Abstract
Purpose:
To review the characteristics and outcomes of patients who underwent pars plana vitrectomy (PPV) with scleral depressed vitreous shaving, 360 degree peripheral endolaser, and 14% C3F8 gas for rhegmatogenous retinal detachment (RRD).
Materials and Methods:
A retrospective review of a consecutive series of patients who underwent primary repair of RRD by PPV with scleral depressed vitreous shaving, 360 degree peripheral endolaser, and 14% perfluoropropane (C3F8) was conducted. Patients with less than 3 months follow-up, previous retinal surgery, and higher than grade B proliferative vitreoretinopathy were excluded.
Results:
Ninety-one eyes were included in the study. The mean age was 60.1 years. The mean follow-up was 13.7 months. The macula was detached in 63% (58/91) of the eyes. The reattachment rate after one surgical procedure was 95% (86/91) while overall reattachment rate was 100%. There was no statistically significant difference between reattachment rates of superior, nasal/temporal, or inferior RRDs. The mean final best corrected visual acuity (BCVA) was 20/40. Of all the patients, 66% of patients with macula-off RRDs had a final BCVA of 20/40 or better.
Conclusions:
PPV with scleral depressed vitreous shaving, 360 degree peripheral endolaser, and 14% C3F8 leads to successful anatomical reattachment with visual improvement in patients with primary RRD.
Keywords: Retinal detachment, vitreous shaving, gas
Rhegmatogenous retinal detachment (RRD)-the separation of the neurosensory retina from the underlying retinal pigment epithelium due to a retinal break-is an important cause of visual disability if left untreated. The precursors of RRD are changes in the vitreous body leading to tractional forces on retina, inducing a break through which fluid gains access to the subretinal space.[1] To achieve successful retinal reattachment, the goal of surgery for RRD is to treat all retinal breaks and relieving vitreous traction (which may also reduce the incidence of new breaks).
Since first introduced by Machemer in 1971, pars plana vitrectomy (PPV) has been shown to be an effective management option for RRDs.[2,3,4] Over the last 40 years, there have been a variety of advancements in vitrectomy surgery including intraocular gases, increased vitreous cutter speeds, wide-angle viewing systems, perfluorocarbon liquids, lighted and curved instruments, and chandelier lights.[5,6,7,8,9] These advancements improve the surgeon's ability to perform a thorough evaluation of the peripheral retina with high magnification and excellent illumination, which is crucial in detecting and treating all retinal breaks and relieving the vitreoretinal traction.
In this study, we evaluated PPV utilitizing the advancements listed above, including vitreous shaving aided with surgeon-assisted scleral depression, 360 degree peripheral endolaser, and 14% perfluoropropane (C3F8) gas in eyes presenting with primary RRDs. This retrospective study reviews the characteristics and outcomes of patients who underwent primary repair of RRD with this surgical technique.
Materials and Methods
Institutional Review Board approval was obtained. A retrospective chart review was performed on all (consecutive) patients from September 2006 to August 2009 diagnosed with primary RRD. The following patients were excluded: Previous vitreoretinal surgery, less than 3 months follow up, and presence of proliferative vitreoretinopathy (PVR) higher than Grade B. Patients with pathologic myopia or less than 50 years of age who were treated with a scleral buckling procedure with or without PPV were also excluded from this study.
Data points collected included age, sex, ocular history, pre-operative visual acuity (VA), lens status, presence of lattice degeneration, location of break (s), extent of retinal detachment, macula status, presence or absence of vitreous hemorrhage (VH), final best corrected visual acuity (BCVA), final anatomic status of retina, and total number of procedures to reattach the retina. Snellen VA measurements were converted and evaluated in their logarithm of the minimum angle of resolution equivalent.
Statistical analysis was performed using SPSS for Microsoft Windows (Version 17.0). The Mann-Whitney test was performed to compare independent groups with respect to non-categorical variables. Fisher's exact test was used to compare independent groups with respect to percentages. Fisher's exact test was used if the expected frequencies were too small to permit use of the chi square test. A 0.05 significance level was used for all statistical tests and no one-sided tests were done.
