Abstract
Fumonisins are one of the most agriculturally significant environmental toxins produced by Fusarium and Aspergillus species that grow on agricultural commodities in the field or during storage. Cereals contaminated with fumonisins causes serious loss to agricultural produce leads to health problems in humans and other farm animals. In the present study, polyclonal hyperimmune sera was raised against FB1 in rabbits immunized with FB1–keyhole limpet haemocyanin (KLH). Purified antibodies were used to establish a sensitive gold nanoparticle based immunochromatographic strip (ICG) for detecting FB1 levels in cereal grains. Effective on-site detection of FB1 was achieved by developing a rapid and sensitive pAb based ICG strip. This strip had a detection limit of 5 ng mL−1 for FB1 in cereal samples and it could be completed within 3 min. Close examination of 150 cereal samples by ICG strip method revealed that 77 were fumonisin-positive. Results obtained by the developed method was further validated with well standardized HPLC method and results of strip method was correlated well with those obtained by HPLC method. In conclusion, the developed method was a better alternative for onsite detection of FB1 in cereal samples intended for human consumption to reduce risk of humans and other farm animals. The high level of FB1 concentrations recorded in present study warrants the need to develop an awareness creation programme to the farmers of India for safe handling of cereal grains at the time of harvesting and storage of grains.
Keywords: Fumonisins, Antibodies, ICG strip, Cereal samples
Introduction
The genus Fusarium is a common fungal contaminant of many economically important plant and plant products and causes problems in human and animal Nutrition. Fusarium spp. infect many important food grains, such as maize, wheat, barley, rice, millet, oats, rye and produce highly toxic secondary metabolites known as mycotoxins. The major classes of Fusarium mycotoxins found in food are trichothecenes and fumonisins (Ramana et al. 2011). Fumonisins are one of the most agriculturally significant environmental toxins produced by Fusarium species that grow on agricultural commodities in the field or during storage (Desai et al. 2002). Over 23 species of Fusarium have been tested for fumonisin production and only F. verticillioides, F. proliferatum, and F. nygamai species produce high levels of fumonisins (Nelson et al. 1983). Fumonisins are water-soluble aminopolyols with a core structure containing 19 or 20 carbon backbones with hydroxyl, methyl, and tricarballylic acid moieties at different positions along with the carbon backbone (Shier et al. 1995). Four main groups of fumonisins occur in nature, among them fumonisin B series contains the most important ones consisting of FB1, FB2, FB3, and FB4, with the most common being FB1 and FB2 (National Toxicology Program 1999; Council for Agriculture Science and Technology 2003). Dietary exposure of fumonisins can cause irreversible tissue damage through biochemical mechanisms that produce pro-oxidative, pro-inflammatory, carcinogenic and immunosuppressive effects at a cellular level (Baumrucker and Prieschl 2002; Gelderblom et al. 2004; Kouadio et al. 2005; Domijan et al. 2007). Studies have also shown that fumonisins are toxic to plants as well (Abbas and Smeda 2000). Because of the recognized mammalian toxicity, many countries have or are about to have specific regulatory limits (guidelines or statutory limits) for fumonisins in food and feed intended for consumption. Only Switzerland has proposed legislation for FB1, and the acceptable limit was determined as 1,000 μg/kg (IARC 2002). The U.S. FDA has issued maximum residue limits in corn and corn byproducts in food as 4 ppm for whole corn grains, 2 ppm for dry milled corn products, 3 ppm for cleaned corn intended for popcorn production and in animal feeds as ranging from 5 to 100 ppm depends on the animal species (USDA 2001).
Fumonisins are typically analyzed by chromatographic methods such as TLC, LC, LC-MS, GCMS and HPLC, these methods requires expensive instrumentation and trained personnel. However, fumonisin levels in contaminated foods and feeds have been analysed and quantified using various immunochemical assays because of their rapidity and sensitivity (Yu and Chu (1996); Yeung et al. 1996; Usleber et al. 1994; Quan et al. 2006; Barna-Vetro et al. 2000; Azcona-Olivera et al. 1992). Most of the established immunoassays are infeasible for on-site detection of toxins owing to the long time incubation, tedious washing steps and need for an electronic microplate reader to read the results. In a recent study Shiu et al. (2010) developed an ICG strip based method for onsite detection of FB1 from contaminated maize samples using polyclonal antibodies raised against FB1-KLH immunogen in rabbits. Commercial immunoassay kits (ELISA: microwell and affinity column) such as the Verotox (Neogen Corp., Lansing, MI) are now available in many countries. Due to high cost and less availability of these test kits in developing countries like India, there is a need to develop a simple cost effective field based detection system for onsite detection of fumonisins from contaminated food and feed samples intended for human and animal consumption. Therefore, the aim of the present study was to generate the antibodies and development of a sensitive gold nanoparticle based immunochromatographic strip for on-site detection of FB1 from contaminated food grains and products intended form consumption. Also to check the reliability and reproducibility of developed method, HPLC validation was carried out.
