Abstract
The surge of interest in naturally occurring phytochemicals with high therapeutic potential has led to the discovery of many molecules, out of which naturally occuring coumarins such as marmelosin, umbelliferone and scopoletin present in Aegle marmelos (Bael) fruit shows good therapeutic potential. The aim of the present work is to develop and validate Reverse Phase-High Performance Liquid Chromatography (RP-HPLC) method for simultaneous determination of marmelosin, umbelliferone and scopoletin in A. marmelos fruit extracts. The chromatographic separation was performed with isocratic elution of 55:45 (%, v/v) methanol–water containing 0.1 % acetic acid as mobile phase. The method used to analyse the extract of A. marmelos showed good resolution with retention time within 12 min. The relative concentrations of above phytoconstituent were determined in A. marmelos fruits. The method was found to give compact peaks for scopoletin, umbelliferone and marmelosin (Rt of 4.6, 6.5 and 11.3 min respectively) and were linear over the range 5–30 μg ml−1 (R2 = 0.9655), 2–10 μg ml−1 (R2 = 0.9964) and 2–10 μg ml−1 (R2 = 0.9862) respectively. The mean recoveries for marmelosin, umbelliferone and scopoletin at three concentrations were in the range of 98.8–102.9, 98.8–101.1 and 94.2–98.3 % respectively. The relative standard deviation of accuracy, precision and repeatability were within 2 %, indicating the method produced highly reproducible results. Therefore this simple, precise and accurate method enables simultaneous separation of this phytoconstituent and hence can be successfully applied in analysis and routine quality control of herbal material and formulation containing A. marmelos.
Keywords: RP-HPLC, Aegel marmelos fruit, Marmelosin, Umbelliferone, Scopoletin, Quantification
Introduction
Aegle marmalos (Correa) Linn., a plant of family Rutaceae is highly reputed Ayurvedic medicinal plant commonly known as Bael, Bengal quince or Golden-apple tree. It is upto 15 m tall with short trunk, thick, soft, flaking bark and spreading spiny branches. It is native to India and is also distributed in deciduous forest on hills and plains of Bangladesh, Burma and Sri Lanka ascending up to 1,200 m (Tiwari et al. 2010). It is found growing wild in Sub-Himalayan tracts from Jhelum eastwards to West Bengal, as well as in central and south India (Gupta and Tandon 2004).
Fruit pulp can be used in traditional Indian drink preparation ‘sharbat’ and preserve food called murrabba which is generally used for stomach disorder or tonic. Bael pulp can also be processed into nectar or squash, jelly, powder or toffee etc. for both food and therapeutic use (Rakesh et al. 2005). Various phytoconstituents like alkaloids, coumarins and steroids have been isolated and identified from different parts of the tree such as leaves, fruits, wood, root and bark. Root and fruit contain alkaloids, coumarins and furocumarins such as aegelenine, aegeline (Sugeng et al. 2001), skimmianine, marmelosin, aurapten, epoxyaurapten, marmin, marmesin, xanthotoxin, scopoletin (Surat et al. 2011), decursinol and haplopine (Basu and Sen 1974), umbelliferone (Sharma et al. 1980), 6-methyl-4-chromanone (Subban et al. 2009), skimmin, psoralen, 6,7-dimethoxycoumarin, tembamide (Shoeb et al. 1973). Fruit in addition, contain xanthotaxol, alloimperatorin and alkaloid like marmeline identified as N-2-hydroxy-2-[4 - (3′,3′-dimethyl allyloxy) phenyl] ethyl cinnamide (Sharma and Sharma 1981). β- sitosterol and its glycoside are also present in the fruit (Saha and Chatterjee 1957). Polysaccharides reported form fruit pulp contained arabinose, galactose and glucose (Basak et al. 1981). The essential oil from the leaves contained (+) α-phellendrene, p-cymene, Cineole, Limonene, citronellal and Eugenol (Bhandari and Gupta 1972).
Fruits of A.marmelos Correa have a numerous uses in Ayurvedic medicine. The unripe fruit is used as an astringent, digestive and stomachic. The fruit is reputed medicine for diarrhea and dysentery and said to act as tonic for the heart and brain. The unripe fruit has been reported to show antiviral activity and proved to be potent hypoglycemic agent. It has also been reported with antifungal and anthelmintic activity (Mookerjee 1943). Marmelosin also called as imperatorin (Fig 1.) is a major chemical constituent of the fruit and has been proved to have an anticancer, antibacterial and anti-inflammatory activity (Pitre and Shrivastav 1987).
Fig. 1.