Description of the procedure
After written informed consent was obtained, the patient underwent the procedure either under retrobulbar anesthesia with monitored anesthesia care or general anesthesia by one surgeon. Three-port 20-gauge PPV was performed using a wide-angle viewing system (BIOM, Insight Instruments, Stuart, FL) with a Carl Zeiss Meditec (Jena, Germany) microscope and the Accurus Surgical System (Alcon Laboratories, Inc., Fort Worth, Texas). The chandelier lights (placed inferotemporally) used for all cases were either the 27-gauge dual chandelier (Dutch Ophthalmic USA, Exter, New Hampshire) or 25-gauge single chandelier (Synergetics Inc, St Charles, Missouri).
After the core vitrectomy was performed, the surgeon confirmed that the posterior vitreous was detached. If not, the posterior vitreous was detached with a vitreous cutter. The mid-peripheral vitreous was then removed, followed by a thorough shaving of the peripheral vitreous with surgeon-assisted scleral depression. To achieve this, one sclerotomy is plugged, allowing the surgeon to use his free hand to scleral depress with illumination provided by the chandelier light. During the scleral depression, the intraocular pressure (IOP) may be lowered to 10-20 mmHg (based on scleral rigidity) to make the scleral indentation easier. High cut rate and low suction avoids the softening of the eye. In phakic patients, the shaft of the vitreous cutter was held parallel to the surface of the retina to avoid touching the lens.
After vitreous shaving, the peripheral retina was examined with scleral depression to localize the retinal breaks. All breaks were marked with endodiathermy. The retina was flattened either with perflurocarbon liquid (PFO) (Perfluoron; Alcon Laboratories, Inc.) or air (77% received PFO). Endolaser treatment was applied with a curved, lighted laser probe from Alcon or Lumenis (Santa Clara, CA). Laser was applied to all retinal breaks with an additional three to four rows applied posterior to the ora serrata for 360 degrees, with surgeon-assisted scleral depression (avoiding the 3 o’clock and 9 o’clock positions, if possible). More confluent laser was applied to previously detached retina. The most posterior row of laser was placed posterior to the vitreous base insertion. All eyes received 14% C3F8 gas as a tamponade agent. Patients were requested to maintain face-down positioning for one week post operatively independent of the location of the break.
Results
Patient demographics
One hundred and thirty eyes of 130 patients were diagnosed with primary RRD without a history of previous retinal surgery or PVR higher than Grade B from September 2006 to August 2009. A total of 39 eyes were excluded from the study for various reasons. Twenty-two eyes underwent a scleral buckling procedure with or without PPV (all eyes were anatomically attached after one procedure at last follow up visit). Nine eyes underwent laser demarcation as primary treatment for asymptomatic shallow RRDs (all eyes were successfully treated with one procedure at last follow up visit. Five eyes underwent pneumatic retinopexy as primary treatment with 3 eyes being anatomically reattached at their last follow-up visit. (2 eyes required subsequent PPV to reattach the retina).
A total of 94 eyes underwent PPV with surgeon assisted scleral depressed vitreous shaving, 360 degree peripheral endolaser, and 14% C3F8 gas. Three eyes were excluded as a result of insufficient follow-up, with all 3 anatomically attached after one procedure at last follow-up visit. Ninety-one eyes of 91 patients were included in the study.
The mean age was 60.1 ± 11.3 years (range, 35-93 years). The mean follow-up was 13.7 ± 9.2 months and a median of 12 months (range, 3-34 months). There were 56% male (51 males) and 44% female (40 females) patients. Out of 91, 65% (59 eyes) were phakic, 35% (32 eyes) were pseudophakic, and 7% (6 eyes) had undergone prior laser in situ keratomileusis surgery. Lattice degeneration was present in 14% (13 eyes) [Table 1].
Table 1.
