Abstract
Fresh leaves of M. oleifera plants were analysed for nutritionally important phytoconstituents and feasible commercially used dehydration method were evaluated to preserve these in dehydrated leaves. Trans-lutein, trans-β-carotene and trans-zeaxanthin were found as the major carotenoids in fresh leaves, accounting for 36.9, 18.2 and 5.5 mg/100 g FW, respectively. Similarly, high amounts of ascorbic acid, α-tocopherol and total phenolic content (271.0, 36.9 and 512.0 mg/100 g FW), respectively were recorded in leaves. α-tocopherol was the most stable vitamin under all drying conditions (86.4 % retention during oven drying), compare to other studied phytoconstituents. Cabinet tray drying was found as efficient as lyophilisation to retain maximum content of total carotenoids (60.1 %), trans-β-carotene (90.1 %), 13-cis-lutein (93.2 %), and DPPH activity, however, lutein (51.3 %) and ascorbic acid (97.8 %) was best preserved by lyophilisation. During dehydration, significant trans to cis isomerization of β-carotene and lutein was also recorded. A ready to eat (RTE) chutney powder (adjunct) was developed from dehydrated leaves. The product was evaluated using Quantitative Descriptive Analysis and was accepted with a high overall quality score. The present investigation explores the nutritional potential of M. oleifera leaves and suitable methods of drying that could be useful for processed food formulation.
Keywords: Moringa oleifera, Dehydration, Drying, Nutrient composition, Antioxidant activity
Introduction
Green leafy vegetables are used as an important food source in all parts of the world and they are rich sources of bioactive compounds, minerals, and dietary fibres (Devadas and Saroja 1980). However, leafy vegetables deteriorate very quickly after harvest due to their perishable nature and become unfit for consumption and furthermore, some of them are not available throughout the year. One way to preserve such plant products is to dry them in order to preserve their desirable qualities, reduce storage volume and to extend shelf-life. In addition, it is also important to retain the biological activity of important phytoconstituents, including antioxidants and nutrients as well as avoid undesirable chemical or physical changes like browning and loss of colour (Korkida et al. 2001). Therefore, the selection of proper drying conditions is of prime importance to minimize nutrient loss and quality deterioation during dehydration. Several investigations have evaluated the influence of drying conditions on the quality characteristics of agricultural commodities and their products such as bay leaves (Demir et al. 2004), and spinach (Ozkan et al. 2007).
The main protective action of leafy vegetables has been attributed to the presence of antioxidants, including ascorbic acid, α-tocopherol, β-carotene and phenolics as these constituents are reported to fight degenerative diseases (Micozi 1989; Kaur and Kapoor 2002). The easiest and healthiest way to maintain body antioxidant status is by consumption of vegetables containing antioxidant compounds (Halliwell 2001).
Leaves, flowers and immature pods of Moringa oleifera tree (family Moringaceae) are considered as rich source of nutritionally important phytoconstituents (Nambiar and Seshadri 1998; Liu et al. 2007; Saini et al. 2012), and the different parts of this tree has an impressive range of medicinal uses (Anwar et al. 2007). Although the nutrient potential of M. oleifera has been reported, information regarding stability of phytoconstituents during dehydration is not available. Therefore, in present investigation, the effects of various drying methods on the retention of carotenoids, α-tocopherol, ascorbic acid, total phenolics and antioxidant properties were evaluated. Similarly, efforts were made to develop a ready to use food product and sensory evaluation was conducted to investigate potential consumer acceptance.
Materials and methods
Sample collection and drying
Fresh leaves (2 kg) were collected from 3 year-old Moringa oleifera (cv. PKM-1) plants grown in the field of the Central Food Technological Research Institute in August 2012 (average temperature 23–25 °C). Recommended cultural practices were followed for raising the plant in field (Saini et al. 2013). Leaves were collected early in the morning, mixed carefully, but thoroughly and divided into six portions for drying, using five different methods, and for analysis of phytoconstituents in fresh leaves. Leaves were dried until reaching a constant dry weight and the moisture content was determined after complete drying. For lyophilisation, leaves were frozen at −35 °C and dried in a freeze dryer for 24 h (Lyodryer LT5B, lyophilization Systems Inc., USA), operated at a pressure of 0.312 mb. The temperature of the plate was −20 °C and the condenser temperature was set at −64 °C. For oven drying, leaves were dried in a laboratory oven drier for 12 h (Memmert GmbH & Co., Germany) at 50 °C. For cabinet tray drying, leaves were dried in a cabinet tray dryer (Armstrong Smith, India), with a capacity of 40 trays of 400 × 800 mm at 50 °C. For sun drying, leaves were spread out on muslin cloth and kept directly in sunlight for 2–3 consecutive days. For microwave (MW) drying, the samples were subjected to intermittent heating (30 s) at 850 W power level to avoid excess heating and overcooking the material. Dehydrated leaves were powdered and stored at −80 °C in amber colour air tight containers until further analysis.
