Abstract
Oil and xanthorrhizol extraction from Curcuma xanthorrhiza Roxb. rhizome by supercritical carbon dioxide was optimized using Taguchi method. The factors considered were pressure, temperature, carbon dioxide flowrate and time at levels ranging between 10–25 MPa, 35–60 °C, 10–25 g/min and 60–240 min respectively. The highest oil yield (8.0 %) was achieved at factor combination of 15 MPa, 50 °C, 20 g/min and 180 min whereas the highest xanthorrhizol content (128.3 mg/g oil) in Curcuma xanthorrhiza oil was achieved at a factor combination of 25 MPa, 50 °C, 15 g/min and 60 min. Soxhlet extraction with n-hexane and percolation with ethanol gave oil yield of 5.88 %, 11.73 % and xanthorrhizol content of 42.6 mg/g oil, 75.5 mg/g oil, respectively. The experimental oil yield and xanthorrhizol content at optimum conditions agreed favourably with values predicted by computational process. The xanthorrizol content extracted using supercritical carbon dioxide was higher than extracted using Soxhlet extraction and percolation process.
Keywords: Curcuma xanthorrhiza Roxb. rhizome, Xanthorrhizol, Extraction, Supercritical carbon dioxide, Optimization
Introduction
Curcuma xanthorrhiza Roxb., a member of the ginger family (Zingiberaceae), is distributed in Southeast Asia region (Suksamrarn et al. 1994). It has been used to treat stomach diseases, liver disorders, constipation, bloody diarrhoea, dysentery, children’s fevers, hypotriglyceridaemic, haemorrhoids and skin eruptions due to its antibacterial, antioxidative, antitumor, anti-inflammatory and hepatoprotective effects (Hwang et al. 2000; Lin et al. 1996; Masuda et al. 1992; Park et al. 2008; Ruslay et al. 2007; Yasni et al. 1994). The traditional benefits of Curcuma xanthorrhiza were further supported by isolation and identification of several active constituents, including xanthorrhizol, curcumin and few volatile substances. Xanthorrhizol has shown medical properties in treatment and prevention of various diseases (Choi et al. 2005; Devaraj et al. 2010a, b; Kang et al. 2009). Pharmacologically active compounds in herbal plants are usually in low concentrations and a number of different effective and selective extraction methods have been developed for extraction of these compounds from raw material. The most widely used method is based on organic solvent extraction (Lang and Wai 2001; Tandrasasmita et al. 2010, 2011; Tjandrawinata et al. 2010). Organic solvent extraction methods have drawbacks such as usage of organic solvent, production of hazardous wastes and few adjustable parameters to control the selectivity of extraction process. Therefore, development of more reliable, simpler, less chemical-intensive and less expensive technique is amenable to commercial-scale production.
In recent years, supercritical fluid carbon dioxide extraction (SCFE-CO2) have been widely been utilized for extraction of oil and bioactive compounds from plant materials (Azmir et al. 2013; Balachandran et al. 2006; Grosso et al. 2008; Huang et al. 2008; Lang and Wai 2001; Salea et al. 2013; Ziemons et al. 2007). SCFE-CO2 offers many advantages in extraction process due to its non toxic nature, unique physicochemical properties including low viscosity, fast diffusion and tunable physical properties with temperature and pressure. The low critical temperature and pressure of carbon dioxide (CO2) (Tc = 31 °C and Pc = 7.38 MPa) offers possibility to operate extraction process at low temperature (below 80 °C) and moderate pressure (10–45 MPa) which may be an ideal condition for thermo labile compound extraction (Azmir et al. 2013; Lang and Wai 2001; Temelli and Guclu-Ustundag 2005). Despite the high potential use of SCFE-CO2 for extraction of oil and bioactive compounds, various parameters of SCFE-CO2 (i.e. temperature, pressure, CO2 flowrate and time of extraction) have been become major obstacle in finding the optimum extraction condition for each plant material (Azmir et al. 2013; Reverchon and Marco 2006; Temelli and Guclu-Ustundag 2005).
