Abstract
Factors released by glioma-associated microglia/macrophages (GAMs) play an important role in the growth and infiltration of tumors. We have previously demonstrated that the co-chaperone stress-inducible protein 1 (STI1) secreted by microglia promotes proliferation and migration of human glioblastoma (GBM) cell lines in vitro. In the present study, in order to investigate the role of STI1 in a physiological context, we used a glioma model to evaluate STI1 expression in vivo. Here, we demonstrate that STI1 expression in both the tumor and in the infiltrating GAMs and lymphocytes significantly increased with tumor progression. Interestingly, high expression of STI1 was observed in macrophages and lymphocytes that infiltrated brain tumors, whereas STI1 expression in the circulating blood monocytes and lymphocytes remained unchanged. Our results correlate, for the first time, the expression of STI1 and glioma progression, and suggest that STI1 expression in GAMs and infiltrating lymphocytes is modulated by the brain tumor microenvironment.
Keywords: glioma, glioma-associated microglia/macrophages, stress-inducible protein 1, glioma progression, brain tumor microenvironment
Introduction
The prognosis of patients with glioblastoma (GBM), the most common primary brain tumor, remains dismal despite aggressive treatment. The high proliferative rate, intense invasiveness, and presence of the blood–brain barrier (BBB), which limits the penetration of large molecules into the central nervous system (CNS), all contribute to poor GBM response to conventional therapies [1]. Besides, the inflammatory function of the active mediators of the innate immune response, namely microglia (MG) and macrophages (MP), seems to be suppressed in gliomas and these cells may even promote tumor progression [2,3]. High infiltration of MG and MP (here referred to as glioma-associated microglia/macrophages or GAMs) into malignant gliomas [4,5], however, suggests that targeted therapies that modulate GAMs function may be an effective immunotherapeutic approach for GBM.
The co-chaperone stress inducible protein 1 (STI1) has been described as a ligand of the cellular prion protein (PrPC), which has several cellular functions and is highly expressed in the brain [6-9]. The interaction between these proteins promotes neuroprotection [9-11], neuronal survival [12], neuritogenesis [11,12], memory formation and consolidation [13], astrocytic survival and differentiation [14,15]. On the other hand, STI1 modulates the proliferation of both wild-type and PrPC-null astrocytes [14], and induces GBM proliferation in a PrPC-independent manner [16]. In fact, we were first to demonstrate a tumor promoting role of STI1 [17].
Recently, we demonstrated that STI1 released by microglia promotes tumor proliferation, modulates MMP-9 activity and favors the migration of human GBM cell lines in vitro [16]. These observations raised the question of the role of STI1 in a physiological context. In order to investigate this, we used a glioma model to evaluate the expression of STI1 in vivo. Here, we show that STI1 expression increases with tumor progression, and is also upregulated in GAMs and infiltrating lymphocytes. In contrast, STI1 expression did not significantly change in circulating leukocytes, and even decreased in leukocytes that infiltrated tumors propagated in the subcutaneous tissue. These observations suggest that STI1 expression is modulated by brain tumor microenvironment.
Materials and Methods
Cell Culture
Luciferase-expressing GL261 murine glioma cell line (GL261-ffluc) was generated as described before [18] and was cultured in Dulbecco's Modified Eagle Medium supplemented with 10% fetal bovine serum, penicillin (100 units/mL), and streptomycin (100 μg/mL) at 37°C in a humidified 5% CO2 atmosphere.
Tumor Implantation
All animals were housed and handled in accordance to the guidelines of City of Hope Institutional Animal Care and Use Committee (IACUC, California, USA). Intracranial tumor implantation was performed as described previously [19]. GL261-ffluc cells were harvested by trypsinization, counted, and resuspended in PBS. Female C57BL/6 or CX3CR1GFP mice that express eGFP under control of the endogenous Cx3cr1 locus (Jackson Laboratory) weighing 15-25 g were anesthetized by intraperitoneal (i.p.) administration of ketamine (132mg/kg) and xylazine (8.8mg/kg), and immobilized in a stereotactic head frame. Through a small burr hole, 3 μl of PBS containing 1 × 105 tumor cells was injected unilaterally as described before [19]. Subcutaneous tumors were generated by injecting 100 μl of PBS containing 1 × 106 tumor cells [20]. Tumor growth was assessed by a Xenogen IVIS In Vivo Imaging System (Xenogen) as previously described [21].