Quantification of carotenoids, chlorophylls and α-tocopherol
Carotenoids, chlorophylls and α-tocopherol were extracted and quantified from dehydrated leaves powder which was dried using different drying methods. For each sample, 1 g dry leaf powder or 5 g fresh leaves were homogenized in chilled acetone and the extraction was repeated until the samples became colourless (total volume 50 ml). The crude extracts were then centrifuged at 8,000×g and filtered through a 0.45 μm membrane (Nupore, India). A volume of 20 μl extract was injected into a HPLC system without saponification and evaporation. The same sample extract was used for quantification of chlorophyll content. Content of carotenoids and α-tocopherol were analysed by HPLC according to Darnoko et al. (2000). Six major carotenoids, i.e., trans-luteoxanthin, 13-cis-lutein, trans-lutein, trans-zeaxanthin, 15-cis-β-carotene and trans-β-carotene was purified from M. oleifera leaves and used as standards (unpublished data). A standard of α-tocopherol was purchased from Sigma Aldrich Bangalore. The HPLC system consisted of a Shimadzu chromatograph (LC 20-AD HPLC), equipped with dual pump and UV detector (SPD 20A). The column was an YMC, C30 carotenoid column, 250 × 4.6 mm, 5 mm (YMC, Wilmington, NC). The mobile phase was 81:15:4 (v/v) methanol : methyl tertiary butyl ether (MTBE) : H2O (solvent A) and 91:9 (v/v) MTBE : methanol (solvent B). The gradient elution was 100 % A to 50 %/50 % A/B in 45 min, followed by 100 % B for 10 min and 100 % A for the last 5 min at a flow rate of 1 ml/min. The same HPLC column and programme was used for quantification of α-tocopherol, except carotenoids were monitored at 450 nm and α-tocopherol at 295 nm.
Content of chlorophylls was measured and characterised by UV–VIS spectroscopy according to Lichtenthaler and Wellburn (1983). In an acetone extract, content of chlorophyll a (Chla) and b (Chlb) were calculated using the following equations, where the pigment concentrations are given in μg/ml extract solution:
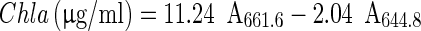 |
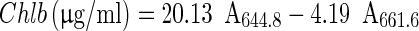 |
Quantification of ascorbic acid
Ascorbic acid was extracted under subdued light according Vanderslice et al. (1990). Fresh leaves (5 g) or dehydrated leaf powder (1 g) of M. oleifera were homogenised with 50 ml cold extraction solution, containing 3 % meta-phosphoric acid (w/v), 0.05 % EDTA (w/v), and 0.8 % glacial acetic acid (v/v). The slurry was centrifuged for 15 min at 8,000×g in a cooling centrifuge (4 °C), and the supernatant was collected. Samples were filtered through 0.22 mm membranes into amber HPLC vials. The 20 μl samples were then directly injected into the HPLC. HPLC quantification was performed using a Shimadzu chromatograph (LC 20-AD HPLC), equipped with dual pump, UV detector (SPD 20A) and a Waters YMC-Pack ODS-AQ column (12 nm, 5 μm, 150 × 4.6 mm). The separation and elution was accomplished by employing a binary gradient mode using solvent A (0.1 % trifluoroacetic acid in water, v/v) and solvent B (acetonitrile) with an injection volume of 20 μl sample at a flow rate of 1.0 ml/min for 20 min. The solvent system was run as follows (% solvent A/solvent B): 0 min (20/80), 15 min (50/50) and 20 min (20/80). Ascorbic acid was detected at 254 nm.
Total phenolic content (TPC) and antioxidant activity
TPC in fresh and dried leaf powder was determined according to the method of Zielinski and Kozlowska (2000) with minor modifications. A methanol extract (50 μl), distilled water (3 ml), Folin-Ciocalteu reagent solution (250 μl) and 7 % sodium carbonate (750 μl) were mixed and incubated for 8 min at room temperature. Then, 950 μl distilled water was added and the mixture allowed standing for 2 h at room temperature. The absorbance was measured at 725 nm using a UV-Visible spectrophotometer (Shimadzu UV 160) against a blank (reaction mixture without sample). The total phenolic content was expressed as gallic acid equivalents (mg of GAE/g sample). The linearity range of the calibration curve was 50–1,000 μg/ml (r = 0.98).
The DPPH radical-scavenging capacity of a methanol extract of M. oleifera was evaluated according to the method of Chen and Ho (1995) with slight modifications. An aliquot of 0.2 ml sample extract was added to 3.8 ml of DPPH solution in absolute ethanol (final concentration 0.1 mM). The mixture was shaken vigorously for 1 min by vortexing and allowed standing at room temperature in the dark for 30 min. Subsequently, the absorbance of the sample was measured using the UV-visible spectrophotometer at 517 nm against an ethanol blank. EC50 values (μg/ml) were calculated by interpolation from linear regression analysis.