Recently, we reported a comparative study of full factorial design with taguchi method for SCFE-CO2 of Nigella sativa seeds oil (Salea et al. 2013). It was shown that taguchi method was able to simplify experimental procedure and maintain experimental cost at minimum level without affecting quality of result. In the other hand, difference between full factorial design and the taguchi methods is insignificant in oil yield (±0.1 %). In this present study, the optimization process for SCFE-CO2 of rhizomes Curcuma xanthorrhiza Roxb. was conducted using the taguchi method. Effect of pressure, temperature, CO2 flowrate and dynamic extraction time will be described as determined parameters to obtain the optimum condition in the SCFE-CO2 process. The experimental Curcuma xanthorrhiza oil yield and xanthorrhizol content at optimum condition were compared with predicted computational results and also compared to conventional extraction methods such as Soxhlet extraction and percolation process.
Material and method
Materials
Dried rhizomes of Curcuma xanthorrhiza Roxb. were supplied by Mitra Keluarga (Cianjur, Indonesia). The dried rhizomes were grounded in milling machine (FFC-15, China) with 2 mm round stainless steel filter. Moisture content was 11.91 % and loss on drying was 17.87 %. Xanthorrhizol standard was purchased from Chromadex (Irvine, CA). Methanol (HPLC grade) and ethanol (HPLC grade) were purchased from J.T. Baker (Phillipsburg, NJ). n-Hexane (ChromAR) was obtained from Merck (Germany). Ethanol (technical grade) was purchased from Brataco (Cikarang, Indonesia). Hydrogen (purity of 99.9 %), air (purity of 99.99 %) and liquid carbon dioxide (food grade) were purchased from PT Inter Gas Mandiri (Cikarang, Indonesia).
Apparatus and procedure
Supercritical fluid extraction
The supercritical fluid carbon dioxide extraction (SCFE-CO2) experiments were conducted using a custom-build SCFE-CO2 apparatus. Figure 1 shows a schematic diagram of the system. Details of apparatus and experimental procedures were described in the previous publication (Salea et al. 2013). Extractor vessel was loaded with 100 g grounded material for each experimental condition. Extraction pressure was varied from 10 to 25 MPa and temperature was varied from 35 to 60 °C. Carbon dioxide (CO2) flow rate was varied from 10 to 25 g/min and static extraction time was fixed to 60 min followed by dynamic extraction time (60–240 min). A Taguchi L-16 orthogonal arrays design was used to investigate optimal conditions and select the most influencing parameters in SCFE-CO2 process, by using four parameters and four levels. The parameters and levels are listed in Table 1 whereas structure of Taguchi’s orthogonal array design is shown in Table 2.
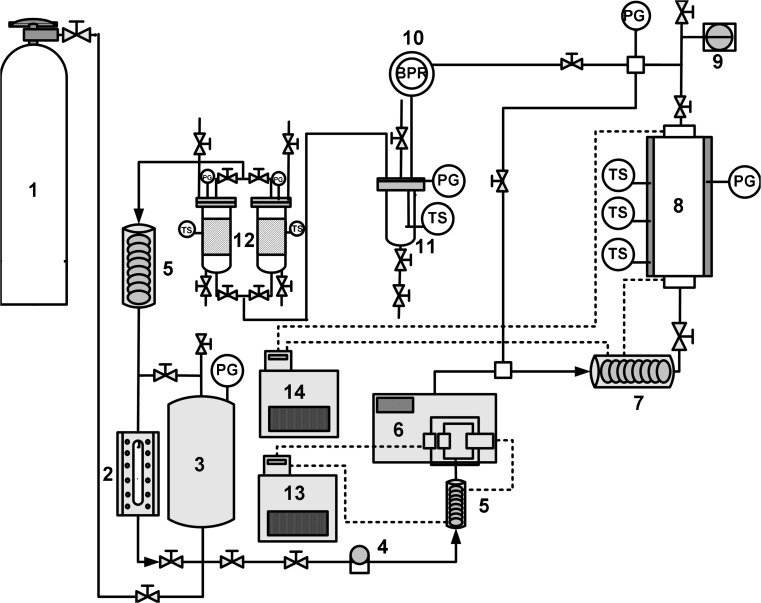
Fig. 1.
Schematic diagram of supercritical CO2 extraction system. Note: 1. CO2 cylinder. 2. Level cell. 3. CO2 Feed tank. 4. Filter. 5. Condenser. 6. CO2 pump. 7. CO2 pre-heater. 8. Extractor. 9. Rupture. 10. Back pressure regulator. 11. Separator. 12. Absorber. 13. Circulator (chiller). 14. Circulator (heater)
Table 1.
