Abstract
Essential oils (EOs) from Ageratum conyzoides L., Coleus aromaticus Benth. and Hyptis suaveolens (L.) Poit were extracted and tested against Tribolium castaneum Herbst, the storage grain insect. The EOs were found effective against Tribolium castaneum during in vitro as well as in vivo fumigant testing. The EOs of H. suaveolens and A. conyzoides showed 100 % mortality of test insect at 250 ppm while C. aromaticus at 350 ppm. During in vivo fumigant testing of wheat samples against Tribolium castaneum, the essential oils of A. conyzoides and C. aromaticus completely checked the damage of wheat grains by the insect at 1000 ppm while essential oil of H. suaveolens checked the grain damage completely even at 500 ppm concentration. There was no adverse effect on seed germination as well as on seedling growth of EOs treated seeds showing non-phytotoxic nature of the oils. Hence, these EOs may be recommended as botanical insecticide against insect invasion of stored food commodities, thereby enhancing their shelf life.
Keywords: Essential oil, Fumigant activity, Phytotoxicity, Tribolium castaneum
Introduction
Stored grain insect pests severely infest agricultural stored products and are responsible for worldwide loss of stored grains up to 10-40 % annually (Rajashekar et al. 2010).The continuous increasing pressure of expanding human population has also created a critical problem of food scarcity. In such a situation, to manage stored grains and other agricultural products from insect infestation, various synthetic insecticides have been used . However, reports on acquired resistance of insects against these synthetics and the residual toxicity of such chemicals have necessitated to search some biodegradable sources of pesticides (Zettler and Cuperus 1990; Jembere et al. 1995).
Earlier attempts to control stored grain pests relied on methods such as mixing gains with dry soil and wood ash for lethal dehydratiotion and fumigation with certain plant materials (Levinson and Levinson 1989). In one form or another, the use of admixture and fumigants for control of storage pests has continued present day also. In addition, growers are moving away from using methyl bromide as a post harvest fumigant because it is an ozone-depleting substance (Zhang and van Epenhuijsen 2004). Plant origin pesticides are much safer than conventionally used synthetic pesticides. The biological activity in some plants may be due to synergistic effects of different active principles. They may impart different mode of action during their pesticidal action (Varma and Dubey 1999). Amongst different plant products, the use of essential oils extracted from aromatic plants to control these pests has been investigated and is well documented (Isman 2006; Devi and Devi 2011). Some studies have assessed the ability of the essential oils and their constituents as fumigants and repellents against a number of insect pests (Clemente et al. 2003; Kumar et al. 2007) and some formulations of essential oils are in use for control of losses of stored food commodities due to biodeterioration caused by insect infestation (Dubey et al. 2010).
The rust-red flour beetle, Tribolium castaneum is a major pest of wheat grain flour (Howe 1965) and other stored cereals in tropical and subtropical regions of the world. The present study has been focused on the in vitro contact and fumigant activity of the chemically characterized essential oils of Ageratum conyzoides L., Hyptis suaveolens (L.) Poit. and Coleus aromaticus Benth. on mortality of Tribolium castaneum as well as on the in vivo fumigation of stored wheat samples by the oils for management of deterioration caused by insects in order to find out their practical efficacy as plant based insecticides. Ageratum conyzoides L. and Hyptis suaveolens (L.) Poit are native to tropical America while Coleus aromaticus Benth is native to East Indies and it has been distributed all over the tropical and subtropical region of India, Pakistan, Sri Lanka, tropical East Africa, Brazil, Egypt, Arabia and Ethiopia. All the three plants are well-known as ethnomedicinal and grow luxuriantly in India (Guha bakshi et al. 2001).
Materials and methods
Rearing of test insects
The storage insect pest Tribolium castaneum Herbst was collected from stored wheat grain flour and its identity was confirmed from Deparment of Entomology, Institute of Agriculture Sciences, Banaras Hindu University, Varanasi, India. The culture of the insects Tribolium castaneum Herbst (family: Tenebrionidae) was maintained on wheat (Triticum aestivum L.) grains following Perez-Mendoza et al. (2004). 200 adult insects were released separately in 250 g of wheat grain flour in plastic containers covered by muslin cloth. After 48 hours, the adult insects were removed from the wheat grain flour and the samples were stored in the laboratory at 28 ± 2 °C to get insects of same age.
