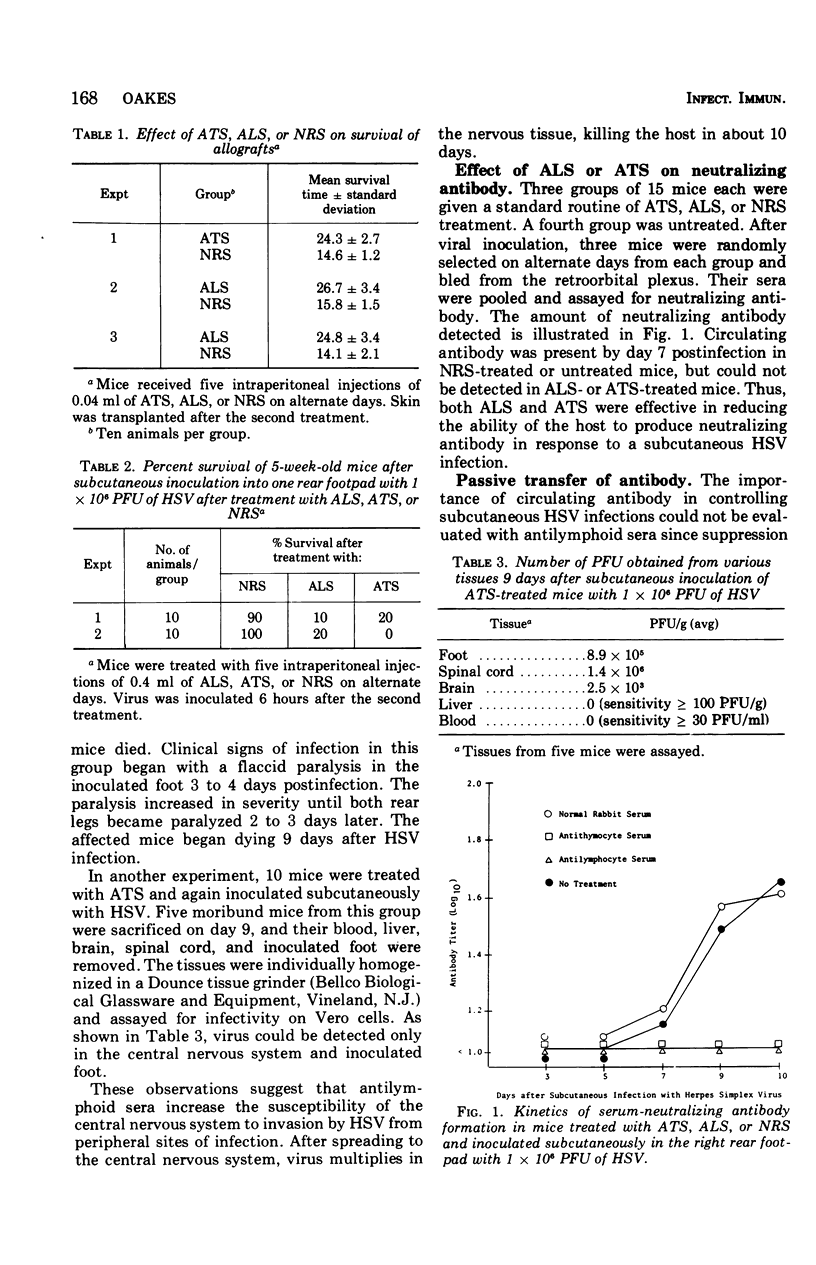
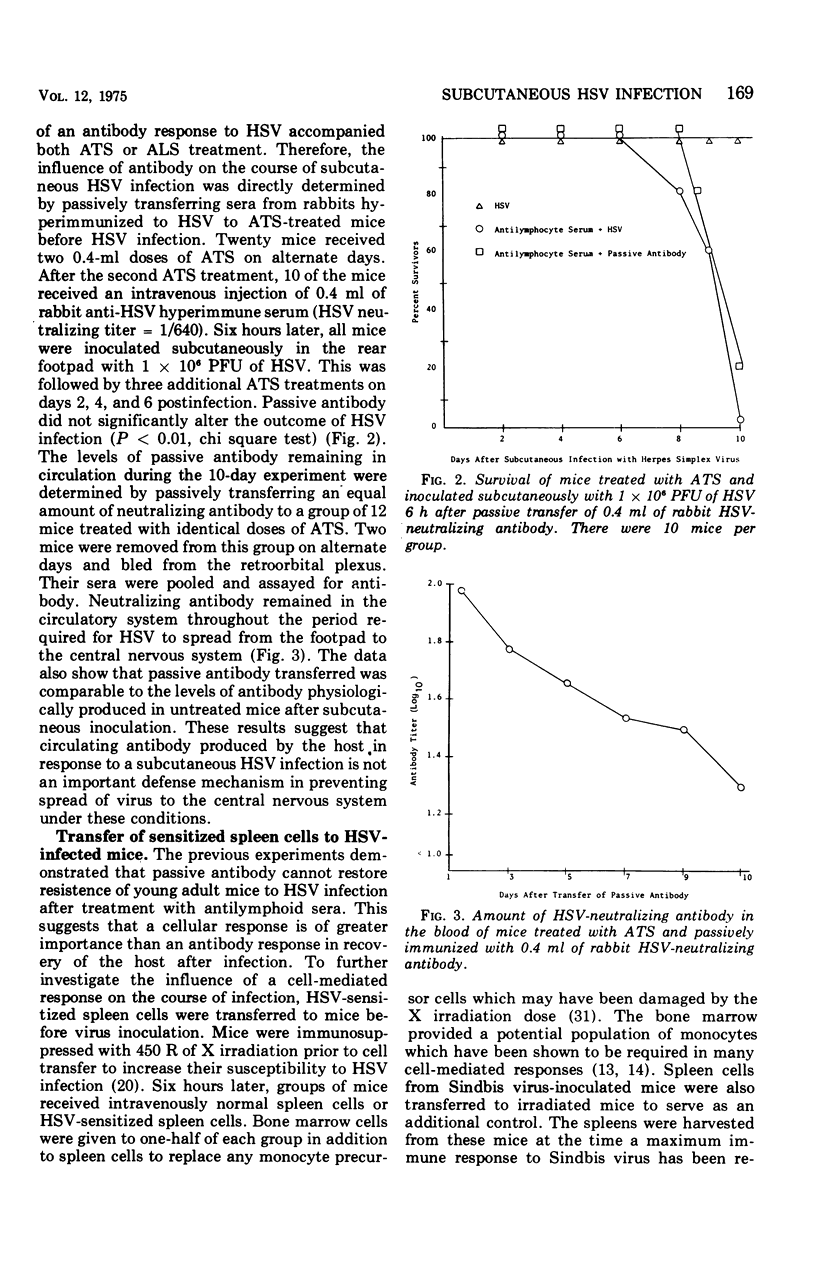
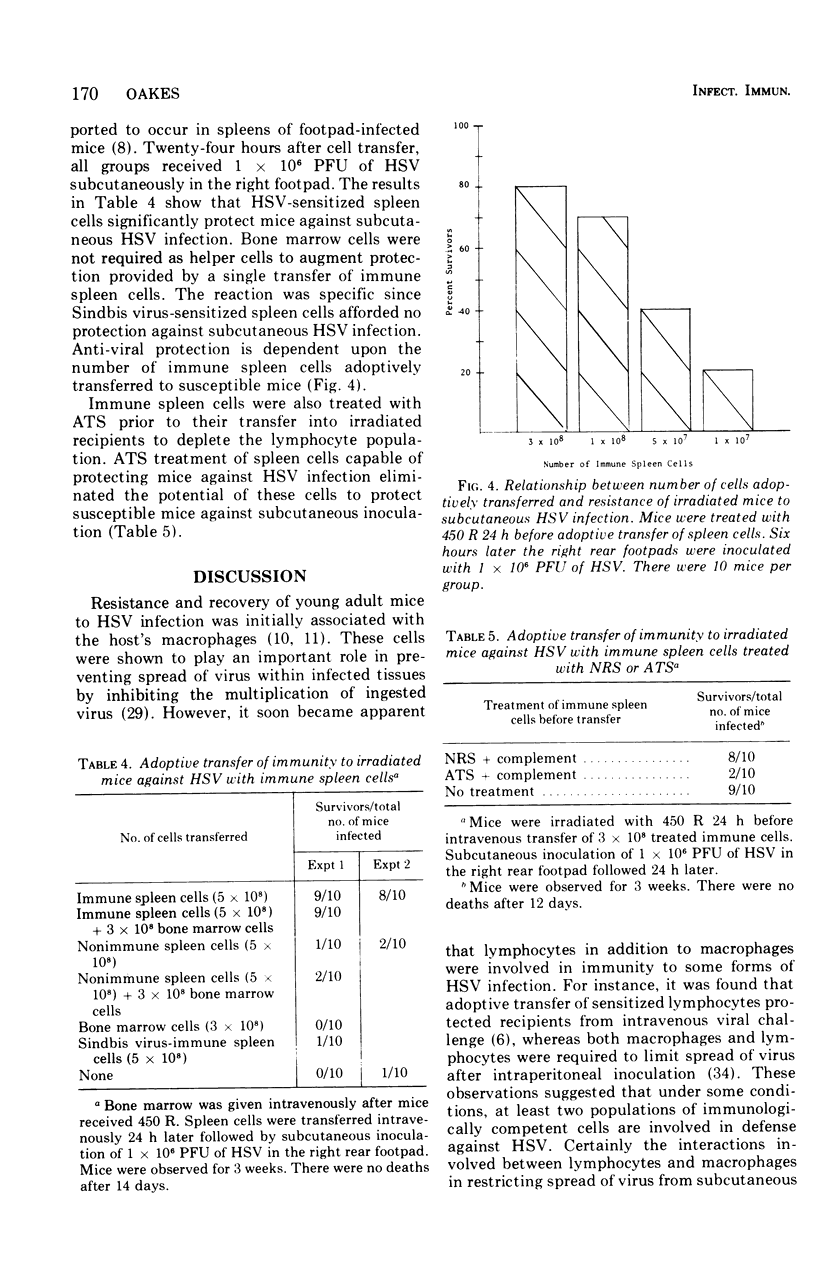
Abstract
The role of cell-mediated immunity in the resistance of young adult mice to subcutaneous herpes simplex virus (HSV) type I infection was studied in mice receiving immunosuppressive doses of antilymphocyte sera (ALS) or antithymocyte sera (ATS). The effectiveness of these treatments to reduce cell-mediated responses was measured by their ability to prolong the life of allografts transplanted to ALS- or ATS-treated mice. It was found that subcutaneous infection of these mice with HSV resulted in spread of virus from the site of inoculation to the central nervous system. Neutralizing antibody could not be detected in the sera of ALS- or ATS-treated mice after HSV inoculation. Passive transfer of neutralizing antibody to ATS-treated mice did not restore resistance to subcutaneous HSV infection. However, adoptive transfer of HSV-sensitized spleen cells did provide significant protection against infection unless the spleen cells were treated with ATS prior to transfer. These experiments suggest that lymphocytes are involved in a cell-mediated response to subcutaneous HSV infection and demonstrate the importance of a noncompromised immune response in controlling spread of HSV from localized areas of infection.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blanden R. V. Mechanisms of recovery from a generalized viral infection: mousepox. II. Passive transfer of recovery mechanisms with immune lymphoid cells. J Exp Med. 1971 May 1;133(5):1074–1089. doi: 10.1084/jem.133.5.