Materials and methods
Materials
Standard toxins of Fumonisin B1 and B2 (FB1 and FB2), Deoxynivalenol (DON), T-2 toxin, Zearalenone (ZON), keyhole limpet haemocyanin (KLH), Ovalbumin (OVA), Carbodiimide (1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) hydrochloride), N-hydroxysuccinimide (NHS), Gold hydrochloride (HAuCl4) and Trisodium citrate, Goat anti-rabbit–peroxidase conjugate were obtained from Sigma Aldrich (St Louis, MO, USA). HRP substrate solution 3,3,5,5-tetramethylbenzidine (TMB) was purchased from Banglore Genei (Bangalore, India), Ammonium sulfate, Syringe filters (0.45 μm) and Tween 20 were obtained from Merck (Mumbai, India). Nitrocellulose membrane plate, Sample pad, Conjugate pad and absorbent pad were procured from Membrane Technologies (Ambala, India). All other chemicals and organic solvents used were of reagent grade or analytical grade purchased from Merk chemicals (Mumbai, India).
Preparation of FB1-protein conjugates
FB1 and KLH was coupled in the presence of carbodiimide (EDC) followed by the earlier report of Shiu et al. (2010). Briefly, EDC (1 mg) was dissolved in 0.1 mL of water and added to FB1 (1 mg) in 0.5 mL of conjugation buffer (0.1 mol L−1 2-N morpholinoethanesulfonic acid (MES), 0.9 mol L−1 NaCl, pH 4.7) and incubated for 15 min at room temperature then 3 mg of KLH was added. The reaction was allowed at room temperature for 2 h, then dialyzed against 2 L of 0.01 mol L−1 phosphate buffer (PBS, pH 7.5) for 72 h with four buffer changes.
Conjugation of FB1 to OVA was achieved by the glutaraldehyde method as used by Yu and Chu (1996). Brifely, FB1 (1 mg) in 1 ml of 25 % ethanol was mixed with 5 mg of OVA, to which 0.05 mL of a 25 % glutaraldehyde solution was added drop wise. The reaction was carried out at 4 °C overnight with constant stirring and stopped with the addition of 0.1 mL lysine (1 mol L−1, pH 7.0) and dialyzed against PBS for 72 h. FB1-protein molar ratio was estimated by TNBS calorimetric assay.
Estimation of Hapten-Carrier conjugation ratio
TNBS assay
The assay protocol was similar to the Sashidhar et al. (1994) for estimation of hapten carrier conjugation ratio. The number of Ɛ-amino groups present in the carrier protein/hapten-protein conjugates was directly determined from a standard curve generated from the difference in the absorbance at 335 nm for TNP-L-lysine and TNP-L-glutamic acid. The difference in absorption accurately accounts for the free Ɛ-amino groups. The standard curve was based on linear regression analysis using the equation y = A + Bx. Furthermore, the percent conjugation of hapten to protein was calculated using the following equation:
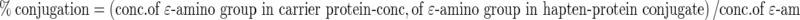 |
Production and purification of antibodies
Antibodies against immunogen (FB1-KLH), was raised in New Zealand white rabbits at the University of Mysore by a standard procedure (Lee et al. 2004). Two female New Zealand white rabbits were each immunized by subcutaneous injection of an emulsion of 0.5 ml immunogen (concentration: 1 mg protein/ml) and 0.5 ml complete Freund’s adjuvant (CFA) at day one. Multiple subcutaneous injections were continued at 2-week intervals, except incomplete Freund’s adjuvant was used instead of CFA. The antibody was purified by the ammonium sulfate precipitation method and protein A column affinity chromatography. Different fractions obtained during the purification of IgG from rabbit serum were subjected to gel electrophoresis using a mini-PROTEAN II Electrophoresis Cell (Bio-Rad, India) to check the purity of the antibody.
Indirect ELISA
The reactivity of immunized rabbit sera was measured by indirect plate-ELISA. FB1-OVA in 0.05 M carbonate/bicarbonate buffer was added to the culture wells and kept at 4 °C overnight. One percent gelatin in PBS solution was used to block the wells for 2 h at 37 °C, and 100 μL of antisera (1:1,000) was added and left to bind for 1 h at 37 °C. Approximately 100 μL of goat anti-rabbit IgG-HRP (1:1,000 in PBST) was added to each well and incubated for 1 h at 37 °C. Between each of the above steps, the wells were washed three times with PBST. Finally, the wells were incubated with TMB/H2O2 substrate for 15 min at 37 °C. The absorbance at 450 nm was measured after the reaction was stopped with 50 μL of 2 M H2SO4.
Specificity of antibody against series of mycotoxins
Specificity of the polyclonal antisera was checked by using series of mycotoxin conjugates including FB1-OVA, FB2-OVA, DON-OVA, T-2 toxin BSA and ZEN-BSA by indirect ELISA as mentioned above. FB2-OVA, DON-OVA conjugates were prepared in lab followed by the method of Yu and Chu (1996) and Chu et al. (1979) method was followed for preparation of T-2 toxin–BSA and ZEN-BSA conjugates.
Preparation of immunochromatographic strip
Synthesis of colloidal gold
All glassware was used in this preparation was thoroughly cleaned and oven dried prior to use. Colloidal gold was prepared by using a previously reported method (Shiu et al. 2010). Briefly, in a 500 ml round bottom flask, 200 ml of 0.01 % (w/w) HAuCl4 in doubled distilled water was added and boiled with vigorous stirring. Four milliliters of 1 % trisodium citrate was then added to the above solution. The solution was turned deep blue within 20 s and the color changed to wine red after 60 s. After continued boil for an additional 10 min, the heating source was removed and the colloid was stirred for 15 min. The colloid gold solution was stored at 4 °C in a dark colored glass bottle until use.