Isolation of essential oils of the selected angiospermic plants
Essential oils were isolated from fresh leaves of Ageratum conyzoides L., Coleus aromaticus Benth. and Hyptis suaveolens (L.) separately through hydrodistillation by Clevanger’s apparatus for 3 h (Prakash et al. 2011). The isolated oils (yield 0.5%V/W, 1.1%V/W and 0.8%V/W respectively) were dried over anhydrous sodium sulphate and the essential oil was stored in clean glass vials.
In vitro insecticidal activity of the essential oils against T. castaneum the stored grain pest by fumigant method
The essential oils were tested for their fumigant activity on mortality of T. castaneum following the method of Kim and Ahn (2001) with slight modification. Plastic containers of 500 mL capacity with screw lids were used as exposure chambers. Requisite amount of EOs were dissolved separately in 0.25 mL acetone and applied to a circular filter paper (WhatmanNo. 1, 3 cm diameter) were introduced into the plastic jars to achieve final concentrations (50–500) ppm with respect to volume of the jars.
Controls received only 0.25 mL acetone. Thereafter allowing the acetone to evaporate by keeping the jars in a fume chamber for 10 min at room temperature, the filter paper was attached to the inner surface of the screw lid of the jar using adhesive tape. 25 adult insects of same age were placed in plastic containers. Treated sets were held at 28 ± 2 °C (80 ± 5 % RH). Insect mortality was determined at 1,2,3,4 and 5 days after treatment. Test insects were considered dead if appendages did not move when podded with brush. The LC50 and LC90 values of essential oils were calculated by Probit analysis (Finney, 1971).
All the experiments were repeated three times and % corrected mortality was recorded by Abott’s formula.
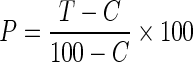 |
Where
- P
% corrected mortality
- T
% kill in treatment
- C
% kill in control
In vivo fumigation of stored wheat samples by the oils for management of deterioration caused by insects
The wheat samples were separately fumigated by the oils by the usual methods of Dubey et al. (1983a), Dikshit et al. (1983) and Shaaya et al. (1997). Prior to experimental, the grain sample were cleaned and sterilized at 45 °C for 6 h in order to kill the eggs and developing larvae of pre infested insect pest (Jayakumar 2010). Different lots, each containing 500 g of wheat samples (moisture content 12 %) were taken separately in closed plastic containers (volume 1 litre). The insect Tribolium castaneum (25 adults of insect) were introduced in each lot. Requisite amount of the essential oils were introduced separately in the plastic containers by soaking in cotton pieces so as to procure concentrations of 250, 500 and 1000 ppm (v/v) for each oils. Control sets contained wheat samples infested by insects (without any treatment with the essential oils). After twelve months of storage at laboratory conditions temperature 28 ± 2 °C and RH 80 ± 5 % the analysis of insects associated with wheat samples of treated and control sets were made. The efficacy of Ageratum conyzoides L., Coleus aromaticus Benth. and Hyptis suaveolens (L.) EOs on insect infestation were determined by calculating grain damage (%), weight loss (%) and feeding deterrence (%) of treated and control sets. The damage was observed as appearance deformation and flour on grains in treatment and control sets.
Grain damage (GD)(%),
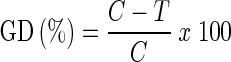 |
Where
- C
number of infested grain (flour appearance) in control set.
- T
number of infested grain (flour appearance) in essential oil treated set
The weight loss (%) of wheat samples in the treated and control sets was calculated by fresh weight basis using the formula suggested by Parkin (1956).
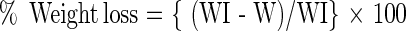 |
Where
- WI
Weight of grains before the experiment.