The effect of M. oleifera leaf extract on the inhibition of lipid peroxidation was estimated according to the procedure described by Jiang et al. (2005). Egg yolk (5 ml) was mixed with 5 ml 50 mM phosphate buffer saline (PBS, pH 7.45) and stirred vigorously using a magnetic stirrer, then diluted 40 times with the same PBS to prepare a yolk suspension. The yolk suspension (0.5 ml) was incubated at 37 °C for 15 min with 1 ml crude extracts in DMSO (0, 25, 50 and 100 mg of dehydrated leaves) and 0.5 ml of 24 mM FeSO4 in water. The reaction was stopped by adding 0.5 ml 20 % trichloroacetic acid (w/v) followed by heating to 100 °C for 15 min with 1 ml 0.8 % (w/v) 2-thiobarbituric acid. The reaction mixture was centrifuged to precipitate protein and the colour of the reaction recorded at 532 nm using the UV-visible spectrophotometer. The EC50 value (μg/ml) is the concentration at which the anti-lipid peroxidation activity was 50 %, as calculated by interpolation from linear regression analysis.
Calculation of percentage true retention
To determine the loss of nutrients during drying, Per cent true retention (TR) was calculated according to Murphy et al. (1975):
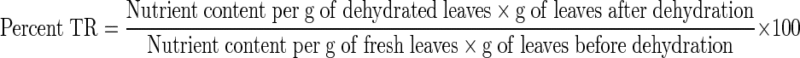 |
Preparation of chutney powder and sensory analysis
Chutney powder is a traditional product used along with main meals as an additive. As it is in a dry mix form, it has a long shelf-life without preservatives. Chutney powder was prepared using roasted peanuts (50 % w/w), chickpea (25 % w/w), chili powder (5 % w/w), garlic (1 % w/w), salt (1 % w/w) and cabinet tray dried Moringa leaf powder (8.1 % w/w). Quantity of different ingredients was fixed for high score of sensory overall quality when evaluated among the trained panelist.
Sensory analyses was carried out by 10–12 trained panelists, selected among scientific staff members familiar with sensory analysis techniques and who had earlier experiences with sensory evaluation of food mixes. The chutney powder was evaluated under white fluorescent light, with the booth area maintained at temperature 22 ± 2 °C and RH 50 ± 5 %. A suitable score card was developed using the ‘Free-Choice Profiling’ method, selecting a suitable terminology specific to the product to trace changes in the sensory qualities during storage. To avoid bias, samples were presented to the panelists in plates coded with 3-digit random numbers. A glass of water was also presented to cleanse the palate in between the samples. Quantitative Descriptive Analysis (QDA) was used to assess the quality of samples (Stone and Sidel 1998). Panelists were asked to mark on a scale of 0–15 cm to indicate the intensity of each attribute listed on the score card. The scale was anchored at 1.25 cm on either end, representing ‘Recognition Threshold’ and ‘Saturation Threshold’ respectively. The scores given for all the attributes for each sample were tabulated. Subsequently, the mean value was calculated for each attribute of a sample, representing the panel’s judgment of the sensory quality of the product. The chutney powder was evaluated as such among the trained panelist, as to avoid interference from other adjunct. For consumer acceptance study, chutney powder was evaluated along with dosa.
Statistical analysis
The sample from fresh leaves and each dehydration method was extracted and analyzed in triplicates. Values from triplicate determinations of each sample were averaged and represented as Mean. The data was analyzed statistically by SPSS 17.0 software by analysis of variance (one way ANOVA), least significant difference (LSD) was calculated between the mean values of different treatments. Different alphabetical letters were assign to demonstrate significant difference between the different treatments.
