B, C and D, respectively. All data reported on dry basis and represent the mean of four replicates
As can be seen from Fig. 1, the 100 % canna or konjac flours had a far greater swelling power than the HPW or LPW. Therefore, it was anticipated that the composite flours possess a greater swelling power than what it has been achieved. This unexpected outcome may be due to the influence of several factors such as the mixing ratio, chemical composition of flour, starch content and characteristics, as well as interaction between starch granules (Nishinari et al. 1992; Ortega-Ojeda and Eliasson 2001; Chen et al. 2003; Ortega-Ojeda et al. 2004). The interactions between starch granules could be attributed to the difference in their granule size and have been observed in canna-rice starch mixtures (Puncha-arnon et al. 2008). Rice starches that were smaller in size compared to that of canna starches, surrounded granules of canna starch and restricted the swelling power of canna flour.
Color characteristics
The results of the analysis for color of the HPWC, LPWC, HPWK and LPWCK composite flours are presented in Table 3. Wheat flours (HPW and LPW) were whiter, more yellow and less red compared to canna flour. The difference in color characteristics between wheat and canna flour could be attributed to the difference in protein content of these flours. Protein content of flours was negatively related with the L* value but positively correlated with a* and b* values (Jamin and Flores 1998; Shandu et al. 2007). In addition, distinct colored pigments that are present in wheat and canna flours may also contribute to this observation (Singh et al. 2003). Substitution of wheat flours with 25 % canna flour in the HPWC and LPWC flour mixtures did not cause a significant (p > 0.05) reduction in terms of the lightness. However, the lightness decreased significantly (p < 0.05) with the presence of 50–75 % of canna flour in the mixtures. In contrast, redness and yellowness of composite flours increased significantly (p < 0.05) with increasing canna flour in the mixtures. Similar to results obtained from wheat/canna mixtures, konjac flour was darker, less yellow, and redder compared to wheat flours. Substitution of wheat flours with konjac flour reduced the whiteness, but increased the redness and yellowness of the mixtures.
Table 3.
Color and hardness of gels created from wheat/canna and wheat/konjac composite flours
| Wheat/canna and wheat/konjac ratio | Wheat/canna composite flours | Wheat/conjac composite flours | ||||||
|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | Firmness (g) | L* | a* | b* | Firmness (g) | |
| HPW | ||||||||
| 100:0 | 67.2 ± 1.8ab | −0.2 ± 0.0g | 8.6 ± 0.2g | 23.2 ± 2.3a | 72.9 ± 1.3a | −0.4 ± 0.1g | 9.1 ± 0.2de | 23.2 ± 2.3a |
| 75:25 | 63.5 ± 1.9bc | 1.5 ± 0.1e | 11.7 ± 0.2ef | 21.4 ± 2.9b | 63.8 ± 2.8b | 1.5 ± 0.2e | 9.5 ± 0.6cde | 18.1 ± 1.1bc |