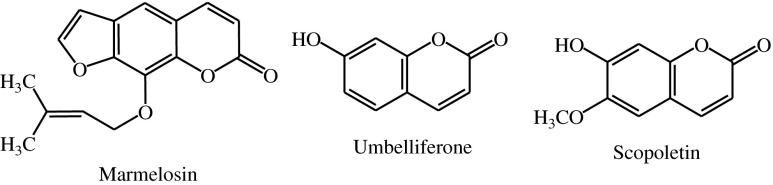
Structure of, Marmelosin, Umbelliferone and Scopoletin
Previously attempt has been made to develop HPLC method for individual phytochemicals and also simultaneous estimation of marmelosin and Psoralen has also been developed (Bhattacherjee et al. 2013) In the present work attempt has been made to develop a sensitive, simple, and reproducible reverse phase-high performance liquid chromatography (RP-HPLC) method with good recovery for determination of three coumarins isolated from methanolic extract of A. marmelos fruit. RP-HPLC method offers a number of advantages for the analysis of herbals extract and is suitable for comparison of samples based on finger prints. It provides flexibility in screening and quantitative analysis of phytoconstituents. The novelty of this investigation is a rapid, precise and accurate gradient RP-HPLC for the simultaneous determination of umbelliferone, scopoletin and a furocoumarin marmelosin.
Experimental
Standard and chemicals
Marmelosin, umbelliferone and scopoletin were purchased from Natural Remedies Pvt. Ltd. (Bangalore, India). All standard used is of above 99 % purity as informed by supplier. All other solvents used were of analytical grade and purchased from Merck India Ltd. (Mumbai, India) and SD Fine chemicals Ltd. (Mumbai, India). Sufficient precautions were taken to ensure the stability of the analytes.
Plant material
Fruits of Aegle marmelos were purchased from local market and authenticated. Sample specimen was deposited in herbarium of Institute of Chemical Technology, Mumbai.
Methanolic extract of Aegle marmelos (MEAM) fruit
About 5 g accurately weighed powdered drug was extracted with methanol in a Soxhlet apparatus for 6 h at temperature 80°c. Filter the extract and transfer to 100 ml volumetric flask and make up the volume with methanol.
HPLC instrumentation
HPLC analysis was performed with a Jasco (Hachioji, Tokyo, Japan) system consisting of an intelligent pump (PU-1580, PU-2080), a high-pressure mixer (MX-2080-31), a manual sample injection valve (Rheodyne 7725i) equipped with a 20-μL loop, and a UV–visible detector (UV-1575). Compounds were separated on a 250 mm × 4.6 mm i.d., 5-μm particle, Hibar Li Chrocart Purospher Star RP-18 end capped column (Merck, Darmstadt, Germany) with 55:45 (%, v/v) methanol–water containing 0.1 % acetic acid as isocratic mobile phase at a flow rate of 1.0 ml min−1. The injection volume was 20 μL and the detection wavelength was 300 nm (appropriate to the UV absorption maxima of the compounds determined). HPLC was performed at ambient temperature and data were analyzed on a computer equipped with Borwin software.
Preparation of standards
A stock solution of marmelosin, umbelliferone and scopoletin (100 μg ml−1) was prepared in methanol by dissolving 10 mg standard in 100 ml volumetric flask, and making up the volume with methanol.
Calibration curve of standards
Calibration standard solutions at six concentrations marmelosin (concentration range 5.0, 10.0, 15.0, 20.0, 25.0, and 30.0 μg ml−1), umbelliferone (concentration range 2.0, 4.0, 6.0, 8.0, and 10.0 μg ml−1) and scopoletin (concentration range 2.0, 4.0, 6.0, 8.0, and 10.0 μg ml−1) were obtained by appropriate dilutions of stock solution.
Method of validation
Calibration plots were constructed in the range of 5–30 μg ml−1 for marmelosin whereas 2–10 μg ml−1 for umbelliferone and scopoletin, measured in triplicate for each calibration solution. Each calibration curve was obtained by plotting AUC (Area under curve) against concentration (μg ml−1) of the corresponding standard solution. High correlation coefficients and wide linear ranges of the calibration curves were found for all the compounds.
Precision
Precision was determined as the intra-day and inter-day variation of results from analysis of six different concentrations of standard solutions. Intra-day precision was determined by triplicate analysis of each solution on a single day. Inter-day precision was determined by triplicate analysis of the solutions on three successive days. The relative standard deviations (RSD) of retention time (Rt) and AUC of three analytes were calculated as measures of precision, repeatability and stability.
Limits of detection and quantitation
To determine the limits of detection (LOD) and quantification (LOQ), standard solutions were further diluted in methanol. LOD and LOQ were defined as the amounts for which signal-to-noise ratios (S/N) were 3 and 10, respectively.