Preparation of colloidal gold –PAb conjugates
Purified PAb raised against FB1 was dialyzed against 2-mM phosphate buffer (pH-7.4) at 4 °C for 2 days with three buffer changes then conjugated with colloidal gold to generate FB1 PAb-CGC colloidal gold conjugate), briefly the pH of colloidal gold solution was adjusted to 8.5 with 0.1 M K2CO3. Five hundred micro liters of dialyzed antibody (0.5 mg/ml) was added drop wise to 50 ml colloidal gold solution with continuous stirring. Further the reaction was allowed for 10 min and blocked the free sites on nanoparticles using 5 ml of 1 % BSA solution for 30 min and centrifuged at 15,000 rpm for 30 min, after centrifugation, the gold pellets were suspended in 5 ml dilution buffer [20 mM Tris/HCl (pH-8.2) containing 1 % BSA (w/w)] and adjusted the optical density to 5.0 at 520 nm with dilution buffer, and then stored at 4 °C until use.
ICG strip development
The test devise was prepared as follows: nitro-cellulose membrane attached onto cellulose acetate matrix was cut into sections (1.5 × 0.5 cm), 1 μg of goat anti-Rabbit IgG (control line) and 1.5 μg of FB1-OVA conjugate (test line) (in 0.05 mM sodium phosphate buffered saline, pH-7.4) were separately applied near to one end of cellulose acetate supported strip of nitrocellulose membrane. The distance between test line and control line was about 5 mm. The test strips were dried at 37 °C for 30 min. the remaining protein binding sites were blocked by immersing the strips with 1 % BSA for 30 min at room temperature. Test strip was washed and dried and then stored in a desiccator at 4 °C. The sample and conjugate pads were treated with 20 mM phosphate buffer containing 2 % BSA, 2.5 % sucrose and 0.1 % SDS, pH-7.4 and dried at 37 °C. The anti FB1 PAb-CGC was applied on to conjugate pad. The absorption pad, conjugate pad and sample pad were assembled on to cellulose acetate supported strip containing nitrocellulose membrane. Test strips were stored at 4 °C until use in a desiccator.
Principle of immunochromatographic assay
The assay is based on the competitive reaction theory. When a sample is applied to the sample pad, it rapidly wets through to the conjugate pad, and the detector reagent (anti FB1 PAb-CGC) is then solubilized. The detector reagent begins to migrate along with the sample flow front up to the nitrocellulose membrane. Absence of FB1 in the test sample, when the sample passes over the test line to which FB1–OVA is immobilized, the detector reagent (anti FB1 PAb-CGC) is bound and the excess detector reagent is trapped by the control line. Two red bands at the test and control lines are then developed. In contrast, when the sample contains FB1, it will bind to the detector reagent and no band or one baby-red band which is weaker than the band of FB1 of the negative control sample at the test line is present. The result can be visualized by naked eyes, and the intensity of the test line is in inversely proportional to the amount of FB1 present in the samples. Several concentrations of FB1 (0, 10, 20, 50, 75, 100, 500 and 1,000 ng/ml) and the negative controls were included in the standardization of test assay.
Assay of fumonisin B1 in cereals with immunochromatographic strip
Diluted test sample solution as well as varied concentrations of FB1 were taken to test the developed method. Diluted test sample or varied concentrations of FB1 (0–1,000 ng mL−1) standard solutions (300 μL) were added to individual wells of tissue culture plate. Consequently, developed test strip was dipped into each well. In turn to characterize and make the cut-off level for each selected concentration, more than three strips were tested. The test samples or FB1 solution migrated up to the membrane. The test strip was allowed for 3 min to develop color and the test results were documented visually (Shiu et al. 2010).
Chemical analysis of fumonisins
Collection of cereal samples
A total of 150 cereal samples comprising maize (n = 50), sorghum (n = 25), wheat (n = 25) and paddy (n = 50) were collected during year 2010–2012 from major cereal growing regions of Southern states of India such as Karnataka, Andhra Pradesh and Tamilnadu. Two hundred fifty grams of each sample was collected and stored in sterile polythene bags. Collected samples were brought to lab for analysis of toxigenic fungal species incidence (results were not shown) and mycotoxin contamination with respective to fumonisins.
Extraction and clean-up of fumonisins from cereal samples
Fumonisins were extracted from samples by the method of Shiu et al. (2010). Briefly, cereal samples (5 g) were extracted with 100 ml methanol/water (3:1, v/v) for 3 min using a homogenizer (blender). After centrifugation at 1,500 g for 10 min at 4 °C, the mixture was filtered through a filter paper and pH was adjusted to 6.0 with 1 M NaOH. Five ml of filtered extract was applied to a strong anion exchange (SAX) solid phase extraction cartridge (Bond-Elut, Varian, Harbor City, CA, USA) containing 500 ng sorbent, at a flow rate of less than 2 ml/min. Prior to use, the cartridge was conditioned by successive passage of 5 ml methanol. Subsequently the cartridge was washed with methanol/water (3:1, 5 ml) and methanol (3 ml). Fumonisins were eluted from the cartridge with 1 % acetic acid in methanol (10 ml) under gravity. Elutes were evaporated to dryness under a steam of nitrogen gas and re-dissolved in 3 ml of methanol immediately prior to derivatisation and HPLC analysis was carried out.
