Abstract
The main cause of perishability of fruits and vegetables are their high water content. To increase the shelf life of these fruits and vegetables many methods or combination of methods had been tried. Osmotic dehydration is one of the best and suitable method to increase the shelf life of fruits and vegetables. This process is preferred over others due to their vitamin and minerals, color, flavor and taste retention property. In this review different methods, treatments, optimization and effects of osmotic dehydration have been reviewed. Studied showed that combination of different osmotic agents were more effective than sucrose alone due to combination of properties of solutes. During the experiments it was found that optimum osmosis was found at approximately 40 °C, 40 °B of osmotic agent and in near about 132 min. Pretreatments also leads to increase the osmotic process in fruits and vegetables. Mass transfer kinetics study is an important parameter to study osmosis. Solids diffusivity were found in wide range (5.09–32.77 kl/mol) studied by Fick’s laws of diffusion. These values vary depending upon types of fruits and vegetables and osmotic agents.
Keywords: Osmotic dehydration, Osmosis, Fruits, Vegetables, Preservation, Mass transfer kinetics, Diffusion
Introduction
About 20–40% percent of the fruit and vegetable production in India goes waste due to lack of proper retailing and adequate storage capacity, The production of vegetables in India is next only to China. The vegetable and fruit production contributes more than 30% of the agriculture GDP (Gross Domestic Product). The crop diversification has led to rise in horticulture production, which has reached 185.2 billion tones in 2010. But the real challenge starts after the production.
Preserving food to extend its shelf-life, with ensuring its safety and quality, is a central preoccupation of the food industry. As a result, there has been a steady stream of new ‘minimal‘ preservation techniques. At the same time, the development of the hurdle concept has led to renewed interest in the use of more traditional preservation methods and the ways they can be combined with newer technologies.
Food preservation is the process of treating and handling food to stop or greatly slow down spoilage (loss of quality, edibility or nutritive value) caused or accelerated by micro-organism. Preservation usually involves preventing the growth of bacterium, fungus, and other microorganism as well as retarding the oxidation of fat which cause rancidity. It also includes processes to inhibit natural ageing and discoloration that can occur during food preparation such as the enzymatic browning reaction in apples after they are cut.
Osmotic dehydration is the phenomenon of removal of water from lower concentration of solute to higher concentration through semi permeable membrane results in the equilibrium condition in both sides of membrane (Tiwari 2005). Osmotic dehydration found wide application in the preservation of food-materials since it lowers the water activity of fruits and vegetables. Osmotic dehydration is preferred over other methods due to their color, aroma, nutritional constituents and flavor compound retention value.
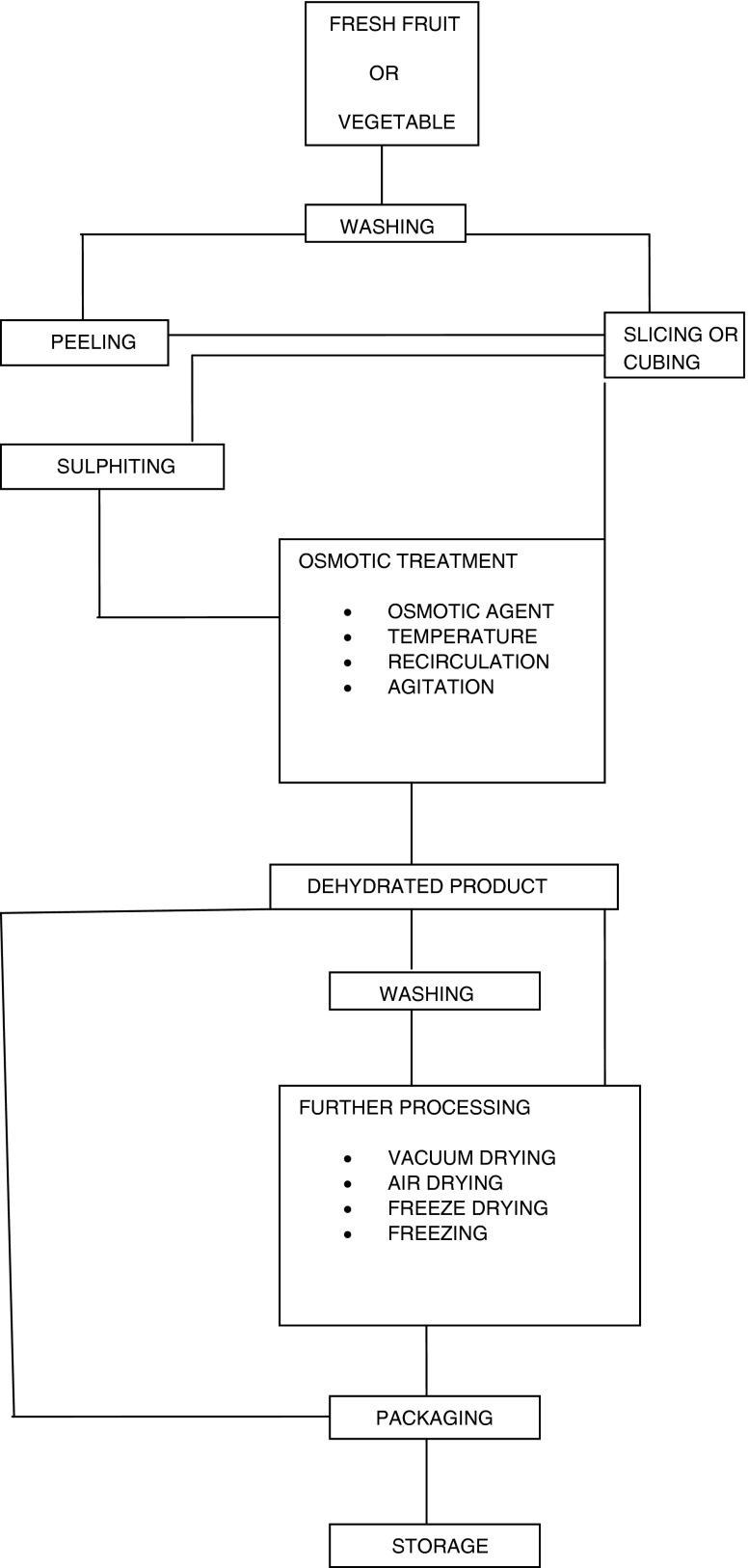
In osmotic dehydration the solutes used are generally sugar syrup with fruit slices or cubes and salt (sodium chloride) or brine with vegetables. This is multicomponent diffusion process. In this process water flow from fruits or vegetables to solution and along with water some components of fruits and vegetables such as minerals, vitamins, fruit acids etc. also move towards solution. The sugar and salt migrate towards the fruits and vegetables. The process of complete osmotic dehydration has been shown by flow diagram.
The most important products of commercial importance available in market made from fruits are murabbas of gooseberry (Aonla), apple, candies of different fruits and vegetables like pethas, sweets of parwal made by osmosis in sugar syrup. In pickles making from raw mango it is treated in brine solution before drying. Various vegetables are treated in brine to reduce their moisture content.
Common methods of applying these processes include drying, spray drying, freeze_drying, freezing, vacuum-packing, canning, preserving in syrup (osmotic dehydration), sugar crystallization, food irradiation and adding preservatives or inert gases such as carbon dioxide.
Sugar is used to preserve fruits, either in syrup with fruit such as apple, pear, peach, apricot, plum or in crystallized form where the preserved material is cooked in sugar to the point of crystallization and the resultant product is then stored dry. This method is used for the skins of citrus fruit (candied peel), angelica and ginger A modification of this process produces glace fruit such as glace cherry where the fruit is preserved in sugar but is then extracted from the syrup and sold, the preservation being maintained by the sugar content of the fruit and the superficial coating of syrup. The use of sugar is often combined with alcohol for preservation of luxury products such as fruit in brandy or other spirits. These should not be confused with fruit flavored spirits such as cherry_brandy or sloe_gin. Work of different researchers on osmotic dehydration on different fruits and vegetables has been reported in Tables 1 and 2. Process for osmotic dehydration is shown in Fig. 1.
Table 1.
Osmotic dehydration of fruits
| Fruits | Osmotic agent and concentration | Temp. °C | Sample to solution ratio | Agitation | Sample size mm | References |
|---|---|---|---|---|---|---|
| Apple | Dry sugar and syrup | 20–49 | 1 to 4 | Yes | 3 | Ponting et al. (1966) |
| Golden | ||||||
| Delicious | ||||||
| Golden delicious | Invert sugar,50% | 30–60 | 25 | – | – | Farkas and Lazar (1969) |
| Sucrose, 55–75% | ||||||
| Golden delicious | Sucrose, 70% | 50 | 4 | – | 3 | Dixon and Jen (1978) |
| Golden delicious | Sucrose, 59% | RT | 5 | – | 6–10 | Lerici et al. (1985) |
| Golden delicious | Sucrose, 60–75% | 40–80 | 26 | – | 15–20 | Videv et al. (1990) |
| Golden delicious | Sucrose, 70% | 50 | 3 | – | 13 | Sharma et al. (1991) |
| Golden delicious and Jersey Mack | Sucrose,70% | 51 | 4 | – | 3 | Dixon et al. (1976) |
| Golden delicious | NaCl | – | – | – | – | Lerici et al. (1985) |
| Red delicious | Sucrose, 30–45% | – | – | – | – | Mandala et al. (2005) |
| Cashew apple | Sucrose/corn syrup 40–60% | 30–35 | – | – | – | Azoubel (2003) |
| Granny Smith cultivar | Glucose,25–34.6% | 30 | – | – | – | Nieto et al. (2004) |
| Fuji apple | Sucrose 50% + NaCl 10% | 27 | – | – | – | Monnerat et al. (2010) |
| Red delicious | Sucrose, 70% | 70 | 4 | – | 15 | Bolin et al. (1983) |
| Apricot | Corn syrup,81% | 49 | 4 | Yes | – | Ponting (1973) |
| Banana | Sucrose, 60% | NK | NK | NK | NK | Hope and Vitale (1972) |
| Sucrose, 60–80% | 49 | 4 | Yes | – | Ponting (1973) | |
| Sucrose, 65% | 60 | NK | NK | NK | Garcia et al. (1974) | |
| Dwarf cavendish | Sucrose, 70% | 27–60 | 1–3 | Yes | 8–10 | Bongirwar and Sreenivasan (1977) |
| Sucrose 40–70 °B | 25–35 | – | – | – | Rastogi et al. (1997) | |
| Sucrose 55–65 °B | – | – | – | – | Oliveira et al. (2006) | |
| Sucrose + salt | 25–55 | – | – | – | Mercali et al. (2011) | |
| Blue berries | Sucrose, 60–80% | 49 | 4 | Yes | – | Ponting (1973) |
| Sucrose | NK | NK | NK | NK | Kim and Toledo (1987) | |
| Cherry | Corn syrup/Sucrose, 70% | NK | NK | NK | NK | Giangiecome et al. (1987) |
| Citrus fruit | Sucrose 60–80% | 49 | 4 | Yes | – | Ponting (1973) |
| Sucrose | – | – | – | – | Mehta and bajaj (1984) | |
| Amla | Sucrose 40–50 °B | 30–50 | – | – | – | Singh et al. (1999) |
| Grapes | Sucrose 60–80% | 49 | 4 | Yes | – | Ponting (1973) |
| Giant kew | Sucrose 40–70% | 20–65 | 10 | – | 6.5 | Rahaman and Lamb (1990) |
| Giant kew | Sucrose 50–70 °B+ 0.2% citric acid and 700 ppm KMS | 60–65 | – | – | – | Rashmi et al. (2005) |
| Mango green | Salt, 25% | 29 | – | – | 10 | Jackson and Mohamed (1971) |
| Ripe | Sucrose, 60% | 29 | – | – | 10 | Jackson and Mohamed (1971) |
| Salt, 25% | NK | NK | NK | NK | Hope and Vitale (1972) | |
| Dashehari | Sucrose, 70% | RT | 1 | – | – | Teotia et al. (1976) |
| Ripe mango | Sucrose, 60% | NK | NK | NK | NK | Moy et al. (1978) |
| Melntosh | Sucrose,75% | 25 | – | – | – | Camirand et al. (1968) |
| Sucrose, 25–50% | 23 | 20 | – | 3–4 | Hawkes and Flink (1978) | |
| NaCl, 5–10% | RT | |||||
| Sucrose, 50–70% | 30–50 | 4 | yes | 10 | Conway et al. (1983) | |
| Papaya | – | – | – | – | – | Jackson and Mohamed (1971) |
| Sucrose 60–80% | 49 | 4 | Yes | – | Ponting (1973) | |
| Sucrose, 65% | 65 | NK | NK | NK | Garcia et al. (1974) | |
| Sucrose, 60% | NK | NK | NK | NK | Moy et al. (1978) | |
| Sucrose, 70% | RT | 2 | – | – | Mehta and Tomar (1980) | |
| Sucrose, 60% | RT | 7 | – | 6 | Moy and Kuo (1985) | |
| Sucrose, 70% | RT | – | – | – | Levi et al. (1985) | |
| Sucrose, 44–56% | 34–46 | – | – | – | Aouar et al. (2006) | |
| Sucrose 40 °B | – | 1:60 | – | – | García et al. (2010) | |
| Peach | Sucrose 65–80% | 49 | 4 | Yes | – | Ponting (1973) |
| Corn syrup/Sucrose, 70% | NK | NK | NK | NK | Giangiecome et al. (1987) | |
| Pineapple | Sucrose 65–80% | 49 | 4 | Yes | – | Ponting (1973) |
| Sucrose,45–65 °B | 30–50 | – | – | 20 | Lombard et al. (2008) | |
| Sucrose | 30–50 | – | – | – | Ramolla et al. (2004) | |
| Sucrose, 60% | 30–50 | – | – | 0.6 | Ramolla and Mascheroni (2005) | |
| Pomegranate | Sucrose 55 °B | – | – | – | – | Bchir et al. (2010a, b) |
| Sucrose 55 °B | 40, 50, 60 °C | – | – | – | Bchir et al. 2010a, b | |
| Sucrose 55 °B | 55 °C | – | – | – | Bchir et al. (2011a, b) | |
| Pumpkin | Sucrose, 45% | 25 | – | – | 15–25 | Mayor et al. (2008) |
| Sucrose, 40–60% | 27 | – | – | – | Garcia et al. (2007) | |
| Sucrose + salt | – | – | – | – | Mayor et al. (2011) | |
| Strawberries | Ethyl oleate + NaOH | – | – | – | – | Venkatachalapathy and Raghavan (1999) |
| – | – | – | – | – | Piotrowski et al. (2004) |
Table 2.