Flow Cytometry Analysis
Brain tumors, subcutaneous tumors and blood samples were harvested and examined by flow cytometry as described previously [18]. Cell suspensions from brain and subcutaneous tissue were forced through a 40-μm filter. Blood samples were incubated in Gey's buffer (pH 7.2) for 10 minutes. For extracellular staining of immune markers, freshly prepared samples were resuspended in 0.1 mol/L PBS containing 1% fetal bovine serum and 2 mmol/L EDTA and incubated with Purified Rat Anti-Mouse CD16/CD32 (BD Pharmingen; #553141) to prevent nonspecific binding. Samples were then stained with CD11b (1:100 dilution; eBioscience; #17-0112-82) and CD45 (1:100 dilution; BD Pharmingen; #557235) antibodies for 15 minutes at 4°C. For intracellular staining, cells were fixed in 4% paraformaldehyde and permeabilized in BD Cytofix/Cytoperm Buffer before incubation with STI1 antibody (1:50 dilution; Abgent; #AP2817b) for 45 minutes at 4°C. Samples were washed with PBS containing 1% bovine serum albumin three times for 5 min and incubated with secondary antibody (1:100 dilution; Santa Cruz; #sc-2012). Fluorescence data were collected on a CyAn fluorescence cell sorter (BDIS). Inflammatory cells were gated base on forward vs. side-scatter analysis and their staining characteristics. FlowJo 9.0 software (Tree Star, Inc.) was used for data analysis. Glioma macrophages (MPs) were gated as CD11b+/CD45high, microglia (MG) as CD11b+/CD45low and lymphocytes as CD11b−/CD45+ on the basis of previously described phenotype characterization [22].
Immunofluorescence Staining
Frozen brain sections were prepared from tumor-bearing mice. Brains were embedded in O.C.T. (Tissue-Tek) and 10-μm sections were cut using cryostat (Leica Microsystem, Bannockburn, IL). Prior to immunofluorescence staining, slides were baked in 37°C and washed with TBS containing 0.05% Triton X-100 (TBS-T) three times for 5 min. After 1 h blocking, the slides were incubated with CD11b antibody (1:10 dilution; BD Pharmingen; #550282) for 1h at room temperature and, after washed with TBS-T three times for 5 min, were incubated with the secondary antibody Alexa Fluor 647 goat anti-rat IgG. Slides were washed with TBS-T three times for 5 min and incubated with STI1 antibody (1:50 dilution; Abgent; #AP2817b) overnight at 4°C followed by three washes with TBS-T and incubation with the secondary antibody Alexa Fluor 555 goat anti-rabbit IgG for another hour. Sections were mounted in Vectashield mounting medium containing 4060-diamidino-2- phenylindole (DAPI) (Vector, Burlingame, CA). Images were obtained by AX-70 fluorescence microscopy (Leica Microsystems, Bannockburn, IL) and were prepared by Image-Pro Plus 6.3 software (Media Cybernetics, Inc.) [18].
Western Blotting
Cells were lysed on the plate (six well plate) with 50 μL lysis buffer (1% Triton X-100, 10% glycerol, 50 mM HEPES pH 7.5, 1 mM EGTA, 150 mM NaCl, 1.5 mM MgCl2, 50 mM sodium fluoride, 1 mM sodium vanadate, 1 mM PMSF, 200 μg mL−1 aprotinin, and 50 μg mL−1 leupeptin). Lysates were cleared by centrifugation at 12,000g and protein concentration was determined with the BioRad protein assay using BSA as standard. Equal amounts of protein were separated on 12.5% SDS-PAGE gels, transferred to PVDF membrane (Millipore Bedford, MA) and probed with primary antibody specific for STI1 (1:500 dilution; Santa Cruz; #sc-134043) followed by detection using the ECL system (Millipore Bedford, MA) [19].
Statistical Analysis
The data were analyzed by ANOVA followed by post hoc comparisons (Tukey's test).
Results
STI1 expression is upregulated in brain tumors, GAMs and infiltrating lymphocytes
To analyze the expression of STI1 in vivo, C57BL/6 mice were inoculated intracranially with glioma GL261 cells and the expression of STI1 was verified at different time points by Western blotting. Tumor growth was monitored by luciferase activity (Figure 1A). As tumors grew (Figure 1B), the relative expression of STI1 also increased (Figure 1 C). Although most of the STI1 expression was seen in tumor cells, some STI1 was also noted in GAMs (Figure 1D, inset).
Figure 1. STI1 expression increases with tumor progression.
