Abstract
The optimal extraction of bioactive compounds from longan fruit pulp using Pectinex® Ultra SP-L pectinase hydrolysis of the fruit homogenate was evaluated. The highest degree of hydrolysis (DH), as determined by the amount of reducing sugars released from the longan pulp, was obtained at a pectinase concentration of 2.5 % (v/w) (257 polygalacturonase units/g fruit) for 4 h. The level of bioactive compounds obtained from the pectinase-treated longan pulp increased with increasing DH to a maximum at the highest DH (21 %) obtained, with an antioxidant activity of 0.083 EC50 μg fresh mass (FM)/μg diphenyl-(2,4,6-trinitrophenyl)iminoazanium and 92.7 μM Trolox equivalent/g FM, respectively. The total phenolic and flavonoid contents in the 21 % DH extract were 196.0 mg gallic acid equivalents/g FM and 19.6 mg catechin equivalents/g FM, respectively. The 21 % DH longan extract showed an enhanced (3.6- to 4.0-fold) inhibition of lipid peroxidation of oil compared to the untreated (0 % DH) extract. In addition, the 21 % DH longan extract had the highest soluble dietary fiber content, which was related to the decreased particle size of 345 μM, and displayed enhanced prebiotic activity scores of 1.69 and 1.44 for Lactobacillus acidophilus La5 and Bifidabacterium lactis Bb12, respectively. Most of the 33 detected volatile compounds differed in their relative proportions after enzymic extraction (15 increased, 15 decreased with three showing no significant change) with the 0 % and 21 % DH hydrolysates exhibiting 25 and 22 different volatile compounds, respectively, with 11 and eight unique compounds between them, respectively.
Keywords: Bioactive compounds, Longan pulp, Pectinase, Enzymatic hydrolysis, Antioxidant activity
Introduction
Bioactive compounds synthesized by plants (phytochemicals) have the potential to be used by humans for a variety of applications. Essential and non-essential bioactive compounds are present in a vast range of foods (such as fruits, vegetables and grains) and are consumed as part of the human diet. The dietary inclusion or supplementation with non-essential bioactive compounds, including coumarins, flavonoids, lignans, phenolic acids, tannins, terpenoids and xanthones, has been linked to promoting optimal health and reducing the risk of some chronic diseases, such as cancer, coronary heart disease, stroke and Alzheimer’s disease that are directly related to oxidative stress (Denny and Buttriss 2007; Biesalski et al. 2009; Iriti and Faoro 2009; Zuchi et al. 2010; Zhou and Raffoul 2012). Accordingly, there is a growing interest in these phytochemical compounds in food due to their potential health- or food-protecting capacity, such as through strong antioxidant and antiproliferative activity or xenohormone activity, (Chu et al. 2002; Sun et al. 2002).
Natural bioactive compounds include a broad diversity of structures and functionalities that theoretically provide a diverse pool of molecules for the production of nutraceuticals, functional foods, and food additives (Shahidi 2009; Ayala-Zavala et al. 2010). One approach to directing searches for likely useful bioactive compounds, either as highly active but scarce or less active but relatively common constituents, is to use existing folklore medicinal applications and screen the plant parts traditionally used for medical ailments or longevity.
Longan (Dimocarpus longan Lour.) from the family Sapindaceae is a famous subtropical fruit in Southeast Asia, especially in China, Vietnam and Thailand. It has been used in traditional Chinese medicines for health benefits for more than a century, such as to promote blood metabolism, increase immunity, relieve insomnia, and to improve learning and memory enhancement (Yang et al. 2008; Park et al. 2010). Polysaccharides extracted from longan pulp with medium and low dose ultrasonication have been reported to exhibit excellent immunomodulatory properties and an antitumor effect in the S180 tumor mice model (Zhong et al. 2010). High pressure-assisted extraction of the polyphenol-rich longan pericarp exhibited strong dose-dependent antioxidant activities, such as inhibiting linoleic acid oxidation and having free-radical scavenging activity against diphenyl-(2,4,6-trinitrophenyl)iminoazanium (DPPH) radicals, superoxide anion and hydroxyl radicals (Prasad et al. 2010). The water extract of longan seeds demonstrated a tyrosinase inhibitor activity that could be developed for use in pharmaceutical, food and cosmetic products (Rangkadilok et al. 2007). Thus, some of the beneficial effect and dietary antioxidants of longan seem to come from its non-essential bioactive compounds, such as polysaccharides, phenolic acids, and flavonoids.
Whilst some non-essential bioactive are found at high concentrations in plants, such as polyphenols, others can only be found at very low levels. Moreover, they are typically synthesized in specialized cell types and only during a particular growth stage or specific season/conditions, making their extraction and purification quite difficult. Therefore, given that their structural diversity and complexity that make chemical synthesis unprofitable, massive harvesting is required to obtain sufficient quantities. Thus, optimization of the extraction of bioactive compounds is an essential logisitic and economic requirement.
Many extraction techniques, such as hot water or other less-polar solvent extractions, and improvements to this, such as ultra-high pressure, ultrasonic and microwave-assisted extraction (Rangkadilok et al. 2007; Pan et al. 2008; Prasad et al. 2010; Zhong et al. 2010; Reddy and Urooj 2013) have been widely used to extract bioactive compounds from plants, but are not without problems. Solvent extraction has a low processing cost and ease of operation but uses toxic solvents, requires an evaporation/concentration step for recovery, calls for large amounts of solvent, a long processing time and gives a low yield (Yang et al. 2011). Moreover, thermal degradation of the desired products can occurr. Improvement by other methods, such as soxhlet, ultrasound, or microwave extraction can give better yields (Szentmihalyi et al. 2002) does not circumvent the requirement to remove the solvent from the product (especially if the product is to be used in food applications), the environmental cost of the solvents, and the laborious extraction conditions (Starmans and Nijhuis 1996; Li et al. 2006; Miron et al. 2010). Likewise, although pressurized liquid extraction, which uses a high temperature (50–200 °C) and pressure (1,450–2,175 psi), provides a rapid extraction rate of compounds with less solvents, it does not circumvent that the solvent must still be removed (unless generally recognized as safe solvents were used), that the compounds must be thermally stabile, and it requires a high energy input and prior knowledge of the compounds to match the polarity of the solvent to that of the compound(s) (Dunford et al. 2010; Miron et al. 2010; Plaza et al. 2010; Ajila et al. 2011). Indeed, thermal stability of the desired bioactive compound limits the application of the more environmental friendly subcritical and supercritical fluid extraction.
Plant cell walls and some internal structures contain polysaccharides, such as cellulose, hemicellulose and pectins, which act as barriers to the release of intracellular substances. Moreover, many bioactive compounds bind to these components. Some enzymes, such as cellulase, β-glucosidase, xylanase, β-gluconase and pectinase, can depolymerize these polysaccharides and degrade the cell wall structure, facilitating the release of linked compounds (Moore et al. 2006; Chen et al. 2010). Hence, these enzymes have been used to optimize the extraction of compounds from the plant matrix (Kim et al. 2005; Wilkins et al. 2007; Wang et al. 2010). For example, flavonoids and phenolic compounds interact with the cell wall cellulose, hemicellulose, and pectin (Kim et al. 2005; Fu et al. 2008), and can be released by cell wall-hydrolyzing enzymes, such as β-glycosidase that breaks the β-1,4 glucosidic linkages in glucosides (flavonoids in conjunction with glucose) (Yang et al. 2010) and xylanases, β-gluconases and cellulases that hydrolyze the ester-linked phenolic acids (Moore et al. 2006).