Osmotic dehydration of vegetables
| Acerolas | Sucrose + salt | 60 | – | yes | – | Alves et al. (2005) |
| Carrot | Sucrose 54+ salt 10% | 29 | – | – | 2 | Jackson and Mohamed (1971) |
| Sucrose 40+ salt 10% | RT | – | Yes | – | Flink (1979) | |
| Sucrose 10% | 20 | NK | NK | NK | Speck et al. (1977) | |
| Salt and ethanol | NK | NK | NK | NK | Biswal and Le Maguer (1989) | |
| Sucrose + salt | 30 | 1:5 | – | 5 | Ghosh et al. (2006) | |
| Sucrose | – | – | – | – | Matuser and Meresz (2002) | |
| NaCl 5–15%+ sucrose 50 °B | 35–55 | 4–6 | – | – | Singh et al. (2007) | |
| Cantaloupe | Sucrose 45–55 °B | 40–50 | – | – | – | Corzo and Gomez (2004) |
| Cayenna lisa | Sucrose 50–70% | 30–50 | 4 | – | 9.8 | Beristain et al. (1990) |
| Chest nut | NaCl 10%+ Sucrose 25% | 25 | – | – | – | Moreira et al. (2011) |
| Dasheen leaves | Sucrose, 20% | 60–80 | – | – | – | Maharaj and Sankat (2000) |
| Hansa | Sucrose 20–60% | 23 | 1–10 | – | 10 | Lenart and Flink (1984a) |
| Onion | Sucrose 54+ salt 10% | 29 | – | – | 6–7 | Jackson and Mohamed (1971) |
| Pepper red, sweet | Sucrose 54+ salt 10% | 29 | – | – | – | Jackson and Mohamed (1971) |
| Potato | NaCl, 10–18% | 25–55 | – | – | 10 | Khin et al. (2006) |
| Potato, Bintjee | Sucrose45/salt15 | RT | 5 | – | – | Islam and Flink (1982) |
| Sugar beet | Sucrose 30–70%+ salt 0–8% | 30–50 | – | – | – | Jokie et al. (2007) |
| Tomato | Salt 10% | 29 | – | – | 10 | Jackson and Mohamed (1971) |
| Sucrose 65–80% | 49 | 4 | Yes | – | Ponting (1973) | |
| NaCl | – | – | – | – | Teles et al. (2004) | |
| Salt 5%+ sucrose 35% | 60 | – | – | – | Souza et al. (2007) | |
| Turkey | Sucrose, 40% | RT | – | – | – | Mehta and Tomar (1980) |
| Corn syrup/Sucrose, 70% | NK | NK | NK | NK | Giangiecome et al. (1987) |
KMS potassium meta by sulphite
NK not known
RT room temperature
°C degree celcius
°B degrre brix
NaCl sodium chloride
Fig. 1.
Flow diagram for osmotic dehydration process
For osmotic treatment of fruits, after washing the fruits are peeled or lye treated. The peeled or lye treated fruits are sliced (small fruits are halved). After size reduction the pretreatments like curing, chlorination etc. are given. After pre treatments the fruit pieces are pricked and boiled with sugar or other osmotic agent at a concentration of about 30 °B. The sugar is added periodically to maintain the concentration level usually 70 °B.
However in general, vegetables after washing and peeling (if required) are reduced in appropriate size by cutting or slicing. After size reduction pretreatment like blanching (dipping the pieces in boiling water) is carried out. After this osmotic treatment is carried out usually in brine or other osmotic agents.
Tiwari (2005) described the different application of osmo-air dehydration of tropical fruits and how this alternative could be beneficial in development of rural areas. Indian tropical fruits have great demand world wide due to their excellent flavor and nutrients. So osmotic dehydration is the best process to preserve them for long duration. Since it results in quality improvement in terms of color, texture, flavor, product stability, nutrient retention and prevention from microbial spoilage. However product quality were influenced by factors like pretreatment, nature and concentration of osmotic solution, quality of raw material, maturity of fruits, shape and size of slices, duration of osmosis, sample to syrup ratio, agitation, temperature and additives added. Osmotic dehydration could be very much beneficial for banana, jackfruit, sapota, mango, guava, and pineapple. He added that the process of osmotic dehydration could be employed in rural areas as entrepreneurs, home scale or with NGO’s (Non Government Organizations) at commercial level since it is economical.
The advantages of osmotic dehydration are as follows:—(Ponting et al. 1966; Jackson and Mohamed 1971; Islam and Flink 1982) -
It is a low temperature water removal process and hence minimum loss of color and flavor take place.
Flavor retention is more when sugar or sugar syrup is used as osmotic agent.
Enzymatic and oxidative browning is prevented as the fruit pieces are surrounded by sugar, thus making it possible to retain good color with little or no use of sulfur di oxide.
Removal of acid and uptake of sugar by the fruit pieces give a sweeter product than conventionally dried product.
It partially removes water and thus reduces water removal load at the dryer.
Energy consumption is much less as no phase change is involved.
It increases solid density due to solid uptake and helps in getting better quality product in freeze drying.
If salt is used as osmotic agent, higher moisture content is allowed at the end of drying as salt uptake influences water sorption behavior of the product.
The textural quality of product is better after reconstitution.
The storage life of product is greatly enhanced.
Simple equipments are required for the process.
The osmotic dehydration of fruits and vegetables is an important subject for studies. Therefore the literature reported on the subject has been reviewed comprehensively.
It has some disadvantages and inconveniences too (Ponting et al. 1966; Jackson and Mohamed 1971) and they are given below
The reduction in acidity level reduces the characteristic taste of some products. This can overcome by adding fruit acid in the solution.
Sugar coating is not desirable in some products and quick rinsing in water may be necessary after the treatment.
Osmotic dehydration with other combined processes such as vacuum drying, air drying or blanching were found expensive.
In osmotic dehydrated products water activity is found higher.
It is a time taking process.
Methods
Osmotic syrup can be concentrated and reused for at least 5 times without adversely affecting the fruit concentration. It was found the penetration rate of HFCS (High Fructose Corn Syrup) is higher as compared to sucrose but on the basis of sensory evaluation, sucrose was preferred over, HFCS for making osmotic solution (Bolin et al. 1983).
Lerici et al. (1983) studied the influence of osmotic agents on drying behavior and quality of apple fruit. They reported that water loss and water activity (aw) of the final product depends not only on the aw (water activity) of osmotic solution but also on the amount of solids in the sample. In addition to this, final product quality also depends on treatment, solid gain, chemical composition of syrup and shape of sample. They added that addition of NaCl increases the drying process.
In osmotic dehydration, a simultaneous flux of water and solutes from and into the material takes place. This method can be used as a pretreatment before air drying in order to reduce from 30% to 70% of the water content of the food. Osmotic dehydration preceding air drying decreases colour changes and increases flavour retention in dried fruits and vegetables (Lenart and Lewicki 1988).
Partial dehydration of banana by osmosis in 70% sugar syrup reduces 50% of its original weight and after drainage, washing and vacuum dried, the product could be preserved up to 1 year depending on condition and packaging material and also gives best result in flavor, color, appearance and texture (Bongirwar and Sreenivasan 1977). Ramanuja and Jayaraman (1980) prepared intermediate moisture banana which was having better flavour and appearance with good storability.
Valle et al. (1998) proved that HTST (High Temperature Short Time) blanching of apple pieces caused PPO (Poly Phenol Oxidase) inactivation and softening. PPO inactivation was minimal during immersion in water at 40 °C, but it increased with temperature after 15 min exposure at 55–65 °C. Softening decreased with adding 0.6% CaCl2 is added to the blanching medium. Vacuum infilteration of apple pieces caused cellular damage that increased with applied pressure. Texture improved by the use of aqueous CaCl2 solution instead of distilled water. HTST blanched apple pieces showed extensive material loss and poor texture on osmotic dehydration.
Rosa and Giroux (2001) studied on the problems related to the solution management in Osmotic treatments. They concluded that implementation of osmotic treatments (OT) of plant or animal materials in concentrated solutions (called also dehydration-impregnation by soaking process, DIS) presents a critical factor due to the management of the concentrated sugar/salt solutions. They studied on the following factors. Solution mass and dilution loss of solutes and particles from food, concentration restoring and solution recycling, reconditioning of solution by membrane processing, microbial contamination of the solution, solution preservation during process stop time, microbial contamination of foodstuffs due to food/solution contact, sanitation of the solution, resistance of the solution to the thermal treatments, condition to determine the end-point of the working solution, possible utilisation of spent solutions for different use and problems related to the discharge of the spent solutions.
Fragoso et al. (2002) proposed a set up of pilot plant for osmotic dehydration of apple cubes of 1 cm3. They showed that suitable pilot plant should consist of novel agitation system, immersion device, a bag filter and vacuum evaporator. The experiment was done at 50 °C in 60 °B of sucrose syrup and sugar/fruit ratio of 5. The concentration was maintained at 60 °B by re-concentrating in the evaporator. The osmo-dehydrated cubes were comparable with laboratory scale products.
According to Sharma et al. (2003) during osmotic dehydration always water loss is favored over solid uptake that leads to mass loss of pear fruit. According to their experiment all these parameters depends on concentration of syrup and syrup to fruit ratio. It was found that with increase in syrup concentration from 35 to 45 °B and syrup fruit ratio from 1 to 2, there was considerable change in water loss from 18.09% to 23.18%, mass loss from 9.26% to 20.06% and solid gain from 13.59 to 16.38%.
70 °B sugar syrup was found effective in removal of moisture from giant kew, a variety of pineapple. On the basis of sensory scores maximum dry fruit yield, lower moisture, higher ascorbic citric acid and carotenoid content, the 60 °Brix sugar syrup with 0.2% critic acid and 700 ppm KMS (Potassium meta bi sulphite) was best for osmotic dehydration for the period of 24 h. The studies were done both before and after storage of 6 month at ambient conditions (Rashmi et al. 2005).
Dehydration of carrot slices were carried out by two different methods viz. hot air drying and osmo air drying after blanching. Hot air drying was done in cabinet drier and osmo air drying was carried out with the help of best cane sugar syrup. It was found that osmo air drying was better since carotene retention was better and reducing sugar increased whereas total sugar decreased (Madan and Dhawan 2005). During osmotic dehydration 100–2,400 kj energy is required for per kg elimination of water while in drying 5,000 kj/kg.
Different parameters were studied after osmotic dehydration viz. water loss, sugar gain, variation in concentration of other natural fruit sugar (glucose and fructose) in 0.6 mm thick slices of pineapple in 60% w/w sucrose solution at 30, 40 and 50 °C. Result showed that with increase in temperature the apparent moisture and sugar diffusivity increased. Glucose and fructose were also lost with increasing temperature. It was also found that moisture content was linearly affected with solute content was independent of temperature up to 600 min of initial dehydration (Ramolla and Mascheroni 2005).
Ghosh et al. (2006) studied many different processes for osmosis. Potassium meta bisulphite (0.1%) were used in the osmotic reagent solution of sucrose (50°B) along with 5,10 and 15% salt solution for osmo hot air drying of 5 mm thick slices of carrot. One important parameter taken into consideration was constant agitation at 150 rpm. Two sets of experiments were performed in which first carrot sliced were osmosed and then air dried at 50, 60 and 70 °C and second experiment consist of only conventionally air drying at 70 °C. It was found that osmo-hot-air carrot slices were received higher sensory scores at 5% significances level. On the basis of organoleptic quality and rehydration ratio the osmotic reagent used for osmo-hot-air drying process for carrot slice should contain 50 °B sugar solution containing 5% salt and 0.1% KMS for 1 h and hot air drying at 50 °C. The rehydration ratio was found 3.3.
