Abstract
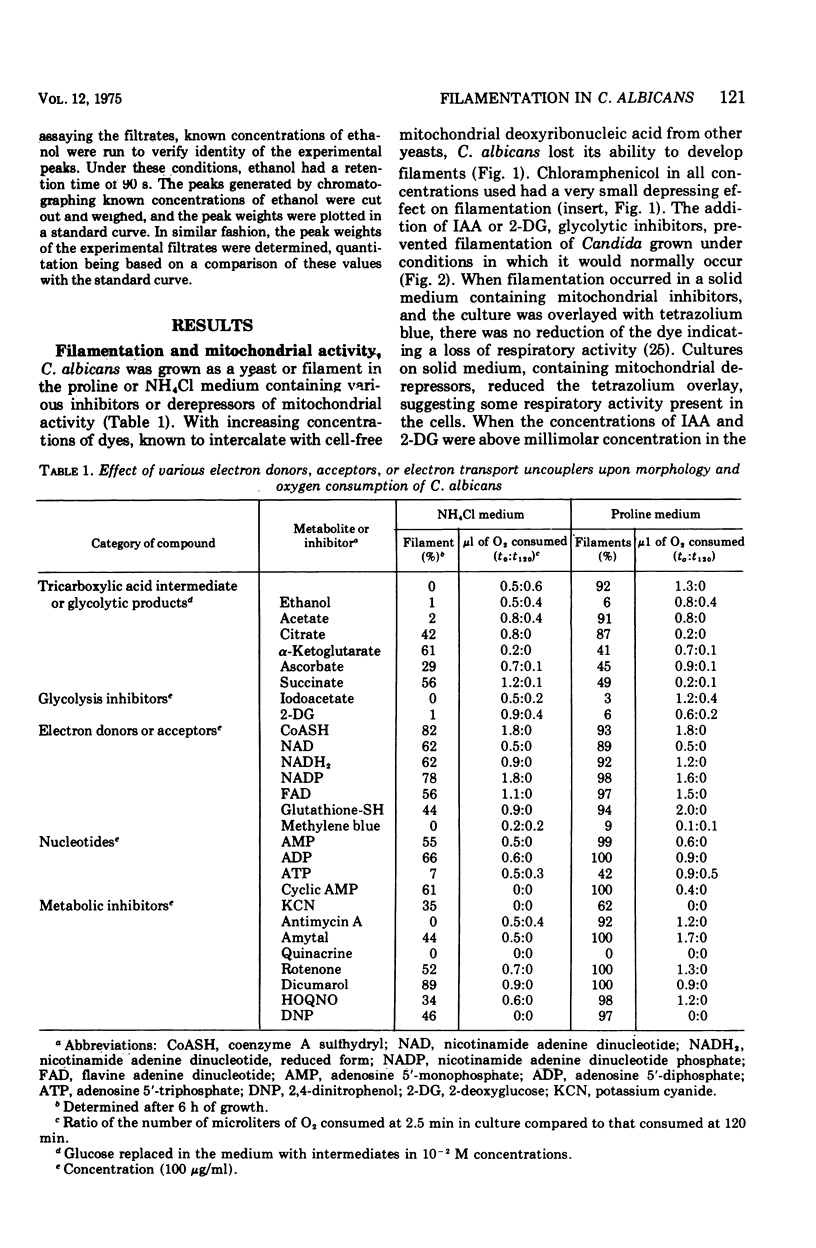
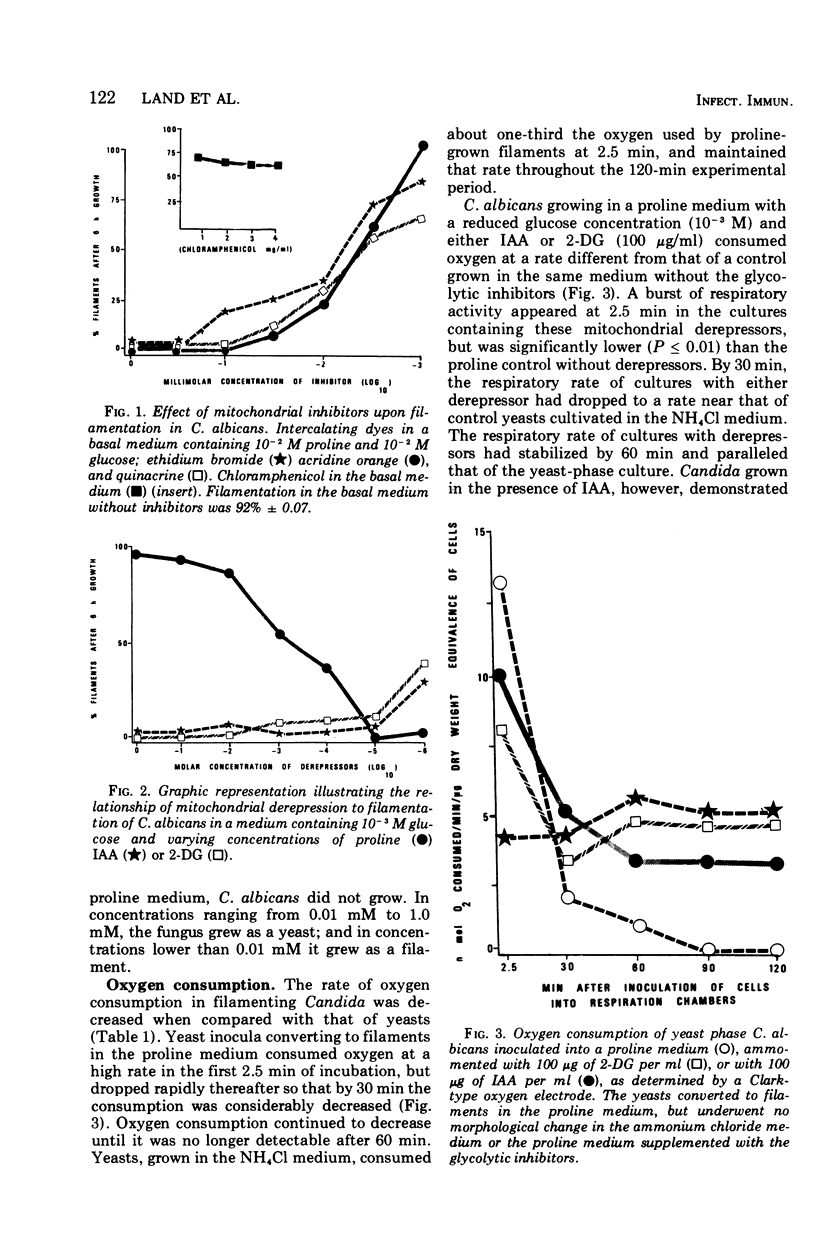
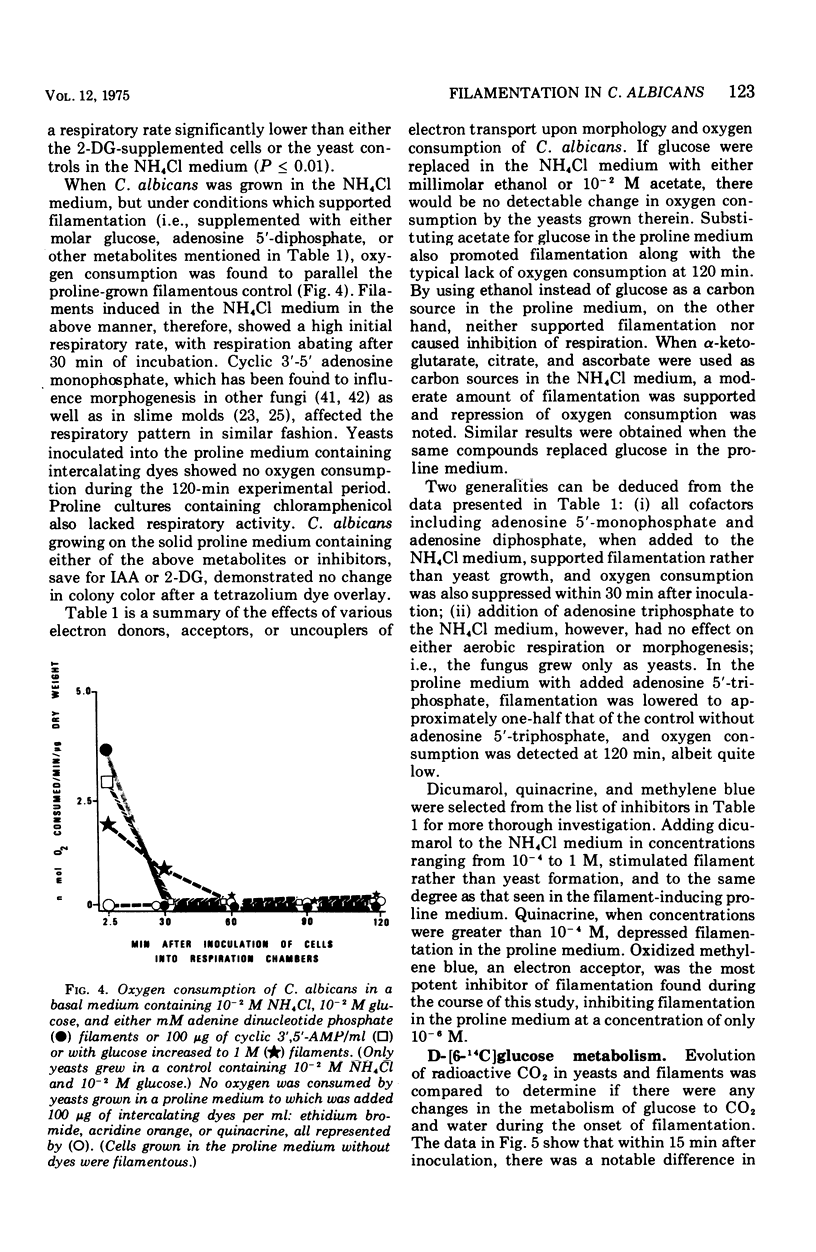
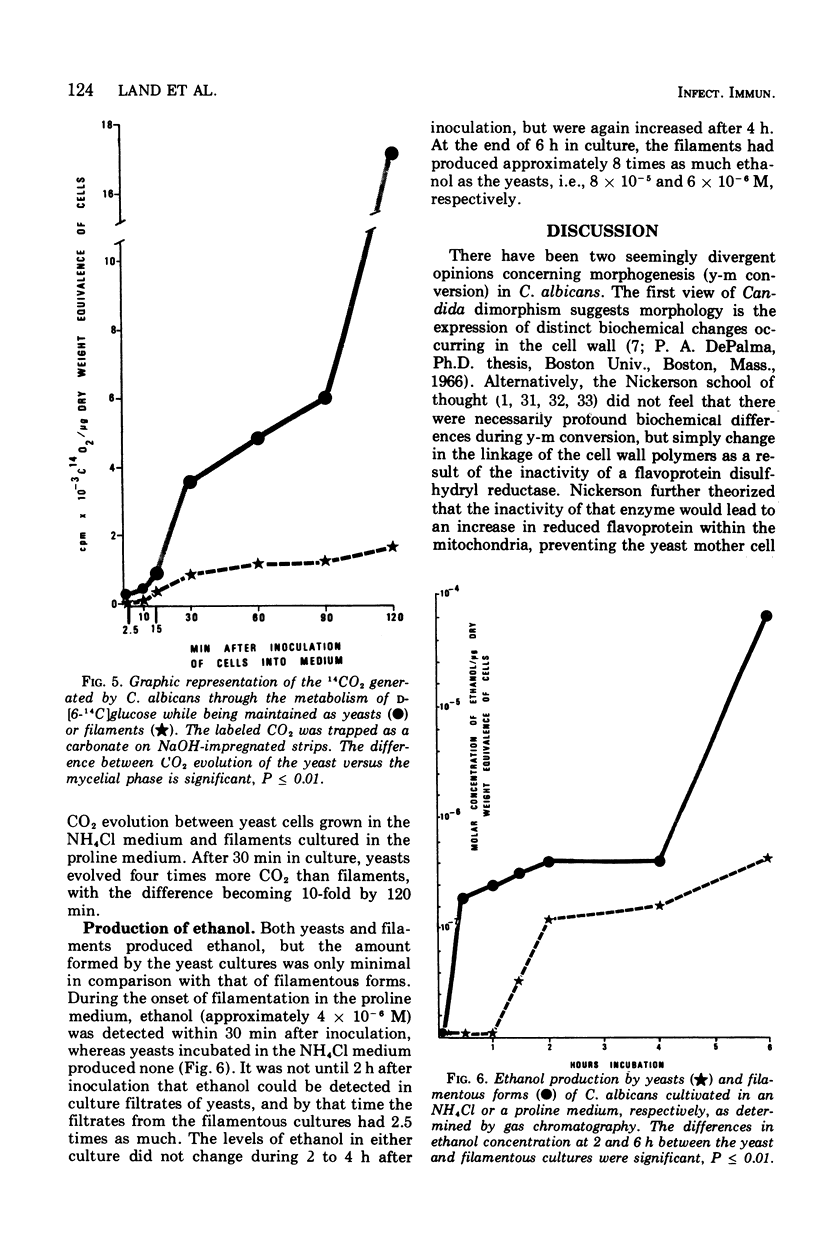
Glucose metabolism and respiration of Candida albicans were compared under conditions which permitted either maximal filamentous or maximal yeast growth. Changes in metabolism were monitored by comparing the quantities of ethanol produced, CO2 evolved, and oxygen consumed. Filamenting cultures produced more ethanol and less CO2 than yeasts, with oxygen consumption in the former concomitantly slower than that of the latter. Studies involving cofactors and inhibitors associated with electron transport imply that a transfer of electrons away from flavoprotein is required for maintenance of yeast morphology. Conditions consistent with a buildup of reduced flavoprotein, however, favored filament formation. These changes were expressed metabolically as a shift from an aerobic to a fermentative metabolism. The results presented are consistent with hypotheses correlating filament production with changes in carbohydrate metabolism and an interruption of electron transfer within the cell.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartnicki-Garcia S. Cell wall differentiation in the phycomycetes. Phytopathology. 1969 Aug;59(8):1065–1071. [PubMed] [Google Scholar]
- Bernander S., Edebo L. Growth and phase conversion of Candida albicans in Dubos medium. Sabouraudia. 1969 Jun;7(2):146–155. [PubMed] [Google Scholar]