| 50:50 | 55.9 ± 1.7d | 2.3 ± 0.1c | 13.1 ± 0.5d | 18.2 ± 2.2bc | 59.3 ± 1.2c | 2.1 ± 0.1cd | 10.0 ± 0.3bc | 10.9 ± 1.1d |
| 25:75 | 59.9 ± 2.1cd | 3.0 ± 0.2b | 16.1 ± 0.6b | 9.9 ± 1.6d | 58.9 ± 1.3c | 2.3 ± 0.3bc | 10.4 ± 0.6ab | 2.3 ± 0.2e |
| 0:100 | 59.5 ± 1.9cd | 3.6 ± 0.2a | 17.9 ± 0.6a | 1.7 ± 0.4e | 59.4 ± 1.3c | 2.7 ± 0.1a | 11.0 ± 0.4a | 1.7 ± 0.4e |
| LPW | ||||||||
| 100:0 | 72.2 ± 2.8a | −0.8 ± 0.1h | 7.7 ± 0.5g | 20.1 ± 1.1abc | 73.8 ± 1.6a | −0.7 ± 0.1g | 7.5 ± 0.3f | 20.0 ± 1.1b |
| 75:25 | 69.8 ± 2.0a | 0.9 ± 0.2f | 10.5 ± 0.2f | 17.9 ± 3.3bc | 71.8 ± 2.1a | 1.1 ± 0.1f | 8.8 ± 0.3e | 15.4 ± 1.8c |
| 50:50 | 59.4 ± 2.1d | 1.8 ± 0.2d | 11.9 ± 0.5de | 17.3 ± 0.9c | 65.7 ± 2.8b | 1.8 ± 0.2de | 9.9 ± 0.3bcd | 8.6 ± 0.7d |
| 25:75 | 58.9 ± 3.1d | 2.7 ± 0.1b | 14.6 ± 0.8c | 8.8 ± 0.8d | 64.7 ± 1.6b | 2.4 ± 0.1abc | 11.1 ± 0.2a | 1.7 ± 0.3e |
| 0:100 | 56.5 ± 2.9d | 3.6 ± 0.3a | 16.9 ± 0.8ab | 1.7 ± 0.4e | 59.3 ± 0.9c | 2.7 ± 0.2ab | 11.2 ± 0.3a | 1.7 ± 0.4e |
All data reported on dry basis and represent the mean of four replicates ± standard deviation. Means followed by a different superscript in each column are significantly different (p < 0.05). HPW: High protein wheat composite flour; LPW: Low protein wheat composite flour
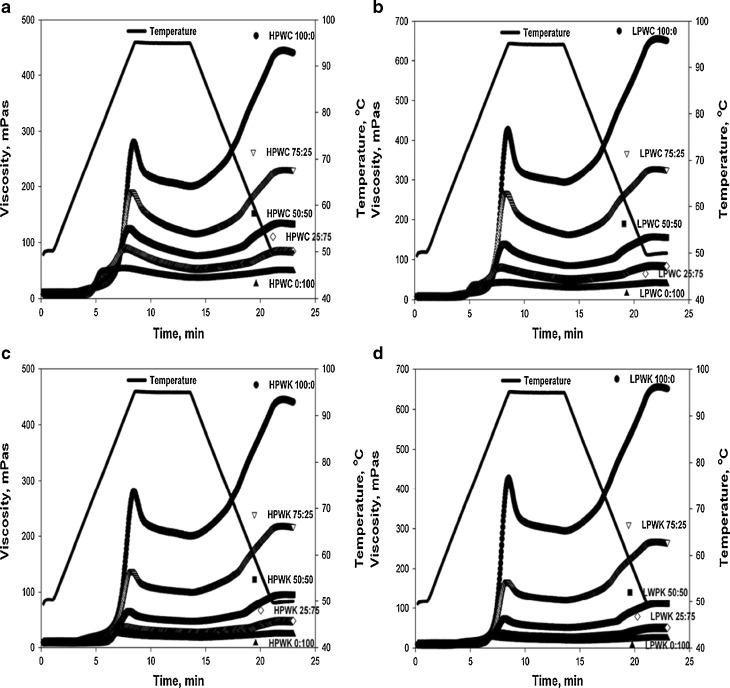
Pasting properties
Pasting properties of HPWC, LPWC, HPWK and LPWCK composite flours are presented in Table 4 and Fig. 2. Wheat flours have a higher peak, holding strength, breakdown, setback, and final viscosity compared to those of canna flour. The LPW flour has a higher peak, breakdown, holding strength, setback from peak, setback from trough and final viscosity values compared to that of HPW flour. This outcome could be partially due to the difference in their protein contents (Table 1). Proteins may form complexes with granule surfaces preventing the release of exudates hence lowering the viscosity (Olkku and Rha 1978). A lowering effect of the protein content in wheat flour on peak viscosity of wheat/sweet potato starch, wheat/cassava starch and wheat/yam starch has been reported previously (Zaidul et al. 2007). The viscosity of canna flour reported in this study was lowered more than what it was reported elsewhere (Srichuwong et al. 2005). This discrepancy might be caused by the activity of amylolytic enzymes (Radley 1976).
Table 4.
Pasting properties of wheat/canna and wheat/konjac composite flours
| Wheat/canna and wheat/konjac ratio | Pasting characteristics | |||||||
|---|---|---|---|---|---|---|---|---|
| *tpeak (sec) | Tpast (°C) | ηpeakv(mPa.s) | Hstrengthv(mPa.s) | Bdown (mPa.s) | SFP (mPa.s) | SFT (mPa.s) | ηfinal (mPa.s) | |
| HPWC: 100:0 | 507 ± 6.0a | 66.3 ± 5.9b | 282 ± 37.5b | 199 ± 20.4b | 82.7 ± 18.1bc | 158 ± 18.4b | 241 ± 30.8b | 441 ± 50.1b |
| 75:25 | 500 ± 5.7ab | 64.4 ± 3.3b | 191 ± 52.2cd | 116 ± 23.7c | 75.4 ± 2.7bcd | 38.7 ± 13.6cd | 114 ± 17.4d | 230 ± 40.8d |