The apparent diffusion coefficients for sucrose and water during osmotic dehydration of jenipapos were determined by Andrade et al. (2007). Equilibrium concentrations inside jenipapos were determined by long time experiment (up to 60 h) and kinetics of water loss and solid gain were determined by short time experiments (up to 4 h). Results showed that mass transfer rates for water and solutes, as well as the apparent diffusion coefficients for sucrose showed to be dependent on sucrose concentration in osmotic solution. Immersion time does not have significant effect over the diffusion coefficients for sucrose and water.
Khin et al. (2007) performed an experiment to study the mass transfer in osmotic dehydration of coated apples by 20% and 50% (w/v) maltodextrin solution. After coating fruits were subsequently dried in an oven at 70 °C for 10 and 40 min respectively to solidify the coating. Osmotic dehydration of both coated and non coated samples were done at 30 °C and 61.5% (w/v) sucrose were used as osmotic agent. Fruit to solution ratio was kept 1:20 for both the samples. Results showed that the coated samples yielded dry matter gain and sugar gain during the osmotic dehydration. In addition, moisture loss of the coated samples was much smaller than that of non coated samples. Hardness, brittleness, springiness and cohesiveness of coated and non coated samples were measured. Results showed that structure of maltodextrin coated samples were altered.
Osmotic dehydration of pomegranate seed was carried out at different temperature (30, 40 and 50 °C) in 50 °Brix sucrose, glucose and mixture of two in 50:50. After 20 min of dewatering water loss was obtained as 46% in sucrose, 37% in glucose and 41% in mixture solution. Temperature increases the water loss. Ratio of frozen and unfrozen water decreased from 5 to 0.5%. It was also found that glass transition temperature depends on type of sugar (Bchir et al. 2009).
Effect of the re-use of the osmotic solution on the stability of osmodehydro-refrigerated grapefruit were studied by Moraga et al. (2011). The osmotic solutions were reused for five osmotic dehydration cycles, with or without pasteurization. The samples obtained in cycles 1, 3 and 5, were stored at 10 °C. Changes in °B, water content, water activity, pH, total acidity, ascorbic acid content, were analyzed and compared to fresh-cut grapefruits. Study showed that osmotic solution could be used up to five times without any reconcentration treatment, but it was advised to pasteurize the solution each time to obtain product with shelf life between 7 and 12 days in refrigeration.
Bellary et al. (2011) studied the osmotic dehydration of assisted impregnation of curcuminoids (curcumin, demethoxycurcumin and bisdemethoxycurcumin) in coconut slices and the rate of mass transfer of moisture, solid and curcuminoids with or without application of ultrasound were also studied. Results showed that increased concentration of osmotic solution beyond 25% resulted in the reversal of direction of moisture and solid gain mass transfer. Ultrasound treatment leads to higher moisture and solid mass transfer due to breaking of cell structure. HPLC analysis revealed that all the curcuminoids were infused into the coconut matrix.
Durrani and Verma (2011) prepared and studied on the quality of honey based Amla murabba. Amla murabba was developed by using honey as natural sweetener instead of white sugar. The overall quality evaluation of honey murabba packed in PET and glass jar were evaluated at different interval for 6 months. Sensory quality scores decreased for samples packed in PET jar and vice versa was the case for sample packed in glass jars. Glass jar was found better packaging material with respect to various physico-chemical properties, microbiological properties and sensory qualities. They concluded that amla murabba can be preserved safely for 180 days.
Durrani et al. (2011) prepared carrot candy using honey by the process of osmotic dehydration. They found that carrot candy could be preserved for 6 months in both glass and LDPE packaging materials.
Treatments
Sometime pre and post treatments were given to increase the extent of osmotic dehydration. These may be blanching, sun or air drying etc.
The product obtained by osmotic dehydration contains moisture in the range of 65–75% with water activity between 0.94 and 0.97, and thus belongs to the class of intermediate moisture foods having moisture content, ranging from 20 to 50% and water activity between 0.65 and 0.90 (Le Maguer 1988). Thus it becomes necessary to further dry the material for extending shelf life. The drying involves air drying, vacuum drying and freeze drying. It has been observed that the flavor retention in case of osmovac drying (osmotic dehydration and vacuum drying) is more than freeze drying (Ponting et al. 1966; Dixon et al. 1976). Osmovac dried fruits are very suitable in storage as the moisture content is quite low (1–3%) and can retain their high quality for 1 year at room temperature. Osmotic air dried fruits are unstable during storage (Ponting 1973). The blanching is practiced after osmotic treatment prior to further processing in order to inactivate enzymes.
Kim and Toledo (1987), showed that lowering of moisture content on high temperature fluidized bed (HTFB) drier of osmotically pretreated sample of Rabbiteye blueberriers as compared to untreated sample. Untreated sample of HTFB at 170 °C for 15 m/s air velocity in 8 min showed reduction in moisture content from 5.8 to 0.7. But when osmotically treated sample were HTFB dried for 4 min at 150 °C showed moisture content reduction to 0.28. The time taken to bring same water activity (0.5) in the sample of HTFB at 60 °C for 4 m/s air velocity is more in untreated sample (2.13 h). Study showed that osmotically dehydrated sample prior to HTFB showed raisin like texture.
Caned apple rings pretreated with 70% sugar solution at 50 °C for half an hour resulted in weight loss, increase in sugar penetration and increase in shrinkage. The results were based on sensory, physico-chemical and economic point of view (Sharma et al. 1991).
Vacuum Osmotic dehydration lead to more water loss but had no effect on solid uptake since vacuum treatment intensify the capillary flow function. Vacuum treatment was suitable in high porous fruit such as pineapple (Shi and Maupoey 1993).
Before pretreatment of strawberries with 2% ethyl oleate and 0.5% NaOH results in better dehydration. These osmotically dehydrated strawberries were convective and microwave dried and compared with freeze dried strawberries with same pretreatments. Comparable studies were done for drying time, rate, dehydration ratio, texture, color and sensory values. All these parameters were found suitable and best in microwave dried sample (Venkatachalapathy and Raghavan 1999).
Maharaj and Sankat (2000) studied on the quality of rehydration at 60, 70, 80 and 100 °C after steam, water or alkali blanching of 2–4% dry basis moisture content of dasheen (Colocasia esculenta) leaves dried at 60 °C. 20% sucrose dipping is required after alkali blanching. Results showed that alkali blanched leaves had highest equilibrium moisture content and hydration rate. When alkali blanched leaves were cooked at 100 °C it was found comparable with freshly cooked leaves however color retention was best in water blanched leaves.
Different pretreatment methods for osmotic dehydration of cranberry had been assessed. Mechanical, chemical and osmotic dehydration were done. In mechanical pretreatment fruits were cut into halves and quarters. In chemical pretreatment difference was in temperature of chemical agent and dipping time of fruits while in osmotic dehydration different osmotic agent in different concentration were used along with varying osmotic dehydration time. On the basis of results according to mass gain, solid gain and moisture, it was conduced that quarter halves showed best result and time and concentration of osmotic agent significantly promote the result however different chemical treatment showed no difference in product quality (Sunjka and Raghavan 2004).
The quality of minimally processed guavas (Psidium guajava L.), osmotically dehydrated and packed under passive modified atmosphere, was evaluated by Pereira et al. (2004) during 24 days of storage at 5 °C. It was found that Modified atmosphere packaging (MAP) in polyethylene terephthalate (PET) containers had a strong influence on color preservation and weight loss of the guavas. Significant changes in the texture was found due to osmotic dehydration, but the color of the fresh fruit remained unchanged. Osmotically dehydrated guavas stored in MAP showed good microbial conditions during storage.
Influence of osmotic dehydration on the microwave convective drying of frozen strawberries was studied. It was found that osmotically dehydrated frozen strawberries took less time (four level from 0 to 1.7 w/g) for microwave drying while those strawberries that were not osmotically dried took 7 levels of microwave doses for drying (Piotrowski et al. 2004).
Impact of modified atmosphere packaging on the osmodehydrated papaya stability were studied by Rodrigues et al. (2006). Papaya pieces were osmotically dehydrated in a 50°Brix sucrose solution containing calcium lactate (0.05 M) and lactic acid (0.02 M) as additives for 1 h at 25 C, packed in polyethylene terephthalate (PET) containers and stored at 5 C for 15 days. Fresh fruit pieces packed in PET containers and under atmosphere condition were used as control samples. Sensory acceptance, microbiological count, CO2 and O2 content inside the packages, color, mechanical properties and weight loss of the product were evaluated. There results showed that PET containers prevented weight loss during storage. The utilization of calcium lactate as additive was effective in maintaining fruit hardness during refrigerated storage. The use of passive modified atmosphere associated with refrigeration was adequate to preserve the osmotically dehydrated fruit for 15 days, showing a good sensory acceptance by the consumers during the storage time.
Influence of pretreatment (blanching, freezing) on osmotic dehydration of pumpkin was analyzed by Kowalska et al. (2008). Pumpkin cubes (10 × 10 × 10 mm) were dehydrated in sucrose, glucose and starch syrup solutions for 180 min. Both pretreatments gave higher water loss and especially increase solid gains in comparison to samples without pretreatment. The highest water loss from blanched osmo-dehydrated during 180 min pumpkin took place by using starch syrup solution (4.5 g H2O/g i.d.m) and the lowest is better than smallest from frozen sample and dehydrated in sucrose solution (1.6 g H2O/g i.d.m). Highest value of WL/SG parameter was obtained equal to 9.4 during osmotic dehydration of raw pumpkin. Results showed that pretreatment such as blanching and freezing did not have significant influence on the diffusion coefficient for water and solids.
Lombard et al. (2008) used osmotic dehydration process as a pretreatment for further drying. They studied the effect of osmotic dehydration on mass fluxes (water loss, solids gain and weight reduction). Pineapple cylinders of 2 cm in diameter and 1 cm thick were immersed in sucrose solutions of 45,55 and 65 °B at 30,40 and 50 °C for 20, 40, 60, 20, 180 and 240 min. Experiments were conducted at both atmospheric pressure and applying a 200 mbar vacuum pulse during the first 10 min. Applied pulse facilitated water loss capacity. Water loss and solid gain were increased with increased concentration and temperature. Water loss was affected by temperature and solid gain was affected by concentration of the solution. The quality was evaluated by water loss and solid gain combination.
Torres et al. (2008) studied on the minimally processed mango that was obtained by applying osmotic treatments and the product quality development was evaluated throughout storage. Osmotic treatments were carried out at atmospheric pressure Osmotic dehydration (OD) and by applying a vacuum pulse osmotic dehydration (PVOD) using 45 °Brix sucrose solutions with and without calcium lactate (2%). Mechanical properties were measured after treatments and after 10 days storage. Color, microbial controls, and respiration rates were measured throughout storage time, in order to evaluate the development of the different parameters which affect product quality. From the microbiological point of view the most stable samples are those treated with calcium in both OD and PVOD treatments. Respiration rates were also reduced to a greater extent in these treatments. Mechanical properties are also better preserved when calcium is applied in PVOD treatments.
Chavan et al. (2010) developed a process for preparation of ripe banana slices using osmotic dehydration. Fully ripe banana fruits were peeled and slices of 8 mm thickness were prepared. The slices were divided into 5 lots and pretreated with sulphur fumigation @ 2 g/kg of slices for 2 h then each lot was soaked in 60 °Brix sugar syrup containing 0.1% KMS + 0.1% citrate, 0.1% KMS + 0.1% citrate + 0.2%, 0.4% and 0.8% ascorbic acid and control respectively. After 16 h soaking, quick washing, blotting and then cabinet drying at 55 °C for 10 h up to 18% moisture content was done. The dried products were packed in 200 gauge polypropylene bags and stored at ambient condition for 6 months. The chemical, microbial and organoleptic changes were monitored for 6 months. The osmo-dried banana slices prepared with sulphur fumigation @ 2 g/kg slices for 2 h followed by soaking in 600Brix sugar syrup containing 0.1% KMS + 0.1% citrate + 0.2% ascorbic acid were found better with respect to colors and appearance, flavour, texture, taste and overall acceptability with non-stickiness of the product. Storage study showed that there was marginal decrease in moisture content and organoleptic quality and increase in TSS, total sugars and reducing sugars content of osmodried banana slices. The products were found microbiologically safe and sensorily acceptable up to 6 months storage at ambient condition.
Bchir et al. (2011b) reported that among all the preservation techniques osmotic dehydration is an economical and interested in nutritional point of view.
Fresh and frozen osmotic dehydrated pomegranate seeds were compared by Bchir et al. (2011a). Process was carried out at 50 °C in 55 °Brix sucrose solution. During first 20 min of process, significant water loss and solid uptake was found i.e. 46 g/100 g water loss and 7 g/100 g solid gain was found after 20 min average water loss and solid gain was observed. Texture modification was also observed in the process.
Osmotic agents
A number of osmotic agent can be used in osmotic dehydration (Table 3) either singly or in combination. The effect of each during osmotic dehydration is also reported in Table 3. The osmotic agent should also reduce water activity of a solution substantially for increasing the driving force. It must be effective, convenient, non toxic and have a good taste. It should be readily dissolved to form a high concentrated solution. It should not react with the product and should be cheap. The concentration of osmotic agent plays an important role in osmotic dehydration. In general, more the concentration of syrup, more the rate of osmosis took place (Chaudhari et al. 1993).