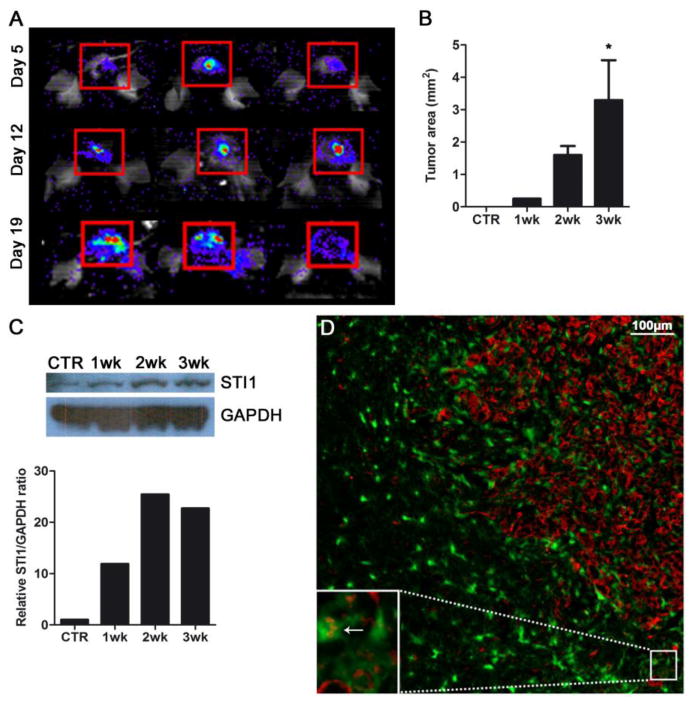
(A) Representative Xenogen images of three mice 5, 12 and 19 days after tumor implantation. (B) Graph illustrating the tumor size of samples used for STI1 analysis; n=4 animals per group; * p < 0.05. (C) Western blot of normal brain (CTR) and brain tumors 1, 2 and 3 weeks after intracranial injection. All samples probed with anti-STI1 antibody showed a band at the expected molecular weight (66 kDa). (D) A two-week old GL261 glioma in a CX3CR1GFP mouse was immunolabeled with anti-STI1 antibody (red) demonstrating the expression of STI1 by tumor and GAMs (inset, arrow); nuclei were stained with DAPI (blue). Bar, 100 μm. Data is representative of two separate experiments.
To confirm if GAMs and/or infiltrating lymphocytes expressed STI1 (besides the tumor cells themselves), we analyzed their STI1 levels at different time points during tumor progression. Flow cytometry showed that the percentage of STI1-expressing MG, MPs and lymphocytes significantly increased 2 and 3 weeks after tumor inoculation (Figure 2A, B, D and F). Similarly, the mean fluorescence intensity of STI1 followed this trend, showing that STI1 was upregulated in individual cells, as well as in the entire tumor (Figure 2C, E and G). This observation confirms a direct correlation between STI1 expression and glioma progression.
Figure 2. STI1 expression in GAMs and infiltrating lymphocytes is upregulated in tumor-bearing brain.
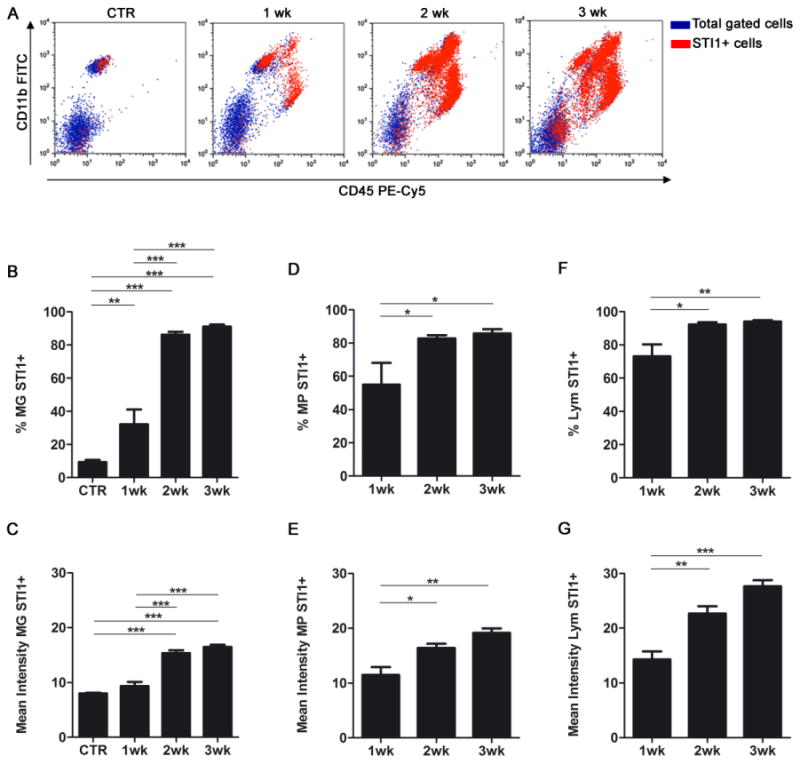
(A) Flow cytometry was performed to identify STI1–positive cells after intracranial inoculation of GL261 gliomas. Representative dot plots demonstrating increase in STI1 expression (red events) in tumor macrophages (MP; CD11b+/CD45high), microglia (MG; CD11b+/CD45low), and lymphocytes (Lym; CD11b−/CD45+) after gating the leukocyte population. Quantification of the percentage of STI1–positive cells (B, D and F) and the mean fluorescence intensity (C, E and G) by cell population; n=4 animals per group; * p < 0.05, ** p < 0.01, *** p < 0.001. Data is representative of two separate experiments.