Results and discussion
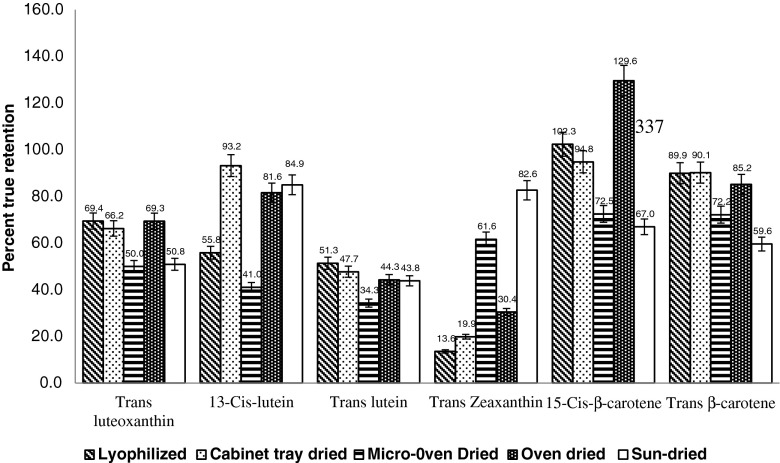
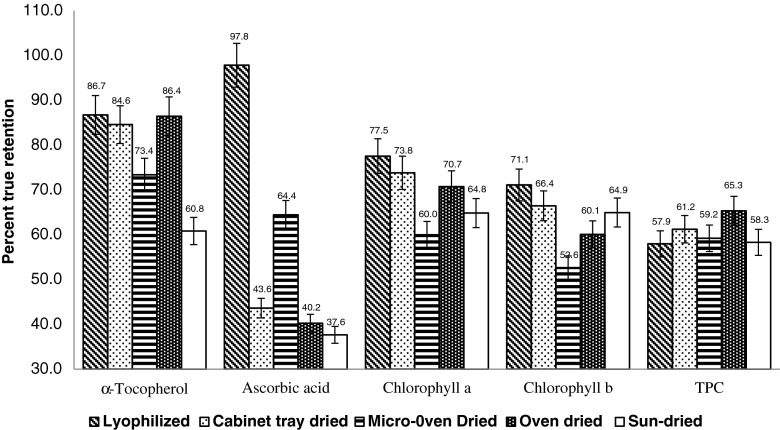
The different drying methods of fresh leaves of M. oleifera (cabinet tray, oven, lyophilisation, sun and microwave drying) had a drastic impact on nutrient composition and antioxidant activity. The content of the studied phytoconstituents are seen in Table 1. Fresh leaves dried by lyophilisation were found best suitable to retain maximum content of trans-luteoxanthin (69.4 %), trans-lutein (51.3 %), trans-β-carotene (89.9 %), total carotenoids (60.6 %), α-tocopherol (86.7 %), ascorbic acid (97.8), chlorophyll a (75.5 %) and chlorophyll b (71.1 %) (Figs. 1 and 2). Interestingly, DPPH and lipid peroxidation activity was best preserved in heat dried leaves (cabinet tray, oven and microwave dried). Cabinet tray drying was also found as efficient as lyophilisation in retaining maximum content of total carotenoids (60.1 %), trans-β-carotene (90.1 %), 13-cis--lutein (93.2 %), α-tocopherol (84.6 %) and DPPH activity. Considerably higher retention of cis-carotenoids (129.6 % for 15-cis-β-carotene) was recorded compare to trans-isomers for all the drying methods.
Table 1.
Effect of different drying methods on content and retention of nutritionally important phytoconstituents in leaves of Moringa oleifera
| Phytoconstituents | Fresh Leaves | Lyophilized | Cabinet tray dried | Micro-oven Dried | Oven dried | Sun-dried |
|---|---|---|---|---|---|---|
| Moisture content (%) | 86.5 | 6.5 | 6.5 | 6.5 | 6.5 | 6.5 |
| Trans- luteoxanthin (mg/100 g) | 5.2 | 18.05(69.4)a | 17.22b | 13c | 18.03(69.3)a | 13.22c |
| 13-Cis--lutein (mg/100 g) | 2.31 | 6.45d | 10.76(93.2)a | 4.74d | 9.42c | 9.81b |
| Trans- lutein (mg/100 g) | 36.88 | 94.63(51.3)a | 87.94b | 63.19d | 81.61c | 80.77c |
| Trans- Zeaxanthin (mg/100 g) | 5.46 | 3.7e | 5.42d | 16.82d | 8.31c | 22.55(82.6)a |
| 15-Cis--β-carotene (mg/100 g) | 0.69 | 3.53b | 3.27c | 2.5d | 4.47(129.6)a | 2.31d |
| Trans- β-carotene (mg/100 g) | 18.27 | 82.13(89.9)a | 82.33(90.1)a | 65.92c | 77.79d | 54.42d |
| Total carotenoids (mg/100 g) | 68.81 | 208.49(60.6)a | 206.94(60.1)a | 164.68c | 199.63(58)a | 183.08b |
| α-Tocopherol (mg/100 g) | 36.94 | 160.2(86.7)a | 156.2b | 135.5c | 159.6(86.4)a | 112.3d |
| Ascorbic acid (mg/100 g) | 271.0 | 1,325.6(97.8)a | 590.8c | 872.9b | 545.0d | 510.0d |
| Chlorophyll a (mg/100 g) | 166.29 | 644.6(75.5)a | 613.7b | 498.6d | 587.9c | 539c |
| Chlorophyll b (mg/100 g) | 49.58 | 176.2(71.1)a | 164.7b | 130.4d | 148.9c | 161b |
| TPC (mg of GAE/100 g) | 512 | 1,483.5c | 1,566.9b | 1,515d | 1,672.1(65.3)a | 1,492.6c |
| DPPH Activity [EC50 (μg/ml)] | 1,230 | 290ab | 272.5c | 280.1c | 277.5bc | 300a |
| Lipid peroxidation Activity [EC50 (μg/ml)] | 1,020 | 312b | 305b | 292.3c | 290c | 348a |
Values are averages of three replications (standard deviation < 5 %). In a row, means marked with the same letters are not significantly different (P < 0.05). Values in parenthesis represent the percent true retention (TR) of maximum stable phytoconstituents
Fig. 1.