Table 3.
Osmotic agents used in osmotic dehydration process
| Osmotic agent | Remark | References |
|---|---|---|
| Calcium chloride | Increase firmness of apple pieces, preserve the texture during storage. Prevents browning because of synergistic effect with ascorbic acid or sulfur di oxide | Ponting et al. (1972) |
| Ethanol | Decrease the viscosity and freezing point of osmotic solution in the de-hydro cooling process | Ponting et al. (1972) |
| Cipolletti et al. (1977) | ||
| Fructose | Solute penetration rate is higher than sucrose, sucrose is preferred over fructose | Bolin et al. (1983) |
| Invert sugar | More effective than sucrose on same concentration because when completely inverted, it has twice as many molecules per unit volume. Practically a little difference in osmotic dehydration rate. | Ponting et al. (1966) |
| Lactose | Has much lower level of sweetness than sucrose. Low solubility in aqueous solution | Hawkes and Flink (1978) |
| Maltodextrin | Can be used at higher total solids concentration or in mixed system | Khin et al. (2007) |
| Sodium chloride | Excellent osmotic agent for vegetables. Retards oxidative and non enzymatic browning. Sometime bleaching effects on colored products, can be prevented using mixtures e.g. salt and sugar. Concentration should be around 10%. Hinders shrinkage | Jackson and Mohamed (1971) |
| Speck et al. (1977) | ||
| Hawkes and Flink (1978) | ||
| Flink (1980) | ||
| Lerici et al. (1985) | ||
| Khin et al. (2006) | ||
| Singh et al. (2007) | ||
| Sucrose/sugar | Dry sugar is unsuitable due to oxidative browning. Difficulty in disposing the sugar syrup formed. Sweetness prevents it for vegetable processing. | Ponting et al. (1966) |
| Farkas and Lazar (1969) | ||
| Ponting (1973) | ||
| Flink (1975) | ||
| Mixture of invert sugar and salt, sucrose and salt, ethanol and salt | More effective than sucrose alone due to combination of properties of both the solutes. | Lenart and Flink (1984a, b) |
Different coefficient during osmotic dehydration of tomato in solution of water, sucrose and salt were studied. Long period (60 h) treatment was given to study equilibrium concentration while short duration (4 h) treatment was given to study kinetics of water losses and solid uptake. Mass transfer was found to be dependent on both sucrose and NaCl. The diffusivity of sucrose and NaCl were also independent. Water losses increased with increase in NaCl concentration while higher percentage of sucrose hinder the penetration of NaCl in the tomato (Teles et al. 2004).
Alves et al. (2005), investigated the osmotic dehydration of frozen mature acerolas in an incubator at temperature 25–60 °C and constant agitation. They performed an experiment in which acerolas were blanched in water (80 °C for 3 min) and dehydrated using binary (water + sucrose) and ternary (water + sucrose + salt) solutions. The concentration of sucrose were varied from 30% to 60% (w/w) in binary solution and from 20% to 50% (w/w) in ternary solution with 10% (w/w) salt. Different parameters viz. water loss, solid gain, solid gain/water loss ratio and water reduction were evaluated. The best results were found at 60 °C for both the solutions but 60% (w/w) sucrose in binary solution and 50% (w/w) sucrose with 10% (w/w) salt in ternary mixture provides optimum result.
Fuji variety of apples were treated in aqueous sucrose (50% w/w) and salt (NaCl, 10% w/w) solutions for 2,4 and 8 h (27 °C). Concentration profiles were determined as a function of the distance more by osmotic solution considering unidirectional movement by Monnerat et al. (2010). The density, water, sugar and salt contents were determined for each piece of apple. Effective diffusion coefficients as a function of concentration were determined, using the material coordinate to consider tissue shrinkage. The coefficients were obtained by simultaneously integrating the three differential equations (for water, sucrose and salt). The sliced apples were also examined by light microscopy to study the cellular behavior.
Mathematical modelling and mass transfer
In osmotic dehydration process, there is a simultaneous counter current mass transfer of water from solution to hypertonic solution and of solute from solution into the sample. Soluble solids of the sample such as organic acids, minerals and vitamins also migrate relatively in the small quantities from sample to solution (Chaudhari et al. 1993). Fundamental aspects of mass transfer in a cell and in aggregate cell has been given in detail by Le Maguer (1988).
Diffusive mass transfer phenomena occur in such type of processes. With the knowledge of effective diffusivity, the rate of mass transfer can be effectively predicted by the appropriate solution of the simplified unsteady state diffusion equation. Crank (1975) has given a detailed discussion on theoretical aspects of the diffusion process.
Hawkes and Flink (1978) reported so-called mass transfer coefficient by plotting the normalized solid content of apple slices (per cent total solid change based on the initial solids) versus square root of time and calculating slopes for various osmotic solutions and their concentrations. Conway et al. (1983) calculated diffusion coefficient, D, and reported the expression for log D as a function of temperature, T, and concentration, B. It was found that log D was in linear function of 1/T and B. Moreover, there was an interaction between 1/T and B.
Lennart and Flink (1984a, b) considered the shrinkage of the material during osmosis for characterization of the process. They used two parameters namely, osmosis effect penetration depth and the relative moisture content of the first slice to discuss the effect of various treatment on osmosis.
Irreversible thermodynamics has also been used to model the overall mass transfer in a multi component system occurring in plant tissue upon osmosis (Le Maguer 1988).
Crank’s equation was found best to analyze the experimental data during study the effect of temperature (30, 40 and 50 °C) and sucrose concentration (50, 60 and 70 °B) on the osmotic dehydration of pineapple at fruit to sucrose ratio as 1:4 (Beristain et al. 1990).
Mass transfer during osmotic dehydration of banana were studied by Rastogi et al. (1997). The effective diffusion coefficient were calculated by applying Fick’s law over a range of temperature (25–35 °C) and osmotic reagent concentration (40–70 °B). the effective diffusion coefficient were empirically correlated with the concentration and temperature by an Arrhenius type equation. The correlation coefficient R2 was found to be 0.67 between predicited and experimental values of effective diffusion coefficient.
Binary osmotic reagent that was mixture of 10% NaCl and 25% sucrose w/w at room temperature (25 °C) for osmotic dehydration of cherry-tomato were used. The kinetics of water loss and solid uptake were determine by two model.
-
I.
Fick’s second law and
-
II.
Spherical geometry
In this case the apparent diffusivity coefficient obtained in the range of 2.17 × 10−10 to 11.69 × 10−10 m2/s (Azoubel and Murr 2000).
Mass transfer in carrot slices was quantitatively investigated. The distribution of water and sugar were determined in longitudinal and radial direction in carrot sample. Effective diffusion coefficient of sugar solution were estimated by Fick’s I and П law depending on the geometric parameters of the slices, the depending of radial and longitudinal geometry was found to be in line if there were uniformity in longitudinal geometry and constant existing difference in radial direction (Matuser and Meresz 2002).
Ferrando and Spiess (2003a, b) applied response surface methodology to study the effect of processing time and concentration on cellular shrinkage and cell destruction of straw berry tissue. 1st order model was followed by kinetics of cell destruction in regards to concentration of sucrose (15–65% w/w). Water diffusivity among the cell is also dependent on concentration and varied in range from 16 ± 2 × 10−12 to 0.6 2 ± 0.6 × 10−12 m2/s. Macroscopic changes were studied in the same experiment by RSM. With increase in dewatering phenomenon, cellular shrinkage and cell destruction increased. The effective diffusivity of water was found to be in the range of 5.1 ± 1.0 × 10−10 to 0.7 ± 0.2 × 10−10 m2/s and it was found higher of microscopic water diffusivity that was of order of magnitude 10−12 m2/s.
In osmotic dehydration process the most important parameter is diffusion coefficient of water and solute. Diffusion coefficients were estimated according to Fick’s law in the slices of pine apple. Extent of water loss and solid gain was studied. In 60% (w/w) sucrose solution the equilibrium water content ranged from 34 to 36% and it was not found to be dependent on temperature. As the temperature rose from 30 to 50 °C, the equilibrium sugar content increased from 45 to 54%, the shrinkage in the thickness was found independent on temperature and dependent only on water content (Ramallo et al. 2004).
Mass transfer kinetics of osmotic dehydration of cherry Tomato in hypertonic solution of NaCl (with or without sucrose) was studied by Azoubel and Murr 2004, according to Peleg, Ficks and Page equation, that determines water loss, effective diffusivity and salt gain respectively. The water loss found to be in the range of 0.43 × 10−9–1.77 × 10−9 m2/s and salt gain was in the range of 0.04 × 10−9–0.54 × 10−9 m2/s. Obviously increased concentration of NaCl increased water loss and salt gain. Addition of sucrose decreased the driving force (Azoubel and Murr 2004).
The effect of two different osmotic pretreatments (Glucose or Sucrose solution at 30–45% w/w) prior to air drying were studied. Sugar gain, water loss, color, porosity, compressive fracture and stress were evaluated after 10 h of immersion. Results showed that sample pretreated with 45% glucose solution showed better behavior in all respects mentioned above (Mandala et al. 2005).
Optimization process of osmosis of Banana followed by air drying was studied by Oliveira et al. 2006. Since banana is a highly perishable fruit and cannot withstand at freezing also, so there must be some alternative to preserve banana. After optimization it was found that moderate to high sucrose concentration (55–65 °B) osmotic reagent at reduced pressure could be used as osmotic treatment for banana that reduces processing time.
The mass transfer kinetics during osmotic dehydration of carrot cubes in ternary solution of sucrose, NaCl salt and water were studied by Singh et al. (2007). The three different osmotic solutions were made by differing NaCl% i.e. 5%, 10%, 15% salt (w/v) in 50 °B sucrose solution. Temperature used were 35, 45, and 55 °C, fruit to solution ratio were 1:4, 1:5, and 1:6 and process duration varied from 0 to 240 min . Azuara model was used to determine water loss while solid gain was estimated by Magee model. Fick’s law of diffusion were used to estimate the effective diffusivity of water as well as solute.
Falade et al. (2007) investigated mass transfer, colour change of fresh, osmosed and osmo-oven dried water melon. Mass transfer during osmotic dehydration was modeled using Fick’s second law of diffusion and found that activation energy for moisture and solids diffusivity ranged from 5.09 to 32.77 kJ/mol and 3.43 to 32.38 kJ/mol, respectively. It was also found that colour intensity and chroma values increased with increase in osmotic solution concentration in osmosed and osmo-oven dried watermelon.
Kinetics of osmotic dehydration (OD) and effects of sucrose impregnation on thermal air-drying of pumpkin slices were investigated by Garcia et al. (2007). A simplified model based on the solution of Fick’s Law was used to estimate effective diffusion coefficients. Pumpkin slices were dehydrated in sucrose solutions (40%, 50% and 60%, w/w, 27 °C). The effective water diffusion coefficients were higher than the sucrose, and low diffusivity dependence with solution concentration was observed. Pretreatment in 60% osmotic solution during one hour before hot air drying at 50 and 70 °C (2 m/s) leads to enhanced mass transfer.
Khoyi and Hesari (2007) found out the best sucrose concentration, temperature and solution to sample ratio for osmotic dehydration of apricot. Dehydration were carried out in three different sucrose concentrations and at four different temperatures and also three different weight of solution to sample ratios. The experimental data were fitted to Peleg and Fick models. Results showed that water loss and solids gain increased with the increase of temperature and concentration. Increased volume of osmotic media caused increased overall mass transfer but the best weight ratio was determined to be 10:1. The most appropriate time calculated was 6 h.
Ferrari and Hubinger (2008) studied on the mass transfer kinetics and mechanical properties of osmodehydrated melon cubes. Samples were immersed in a hypertonic solution of sucrose or maltose up to 8 h under controlled temperature and agitation. Water loss, sugar gain and mechanical properties were analysed throughout the process. Mass transfer kinetics was modelled according to Peleg and Fick equations. Higher water loss, lower sugar uptake and stress at rupture values more similar to fresh fruit were observed in samples treated with maltose solutions. Peleg’s model showed the best adjustment to all the experimental data. The effective diffusivity obtained using Fick’s equation varied from 3.93 × 10−9 to 6.45 × 10−9 m2 s−1 for water loss and from 7.57 × 10−10 to 3.14 × 10−9 m2 s−1 for sugar gain.
The equilibrium moisture contents were determined for carrot cubes osmotically pretreated in salt, sucrose and salt plus sucrose combined solution using static method at 10, 25, 40 and 50 °C over a range of relative humidities from 14% to 95% by Singh and Mehta (2008). It was found that modified exponential equation, is the best equation for predicting the equilibrium moisture contents (EMC) of the dehydrated carrot cubes preosmosed in salt and sucrose plus salt solution, whereas modified Hasley equation is suitable for dehydrated carrot cubes preosmosed in sucrose solution over the relative humidity range of 14–95%.