Accuracy and recovery studies
The accuracy of the method was determined by application of the standard addition method (Joshi and Sharma 2008). Accurately known amounts of the standards were added to 1 ml of preanalyzed MEAM and then analyzed in duplicate as described above. The total amount of each compound was calculated from the corresponding calibration plot and the recovery of each compound was calculated by use of the equation:
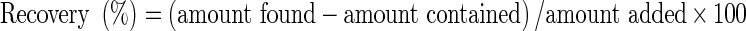 |
Estimation of scopoletin, marmelosin and umbelliferone in MEAM
To quantify coumarins in MEAM, an accurately measured 1 ml of stock solution was transferred into a 10 ml volumetric flask and volume was made up with methanol. The solution was filtered using a G-3 glass sintered funnel. 20 μL of the filtered solution was injected for RP-HPLC analysis. The analysis was repeated in triplicate.
Results and discussion
Optimization of the chromatographic condition
The method was optimized by varying the flow rate and the relative amounts of methanol and water. Use of a high concentration of methanol and a flow rate of 1.0 ml min−1 resulted in rapid elution but with distortion of the chromatographic peaks. Reducing the amount of methanol and addition of 0.1 % acetic acid in water resulted in sharp peaks and better resolution. When methanol: water 55:45 (%, v/v) containing 0.1 % acetic acid was used at a flow rate of 1.0 ml min−1, scopoletin, umbelliferone and marmelosin were eluted at 4.6, 6.5 and 11.3 min, respectively. Peak symmetries were <1.2 for three marker compounds under the optimized conditions. The absorption maxima (λmax) of marmelosin, umbelliferone and scopoletin were 302, 305 and 290 nm respectively, so the UV detector was programmed at 300 nm. Chromatograms obtained from a mixed standard solution and from the sample (Figure. 2) revealed that separation of the selected marker constituents was good. Chromatographic peaks were identified by comparing retention times of standard under the same operating conditions. Spiking the sample solution with the standard compounds was also used to assist confirmation of peak identity.
Fig. 2.
RP-HPLC chromatogram of Marker compounds and Aegle marmelos extract. a. HPLC chromatogram of Aegle marmelos extract. b HPLC chromatogram of three marker compounds scopoletin, umbelliferone and marmelosin respectively
Linearity and calibration curves
The calibration plots for marmelosin, umbelliferone and scopoletin were linear in the ranges 5–30, 2–10 and 2–10 μg ml−1, respectively. The regression equations were y = 41438.1x – 104008.0 (R2 = 0.9655), y = 71440.9x + 15954.3 (R2 = 0.9862) and y = 67644.0x + 2202.3(R2 = 0.9964) respectively for marmelosin, umbelliferone and scopoletin. Figure 2 displays RP-HPLC chromatograms of three marker compounds. The regression data is shown in Table 1.
Table 1.
Calibration curves, linear ranges, detection limit and precisions of three coumarins
| Coumarins | Eq. of calibration curve | Correlation coefficient (R2) | Linear range (μg ml−1) | LODa (μg ml−1) | LOQb (μg ml−1) | Precision (RSD,%, n = 6) | |
|---|---|---|---|---|---|---|---|
| Intra-day AUCc | Inter-day AUCc | ||||||
| Marmelosin | y = 41438.1x – 104008.0 | 0.9655 | 5–30 | 063 | 2.12 | 1.44 | 1.14 |
| Umbelliferone | y = 71440.9x + 15954.3 | 0.9862 | 2–10 | 0.69 | 2.31 | 1.86 | 1.76 |
| Scopoletin | y = 67644.0x + 2202.3 | 0.9964 | 2–10 | 0.39 | 1.31 | 1.80 | 1.67 |
aLimit of detection
bLimit of quantification
cArea under curve
Precision
Intra-day and inter-day RSD of AUC were less than 1.9 %, showing precision was good (Table 1). Recovery of marmelosin, umbelliferone and scopoletin was in the range 98.8–102.9, 98.8–101.1 and 94.2–98.3 %, respectively, with RSD < 2.17 %, indicating the analysis was accurate.
Limits of detection and quantitation
The LOD and LOQ were 0.63 and 2.12 μg ml−1 for marmelosin, 0.69 and 2.30 μg ml−1 for umbelliferone and 0.39 and 1.31 μg ml−1 for scopoletin.
Estimation of scopoletin, marmelosin and umbelliferone
These results revealed the method enables rapid, precise, and accurate simultaneous detection and quantification of marmelosin, umbelliferone and scopoletin. When the method was subsequently used for analysis of methanolic extract of A. marmelos, the amounts of marmelosin, umbelliferone and scopoletin were found to be 0.3546, 0.005 and 0.014 % respectively (Table 2).