The impact of different drying methods on % true retention of carotenoids and their isomers in dehydrated leaves of M. oleifera. Values above bars displaying the data labels
Fig. 2.
Impact of different drying methods on % true retention of α-tocopherol, ascorbic acid and chlorophyll a and b in dehydrated leaves of M. oleifera. Values above bars displaying the data labels
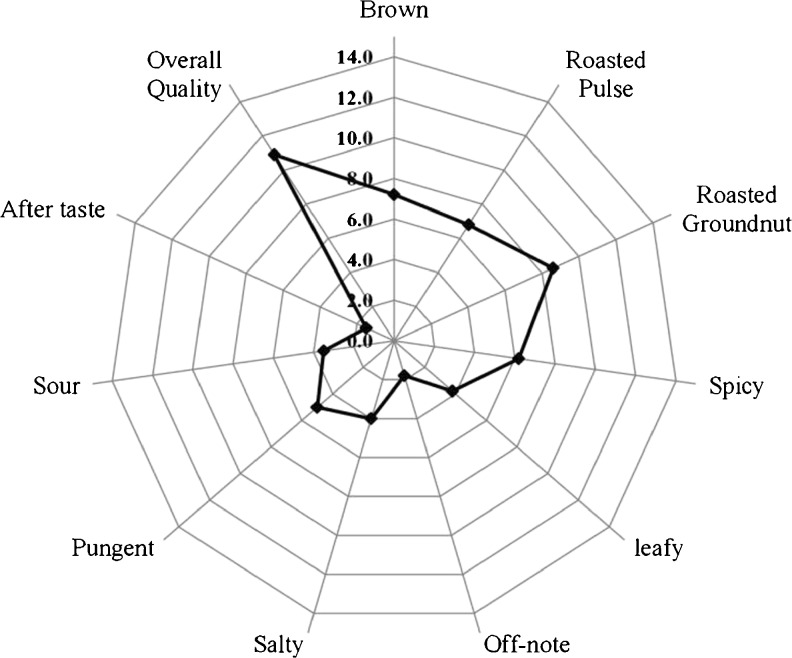
The product (chutney powder) developed from dehydrated leaves had high quality score for desirable attributes such as ‘roasted pulse-like’ and ‘groundnut-like’ aroma, as shown in Fig. 3. Although the product had a slight leafy and off-note characteristic of Moringa leaves, this was masked by addition of spices and other ingredients which resulted in high overall quality score (11) for the Chutney powder.
Fig. 3.
Sensory profile of Moringa chutney powder, showing the quality score of desirable attributes
In present investigation, the effects of various drying methods on the retention of carotenoids, α-tocopherol, ascorbic acid, total phenolics and antioxidant properties were evaluated. M. oleifera leaves processed by different methods retained a high phenolic content and exhibited strong free radical scavenging and lipid peroxidation activity. Potent free radical scavenger activity has been recorded in M. oleifera leaves, indicating a significant source for natural antioxidants to prevent free radical mediated oxidative damage. Different drying methods were shown to reduce all the studied phytoconstituents significantly, with sun- and microwave giving the highest decrease in the studied nutrients and antioxidant activity. Different drying methods least affect the TPC and α-tocopherol, which accounted 57.9–65.3 and 60.8–86.7 % retention, respectively, indicating that they are rather stable to different dehydration conditions. The low retention of nutrients and antioxidant activity in sun-dried leaves might have been caused by enzymatic processes (Mueller-Harvey 2001) that may lead to significant changes in the composition of phytochemicals (Capecka et al. 2005). A number of dehydration procedures are employed for fruits and vegetables, i.e., sun-, cross flow-, drum-, spray-, puff-, freeze- and microwave-drying (Chong and Law 2010). For most vegetables, drying resulted in 10–20 % loss of carotenoids (Clydesdale et al. 1991), with the increased surface area of dried or powdered products leading to further losses (through autoxidation) unless they were protected from air and light. Specially, dehydration (heating) cause significant quantitative changes in carotenoid isomers, which was also observed in present investigation. Due to possible trans- to cis- somerization of β-carotene and lutein, higher retention of cis-isomers were recorded in oven and cabinet tray dried leaves. In general, cis-isomers of carotenoids exhibit less potent provitamin activity compare to trans-isomers, which cause further loss of provitamin activity in dehydrated leaves (Castenmiller and West 1998). Degradation of β-carotene and lutein and formation of cis-isomers (4–40 %) during thermal dehydration studied in number of fruits and vegetables including peas, broccoli, kale, spinach, and corn (Updike and Schwartz 2003).