| 50:50 | 484 ± 2.8cd | 66.0 ± 1.1b | 125 ± 6.8def | 75.2 ± 1.3d | 50.3 ± 6.4cde | 7.8 ± 3.2e | 58.1 ± 4.4e | 133 ± 4.9e |
| 25:75 | 465 ± 4.2ef | 66.6 ± 0.7b | 90.7 ± 9.6ef | 51.7 ± 1.8de | 38.6 ± 8.8de | 6.1 ± 7.9e | 32.6 ± 0.7ef | 84.3 ± 1.8ef |
| 0:100 | 456 ± 10.1f | 65.3 ± 1.2b | 53.4 ± 6.3f | 34.9 ± 2.6e | 18.4 ± 4.6e | 3.8 ± 4.9e | 14.6 ± 1.3f | 49.5 ± 3.7f |
| LPWC: 100:0 | 509 ± 2.0a | 74.1 ± 0.8a | 428 ± 40.5a | 2934 ± 24.0a | 134 ± 16.7a | 223 ± 15.9a | 357 ± 30.7a | 651 ± 54.2a |
| 75:25 | 501 ± 3.5ab | 64.8 ± 1.8b | 267 ± 65.4bc | 163 ± 32.2b | 103 ± 33.4ab | 58.9 ± 8.5c | 162 ± 27.4c | 326 ± 59.6c |
| 50:50 | 490 ± 3.0bc | 65.6 ± 0.9b | 140 ± 18.5de | 84.1 ± 10.1cd | 56.2 ± 9.1cde | 15.6 ± 4.4de | 71.8 ± 6.8e | 155 ± 16.9de |
| 25:75 | 474 ± 0.0de | 66.2 ± 0.9b | 80.6 ± 5.9ef | 51.3 ± 0.8de | 29.3 ± 5.2e | 2.9 ± 1.8e | 32.2 ± 3.9ef | 83.6 ± 4.6ef |
| 0:100 | 456 ± 10.1f | 65.3 ± 1.2b | 53.4 ± 6.3f | 34.9 ± 2.6e | 18.4 ± 4.6e | 3.8 ± 4.9e | 14.6 ± 1.3f | 49.5 ± 3.7f |
| HPWK: 100:0 | 507 ± 6.0a | 66.3 ± 5.9b | 282 ± 37.5b | 199 ± 20.4b | 82.7 ± 18.1b | 158 ± 18.4b | 2410 ± 30.8b | 441 ± 50. b |
| 75:25 | 494 ± 3.0a | 68.8 ± 3.5ab | 138 ± 21.9c | 100 ± 1.7c | 37.9 ± 10.4cd | 78.9 ± 7.8c | 116 ± 14.5c | 2170 ± 25.8c |
| 50:50 | 483 ± 3.5a | 68.6 ± 1.6ab | 65.2 ± 4.6 de | 46.0 ± 2.1de | 19.2 ± 2.4 de | 30.5 ± 2.1de | 49.6 ± 1.1de | 95.6 ± 2.7 de |
| 25:75 | 462 ± 4.9a | 69.8 ± 0.9ab | 36.5 ± 2.5 de | 25.5 ± 1.2ef | 10.9 ± 1.3e | 10.9 ± 0.4ef | 21.8 ± 1.6ef | 47.3 ± 2.8ef |
| 0:100 | 411 ± 5.7a | 64.7 ± 3.3b | 31.3 ± 3.9 de | 17.7 ± 2.5f | 13.6 ± 1.4e | −4.6 ± 1.2f | 8.9 ± 1.2f | 26.6 ± 3.3f |
| LPWK: 100:0 | 509 ± 2.0a | 74.2 ± 0.8ab | 428. ± 40.5a | 293 ± 24.0a | 134 ± 16.7a | 223 ± 15.9a | 357 ± 30.7a | 651 ± 54.2a |
| 75:25 | 499 ± 3.0a | 71.9 ± 0.8ab | 167.2 ± 5.4c | 122 ± 2.9c | 45.2 ± 3.6c | 98.4 ± 3.6c | 143 ± 6.9c | 265 ± 7.9c |
| 50:50 | 472 ± 36.2a | 71.6 ± 0.3ab | 73.3 ± 6.9d | 52.0 ± 2.9d | 21.3 ± 5.3de | 38.4 ± 0.7d | 59.6 ± 5.9d | 111 ± 7.6d |
| 25:75 | 468 ± 6.9a | 76.0 ± 6.8a | 35.9 ± 3.2de | 23.8 ± 1.7ef | 12.1 ± 1.6e | 14.3 ± 3.4ef | 26.4 ± 1.8def | 50.3 ± 1.4ef |
| 0:100 | 411 ± 5.7a | 65.8 ± 3.4b | 26.6 ± 6.9e | 16.8 ± 2.6f | 9.8 ± 5.9e | −1.1 ± 5.1f | 8.7 ± 1.1f | 25.5 ± 3.5f |
All data reported on dry basis and represent the mean of four replicates ± standard deviation. Means followed by a different superscript in each column are significantly different (p < 0.05). HPWC: High protein wheat/canna composite flour; LPWC: Low protein wheat/canna composite flour, HPWK: High protein wheat/konjac composite flour; LPWK: Low protein wheat/konjac composite flour. *tpeak - peak time; Tpast - pasting temperature; ηpeak - peak viscosity; Hstrength - holding strength; Bdown - breakdown; SFP - setback from peak; SFT - set back from through; ηfinal - final viscosity.
Fig. 2.