- W
Weight of grains after experiment.
Feeding deterrence was calculated using the feeding deterrent index following Isman et al. (1990)
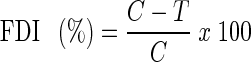 |
Where
- C
Weight loss in control set.
- T
Weight loss in fumigated set.
Phytotoxicity of the essential oils
The phytotoxicity of the EOs in terms of seed germination and seedling growth of wheat was assayed with respect to control sets following Mishra and Dubey (1994). Requisite quantity of the oils were dissolved in one ml of 0.01 % of Tween-80 and it was then pipetted in a flask containing 19 ml water so as to procure the final concentration of 500 ppm for H. suaveolense and 1000 ppm for A. conyzoides and C. aromaticus essential oils. 50 g seeds of wheat were soaked separately in each of the solution for 30 and 60 minutes. After soaking for requisite period 20 seeds were placed on two layered Whatman No.1 moistened filter paper in sterilized Petri plates. For controls, requisite amount of sterilized water was used in place of the essential oils. The experiment was repeated for five times. Percent germination of wheat seeds as well as length of radicle and plumule was recorded at 24, 48, 72 and 96 h interval.
Data analysis
Experiments were performed in triplicate and data analysis was done on mean ± SE subjected to one way ANOVA. Means are separated by the Tukey's multiple range test when ANOVA was significant (p < 0.05) (SPSS 10.0; Chicago, IL, USA).
Results and discussion
In recent years, many essential oils have been shown to be effective against stored product insects (Don Pedro 1996; Ranaweera 1996; Jayasingha et al. 1999; Elhag 2000; Adhikari et al. 2002; Bandara et al. 2005) but the practical efficacy of only few oils have been observed. The literature is mostly silent on the in vivo pest controlling potency of the oils.
Fumigation has been considered to be the most effective, practical and quick method of eradicating storage pests. The tiny gas molecules easily penetrate large stacks right into the individual grains, reaching and killing all stages of development of the pest (Bond 1984). Hence, for practical application in management of biodeterioration of stored wheat samples by the insects, the efficacy of essential oils of Ageratum conyzoides, Coleus aromaticus and Hyptis suaveolens were evaluated as fumigant in the present investigation.
In the present investigation, the essential oils of A. conyzoides, H. suaveolens and C. aromaticus were found absolute insecticidal in nature to the test insect during in vitro testing by filter paper method. All the three oils showed 100 % mortality of the test insect Tribolium castaneum. During in vitro fumigation, the insecticidal activity of the oils was found to vary against Tribolium castaneum, with their concentration and exposure period. The essential oil of H. suaveolens and A. conyzoides could show 100 % mortality of Tribolium castaneum on 2nd day of exposure at 250 ppm while essential oil of C. aromaticus showed 100 % mortality on 2nd day of exposure at 350 ppm. The LC50 value of EO of H. suaveolens A. conyzoides and C. aromaticus were found to be 229.33, 213.02, 256.71 ppm respectively while the corresponding LC90 values were 414.39, 351.12 and 414.2 ppm (Table 1).The essential oils showed absolute insecticidal potency on prolonged exposure after fumigation and at enhanced concentrations. Their efficacy increased with increased concentration of doses.
Table 1.