Parameters and levels used in experimental design
| Level | Parameters | |||
|---|---|---|---|---|
| Pressure (MPa) | Temperature (°C) | CO2 flowrate (gr/min) | Dynamic time (min) | |
| A | B | C | D | |
| 1 | 10 | 35 | 10 | 60 |
| 2 | 15 | 40 | 15 | 120 |
| 3 | 20 | 50 | 20 | 180 |
| 4 | 25 | 60 | 25 | 240 |
Table 2.
Standard L16 orthogonal arrays
| Exp. no. | Independent variables | |||
|---|---|---|---|---|
| A | B | C | D | |
| 1 | 1 | 1 | 1 | 1 |
| 2 | 1 | 2 | 2 | 2 |
| 3 | 1 | 3 | 3 | 3 |
| 4 | 1 | 4 | 4 | 4 |
| 5 | 2 | 1 | 2 | 3 |
| 6 | 2 | 2 | 1 | 4 |
| 7 | 2 | 3 | 4 | 1 |
| 8 | 2 | 4 | 3 | 2 |
| 9 | 3 | 1 | 3 | 4 |
| 10 | 3 | 2 | 4 | 3 |
| 11 | 3 | 3 | 1 | 2 |
| 12 | 3 | 4 | 2 | 1 |
| 13 | 4 | 1 | 4 | 2 |
| 14 | 4 | 2 | 3 | 1 |
| 15 | 4 | 3 | 2 | 4 |
| 16 | 4 | 4 | 1 | 3 |
Soxhlet extraction
Soxhlet extraction was carried out using stainless steel soxhlet apparatus (KIST, Korea). Fifty gram of dried material and 600 mL of hexane were used. The extraction was carried out at the normal pressure of hexane for 6 h. After extraction, hexane was removed with evaporation at reduced pressure using Rotavapor R-215 (Buchi, Switzerland). The amount of remaining extract was determined as yield of experiment.
Percolation
Percolation was carried out by using 2,400 mL custom built stainless steel percolator. About 150 g of dried Curcuma xanthorrhiza rhizomes were loaded into a percolator. Ethanol (96 %-v) with total volume of 2,600 ml was used as solvent. A HKS-12000 Pump (Korea) was used to continuously deliver solvent through the system with rate 180 ml/min. After 4 h extraction time, solvent then removed with evaporation at reduced pressure using Rotavapor R-215 (Buchi, Switzerland). The amount of remaining extract was determined as yield of experiment.
Analytical methods
Moisture content in the raw material was measured using a V30 compact volumetric Karl Fischer titrator (Mettler Toledo, OH). Loss on drying was measured using a HR83 halogen moisture analyzer (Mettler Toledo, OH). Xanthorrhizol content was quantified using Perkin-Elmer Gas chromatography model Clarus 680 equipped with a flame ionization detector (FID) and Elite-5 column (30 m × ID 0.25 mm, 0.25 μm film thickness). Initial column temperature was 160 °C, followed by ramping up to 230 °C with a rate of 5 °C/min. Temperature of detector and injector was maintained at 240 °C. The carrier gas was nitrogen at a flow rate of 1.0 mL/min. Samples were prepared by dissolving oil with concentration of 500 ppm. Methanol and ethanol was used as solvent with 1:1 ratio. The injection volume was 1 μL with a split ratio of 10:1.
Statistical analysis
Data obtained from SCFE-CO2 were subjected to analysis of variance (ANOVA) and signal-to-noise (S/N) ratio calculation. ANOVA was used to determine the significant differences among extractions yields. P-value less than 0.05 were considered significant. The S/N ratio calculation is an evaluation of output performance stability which measures level of performance and effect of noise parameters on performance with three different target values: “larger is better”, “smaller is better” or “nominal is better” (Nuruddin and Bayuaji 2009). In this study, target values of ‘larger is better’ was used since the purpose of this study was the highest oil yield and xanthorrhizol content. The larger difference (Δ) value in parameter indicated a significant effect on the process since changes in signal cause a larger effect on the output factor being measured. The S/N ratio is calculated using the following Eq. (1):
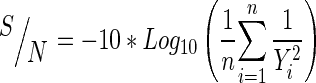 |
1 |
where, n is the number trials for experiments i, i is the experiment number and Y is the response. All statistical analysis were performed using MINITAB v.15 (Minitab Inc., USA) statistical software package.