To evaluate if STI1 upregulation in GAMs and lymphocytes was caused by local tumor microenvironment, we also analyzed STI1 expression in circulating monocytes and lymphocytes in the same animals. Despite a modest change in the STI1 mean fluorescence intensity, the percentage of STI1-positive cells in the circulation remained low (Figure 3), indicating that modulation of STI1 expression in brain tumor inflammatory cells was mostly due to local tumor factors and not systemic promotion of STI1 expression in circulating leukocytes that migrated into brain tumors.
Figure 3. STI1 expression in circulating blood monocytes and lymphocytes in glioma-bearing mice.
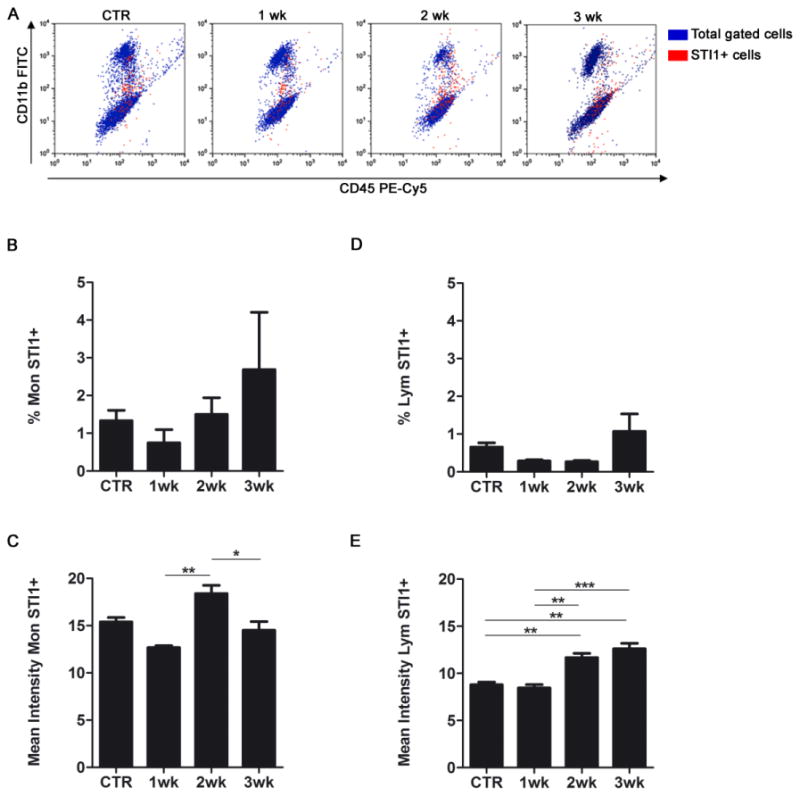
(A) Flow cytometry was performed to identify STI1–positive cells (red events) in blood after intracranial GL261 glioma inoculation. Representative dot plots demonstrating the expression of STI1 in monocytes (Mon; CD11b+/CD45high) and lymphocytes (Lym; CD11b−/CD45+) after gating the CD45+ (leukocyte) population. Quantification of the percentage of STI1–positive cells (B and D) and the mean fluorescence intensity (C and E) by cell type; n=4 animals per group; * p < 0.05, ** p < 0.01, *** p < 0.001. Data is representative of two separate experiments.
STI1 expression in GAMs and infiltrating lymphocytes is modulated by the brain tumor microenvironment
To evaluate if upregulation of STI1 was dependent on the location of the tumor, the expression of STI1 in MPs, MG and lymphocytes was analyzed in GL261 tumors that were either inoculated intracranially or subcutaneously (Figure 4). Flow cytometry analysis confirmed that STI1 expression in the circulating monocytes and lymphocytes was not altered during tumor progression either in the brain or subcutaneous tissue (Figure 4D-G, blue lines). Furthermore, during brain tumor progression, the expression of STI1 was upregulated in GAMs and lymphocytes as before (Figure 4B, D and F). In contrast, during the progression of subcutaneous tumors, the expression of STI1 was slightly downregulated in macrophages and lymphocytes (Figure 4E and G, green lines). These observations strongly suggest that brain-specific microenvironmental factors were responsible for STI1 upregulation in infiltrating GAMs and lymphocytes in brain tumors.