LC50 and LC90 value of essential oils of Ageratum conyzoides, Hyptis suaveolens and Coleus aromaticus oils against T. castaneum after 24 h exposure time
| Essential oil | LC 50(ppm) (95 % fiducial limits) | LC90 (ppm (95 % fiducial limits) | Slope ± S.E. | Chi Square (χ2) | df |
|---|---|---|---|---|---|
| Ageratum conyzoides | 213.02 (192.81 - 232.95) | 351.12 (312.43 - 416.76) | 5.91 ± 0.37 | 15.27 | 6 |
| Hyptis suaveolens | 229.33 (201.03 – 259.47) | 414.39 (349.14 – 557.16) | 4.98 ± 0.34 | 22.43 | 6 |
| Coleus aromaticus | 256.71 (238.99-275.32) | 414.20 (372.97-482.30) | 6.17 ± 0.43 | 10.19 | 6 |
During in vivo fumigant testing, the essential oils were highly effective in checking infestation of wheat samples by Tribolium castaneum. At 1000 ppm essential oils of Ageratum conyzoides and Coleus aromaticus completely checked the damage of wheat grains while essential oil of H. suaveolens checked the grain damage completely even at 500 ppm showing its more efficacy than other two oils. The essential oils showed zero % weight loss and 100 % FDI index, at 1000 ppm against test insect. But the efficacy of the oils declined at lower concentrations. However, the essential oil of Hyptis suaveolens was strongly efficacious even at 500 ppm showed zero % weight loss and 100 % FDI index (Table 2). The mycoflora analysis of the wheat grains has not been observed in the present investigation, although, the samples of control and treatment sets were procured from same source.
Table 2.
Effect of essential oil s on grain damage, Weight loss and Feeding deterent index in treated wheat during storage (Insect: Tribolium castaneum) (n = 3)
| Insect Tribolium castaneum | Concentrations of oil (ppm) | Hyptis suaveolens (L.) Poit. | Ageratum conyzoides L. | Coleus aromaticus Benth. |
|---|---|---|---|---|
| % grain damage | 250 | 8.0 ± 0.11b | 8.3 ± 0.13c | 12.5 ± 0.08c |
| 500 | 0.0 ± 0.00a | 3.8 ± 0.05b | 8.8 ± 0.11b | |
| 1000 | 0.0 ± 0.00a | 0.0 ± 0.00a | 0.0 ± 0.00a | |
| Control | 96 ± 0.00c | 96 ± 0.00d | 96 ± 0.00d | |
| % weight loss | 250 | 5.2 ± 0.05b | 7.1 ± 0.21c | 10.0 ± 0.17c |
| 500 | 0.0 ± 0.00a | 3.2 ± 0.11b | 6.3 ± 0.10b | |
| 1000 | 0.0 ± 0.00a | 0.0 ± 0.00a | 0.0 ± 0.00a | |
| Control | 23.2 ± 0.15c | 23.2 ± 0.15d | 23.2 ± 0.15d | |
| % Feeding Deterent Index (FDI) | 250 | 77.42 ± 0.11a | 68.86 ± 0.35a | 56.39 ± 0.25a |
| 500 | 100.0 ± 0.0b | 86.11 ± 0.06b | 72.31 ± 0.26b | |
| 1000 | 100.0 ± 0.0b | 100.0 ± 0.0c | 100.0 ± 0.00c |
Means followed by the same letter in the same column for each parameter do not significantly (p < 0.05) different according to ANOVA and Tukey’s multiple comparison tests
Estimation of weight loss of the fumigated commodities in comparison with untreated samples of control sets gives a clear picture of the efficacy of a product used as fumigant. Hence, this parameter was observed in the control and treatment sets before observing the efficacy of oils as feeding deterrent. Results of present study showed the efficacy of essential oils against the infestation of wheat samples by T. castaneum. The essential oils were found to be equally and absolutely effective at 1000 ppm showing remarkable control of the infestation of the wheat samples with respect to grain damage, weight loss as well as FDI during in vivo investigation. Such findings of the present investigation are in accordance with earlier reports made with essential oil of Cymbopogon martini (Kumar et al. 2007).
A product may be recommended in the management of post harvest biodeterioration of food commodities only when it is non phytotoxic in nature. The germination and seedling growth of the treated commodities should not be adversely affected by treatment with the pesticidal product. In the present investigation the three essential oils neither showed any adverse effect on germination of wheat seeds nor exhibited any deleterious effect on seedling growth of wheat indicating their nonphytotoxic nature (Tables 3 and 4). At 72 h period of exposure absolute germination of wheat seeds for all the treatments. It was also found that there was no visual abnormality in morphology of the plants of all the treatment sets and the plants appeared as healthy as those of control sets, thereby, further indicating the nonphytotoxic nature of the essential oils showing their prospectives as plant based insecticide. During practical application the farmer and growers may store the treated wheat samples for different period. Hence, direct phytotoxic testing of the oils was performed to know their effect on wheat germination immediately after treatment. Moreover, essential oils are tested as fumigant .The oils being volatile in nature would easily emit the vapours during sun drying. In addition, the ethnomedicinal uses of these plants may strengthen the possibility of their exploitation as safe insecticides. However, before large scale trial their safety profile on mammals should be investigated.