- Bird D. C., Sheagren J. N. Evaluation of reticuloendothelial system phagocytic activity during systemic Candida albicans infection in mice. Proc Soc Exp Biol Med. 1970 Jan;133(1):34–37. doi: 10.3181/00379727-133-34401. [DOI] [PubMed] [Google Scholar]
- Borowski J., Brylińska A., Buluk H., Naruszewicz S. Studies on mycelial transformation of Candida albicans cells in the presence of human sera. Pol Med J. 1969;8(5):1191–1196. [PubMed] [Google Scholar]
- Cecchini G. L., O'Brien R. T. Detection of Escherichia coli by gas chromatography. J Bacteriol. 1968 Mar;95(3):1205–1206. doi: 10.1128/jb.95.3.1205-1206.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattaway F. W., Bishop R., Holmes M. R., Odds F. C., Barlow A. J. Enzyme activities associated with carbohydrate synthesis and breakdown in the yeast and mycelial forms of Candida albicans. J Gen Microbiol. 1973 Mar;75(1):97–109. doi: 10.1099/00221287-75-1-97. [DOI] [PubMed] [Google Scholar]
- Chattaway F. W., Holmes M. R., Barlow A. J. Cell wall composition of the mycelial and blastospore forms of Candida albicans. J Gen Microbiol. 1968 May;51(3):367–376. doi: 10.1099/00221287-51-3-367. [DOI] [PubMed] [Google Scholar]
- Chilgren R. A., Hong R., Quie P. G. Human serum interactions with Candida albicans. J Immunol. 1968 Jul;101(1):128–132. [PubMed] [Google Scholar]
- Clark-Walker G. D., Linnane A. W. The biogenesis of mitochondria in Saccharomyces cerevisiae. A comparison between cytoplasmic respiratory-deficient mutant yeast and chlormaphenicol-inhibited wild type cells. J Cell Biol. 1967 Jul;34(1):1–14. doi: 10.1083/jcb.34.