Enzyme-based extraction can function under mild processing conditions in aqueous solutions (Gardossi et al. 2009), and the degradation or disruption of the cell walls and membranes typically leads to a more efficient extraction of bioactive components (Pinelo et al. 2006), including polysaccharides, oils, natural pigments, flavors and medicinal compounds (Barzana et al. 2002; Wu et al. 2007; Passos et al. 2009; Sowbhagya and Chitra 2010; Yang et al. 2010). Recent studies on enzyme-assisted extraction have shown faster extraction, higher recovery, reduced solvent usage and lower energy consumption when compared to non-enzymatic methods (e.g. Barzana et al. 2002; Yang et al. 2010; Dehghan-Shoar et al. 2011). For example, the enzyme-aided extraction of lycopene from tomato tissues using cellulases and pectinases under optimized conditions resulted in an over two-fold higher lycopene yield (Choudhari and Ananthanarayan 2007).
With respect to tropical fruits, that are of interest due to their medicinal application and typically low levels of lignocelluloses, simple enzyme-assisted extraction has been shown to be one of the more effective techniques that can enhance the extraction of antioxidant activity, soluble dietary fiber (SDF) and volatile compounds from tropical fruits (Charoensiddhi and Anprung 2010; Wuttisit and Anprung 2011; Anprung and Sangthawan 2012).
This research used Pectinex® Ultra SP-L, a commercial pectinase, for the enzyme-assisted extraction to enhance the yields of extracted bioactive compounds from longan fruits. The effect of the degree of hydrolysis (DH) on the level of antioxidant activity, total SDF, prebiotic activity score, particle size, volatile compound composition and lipid peroxidation inhibition levels were evaluated and then compared with that from longan extracts without enzyme treatment (0 % DH). The pectinase-mediated biological extraction is somewhat similar to the natural ripening of fruit and is easy to perform. The application of pectinase-assisted extraction helps to release bioactive compounds from plant cell walls because the pectinase-mediated hydrolysis of the pectin glycosidic bonds leads to the release of smaller bioactive or prebiotic polysaccharides and the bound bioactive components so gives a higher extraction yield (Karunasawat and Anprung 2010).
Materials and methods
Materials
Mature longan fruits, Dimocarpus longan Lour. Cv. Edor, were purchased from Jatujak market (Bangkok, Thailand). Pectinex® Ultra SP-L, a commercial enzyme, with a stated enzyme activity of 10,292 polygalacturonase units (PGU)/mL, was purchased from Novozyme Co. (Bagsvacrd DK-2880, Denmark). All other chemicals and reagents used were of analytical grade and were purchased from Sigma Chemical Co., Ltd. (St. Louis, Mo, USA) or Sigma Aldrich Co., Ltd. (Steinheim, Germany).
Methods
Longan pulp preparation
Longan fruit pulp was homogenized in a blender for 3 min and then, to inhibit the browning reaction, was steam blanched until the center reached 85 °C and held for 5 min before being rapidly cooled and stored at 4 °C in the dark condition until used.
Enzymatic hydrolysis
Homogenized longan fruit pulp was treated by the commercial Pectinex® Ultra SP-L pectinase at 0, 0.5, 1, 1.5, 2 and 2.5 % (v/w) (equivalent to 51.5, 103, 154, 203 and 257 PGU/g fresh mass (FM)) at 32 °C for 0, 1, 2, 3, 4 and 5 h. The reaction was then stopped by boiling at 100 °C for 5 min. The released reducing sugar concentration was analyzed using the Nelson-Somogyi method (Somogyi 1952; Nelson 1944) and the DH was determined using Eq. (1),
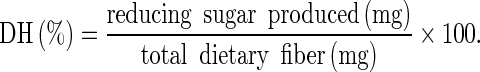 |
1 |
Determination of antioxidant activity
Samples were prepared by the modified method of Velioglu et al. (1998). The free radical-scavenging activity of longan extract was measured using the DPPH assay according to the method of Maisuthisakul et al. (2007). The antioxidant activity, as a percentage of the scavenging activity on the DPPH radical, was evaluated from Eq. (2);
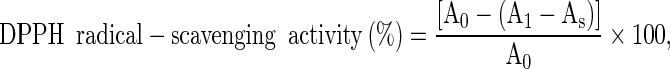 |
2 |
Where A1 and As are the absorbance (measured at 517 nm) for the diluted sample with and without DPPH, respectively, and A0 is that for the DPPH solution without longan extract (control). The percentage of DPPH radical-scavenging activity was plotted against the longan extract concentration (μg/mL) to determine the EC50, the amount of extract necessary to decrease the DPPH radical concentration by 50 %.
The ferric reducing antioxidant power (FRAP) assay was performed as reported (Anprung and Sangthawan 2012). The standard curve was linear between 82 and 625 μm Trolox. The results are expressed in μm Trolox equivalent (TE)/g FM.
Determination of total phenolic and flavonoid compounds
The total level of phenolics in each longan extract was determined using the Folin-Ciocalteu reagent according to method of Waterhouse (2005) and measuring the absorbance at 756 nm. The standard curve was linear between 50 and 500 ng gallic acid equivalent (GAE)/mL. The results are expressed as mg GAE/g FM.
The total flavonoid content in each longan extract was evaluated by the method of Zhishen et al. (1999), measuring the absorbance at 510 nm. Measurements were calibrated to a catechin standard curve (20–100 ng/mL). The results are expressed as mg catechin equivalent (CE)/g FM.
Determination of the dietary fiber level
The total dietary fiber (TDF), SDF and insoluble dietary fiber (IDF) levels in each longan extract were analyzed according to standard AOAC methods (AOAC 1995). The results are expressed as g/100 g FM.
Determination of prebiotic activity
The prebiotic activity assay was performed as reported (Anprung and Sangthawan 2012). Lactobacillus acidophilus La5 and Bifidobacterium lactis Bb12 (Christian Hansen, Denmark) were used as the representative probiotic cultures (P), while Escherichia coli (ATCC 25922) (Culture Collection Unit, Chulalongkorn hospital, Thailand) was used as the representative enteric species (E). Inulin, a commercial prebiotic, was used as the reference standard for comparison. Each assay was performed in triplicate, measuring the number of viable colony forming units (CFU)/mL before (P0 and E0) and after (P24 and E24) incubation for 24 h on 1 % (w/v) glucose (PG and EG), 1 % (w/v) of the test longan hydrolysate or 10 mg/mL inulin as the prebiotic (Px and Ex) as reported (Anprung and Sangthawan 2012). The prebiotic activity score was then determined using Eq. (3),
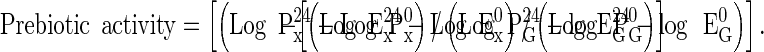 |
3 |
Volatile compound analysis
Solid-phase microextraction (SPME), as reported by Chen et al. (2006), was used to isolate the volatile compounds from longan fruits and analyzed by gas chromatography–mass spectrometry (GC-MS) using an Agilent 6890 GC equipped with an Agilent 5973 mass-selective detector (Agilent Technologies). The HP-Innowax column dimension were 0.25 mm × 30 m × 0.25 μm film thickness. The carrier gas was Helium which had flow rate of 13.7 ml/min. The column temperature program was isothermal for 10 min at 50 °C, rised to 240 °C for 10 min with the rate of 15 °C/min. The injection port temperature is 200 °C while the detector port temperature is 250 °C. Eletron impact mass spectra were recorded at 70 eV. Em voltage was 1576.5 V. The scan range was 10–300 amu. The volatile identification was obtained by matching the mass spectra (quality match >80 %) against the system library (Wiley 7).