Result and discussion
Summary of supercritical fluid carbon dioxide extraction (SCFE-CO2) experiments using Taguchi method are listed in Table 3. The results show that Curcuma oil yield vary from 4.7 to 7.6 % with xantorrhizhol content 75.7–125.9 mg/g oil.
Table 3.
Experiment results for SCFE-CO2 process of Curcuma xanthorrhiza with Taguchi Method
| Run | Pressure (MPa) | Temperature (°C) | CO2 flowrate (gr/min) | Dynamic time (min) | Yield oil (%) | Xanthorrhizol (mg/g oil) |
|---|---|---|---|---|---|---|
| 1 | 10 | 35 | 10 | 60 | 4.7 | 92.6 |
| 2 | 10 | 40 | 15 | 120 | 6.8 | 90.1 |
| 3 | 10 | 50 | 20 | 180 | 6.6 | 82.6 |
| 4 | 10 | 60 | 25 | 240 | 5.4 | 75.7 |
| 5 | 15 | 35 | 15 | 180 | 7.0 | 94.7 |
| 6 | 15 | 40 | 10 | 240 | 7.2 | 96.0 |
| 7 | 15 | 50 | 25 | 60 | 7.6 | 115.7 |
| 8 | 15 | 60 | 20 | 120 | 6.9 | 105.8 |
| 9 | 20 | 35 | 20 | 240 | 7.3 | 100.9 |
| 10 | 20 | 40 | 25 | 180 | 6.6 | 117.1 |
| 11 | 20 | 50 | 10 | 120 | 5.9 | 106.8 |
| 12 | 20 | 60 | 15 | 60 | 6.0 | 103.1 |
| 13 | 25 | 35 | 25 | 120 | 6.2 | 99.4 |
| 14 | 25 | 40 | 20 | 60 | 6.4 | 124.4 |
| 15 | 25 | 50 | 15 | 240 | 7.3 | 125.9 |
| 16 | 25 | 60 | 10 | 180 | 7.5 | 107.1 |
Pressure effect
Figure 2a shows effect of extraction pressure on oil yield and xanthorrhizol content. The amount of xantorrhizhol extracted from Curcuma xanthorrhiza material increased as the extraction pressure increased within the selected range of pressure. Generally, by using higher pressure at isothermal conditions resulted in increasing solvent density and subsequently solvent power and solubility of compounds. As the density increased, distance between molecules decreased and interaction between compounds and CO2 increased, which led to greater solubility of compounds in CO2 and higher solvent strength of supercritical CO2. Therefore, increase in density of CO2 will also accelerate mass transfer of analytes and solvent in supercritical extractor vessel system and improve extraction rate (Reverchon and Marco 2006). Effect of the extraction pressure on oil yield showed no particular trend. Since the difference of oil yield on pressures higher than 15 MPa was not significant, it was concluded that the optimum pressure for extraction of Curcuma xanthorrhiza oil is 15 MPa.
Fig. 2.
Main effects plot for oil yield and xanthorrhizol content: a pressure effect; b temperature effect; c CO2 flowrate effect; d dynamic extraction time effect
Temperature effect
Figure 2b shows that oil yield and xanthorrhizhol content are not strongly dependent on extraction temperature. As the extraction temperature increased, two competing factors namely solvent density and solid vapor pressure are influenced. Increase in temperature reduces the density of supercritical CO2 thus reducing solvent power of the supercritical solvent; but it increases vapor pressure of the compounds to be extracted (Danh et al. 2009). As result, there is a high possibility that density and solid vapor effects negate each other and give insignificant trend.
CO2 flowrate effect
Effect of CO2 flowrate were investigated at rate 10–25 g/min. As shown in Fig. 2c, both oil yield and xanthorrhizol content relatively constant on CO2 flowrate variation. CO2 flowrate is a relevant parameter only if the process is controlled by an external mass transfer resistance or by equilibrium: the amount of supercritical solvent feed to the extraction vessel, in this case, determines the extraction rate. In this study, extraction process probably controlled by internal mass transfer that related to particle size. Particle size plays a determining role in extraction processes that control by internal mass transfer resistances since a smaller mean particle size reduces the length of diffusion of the solvent (Reverchon and Marco 2006).