High performance liquid chromatography (HPLC) analysis of fumonisins
The HPLC method allows detection and quantification of much smaller quantities of fumonisins. FB1 analysis was performed according to the method described by Sydenham et al. (1992). Briefly, aliquots (25 μl) of the purified extract was derivatised with 225 μl O-phthaldialdehyde (OPA) solution prepared by dissolving 40 mg OPA in 1 ml methanol and adding 5 ml of 0.1 M sodium tetraborate and 50 μl of 2-mercaprtoethanol. The FB1-OPA derivatives were analyzed using HPLC with fluorescence detection. The HPLC consisted of a Waters model 590 pump (Milford, MA, USA) connected to a Waters 470 scanning fluorescent detector. The separations were performed on a reversed-phase stainless steel Ultracarb 5 ODS column (150 × 4.60 mm id, Phenomenex, Torrance, CA, USA). The mobile phase was methanol/0.1 M sodium dihydrogen phosphate (75:25, v/v) adjusted to pH: 3.35 with orthophosphoric acid and pumped at a flow rate of 1 ml/min.
Derivatisation and LC determination
The auto injector was programmed to mix 50.0 μL standards or sample solution with 200 μL OPA reagent, and 50 μL was injected immediately into the LC. The excitation was set at 335 nm, emission: 440 nm, flow rate: 1.0 mL/min. FB1 quantification were performed by peak height and area measurement comparing with reference standard calibration curve with known concentrations. If peak area of sample FB1 exceeded that of the standard, the extract was diluted and re-injected. The standard calibration curve was made using different dilutions of FB1 standard toxin ranging from 10 ng to 1 μg. The lowest detection limit (LOD)/LOQ for FB1 was determined as 10 ng by HPLC method (results were not shown).
Results and discussion
Preparation of antigen
FB1 is too a low molecular to be immunogenic. In order to overcome the limit of small molecule recognition in an immune response, the FB1–protein conjugate was constructed and used as the immunogen instead of FB1 itself. In this present study, FB1–KLH and FB1-OVA conjugates were prepared and used as the immunogen and solid phase antigen respectively.
Conjugation of FB1 to KLH/OVA
Conjugation of FB1 with KLH or OVA was accomplished by a carbodiimide method. The carrier protein was KLH and OVA. The scheme of different conjugate proteins and synthesis methods for the immunization antigen (FB1-KLH) and the coating antigen (FB1-OVA) was beneficial for screening for specific antibody (Clarke et al. 1993). In the present study, the molar ratio of FB1: KLH and FB1: OVA were measured as 30:1 and 33:1 respectively by TNBS method.
Production of antibody
In the present study, the protocol used for immunization achieved an antibody titer of 1:32,000 after 4–5 weeks initiation of immunization. FB1-OVA conjugate was tested at concentrations ranging from 1 to 10 μg mL−1 in 10-fold intervals. In five independent tests, an FB1-OVA conjugate concentration of 5 μg mL−1 was found to be optimum for coating the plates. The different sensitivities of antibodies are the result of the conjugation ratio of toxin to protein molecule, the immunization procedure, and the ELISA conditions, which include the concentration of antigen and antibody and the time and temperature of reaction. First, a suitable ratio of toxin to carrier protein favors the promotion of an intense immune response and the production of an antibody with good characteristics. However, a suitable ratio might depend on the type of carrier protein, as discussed above. Second, the immunization procedure of FB1 has been investigated in several previous studies (Yeung et al. 1996; Usleber et al. 1994). In general, the correct dose of antigen (50–200 μg) is administered for animal immunization at least four times at intervals of 2 weeks, because FB1 is a hapten with weaker immunity.
Characterization of antibodies
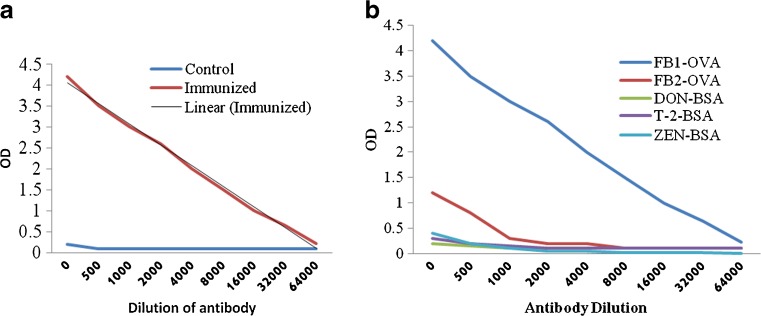
Antibody titers were determined using an indirect ELISA with FB1-OVA coated onto ELISA plates. Antibodies against FB1 were initially detected with the sera of rabbits at 6 weeks after the first immunization. The antibody titer increased gradually over time and the highest antibody titer was found with the sera of rabbits at 16 weeks after two subsequent immunizations Fig. 1a.
Fig. 1.
Immuno reactivity and cross reactivity of anti FB1- polyclonal hyper immune sera raised against FB1-KLH immunogen. a titration of the PAb against FB1 b Cross reactivity of anti FB1 Pab- against series of mycotoxins
Therefore, specificity of the antibody was evaluated using the 16th week of antiserum by indirect ELISA, Fig. 1a shows the antibodies exhibited the highest affinity for FB1 than FB2. When the specificity of anti-FB1 PAbs were evaluated on to different toxin conjugates by indirect ELISA, none of the toxins shown significant reactivity (Fig. 1b).