Table 3.
Effect of Hyptis oil, Ageratum oil and Coleus oil on germination (%) of seeds of treated wheat (n = 3)
| Essential oil | Germination period (h) | Control | ½ h treatment (500 ppm) | 1 h treatment(500 ppm) |
|---|---|---|---|---|
| Hyptis oil | 24 | 90 ± 0.28c | 73 ± 0.28c | 68 ± 0.60c |
| 48 | 97.5 ± 0.40b | 87 ± 0.34b | 86 ± 0.15b | |
| Ageratum oil | 24 | 90 ± 0.17b | 80 ± 0.40c | 75 ± 0.00c |
| 48 | 100 ± 0.00a | 97.5 ± 0.17b | 89 ± 0.20b | |
| Coleus oil | 24 | 93.2 ± 0.11c | 90 ± 0.850c | 73 ± 0.32c |
| 48 | 97.5 ± 0.17b | 96 ± 0.11b | 90 ± 0.00b | |
| * | 72 | 100 ± 0.00a | 100 ± 0.00a | 100 ± 0.00a |
| 96 | 100 ± 0.00a | 100 ± 0.00a | 100 ± 0.00a |
* absolute germination for all the treatment. Means followed by the same letter in the same column for each parameter do not significantly p < 0.05) different according to ANOVA and Tukey’s multiple comparison tests
Table 4.
Effect of Hyptis oil, Ageratum oil and Coleus oil on seedling growth of wheat (n = 3)
| Age of Seedlings (h) | Radical (cm) | 1/2 h | 1 h | Plumule (cm) | 1/2 h | 1 h |
|---|---|---|---|---|---|---|
| Hyptis oil | Control | 500 ppm | 500 ppm | Control | 500 ppm | 500 ppm |
| 48 | 1.17 ± 0.01c | 1.02 ± 0.12c | 0.85 ± 0.01c | 0.36 ± 0.12c | 0.28 ± 0.04c | 0.26 ± 0.08c |
| 72 | 2.10 ± 0.2b | 2.1 ± 0.15b | 1.86 ± 0.08b | 0.53 ± 0.08b | 0.34 ± 0.17b | 0.32 ± 0.13b |
| 96 | 2.53 ± 0.14a | 2.4 ± 0.12a | 2.13 ± 0.20a | 1.12 ± 0.14a | 0.93 ± 0.17a | 0.78 ± 0.13a |
| Ageratum oil | Radicle (Control) | 1000 ppm | 1000 ppm | Plumule cm | 1000 ppm | 1000 ppm |
| 48 | 2.13 ± 0.13c | 1.80 ± 0.05c | 1.75 ± 0.10c | 1.27 ± 0.05c | 1.17 ± 0.14c | 1.03 ± 0.03c |
| 72 | 2.60 ± 0.11b | 2.00 ± 0.00b | 1.94 ± 0.15b | 1.45 ± 0.02b | 1.39 ± 0.17b | 1.28 ± 0.03b |
| 96 | 3.13 ± 0.13a | 2.50 ± 0.05a | 2.23 ± 0.14a | 2.23 ± 0.08a | 1.96 ± 0.08a | 1.85 ± 0.02a |
| Coleus oil | Radicle (Control) | 1000 ppm | 1000 ppm | Plumule cm | 1000 ppm | 1000 ppm |
| 48 | 2.10 ± 0.10c | 2.01 ± 0.11c | 1.93 ± 0.11c | 0.99 ± 0.05c | 0.90 ± 0.15c | 0.84 ±0.20c |
| 72 | 2.50 ± 0.10b | 2.32 ± 0.00b | 2.18 ± 0.05b | 1.32 ± 0.10b | 1.10 ± 0.05b | 1.07 ± 0.20b |
| 96 | 2.80 ± 0.00a | 2.50 ± 0.17a | 2.30 ± 0.11a | 1.60 ± 0.11a | 1.40 ± 0.15a | 1.23 ± 0.88a |
Means followed by the same letter in the same column for each parameter do not significantly (p < 0.05) different according to ANOVA and Tukey’s multiple comparison tests
The essential oils as such contain different types of major and minor components in their chemical profile. Their activity may be mostly because of synergism between the components present in them (Desmarchelier 1994; Isman 2000). The intimate knowledge of essential oil composition leads to its proper exploitation in pest management. The chemical profiles of essential oils have been reported by our research group (Jaya et al. 2011). Currently some essential oils formulations have been prepared through microencapsulation technologies and are used as fumigants against storage grain pests (Dubey et al. 2010). A few EO based preservatives are already commercially available. ‘DMC Base Natural’ is a food preservative comprising 50 % EO from rosemary, sage and citrus