Pasting properties of composite flours created by mixing high protein wheat (HPW) or low protein wheat flour (LPW) with canna (C) flour and with konjac (K) flour in different proportions. High protein wheat/canna (HPWC), low protein wheat/canna (LPWC), high protein wheat/konjac (HPWK) and low protein wheat/konjac (LPWK) flours and their proportions are presented as figure A, B, C and D, respectively. All data reported on dry basis and represent the mean of four replicates flours and their proportions are presented as figure A and B, respectively
In general, viscosities (peak, breakdown, setback from peak, setback from trough and final viscosities) of HPWC and LPWC composite flours decreased significantly (p < 0.05) with the presence of canna flour at 25 % and 50 % (Table 4). After this point, viscosities tended to decrease gradually with the increase of the canna flour in the mixture. The high setback and final viscosities of the wheat flour pastes indicated their weak resistance against retrogradation. Substitution of wheat flour with canna flour might be used to decrease retrogradation tendency of wheat flours indicated by lower setback viscosities of these mixtures, as compared to that of the wheat flour alone (Leon et al. 2006; Zaidul et al. 2007). In addition, substitution of wheat flour with 50 % canna flour in LPWC composites increased the heat stability of this mixture indicated by the lowered value of its breakdown viscosity. In contrast, substitution of wheat flour with canna flour in HPWC composites did not improve the heat stability of wheat flours (Table 4) (Leon et al. 2006; Zaidul et al. 2007).
For HPWK mixtures, the overall viscosity decreased significantly (p < 0.05) with the presence of 25 and 50 % of konjac flour in the mixtures. Further increments in konjac flour did not cause a significant (p > 0.05) decrease in viscosity. The same trend was observed in LPWK composite flours. As opposed to the individual konjac flour paste, the high setback and final viscosity of the individual wheat flour paste indicated its high retrogradation tendency. Setback viscosity of the mixture tended to decrease significantly with increasing amounts of konjac flour up to 50 % in the mixture. Thus, substitution of wheat flour with konjac flour may be used to reduce the retrogradation tendency of wheat flours. Similar effects to the konjac flour in reducing retrogradation have been observed in maize and potato starches (Khanna and Tester 2006).
Retrogradation occurs due to the formation and subsequent aggregation of double helix of amylose and amylopectin. The process of retrogradation involves the loss of fine stranded amylose network, development of amylose aggregates and the assembly of amylose aggregates. Meanwhile, amylopectin retrogradation occurs more slowly. Therefore, amylose is proposed to be responsible for the short-term (less than one day) retrogradation while amylopectin contributes to the long term retrogradation (Bao 2004). The interaction of konjac or other hydrocolloids with amylose and amylopectin could reduce retrogradation (Funami et al. 2005; Khanna and Tester 2006). Konjac might act as a physical barrier that prevents amylose and amylopectin chain association, which is a perquisite to retrogradation. (Khanna and Tester 2006). In addition, hydrocolloids might also act as a water binder and limit the availability of water for amylose and amylopectin, which subsequently prevents crystallization. Substitution of wheat flour with konjac flour also increased the shear stability of the composite flour indicated by the decrease of their breakdown viscosity (Leon et al. 2006; Zaidul et al. 2007).
Based on the pasting properties of HPWC and LPWC as well as the HPWK and LPWK composite flours discussed above, partial replacement of wheat flours with canna or konjac flours might offer advantages for some food applications. Due to their low retrogradation tendency, these composite flours might be advantageously used in frozen products, for example, frozen pie fillings or cold-storage foods such as canned sauces and gravies (Cornell 2004). In bread making, the low retrogradation tendency of these composite flours could reduce bread staling, thus enhancing shelf-storage in this product (Radley 1976; Taggart 2004).
The low viscosity of these composite flours might bring advantages to the confectionary industry especially in the processing of gumdrops by allowing better evaporation and pouring (Radley 1976). Having a low viscosity value, these composite flours could be incorporated into certain food products in higher concentrations without becoming too viscous (Cornell 2004). The low viscosity of these flours is also beneficial to infant nutrition since infants cannot tolerate a solid diet due to their digestive system and undeveloped eating skills (Walker and Rolls 1999; Muyonga et al. 2001). There is an inverse relationship between viscosity and energy density of infant food. The low viscosity value might eliminate the need for dilution thus increasing the energy intake of infants (Walker and Rolls 1999). In addition, the lower breakdown of these composite flours may produce a less cohesive paste being beneficial during food processing (Moorthy 2002). Finally, the increase of heat stability in these composite flours is important for food products prepared by heat-moisture or steam pressure, for example, canned foods (Moorthy 2002). In this study, viscosity of the composite flours was lower than what it was anticipated. This is in agreement with results obtained from the swelling power experiments. It also confirmed the presence of interference patterns in the viscoelasticity of canna and wheat granules or with other components such as the leached amylose and amylopectin discussed previously.