Patient demographics
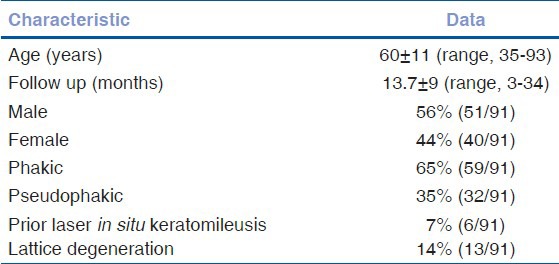
Initial retinal detachment characteristics
The mean extent of the RRD was 7.3 clock hours (range, 1.5-12 clock hours). The mean number of retinal breaks was 2.2 (range, 1-9 breaks). Among the 91 eyes, 16% (15 eyes) had vitreous hemorrhage; 62% (56 eyes) had breaks located superiorly (from 10-2 o’clock); 20% (18 eyes) had breaks located nasally or temporally (between 2-4 o’clock or 8-10 o’clock); 18% (17 eyes) had breaks located inferiorly (from 4-8 o’clock); 36% (33 eyes) had macula-on RRDs with the average time from loss of vision to presentation was 9 days (range, 1-60 days); and 64% (58 eyes) had macula-off RRDs with the average duration of macula-off status (from loss of VA based on patient history until time of presentation) was 13 days (range, 1-150 days) [Table 2].
Table 2.
Retinal detachment characteristics
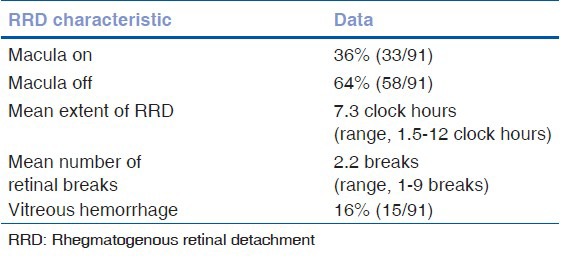
Anatomical and visual outcomes
The single surgical re-attachment rate was 95% (86/91). This was defined as anatomically flat retina (no subretinal fluid after a minimum of 3 months of follow up) without the need for additional procedures (laser, cryotherapy, or additional surgery). The single surgical re-attachment rate in phakic eyes was 93% (55/59) and in pseudophakic eyes was 97% (31/32) (P = 0.442). The single surgical re-attachment rate was 100% (56/56) for eyes with superior breaks, 89% (16/18) for eyes with nasal or temporal breaks, and 82% (14/17) for eyes with inferior breaks. The location of the break did not have any significant impact on the successful re-attachment of the retina (P = 0.629). The single surgical re-attachment rate was 94% (31/33) for macula-on RRDs and 93% (55/59) for macula-off RRDs (P = 0.621) [Table 3].
Table 3.
Percentage of anatomic success with a single procedure
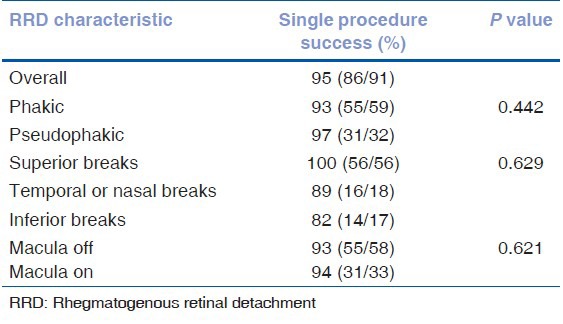
The mean pre-operative VA was 20/300 (range, 20/20-hand movements, HM) while the mean final BCVA was 20/40 (range, 20/20-5/200). The mean pre-operative VA in macula-on RRDs was 20/50 (range, 20/20-HM; vision was HM in those eyes with vitreous hemorrhage but were still macula-on RRDs) while the mean final BCVA was 20/30 (range, 20/20-5/200). The mean pre-operative VA in macula-off RRDs was 5/200 (range, 20/70-HM) while the mean final BCVA was 20/40 (range, 20/20-HM).