Dynamic extraction time effect
Figure 2d shows that Curcuma oil yield increased with increasing time up to 180 min and did not vary with further increase in the extraction time. Most parts of xanthorrhizhol was extracted in the first (rapid) stage of extraction during static time. Xanthorrhizhol is volatile compound and easily extracted at rapid extraction stage. Less volatile and more polar compounds were extracted later and diluted xanthorrhizhol in the Curcuma oil. Yonei et al. (1995) and Sonsuzer et al. (2004) found similar results for observed volatile compound extraction from ginger and Thymbra spicata.
Optimum condition
The signal-to-noise (S/N) ratio calculation result in terms of oil yield and xanthorrhizol content are shown in Tables 4 and 5. The S/N values indicated the effect of oil yield and xanthorrhizol content when level of factor in parameters is changed. Based on the S/N ratio calculation, the most influencing parameters in this process is extraction pressure. Influencing parameters decreased in the order: extraction pressure > dynamic extraction time > extraction temperature > carbon dioxide flowrate. Similar phenomena were observed by Sonsuzer et al. (2004) for SCFE-CO2 of Thymbra spicata oil and Danh et al. (2009) for SCFE-CO2 of Vetiveria zizanioides.
Table 4.
S/N value calculated for each parameters and level in terms of oil yield
| Level | Pressure | Temperature | CO2 flowrate | Dynamic time |
|---|---|---|---|---|
| 1 | 15.28 | 15.86 | 15.88 | 15.69 |
| 2 | 17.11 | 16.58 | 16.60 | 16.17 |
| 3 | 16.16 | 16.67 | 16.64 | 16.80 |
| 4 | 16.68 | 16.12 | 16.13 | 16.58 |
| Δ (max-min) | 1.83 | 0.81 | 0.76 | 1.11 |
| Rank | 1 | 3 | 4 | 2 |
Table 5.
S/N value calculated for each parameter and level in terms xanthorrhizol content
| Level | Pressure | Temperature | CO2 flowrate | Dynamic time |
|---|---|---|---|---|
| 1 | 38.59 | 39.72 | 40.04 | 40.69 |
| 2 | 40.23 | 40.50 | 40.22 | 40.03 |
| 3 | 40.57 | 40.54 | 40.20 | 39.96 |
| 4 | 41.11 | 39.73 | 40.04 | 39.83 |
| Δ (max-min) | 2.52 | 0.82 | 0.19 | 0.86 |
| Rank | 1 | 3 | 4 | 2 |
Tables 6 and 7 listed analysis of variance (ANOVA) of four parameters for SCFE-CO2 of Curcuma xanthorrhiza Roxb. with 95 % confidence. Results showed that different level of parameters had no significant effect on extraction result (P > 0.05). However, it was shown that extraction pressure as a main factor effect on extraction process and was in a good agreement with S/N ratio calculation results.
Table 6.
Analysis of variance (ANOVA) for oil yield
| Source | DF | Seq SS | Adj SS | Adj MS | F | P |
|---|---|---|---|---|---|---|
| Pressure | 3 | 7.437 | 7.437 | 2.4789 | 1.42 | 0.390 |
| Temperature | 3 | 1.748 | 1.748 | 0.5826 | 0.33 | 0.804 |
| CO2 flowrate | 3 | 1.647 | 1.647 | 0.5489 | 0.31 | 0.816 |
| Dynamic time | 3 | 2.873 | 2.837 | 0.9576 | 0.55 | 0.683 |
| Residual error | 3 | 5.240 | 5.240 | 1.7467 | ||
| Total | 15 | 18.944 |
Table 7.