and 50 % glycerol (Mendoza-Yepes et al. 1997). Recently some herbal products showing pesticidal activity are recognizes as ‘generally recognized as safe’ (GRAS) as food additives in some of the developed countries and modern society is attracting towards ‘green consumarism’ (Tuley de Silva 1996; Smid and Gorris 1999) desiring fewer synthetic ingredients in foods. Hence, EOs are currently regarded as potentially useful additives for food preservation. Moreover, recommendation of herbal products as ‘generally recognized as safe’ (GRAS) as food additives in the developed countries may lead scientific interest in the exploitation of essential oils as plant based antimicrobials.
Based on the findings of the present investigation the large scale trials are required with these oils so as to recommend them as gain protectant and the treated samples should be subjected to pharmacological investigations in order to find out the safety profile of the oils. Being abundantly growing in tropical and subtropical countries, sufficient raw materials would be available for formulation of insecticides from the oils of these plants.
Conclusion
The essential oils of Ageratum conyzoides, Coleus aromaticus and Hyptis suaveolens based on their in vivo and in vitro insecticidal efficacy may be recommended as nonphytotoxic herbal insecticides against Tribolium castaneum contamination.
Acknowledgement
This work was financially supported by University Grant Commission (UGC), New Delhi, India.
References
- Adhikari AACK, Paranagama PA, Abeywickrama KP, Bandara KANP. Behavioral studies of Cowpea bruchid, Callosobruchus maculates (F.) against volatile extracts from leaves of Lemongrass (Cymbopogon citrates D.C.Stapf), Neem (Azadirachta indica A. Juss) and Curry leaf (Murraya koenigii Spreng) J Trop Agricul Res. 2002;14:138–145. [Google Scholar]
- Bandara KANP, Kumar V, Saxena RC, Ramdas PK. Bruchid (Coleoptera: Bruchidae) Ovicidal Phenylbutanoid from Zingiber purpureum. J Econ Entomol. 2005;98:1163–1169. doi: 10.1603/0022-0493-98.4.1163. [DOI] [PubMed] [Google Scholar]
- Bond EJ. Manual of fumigation for insect control. FAO: Rome; 1984. p. 432. [Google Scholar]
- Clemente S, Mareggiani G, Broussalis A, Martino V, Ferraro G. Insecticidal effects of Lamiaceae species against stored products insects. Bol San Veg Plagas. 2003;29:1–8. [Google Scholar]
- Desmarchelier JM. Grain protectants: trends and developments. In: Highley E, Wright EJ, Banks HJ, Champ BR, editors. Stored product protection. Wallingford: CAB International; 1994. pp. 722–728. [Google Scholar]
- Devi CK, Devi SS (2011) Insecticidal and oviposition deterrent properties of some spices against coleopteran beetle, Sitophilus oryzae. J Food Sci Technol. doi:10.1007/s13197-011-0377-1 [DOI] [PMC free article] [PubMed]
- Dikshit A, Dubey NK, Tripathi NN, Dixit SN. Cedrus oil- A promising storage fungitoxicant. J Stored Prod Res. 1983;19:159–162. doi: 10.1016/0022-474X(83)90003-6. [DOI] [Google Scholar]
- Guha bakshi DN, Sensarma P, Pal DC. A Lexicon of Medicinal Plants in India. Vol. 2. Calcutta: Naya Prakash; 2001. pp. 386–387. [Google Scholar]