Construction and characterization of immunochromatographic assay strip (ICA)
The main objective of the ICA test was qualitative detection of FB1 contamination at threshold levels, and used in on-site screening of maize, sorghum, wheat and paddy samples. For the development of a sensitive ICA test, optimization of various parameters like size of gold nanoparticles, purity of antibody, concentration of capture reagent and NC membrane selection was carried out. In ICA assay, there is no need for complex operations and therefore the detection time is remarkably shortened. Furthermore, semi-quantitative detection can be realized by the intensity of signals as a response to an analyte concentration. In particular, the results can be read directly by naked eyes, ensuring the convenience of assay onsite. Therefore, ICA can accelerate the analytical procedure and also provide a means for performing the test without reagent handling, allowing a one-step assay (Paek et al. 2000). Moreover, the rapidity of test strip mainly depends on the size of the colloidal gold particles used. The particle size must be large enough to be seen. The most common size used is 40 nm. Previous reports observed that 40 nm colloidal gold particles offered maximum visibility due to the low steric hindrance when conjugated with IgG (Christopher et al. 2005). Therefore, spherical gold particles with a diameter of 40–50 nm colloidal gold were used in the present study. In addition to IgG, the unpurified fraction will contain a mass of proteins. All of these proteins will compete for binding sites on surface of the labeled gold colloid. This will result in a low degree of sensitivity of the detector reagent in the assay (Christopher et al. 2005). In this present study, the anti-FB1 PAb from the rabbit sera was purified by ammonium sulfate precipitation (50 % saturation for the final solution) twice and then was purified again by affinity chromatography with a Protein-A Sepharose Fast Flow Column.
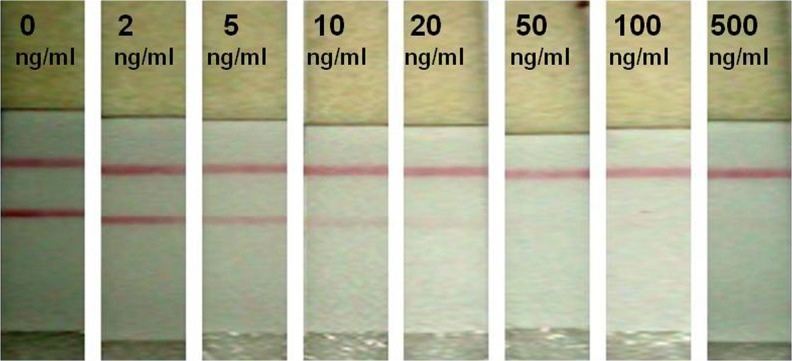
In this study, various types FB1–carrier protein conjugates such as FB1–OVA and FB1–KLH on NC membrane were compared. The application of FB1–OVA for capture reagents has several advantages. Firstly, the OVA give higher stability in coupling with FB1. Secondly, the OVA show higher coupling ratio than other protein in the coupling reaction with FB1. Finally, the FB1–OVA for capture reagents improve the assay sensitivity. However, it was also found the non-specific bindings were high between FB1-OVA and colloidal gold due to electrostatic action. To remove the nonspecific adsorption, a range of blocking reagents such as polymers (PVP, PVA, and PEG), surfactant (Tween-20, Triton-X), and protein (BSA, skimmed milk, casein) were tested to demonstrate the minimum non-specific binding in ICA test applications (Kolosova et al. 2008). However, compared with the NC membrane unblocked, blocked membrane with these proteins allows both sample solution and gold-labeled antibody to flow more slowly, and hence are not recommended as suitable blocking materials for ICA test applications (Christopher et al. 2005). In this study, blocking buffer (0.005–3 % BSA, 0.01 % SDS and 0.005 % sucrose) was used to reduce the non specific interactions between FB1-OVA and colloidal gold. In order to develop sensitive and rapid detection methods for FB1, we adopted the competitive reaction format. FB1–OVA conjugate was immobilized to a defined detection zone on the porous nitrocellulose membrane and anti FB1-PAb-CGC served as a detection reagent for the preparation of an immunochromatographic strip. Sensitivity of the developed method was evaluated onto different concentrations of FB1 analytical standard obtained from Sigma Aldrich, USA. The cut off levels of the developed method was determined as 5 ng/ml of the toxin (Fig. 2), moreover the developed test could be completed within 3 mins. The developed method could achieve moderate sensitivity and high rapidity than the previous reported methods for the detection of FB1 from cereal based food matrices (Shiu et al. 2010; Wang et al. 2006; Scott et al. 1997). Many of the reported ICG methods were limited to use in one kind of food matrix. In present study, developed method was evaluated on to different cereal based matrices to check the reliability, reproducibility and also to know the matrix interference. Further developed method was validated using well standardized quantitative HPLC method for detection of FB1. Moreover developed method had advantageous over existing methods in terms of rapidity, adaptability and reproducibility for detection of fuminisnB1 from contaminated food and feed products intended for human and farm animal consumption.
Fig. 2.