Thermal properties
The gelatinization temperature and enthalpy for the HPWC, LPWC, HPWK and LPWCK are presented in Table 5. Each individual wheat and canna flour has a single endothermic peak. Despite having different protein contents, HPW and LPW flours used in this study have similar gelatinization temperatures and enthalpies. A similar onset (57.0 °C) and peak (62.3 °C) temperature as well as enthalpy value (5.3 J/g dry starch) of wheat flour has been reported previously (Zaidul et al. 2007). Gelatinization temperature and enthalpy of the canna flour was higher than that of wheat flour, which indicates the higher stability of canna flour compared to that of wheat flour (Srichuwong et al. 2005).
Table 5.
Differential scanning calorimetric data* for wheat/canna and wheat/konjac composite flours dispersed in deionized water
| Wheat/canna and wheat/konjac ratio | Gelatinization characteristics | ||||
|---|---|---|---|---|---|
| To (°C) | Tp1 (°C) | Tp2 (°C) | Tc (°C) | ΔH (J/g dry starch) | |
| HPWC | |||||
| 100:0 | 57.8 ± 1.5b | 63.9 ± 1.1b | – | 70.3 ± 1.5b | 5.0 ± 0.9d |
| 75:25 | 58.0 ± 3.4b | 63.4 ± 2.4b | 71.8 ± 1.2a | 75.3 ± 1.4a | 8.3 ± 1.1cd |
| 50:50 | 57.8 ± 2.3b | 65.1 ± 3.3 b | 72.4 ± 0.7a | 77.6 ± 1.8a | 10.5 ± 0.9abc |
| 25:75 | 58.1 ± 1.4b | 64.7 ± 2.1b | 71.9 ± 0.7a | 76.9 ± 1.1a | 11.6 ± 1.0ab |
| 0:100 | 68.6 ± 2.7a | 72.5 ± 1.2a | – | 77.7 ± 1.8a | 13.0 ± 1.8a |
| LPWC | |||||
| 100:0 | 60.6 ± 1.8b | 64.1 ± 1.4 b | – | 69.6 ± 2.5b | 5.7 ± 0.8d |
| 75:25 | 60.9 ± 1.4b | 63.6 ± 0.7 b | 71.8 ± 1.4a | 77.9 ± 1.0a | 8.8 ± 1.4bc |
| 50:50 | 59.4 ± 1.8b | 65.8 ± 0.9 b | 72.5 ± 0.8a | 77.3 ± 1.8a | 10.1 ± 0.9abc |
| 25:75 | 61.5 ± 1.7b | 63.5 ± 2.1 b | 72.2 ± 0.6a | 78.0 ± 1.5a | 11.9 ± 1.8a |
| 0:100 | 68.5 ± 2.2a | 73.5 ± 0.8a | – | 77.6 ± 1.5a | 13.1 ± 1.2a |
| HPWK | |||||
| 100:0 | 57.7 ± 1.6bc | 63.9 ± 0.5b | _ | 69.1 ± 0.7 b | 5.0 ± 1.6d |
| 75:25 | 56.7 ± 1.1c | 64.2 ± 1.3b | 69.1 ± 1.5a | 76.9 ± 1.3a | 9.7 ± 0.9c |
| 50:50 | 58.2 ± 3.0bc | 64.4 ± 2.3b | 70.2 ± 0.6a | 76.6 ± 1.1a | 11.8 ± 1.5bc |
| 25:75 | 57.7 ± 1.2bc | 64.2 ± 1.2b | 70.1 ± 1.2a | 78.3 ± 0.9a | 13.0 ± 1.3b |
| 0:100 | 64.9 ± 1.3a | 69.9 ± 1.7a | _ | 77.5 ± 1.0a | 16.6 ± 1.1a |
| LPWK | |||||
| 100:0 | 60.6 ± 1.8bc | 64.1 ± 1.1b | _ | 69.6 ± 2.5b | 5.7 ± 0.8d |
| 75:25 | 60.9 ± 1.9ab | 63.9 ± 1.1b | 69.9 ± 1.6a | 78.7 ± 1.1a | 11.1 ± 0.9bc |
| 50:50 | 60.8 ± 1.1abc | 64.8 ± 1.3b | 69.3 ± 1.4a | 77.9 ± 2.3a | 12.4 ± 1.2bc |
| 25:75 | 59.4 ± 2.4bc | 64.6 ± 1.7b | 69.1 ± 1.3a | 77.7 ± 1.3a | 12.9 ± 0.9b |
| 0:100 | 64.9 ± 1.3a | 69.1 ± 1.1a | _ | 77.5 ± 1.0a | 13.6 ± 1.0b |
*All data reported on dry basis and represent the mean of four replicates ± standard deviation. Means followed by a different superscript in each column are significantly different (p < 0.05). HPWC: High protein wheat/canna composite flour; LPWC: Low protein wheat/canna composite flour, HPWK: High protein wheat/konjac composite flour; LPWK: Low protein wheat/konjac composite flour. To, Tp, and Tc indicate temperature of onset, midpoint and end of gelatinization, respectively; ΔH (J/g dry starch) - enthalpy of gelatinization
The X-ray diffraction patterns reflect the packing of amylopectin side chains. Based on their diffraction patterns, native starches can be categorized into A, B, and C type starches (Jane et al. 1997). The A type starch has a closely packaged double helices, and formed from short-chain amylopectin. This type is the characteristic of cereal starches. In contrast, the B type starch has a less dense packaging and formed from long-chain amylopectin. Root starches, amylomaize starches, and retrograded starches belong to B-type starches, while, C type starch is a combination between the A and B-type starches (Cui 2005). Canna starch is considered to be a B-type starch and therefore has a high proportion of longer chains of amylopectin (Srichuwong et al. 2005), as opposed to wheat starch with shorter amylopectin chains (Blazek and Copeland 2008). Thus, the higher gelatinization temperature and enthalpy of canna flour compared to that of wheat flours could be attributed to the higher amount of long-chain amylopectin in canna starch. Yuan et al. (1993) reported that there was a positive correlation between the proportion of longer chains of amylopectin and the increase in enthalpy or temperature of gelatinization. Compared to the short-chain amylopectin, the long-chain counterpart is more stable to heat and requires more energy to initiate gelatinization. As a consequence, a higher gelatinization temperature is recorded (Hoover and Ratnayake 2005).