Figure 4. STI1 expression in GAMs and infiltrating lymphocytes is modulated by the brain tumor microenvironment.
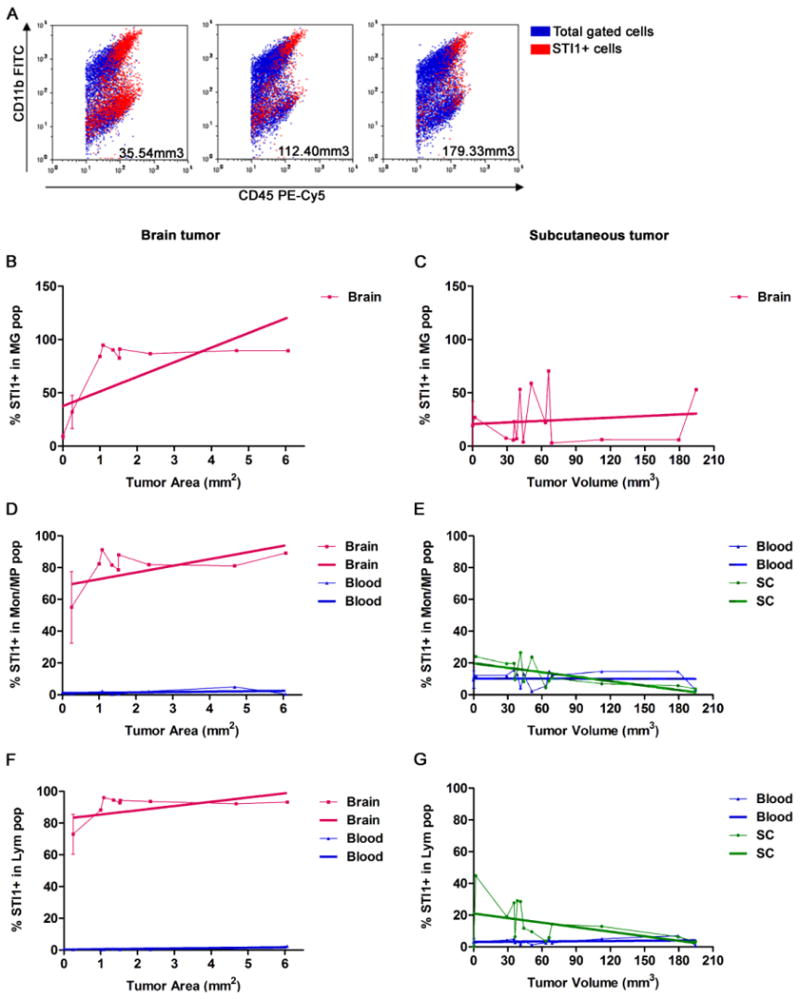
(A) Flow cytometry wasperformed to identify STI1–positive inflammatory cells in GL261 tumors propagated in either the brain or subcutaneous tissue. Representative dot plots demonstrating the expression of STI1 in monocytes/macrophages (Mon/MP; CD11b+/CD45high) and lymphocytes (Lym; CD11b−/CD45+) after gating the CD45+ (leukocyte) population. The size of the tumors is identified in each dot plot. B, D and F: brain tumors (n=15 mice in total); C, E and G: subcutaneous tumors (n=13 mice in total). Y axis: percentage of microglia (MG), monocytes/macrophages (Mon/MP) and lymphocytes (Lym) positives for STI1; X axis: tumor area (mm2) or volume (mm3). Brain, blood and subcutaneous tissue (SC) were analyzed. The thicker lines are the result of a linear regression from each of the curves.
Discussion
Glioma-associated microglia/macrophages compose approximately 30% of tumor inflammatory cells and participate in glioma growth, invasion, angiogenesis and local immunosuppression [3]. We have previously demonstrated that the co-chaperone STI1 is expressed and released by MG and promotes proliferation and migration of human GBM cell lines in vitro [16]. STI1 had already been related to neuronal and astrocytic survival [12,14], among other important biological effects. This study investigated STI1 expression in an in vivo glioma model, and demonstrated that STI1 expression is upregulated with tumor progression, and its expression is also increased in GAMs and infiltrating lymphocytes.
In contrast with the traditionally accepted notion that the BBB restricts invasion of inflammatory cells into the CNS, it is now believed that continuous surveillance of CNS tissue takes place by both resident MG and migrating lymphocytes [23,24]. Furthermore, under pathologic conditions, such as inflammation, autoimmune diseases, and cancers, immune-competent cells and monocytes can readily cross the disrupted BBB to participate in repair or exacerbate injury [24,25].