Sensitivity evaluation of developed test strip on to several concentrations of FB1 standard toxin (Dilution study) Note- Detection limit of the developed strip was determined as 5 ng/ml of standard toxin
Analysis of fumonisins from cereal samples by immunochromatographic strip assay
In the present study, close examination of 150 cereal samples (50 maize, 50 paddy, 25 wheat and 25 sorghum) collected from different cereal grain growing regions of India by the developed test method reveals that 77 samples were stayed as positive for fumonisin contamination and rest stayed as negative (Fig. 3). Employing a one-step assay method that is promising for extensive use in on-site detection of fumonisins, this study focuses on developing an antibody–gold nanoparticle immunochromatographic strip that is simpler and less time-consuming than competitive direct ELISA. Although both R-Biopharm Inc. (Germany) and Neogen Corporation (USA) have commercialized immunochromatographic strips for FB1 detection, their strips, with detection limit of 2 μg g−1, are not sensitive enough to comply with practical requirements. However, Wang et al. (2006) also described a colloidal gold immunoassay method for analysing FB1. In their assay system, antibody–colloidal gold conjugates in a test tube, but not on the conjugate pad of the immune-strip, were premixed with toxin-containing sample solution before being applied to the sample pad of the immunostrip. This premixing procedure is extremely difficult to adapt for practical onsite analysis toxins from contaminated samples. Therefore, to overcome this limitation, in this study, we established an immune-strip with antibody–gold conjugates absorbed onto the conjugate pad of the strip, leading to high sensitivity and feasibility of one-step on-site detection of fumonisins. For one-step detection of fumonisins by immune-strip, toxins were extracted from food and feed samples efficiently using a mixed methanol/water solution (70:30 v/v). Nevertheless, more than 30 % (v/v) of methanol in the sample solution may lead to solvent interference in the immune-strip assay procedure. Therefore, the sample solution was diluted only two fold with PBS before being subjected to the strip test. Compared with the immune-strip, enzyme immunoassays (ELISA), which can only tolerate less than 10 % (v/v) of methanol in the sample, are more susceptible to solvent interference. The antibody–enzyme (HRP) conjugates used in ELISA may be more sensitive to the adverse effect of methanol, whereas the gold nanoparticles conjugated with a red colour are more tolerant to methanol, serving as a detection marker in the immune-strip.
Fig. 3.
Evaluation of the developed test strip on to cereal samples collected from different regions of Southern States of India. Note- Sample no- S1-S18 were positive for FB1 toxin; S19-S21 were negative for toxin contamination
Comparative evaluation of ICS test with HPLC
Further the results of developed method were confirmed with existing HPLC method for FB1 contamination. Results of FB1 analysis from contaminated samples obtained by the test strip were in a good agreement with those obtained from HPLC. This results show that the two methods adopted in this study corresponded well, and the ICA test gave neither false-positive nor false-negative results. In the earlier study Ramana et al. (2011, 2012), Chandra Nayaka et al. (2010) observed that the presence of fumonisins in maize and paddy samples of southern India. To extending the previous reports in the present study assessment of fumonisin contamination from other cereal grains such as sorghum and wheat were carried out among the major cereal grain growing regions of Southern states of India. The efficacy of immunochromatographic test for FB1 detection was confirmed by conducting HPLC. The HPLC retention time for the FB1 standard was found to be 20 min under the designated gradient elution. Out of 150 samples were analyzed 77 samples found to have FB1 contamination (Table 1) with varied levels of toxin concentrations among the studied regions. Among the samples were analyzed maize sample (TNM3) collected from Tamilnadu and paddy sample (KAP22) collected from Karnataka found to have maximum levels of FB1 contamination as 2,357 ng gm−1 and 2,746 ng gm−1 respectively. The minimum amount of FB1 concentrations were recorded ranging from 20 to 40 ng gm−1 in wheat (APW-7) and sorghum (APS-3), sample of Andhra Pradesh and maize (TNM6), paddy (TNP-8) from Tamilnadu as well as paddy sample of Karnataka (KAP-7). The recorded mean levels concentrations of FB1 from maize, wheat, sorghum and paddy was 1,257 ng gm−1, 942 ng gm−1, 536 ng gm−1 and 1,746 ng gm−1 respectively. Results showed that most of the studied cereal grain samples collected from Southern India was posing high levels of fumonisins contamination. It may be due to the several climatic factors influencing the growth of the fumonisin producing Fusarium species and the availability of the favorable conditions for production of large quantities of fumonisins in studied states of India.
Table 1.
Analysis of cereal samples for FB1 by Immuno chromatographic strip and HPLC methods
| S.No | Sample | Source | ICS analysis | Mean FB1 toxin concentration by HPLC (ng/gm) |
|---|---|---|---|---|
| 1 | Maize A1-A15 | Andhra Pradesh | A1, A2, A5, A6, A8, A10, A11, A13, A15 | 1,126 ± 3.5 |
| 2 | Maize A16-A35 | Karnataka | A18, A21, A24, A25, A27, A28, A29, A33, A35 | 1,345 ± 5.4 |
| 3 | Maize A36-A50 | Tamilnadu | A36, A37, A39, A40, A42, A45, A49, A50 | 1,301 ± 4.2 |
| 4 | Paddy-P1- P15 | Andhra Pradesh | P2, P3, P5, P7 P8 P11, P13 | 1,536 ± 4.7 |
| 5 | Paddy P16-P30 | Karnataka | P16, P17, P19, P22, P25, P28, P29 | 1,786 ± 3.2 |
| 6 | Paddy P31-P50 | Tamilnadu | P32, P33, P35, P37, P38, P41, P44, P45, P47, P49 | 1,916 ± 6.3 |
| 7 | Sorghum S1-S10 | Andhra Pradesh | S1, S4, S5, S7, S9, S10 | 653 ± 6.3 |
| 8 | Sorghum S11-S17 | Karnataka | S13, S14, S16 | 472 ± 5.5 |
| 9 | Sorghum S18-S25 | Tamilnadu | S18, S21, S25 | 483 ± 4.2 |
| 10 | Wheat W1-W10 | Andhra Pradesh | W1, W3, W4, W6, W8, W9 | 1,042 ± 6.5 |
| 11 | Wheat W11-W18 | Karnataka | W11, W13, W14, W16, W17 | 835 ± 3.1 |
| 12 | Wheat W19-W25 | Tamilnadu | W20, W21, W23, W25 | 949 ± 5.7 |
The present study was intended to develop a rapid and simple to use detection test for onsite detection of FB1 contamination in food samples intended for armed forces consumption in India. Hence, the present study, developed method was converted to a simple to use rapid dipstick test kit for detection of FB1 from food samples. Due to its simplicity, rapidity, cost effective nature, developed test is more use full in routine food testing laboratories in developing countries like India. Moreover, results obtained in present study are very much useful for the regulatory authorities or policy makers to make stringent regulatory limits of FB1 in foods and food grain matrices of Indian origin.