In contrast to the individual wheat or canna flours that have a single endothermic peak, the presence of canna flour at 25, 50, and 75 % in the composite systems led to the presence of two endothermic peaks (Table 5). The lower Tp (midpoint temperature) corresponded to the gelatinization of the wheat flour while the higher Tp reflected that of the canna flour. The To (onset temperature) of these composites was similar to temperatures observed for the wheat flours, while their Tc (end temperature) was close to that of canna flour. The presence of two endothermic peaks indicated that wheat and canna starches present in the composite flours were gelatinized independently, which argues for the formation of micro phase separated systems that don’t interact directly at the molecular level. Similar phenomena have been reported from mixtures of canna/potato starch (Puncha-arnon et al. 2008), potato/waxy maize and waxy maize/barley starches (Ortega-Ojeda and Eliasson 2001), as well as yam/cassava/maize starch blends (Karam et al. 2006).
A similar gelatinization phenomenon was observed for HPWK and LPWK composite flours (Table 5). Starches within the konjac flour have a higher stability compared to that of starches in wheat flour. This was surmised by the higher gelatinization temperature and enthalpy values of konjac flour compared to those of wheat flour. The presence of canna or konjac flour in their respective composite flours increased the gelatinization temperature range (Tc–To) of the composite flours in comparison with the single systems. In other words, partial substitution of wheat flour with canna or konjac flour increased the heterogeneity of starch crystallites within the composite flours, which also means that additional energy is required to fully gelatinize these materials.
Conclusions
There is a strong indication from this work that substitution of wheat flour for that from canna and konjac tubers should be beneficial in the production of various food products. Specific improvements include health benefits by increasing the amount of resistant starch and decreasing carbohydrate digestibility, and improve the textural properties of snack foods. In addition, substitution of wheat with canna flour might improve the pasting behavior of the former by reducing its retrogradation tendency. Such beneficial outcome is a must in the productions of frozen and cold-storage foods. A low retrogradation tendency is also important in the improvement of shelf-storage of bread products by reducing the occurrence of bread staling. The increase of canna or konjac flours in composite flours decreased wheat flour viscosity, which is suitable for the production of weaning food, confectionary (soft candy and gum drops) and other liquid-like embodiments. Substitution of HPW flour with 50 % of canna flour or replacement of LPW with 25 % of canna flour can produce composite flours that are suitable for sweet biscuit production, when substituting HPW flour with canna flour at 75:25 ratio is suitable for sponge biscuit making. Further investigation into the applications of such composite flours should be undertaken with a view to extending the knowledge gained presently to specific product concepts with improved nutritional profile and mouthfeel.