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brier A. M., Wohlenberg C., Rosenthal J., Mage M., Notkins A. L. Inhibition or enhancement of immunological injury of virus-infected cells. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3073–3077. doi: 10.1073/pnas.68.12.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas R. G., Jr, Couch R. B. A prospective study of chronic herpes simplex virus infection and recurrent herpes labialis in humans. J Immunol. 1970 Feb;104(2):289–295. [PubMed] [Google Scholar]
- Ennis F. A. Host defense mechanisms against Herpes simplex virus. I. Control of infection in vitro by senstized spleen cells and antibody. Infect Immun. 1973 Jun;7(6):898–904. doi: 10.1128/iai.7.6.898-904.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis F. A. Host defense mechanisms against herpes simplex virus. II. Protection conferred by sensitized spleen cells. J Infect Dis. 1973 Jun;127(6):632–638. doi: 10.1093/infdis/127.6.632. [DOI] [PubMed] [Google Scholar]
- Freedman L. R., Cerottini J. C., Brunner K. T. In vivo studies of the role of cytotoxic T cells in tumor allograft immunity. J Immunol. 1972 Dec;109(6):1371–1378. [PubMed] [Google Scholar]
- Griffin D. E., Johnson R. T. Cellular immune response to viral infection: in vitro studies of lymphocytes from mice infected with Sindbis virus. Cell Immunol. 1973 Dec;9(3):426–434. doi: 10.1016/0008-8749(73)90057-9. [DOI] [PubMed] [Google Scholar]
- Hampar B., Notkins A. L., Mage M., Keehn M. A. Heterogeneity in the properties of 7 S and 19S rabbit-neutralizing antibodies to herpes simplex virus. J Immunol. 1968 Mar;100(3):586–593. [PubMed] [Google Scholar]