Of the 91 eyes, 82% (75/91) achieved mean final BCVA ≥20/60 and 75% (68/91) achieved mean final BCVA ≥ 20/40. In the macula-on RRD eyes, 97% (32/33) and 91% (30/33) achieved mean final BCVA ≥ 20/60 and ≥ 20/40, respectively. In the macula-off RRD eyes, 74% (43/59) and 66% (38/59) achieved mean final BCVA ≥ 20/60 and ≥ 20/40, respectively.
Subsequent surgeries
In this study, 5.5% (5/91) of the eyes required additional retinal re-attachment surgery. Two eyes developed PVR Grade C or higher and 3 eyes developed new RRDs secondary to new retinal breaks (posterior to laser). All 5 of these eyes underwent a second PPV with scleral buckling. All eyes were anatomically flat after second retinal reattachment surgery at last follow-up visit. The average number of surgical procedures to reattach the RRD in our study was 1.05. The overall reattachment rate was 100%.
Of the 91 eyes, 3.2% (3 eyes) eyes had subsequent PPV for other retinal pathology. Two eyes had PPV for epiretinal membrane (ERM) (each 4 months after initial PPV for RRD), and 1 eye had PPV for macular hole (MH) 16 months after initial PPV for RRD.
All 59 phakic eyes had progression of cataract during follow up; 66% (39/59) had uncomplicated cataract extraction as of last follow-up visit.
Discussion
The importance of vitreous base shaving for the repair of pseudophakic RRDs and RRDs with PVR has been documented previously.[10,11] The development of new technologies allows the surgeon to gain excellent access to the vitreous base. Chandelier lighting provides superior illumination of the peripheral retina, alleviating the need for endoilluminator. The chandelier light allows direct visualization of the vitreous base while the vitreous farther away can be identified with retroillumination. The chandelier permits the surgeon to use his other hand for controlled scleral depression, making him independent from support staff (in both phakic and pseudophakic eyes). To avoid touching the lens in phakic eyes, we did not perform vitrectomy anterior to the ora serrata. A temporary reduction of intraocular pressure also helped anterior scleral indentation by reducing counter pressure. In these cases, a high cut rate and low suction with gentle movement of scleral depressor prevented softening of the eye.
Meticulous shaving of the peripheral vitreous may reduce the risk for the formation of new retinal breaks post-operatively, since it has been thought that post-operative breaks are caused by vitreous base traction.[12] Peripheral vitreous shaving theoretically reduces the vitreous scaffold needed for anterior PVR formation as described by Cowley et al. when they demonstrated the use of PPV for RRD was the single strongest predictor of PVR.[13] Veckeneer and Wong demonstrated the benefit of vitreous base shaving using transscleral, diaphonoscopic illumination in eyes with RRDs.[14] Their modified light source (“lightindentor”) allowed transscleral illumination of the vitreous base, allowing for controlled vitreous base removal. Martinez-Castillo et al. also found that “meticulous” peripheral vitreous dissection (n = 61) leads to high re-attachment rate in phakic, pseudophakic, and aphakic RRDs with unseen breaks (98%).[15] In the current study, 2 eyes developed PVR leading to re-detachment and 3 eyes developed new breaks leading to re-detachment. Peripheral vitreous shaving may play a role in increasing the single surgical success rate for PPV for RRD.
Another step in reducing the rate of post-operative complications (new retinal breaks and recurrent RRDs) is the placement of 360 degree peripheral retinopexy which effectively produces a second ora serrata. A curved, lighted endolaser probe allows treatment near the ora serrata in both phakic and pseudophakic patients. The 360 degree laser has been shown to be successful in a variety of conditions, including PPV, for retained lens fragments and after removal of silicone oil.[16,17,18] Koh et al. showed that intraoperative 360 degree retinopexy was associated with a three-fold reduction in the incidence of RRD after PPV.[18]
The 360 degree peripheral endolaser with PPV may promote the formation of ERM postoperatively. Ryan Jr. and Mittra suggested that the development of PVR and ERM is a disadvantage of PPV for RRD.[19] Of the 91 patients, 3.2% (3/91) of the patients in study underwent a second PPV for repair of either ERM (n = 2) or MH (n = 1). Two eyes in this study developed PVR leading to re-detachment. Katira et al. reported that 12.8% of eyes (n = 141) undergoing PPV (All patients underwent standard three-port PPV, posterior vitreous detachment with laser or cryotherapy for retinopexy, internal drainage of subretinal fluid, air–fluid exchange, and placement of an intraocular gas tamponade) for RRD develop post-operative ERM, and 4.2% underwent second PPV with membrane peeling for ERM.[20] The rate of ERM formation requiring PPV and membrane peeling in our study (3.2%) was similar to Katira et al. (4.2%). Peripheral laser might increase the rate of ERM formation.