Particle size measurement
Particle size was evaluated using a Mastersizer 2000 analyzer (Malvern) with a refractive index of the hydrolysate of 1.353, a laser obscuration of 5.17 ± 0.13 %, pump speed of 2,500 rpm, absorption of 0 and sample dispersant in distilled water (Worrasinchai et al. 2006). The results are reported as volume weighted mean d[4,3] (μm). Each analyzed sample was replicated four times.
Determination of lipid peroxidation
Each hydrolysate was screened for the ability to inhibit lipid peroxidation of soybean oil, olive oil and lard using the modified thiobarbituric acid reactive substances (TBARS) assay (Cruz et al. 2000). The standard curve was obtained using malondialdehyde-bis-diethyl-acetal, whilst 1 mg/mL butylated hydroxytoluene (BHT), a synthetic antioxidant agent, was used as a comparative standard. The results are expressed as mg malondialdehyde (MDA)/g FM.
Statistical analysis
SPSS version 19 software was used for the statistical analyses. The results are shown as the mean ± 1 standard deviation (SD), derived from three replications. The significance between different means was tested for using one-way analysis of variance (ANOVA) and Duncan’s new multiple range test (DMRT), with significance accepted at the p ≤ 0.05 level.
Results and discussion
Enzyme hydrolysis
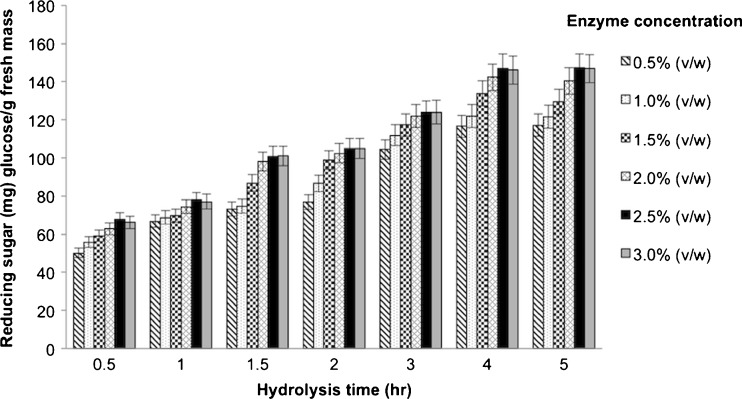
Increasing the pectinase concentration (0.5–3.0 % (v/w); 51–257 PGU/g FM) and hydrolysis time (0.5–5 h) both significantly increased the level of released reducing sugar level, and so the DH, up to 2.5 % (v/w) pectinase and a 4 h reaction time (Fig. 1). Further increasing either the enzyme concentration or the reaction time had no significant effect upon the DH obtained (maximal at 21 % DH). The increased reducing sugar level represents the hydrolysis of the glycosidic bonds in the longan pulp. From the reducing sugar yield (% DH) over time with 2.5 % (v/w) of pectinase (Fig. 1), a DH of longan pulp of 9, 13, 17 and 21 % was obtained after 1, 2, 3 and 4 h digestion, respectively, and these hydrolysates were selected, along with the 0 % DH (no enzyme treatment) hydrolysate, to screen for bioactive compounds.
Fig. 1.
Changes in the reducing sugar content during enzyme hydrolysis. Data are shown as the mean ± 1 SD and are derived from three independent repeats
Antioxidant activity
The 0, 9, 13, 17 and 21 % DH longan hydrolysates were screened for antioxidant activity using the DPPH and FRAP assays, with the results summarized in Table 1. Longan extract with a 21 % DH exhibited the highest level of antioxidant activity, with EC50 values of 0.083 μg FM/μg DPPH and 92.7 μM TE/g FM. These results agree with a previous study on the DPPH antioxidant activity of Thai bael fruit pulps (Aegle marmelos (L.) Corr. Serr.), where extracts with a higher DH had a higher antioxidant activity (Charoensiddhi and Anprung 2010). This presumably reflects the release of bound antioxidants from the plant cell walls as the protopectin is hydrolyzed by pectinase (Cinar 2005; Puupponen-Pimia et al. 2008). Therefore, pectinase-assisted extraction of plant homogenates can improve the extraction efficiency of bioactive compounds and give higher yields (Wang et al. 2011).
Table 1.
Bioactive compounds and antioxidant capacity of longan pulp with different degrees of hydrolysis (DH)
| Longan fruit hydrolysate with a DH of: | |||||
|---|---|---|---|---|---|
| 0 % | 9 % | 13 % | 17 % | 21 % | |
| Antioxidant activity: | |||||
| DPPH (EC50 μg FM1/ng DPPH) | 254a ± 22 | 184b ± 4 | 153c ± 4 | 126d ± 2 | 83e ± 1 |
| FRAP (μM TE2/g FM1) | 38.3e ± 1.8 | 49.5d ± 0.0 | 63.1c ± 1.8 | 73.0b ± 1.8 | 92.7a ± 1.8 |
| Total phenolic (mg GAE3/g FM1) | 111.7e ± 0.1 | 126.3d ± 0.1 | 156.6c ± 0.3 | 181.2b ± 0.1 | 196.0a ± 0.2 |
| Total flavonoid (mg CE4/g FM1) | 6.32e ± 0.10 | 9.89d ± 0.16 | 11.1c ± 0.3 | 15.3b ± 0.2 | 19.6a ± 0.2 |
Data are shown as the mean ± 1 SD, and are derived from three independent repeats. Means in the same row with a different letter are significantly different (p ≤ 0.05; ns non-significant; ANOVA and DMRT)
1 FM fresh mass, 2 TE Trolox equivalents, 3 GAE gallic acid equivalents, 4 CE catechin equivalents
Total phenolic and flavonoid contents
Longan fruit is known to be relatively abundant in polyphenolic compounds, such as corilagin, gallic acid and ellagic acid (Rangkadilok et al. 2005). The total phenolic and flavonoid contents in the longan fruit hydrolysates increased in a dose-dependent manner with an increasing DH, reaching a total phenolic and flavonoid content of 196.0 mg GAE/g FM and 19.6 mg CE/g FM, respectively, in the 21 % DH hydrolysate (Table 1). This was 1.75- and 3.1-fold higher, respectively, than in the untreated (0 % DH) hydrolysate (Table 1). In accord, the total phenolic and flavonoid contents in cantaloupe hydrolysates were reported to be higher in the enzyme treated samples than in the non-treated ones (Wuttisit and Anprung 2011).