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree H. G. Observations on the carbohydrate metabolism of tumours. Biochem J. 1929;23(3):536–545. doi: 10.1042/bj0230536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Deken R. H. The Crabtree effects and its relation to the petite mutation. J Gen Microbiol. 1966 Aug;44(2):157–165. doi: 10.1099/00221287-44-2-157. [DOI] [PubMed] [Google Scholar]
- Evans E. G., Odds F. C., Richardson M. D., Holland K. T. The effect of growth medium of filament production in Candida albicans. Sabouraudia. 1974 Mar;12(1):112–119. doi: 10.1080/00362177485380151. [DOI] [PubMed] [Google Scholar]
- Graafmans W. D. The influence of carbon dioxide on morphogenesis in Penicillium isariiforme. Arch Mikrobiol. 1973 Apr 8;91(1):67–76. doi: 10.1007/BF00409539. [DOI] [PubMed] [Google Scholar]
- Gupta R. K., Howard D. H. Comparative physiologial studies of the yeast and mycelial forms of Histoplasma capsulaum: uptake and incorporation of L-leucine. J Bacteriol. 1971 Mar;105(3):690–700. doi: 10.1128/jb.105.3.690-700.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasilik A. Perturbation of growth and metabolism in Candida albicans by 4-bromobenzyl isothiocyanate and iodoacetate. Z Naturforsch C. 1973 Jan-Feb;28(1):21–31. doi: 10.1515/znc-1973-1-205. [DOI] [PubMed] [Google Scholar]
- Houston M. R., Meyer K. H., Thomas N., Wolf F. T. Dimorphism in Cladosporium werneckii. Sabouraudia. 1969 Oct;7(3):195–198. [PubMed] [Google Scholar]
- Iralu V. Formation of aerial hyphae in Candida albicans. Appl Microbiol. 1971 Sep;22(3):482–488. doi: 10.1128/am.22.3.482-483.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapell J., Urbach H. Möglichkeiten des Nachweises einer hemmenden Wirkung des Serums auf die Atmung von Candida albicans mit Hilfe des Warburg-Apparates. Arch Hyg Bakteriol. 1971 Apr;154(5):524–532. [PubMed] [Google Scholar]