STI1 expression has been correlated with tumor progression in a few types of cancer, such as ovary [26] and pancreas [27]. In our model, the analysis of brain tumors at different time points showed that larger gliomas expressed higher levels of STI1. Moreover, the specific characterization of STI1 expression in GAMs and lymphocyte populations by flow cytometry revealed a significant increase in the expression of this protein when tumors grew larger. Although STI1 expression by MG and MPs as detected by immunohistochemistry was not as strong as tumor cells, and possibly masked by the GFP signal, flow cytometry confirmed the expression of STI1 expression by these cells in brain tumors. To the best of our knowledge, we are the first to show STI1 expression in these leukocytes populations and to directly correlate its expression to glioma progression.
Brain microenvironment has significant impact over glioma growth and invasion. Several cell types, such as astrocytes and endothelial cells, interact with tumor cells possibly favoring its progression [2,28]. Our results show higher expression of STI1 in brain tumor-infiltrating monocytes and lymphocytes compared to the same cells circulating in the blood. In contrast, the expression of STI1 in MPs and lymphocytes in subcutaneous tumors did not increase with tumor progression as it did in brain tumors. Although the mechanism responsible for this differential STI1 expression in tumor-associated inflammatory cells is unclear, it appeared that the brain milleu, rather than the glioma itself, directly influenced the STI1 expression during tumor progression.
The exact mechanism by which STI1 was upregulated in gliomas was not addressed here. One possibility is that STI1 increased expression in GAMs due to its co-chaperone role, since GAMs are (alternatively) activated in the tumor microenvironment [3,29,30] and therefore express a variety of proteins that are not detectable in resident MG [31]. Further, STI1 is highly expressed in the CNS [32] and can be secreted [12,17,33]. It is possible that the presence of STI1 in the brain tumor microenvironment induces more STI1 expression, a well-known feed-forward process by which several growth factors, transcriptional factors and hormones regulate their own synthesis [34-37].
Based on our present and previous study [16], we hypothesize that the upregulation of STI1 increases its release into the microenvironment and consequently enhance tumor growth and invasion. STI1 is a ligand of the cellular prion protein (PrPc) [7,9] that is also expressed by glioma cells [16]. PrPc is a GPI-anchored receptor with several biological effects, yet its signaling is not fully understood. It is suggested that PrPc acts in a dynamic cell surface platform for the assembly of signaling molecules [6]. But, the fact that STI1 increases the proliferation of GBM cells in a PrPc-independent manner may also suggest that its binding to PrPc may not be necessary, and other receptors may be involved in STI1-mediated cell proliferation [16].
The role of the tumor microenvironment has been a major focus of research recently. Particularly in gliomas, the influence of stromal cells on tumor progression is being actively investigated [1,2,28]. Considering that the STI1 protein has recently been associated with other cancers (such as ovary [26,38,39], colon [40] and pancreatic [27,41], confirming its function in gliomagenesis is warranted and developing inhibitors against this protein may have potential therapeutic value against GBM.
Highlights.
We evaluated STI1 expression in a glioma model.
STI1 expression significantly increased with tumor progression.
We correlate, for the first time, the expression of STI1 and glioma progression.
We suggest that STI1 expression is modulated by the brain tumor microenvironment.
Acknowledgments
Grant Support: This work was supported by R01CA155769, James S. McDonnell Foundation and ThinkCure Foundation. The City of Hope Flow Cytometry Core was equipped in part through funding provided by ONR N00014-02-1 0958, DOD 1435-04-03GT-73134, and NSF DBI-9970143.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lima FR, et al. Glioblastoma: therapeutic challenges, what lies ahead. Biochim Biophys Acta. 2012;1826:338–49. doi: 10.1016/j.bbcan.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Charles NA, Holland EC, Gilbertson R, Glass R, Kettenmann H. The brain tumor microenvironment. Glia. 2011;59:1169–80. doi: 10.1002/glia.21136. [DOI] [PubMed] [Google Scholar]