- Hirsch M. S., Zisman B., Allison A. C. Macrophages and age-dependent resistance to Herpes simplex virus in mice. J Immunol. 1970 May;104(5):1160–1165. [PubMed] [Google Scholar]
- JOHNSON R. T. THE PATHOGENESIS OF HERPES VIRUS ENCEPHALITIS. II. A CELLULAR BASIS FOR THE DEVELOPMENT OF RESISTANCE WITH AGE. J Exp Med. 1964 Sep 1;120:359–374. doi: 10.1084/jem.120.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane F. C., Unanue E. R. Requirement of thymus (T) lymphocytes for resistance to listeriosis. J Exp Med. 1972 May 1;135(5):1104–1112. doi: 10.1084/jem.135.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubaroff D. M., Waksman B. H. Bone marrow as a source of cells in reactions of cellular hypersensitivity. I. Passive transfer of tuberculin sensitivity in syngeneic systems. J Exp Med. 1968 Dec 1;128(6):1425–1435. doi: 10.1084/jem.128.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCCULLOCH E. A., TILL J. E. The radiation sensitivity of normal mouse bone marrow cells, determined by quantitative marrow transplantation into irradiated mice. Radiat Res. 1960 Jul;13:115–125. [PubMed] [Google Scholar]
- Mackaness G. B. The monocyte in cellular immunity. Semin Hematol. 1970 Apr;7(2):172–184. [PubMed] [Google Scholar]
- Marker O., Volkert M. Studies on cell-mediated immunity to lymphocytic choriomeningitis virus in mice. J Exp Med. 1973 Jun 1;137(6):1511–1525. doi: 10.1084/jem.137.6.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland H. F. In vitro studies of cell-mediated immunity in an acute viral infection. J Immunol. 1974 Jul;113(1):173–180. [PubMed] [Google Scholar]
- Nahmias A. J., Roizman B. Infection with herpes-simplex viruses 1 and 2. 3. N Engl J Med. 1973 Oct 11;289(15):781–789. doi: 10.1056/NEJM197310112891505. [DOI] [PubMed] [Google Scholar]