Moraga et al. (2009) evaluated the effect of calcium lactate (2%) on osmotic dehydration kinetics and on the respiration rate, mechanical properties and shelf life of fresh, vacuum impregnated and pulse vacuum osmo-dehydrated (PVOD) grapefruits. The shelf life of grapefruits were increased from 5 to 8 days if they were osmodehydrated and to 11 days if calcium is added to osmotic solution. The result was related with the decrease in the cellular respiration rate caused by dehydration and enhanced with the presence of this ion. With the addition of calcium lactate water effective diffusion coefficient was reduced from 3.64 8 × 10−11 to 1.80 × 10−11 m2/s.
Dermesonlouoglou et al. (2005) in the Шrd international symposium of Application of modeling as an innovative technology in the Agri food chain model-IT, gave the analytical solution of Fick’s law for calculation of effective diffusion coefficient over temperature range (5, 35, 55 °C) and 40–60% w/w concentration of osmotic solution. Arrhenius type equation was modeled to study the dependency of concentration and temperature on effective diffusion coefficient which is directly proportional to concentration and temperature. Mass transfer was found higher if this molecular weight of the carbohydrate was lower and same treatment exhibited significantly improved quality such as color, flavor, texture and stabilization during frozen storage.
Pomegranate seeds were osmo dehydrated using date juice with 55 °Brix sucrose. The kinetics of osmotic dehydration showed that mass transfer took place significantly during first 20 min of the process. During the process water loss and solid gain was found around 39 and 6% respectively. Scanning electron microscopy revealed texture improvement by the process. Natural sugar present in the date juice permits in substituting the 35% sucrose content of the osmotic solution (Bchir et al. 2010a, b).
The drying of pomegranate seeds was investigated at 40, 50 and 60 °C with air velocity 2 m/s. Prior to drying seeds were osmo dehydrated in 55 °Brix sucrose solution for 20 min at 50 °C. Drying kinetics, antioxidant capacity, total phenolics, color and texture were determined by Bchir et al. (2010a, b). The results showed that 40 °C is the best temperature on which less impact on the quality of seed was found.
According to Mancilla et al. (2011) high hydrostatic pressure treatment improved the diffussion coefficient of water and soluble solids compared to atmospheric pressure in strawberries. Effect of these pressure were achieved by Arrhenius type equation. Based on statistical data, the Weibull model gave the best goodness of fit on the experimental data out of Newton, Henderson–Pabis, Page and Weibull models. Results indicated that application of this innovative technology improved strawberries dehydration rates, resulting in a dried fruit with intermediate moisture content.
Mercali et al. (2011) worked on optimization of osmotic dehydration of banana with respect to temperature (25–55 °C), salt (0–10 g/100 g) and sucrose (30–60 g/100 g) concentrations through response surface methodology. Effective diffusivities of water, sucrose and NaCl were calculated by Fick’s Law. Water loss and solute uptake were predicted by Peleg’s model. The final result showed the effective diffusivity of water to be in the range of 5.19–6.47 × 10−10 m2 s−1, the sucrose effective diffusivity between 4.27 and 6.01 × 10−10 m2 s−1 and that of NaCl between 4.32 and 5.42 × 10−10 m2 s−1. The optimized level of three variables viz. temperature (25 °C) with a solution composed of 30 g/100 g of sucrose and 10 g/100 g of sodium chloride was found and the value of effective diffusivity at these levels were 4.80 × 10−10 m2s−1 for water, 3.21 × 10−10m2s−1 for sucrose and 4.49 × 10−10 m2 s−1 for NaCl.
Process optimization
Azoubel (2003) studied on the optimization of sucrose and osmotic dehydration of cashew Apple in two different solution of corn syrup using response surface methodology. Three different factors were temperature (30–50 °C), sugar syrup concentration (40–60%w/w) and immersion time (90–240 min) and responses were water loss (%) and solid gain (%) studied in polynomial equation with multiple correlation coefficient ranging between 0.92 and 0.99. It was tried to obtain maximum water loss and minimum incorporation of solid so that the fruit resemble fresh. The ascorbic acid was found almost same in both different osmotic reagent solutions.
Optimization of Process was done for the osmotic dehydration of Cantaloupe by using desire function methodology. The factors that were taken into account were temperature (40–50 °C), concentration (45–55 °B) and time (60–120 min) and the responses were weight, humidity and °B increases respectively. The response variables were fitted to predictive model applying multiple linear regressions. The conclusion was that the optimized dehydration process consist of 37.39 °C temperature, 41.6 °B osmotic reagent solution and 132.30 min of time that result in weight loss equal to 0.11 (g/g), water loss equal to 0.33 (g/g) and °B increases equal to 12.3 °B/g (Corzo and Gomez 2004).
Optimization of osmotic of melons followed by air drying was done. Osmotic dehydration is the best alternative to reduce the post harvest losses of fruit. Osmotic solution to fruit weight ratio were examined of the melon sample. Models were developed and data were analyzed to search the best operating condition in less time (Teles et al. 2006).
Fernandes et al. (2006) studied the optimization of osmotic dehydration of Papaya followed by air drying. They put the data of loss of papaya fruit ranges between 10% and 40% and could be reduced by drying. The process of osmotic dehydration followed by air drying was studied and modeled for papaya preservation. An optimization was done using the model in order to search for the best operating condition that would reduce the processing time.
Aouar et al. (2006) studied the influence of the osmotic agent on osmotic dehydration of papaya. The study was carried out using two factorial experimental design with three independent variables whose levels varied from 44% to 56% w/w for concentration, from 34 to 46 °C for temperature and from 120 to 210 min for immersion, time. The responses were weight reduction, water loss, solid gain and water activity. The results showed that on same osmotic pressure for two osmotic reagents (sucrose and corn syrup) the value obtained for water loss, weight reduction and solid gain for dehydration in sucrose solution were higher than those obtained in corn syrup due to high viscosity and polysaccharide content. For water activity opposite results were observed.
Response surface methodology was used for quantitative investigation of water and solids transfer during osmotic dehydration of sugar beet cossettes in combined aqueous solutions of sucrose and sodium chloride by Jokie et al. (2007). Effects of immersion time (30–240 min), sucrose concentration (30–70%, w/w), sodium chloride concentration (0–8%, w/w), and temperature of the osmotic solution (30–50 °C) were estimated. It was found that effects of immersion time and sucrose concentrations were more significant than sodium chloride concentration and temperature on water loss and for solid gain immersion time and sodium chloride concentration were the most significant.
Ozdemir et al. (2008) used response surface methodology for the optimization of osmotic dehydration of diced green pepper. Optimization was done with respect to temperature (20–40 °C), time (15–600 min), salt (0–10 g/100 g) and sorbitol (0–10 g/100 g) concentrations. Water loss, solids gain, salt uptake and sorbitol uptake were kept the responses in a 24 central composite rotatable design. All models were significant (P = 0.001) with significant lack of fit. Results suggested that optimum processing condition of 5.5 g salt/100 g and 6 g sorbitol/100 g at 30 °C after 240 min would result in water loss of 23.3%, solid gain of 4.1%, salt uptake 8 g/100 g dry pepper and sorbitol uptake 2.4 g/100 ml extract.
Optimization of the osmotic dehydration process of the carrot cubes in mixtures of sucrose and sodium chloride by response surface methodology, using face-centered central composite design was done by Singh et al. (2010). Variables used were osmotic solution concentrations (5–15% w/v sodium chloride in 50 °B sucrose syrup), temperature (35–55 °C) and process duration (120–240 min). Results showed that all the variables had a significant effect on all the responses. Maximum water loss, minimum solute gain, maximum retention of color, and sensory score were obtained at 50 °B + 15% w/v sodium chloride solution, 54.8 °C solution temperature and 120 min process duration.
Effects of osmotic dehydration
Shi et al. (1997) studied the effect of different treatments on mass transfer in osmotic dehydration of tomato. They concluded that cut tomatoes are poorly suited for osmotic dehydration due to their heterogenous texture and potential excessive loss of liquid components. According to their experiment a NaOH solution mixed with ethyle oleate had less skin damage and more water removal as compared to lonely NaOH solution. Alkali are more effective than acids for water removal. More NaOH concentration (7–8%) leads to undesirable texture of tomato. They preferred physical skin puncturing over chemical treatments.
In a study by Mehta and Bajaj (1984), it was found that there is decrease in the content of ascorbic acid, sugar acid and carotenoids, flavonoids and phenols during curing of candied peels. In their study they found that kinnow peels candy was best as comparable to other citrus fruits candies.
Different tests viz. moisture content, citric acid, non reducing sugar, reducing sugar, total sugar and total soluble solids were analysed for Aonla preserve on different interval of days. It was concluded that minimum sugar concentration required for making Aonla preserve is 60 °B and temperature should be 40 °C or below in order to minimize non enzymatic browning and loss of ascorbic acid. It was also advised to store preserve for 60 days before consumption (Singh et al. 1999).
The combined effect of blanching [steam (S) or microwave (MW)] and osmotic dehydration at atmospheric pressure (OD) or pulsed vacuum treatments (PVOD), on some physiochemical and quality parameters (aw water activity, pH, color, firmness, poly-phenoloxidase enzyme activity and microstructure), of strawberry as well as on microbial stability of processed samples, was analyzed by Moreno et al. (2000). Water activity decreased when the sample was pulsed vacuum osmotic dehydrated with 65 °B sucrose solution after steam blanching due to higher sucrose gain, but this treatment tends to loss of firmness and colour change but at the same time it induces great microbial stability. Cryo SEM observations showed that, steam treated samples suffer a great degree of cell decompartmentation near the fruit skin, as compared with the well preserved cells in MW treated samples.
Light microscope and Environmental electron scanning microscopy explained osmotic dehydration as a multi component diffusion process. Micro and macro structural study was done in apple slices after immersing in 25.0% (w/w) glucose or 34.6% (w/w) sucrose aqueous solution for approx. 350 min. Different parameters viz. thickness, volume, bulk, solid-liquid densities, porosity, water acidity, water loss, solid gain were evaluated. Weight loss and volume loss were observed in both treatment, solid or liquid density were also increased along with time, however bulk density increased up to a certain limit then fluctuate with time. Porosity value decreased as the osmotic dehydration time increases (Nieto et al. 2004).
Tissue structure was examined after dewatering in apple cubes in 61.5% sucrose solution under light microscope. Computer image analysis revealed that no rupture of cell took place due to osmotic dehydration. Cell get smaller and inter cellular space increased on osmotic dehydration. The increase was found more than 30% until 120 min of osmosis. This study suggest that prolong osmotic dehydration disintegrate the tissue of apple and destroy its continuity (Lewicki and Pawlak 2005).
The effect on strawberry tissue of an osmotic step applied at atmospheric pressure for different lengths of time, was analysed by Prinzivalli et al. (2006). In their work the specific influence of osmotic treatments with sucrose solutions at 25 °C on cellular structure, texture, pectin composition and their relationship are discussed. Results showed that the longer the immersion time, the higher the texture loss. From 1 h onwards of osmosis there was a gradual disconnection and breakdown of the tissue, with a loss of shape of cellular walls together with loss of turgor pressure. A good agreement was obtained between texture, structure changes and pectic composition modifications. Besides a pectin solubilization, there was an hemicelluloses solubilization, most likely due to an accelerated ripening of strawberry fruit, linked to the anaerobic conditions, caused by a sugar peripheral layer formation.
Changes in the mechanical properties of pumpkin (Cucurbita pepo L.) fruit when submitted to osmotic dehydration were studied by Mayor et al. (2007). Pumpkin cylinder were dehydrated with different concentration of sucrose solution (30–60% w/w), temperature (12–38 °C) and process time (0–9 h). Four parameters were analyzed: apparent modulus of elasticity, true stress of failure, Hencky strain at failure and failure work (toughness). Mechanical properties of osmo-dehydrated sample showed no dependence on concentration of the osmotic solution and process temperature, whereas they were found to be dependent on moisture content, apparent elastic modulus decreased and failure strain increased during dehydrating, toughness and failure stress initially decreased with moisture content and increased at advanced stages of the process.
The effect of osmotic dehydration on the volatile fraction of mango fruit was studied by Torres et al. (2007). Along with osmotic treatment atmospheric pressure and vacuum pulse is also provided. Sucrose of 35, 45, 55 and 65 °B was used and liquid phase of dehydrated mango were added to these osmotic solution until the brix reaches to 20 or 30. Volatile compounds of mango were extracted and analyzed by GC-MS. The results showed that with high concentration of osmotic reagent the volatile compounds lost also increased. In these cases it was found that sample’s mass loss reduced during treatment since sugar gain was promoted against water loss.
Souza et al. (2007) examined the influence of osmotic solution composition and temperature on osmotic dehydration of tomato. Both type of tomato (skinned and without skinned) were taken and were optimized aiming the reduction of total processing time. These results showed that tomatoes without skin were processed faster using air drying without osmotic dehydration while tomatoes with skin dry faster when treated with osmotic solution of 35% sucrose, 5% salt at 60 °C.
Osorio et al. (2007) studied on colour and flavor change during osmotic dehydration of Andes Berry and Tamarillo with three different osmotic agents: sucrose (70%), sucrose (70%)-glycerol (65%) 1:1, and ethanol. This process leads to transfer of anthocyanin pigment and flavoring constituents to the osmotic solution, that is proved by successive DIS (Dewatering Impregnation- Soaking) cycles, it was advisable to obtain high Cab color parameter values, as criteria for the evaluation of osmotic solutions. They also used osmotic solutions containing pigments and volatile constituents as raw materials for developing natural additives.