References
- Bao J. The functionality of rice starch. In: Elliason AC, editor. Starch in food. London: Woodhead Publishing Limited; 2004. pp. 258–294. [Google Scholar]
- Blazek J, Copeland L. Pasting and swelling properties of wheat flour and starch in relation to amylase content. Carbohyd Polym. 2008;71:380–387. doi: 10.1016/j.carbpol.2007.06.010. [DOI] [Google Scholar]
- Charles AL, Huang TC, Lai PY, Chen CC, Lee PP, Chang YH. Study of wheat flour-cassava starch composite mix and the function of cassava mucilage in Chinese noodles. Food Hydrocolloids. 2007;21:368–78. doi: 10.1016/j.foodhyd.2006.04.008. [DOI] [Google Scholar]
- Chen HI, Sheu WHH, Tai TS, Liaw YP. Konjac supplement alleviated hypercholesterolemia and hyperglycemia in type 2 diabetic subjects - a randomized double-blind trial. J Am Coll Nutr. 2003;22:36–42. doi: 10.1080/07315724.2003.10719273. [DOI] [PubMed] [Google Scholar]
- Cornell H. The functionality of wheat starch. In: Elliason AC, editor. Starch in food. London: Woodhead Publishing Limited; 2004. pp. 211–38. [Google Scholar]
- Cui SW. Understanding starch and their role in foods. In: Cui SW, editor. Food carbohydrates: chemistry, physical properties, and applications. Florida: CRC Press; 2005. pp. 310–49. [Google Scholar]
- Deshaliman (2003) Attempts to strengthen food stability with tubers and roots. Badan Pengkajian dan Penerapan Technology: http://www.bppt.go.id. Accessed 13 November, 2008
- Fu BX. Asian noodles: history, classification, raw materials, and processing. Food Res Int. 2008;41:888–902. doi: 10.1016/j.foodres.2007.11.007. [DOI] [Google Scholar]
- Funami T, Kataoka Y, Omoto T, Goto Y, Asai I, Nishinari K. Food hydrocolloids control the gelatinization and retrogradation behavior of starch. 2b. Functions of guar gums with different molecular weights on the retrogradation behavior of corn starch. Food Hydrocolloid. 2005;19:25–36. doi: 10.1016/j.foodhyd.2004.04.009. [DOI] [Google Scholar]
- Hoover R, Ratnayake WS. Determination of total amylose content of starch. In: Wrolstad RE, Acree TE, Deckeret EA, editors. Handbook of food analytical chemistry: water, proteins, enzymes, lipids, and carbohydrates. New York: Wiley; 2005. pp. 689–693. [Google Scholar]
- Hung PV, Morita N. Physicochemical properties and enzymatic digestibility of starch from edible canna (Canna edulis) grown in Vietnam. Carbohyd Polym. 2005;61:314–21. doi: 10.1016/j.carbpol.2005.04.021. [DOI] [Google Scholar]
- Jamin FF, Flores RA. Effect of additional separation and grinding on the chemical and physical properties of selected corn-dry milled streams. Cereal Chem. 1998;75:166–70. doi: 10.1094/CCHEM.1998.75.1.166. [DOI] [Google Scholar]
- Jane J, Wong KS, McPherson AE. Branch-structure difference in starches of A- and B-type X-ray patterns revealed by their Naegali dextrins. Carbohyd Res. 1997;300:219–27. doi: 10.1016/S0008-6215(97)00056-6. [DOI] [Google Scholar]
- Jayakody L, Hoover R, Liu Q, Donner E. Studies on tuber starches. II. Molecular structure, composition and physicochemical properties of yam (Dioscorea sp) starches grown in Sri Lanka. Carbohyd Polym. 2007;69:148–63. doi: 10.1016/j.carbpol.2006.09.024. [DOI] [Google Scholar]
- Karam LB, Ferrero C, Martino MN, Zaritzky NE, Grossmann MVE. Thermal, microstructural and textural characterization of gelatinized corn, cassava and yam starch blends. Int J Food Sci Tech. 2006;41:803–12. doi: 10.1111/j.1365-2621.2005.01110.x. [DOI] [Google Scholar]
- Khanna S, Tester RF. Influence of purified konjac glucomannan on the gelatinization and retrogradation properties of maize and potato starches. Food Hydrocolloid. 2006;20:567–76. doi: 10.1016/j.foodhyd.2005.05.004. [DOI] [Google Scholar]
- Leon AE, Barrera GN, Perez GT, Ribotta PD, Rosell CM. Effect of damaged starch levels on flour-thermal behavior and bread staling. Eur Food Res Technol. 2006;224:187–92. doi: 10.1007/s00217-006-0297-x. [DOI] [Google Scholar]
- Liu Q, Donner E, Yin Y, Huang RL, Fan MZ. The physicochemical properties and in vitro digestibility of selected cereals, tubers, and legumes grown in China. Food Chem. 2006;99:470–77. doi: 10.1016/j.foodchem.2005.08.008. [DOI] [Google Scholar]
- Moorthy SN. Physicochemical and functional properties of tropical tuber starches: a review. Starch. 2002;54:559–92. doi: 10.1002/1521-379X(200212)54:12<559::AID-STAR2222559>3.0.CO;2-F. [DOI] [Google Scholar]
- Muyonga JH, Ramteke RS, Eipeson WE. Prehydration steaming changes-physicochemical properties of unripe banana flour. J Food Process Pres. 2001;27:153–64. [Google Scholar]
- Nasution. (2003). The strategy to overcome food instability in Indonesia. Badan Pengkajian dan Penerapan Technology: http://www.bppt.go.id. Accessed July, 2008.