- North R. J. The relative importance of blood monocytes and fixed macrophages to the expression of cell-mediated immunity to infection. J Exp Med. 1970 Sep 1;132(3):521–534. doi: 10.1084/jem.132.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes J. E. Invasion of the central nervous system by herpes simplex virus type 1 after subcutaneous inoculation of immunosuppressed mice. J Infect Dis. 1975 Jan;131(1):51–57. doi: 10.1093/infdis/131.1.51. [DOI] [PubMed] [Google Scholar]
- Rawls W. E., Laurel D., Melnick J. L., Glicksman J. M., Kaufman R. H. A search for viruses in smegma, premalignant and early malignant cervical tissues. The isolation of Herpesviruses with distinct antigenic properties. Am J Epidemiol. 1968 May;87(3):647–655. doi: 10.1093/oxfordjournals.aje.a120855. [DOI] [PubMed] [Google Scholar]
- Rosenberg G. L., Snyderman R., Notkins A. L. Production of chemotactic factor and lymphotoxin by human leukocytes stimulated with Herpes simplex virus. Infect Immun. 1974 Jul;10(1):111–115. doi: 10.1128/iai.10.1.111-115.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell A. S. Cell-mediated immunity to herpes simplex virus in man. J Infect Dis. 1974 Feb;129(2):142–146. doi: 10.1093/infdis/129.2.142. [DOI] [PubMed] [Google Scholar]
- Schluter B., Bellomy B., Brown A. Pathogenesis of temperature-sensitive mutants of sindbis virus in the embryonated egg. I. Characterization and kinetics of viral multiplication. Infect Immun. 1974 Jan;9(1):68–75. doi: 10.1128/iai.9.1.68-75.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore S. L., Nahmias A. J., Starr S. E., Wood P. A., McFarlin D. E. Detection of cell-dependent cytotoxic antibody to cells infected with herpes simplex virus. Nature. 1974 Sep 27;251(5473):350–352. doi: 10.1038/251350a0. [DOI] [PubMed] [Google Scholar]