Impact of heat dehydration on content of tocopherol in vegetables was not studied previously. In the present investigation, α-tocopherol was found comparatively more stable under all the drying conditions then other studied vitamins, which can help in preserving the antioxidant potential of vegetables during dehydration, since, α-tocopherol is known as potent antioxidant.
Moringa leaves are a rich source of ascorbic acid, but dehydration has been reported to have a very unfavourable effect on its retention (Toor and Savage 2006). Recently, Demiray et al. (2013) studied the kinetics of degradation of lycopene, β-carotene and ascorbic acid in tomato during hot air drying and suggested the drying temperature should be less than 70 °C to preserve the ascorbic acid at maximum extent. Similarly, retention of antioxidant capacity is also decreased with increasing dehydration temperature (Demarchi et al. 2012). In the present study, only lyophilisation was found to be effective to retain the maximum amount of ascorbic acid (97.8 %), followed by oven drying (64.4 %), suggest the use of lyophilisation based dehydration method to preserve the ascorbic acid in dehydrated leaves.
A high correlation has been reported between free radical scavenging and the phenolic contents in various culinary herbs (Zheng and Wang 2001). Similar results were also seen in our study (Table 1), where, DPPH and lipid peroxidation activity was decreased with decrease in TPC. High temperatures or long drying times in conventional air drying may also cause serious damage to the quality of the dried product such as flavour and colour (Vadivambal and Jayas 2007). Similar results were found in our study, where significantly lower retention of the studied phytoconstituents were recorded for the sun-drying (prolonged thermal treatment) and micro wave drying (intense heating), compare to cabinet tray, oven drying and lyophilisation.
Chlorophylls and carotenoids are very common pigments, which give colour to vegetables and several fruits. Because of their colour and nutritional properties, they are also used as additives to food products (Schoefs 2003). In our study, higher retention of chlorophylls a (73.8 %) and b (66.4 %) was recorded for cabinet tray drying, which explains the quality of (dark green colour) of the dehydrated powder and favour better consumer acceptance. Chlorophyll a was reported to be thermally less or equally stable than chlorophyll b (King et al. 2001), which is largely due to a function of water activity in the material (Schwartz and Lorenzo 1991). Interestingly, higher retention of chlorophyll a than chlorophyll b was observed for all the drying methods and this has not been pointed out previously. Park (1987) observed that processing, regardless of drying method, significantly reduced the carotene content in vegetables. However, carotene levels in vacuum dried (16 h at 55 °C, 15 in. mm Hg) broccoli and spinach were significantly higher than those of the microwave dried (high heat setting, 750 W) vegetables. In contrast, in industrial dehydration (hot air drying at 65 °C) and lyophilisation (freezing at −30 °C and lyophilisation at −10 °C) of spinach, only a 12 % loss of α-carotene was reported (Ramos and Rodriguez-Amaya 1993). However, adoption of lyophilisation is difficult by the industries due to high operational and set-up cost (Liapis and Bruttini 2006). Compare to oven drying, better retention of studied phytoconstituents in cabinet tray drying is mostly due to forced convection of heated air in cabinet tray dryer. In the present study, for oven and cabinet tray drying, drying time was 12 h and 6 h, respectively at temperature of 50 °C. This reduced drying time is mostly attributed to better retention of phytoconstituents in cabinet tray dried leaves. Influence of natural and forced convection on retention of ascorbic acid was clearly observed in dasheen (Colocasia esculenta) leaves (Maharaj and Sankat 1996). In this study, the losses of ascorbic acid were higher than 90 % in leaves dried under natural convection. Whereas, the losses of ascorbic acid was ranged from 81.8 to 72.6 % in forced convection drying. (Muratore et al. 2008), studied the effect of drying temperature and drying time on retention of lycopene and β-carotene in tomatoes slices, and concluded that length of drying is more crucial then drying temperature.
Conclusion
In conclusion, high amount of carotenoids, tocopherol, ascorbic acid and antioxidant potential were recorded in fresh leaves of M. oleifera and cabinet tray drying, also known as cross flow drying, can be recommended to preserve these phytoconstituents and antioxidant potential, with minor loss of constituents in dehydrated leaves of M. oleifera in place of lyophilisation. For household purposes, it may be better to use oven-drying instead of sun-drying, since sun-drying was not suitable to preserve nutrients and antioxidant activity even though it is a commonly used method to dry leafy vegetables in many households in tropical countries. A ready to use chutney powder (additive) was prepared from cabinet tray dried leaves of M. oleifera (retain maximum nutrients) and it was well accepted with overall high quality score.
Acknowledgments
The authors wish to thank the Council of Scientific and Industrial Research, New Delhi (India) for financial support.