Yoon and Marmor reported that it takes 24 hours to 3 days to achieve chorioretinal adhesion following laser photocoagulation.[21] During this time, the subretinal fluid can re-detach the retina. To avoid retinal re-detachment by formation of new inferior breaks, all patients in this study received 14% C3F8 gas and positioned face down for one week post-operatively. This theoretically would tamponade the inferior retina during while chorioretinal adhesions are forming from the endolaser treatment. This may reduce retinal re-detachment during this critical time period. The use of C3F8, however, is associated with increased risk of cataract progression, angle closure glaucoma, transient increased intraocular pressure, and fibrinous pupillary membranes.[22] All phakic eyes in our study had progression of their cataract. Thirty-nine of the 59 eyes (66%), who were phakic at initial visit, had uncomplicated cataract extraction by the last follow up visit. This is comparable to previous reports where 53–58% of phakic patients undergoing PPV for RRD had cataract surgery at their last follow up.[23,24] None of the eyes had elevated intraocular pressure requiring treatment one month after PPV. The major drawbacks for utilizing C3F8 gas were progression of cataract and delay of air travel.
In this study, surgeon-assisted vitreous shaving, 360 endolaser, and C3F8 gas yielded an overall 95% (86/91) single surgical success rate. In literature, PPV for primary RRD has a single surgical re-attachment rate of 70-98%.[25,26,27,28,29,30,31] The single surgical re-attachment rate in our study was 97% (31/32) of pseudophakic eyes, 93% (55/59) of phakic eyes, 82% (14/17) of eyes with inferior breaks, 89% (16/18) of eyes with nasal or temporal breaks, 100% (56/56) of eyes with superior breaks, 93% (55/59) of macula-off RRDs, and 94% (31/33) of macula-on RRDs. Although we did not find a statistically significant difference between the superior, nasal/temporal, or inferior RRDs (P = 0.629), the study's small sample size of RRDs with inferior breaks (n = 17) does not give the study enough power to fully understand the difference in outcome. Further studies comparing vitreous shaving for superior versus inferior RRDs will be helpful.
PPV alone for RRDs in phakic eyes has been shown to have a single surgical re-attachment rate of 63.8% to 92%.[24,32] Miki et al. showed a 92% success rate (n = 87) for phakic superior RRDs with flap tears.[32] Our results for RRDs in phakic eyes (93%) were higher than these numbers. The anatomical success of PPV for phakic RRDs in our study may be explained by the extensive peripheral vitreous shaving, 360 degree retinopexy, and use of C3F8 to help reduce the risk of post-operative breaks and recurrent RRD (with or without PVR).
Visual rehabilitation following RRD, particularly with macula-off RRDs, can be limited. The difficulty in visual recovery depends on outer retinal changes in the detached retina.[33,34,35,36] In this study, the mean pre-operative VA in macula-off eyes was 5/200 (range, 20/70-HM) while the mean final BCVA in macula-off eyes was 20/40 (range, 20/20-HM). Ross and Kozy reported 59% of macula-off RRDs regain 20/50 or better VA while Speicher et al. showed 80% of eyes achieve 20/40 vision or better.[37,38] Bourlh et al. found the mean final BCVA in macula-off eyes to be 20/73.[39] In this study, 66% of the macula-off eyes achieved mean final BCVA of better than 20/40 (mean final BCVA 20/40). Our results are consistent with previous findings that although eyes with macula-off RRDs have poorer visual outcome than eyes with macula-on RRDs, the majority (66%) achieve visual rehabilitation to 20/40 or better.