- 3.Fonseca ACC, Badie B. Microglia and Macrophages in Malignant Gliomas: Recent Discoveries and Implications for Promising Therapies. Clin Dev Immunol. 2013 doi: 10.1155/2013/264124. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Badie B, Schartner JM. Flow cytometric characterization of tumor-associated macrophages in experimental gliomas. Neurosurgery. 2000;46:957–61. doi: 10.1097/00006123-200004000-00035. discussion 961-2. [DOI] [PubMed] [Google Scholar]
- 5.Graeber MB, Scheithauer BW, Kreutzberg GW. Microglia in brain tumors. Glia. 2002;40:252–9. doi: 10.1002/glia.10147. [DOI] [PubMed] [Google Scholar]
- 6.Linden R, Martins VR, Prado MA, Cammarota M, Izquierdo I, Brentani RR. Physiology of the prion protein. Physiol Rev. 2008;88:673–728. doi: 10.1152/physrev.00007.2007. [DOI] [PubMed] [Google Scholar]
- 7.Martins VR, et al. Complementary hydropathy identifies a cellular prion protein receptor. Nat Med. 1997;3:1376–82. doi: 10.1038/nm1297-1376. [DOI] [PubMed] [Google Scholar]
- 8.Prusiner SB. Prions. Proc Natl Acad Sci U S A. 1998;95:13363–83. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zanata SM, et al. Stress-inducible protein 1 is a cell surface ligand for cellular prion that triggers neuroprotection. EMBO J. 2002;21:3307–16. doi: 10.1093/emboj/cdf325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiarini LB, Freitas AR, Zanata SM, Brentani RR, Martins VR, Linden R. Cellular prion protein transduces neuroprotective signals. EMBO J. 2002;21:3317–26. doi: 10.1093/emboj/cdf324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lopes MH, et al. Interaction of cellular prion and stress-inducible protein1 promotes neuritogenesis and neuroprotection by distinct signaling pathways. J Neurosci. 2005;25:11330–9. doi: 10.1523/JNEUROSCI.2313-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lima FR, Arantes CP, Muras AG, Nomizo R, Brentani RR, Martins VR. Cellular prion protein expression in astrocytes modulates neuronal survival and differentiation. J Neurochem. 2007;103:2164–76. doi: 10.1111/j.1471-4159.2007.04904.x. [DOI] [PubMed] [Google Scholar]
- 13.Coitinho AS, et al. Short-term memory formation and long-term memory consolidation are enhanced by cellular prion association to stress-inducible protein 1. Neurobiol Dis. 2007;26:282–90. doi: 10.1016/j.nbd.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Arantes C, Nomizo R, Lopes MH, Hajj GN, Lima FR, Martins VR. Prion protein and its ligand stress inducible protein 1 regulate astrocyte development. Glia. 2009;57:1439–49. doi: 10.1002/glia.20861. [DOI] [PubMed] [Google Scholar]
- 15.Hartmann CA, Martins VR, Lima FR. High levels of cellular prion protein improve astrocyte development. FEBS Lett. 2013;587:238–44. doi: 10.1016/j.febslet.2012.11.032. [DOI] [PubMed] [Google Scholar]
- 16.Fonseca AC, et al. Microglial stress inducible protein 1 promotes proliferation and migration in human glioblastoma cells. Neuroscience. 2012;200:130–41. doi: 10.1016/j.neuroscience.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 17.Erlich RB, et al. STI1 promotes glioma proliferation through MAPK and PI3K pathways. Glia. 2007;55:1690–8. doi: 10.1002/glia.20579. [DOI] [PubMed] [Google Scholar]
- 18.Zhang L, Liu W, Alizadeh D, Zhao D, Farrukh O, Lin J, Badie SA, Badie B. S100B attenuates microglia activation in gliomas: possible role of STAT3 pathway. Glia. 2011;59:486–98. doi: 10.1002/glia.21118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang L, Alizadeh D, Van Handel M, Kortylewski M, Yu H, Badie B. Stat3 inhibition activates tumor macrophages and abrogates glioma growth in mice. Glia. 2009;57:1458–67. doi: 10.1002/glia.20863. [DOI] [PubMed] [Google Scholar]
- 20.Fan H, et al. Intracerebral CpG immunotherapy with carbon nanotubes abrogates growth of subcutaneous melanomas in mice. Clin Cancer Res. 2012;18:5628–38. doi: 10.1158/1078-0432.CCR-12-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao D, Alizadeh D, Zhang L, Liu W, Farrukh O, Manuel E, Diamond DJ, Badie B. Carbon nanotubes enhance CpG uptake and potentiate antiglioma immunity. Clin Cancer Res. 2011;17:771–82. doi: 10.1158/1078-0432.CCR-10-2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Badie B, Bartley B, Schartner J. Differential expression of MHC class II and B7 costimulatory molecules by microglia in rodent gliomas. J Neuroimmunol. 2002;133:39–45. doi: 10.1016/s0165-5728(02)00350-8. [DOI] [PubMed] [Google Scholar]