References
- Anwar F, Latir S, Ashraf M, Gilani AH. Moringa oleifera: a food plant with multiple medicinal uses. Phytother Res. 2007;21:17–25. doi: 10.1002/ptr.2023. [DOI] [PubMed] [Google Scholar]
- Capecka E, Mareczeek A, Leja M. Antioxidant activity of fresh and dry herbs of some Lamiaciae species. Food Chem. 2005;93:223–226. doi: 10.1016/j.foodchem.2004.09.020. [DOI] [Google Scholar]
- Castenmiller JJM, West CE. Bioavailability and bioconversion of carotenoids. Annu Rev Nutr. 1998;18:19–38. doi: 10.1146/annurev.nutr.18.1.19. [DOI] [PubMed] [Google Scholar]
- Chen CW, Ho CT. Antioxidant properties of polyphenols extracted from green and black teas. J Food Lipids. 1995;2:35–46. doi: 10.1111/j.1745-4522.1995.tb00028.x. [DOI] [Google Scholar]
- Chong CH, Law CL (2010) Drying of exotic fruits. In: Jangam SV, Law CL, Mujumdar AS (eds) Drying of exotic fruits, vegetables and fruits, vol 2. Singapore, pp 1–42
- Clydesdale FM, Ho C-T, Lee CY, Mondy NI, Shewfelt RL, Lee K. The effects of postharvest treatment and chemical interactions on the bioavailability of ascorbic acid, thiamin, vitamin A, carotenoids, and minerals. CRC Crit Rev Anal Chem. 1991;30:599–638. doi: 10.1080/10408399109527558. [DOI] [PubMed] [Google Scholar]
- Darnoko D, Cheryan M, Moros E, Jerrel J, Perkins EG. Simultaneous HPLC analysis of palm carotenoids and tocopherols using a C-30 column and photodiode array detector. J Liq Chromatogr Relat Technol. 2000;23:1873–1885. doi: 10.1081/JLC-100100459. [DOI] [Google Scholar]
- Demarchi SM, Quintero Ruiz NA, Concellon A, Giner SA. Effect of temperature on hot-air drying rate and on retention of antioxidant capacity in apple leathers. Food Bioprod Process. 2012;91:310–318. doi: 10.1016/j.fbp.2012.11.008. [DOI] [Google Scholar]
- Demir V, Gunhan T, Yagcioglu AK, Degirmencioglu A. Mathematical modelling and the determination of some quality parameters of air-dried bay leaves. Biosyst Eng. 2004;88:325–335. doi: 10.1016/j.biosystemseng.2004.04.005. [DOI] [Google Scholar]
- Demiray E, Tulek Y, Yilmaz Y. Degradation kinetics of lycopene, β-carotene and ascorbic acid in tomatoes during hot air drying. LWT-Food Sci Technol. 2013;50:172–176. doi: 10.1016/j.lwt.2012.06.001. [DOI] [Google Scholar]
- Devadas RP, Saroja S. Proceedings of the second Amaranthus conference. Emmaus: Rodale Press Inc; 1980. Availability of Fe and β-carotene from Amaranthus to children; pp. 15–21. [Google Scholar]
- Halliwell B. Role of free radicals in the neurodegenerative diseases: therapeutic implications for antioxidant treatment. Drug Aging. 2001;18:685–716. doi: 10.2165/00002512-200118090-00004. [DOI] [PubMed] [Google Scholar]
- Jiang YH, Jiang XL, Wang P, Hu XK. In vitro antioxidant activities of water-soluble polysaccharides extracted from Isaria Farinosa B05. J Food Biochem. 2005;29:323–335. doi: 10.1111/j.1745-4514.2005.00040.x. [DOI] [Google Scholar]
- Kaur C, Kapoor HC. Anti-oxidant activity and total phenolic content of some Asian vegetables. Int J Food Sci Tech. 2002;37:153–161. doi: 10.1046/j.1365-2621.2002.00552.x. [DOI] [Google Scholar]
- King VA, Liu CF, Liu YJ. Chlorophyll stability in spinach dehydrated by freeze-drying and controlled low-temperature vacuum dehydration. Food Res Int. 2001;34:167–175. doi: 10.1016/S0963-9969(00)00148-4. [DOI] [Google Scholar]
- Korkida MK, Maroulis ZB, Saravacos GD. The effect of method of drying on the colour of dehydrated products. Int J Food Sci Tech. 2001;36:53–59. doi: 10.1046/j.1365-2621.2001.00426.x. [DOI] [Google Scholar]
- Liapis AI, Bruttini R (2006) Freeze drying. In: Mujumdar AS (ed) Handbook of industrial drying, 3rd edn. CRC Press, Boca Raton, pp 257–283
- Lichtenthaler HK, Wellburn AR. Determination of total carotenoids and chlorophylls a and b of leaf in different solvents. Biochem Soc T. 1983;11:591–592. [Google Scholar]
- Liu Y, Perera CO, Suresh V. Comparison of three chosen vegetables with others from South East Asia for their lutein and zeaxanthin content. Food Chem. 2007;101:1533–1539. doi: 10.1016/j.foodchem.2006.04.005. [DOI] [Google Scholar]