This study design is limited by its retrospective and non-comparative nature. The primary limitations of this technique are cataract progression and delay in air travel.
In conclusion, PPV with surgeon assisted scleral depression, 360 degree peripheral endolaser, and C3F8 gas leads to successful anatomic outcome and functional improvement in phakic, pseudophakic, inferior, superior, macula-on, and macula-off primary RRDs, as highlighted by Chong and Fuller.[40] The single surgical re-attachment rate was 95%. Overall, 82% of eyes achieved mean final BCVA ≥ 20/60, and 75% achieved mean final BCVA ≥ 20/40.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
References
- 1.Ghazi NG, Green WR. Pathology and pathogenesis of retinal detachment. Eye (Lond) 2002;16:411–21. doi: 10.1038/sj.eye.6700197. [DOI] [PubMed] [Google Scholar]
- 2.Machemer R, Buettner H, Norton EW, Parel JM. Vitrectomy: A pars plana approach. Trans Am Acad Ophthalmol Otolaryngol. 1971;75:813–20. [PubMed] [Google Scholar]
- 3.SPR Study Group. View 2: The case for primary vitrectomy. Br J Ophthalmol. 2003;87:784–7. doi: 10.1136/bjo.87.6.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwartz SG, Flynn HW. Primary retinal detachment: Scleral buckle or pars plana vitrectomy? Curr Opin Ophthalmol. 2006;17:245–50. doi: 10.1097/01.icu.0000193097.28798.fc. [DOI] [PubMed] [Google Scholar]
- 5.Norton EW. Intraocular gas in the management of selected retinal detachments. Trans Am Acad Ophthalmol Otolaryngol. 1973;77:OP85–98. [PubMed] [Google Scholar]
- 6.Lincoff H, Coleman J, Kreissig I, Richard G, Chang S, Wilcox LM. The perfluorocarbon gases in the treatment of retinal detachment. Ophthalmology. 1983;90:546–51. doi: 10.1016/s0161-6420(83)34525-5. [DOI] [PubMed] [Google Scholar]
- 7.Chang S. Low viscosity liquid fluorochemicals in vitreous surgery. Am J Ophthalmol. 1987;103:38–43. doi: 10.1016/s0002-9394(14)74166-2. [DOI] [PubMed] [Google Scholar]
- 8.Brazitikos PD, Androudi S, D’Amico DJ, Papadopoulos N, Dimitrakos SA, Dereklis DL, et al. Perfluorocarbon liquid utilization in primary vitrectomy repair of retinal detachment with multiple breaks. Retina. 2003;23:615–21. doi: 10.1097/00006982-200310000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Oshima Y, Awh CC, Tano Y. Self-retaining 27-gauge transconjunctival chandelier endoillumination for panoramic viewing during vitreous surgery. Am J Ophthalmol. 2007;143:166–7. doi: 10.1016/j.ajo.2006.07.051. [DOI] [PubMed] [Google Scholar]
- 10.Martínez-Castillo V, Zapata MA, Boixadera A, Fonollosa A, García-Arumí J. Pars plana vitrectomy, laser retinopexy, and aqueous tamponade for pseudophakic rhegmatogenous retinal detachment. Ophthalmology. 2007;114:297–302. doi: 10.1016/j.ophtha.2006.07.037. [DOI] [PubMed] [Google Scholar]
- 11.Oyagi T, Emi K. Vitrectomy without scleral buckling for proliferative vitreoretinopathy. Retina. 2004;24:215–8. doi: 10.1097/00006982-200404000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Kreiger AE. Wound complications in pars plana vitrectomy. Retina. 1993;13:335–44. doi: 10.1097/00006982-199313040-00012. [DOI] [PubMed] [Google Scholar]