Analysis of variance (ANOVA) for xanthorrhizol content
| Source | DF | Seq SS | Adj SS | Adj MS | F | P |
|---|---|---|---|---|---|---|
| Pressure | 3 | 14.1858 | 14.1858 | 4.72862 | 4.14 | 0.137 |
| Temperature | 3 | 2.5386 | 2.5386 | 0.84621 | 0.74 | 0.594 |
| CO2 flowrate | 3 | 0.1197 | 0.1197 | 0.03990 | 0.03 | 0.990 |
| Dynamic time | 3 | 1.7844 | 1.7844 | 0.59480 | 0.52 | 0.697 |
| Residual error | 3 | 3.4233 | 3.4233 | 1.14111 | ||
| Total | 15 | 22.0519 |
Based on the taguchi method, it was established that the conditions (pressure, temperature, carbon dioxide flow rate and time) for optimum oil yield from Curcuma xanthorrhiza Roxb. rhizome were 15 MPa, 50 °C, 20 g/min and 180 min; and for extraction of xanthorrhizol from Curcuma xanthorrhiza Roxb. oil were 25 MPa, 50 °C, 15 g/min and 60 min respectively. These data were subjected to Minitab v.15 statistical software package to predict the oil yield and xanthorrhizol content at proposed optimum conditions. The predicted oil yield was 7.9875 % at proposed optimum conditions for oil yield whereas the predicted xanthorrhizol content was 127.2 mg/g oil at these optimum conditions for xanthorrhizol content as mentioned above. The experimental oil yield was 8.0 % (xanthorrhizol content 94.0 mg/g oil) whereas the experimental xanthorrhizol content was 128.3 mg/g oil (oil yield 5.5 %) at optimum condition as mentioned above. Experimental results have similar value with the predicted results. The application taguchi method in SCFE-CO2 of Curcuma xanthorrhiza Roxb. provides an alternative way to simplify experimental design required to optimize oil yield and xanthorrhizol content. This method can significantly reduce the number of experiments from 256 (44) experiment trials when using full factorial design, to those of only 16 using L16 arrays, without influencing the quality of results. These results proved an example of how an experimental design could be beneficial for a comprehensive determination of the best experimental conditions as in the case of SCFE-CO2 of Curcuma xanthorrhiza Roxb.
Comparison SCFE-CO2 and conventional methods
Table 8 shows experimental results for three different extraction techniques in order to compare extraction methods for Curcuma xanthorrhiza. SCFE-CO2 has the highest xanthorrhizhol content compared to both conventional extraction methods. However, for oil yield, SCFE-CO2 have lower value compared to percolation. This result suggests Curcuma xanthorrhiza has high content of ethanol soluble compound. As result, extraction yield from percolation is higher and xanthorrhizhol is diluted with other compound. This comparation study shows the ability of SCFE-CO2 to specifically extract volatile compound.
Table 8.
Results from SCFE-CO2, Soxhlet extraction and percolation
| Methods | Yield (%) | Xanthorrhizol (mg/g oil) |
|---|---|---|
| Conventional methods: | ||
| Soxhlet extraction with n-hexane | 5.88 | 42.6 |
| Percolation with ethanol (96 %) | 11.73 | 75.5 |
| SCFE-CO2: | ||
| Optimum oil | 8.0 | 94.0 |
| Optimum xanthorrhizol | 5.5 | 128.3 |
Conclusion
Optimization process for oil yield and xanthorrhizol content from SCFE-CO2 of rhizomes Curcuma xanthorrhiza Roxb. was successfully performed using L-16 orthogonal arrays design of taguchi method. Optimum conditions of oil yield (8.0 %) was achieved at extraction pressure of 15 MPa, extraction temperature of 50 °C, carbon dioxide flowrate of 20 g/min and dynamic extraction time of 180 min. Optimum conditions of xanthorrhizol content (128.3 mg/g oil) was achieved at extraction pressure of 25 MPa, extraction temperature of 50 °C, carbon dioxide flowrate of 15 g/min and dynamic extraction time of 60 min. The experimental oil yield and xanthorrhizol content at optimum conditions was in good agreement with the predicted computational results. The S/N ratio calculation and ANOVA identified pressure as a main factor that has the largest effect on SFCE-CO2 of Curcuma xanthorrhiza Roxb. SCFE-CO2 has the highest xanthorrhizhol content compare to soxhlet and percolation extraction system.
Acknowledgments
Authors thank to Diena Arsyiana Hidayath for supporting data on GC analysis. Authors also thank to Sherly Juliani for critical review on this manuscript.
Conflict of interest
The author(s) declared no conflict of interests with respect to the authorship and/or publication. This research was supported by PT Dexa Medica, Indonesia.