Conclusion
Fumonisin contamination is common in cereal based foods of developing countries like India. The developed method/test may find application in rapid detection of FB1 from contaminated cereals before entering in to the food chain to minimize the risk of Fumonisins to humans and other farm animals. Also, the developed system provides an accurate, rapid, sensitive detection of FB1. In addition to this developed method is free from enzymatic reaction leads to its more stability and increased storage time.
Acknowledgments
Authors are thankful to the Director, DFRL, Mysore, for his support to the present study.
Conflict of interest
Authors are declared that, there is no conflict of interest in present study.
References
- Abbas HK, Smeda RJ. Fumonisins B1 from the fungus Fusarium moniliforme causes contact toxicity in plants: evidence from studies with biosynthetically labeled toxin. J Nat Toxins. 2000;9:85–100. [PubMed] [Google Scholar]
- Azcona-Olivera JI, Abouzid MM, Plattner RD, Pestka JJ. Production of monoclonal antibodies to the mycotoxins fumonisin B1, B2, B3. J Agric Food Chem. 1992;40:531–534. doi: 10.1021/jf00015a034. [DOI] [Google Scholar]
- Barna-Vetro I, Szabo E, Fazekas B, Solti L. Development of a sensitive ELISA for the determination of fumonisin B1 in cereals. J Agric Food Chem. 2000;48:2821–2825. doi: 10.1021/jf990731m. [DOI] [PubMed] [Google Scholar]
- Baumrucker T, Prieschl EE. Sphingolipids and the regulation of immune response. Semin Immunol. 2002;14:57–63. doi: 10.1006/smim.2001.0342. [DOI] [PubMed] [Google Scholar]
- Chandra Nayaka S, Udaya Shankar AC, Niranjana SR, Ednar GW, Mortensen CN, Prakash HS. Detection and quantification of fumonisins from Fusarium verticillioides in maize grown in southern India. World J Microbiol Biotechnol. 2010;26:71–78. doi: 10.1007/s11274-009-0144-x. [DOI] [Google Scholar]
- Christopher P, Robinson N, Shaw MK (Eds) (2005) Antibody–label conjugates in lateral-flow assays. In: Forensic science and medicine: Drugs of abuse: body fluid
- Chu FS, Grossman S, Wei RD, Mirocha CJ. Production of antibody against T-2 toxin. Appl Environ Microbiol. 1979;37:104–108. doi: 10.1128/aem.37.1.104-108.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke JR, Marquardt RR, Oosterveld A, Frohlich AA, Madrid FJ, Dawood M. Development of a quantitative and sensitive enzyme-linked immunosorbent assay for ochratoxin A using antibodies from the yolk of the laying hen. J Agric Food Chem. 1993;41:1784–1789. doi: 10.1021/jf00034a050. [DOI] [Google Scholar]
- Council for Agriculture Science and Technology (2003) Mycotoxins risks in plant, animal, and human systems. Ames (IA): Council for Agriculture Science and Technology. Task Force Report No.139
- Desai K, Sullards MC, Allegood J, Wang E, Schmelz EM, Hartl M, Humpf HU, Liotta DC, Peng Q, Merril AH. Fumonisins and fumonisin analogs as inhibitors of ceramide synthase and inducers of apoptosis. Biochem Biophys Acta. 2002;1585:188–192. doi: 10.1016/s1388-1981(02)00340-2. [DOI] [PubMed] [Google Scholar]
- Domijan AM, Mili M, Peraica M. Fumonisin B1: oxidative status and DNA damage in rats. Toxicology. 2007;232:163–169. doi: 10.1016/j.tox.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Gelderblom WCA, Rheeder JP, Leggott N, Stockenstrom S, Humphreys J, Shephard GS, Marasas WFO. Fumonisin contamination of a corn samples associated with the induction of hepatocarcinogenesis in rat’s role of dietary deficiencies. Food Chem Toxicol. 2004;42:471–479. doi: 10.1016/j.fct.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Some traditional herbal medicines, some mycotoxins, naphthalene and styrene, vol. 82 of IARC monographs on the evaluation of carcinogenic risks to humans. Lyon: IARC Press; 2002. p. 301. [PMC free article] [PubMed] [Google Scholar]
- Kolosova AY, Sibanda L, Dumoulin F, Lewis J, Duveiller E, VanPeteghem C. Lateral-flow colloidal gold-based immunoassay for the rapid detection of deoxynivalenol with two indicator ranges. Anal Chim Acta. 2008;616:235–244. doi: 10.1016/j.aca.2008.04.029. [DOI] [PubMed] [Google Scholar]