Table 2.
Percentage of three coumarins in A. marmelos root extract
| Constituent | Amount present (%) |
|---|---|
| Marmelosin | 0.3546 ± 0.02 |
| Umbelliferone | 0.005 ± 0.001 |
| Scopoletin | 0.014 ± 0.005 |
Conclusion
The above mentioned RP-HPLC method was successfully used for estimation of coumarins and furocoumarin in MEAM. The method enabled rapid, highly accurate and reproducible quantification of these marker compounds in herbal extract. It can be used for quantitative analysis and quality control of polyherbal formulations containing Bael and other plant extracts which consist of coumarins on the basis of analysis of marmelosin, umbelliferone and scopoletin as marker compounds.
Acknowledgments
Authors are thankful to University Grant Commission, New Delhi for providing financial assistance for the project.
References
- Basak RK, Mandal PK, Mukherjee AK. Studies on a neutral polysaccharide isolated from Bael (Aegle marmelos) fruit pulp. Carbohy Res. 1981;97:315–321. doi: 10.1016/S0008-6215(00)80677-1. [DOI] [Google Scholar]
- Basu D, Sen R. Alkaloids and coumarins from Aegle marmelos. Phytochem. 1974;13:2329–2330. doi: 10.1016/0031-9422(74)85057-0. [DOI] [Google Scholar]
- Bhandari KS, Gupta YN. Chemical examination of essential oil from leaves of Aegle marmelos. Indian Oil Soap J. 1972;37:301–304. [Google Scholar]
- Bhattacherjee AK, Dikshit A, Pandey D, Tandon DK. High performance liquid chromatographic determination of marmelosin and Psoralen in bael (Aegle marmelos(L.) Correa) fruit. J Food Sci Technol. 2013 [Google Scholar]
- Gupta AK, Tandon N. Reviews on Indian medicinal plants, Vol-1(Abe-Alle) New Delhi: Indian council of medical research; 2004. pp. 310–336. [Google Scholar]
- Joshi R, Sharma R. Development and validation of RP-HPLC method for simultaneous estimation of three- component tablet formulation containing acetaminophen, chlorzoxazone, and aceclofenac. Anal Lett. 2008;41:3297–3308. doi: 10.1080/00032710802515086. [DOI] [Google Scholar]
- Mookerjee A. On the active principles of bark of Aegle marmelos Correa. Curr Sci. 1943;20:209. [Google Scholar]
- Pitre S, Shrivastav SK. Pharmacological, microbiological and phytochemical studies on roots of Aegle marmelos. Fitoterapia. 1987;58:194–197. [Google Scholar]
- Rakesh S, Dhawan S, Arya SS. Processed products of Bael. Process Food Industry. 2005;8(12):25–27. [Google Scholar]
- Saha SK, Chatterjee A. Isolation of Allo-imperatorin and β-Sitosterol from the fruits of Aegle marmelos Correa. J Indian Chem Soc. 1957;34:228–230. [Google Scholar]
- Sharma BR, Sharma P. Constituents of Aegle marmelos II alkaloids and coumarins from the fruits. Planta Med. 1981;43:102–103. doi: 10.1055/s-2007-971486. [DOI] [PubMed] [Google Scholar]
- Sharma BR, Rattan RK, Sharma P. Constituents of leaves and fruits of Aegle marmelos. Indian J Chem. 1980;19B:162. [Google Scholar]
- Shoeb A, Kapil RS, Popli SP. Coumarins and alkaloids of Aegle marmelos. Phytochem. 1973;12:2071–2073. doi: 10.1016/S0031-9422(00)91550-4. [DOI] [Google Scholar]
- Subban R, Sadashiva CT, Tamizmani T, Balasubramanian T, Rupeshkumar M, Balachandran I. In vitro glucose uptake by isolated rat hemi-diaphragm study of Aegle marmelos Correa root. Bangladesh J Pharmacol. 2009;4:65–68. [Google Scholar]
- Sugeng R, Mohd.Aspollah S, Mawardi R, Gwendoline CLE, Taufiq-Yap YH, Norio A, Mariko K. Alkaloids from Aegle marmelos (Rutaceae) Mal J Anal Sci. 2001;7:463–465. [Google Scholar]
- Surat L, Chalita P, Cholpisut T, Somsak T, Suphara TI, Wanwasan N. Chemical constituents from Aegle marmelos. J Braz Chem Soc. 2011;22:176–178. [Google Scholar]
- Tiwari BN, Khatri P, Ali J, Soni ML, Patel R. Tissue culture of endangered Bael tree (Aegle marmelos): a review. J Adv Sci Res. 2010;1:34–40. [Google Scholar]