- Knight L., Fletcher J. Growth of Candida albicans in saliva: stimulation by glucose associated with antibiotics, corticosteroids, and diabetes mellitus. J Infect Dis. 1971 Apr;123(4):371–377. doi: 10.1093/infdis/123.4.371. [DOI] [PubMed] [Google Scholar]
- Konijn T. M., Van De Meene J. G., Bonner J. T., Barkley D. S. The acrasin activity of adenosine-3',5'-cyclic phosphate. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1152–1154. doi: 10.1073/pnas.58.3.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koobs D. H. Phosphate mediation of the Crabtree and Pasteur effects. Science. 1972 Oct 13;178(4057):127–133. doi: 10.1126/science.178.4057.127. [DOI] [PubMed] [Google Scholar]
- Krichevsky M. I., Love L. L., Chassy B. M. Acceleration of morphogenesis in Dictyostelium discoideum by exogenous mononucleotides. J Gen Microbiol. 1969 Aug;57(3):383–389. doi: 10.1099/00221287-57-3-383. [DOI] [PubMed] [Google Scholar]
- Land G. A., McDonald W. C., Stjernholm R. L., Friedman T. L. Factors affecting filamentation in Candida albicans: relationship of the uptake and distribution of proline to morphogenesis. Infect Immun. 1975 May;11(5):1014–1023. doi: 10.1128/iai.11.5.1014-1023.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Cline M. J. Interaction of Candida albicans with human leukocytes and serum. J Bacteriol. 1969 Jun;98(3):996–1004. doi: 10.1128/jb.98.3.996-1004.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie D. W. Morphogenesis of Candida albicans in vivo. Sabouraudia. 1964 Jun;3(3):225–232. [PubMed] [Google Scholar]