Abbreviations
- Adj MS
Adjusted means of squares
- Adj SS
Adjusted sums of squares
- ANOVA
Analysis of variance
- CO2
Carbon dioxide
- DF
Degree of freedom
- F
F-value
- FID
Flame ionization detector
- GC
Gas chromatography
- HPLC
High performance liquid chromatography
- i
Experiment number
- n
Number trials for experiments i
- P
P-value
- Pa
Pascal
- Pc
Critical pressure
- SCFE
Supercritical fluid extraction
- SCFE–CO2
Supercritical fluid carbon dioxide extraction
- Seq SS
Sequentials sum of squares
- S/N ratio
Signal-to-noise ratio
- Tc
Critical temperature
- Y
Response
References
- Azmir J, Zaidul ISM, Rahman MM, Sharif KM, Mohamed A, Sahena F, Jahurul MHA, Ghafoor K, Norulaini NAN, Omar AKM. Techniques for extraction of bioactive compounds from plant materials: a review. J Food Eng. 2013;117:426–436. doi: 10.1016/j.jfoodeng.2013.01.014. [DOI] [Google Scholar]
- Balachandran S, Kentish SE, Mawson R. The effects of both preparation method and season on the supercritical extraction of ginger. Sep Purif Technol. 2006;48:94–105. doi: 10.1016/j.seppur.2005.07.008. [DOI] [Google Scholar]
- Choi MA, Kim SH, Chung WY, Hwang JK, Park KK. Xanthorrhizol, a natural sesquiterpenoid from Curcuma xanthorrhiza, has an anti-metastatic potential in experimental mouse lung metastasis model. Biochem Biophys Res Commun. 2005;326:210–217. doi: 10.1016/j.bbrc.2004.11.020. [DOI] [PubMed] [Google Scholar]
- Danh LT, Mammucari R, Truong P, Foster N. Response surface method applied to supercritical carbon dioxide extraction of Vetiveria zizanioides essential oil. Chem Eng J. 2009;155:617–626. doi: 10.1016/j.cej.2009.08.016. [DOI] [Google Scholar]
- Devaraj S, Esfahani AS, Ismail S, Ramanathan S, Yam MF. Evaluation of the antinociceptive activity and acute oral toxicity of standardized ethanolic extract of the rhizome of Curcuma xanthorrhiza Roxb. Molecules. 2010;15:2925–2934. doi: 10.3390/molecules15042925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaraj S, Ismail S, Ramanathan S, Santhini M, Yam MF. Evaluation of the hepatoprotective activity of standardized ethanolic extract of Curcuma xanthorrhiza Roxb. J Med Plants Res. 2010;4(23):2512–2517. [Google Scholar]
- Grosso C, Ferraro V, Figueiredo AC, Barroso JG, Coelho JA, Palavra AM. Supercritical carbon dioxide extraction of volatile oil from Italian coriander seeds. Food Chem. 2008;111:197–203. doi: 10.1016/j.foodchem.2008.03.031. [DOI] [Google Scholar]
- Huang W, Li Z, Niu H, Zhang J. Optimization of operating parameters for supercritical carbon dioxide extraction of lycopene by response surface methodology. J Food Eng. 2008;89:298–302. doi: 10.1016/j.jfoodeng.2008.05.006. [DOI] [Google Scholar]
- Hwang JK, Shim JS, Pyun YR. Antibacterial activity of xanthorrhizol from Curcuma xanthorrhiza against oral pathogens. Fitoterapia. 2000;71:321–323. doi: 10.1016/S0367-326X(99)00170-7. [DOI] [PubMed] [Google Scholar]
- Kang YJ, Park KK, Chung WY, Hwang JK, Lee SK. Xanthorrhizol, a natural sesquiterpenoid, induces apoptosis and growth arrest in HCT116 human colon cancer cells. J Pharmacol Sci. 2009;111:276–284. doi: 10.1254/jphs.09141FP. [DOI] [PubMed] [Google Scholar]
- Lang Q, Wai CM. Supercritical fluid extraction in herbal and natural product studies—a practical review. Talanta. 2001;53:771–782. doi: 10.1016/S0039-9140(00)00557-9. [DOI] [PubMed] [Google Scholar]
- Lin SC, Teng CW, Lin CC, Lin YH, Supriyatna S. Protective and therapeutic effect of the Indonesian medicinal herb Curcuma xanthorrhiza on β-D-galactosamine-induced liver damage. Phytother Res. 1996;10:131–135. doi: 10.1002/(SICI)1099-1573(199603)10:2<131::AID-PTR786>3.0.CO;2-0. [DOI] [Google Scholar]