Determination of dietary fiber levels
The TDF, SDF and IDF levels in the 0 % and 21 % DH longan hydrolysates are shown in Table 2. Pectinase treatment to a 21 % DH increased the SDF level by 0.176 g/100 g (2.6-fold) and correspondingly decreased the IDF by 0.161 g/100 g (1.61-fold) with no significant change in the TDF level. This is because the pectinase breaks down pectin in the longan pulp by cleaving the α-1,4- glycosidic bonds between galacturonic acid molecules forming smaller more soluble pectins and carbohydrates (Karunasawat and Anprung 2010), and so increases the SDF level whilst concomitantly decreasing the IDF level. The SDF level of the 21 % DH longan hydrolysate found here was 1.43-fold higher than that reported in the pectinase-treated bael fruit hydrolysate (Charoensiddhi and Anprung 2010), reflecting the greater TDF content of longan fruits.
Table 2.
Total dietary fiber (TDF), soluble dietary fiber (SDF) and insoluble dietary fiber (IDF) of longan pulp hydrolysates without (0 % DH) or with (21 % DH) pectinase treatment
| Dietary fiber (g/100 g) | 0 % DH | 21 % DH |
|---|---|---|
| TDF | 0.575ns ± 0.01 | 0.571ns ± 0.00 |
| SDF | 0.110b ± 0.00 | 0.286a ± 0.01 |
| IDF | 0.460a ± 0.00 | 0.286b ± 0.01 |
Data are shown as the mean ± 1 SD, and are derived from three independent repeats. Means in the same row with a different letter are significantly different (p ≤ 0.05; ns non-significant; ANOVA; DMRT)
Prebiotic activity score
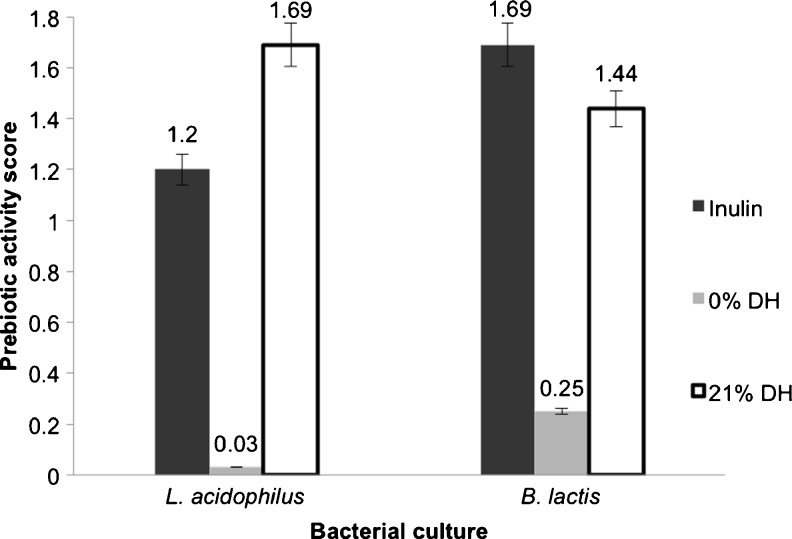
The prebiotic activity scores for L. acidophilus La5 and B. lactis Bb12 were significantly higher (56.3- and 5.76-fold, respectively) in the 21 % DH hydrolysate than the corresponding values for the untreated (0 % DH) hydrolysate (Fig. 2). Indeed, the 21 % DH longan hydrolysate had a 1.4-fold higher prebiotic score than inulin for L. acidophilus and was only slightly (1.17-fold) lower than that for inulin for B. lactis. Thus, the 21 % DH longan fruit hydrolysate has a good potential as a prebiotic, especially for L. acidophilus La5. The pectinase hydrolyzes pectin to galacturonic acid and pectic oligosaccharides that have prebiotic potential (GullÓn et al. 2013). Furthermore, the hydrolysis of pectin also enhances the release of bound bioactive compounds from the cell wall, and these can affect a higher growth of the probiotic bacterial strain, as seen here (Table 3). The 21 % DH longan hydrolysate showed a higher prebiotic value and bacterial growth level for L. acidophilus and B. lactis than that previously reported for the enzymatically extracted mangosteen aril hydrolysate (Anprung and Sangthawan 2012). This presumably reflects the different pharmaceutical properties of longan, and highlights the potential medical importance and interest in this fruit.
Fig. 2.
Prebiotic activity score of bacterial cultures grown in MRS supplemented with 1 % (v/w) of the untreated (0 % DH) or pectinase treated (21 % DH) longan fruit hydrolysates in comparison to that with 10 mg/mL of inulin. Data are shown as the mean ± 1 SD and are derived from three independent repeats
Table 3.
Cell density after 0 and 24 h culture in MRS/TSA with various prebiotics
| Bacterial culture | Cell density (log10 [CFU/mL]) | |||
|---|---|---|---|---|
| Glucose | Inulin | 0 % DH longan hydrolysate | 21 % DH longan hydrolysate | |
| (10 mg/mL) | (10 mg/mL) | (1 % (v/w)) | (1 % (v/w)) | |
| L. acidophilus La5 | 8.79c ± 0.01 | 8.87b ± 0.01 | 7.42d ± 0.02 | 9.02a ± 0.06 |
| B. lactis Bb12 | 10.10c ± 0.06 | 10.33a ± 0.06 | 9.53d ± 0.06 | 10.26b ± 0.01 |
| E. coli ATCC 29922 | 9.96a ± 0.06 | 8.91c ± 0.06 | 9.25b ± 0.06 | 8.85d ± 0.06 |
Data are shown as the mean ± 1 SD, and are derived from three independent repeats. Means in the same row with a different letter are significantly different (p ≤ 0.05; ns non-significant; ANOVA; DMRT)
Volatile compounds
In total, 33 volatile compounds were identified from within the 0 % and 21 % DH longan fruit hydrolysates after enrichment and characterization by SPME/GC/MS analysis (Table 4). Of these, β-ocimene, ethanol, acetaldehyde, ethyl acetate, and (E)-2,6-dimethyl-1,3,5,7-octatetraene were the main volatile compounds (in decreasing order) in the 21 % DH hydrolysate and the same top three compounds were also the most common in the 0 % DH hydrolysate. In both the 0 % and 21 % DH hydrolysates, β-ocimene had by far the highest content, followed by ethanol, with much lower levels of the others. In some but not total accord, it was reported that the major volatile compounds in longan were ethanol, ethyl acetate and cis-ocimene (Susawaengsup et al. 2005). β-Ocimene is reported to have a flowery, sweet scent (Chang et al. 1998) or a herbal, weak floral scent (Jirovetz et al. 2002).
Table 4.