- Kouadio JH, Mobio TA, Maudromont I, Moukha S, Dano SD, Crepp EE. Comparative study of cytotoxicity and oxidative stress induced by deoxynivalenol, zearalenone of fumonisin B1 in human intestinal cell line Caco-2. Toxicology. 2005;213:56–65. doi: 10.1016/j.tox.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Lee NA, Wang S, Allan RD, Kennedy IR. A rapid aflatoxin B1 ELISA: development and validation with reduced matrix effects for peanuts, corn, pistachio, and soybeans. J Agric Food Chem. 2004;52:2746–2755. doi: 10.1021/jf0354038. [DOI] [PubMed] [Google Scholar]
- Toxicology and carcinogenesis studies on fumonisin B1 in F344/N rats and B6CF1 mice (feed studies) Research Triangle Park: US Department of Health and Human Services, National Institutes of Health; 1999. [Google Scholar]
- Nelson PE, Toussoun TA, Marasas WFO. Fusarium species: an illustrated manual for identification. University Park: Pennsylvania State University Press; 1983. [Google Scholar]
- Paek SH, Lee SH, Cho JH, Kim YS. Development of rapid one-step immunochromatographic assay. Methods. 2000;22:53–60. doi: 10.1006/meth.2000.1036. [DOI] [PubMed] [Google Scholar]
- Quan Y, Zhang Y, Wang S, Lee N, Kennedy IR. A rapid and sensitive chemiluminescence enzyme-linked immunosorbent assay for the determination of fumonisin B1 in food samples. Anal Chim Acta. 2006;580:1–8. doi: 10.1016/j.aca.2006.07.063. [DOI] [PubMed] [Google Scholar]
- Ramana MV, Balakrishna K, Murali HS, Batra HV. Multiplex PCR-based strategy to detect contamination with mycotoxigenic Fusarium species in rice and finger millet collected from southern India. J Sci Food Agric. 2011;91:1666–1673. doi: 10.1002/jsfa.4365. [DOI] [PubMed] [Google Scholar]
- Ramana MV, Nayaka SC, Balakrishna K, Murali HS, Batra HV. A novel PCR–DNA probe for the detection of fumonisin-producing Fusarium species from major food crops grown in southern India. Mycology. 2012;3:167–174. [Google Scholar]
- Sashidhar RB, Capoor AK, Ramana D. Quantitation of Ɛ-amino group using mino acids as reference standards by trinitrobenzene sulfonic acid: a simple spectrophotometric method for the estimation of hapten to carrier protein ratio. J Immunol Methods. 1994;167:121–127. doi: 10.1016/0022-1759(94)90081-7. [DOI] [PubMed] [Google Scholar]
- Scott PM, Yeung JM, Lawrence GA, Prelusky DB. Evaluation of enzyme-linked immunosorbent assay for analysis of beer for fumonisins. Food Addit Contam. 1997;14:445–450. doi: 10.1080/02652039709374550. [DOI] [PubMed] [Google Scholar]
- Shier WT, Abbas HK, Badria FA. Complete structure of the sphingosine analog mycotoxins fumonisin B1 and AAL-toxin TA: absolute configuration of the side chains. Tetrahedron Lett. 1995;36:1571–1574. doi: 10.1016/0040-4039(95)00090-Y. [DOI] [Google Scholar]
- Shiu C-M, Wang J-J, Yu F-Y. Sensitive enzyme-linked immunosorbent assay and rapid one-step immunochromatographic strip for fumonisin B1 in grain-based food and feed samples. J Sci Food Agric. 2010;90:1020–1026. doi: 10.1002/jsfa.3911. [DOI] [PubMed] [Google Scholar]
- Sydenham EW, Shephard GS,Thiel PG (1992) Liquid chromatographic determination of fumonisins B1, B2, and B3 in foods and feeds. J AOAC International 75:313–317. [PubMed]
- US Food and Drug Administration, Center for Food Safety and Applied Nutrition, Fumonisin Levels in Human Foods and Animal Feeds (2001) Available: http://www.cfsan.fda.gov
- Usleber E, Straka M, Terplan G. Enzyme immunoassay for fumonisin B1 applied to cornbased food. J Agric Food Chem. 1994;42:1392–1396. doi: 10.1021/jf00042a027. [DOI] [Google Scholar]
- Wang S, Quan Y, Lee N, Kennedy IR. Rapid determination of fumonisin B1 in food samples by enzyme-linked immunosorbent assay and colloidal gold immunoassay. J Agric Food Chem. 2006;54:2491–2495. doi: 10.1021/jf0530401. [DOI] [PubMed] [Google Scholar]
- Yeung JM, Prelusky DB, Savard ME, Dang BDM, Robinson LA. Sensitive immunoassay for fumonisin B1 in corn. J Agric Food Chem. 1996;44:3582–3586. doi: 10.1021/jf960078s. [DOI] [Google Scholar]
- Yu FY, Chu FS. Production and characterization of antibodies against fumonisin B1. J Food Prot. 1996;59:992–997. doi: 10.4315/0362-028X-59.9.992. [DOI] [PubMed] [Google Scholar]