- Nishinari K, Williams PA, Phillips GO. Review of the physicochemical characteristics and properties of konjac mannan. Food Hydrocolloid. 1992;6:199–222. doi: 10.1016/S0268-005X(09)80360-3. [DOI] [Google Scholar]
- Olkku J, Rha C. Gelatinization of starch and wheat flour starch- a review. Food Chem. 1978;3:293–17. doi: 10.1016/0308-8146(78)90037-7. [DOI] [Google Scholar]
- Ortega-Ojeda FE, Eliasson AC. Gelatinization and retrogradation behaviors of some starch mixtures. Starch. 2001;53:520–29. doi: 10.1002/1521-379X(200110)53:10<520::AID-STAR520>3.0.CO;2-D. [DOI] [Google Scholar]
- Ortega-Ojeda FE, Larsson H, Eliasson AC. Gel formation in mixtures of high amylopectin potato starch and potato starch. Carbohyd Polym. 2004;56:505–14. doi: 10.1016/j.carbpol.2004.03.021. [DOI] [Google Scholar]
- Piyachomkwan K, Chotineeranat S, Kijkunasatian C, Tonwitowat R, Prammanee S, Oates CG, Sriroth K. Edible canna (Canna edulis) as a complementary starch source to cassava for the starch industry. Ind Crop Prod. 2002;16:11–21. doi: 10.1016/S0926-6690(02)00003-1. [DOI] [Google Scholar]
- Puncha-arnon S, Pathipanawat W, Puttanlek C, Rungsardthong V, Uttapap D. Effects of relative granule size and gelatinization temperature on paste and gel properties of starch blends. Food Res Int. 2008;41:552–61. doi: 10.1016/j.foodres.2008.03.012. [DOI] [Google Scholar]
- Radley JA. Industrial uses of starch and its derivatives. London: Applied Science Publishers; 1976. [Google Scholar]
- Ratnayake WS, Hoover R, Shahidi F, Parera C, Jane J. Composition, molecular structure, and physicochemical properties of starches from four field pea (Pisum sativum L.) cultivars. Food Chem. 2001;74:189–202. doi: 10.1016/S0308-8146(01)00124-8. [DOI] [Google Scholar]
- Santacruz S, Rualesa J, Eliasson AC. Three under-utilised sources of starch from the Andean region in Ecuador. Part II. Rheological characterization. Carbohyd Polym. 2003;51:85–92. doi: 10.1016/S0144-8617(02)00140-6. [DOI] [Google Scholar]
- Shandu KS, Singh N, Malhi NS. Some properties of corn grains and their flours I: physicochemical, functional and chapatti-making properties of flours. Food Chem. 2007;101:938–46. doi: 10.1016/j.foodchem.2006.02.040. [DOI] [Google Scholar]
- Shiau S, Yeh A. Effects of alkali and acid on dough rheological properties and characteristics of extruded noodles. J Cereal Sci. 2001;33:27–37. doi: 10.1006/jcrs.2000.0344. [DOI] [Google Scholar]
- Singh N, Singh J, Kaur L, Sodhi NS, Gill BS. Morphological, thermal and rheological properties of starches from different botanical sources. Food Chem. 2003;81:219–31. doi: 10.1016/S0308-8146(02)00416-8. [DOI] [Google Scholar]
- Srichuwong S, Sunarti TC, Mishima T, Isono N, Hisamatsu M. Starches from different botanical sources II: contribution of amylopectin fine structure to thermal properties and enzyme digestibility. Carbohyd Polym. 2005;62:25–34. doi: 10.1016/j.carbpol.2005.07.003. [DOI] [Google Scholar]
- Taggart P. Starch as an ingredient: manufacture and implication. In: Elliason AC, editor. Starch in food. London: Woodhead Publishing Limited; 2004. pp. 363–92. [Google Scholar]
- Thitipraphunkul K, Uttapap D, Piyachomkwan K, Takeda Y. A comparative study of edible canna (Canna edulis) starch from different cultivars. Part I. Chemical composition and physicochemical properties. Carbohyd Polym. 2003;53:317–24. doi: 10.1016/S0144-8617(03)00081-X. [DOI] [Google Scholar]
- Walker AF, Rolls BA. Infant Nutrition. In: Walker AF, Rolls BA, editors. The issues in nutrition and toxicology. New York: Jones and Bartlett Publishers; 1999. pp. 228–314. [Google Scholar]
- Yuan RC, Thompson DB, Boyer CD. Fine structure of amylopectin in relation to gelatinization and retrogradation behavior of maize starch from three wx-containing genotypes in two inbred lines. Cereal Chem. 1993;70:81–89. [Google Scholar]
- Zaidul ISM, Norulaini NAN, Omar AKM, Yamauchi H, Noda T. RVA analysis of mixtures of wheat flour and potato, sweet potato, yam and cassava starches. Carbohyd Polym. 2007;69:784–91. doi: 10.1016/j.carbpol.2007.02.021. [DOI] [Google Scholar]