Volatile compounds in the untreated (0 % DH) and pectinase treated (21 % DH) longan pulp hydrolysate
| Peak | Compound name | (%)area | ||
|---|---|---|---|---|
| 0 % DH | 21 % DH | RIs | ||
| 1 | Acetaldehyde | 4.39 | 6.78 | 714 |
| 2 | Ethyl acetate | ND | 2.42 | 807 |
| 3 | Ethanol | 20.16 | 28.50 | 857 |
| 4 | Butanoic acid ethyl ester | 0.73 | ND | 1,022 |
| 5 | 2-butenal | ND | 0.27 | 1,040 |
| 6 | (E)-2-butenal | 0.31 | ND | 1,041 |
| 7 | DL-limonene | ND | 0.50 | 1,194 |
| 8 | α-ocimene | 1.92 | 1.25 | 1,245 |
| 9 | β-ocimene | 60.34 | 49.72 | 1,270 |
| 10 | 3-hydroxy-2-butanone | ND | 0.47 | 1,295 |
| 11 | Alloocimene | 0.50 | ND | 1,378 |
| 12 | Ethyl octanoate | 0.87 | ND | 1,427 |
| 13 | (E)-2,6-dimethyl-1,3,5,7-octatetraene | 2.31 | 2.00 | 1,460 |
| 14 | Ethyl sorbate | 0.17 | ND | 1,478 |
| 15 | 2-ethyl-1-hexanol | ND | 0.53 | 1,492 |
| 16 | Ethyl 3-hydroxybutyrate | 0.85 | 1.25 | 1,524 |
| 17 | Linalool | 0.73 | 0.92 | 1,554 |
| 18 | Ethyl E-2-octenoate | 0.31 | ND | 1,585 |
| 19 | Nonylcyclopropane | 0.17 | ND | 1,621 |
| 20 | Ethyl benzoate | 1.83 | 0.82 | 1,675 |
| 21 | alpha-caryophyllene | 0.18 | ND | 1,680 |
| 22 | 2,6-octadienoic acid, 3,7-dimethyl-, methyl ester | 0.34 | ND | 1,698 |
| 23 | 2,6-dimethyl-6-(4-methyl-3-pentenyl)bicyclo[3.1.1]hept-2-ene | 0.21 | 0.26 | 1,712 |
| 24 | E,E-alpha-farnesene | 0.55 | 0.72 | 1,725 |
| 25 | Methyl salicylate | 0.25 | ND | 1.782 |
| 26 | Dodecanoic acid, ethyl ester | 0.71 | 0.85 | 1,822 |
| 27 | Carbamodithioic acid, diethyl-, methyl ester | 0.18 | 0.40 | 1,956 |
| 28 | Ethyl tetradecanoate | 0.36 | 0.46 | 2,029 |
| 29 | Methyl 2-methoxybenzoate | ND | 0.33 | 2,114 |
| 30 | Eugenol | ND | 0.63 | 2,215 |
| 31 | Hexadecanoic acid, ethyl ester | 0.19 | 0.21 | 2,229 |
| 32 | Phenol, 2,4-bis(l,l-dimethylethyl)- | 1.45 | ND | 2,243 |
| 33 | Benzophenone | ND | 0.70 | 2,545 |
ND not detected
RIs retention indices
The enzyme hydrolysis of pectin is somewhat similar to the natural fruit ripening in response to ethylene where an increase in the activity of cell wall hydrolyzing enzymes, such as pectinases and pectinesterases, is noted (Chourasia et al. 2006). The volatile profiles of Alphonso mango fruit were observed to significantly change upon exogenous ethylene treatment (Chidley et al. 2013), whilst after enzyme hydrolysis, free and glycosidically-bound volatile compounds were both found to change. This is because they were easily released from the cell wall, leading to changes in the proportion of volatile compounds, and was also caused by the subsequent isomerization of some of the volatile compounds via oxidation (Chyau et al. 2003; Moreira et al. 2010). Here, compared to the 0 % DH hydrolysate, the 21 % DH longan hydrolysate showed 15 and 15 compounds with a reduced and increased proportion, respectively, whilst 11 and eight volatiles were unique to the 0 % and 21 % DH hydrolysates, respectively. Of these proportional changes in the volatile composition, a significantly reduced proportion of β-ocimene and (E)-2,6-dimethyl-1,3,5,7-octatetraene (1.2-fold, respectively) were noted after enzyme treatment, whilst conversely, acetaldehyde and ethanol were both increased (1.5- and 1.4-fold, respectively). Longan also has other volatile compounds which occurred after hydrolysis, such as ethyl acetate, which is described as being of a fruity and pineapple flavor (Chen et al. 2006; Guillot et al. 2006), eugenol, which is described as having a herbal scent (Ashurst 1995), and limonene, which is mostly found in oranges, grapes and lemons (Sawamura et al. 1991).
Particle size
The average particle sizes of the longan pulp was decreased 2.26-fold by the pectinase treatment, from 781 ± 2.7 μm in the 0 % DH hydrolysate to 345 ± 2.7 μm in the 21 % DH hydrolysate, which was significantly smaller (p < 0.05; DMRT). The particles from the 21 % DH hydrolysate were, however, within the average particle sizes seen in some commercial concentrated fruit essences of 86 ± 3.1 to 395 ± 1.0 μm that require a longer and more energy-utilizing manufacturing process than this pectinase treatment. The decreased particle size between the 0 and 21 % DH hydrolysates simply reflects the hydrolyzed pectin glycosidic bonds leading to increased TSF contents and less intermolecular interactions and so a smaller particle size (Karunasawat and Anprung 2010). The smaller particle size can further aid digestion and so might make it quicker or easier to absorb the bioactive compounds.
Lipid peroxidation
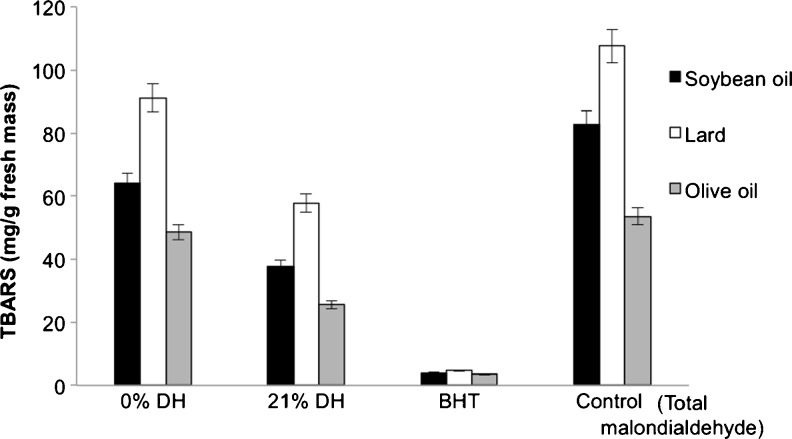
Using the TBARS assay, the 21 % DH longan hydrolysate was found to be able to reduce the lipid peroxidation of lard, soybean oil and olive oil better than the non-enzyme treatment (Fig. 3). That is the 0 % DH hydrolysate decreased the lipid peroxidation of soya oil, lard and olive oil by 1.3-, 1.18- and 1.15-fold, respectively, compared to the MDA control, whilst that for the 21 % DH hydrolysate was 2.19-, 1.90- and 2.1-fold lower than MDA, respectively. However, even the 21 % DH longan hydrolysate was still far less effective than BHT. The inhibition of lipid peroxidation is associated with the total phenolic and flavonoid contents (Thitilertdecha et al. 2010; Wu et al. 2013), which act as antioxidants, and in accord the 21 % DH longan hydrolysate had the highest levels of phenolics and flavonoids (Table 1).
Fig. 3.