- 23.Galea I, Bechmann I, Perry VH. What is immune privilege (not)? Trends Immunol. 2007;28:12–8. doi: 10.1016/j.it.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 24.Wraith DC, Nicholson LB. The adaptive immune system in diseases of the central nervous system. J Clin Invest. 2012;122:1172–9. doi: 10.1172/JCI58648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Djukic M, Mildner A, Schmidt H, Czesnik D, Bruck W, Priller J, Nau R, Prinz M. Circulating monocytes engraft in the brain, differentiate into microglia and contribute to the pathology following meningitis in mice. Brain. 2006;129:2394–403. doi: 10.1093/brain/awl206. [DOI] [PubMed] [Google Scholar]
- 26.Chao A, et al. Tumor stress-induced phosphoprotein1 (STIP1) as a prognostic biomarker in ovarian cancer. PLoS One. 2013;8:e57084. doi: 10.1371/journal.pone.0057084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walsh N, Larkin A, Swan N, Conlon K, Dowling P, McDermott R, Clynes M. RNAi knockdown of Hop (Hsp70/Hsp90 organising protein) decreases invasion via MMP-2 down regulation. Cancer Lett. 2011;306:180–9. doi: 10.1016/j.canlet.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 28.Alves TR, Lima FR, Kahn SA, Lobo D, Dubois LG, Soletti R, Borges H, Neto VM. Glioblastoma cells: a heterogeneous and fatal tumor interacting with the parenchyma. Life Sci. 2011;89:532–9. doi: 10.1016/j.lfs.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 29.Gabrusiewicz K, Ellert-Miklaszewska A, Lipko M, Sielska M, Frankowska M, Kaminska B. Characteristics of the alternative phenotype of microglia/macrophages and its modulation in experimental gliomas. PLoS One. 2011;6:e23902. doi: 10.1371/journal.pone.0023902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Komohara Y, Ohnishi K, Kuratsu J, Takeya M. Possible involvement of the M2 anti-inflammatory macrophage phenotype in growth of human gliomas. J Pathol. 2008;216:15–24. doi: 10.1002/path.2370. [DOI] [PubMed] [Google Scholar]
- 31.Li W, Graeber MB. The molecular profile of microglia under the influence of glioma. Neuro Oncol. 2012;14:958–78. doi: 10.1093/neuonc/nos116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hajj GN, Santos TG, Cook ZS, Martins VR. Developmental expression of prion protein and its ligands stress-inducible protein 1 and vitronectin. J Comp Neurol. 2009;517:371–84. doi: 10.1002/cne.22157. [DOI] [PubMed] [Google Scholar]
- 33.Eustace BK, et al. Functional proteomic screens reveal an essential extracellular role for hsp90 alpha in cancer cell invasiveness. Nat Cell Biol. 2004;6:507–14. doi: 10.1038/ncb1131. [DOI] [PubMed] [Google Scholar]
- 34.Cargnin F, Flora A, Di Lascio S, Battaglioli E, Longhi R, Clementi F, Fornasari D. PHOX2B regulates its own expression by a transcriptional auto-regulatory mechanism. J Biol Chem. 2005;280:37439–48. doi: 10.1074/jbc.M508368200. [DOI] [PubMed] [Google Scholar]
- 35.Leibiger B, Moede T, Muhandiramlage TP, Kaiser D, Vaca Sanchez P, Leibiger IB, Berggren PO. Glucagon regulates its own synthesis by autocrine signaling. Proc Natl Acad Sci U S A. 2012;109:20925–30. doi: 10.1073/pnas.1212870110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Obberghen-Schilling E, Roche NS, Flanders KC, Sporn MB, Roberts AB. Transforming growth factor beta 1 positively regulates its own expression in normal and transformed cells. J Biol Chem. 1988;263:7741–6. [PubMed] [Google Scholar]
- 37.Cheng PL, Song AH, Wong YH, Wang S, Zhang X, Poo MM. Self-amplifying autocrine actions of BDNF in axon development. Proc Natl Acad Sci U S A. 2011;108:18430–5. doi: 10.1073/pnas.1115907108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang TH, et al. Stress-induced phosphoprotein 1 as a secreted biomarker for human ovarian cancer promotes cancer cell proliferation. Mol Cell Proteomics. 2010;9:1873–84. doi: 10.1074/mcp.M110.000802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim S, Cho H, Nam EJ, Kim SW, Kim YT, Park YW, Kim BW, Kim JH. Autoantibodies against stress-induced phosphoprotein-1 as a novel biomarker candidate for ovarian cancer. Genes Chromosomes Cancer. 2010;49:585–95. doi: 10.1002/gcc.20769. [DOI] [PubMed] [Google Scholar]
- 40.Kubota H, et al. Increased expression of co-chaperone HOP with HSP90 and HSC70 and complex formation in human colonic carcinoma. Cell Stress Chaperones. 2010;15:1003–11. doi: 10.1007/s12192-010-0211-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walsh N, O'Donovan N, Kennedy S, Henry M, Meleady P, Clynes M, Dowling P. Identification of pancreatic cancer invasion-related proteins by proteomic analysis. Proteome Sci. 2009;7:3. doi: 10.1186/1477-5956-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