- Don Pedro KN. Fumigant toxicity is the major role of insect activity of Citrus peel essential oils. Pestic Sci. 1996;46:71–78. doi: 10.1002/(SICI)1096-9063(199601)46:1<71::AID-PS318>3.0.CO;2-J. [DOI] [Google Scholar]
- Dubey NK, Bhargava KS, Dixit SN. Protection of some stored food commodities from fungi by essential oils of Ocimum canum and Citrus medica. Int J Trop Plant Diseases. 1983;1:177–179. [Google Scholar]
- Dubey NK, Kumar A, Singh P, Shukla R. Exploitation of natural compounds in ecofriendly management of plant pests. In: Gisi U, Chet I, Gullino ML, editors. Recent developments in management of plant diseases. Netherlands: Springer; 2010. pp. 181–198. [Google Scholar]
- Elhag EA. Deterrent effect of some botanical products on oviposition of the cowpea bruchid Callosobruchus maculates (F.) (Coleoptera: Bruchidae) Int J Pest Manag. 2000;46:109–113. doi: 10.1080/096708700227462. [DOI] [Google Scholar]
- Finney JD (1971) Probit analysis, 3rd edn. Cambridge University Press, Cambridge, UK
- Howe RW. Losses caused by insects and mites in stored foods and foodstuffs. Nutr Abstr Rev. 1965;35:285–302. [PubMed] [Google Scholar]
- Isman MB. Plant essential oils for pest and disease management. Crop Prot. 2000;19:603–608. doi: 10.1016/S0261-2194(00)00079-X. [DOI] [Google Scholar]
- Isman MB. Botanical insecticides, deterrents and repellents in modern agriculture and an increasingly regulated world. Annu Rev Entomol. 2006;51:45–66. doi: 10.1146/annurev.ento.51.110104.151146. [DOI] [PubMed] [Google Scholar]
- Isman MB, Koul O, Luczynski A, Kaminski A. Insecticidal and antifeedant bioactivities of neem oils and their relationship to Azadirachtin content. J Agricul Food Chem. 1990;38:1406–1411. doi: 10.1021/jf00096a024. [DOI] [Google Scholar]
- Jaya, Dubey NK. Evaluation of chemically characterised essential oils of Coleus aromaticus, Hyptis suaveolens and Ageratum conyzoides against storage fungi and aflatoxin contamination of food commodities. Int J Food Sci Technol. 2011;46:754–760. doi: 10.1111/j.1365-2621.2011.02557.x. [DOI] [Google Scholar]
- Jayakumar M. Oviposition deterrent and adult emergence activities of some plant aqueous extracts against Callosobruchus maculates F. (Coleoptera : Bruchidae) J Biopesticides. 2010;3:325–329. [Google Scholar]
- Jayasingha P, Warnasooriya D, Dissanayake H (1999) Lemongrass (Cymbopogon citrates) –A literature survey. Medicinal and Aromatic plant series. No 9. ITI, Colombo, Sri Lanka
- Jembere B, Obeng-Ofori D, Hassanali A, Nyamasyo GNN. Products derived from the leaves of Ocimum kilimanndscharium (Labiatae) as post-harvest grain protectants against the infestation of three major stored product insect pests. Bull Entomol Res. 1995;85:361–367. doi: 10.1017/S0007485300036099. [DOI] [Google Scholar]
- Kim DH, Ahn YJ. Contact and fumigant activities of constituents of Foeniculum vulgare fruit against three coleopteran stored - product insects. Pest Manag Sci. 2001;57(3):301–306. doi: 10.1002/ps.274. [DOI] [PubMed] [Google Scholar]
- Kumar R, Srivastava M, Dubey NK. Evaluation of Cymbopogon martinii oil extract for control of postharvest insect deterioration in cereals and legumes. J Food Prot. 2007;70:172–178. doi: 10.4315/0362-028x-70.1.172. [DOI] [PubMed] [Google Scholar]