Thiobarbituric acid (TBARS) formation in the three different lipid sources in the presence of 1 % (w/v) longan pulp hydrolysate without (0 % DH) or with (21 % DH) pectinase treatment compared to that with 1 mg/mL BHT or no addition (control). The 0 % DH hydrolysate contained 111.7 mg GAE/g FM and 6.32 mg CE/g FM, whilst the 21 % DH hydrolysate contained 196.0 mg GAE/g FM and 19.6 mg CE/g FM. Data are shown as the mean ± 1 SD and are derived from three independent repeats
Conclusion
The pectinase-assisted extraction of longan fruit pulp significantly increased the level of antioxidant activity, total phenolics, total flavonoids, SDF, prebiotic activity score and lipid peroxidation inhibition. These were all increased in a DH-dependent manner, being highest with the highest DH (21 %) of the hydrolysate. The optimal 21 % DH hydrolysate degraded pectin into short molecules leading to the release of the highest amount of phenolics and flavonoids from both the cytoplasm and the structural polysaccharides in plant cell wall, which are linked to the antioxidant capacity and lipid peroxidation inhibition. Changes in the proportion of volatile compounds, including those associated with food-associated human olfactory responses, were also detected, and are likely to reflect both changes in volatilization and oxidation. β-Ocimene was the major volatile compound in both the treated (21 % DH) and non-treated (0 % DH) longan hydrolysates. Also the 21 % DH longan hydrolysates exhibited a good prebiotic potential on L. acidophilus La5 and increased the SDF and decreased the average particle size to 345 μm, which is within the range of available concentrated fruit essences. Therefore, subject to the caveats of the commercial and technical limitations of industrial scale processing of (i) the enzyme cost and the (ii) difficulty in scaling up enzyme-assisted extractions due to the different environmental conditions, pectinase-treated longan fruit extract can be developed and applied as concentrated fruit essences or as a food additive for added nutritional value and shelf-life extension.
Acknowledgement
The authors gratefully acknowledge the financial support from the Research Scholarships, Graduate School of Chulalongkorn University and the Integration Project: Innovation for the Improvement of Food Safety and Food Quality for New World Economy. We thank Dr. Robert Butcher for proof reading and correcting the manuscript and for suggestions.
Contributor Information
Boossara Thitiratsakul, Phone: +66-896990014, Email: newlife.boossara@gmail.com.
Pranee Anprung, Phone: +66-22185530, Email: Pranee.a@chula.ac.th.
References
- Ajila CM, Brar SK, Verma M, Tyagi RD, Godbout S, Valero JR. Extraction and analysis of polyphenols: recent trends. Crit Rev Biotechnol. 2011;31(3):227–249. doi: 10.3109/07388551.2010.513677. [DOI] [PubMed] [Google Scholar]
- Anprung P, Sangthawan S. Prebiotic activity and bioactive compounds of the enzymatically depolymerized Thailand-grown mangosteen aril. J Food Res. 2012;1:268–276. doi: 10.5539/jfr.v1n1p268. [DOI] [Google Scholar]
- Official methods of analysis of the AOAC International. Washington: Association of Official Analytical Chemists; 1995. [Google Scholar]
- Ashurst PR (ed) (1995) Production and packaging of non-carbonated fruit juices and fruit beverages, 2nd edn. Blackie Acadamic and Professional. Chapman and Hall, London
- Ayala-Zavala J, Rosas-Domínguez C, Vega-Vega V, González-Aguilar G. Antioxidant enrichment and antimicrobial protection of fresh-cut fruits using their own byproducts: looking for integral exploitation. J Food Sci. 2010;75(8):R175–R181. doi: 10.1111/j.1750-3841.2010.01792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzana E, Rubio D, Santamaria RI, Garcia F, Ridaura Sanz VE, Lopez-Mungula A. Enzyme-mediated solvent extraction of carotenoids from marigold flower (Tagetes erecta) J Agric Food Chem. 2002;50:4491–4496. doi: 10.1021/jf025550q. [DOI] [PubMed] [Google Scholar]
- Biesalski HK, Dragsted LO, Elmadfa I, Grossklaus R, Müller M, Schrenk D, Walter P, Weber P. Bioactive compounds: definition and assessment of activity. Nutrition. 2009;25:1202–1205. doi: 10.1016/j.nut.2009.04.023. [DOI] [PubMed] [Google Scholar]
- Chang CY, Chang CH, Yu TH, Lin LY, Yen YH. The effect of drying treatment on the flavor and quality of longan fruit. Dev Food Sci. 1998;40:353–367. doi: 10.1016/S0167-4501(98)80059-9. [DOI] [Google Scholar]
- Charoensiddhi S, Anprung P. Characterization of bael fruit (Aegle marmeols [L.] Correa) hydrolysate as affected by enzyme treatment. J Food Biochem. 2010;34:1249–1267. doi: 10.1111/j.1745-4514.2009.00333.x. [DOI] [Google Scholar]
- Chen JL, Yan S, Feng Z, Xiao L, Hu XS. Changes in the volatile compounds and chemical and physical properties of Yali pear (Pyrus bertschneideri Reld) during storage. Food Chem. 2006;97:248–255. doi: 10.1016/j.foodchem.2005.03.044. [DOI] [PubMed] [Google Scholar]
- Chen S, Xing XH, Huang JJ, Xu MS. Enzyme-assisted extraction of flavonoids from Ginkgo biloba leaves: improvement effect of flavonol transglycosylation catalyzed by Penicillium decumbens cellulase. Enzym Microb Technol. 2010;48(1):100–105. doi: 10.1016/j.enzmictec.2010.09.017. [DOI] [PubMed] [Google Scholar]
- Chidley HG, Kulkarni RS, Pujari KH, Giri AP, Gupta VS. Spatial and temporal changes in the volatile profile of Alphonso mango upon exogenous ethylene treatment. Food Chem. 2013;136:585–594. doi: 10.1016/j.foodchem.2012.08.029. [DOI] [PubMed] [Google Scholar]
- Choudhari SM, Ananthanarayan L. Enzyme aided extraction of lycopene from tomato tissues. Food Chem. 2007;102:77–81. doi: 10.1016/j.foodchem.2006.04.031. [DOI] [Google Scholar]
- Chourasia A, Sane VA, Nath P. Differential expression of pectatelyase during ethylene-induced postharvest softening of mango (Mangifera indicavar Dashehari) Physiol Plant. 2006;128(3):546–555. doi: 10.1111/j.1399-3054.2006.00752.x. [DOI] [Google Scholar]
- Chu Y-F, Sun J, Wu X, Liu RH. Antioxidant and antiproliferative activities of common vegetables. J Agric Food Chem. 2002;50:6910–6916. doi: 10.1021/jf020665f. [DOI] [PubMed] [Google Scholar]
- Chyau CC, Ko PT, Chang CH, Mau JL. Free and glycosidically bound aroma compounds in lychee (Litchi chinensis Sonn.) Food Chem. 2003;80:387–392. doi: 10.1016/S0308-8146(02)00278-9. [DOI] [Google Scholar]