- Speel L. F., Osborn J. E., Walker D. L. An immuno-cytopathogenic interaction between sensitized leukocytes and epithelial cells carrying a persistent noncytocidal myxovirus infection. J Immunol. 1968 Sep;101(3):409–417. [PubMed] [Google Scholar]
- Stevens J. G., Cook M. L. Restriction of herpes simplex virus by macrophages. An analysis of the cell-virus interaction. J Exp Med. 1971 Jan 1;133(1):19–38. doi: 10.1084/jem.133.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkman A., Collins F. M. Recovery of delayed-type hypersensitivity in mice following suppressive doses of X-radiation. J Immunol. 1968 Nov;101(5):846–859. [PubMed] [Google Scholar]
- Wood M. L., Vriesendorp H. M. A comparative study of heterologous anti-mouse lymphocyte and antimouse thymocyte sera prepared by two different immunization methods. Transplantation. 1969 Jun;7(6):522–533. doi: 10.1097/00007890-196906000-00008. [DOI] [PubMed] [Google Scholar]
- YOSHINO K., TANIGUCHI S. STUDIES ON THE NEUTRALIZATION OF HERPES SIMPLEX VIRUS. I. APPEARANCE OF NEUTRALIZING ANTIBODIES HAVING DIFFERENT GRADES OF COMPLEMENT REQUIREMENT. Virology. 1965 May;26:44–53. doi: 10.1016/0042-6822(65)90024-3. [DOI] [PubMed] [Google Scholar]
- Zisman B., Hirsch M. S., Allison A. C. Selective effects of anti-macrophage serum, silica and anti-lymphocyte serum on pathogenesis of herpes virus infection of young adult mice. J Immunol. 1970 May;104(5):1155–1159. [PubMed] [Google Scholar]