Khin et al. (2007) investigated the effect of coating with osmotic dehydration of Potato cubes under various processing conditions. Solutions of 1% sodium alginate and 2% low methoxyl pectinate (LMP, degree of esterification: 31.5%) were chosen as the coating materials and NaCl as osmotic agent used. Potatoes were cut into cubes of 1 cm3. 10%, 14% and 18% salt concentration and temperature of 25, 40 and 55 °C were used as the process variables. Mass transfer kinetics was modeled by using Peleg’s proposed equation. Diffusivities of both water and solute were evaluated by using Crank’s analytical solution to Fick’s second law. These two models were very much helpful in describing how the considered variables influenced the mass diffusion and kinetic rate constants during the osmotic dehydration of both non-coated and coated potatoes cubes.
Effect of osmotic dehydration on the dielectric properties of carrot and strawberries were studied by Changrue et al. (2008). Two osmotic agents, sucrose and salt, were used for carrots but only sucrose was used for strawberries. The effects of variations in sucrose and salt concentrations, solution temperature, and length of immersion time on the dielectric constant (e′) and the loss factor (e″) were measured. In general it was found that dielectric constant decreased with an increase in osmotic conditions.
The effect of osmotic pretreatment with alternative osmotic solutes oligofructose and a high DE maltodextrin on the quality and functional properties of frozen cucumber tissue by Dermesonlouoglou et al. (2008). Color, texture and sensory characteristics of pretreated and conventionally frozen sample were measured at 3 months. When osmo-dehydrofrozen samples were compared with conventionally frozen slice, dehydrofrozen samples showed improved firmness for prolong storage period and better quality. They put a point forward that poor quality of frozen cucumber can significantly be improved by means of the cryoprotection accomplished by a prefreezing osmotic dehydration step.
The effect of solute on osmotic dehydration and rehydration of vacuum impregnated apple cylinders was studied by Atarés et al. (2008). Apple cylinders of 10 mm diameter and 10 mm height were vacuum impregnated, osmodehydrated and rehydrated using solutions of glucose, sucrose and trehalose. Water activity for dehydration solution was 0.96 and rehydration solution was 0.99. Total and component mass variations and sample shrinkage were quantified throughout dehydration. Peleg’s equation was used to model changes in water and solute contents. Better liquid phase retention was showed by sample treated with trehalose that showed the protecting role of this sugar on cell membrane of apple.
The effect of pectinmethylesterase (PME) and calcium (Ca++) added to the osmotic solutions on several compositional parameters and the textural/structural quality of dehydrated and osmodehydrofrozen-then-thawed strawberries was studied by Van Buggenhout et al. (2008). Due to the presence of PME and Ca++ in the osmotic solutions, weight reduction upon dehydration was slightly decreased, which was correlated to a small positive effect on the net uptake of sugars and depression of the initial freezing point. No cell wall damage and tissue particle alterations were observed upon dehydration. The effect of osmotic dehydration (OD) using different sugar solutions without PME and Ca++ on the texture and structure of frozen-then-thawed samples was limited and sometimes negative. The added PME and Ca++ however positively influenced the volume and shape of the thawed samples. They concluded that tissue distortion caused by freezing and indicated by a decrease in particle size, convexity and roundness, was compensated by the use of PME and Ca++ during Osmotic dehydration.
The internal change and kinetics of both moisture change and mobility during osmotic dehydration of apples were reported by Derossi et al. (2008). It was found that the effective diffusion coefficient of water was not constant during dehydration treatment. Results showed that there was existence of an osmotic dehydration front that moves from the surface to the core of apple. According to them all layers of cell appeared to be involved in the moisture transport process at the same time.
Mayor et al. (2008) studied the microstructural changes during osmotic dehydration of parenchymatic pumpkin tissue. First of all pumpkin cylinders (Diameter 1.5 cm; length 2.5 cm) were dehydrated with 45% (w/w) sucrose solution at 25 °C. After dehydration main modifications observed were shrinkage of cells, plasmolysis and folding of the cell wall. These changes leads to decrease of cellular area, equivalent diameter, roundness and compactness; elongation of cells whereas perimeter was maintained constant. Empirical quadratic functions were used to relate the average cellular shape and size parameters with the dehydration parameters. Water loss, weight reduction and normalized moisture content. The equation showed a good fit of the experimental data, leading to correlation coefficient ranging 0.93–0.99 and average relative deviation ranging 0.7–2.8%.
Heredia et al. (2009) studied the influence of different variables (lycopene and b-carotene content) on osmotically treated cherry tomato (L. esculentum var. cerasiforme cv. cocktail) by immersing in different solution (10% NaCl and 27.5% sucrose), (20% w/w NaCl solution and 55 °B sucrose solution) at 30,40, and 50 °C for 24 h. The results showed that lycopene and β-carotene content increased at moderate temperature (30 °C and 40 °C) with solution that includes sucrose in its composition.
Ispir and Togrul (2009), investigated the effect the different parameters on the osmotic dehydration of apricot in terms of water loss and solid gain. They found that increased temperature and concentration of osmotic medium leads to increased water loss and solid gain. These also increased when dimensions of apricot were decreased. The study was also made on effective diffusion and mass transfer coefficient for water loss and solid gain. Finally they concluded that water loss and solid gain were dependent on temperature and osmotic solution concentration.
Castelló et al. (2009) studied the effect of vacuum pulse on mechanical properties, respiration rates and microbial stability of osmo-dehydrated apple slices at 10 °C. Treatments were carried out until reaching 20 and 30 °B in the samples. Values of the force dependent mechanical parameters were decreased, small increase was observed when 1% calcium was applied. These parameters were better preserved during storage. When sample concentration was 20 °B oxygen consumption was reduced due to osmotic dehydration . When the sample had 30 °B, CO2 generation increased. Results showed that dehydrated and vacuum pulsed samples had greater microbial stability.
The effect of osmotic dehydration on the respiration rate (R) and the mechanical and optical properties of strawberry halves were evaluated throughout six days at 10 °C by Castello et al. (2010). Dehydrated samples showed a faster drop in R than non-treated samples, thus indicating a faster development of senescence. According to them PVOD (Pulse Vacuum osmotic dehydration) implied a greater reduction of O2 consumption. Calcium addition slightly reduced R. Osmotic treatments provoked a decrease in the puncture forces, especially in samples with 20 °Brix, as a consequence of the structural collapse caused by treatments. After storage, calcium addition and PVOD treatments had beneficial effects on the maintenance of the sample texture. Color of treated strawberries was modified, mainly in the parenchyma zone, when changes in the sample porosity were greater due to the treatment.
The effect of calcium lactate on the cellular structure and mechanical properties of osmodehydrated melon pieces with sucrose solutions was studied by Ferrari et al. (2010). Samples were treated with sucrose solutions (40 or 60°Brix) containing calcium lactate (0–20 g kg−1). Salt concentrations above 15 g kg−1 as well as the treatment performed with sucrose solution alone promoted cytoplasm plasmolysis and cell wall damages. Fruits processed with sucrose solutions at 60°Brix with or without calcium lactate showed good sensory acceptance.
García et al. (2010) investigated the effect of chitosan coating on mass transfer during osmotic dehydration of papaya. The study was done on scalded cut papaya on both green and riped fruit. Papaya cut cubics (1 cm3) were divided into 3 groups depending on the treatments i.e. without chitosan coatings; with chitosan coatings at 1% (w/v) in lactic acid 1% (v/v) and Tween 80 at 0.1% (v/v); and with chitosan coatings at 1% (w/v) in lactic acid 1% (v/v), Tween 80 at 0.1% (v/v) and oleic acid at 2% (v/v).Osmotic dehydration was carried out in sucrose solution at 40 °B in fruit/solution ratio of 1:60. Weight reduction, water loss and solid gain were best measured in sample with chitosan coatings that showed increased water loss and decreased solid gain.
Mayor et al. (2011) studied the changes in shrinkage, density, porosity and shape during osmotic dehydration of Pumpkin. Pumpkin slices with length/diameter ratio of 5/3 were used. Air drying was conducted at 70 °C. Volume of samples decreased linearly with weight reduction. Bulk density varied in a restricted range (5–13%). Particle density was also found to be increased. Porosity was found to be decreased at advanced degree of dehydration, showing a minimum value at starting of osmotic dehydration and air drying. Image analysis showed that shrinkage of samples during OD was isotropic. Osmotic dehydration increased elongation and decreased roundness and compactness.
Effect of the major parameters on osmotic dehydration
Process temperature
An increase in temperature up to a certain limit is known to increase the rate of osmosis. Further increase in temperature affects the semipermeability of the cell walls and reduces the rate of osmosis. The reported temperature limit is 60 °C (Le Maguer 1988). Ponting et al. 1966 have however reported enzymatic browning and flavor deterioration above 49 °C.
Agitation/circulation
The osmotic dehydration is faster in fruits, when syrup is agitated/circulated. This is because of reduced mass transfer resistance at the surface, in other words, localized dilution which affects the water removal rate is avoided. However, agitation may be difficult and may cause damage to the sample. The circulation of syrup with centrifugal pump is simple and quite effective. It has also been observed advantageous in case of apple that little sugar uptake took place when the syrup was circulated and pieces were kept stationary. Sugar uptake was rapid, reaching the maximum level after half an hour of treatment at which it remained constant (Karel 1976). The gentle agitation has little effect on osmosis rate at low syrup concentration (Hawkes and Flink 1978).
Solution to sample ratio
It is important to use an optimum ratio of solution to sample for the economic considerations. In general, as the solution to sample ratio increased, the osmosis rate increased up to a certain level and then level off (Chaudhari et al. 1993).
Ponting et al. (1966) observed that increase in dry sugar to fruit ratio above 1 neither increased the rate nor the extent of osmotic dehydration when sample was agitated. Bongirwar and Sreenivasan (1977) reported that ratio 2 gave an optimum dehydration rate in osmotic dehydration of banana. However, they experienced the difficulty in draining the syrup from fruit because some undissolved sugar was present. With the use of sugar syrup, the rate of osmosis increased with increase in ratio, but the increase was small. Besides, larger ratios offer practical difficulties in handling the syrup-fruit mixture for processing (Bongirwar and Sreenivasan 1977; Conway et al. 1983). Lenart and Flink (1984a, b) conducted studies on potato cubes at different sugar concentrations and sugar syrup to potato ratios. They reported that ratio 4 was the best for the optimal condition for water loss, solid gain, change in water activity and economics.
Time of treatment
In general, as the time of treatment increases, the weight loss increases, but the rate at which it occurs decreases. The treatment time can be selected in such a way that the amount of water removal is maximum with no appreciable uptake of solids. At this point, the rate of moisture removal is much less as compared to the rate of solid uptake (Chaudhari et al. 1993).
Size and shape of sample
Size and shape of sample has a definite effect on dehydration characteristics of material. Islam and Flink (1982) reported that the net solid content of the sample was more for the sample size of 5 mm slices than 10 mm and 10 × 10 mm French cut samples after 4 h. But it is remained almost same after 18 h of treatment.
Lerici et al. (1985) studied the effect of sample on water loss and solid gain and reported that the water loss increased with increase in surface area to minimum half thickness ratio up to a maximum (ring shaped) and then decreased for a cube even though surface area/thickness ratio is higher. On the other hand, solid gain increased continuously with increase in area/thickness ratio.
Conclusion
Osmotic dehydration can be used successfully for 50% weight reduction in the material and require further drying or processing to enhance the shelf life. It is an energy saving and quality improvement process, it is predicted that osmo-air drying process has good potential for fruits such as banana, jackfruit, sapota, guava, mango and pineapple. This process could be used on small scale for development of self-entrepreneurs and home scale industries. Consumption of such nutritional and valued products could be popularized through exhibition and media.
During the last three decades a lot of work had been done on osmotic dehydration and found that it is one of the best method for preservation because it does not destroy much nutritional parameters, color, flavor and texture etc. On the other hand this process is too economical and since no preservative were used, it does not adversely affect the human body. It can be used to decrease the post harvest losses of fruits and vegetables.
Acknowledgement
Authors are thankful to UGC for funding through the reference no. 37-63/2009 (SR); Dec 19, 2009.
Contributor Information
Ashok Kumar Yadav, Email: ashokbhu99@gmail.com.
Satya Vir Singh, Phone: +91-5426702031, FAX: +91-5422368092, Email: satyavirsingh59@rediffmail.com.