- Cinar I. Stability studies on the enzyme extracted sweet potato carotenoproteins. Food Chem. 2005;89:397–401. doi: 10.1016/j.foodchem.2004.02.048. [DOI] [Google Scholar]
- Cruz PDL, Quintero L, Villalobos A, Cuesta NDL. Lipid peroxidation and glutathione system in hyperlipemic rabbits: influence of olive oil administration. Biochim Biophys Acta. 2000;1485:36–44. doi: 10.1016/S1388-1981(00)00027-5. [DOI] [PubMed] [Google Scholar]
- Dehghan-Shoar Z, Hardacre AK, Meerdink G, Brennan CS. Lycopene extraction from extruded products containing tomato skin. Int J Food Sci Technol. 2011;46:365–371. doi: 10.1111/j.1365-2621.2010.02491.x. [DOI] [Google Scholar]
- Denny A, Buttriss J (2007) Plant foods and health: focus on plant bioactives. European Food Information Resource (EuroFIR) Consortium. Funded under the EU 6th Framework Food Quality and Safety Thematic Priority. Contract FOOD-CT-2005-513944
- Dunford N, Irmak S, Jonnala R. Pressurised solvent extraction of policosanol from wheat straw, germ and bran. Food Chem. 2010;119(3):1246–1249. doi: 10.1016/j.foodchem.2009.07.039. [DOI] [Google Scholar]
- Fu YJ, Liu W, Zu YG, Tong MH, Li SM, Yan MM, Efferth T, Luo H. Enzyme-assisted extraction of luteolin and apigenin from pigeon pea [Cajanus cajan (L.) Millsp.] leaves. Food Chem. 2008;111(2):508–512. doi: 10.1016/j.foodchem.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Gardossi L, Poulsen PB, Ballesteros A, Hult K, Svedas VK, Vasić-Racki D, Carrea G, Magnusson A, Schmid A, Wohlgemuth R, Halling PJ. Guidelines for reporting of biocatalytic reactions. Trends Biotechnol. 2009;28:171–180. doi: 10.1016/j.tibtech.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Guillot S, Peytavi L, Bureau S, Boulanger R, Lepoutre JP, Crouzet J, Schorr-Galindo S. Aroma characterization of various apricot varieties using headspace solid phase microextraction combined with gas chromatography–mass spectrometry and gas chromatography-olfactometry. Food Chem. 2006;96:147–155. doi: 10.1016/j.foodchem.2005.04.016. [DOI] [Google Scholar]
- GullÓn B, Gómez B, Sabajanes MM, Yáñez R, Rarajó JC, Alonso JL. Pectic oligosaccharides: manufacture and functional properties. Trends Food Sci Technol. 2013;30:153–161. doi: 10.1016/j.tifs.2013.01.006. [DOI] [Google Scholar]
- Iriti M, Faoro F. Bioactivity of grape chemicals for human health. Nat Prod Commun. 2009;4:611–634. [PubMed] [Google Scholar]
- Jirovetz L, Buchbauer G, Ngassoum MB, Geissler M. Aroma compound analysis of Piper nigrum and Piper guineense essential oils from Cameroon using solid-phase microextraction-gas chromatography, solid-phase microextraction-gas chromatography–mass spectrometry and olfactometry. J Chromatogr A. 2002;976:265–275. doi: 10.1016/S0021-9673(02)00376-X. [DOI] [PubMed] [Google Scholar]
- Karunasawat K, Anprung P. Effect of depolymerized mango pulp as a stabilizer in, oil in water emulsion containing sodium caseinate. Food Bioprod Process. 2010;88:202–208. doi: 10.1016/j.fbp.2010.01.004. [DOI] [Google Scholar]
- Kim YJ, Kim DO, Chun OK, Shin DH, Jung H, Lee CY, Wilson DB. Phenolic extraction from apple peel by cellulases from Thermobifida fusca. J Agric Food Chem. 2005;53(24):9560–9565. doi: 10.1021/jf052052j. [DOI] [PubMed] [Google Scholar]
- Li B, Smith B, Hossain M. Extraction of phenolics from citrus peels: I. Solvent extraction method. Sep Purif Technol. 2006;48(2):182–188. doi: 10.1016/j.seppur.2005.07.005. [DOI] [Google Scholar]
- Maisuthisakul P, Suttajit M, Pongsawatmanit R. Assessment of phenolic content and free radical-scavenging capacity of some Thai indigenous plants. Food Chem. 2007;100:1409–1418. doi: 10.1016/j.foodchem.2005.11.032. [DOI] [Google Scholar]
- Miron T, Plaza M, Bahrim G, Ibanez E, Herrero M. Chemical composition of bioactive pressurized extracts of Romanian aromatic plants. J Chromatogr. 2010;1218(30):4918–4927. doi: 10.1016/j.chroma.2010.11.055. [DOI] [PubMed] [Google Scholar]
- Moore J, Cheng Z, Su L, Yu L. Effects of solid-state enzymatic treatments on the antioxidant properties of wheat bran. J Agric Food Chem. 2006;54(24):9032–9045. doi: 10.1021/jf0616715. [DOI] [PubMed] [Google Scholar]
- Moreira RFA, Maria CAB, Pietroluongo M, Trugo LC. Chemical changes in the volatile fractions of Brazilian honeys during storage under tropical conditions. Food Chem. 2010;121:697–704. doi: 10.1016/j.foodchem.2010.01.006. [DOI] [Google Scholar]
- Nelson N. A photometric adaptation of the Somogyi method for determination of glucose. J Biol Chem. 1944;153:375–380. [Google Scholar]
- Pan Y, Wang K, Huang S, Wang H, Mu X, He C, Ji X, Zhang J, Huang F. Antioxidant activity of microwave assisted extract of longan (Dimorcarpus longan Lour.) peel. Food Chem. 2008;106:1264–1270. doi: 10.1016/j.foodchem.2007.07.033. [DOI] [Google Scholar]
- Park SJ, Park DH, Kim DH, Lee S, Yoon BH, Jung WY, Lee KT, Cheong JH, Ryu JH. The memory enhancing effects of Euphoria longan fruit extract in mice. J Ethnopharmacol. 2010;128:160–165. doi: 10.1016/j.jep.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Passos CP, Yilmaz S, Silva CM, Cuimbra MA. Enhancement of grape seed oil extraction using a cell wall degrading enzyme cocktail. Food Chem. 2009;115:48–53. doi: 10.1016/j.foodchem.2008.11.064. [DOI] [Google Scholar]
- Pinelo M, Arnous A, Meyer AS. Upgrading of grape skins: significance of plant cell-wall structural components and extraction techniques for phenol release. Trends Food Sci Technol. 2006;17:579–590. doi: 10.1016/j.tifs.2006.05.003. [DOI] [Google Scholar]
- Plaza M, Amigo-Benavent M, del Castillo M, Ibanez E, Herrero M. Facts about the formation of new antioxidants in natural samples after subcritical water extraction. Food Res Int. 2010;43(10):2341–2348. doi: 10.1016/j.foodres.2010.07.036. [DOI] [Google Scholar]
- Prasad KN, Yang B, Shi J, Yu C, Zhao M, Xue S, Jiang Y. Enhanced antioxidant and antityrosinase activities of longan fruit pericarp by ultra-high-pressure-assisted extraction. J Pharm Biomed Anal. 2010;51:471–477. doi: 10.1016/j.jpba.2009.02.033. [DOI] [PubMed] [Google Scholar]
- Puupponen-Pimia R, Nohynek L, Ammann S, Oksman-Caldentey KM, Buchert J. Enzyme-assisted processing increases antimicrobial and antioxidant activity of bilberry. J Agric Food Chem. 2008;56:681–688. doi: 10.1021/jf072001h. [DOI] [PubMed] [Google Scholar]