- Levinson H, Levinson AR. Food storage and storage protection in ancient Egypt. Boletin de Salidad vegetal. 1989;17:475–482. [Google Scholar]
- Mendoza-Yepes MJ, Sanchez-Hidalgo LE, Maertens G, Marinlniesta F. Inhibition of Listeria monocytogenes and other bacteria by a plant essential oil (DMC) In Spanish soft cheese. J Food Safety. 1997;17:47–55. doi: 10.1111/j.1745-4565.1997.tb00175.x. [DOI] [Google Scholar]
- Mishra AK, Dubey NK. Evaluation of some essential oils for their toxicity against fungi causing deterioration of stored food commodities. Appl Environ Microbiol. 1994;60(1101–1):105. doi: 10.1128/aem.60.4.1101-1105.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin EA. Stored product entomology (the assessment and reduction of losses caused by insects to stored food stuffs) Annu Rev Entomol. 1956;1:223–240. doi: 10.1146/annurev.en.01.010156.001255. [DOI] [Google Scholar]
- Perez-Mendoza J, Thorne JE, Dowell FE, Baker JE. Chronological agegrading of three species of stored-product beetles by using near-infrared spectroscopy. J Econ Entomol. 2004;97:1159–1167. doi: 10.1603/0022-0493(2004)097[1159:CAOTSO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Prakash B, Shukla R, Singh P, Mishra PK, Dubey NK, Kharwar RN. Efficacy of chemically characterized Ocimum gratissimum L. essential oil as an antioxidant and a safe plant based antimicrobial against fungal and aflatoxin B1 contamination of spices. Food Res Int. 2011;44:385–390. doi: 10.1016/j.foodres.2010.10.002. [DOI] [Google Scholar]
- Rajashekar Y, Gunasekaran N, Shivanandappa T. Insecticidal activity of the root extract of Decalepis hamiltonii against stored-product insect pests and its application in grain protection. J Food Sci Technol. 2010;47:310–314. doi: 10.1007/s13197-010-0049-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranaweera SS. Mosquito-larvicidal activity of some Sri Lankan plants. J Natl Sci Counc Sri Lanka. 1996;24:63–69. [Google Scholar]
- Shaaya E, Kostjukovski M, Eilberg J, Sukprakarn C. Plant oils as fumigants and contact insecticides for the control of stored- product insects. J Stored Prod Res. 1997;33:7–15. doi: 10.1016/S0022-474X(96)00032-X. [DOI] [Google Scholar]
- Smid EJ, Gorris LGM. Natural antimicrobials for food preservation. In: Rehman MS, editor. Handbook of food preservation. New York: Marcel Dekker; 1999. pp. 285–308. [Google Scholar]
- Tuley de Silva K. A manual of the essential oils industry. Viena: United nations Industrial Development Organization; 1996. [Google Scholar]
- Varma J, Dubey NK. Insecticidal and repellent activity of some essential oils against Tribolium castaneum. Natl Acad Sci Lett. 1999;20:143–147. [Google Scholar]
- Zettler JL, Cuperus GW. Pesticide resistance in Tribolium castaneum (Coleoptera: Tenebrionidae) and Rhyzopertha dominica (Coleoptera: Bostrichidae) in wheat. J Econ Entomol. 1990;83:1677–1681. [Google Scholar]
- Zhang Z, Van Epenhuijsen CW. Improved Envirosol fumigation methods for disinfesting export cut Xowers and foliage crops. Palmerston North: New Zealand Institute for Crop Food Res Limited; 2004. [Google Scholar]