- Masuda T, Isobe J, Jitoe A, Nakatani N. Antioxidative curcuminoids from rhizomes of Curcuma xanthorrhiza. Phytochemistry. 1992;31:3645–3647. doi: 10.1016/0031-9422(92)83748-N. [DOI] [Google Scholar]
- Nuruddin MF, Bayuaji R. Application of Taguchi’s approach in the optimization of mix proportion for Microwave Incinerated Rice Husk Ash foamed concrete. IJCEE. 2009;9:121–129. [Google Scholar]
- Park JH, Park KK, Kim MJ, Hwang JK, Lee SK, Chung WY. Cancer chemoprotective effects of Curcuma xanthorrhiza. Phytother Res. 2008;22:695–698. doi: 10.1002/ptr.2418. [DOI] [PubMed] [Google Scholar]
- Reverchon E, Marco ID. Review: supercritical fluid extraction and fractionation of natural matter. J Supercrit Fluids. 2006;38:146–166. doi: 10.1016/j.supflu.2006.03.020. [DOI] [Google Scholar]
- Ruslay S, Abas F, Shaari K, Zainal Z, Maulidiani SH, Israf DA, Lajis NH. Characterization of the components present in the active fractions of health gingers (Curcuma xanthorrhiza and Zingiber zerumbet) by HPLC-DAD-ESIMS. Food Chem. 2007;104:1183–1191. doi: 10.1016/j.foodchem.2007.01.067. [DOI] [Google Scholar]
- Salea R, Widjojokusumo E, Hartanti AW, Veriansyah B, Tjandrawinata RR. Supercritical fluid carbon dioxide extraction of Nigella sativa (black cumin) seeds using taguchi method and full factorial design. Biochem Compd. 2013 [Google Scholar]
- Sonsuzer S, Sahin S, Yilmaz L. Optimization of supercritical CO2 extraction of Thymbra spicata oil. J Supercrit Fluids. 2004;30:189–199. doi: 10.1016/j.supflu.2003.07.006. [DOI] [Google Scholar]
- Suksamrarn A, Eiamong S, Piyachaturawat P, Charoenpiboonsin J. Phenolic diarylheptanoids from Curcuma xanthorrhiza. Phytochemistry. 1994;36:1505–1508. doi: 10.1016/S0031-9422(00)89751-4. [DOI] [Google Scholar]
- Tandrasasmita OM, Lee JS, Baek SH, Tjandrawinata RR. Induction of cellular apoptosis in human breast cancer by DLBS1425, a Phaleria macrocarpa compound extract, via downregulation of PI3-kinase/AkT pathway. Cancer Biol Ther. 2010;10:814–823. doi: 10.4161/cbt.10.8.13085. [DOI] [PubMed] [Google Scholar]
- Tandrasasmita OM, Wulan DD, Nailufar F, Sinambela J, Tjandrawinata RR. Glucose-lowering effect of DLBS3233 is mediated through phosphorylation of tyrosine and upregulation of PPARγ and GLUT4 expression. Int J Gen Med. 2011;4:345–357. doi: 10.2147/IJGM.S16517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temelli F, Guclu-Ustundag O. Bailey’s industrial oil and fat products. New Jersey: Wiley; 2005. Supercritical technologies for further processing of edible oils. [Google Scholar]
- Tjandrawinata RR, Arifin PF, Tandrasasmita OM, Rahmi D, Aripin A. DLBS1425, a Phaleria macrocarpa (Scheff.) Boerl. Extract confers anti proliferative and proapoptosis effects via eicosanoid pathway. J Exp Ther Oncol. 2010;8:187–201. [PubMed] [Google Scholar]
- Yasni S, Imaizumi K, Sin K, Sugano M, Nonaka G, Sidik Identification of an active principle in essential oils and hexane-soluble fractions of Curcuma xanthorrhiza Roxb. showing triglyceride-lowering action in rats. Food Chem Toxicol. 1994;32:273–278. doi: 10.1016/0278-6915(94)90200-3. [DOI] [PubMed] [Google Scholar]
- Yohei Y, Ohinata H, Yoshida R, Shimizu Y, Yokoyama C. Extraction of ginger flavor with liquid or supercritical carbon dioxide. J Supercrit Fluids. 1995;8:156–161. doi: 10.1016/0896-8446(95)90028-4. [DOI] [Google Scholar]
- Ziemons E, Mbakop NW, Rozet E, Lejeune R, Angenot L, Thunus L, Hubert P. Optimisation of SFE method on-line coupled to FT-IR spectroscopy for the real-time monitoring of the extraction of tagitinin C in T. diversifolia. J Supercrit Fluids. 2007;40:368–375. doi: 10.1016/j.supflu.2006.07.009. [DOI] [Google Scholar]