- Rangkadilok N, Worasuttayangkurn L, Bennett RN, Satayavivad J. Identification and quantification of polyphenolic compounds in longan (Euphoria longana Lam.) fruit. J Agric Food Chem. 2005;56:1387–1392. doi: 10.1021/jf0403484. [DOI] [PubMed] [Google Scholar]
- Rangkadilok N, Sitthimonchai S, Worasuttayangkurn L, Mahidol C, Ruchirawat M, Satayavivad J. Evaluation of free radical scavenging and antityrosinase activities of standardized longan fruit extract. Food Chem Toxicol. 2007;45:328–336. doi: 10.1016/j.fct.2006.08.022. [DOI] [PubMed] [Google Scholar]
- Reddy VP, Urooj A. Antioxidant properties and stability of Aegle marmelos leaves extracts. J Food Sci Technol. 2013;50(1):135–140. doi: 10.1007/s13197-010-0221-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawamura M, Shichiri K, Ootani Y, Zhang XH. Volatile constituents of several varieties of pummelos and characteristics among citrus species. Agric Biol Chem. 1991;55(10):2571–2578. doi: 10.1271/bbb1961.55.2571. [DOI] [Google Scholar]
- Shahidi F. Nutraceuticals and functional foods: whole versus processed foods. Trends Food Sci Technol. 2009;20(9):376–387. doi: 10.1016/j.tifs.2008.08.004. [DOI] [Google Scholar]
- Somogyi M. Notes on sugar determination. J Biol Chem. 1952;195:19–23. [PubMed] [Google Scholar]
- Sowbhagya HB, Chitra VN. Enzyme-assisted extraction of flavorings and colorants from plant materials. Crit Rev Food Sci Nutr. 2010;50:146–161. doi: 10.1080/10408390802248775. [DOI] [PubMed] [Google Scholar]
- Starmans D, Nijhuis H. Extraction of secondary metabolites from plant material: a review. Trends Food Sci Technol. 1996;7(6):191–197. doi: 10.1016/0924-2244(96)10020-0. [DOI] [Google Scholar]
- Sun J, Chu Y-F, Wu X, Liu RH. Antioxidant and antiproliferative activities of common fruits. J Agric Food Chem. 2002;50:7449–7454. doi: 10.1021/jf0207530. [DOI] [PubMed] [Google Scholar]
- Susawaengsup C, Rayanakorn M, Wangkam S (2005) Gas chromatography–mass spectrometry of volatile components of some local fruits in Northern Thailand, Proceedings of the 31st Congress on Science and Technology of, Thailand P.3
- Szentmihalyi K, Vinkler P, Lakatos B, Illes V, Then M. Rose hip (Rosa canina L.) oil obtained from waste hip seeds by different extraction methods. Bioresour Technol. 2002;82(2):195–201. doi: 10.1016/S0960-8524(01)00161-4. [DOI] [PubMed] [Google Scholar]
- Thitilertdecha N, Teerawutgulrag A, Kilburn JD, Rakariyatham N. Identification of major phenolic compounds from Nephelium lappaceum L. and their antioxidant activities. Molecules. 2010;15:1453–1465. doi: 10.3390/molecules15031453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velioglu YS, Massa G, Gao L, Oomah BD. Antioxidant activity and total phenolics in selected fruits, vegetables and grain products. J Agric Food Chem. 1998;46:4113–4117. doi: 10.1021/jf9801973. [DOI] [Google Scholar]
- Wang T, Jonsdottir R, Kristinsson HG, Hreggvidsson GO, Jonsson JO, Thorkelsson G, Olafsdottir G. Enzyme-enhanced extraction of antioxidant ingredients from red algae Palmaria palmata. LWT Food Sci Technol. 2010;43(9):1387–1393. doi: 10.1016/j.lwt.2010.05.010. [DOI] [Google Scholar]
- Wang Y, Zu Y, Long J, Fu Y, Li S, Zhang D. Enzymatic water extraction of taxifolin from wood sawdust of Larixgmelini (Rupr.) Rupr. and evaluation of its antioxidant activity. Food Chem. 2011;126:1178–1185. doi: 10.1016/j.foodchem.2010.11.155. [DOI] [Google Scholar]
- Waterhouse AL (2005) Determination of total phenolics. In: Wrolstad RE, Acree TE, Decker EA, Penner MH, Reid DS, Schwartz SJ, Shoemaker CF, Smith D, Sporns P (eds) Handbook of food analytical chemistry: pigments, colorants, flavors, texture, and bioactive food components. John Wiley and Sons, New Jersey, pp 463–481
- Wilkins MR, Widmer WW, Grohmann K, Cameron RG. Hydrolysis of grapefruit peel waste with cellulase and pectinase enzymes. Bioresour Technol. 2007;98(8):1596–1601. doi: 10.1016/j.biortech.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Worrasinchai S, Suphantharika M, Pinjai S, Jamnong P. ß-glucan prepared from spent brewer’s yeast as a fat replacer in mayonnaise. Food Hydrocoll. 2006;20:68–78. doi: 10.1016/j.foodhyd.2005.03.005. [DOI] [Google Scholar]
- Wu Y, Cui SW, Tang J, Gu X. Optimization of extraction process of crude polysaccharides from boat-fruited sterculia seeds by response surface methodology. Food Chem. 2007;105:1599–1605. doi: 10.1016/j.foodchem.2007.03.066. [DOI] [Google Scholar]
- Wu LY, Xiao H, Zhao WJ, Shang H, Zhang MZ, Lin YD, Sun P, Ge GP, Lin JK. Effect of instant tea powder with high ester-catechins content on shelf life extension of sponge cake. J Agric Sci Technol. 2013;15:1–8. [Google Scholar]
- Wuttisit N, Anprung P. Effect of pectinase on volatile and functional bioactive compounds in the flesh and placenta of Sunlady cantaloupe. Int Food Res J. 2011;18:792–800. [Google Scholar]
- Yang C, He N, Ling X, Ye M, Zhang C, Shao W, Yao C, Wang Z, Li Q. The isolation and characterization of polysaccharides from longan pulp. Sep Purif Technol. 2008;63:226–230. doi: 10.1016/j.seppur.2008.05.004. [DOI] [Google Scholar]
- Yang YC, Li J, Zu YG, Fu YJ, Luo M, Wu N, Liu XL. Optimisation of microwave-assisted enzymatic extraction of corilagin and geraniin from Geranium sibiricum Linne and evaluation of antioxidant activity. Food Chem. 2010;122(1):373–380. doi: 10.1016/j.foodchem.2010.02.061. [DOI] [Google Scholar]
- Yang B, Jiang Y, Shi J, Chen F, Ashraf M. Extraction and pharmacological properties of bioactive compounds from (Dimocarpus longan Lour.) longan fruit: a review. Food Res Int. 2011;44:1837–1842. doi: 10.1016/j.foodres.2010.10.019. [DOI] [Google Scholar]
- Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555–559. doi: 10.1016/S0308-8146(98)00102-2. [DOI] [Google Scholar]
- Zhong K, Wang Q, He Y, He X. Evaluation of radicals scavenging, immunity-modulatory and antitumor activities of longan polysaccharides with ultrasonic extraction on in S180 tumor mice models. Int J Biol Macromol. 2010;47:356–360. doi: 10.1016/j.ijbiomac.2010.05.022. [DOI] [PubMed] [Google Scholar]
- Zhou K, Raffoul JJ. Potential anticancer properties of grape antioxidants. J Oncol. 2012;2012:803294. doi: 10.1155/2012/803294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuchi C, Ambrosio G, Lüscher TF, Landmesser U. Nutraceuticals in cardiovascular prevention: lessons from studies on endothelial function. Cardiovasc Ther. 2010;28:187–201. doi: 10.1111/j.1755-5922.2010.00165.x. [DOI] [PubMed] [Google Scholar]