- Mackenzie D. W. Studies on the morphogenesis of Candida albicans. II. Growth in organ extract. Sabouraudia. 1965 Jun;4(2):126–130. [PubMed] [Google Scholar]
- Mardon D., Balish E., Phillips A. W. Control of dimorphism in a biochemical variant of Candida albicans. J Bacteriol. 1969 Nov;100(2):701–707. doi: 10.1128/jb.100.2.701-707.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICKERSON W. J. Experimental control of morphogenesis in microorganisms. Ann N Y Acad Sci. 1954 Oct 29;60(1):50–57. doi: 10.1111/j.1749-6632.1954.tb39997.x. [DOI] [PubMed] [Google Scholar]
- NICKERSON W. J. SYMPOSIUM ON BIOCHEMICAL BASES OF MORPHOGENESIS IN FUNGI. IV. MOLECULAR BASES OF FORM IN YEASTS. Bacteriol Rev. 1963 Sep;27:305–324. doi: 10.1128/br.27.3.305-324.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OGUR M., ST. JOHN R., NAGAI S. Tetrazolium overlay technique for population studies of respiration deficiency in yeast. Science. 1957 May 10;125(3254):928–929. doi: 10.1126/science.125.3254.928. [DOI] [PubMed] [Google Scholar]
- Saltarelli C. G. Growth stimulation and inhibition of Candida albicans by metabolic by-products. Mycopathol Mycol Appl. 1973 Sep 28;51(1):53–63. doi: 10.1007/BF02141285. [DOI] [PubMed] [Google Scholar]
- Slonimski P. P., Perrodin G., Croft J. H. Ethidium bromide induced mutation of yeast mitochondria: complete transformation of cells into respiratory deficient non-chromosomal "petites". Biochem Biophys Res Commun. 1968 Feb 15;30(3):232–239. doi: 10.1016/0006-291x(68)90440-3. [DOI] [PubMed] [Google Scholar]