References
- Alves DG, Barbosa JL, Jr, Antonio GC, Murr FEX. Osmotic dehydration of acerola fruit (Malpighia punicifolia L.) J Food Eng. 2005;68:99–103. [Google Scholar]
- Andrade SAC, Neto BB, Nóbreg AC, Azoubel PM, Guerra NB. Evaluation of water and sucrose diffusion coefficients during osmotic dehydration of jenipapo (Genipa americana L.) J Food Eng. 2007;78:551–555. [Google Scholar]
- Aouar AAE, Azoubel PM, Barbosa JL, Jr, Murr FEX. Influence of the osmotic agent on the osmotic dehydration of papaya (Carica papaya L.) J Food Eng. 2006;75:267–274. [Google Scholar]
- Atarés L, Chiralt A, Martínez CG. Effect of solute on osmotic dehydration and rehydration of vacuum impregnated apple cylinders (cv. Granny Smith) J Food Eng. 2008;89:49–56. [Google Scholar]
- Azoubel PM. Optimization of osmotic dehydration of cashew apple (Anacardium Occidentale L.) in sugar solution. Food Sci Technol Int. 2003;9(6):427–433. [Google Scholar]
- Azoubel PM, Murr FEX. Mathematical modeling of the osmotic dehydration of cherry tomato (Lycopersicon esculentum var.cerasiforme) Cienc Tecnol Aliment. 2000;20(2):228–232. [Google Scholar]
- Azoubel PM, Murr FEX. Mass transfer kinetics of osmotic dehydration of cherry tomato. J Food Eng. 2004;61:291–295. [Google Scholar]
- Bchir B, Besbes S, Attia H, Blecker C. Osmotic dehydration of pomegranate seeds: mass transfer kinetics and DSC characterization. Int J Food Sci Technol. 2009;44:2208–2217. [Google Scholar]
- Bchir B, Besbes S, Karoui R, Paquot M, Attia H, Blecker C. Osmotic dehydration of pomegranate seeds using date juice as an immersion solution base. Food Bioprocess Technol. 2010;10:442–445. [Google Scholar]
- Bchir B, Besbes S, Karoui R, Attia H, Paquot M, Blecker C. Effect of air drying condition on physico-chemical properties of osmotically pre-treated pomegranate seeds. Food Bioprocess Technol. 2010;10:469–473. [Google Scholar]
- Bchir B, Besbes S, Attia H, Blecker C (2011a) Osmotic dehydration of pomegranate seeds (punica granatum L.): effect of freezing pre treatment. J Food Process Eng 1745–4530
- Bchir B, Besbes S, Giet GM, Attia H, Paquot M, Blecker C (2011b) Synthese des connaissances sur la deshydration osmotique 15:129–142
- Bellary AN, Sowbhagya HB, Rastogi NK. Osmotic dehydration assisted impregnation of curcuminoids in coconut slices. J Food Eng. 2011;105:453–459. [Google Scholar]
- Beristain CI, Azuara E, Cortes R, Garcia HS. Mass transfer during osmotic dehydration of pineapple rings. Intl J Food Sci Technol. 1990;25(5):576–582. [Google Scholar]
- Biswal RN, Le Maguer M (1989) Mass transfer in plant materials in aqueous solutions of ethanol and sodium chloride: equilibrium data. J Food Process Eng 11(3):159–176
- Bolin HR, Huxsoll CC, Jackson R, Ng KC. Effect of osmotic agents and concentration on fruit quality. J Food Sci. 1983;48(1):202–205. [Google Scholar]
- Bongirwar DR, Sreenivasan A. Studies on osmotic dehydration of banana. J Food Sci Technol. 1977;14(3):104–112. [Google Scholar]
- Camirand WM, Forrey RR, Popper K, Boyle FP, Stanley WL. Dehydration of membranes coated foods by osmosis. J Sci Food Agric. 1968;19(8):472–474. doi: 10.1002/jsfa.2740190813. [DOI] [PubMed] [Google Scholar]
- Castelló ML, Igual M, Fito PJ, Chiralt A. Influence of osmotic dehydration on texture, respiration and microbial stability of apple slices (Var. Granny Smith) J Food Eng. 2009;91:1–9. [Google Scholar]
- Castello ML, Fito PJ, Chiralt A. Changes in respiration rate and physical properties of strawberries due to osmotic dehydration and storage. J Food Eng. 2010;97(1):64–71. [Google Scholar]
- Changrue V, Orsat V, Raghavan GSV, Lyew D. Effect of osmotic dehydration on the dielectric properties of carrots and strawberries. J Food Eng. 2008;88:280–286. [Google Scholar]
- Chaudhari AP, Kumbhar BK, Singh BPN, Narain M. Osmotic dehydration of fruits and vegetables—a review. Indian Food Ind. 1993;12(1):20–27. [Google Scholar]
- Chavan UD, Prabhukhanolkar AE, Pawar VD. Preparation of osmotic dehydrated ripe banana slices. J Food Sci Tech. 2010;47(4):380–386. doi: 10.1007/s13197-010-0063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolletti JC, Robertson GH, Farkas DF. Freezing of vegetables by direct contact with aqueous solutions of ethanol and sodium chloride. J Food Sci. 1977;42(4):911–916. [Google Scholar]
- Conway J, Castaigne F, Picard G, Vevan X. Mass transfer consideration in the osmotic dehydration of apple. Can Inst Food Sci Technol J. 1983;16(1):25–29. [Google Scholar]
- Corzo O, Gomez ER. Optimization of osmotic dehydration of cantaloupe using desired function methodology. J Food Eng. 2004;64:213–219. [Google Scholar]
- Crank J. The mathematics of diffusion. Oxford: Clarenden Press; 1975. [Google Scholar]
- Dermesonlouoglou EK, Giannakourou MC, Bakalis S, Taoukis PS (2005) Mass transfer properties of water melon tissue in osmotic solution and effect of osmotic dehydration on frozen water melon quality, III International symposium of Application of modeling as an innovative technology in the Agri food chain Model IT held on 29 May at Leuven, Belgium ISBN-978-90-66055-18-6
- Dermesonlouoglou EK, Pourgouri S, Taoukis PS. Kinetic study of the effect of the osmotic dehydration pre-treatment to the shelf life of frozen cucumber. Innov Food Sci Emerg Technol. 2008;9:542–549. [Google Scholar]
- Derossi A, Pilli TD, Severini C, McCarthy MJ. Mass transfer during osmotic dehydration of apples. J Food Eng. 2008;86:519–528. [Google Scholar]
- Dixon GM, Jen JJ. Changes of sugar and acids of osmovac dried apple slices. J Food Sci. 1978;42(4):1126–1131. [Google Scholar]
- Dixon GM, Jen JJ, Paynter VP. Tasty apple slices result from combined osmotic dehydration and vacuum drying process. Food Prod Dev. 1976;10(7):60–66. [Google Scholar]
- Durrani AM, Verma S. Preparation and Quality evaluation of honey Amla Murabba. J Ind Res Tech. 2011;1(1):40–45. [Google Scholar]
- Durrani AM, Srivastava PK, Verma S. Development and Quality Evaluation of honey based carrot candy. J Food Sci Technol. 2011;48(4):502–505. doi: 10.1007/s13197-010-0212-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falade KO, Igbeka JC, Ayanwuyi FA. Kinetics of mass transfer and colors changes during osmotic dehydration of watermelon. J Food Eng. 2007;80:979–985. [Google Scholar]
- Farkas DF, Lazar ME. Osmotic dehydration of apple pieces: effect of temperature and syrup concentration of rates. Food Technol. 1969;23(5):90–92. [Google Scholar]
- Fernandes FAN, Rodrigues S, Gaspareto OCP, Oliveira EL. Optimization of osmotic dehydration of papaya followed by air-drying. Food Res Int. 2006;39:492–498. [Google Scholar]
- Ferrando M, Spiess WEL. Mass transfer in strawberry tissue during osmotic treatment I: microstructural changes. J Food Sci. 2003;68(4):1347–1355. [Google Scholar]
- Ferrando M, Spiess WEL. Mass transfer in strawberry tissue during osmotic treatment II: structure function relationship. J Food Sci. 2003;68(4):1356–1364. [Google Scholar]
- Ferrari CC, Hubinger MD. Evaluation of the mechanical properties and diffusion coefficients of osmodehydrated melon cubes. Int J Food Sci Technol. 2008;43(11):2065–2074. [Google Scholar]
- Ferrari CC, Carmello-Guerreiro SM, Bolini HMA, Hubinger MD. Structural changes, mechanical properties and sensory preference of osmodehydrated melon pieces with sucrose and calcium lactate solutions. Int J Food Prop. 2010;13(1):112–130. [Google Scholar]
- Flink JM. Process condition for improved flavor quality of freeze dried foods. J Agric Food Chem. 1975;23(6):1019–1026. [Google Scholar]
- Flink JM (1979) Dehydrated carrot slices: influence of osmotic concentration on drying behaviour and product quality. In: Linko P, Malki Y, Olkku J, Larinkari J, Fito P, Ortega E, Barbosa G (eds)Food process engineering. Applied Science Publishers, London, pp 412–418
- Flink JM. Dehydrated carrot slices: influence of osmotic concentration on drying behavior and product quality. Food Proc Eng. 1980;1:412–416. [Google Scholar]
- Fragoso AV, Paz HM, Giroux F, Chanes JW. Pilot plant for osmotic dehydration of fruits: design and evaluation. J Food Proc Eng. 2002;25:189–199. [Google Scholar]
- Garcia R, Menchu JF, Rolz C (1974) Tropical fruit drying : comparative study. Proc. Intl. congress Food Sci Technol 4:32–40
- Garcia CC, Mauro MA, Kimura M. Kinetics of osmotic dehydration and air-drying of pumpkins (Cucurbita moschata) J Food Eng. 2007;82:284–291. [Google Scholar]
- García M, Díaz R, Martínez Y, Casariego A. Effects of chitosan coating on mass transfer during osmotic dehydration of papaya. Food Res Int. 2010;43:1656–1660. [Google Scholar]
- Ghosh PK, Agrawal YC, Jayas DS, Kumbhar BK. Process development for osmo-hot air drying of carrot. J Food Sci Technol. 2006;43(1):65–68. [Google Scholar]
- Giangiecome R, Torreggiani D, Abbe E. Osmotic dehydration of fruit part I. Sugar exchange between fruit and extraction syrup. Food Nutr Preserv. 1987;11(3):183–195. [Google Scholar]
- Hawkes J, Flink JM. Osmotic concentration of fruit slices, prior to freeze dehydration. Food Proc Preserv. 1978;2:265–284. [Google Scholar]
- Heredia A, Peinado I, Barrera C, Grau AA. Influence of process variables on colour changes, carotenoids retention and cellular tissue alteration of cherry tomato during osmotic dehydration. J Food Compos Anal. 2009;22:285–294. [Google Scholar]
- Hope GW, Vitale DG (1972) Osmotic dehydration: a cheap and simple method of preserving mangoes, bananas and plantains. Contribution 175 Food Res. Inst. Can Dept. of Agric Ottawa, Canada
- Islam MN, Flink JM. Dehydration of potato II. Osmotic concentration and its effect on air drying behavior. J Food Technol. 1982;17:387–403. [Google Scholar]
- Ispir AE, Togrul IT. Osmotic dehydration of apricot: kinetics and the effect of process parameters. Chem Eng Res Des. 2009;87:166–180. [Google Scholar]
- Jackson TH, Mohamed BB. The shambat process: new development arising from the osmotic dehydration of fruits and vegetables. Sudan J Food Sci Technol. 1971;3:18–22. [Google Scholar]
- Jokie A, Gyura J, Levic L, Zavargo Z. Osmotic dehydration of sugar beet in combined aqueous solutions of sucrose and sodium chloride. J Food Eng. 2007;78:47–51. [Google Scholar]
- Karel M. Technology and application of new intermediate moisture foods. In: Davies R, Birch GG, Parker KJ, editors. Intermediate moisture foods. London: Applied Science Publishers; 1976. pp. 4–31. [Google Scholar]
- Khin MM, Zhou W, Perera CO. A study of the mass transfer in osmotic dehydration of coated potato cubes. J Food Eng. 2006;77:84–95. [Google Scholar]
- Khin MM, Zhou W, Yeo SY. Mass transfer in the osmotic dehydration of coated apple cubes by using maltodextrin as the coating material and their textural properties. J Food Eng. 2007;81:514–522. [Google Scholar]
- Khoyi MR, Hesari J. Osmotic dehydration kinetics of apricot using sucrose solution. J Food Eng. 2007;78:1355–1360. [Google Scholar]
- Kim MH, Toledo RT. Effect of osmotic dehydration and high temperature fluidized bed drying on properties of dehydrated rabbiteye blueberries. J Food Sci. 1987;52(4):980–984. [Google Scholar]
- Kowalska H, Lenart A, Leszczyk D. The effect of blanching and freezing on osmotic dehydration of pumpkin. J Food Eng. 2008;86:30–38. [Google Scholar]
- Le Maguer M (1988) Osmotic dehydration: review and future directions. Proc. Int. Symp. On progress in food preservation organized by CERIA, Centre of Education and Research on Food and Chemical Industries, Brussels, Belgium, April 12–14