- Maharaj V, Sankat CK. Quality changes in dehydrated dasheen leaves: effects of blanching pre-treatments and drying conditions. Food Res Int. 1996;29:563–568. doi: 10.1016/S0963-9969(96)00021-X. [DOI] [Google Scholar]
- Micozi MS (1989) Foods, micronutrients, and reduction of human cancer rates. In: Moon TE, Micozzi MS (eds) Nutrition and cancer prevention: investigating the role of micronutrients. Marcel Decker, New York, pp 213–242
- Mueller-Harvey I. Analysis of hydrolysable tannins. Anim Feed Sci Tech. 2001;91:3–20. doi: 10.1016/S0377-8401(01)00227-9. [DOI] [Google Scholar]
- Muratore G, Rizzo V, Licciardello F, Maccarone E. Partial dehydration of cherry tomato at different temperature, and nutritional quality of the products. Food Chem. 2008;111:887–891. doi: 10.1016/j.foodchem.2008.05.001. [DOI] [Google Scholar]
- Murphy EW, Criner PE, Gray BC. Comparisons of methods for calculating retentions of nutrients in cooked foods. J Agric Food Chem. 1975;23:1153–1157. doi: 10.1021/jf60202a021. [DOI] [PubMed] [Google Scholar]
- Nambiar VS, Seshadri S. A study on β-carotene content of some green leafy vegetables of Western India by high performance liquid chromatography. J Food Sci Tech. 1998;35:365–367. [Google Scholar]
- Ozkan IA, Akbudak B, Akbudak N. Microwave drying characteristics of spinach. J Food Eng. 2007;78:577–583. doi: 10.1016/j.jfoodeng.2005.10.026. [DOI] [Google Scholar]
- Park YW. Effect of freezing, thawing, drying and cooking on carotene retention in carrots, broccoli and spinach. J Food Sci. 1987;52:1022–1025. doi: 10.1111/j.1365-2621.1987.tb14266.x. [DOI] [Google Scholar]
- Ramos DMR, Rodriguez-Amaya DB. Assessing losses of carotenoids and vitamin A value during industrial dehydration and freeze drying of spinach. Arq Biol Tecnol. 1993;36:83–94. [Google Scholar]
- Saini RK, Shetty NP, Giridhar P, Ravishankar GA. Rapid in vitro regeneration method for Moringa oleifera and performance evaluation of field grown nutritionally enriched tissue cultured plants. 3. Biotech. 2012;2:187–192. [Google Scholar]
- Saini RK, Saad KR, Ravishankar GA, Giridhar P, Shetty NP. Genetic diversity of commercially grown Moringa oleifera Lam. cultivars from India by RAPD, ISSR and cytochrome P450-based markers. Plant Syst Evol. 2013;299:1205–1213. doi: 10.1007/s00606-013-0789-7. [DOI] [Google Scholar]
- Schoefs B. Chlorophyll and carotenoid analysis in food products. A practical case-by-case view. Trends Anal Chem. 2003;22:335–339. doi: 10.1016/S0165-9936(03)00602-2. [DOI] [Google Scholar]
- Schwartz SJ, Lorenzo TV. Chlorophyll stability during continuous aseptic processing and storage. J Food Sci. 1991;56:1059–1062. doi: 10.1111/j.1365-2621.1991.tb14641.x. [DOI] [Google Scholar]
- Stone S, Sidel JL. Sensory evaluation by Quantitative descriptive analysis, developments, application and future. Food Technol. 1998;52:48–52. [Google Scholar]
- Toor RK, Savage GP. Effect of semi-drying on the antioxidant components of tomatoes. Food Chem. 2006;94:90–97. doi: 10.1016/j.foodchem.2004.10.054. [DOI] [Google Scholar]
- Updike AA, Schwartz SJ. Thermal processing of vegetables increases cis-isomers of lutein and zeaxanthin. J Agric Food Chem. 2003;51:6184–6190. doi: 10.1021/jf030350f. [DOI] [PubMed] [Google Scholar]
- Vadivambal R, Jayas DS. Changes in quality of microwave-treated agricultural products - a review. Biosyst Eng. 2007;98:1–16. doi: 10.1016/j.biosystemseng.2007.06.006. [DOI] [Google Scholar]
- Vanderslice JT, Higgs DJ, Hayes JM, Block G. Ascorbic acid and dehydroascorbic acid content of foods-as-eaten. J Food Compos Anal. 1990;3:105–118. doi: 10.1016/0889-1575(90)90018-H. [DOI] [Google Scholar]
- Zheng W, Wang SY. Antioxidant activity and phenolic compounds in selected herbs. J Agric Food Chem. 2001;49:5165–5170. doi: 10.1021/jf010697n. [DOI] [PubMed] [Google Scholar]
- Zielinski H, Kozlowska H. Antioxidant activity and total phenolics in selected cereal grains and their different morphological fractions. J Agric Food Chem. 2000;48:2008–2016. doi: 10.1021/jf990619o. [DOI] [PubMed] [Google Scholar]