- Smith J. K., Louria D. B. Anti-Candida factors in serum and their inhibitors. II. Identification of a Candida-clumping factor and the influence of the immune response on the morphology of Candida and on anti-Candida activity of serum in rabbits. J Infect Dis. 1972 Feb;125(2):115–122. doi: 10.1093/infdis/125.2.115. [DOI] [PubMed] [Google Scholar]
- Stanley V. C., Hurley R. Growth of Candida species in cultures of mouse epithelial cells. J Pathol Bacteriol. 1967 Oct;94(2):301–315. doi: 10.1002/path.1700940207. [DOI] [PubMed] [Google Scholar]
- Terenzi H. F., Storck R. Stimulation of fermentation and yeast-like morphogenesis in Mucor rouxii by phenethyl alcohol. J Bacteriol. 1969 Mar;97(3):1248–1261. doi: 10.1128/jb.97.3.1248-1261.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uno I., Ishikawa T. Purification and identification of the fruiting-inducing substances in Coprinus macrorhizus. J Bacteriol. 1973 Mar;113(3):1240–1248. doi: 10.1128/jb.113.3.1240-1248.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wijk R., Konijn T. M. Cyclic 3', 5'-amp in Saccharomyces carlsbergensis under various conditions of catabolite repression. FEBS Lett. 1971 Mar 5;13(3):184–186. doi: 10.1016/0014-5793(71)80231-4. [DOI] [PubMed] [Google Scholar]
- WELD J. T. Candida albicans; rapid identification in pure cultures with carbon dioxide on modified eosin-methylene blue medium. AMA Arch Derm Syphilol. 1952 Dec;66(6):691–694. doi: 10.1001/archderm.1952.01530310029003. [DOI] [PubMed] [Google Scholar]