- Lenart A, Flink JM. Osmotic concentration of potato I: criteria for the end point of the osmasia process. J Food Technol. 1984;19(1):45–60. [Google Scholar]
- Lenart A, Flink JM. Osmotic concentration of potato II: special distribution of the osmotic effect. J Food Technol. 1984;19(1):65–89. [Google Scholar]
- Lenart A, Lewicki PP. Osmotic preconcentration of carrot tissue followed by convention drying. J Food Proc Eng. 1988;14:163–171. [Google Scholar]
- Lerici CR, Pinnavaia G, Dalla Rosa M, Mastrocola D (1983) Applicazione dell’ osmosi diretta nella disidratazione della frutta. Industrie Alimentari 3:184–190
- Lerici CR, Pinnavala G, Daliarosa M, Bartolucci L. Osmotic dehydration of fruit: influence of osmotic agents on drying behavior and product quality. J Food Sci. 1985;50:1217–1220. [Google Scholar]
- Levi A, Gagel S, Juven BJ. Intermediate moisture tropical food products for developing countries II. Quality characteristics of papaya. Int J Food Sci Technol. 1985;20(2):163–175. [Google Scholar]
- Lewicki PP, Pawlak RP. Effect of osmotic dewatering on apple tissue structure. J Food Eng. 2005;66:43–50. [Google Scholar]
- Lombard GE, Oliveira JC, Fito P, Andrés A. Osmotic dehydration of pineapple as a pre-treatment for further drying. J Food Eng. 2008;85:277–284. [Google Scholar]
- Madan S, Dhawan SS. Development of value added product ‘CANDY’ from carrots. Process Food Ind. 2005;8(3):26–29. [Google Scholar]
- Maharaj V, Sankat CK. The rehydration characteristics and quality of dehydrated dasheen leaves. Can Agric Eng. 2000;42(2):81–85. [Google Scholar]
- Mancilla YN, Won MP, Gálvez AV, Arias V, Munizaga GT, Labarca VB, Mondaca RL, Scala KD. Modeling mass transfer during osmotic dehydration of strawberries under high hydrostatic pressure conditions. Innov Food Sci Emerg Technol. 2011;12:338–343. [Google Scholar]
- Mandala IG, Anagnostaras EF, Oikonomou CK. Influence of osmotic dehydration conditions on apple air drying kinetics and their quality characterstics. J Food Eng. 2005;69:307–316. [Google Scholar]
- Matuser A, Meresz P. Modelling of sugar transfer during osmotic dehydration of carrot. Period Polytech Ser Chem Eng. 2002;46(1–2):83–92. [Google Scholar]
- Mayor L, Cunha RL, Sereno AM. Relation between mechanical properties and structural changes during osmotic dehydration of pumpkin. Food Res Int. 2007;40:448–460. [Google Scholar]
- Mayor L, Pissarra J, Sereno AM. Microstructural changes during osmotic dehydration of parenchymatic pumpkin tissue. J Food Eng. 2008;85:326–339. [Google Scholar]
- Mayor L, Moreira R, Sereno AM. Shrinkage, density, porosity and shape changes during dehydration of pumpkin (Cucurbita pepo L.) fruits. J Food Eng. 2011;103:29–37. [Google Scholar]
- Mehta U, Bajaj S. Change in the chemical composition and organoleptic quality of citrus peel candy during preparation and storage. J Food Sci Technol. 1984;21:422–424. [Google Scholar]
- Mehta GL, Tomar MC. Studies on dehydration of tropical fruits in Uttar Pradesh. III. Papaya (Carica papaya L.) Indian Food Pack. 1980;8(1):12–15. [Google Scholar]
- Mercali GD, Marczak LDF, Tessaro IC, Noreña CPZ. Evaluation of water, sucrose and NaCl effective diffusivities during osmotic dehydration of banana (Musa sapientum, shum.) LWT—Food Sci Technol. 2011;44:82–91. [Google Scholar]
- Monnerat SM, Pizzi TRM, Mauro MA, Menegalli FC. Osmotic dehydration of apples in sugar/salt solutions: concentration profiles and effective diffusion coefficients. J Food Eng. 2010;100:604–612. [Google Scholar]
- Moraga MJ, Moraga G, Fito PJ, Martínez-Navarrete N. Effect of vacuum impregnation with calcium lactate on the osmotic dehydration kinetics and quality of osmodehydrated grapefruit. J Food Eng. 2009;90:372–379. [Google Scholar]
- Moraga MJ, Moraga G, Navarrete NM. Effect of the re-use of the osmotic solution on the stability of osmodehydro-refrigerated grapefruit. LWT—Food Sci Technol. 2011;44:35–41. [Google Scholar]
- Moreira R, Chenlo F, Chaguri L, Vázquez G. Air drying and colour characteristics of chestnuts pre-submitted to osmotic dehydration with sodium chloride. Food Bioproducts Process. 2011;89:109–115. [Google Scholar]
- Moreno J, Chiralt A, Escriche I, Serra JA. Effect of blanching/osmotic dehydration combined methods on quality and stability of minimally processed strawberries. Food Res Int. 2000;33:609–616. [Google Scholar]
- Moy JH, Kuo MJL. Solar osmovac dehydration of papaya. J Food Eng. 1985;8:23–32. [Google Scholar]
- Moy JH, Lau NBM, Dollar AM. Effect of sucrose and acids on osmotic dehydration of tropical fruits. J Food Process Preserv. 1978;2(2):131–135. [Google Scholar]
- Nieto AB, Salvatori DM, Castro MA, Alzamora SM. Structural changes in apple tissue during glucose and sucrose osmotic dehydration: shrinkage, porosity, density and microscopic features. J Food Eng. 2004;61:269–278. [Google Scholar]
- Oliveira IM, Fernandes FAN, Rodrigues S, Sousa PHM, Maia GA, Figueiredo W. Modelling and optimization of osmotic dehydration of banana followed by air drying. J Food Process Eng. 2006;29:400–406. [Google Scholar]
- Osorio C, Franco MS, Castaño MP, González-Miret ML, Heredia FJ, Morales AL. Colour and flavour changes during osmotic dehydration of fruits. Innov Food Sci Emerg Technol. 2007;8:353–359. [Google Scholar]
- Ozdemir M, Ozen BF, Dock LL, Floros JD. Optimization of osmotic dehydration of diced green peppers by response surface methodology. LWT—Food Sci Technol. 2008;41:2044–2050. [Google Scholar]
- Pereira LM, Rodrigues ACC, Sarantópoulos CIGL, Junqueira VCA, Cunha RL, Hubinger MD. Influence of modified atmosphere packaging and osmotic dehydration of minimally processed guavas. J Food Sci. 2004;69(4):172–177. [Google Scholar]
- Piotrowski D, Lenart A, Wardzynski A. Influence of osmotic dehydration on microwave convective drying of frozen strawberries. J Food Eng. 2004;65:519–525. [Google Scholar]
- Ponting JD. Osmotic dehydration of fruits—recent modifications and applications. Process Biochem. 1973;8:18–20. [Google Scholar]
- Ponting JD, Watters GG, Forrey RR, Jackson R, Stanley WL. Osmotic dehydration of fruits. Food Technol. 1966;20:125–128. [Google Scholar]
- Ponting JD, Jackson R, Watters G. Refrigerated apples: preservative effect of ascorbic acid, calcium and sulfites. J Food Sci. 1972;37(3):434–436. [Google Scholar]
- Prinzivalli C, Brambilla A, Maffi D, Lo Scalzo R, Torreggiani D. Effect of osmosis time on structure, texture and pectic composition of strawberry tissue. Eur Food Res Technol. 2006;224:119–127. [Google Scholar]
- Rahaman MS, Lamb J. Osmotic dehydration of pineapple. J Food Sci Technol. 1990;27(3):150–152. [Google Scholar]
- Ramallo LA, Schvezov C, Mascheroni RH. Mass transfer during osmotic dehydration of pineapple. Food Sci Technol Int. 2004;10(5):323–332. [Google Scholar]
- Ramanuja MN, Jayaramank J. Studies on the preparation and storage of stability of intermediate moisture banana. J Food Sci. 1980;17(4):183–185. [Google Scholar]
- Ramolla LA, Mascheroni RH. Rate of water loss and sugar uptake during the osmotic dehydration of pineapple. Braz Arch Biol Technol. 2005;48(5):761–770. [Google Scholar]
- Rashmi HB, Doreyappa GI, Mukanda GK. Studies on osmo-air dehydration of pineapple fruit. J Food Sci Technol. 2005;42(1):64–67. [Google Scholar]
- Rastogi NK, Raghavarao KSMS, Niranjan K. Mass transfer during osmotic dehyadration of banana: Fickian diffusion in cylindrical configuration. J Food Eng. 1997;31(4):423–432. [Google Scholar]
- Rodrigues ACC, Pereira LM, Sarantópoulos CIGL, Bolini HMA, Cunha RL, Junqueira VCA, Hubinger MD. Impact of modified atmosphere packaging on the osmodehydrated papaya stability. J Food Process Preserv. 2006;30(5):563–581. [Google Scholar]
- Rosa MD, Giroux F. Osmotic treatments (OT) and problems related to the solution management. J Food Eng. 2001;49(2–3):223–236. [Google Scholar]
- Sharma RC, Joshi VK, Chauhan SK, Chopra SK, Lal BB. Application of osmosis–osmo canning of apple rings. J Food Sci Technol. 1991;28(2):86–88. [Google Scholar]
- Sharma HK, Pandey H, Kumar P. Osmotic dehydration of sliced pears. J Agric Eng. 2003;40(1):65–68. [Google Scholar]
- Shi XQ, Maupoey PF. Vacuum osmotic dehydration of fruits. Drying Technol. 1993;11(6):1429–1442. [Google Scholar]
- Shi JX, Maguer ML, Wangb SL, Liptayc A. Application of osmotic treatment in tomato processing effect of skin treatments on mass transfer in osmotic dehydration of tomatoes. Food Res Int. 1997;30(9):669–674. [Google Scholar]
- Singh B, Mehta S. Effect of osmotic pretreatment on equilibrium moisture content of dehydrated carrot cubes. Intl J Food Sci Technol. 2008;43(3):532–537. [Google Scholar]
- Singh M, Shivhare US, Singh H, Bawa AS. Osmotic concentration kinetics of Amla preserve. Indian Food Pack. 1999;53(1):13–15. [Google Scholar]
- Singh B, Kumar A, Gupta AK. Study of mass transfer kinetics and effective diffusivity during osmotic dehydration of carrot cubes. J Food Eng. 2007;79:471–480. [Google Scholar]
- Singh B, Panesar PS, Nanda V, Kennedy JF. Optimization of osmotic dehydration process of carrot cubes in mixtures of sucrose and sodium chloride solutions. Food Chem. 2010;123:590–600. [Google Scholar]
- Souza JS, Medeiros MFD, Magalhães MMA, Rodrigues S, Fernandes FAN. Optimization of osmotic dehydration of tomatoes in a ternary system followed by air-drying. J Food Eng. 2007;83:501–509. [Google Scholar]
- Speck P, Escher F, Solms J. Effect of salt pretreatment on quality and storage stability of air dried carrots. LWT. 1977;10:308–313. [Google Scholar]
- Sunjka PS, Raghavan GS. Assesment of pretreatment methods and osmotic dehydration for cranberries. Can Biosyst Eng. 2004;46:35–40. [Google Scholar]
- Teles VRN, Murari RCBDL, Yamashita F. Diffussion coefficient during osmotic dehydration of tomatoes in ternary solutions. J Food Eng. 2004;61:253–259. [Google Scholar]
- Teles UM, Fernandes FAN, Rodrigues S, Lima AS, Maia GA, Figueiredo RW. Optimization of osmotic dehydration of melons followed by air drying. Intl J Food Sci Technol. 2006;41:674–677. [Google Scholar]
- Teotia SS, Gl M, Tomar MC, Garg RC. Studies on dehydration of tropical fruits in Uttar Pradesh I Mango. Indian Food Packer. 1976;30(6):15–19. [Google Scholar]
- Tiwari RB. Application of osmo-air dehydration for processing of tropical fruits in rural areas. Indian Food Ind. 2005;24(6):62–69. [Google Scholar]
- Torres JD, Talens P, Carot JM, Chiralt A, Escriche I. Volatile profile of mango (Mangifera indica L.), as affected by osmotic dehydration. Food Chem. 2007;101:219–228. [Google Scholar]
- Torres JD, Castelló ML, Escriche I, Chiralt A. Quality characteristics, respiration rates, and microbial stability of osmotically treated mango tissue (Mangifera indica L.) with or without calcium lactate. Food Sci Technol Int. 2008;14(4):355–365. [Google Scholar]
- Valle JMD, Aranguiz V, Leoan H. Effects of blanching and calcium infiltration on PPO activity, texture, microstructure and kinetics of osmotic dehydration of apple tissue. Food Res Int. 1998;31(8):557–569. [Google Scholar]
- Van Buggenhout S, Grauwet T, Loey AV, Hendrickx M. Use of pectinmethylesterase and calcium in osmotic dehydration and osmodehydrofreezing of strawberries. Eur Food Res Technol. 2008;226:1145–1154. [Google Scholar]
- Venkatachalapathy K, Raghavan GSV. Combined osmotic and microwave drying of strawberries. CAO LA Technol. 1999;17(4&5):837–853. [Google Scholar]
- Videv K, Tanchev S, Sharma RC, Joshi VK. Effect of sugar syrup concentration and temperature on the rate of osmotic dehydration of apples. J Food Sci Technol. 1990;27(5):307–308. [Google Scholar]