- 13.Cowley M, Conway BP, Campochiaro PA, Kaiser D, Gaskin H. Clinical Risk factors for proliferative vitreoretinopathy. Arch Ophthalmol. 1989;107:1147–51. doi: 10.1001/archopht.1989.01070020213027. [DOI] [PubMed] [Google Scholar]
- 14.Veckeneer M, Wong D. Visualising vitreous through modified trans-scleral illumination by maximizing the Tyndall effect. Br J Ophthalmol. 2009;93:268–70. doi: 10.1136/bjo.2008.147306. [DOI] [PubMed] [Google Scholar]
- 15.Martinez-Castillo V, Boixadera A, Garcia-Arumi J. Pars plana vitrectomy alone with diffuse illumination and vitreous dissection to manage primary retinal detachment with unseen breaks. Arch Ophthalmol. 2009;127:1297–304. doi: 10.1001/archophthalmol.2009.254. [DOI] [PubMed] [Google Scholar]
- 16.Morris RE, Shere JL, Witherspoon CD, Segal ZK, Tehranchi L, Kuhn F, et al. Intraoperative retinal detachment prophylaxis in vitrectomy for retain cataract fragments. J Cataract Refract Surg. 2009;35:491–5. doi: 10.1016/j.jcrs.2008.11.037. [DOI] [PubMed] [Google Scholar]
- 17.Laidlaw DA, Karia N, Bunce C, Aylward GW, Gregor ZJ. Is prophylactic 360° laser retinopexy protective? Risk factors for retinal redetachment after removal of silicone oil. Ophthalmology. 2002;109:153–8. doi: 10.1016/s0161-6420(01)00848-x. [DOI] [PubMed] [Google Scholar]
- 18.Koh HJ, Cheng L, Kosobucki B, Freeman WR. Prophylactic intraoperative 360 degrees of laser retinopexy for prevention of retinal detachment. Retina. 2007;27:744–9. doi: 10.1097/IAE.0b013e318030ebd7. [DOI] [PubMed] [Google Scholar]
- 19.Ryan EH, Jr, Mittra RA. Scleral buckling vs vitrectomy: The continued role for scleral buckling in the vitrectomy era. Arch Ophthalmol. 2010;128:1202–5. doi: 10.1001/archophthalmol.2010.192. [DOI] [PubMed] [Google Scholar]
- 20.Katira RC, Zamani M, Beristein DM, Garfinkel RA. Incidence and characteristics of macular pucker formation after primary retinal detachment repair by pars plana vitrectomy alone. Retina. 2008;28:744–8. doi: 10.1097/IAE.0b013e318162b031. [DOI] [PubMed] [Google Scholar]
- 21.Yoon YH, Marmor MF. Rapid enhancement of retinal adhesion by laser photocoagulation. Ophthalmology. 1988;95:1385–8. doi: 10.1016/s0161-6420(88)33000-9. [DOI] [PubMed] [Google Scholar]
- 22.Sabates NR, Tolentino FI, Arroyo M, Freeman HM. The complications of perfluoropropane gas use in complex retinal detachments. Retina. 1996;16:7–12. doi: 10.1097/00006982-199616010-00003. [DOI] [PubMed] [Google Scholar]
- 23.Oshima Y, Yamanishi S, Sawa M, Motokura M, Harino S, Emi K. Two-year follow-up study comparing primary vitrectomy with scleral buckling for macula-off rhegmatogenous retinal detachment. Jpn J Ophthalmol. 2000;44:538–49. doi: 10.1016/s0021-5155(00)00205-7. [DOI] [PubMed] [Google Scholar]
- 24.Heimann H, Bartz-Schmidt KU, Bornfeld N, Weiss C, Hilgers RD, Foerster MH. Scleral Buckling versus Primary Vitrectomy in Rhegmatogenous Retinal Detachment Study Group. Scleral buckling versus primary vitrectomy in rhegmatogenous retinal detachment: A prospective randomized multicenter clinical study. Ophthalmology. 2007;114:2142–54. doi: 10.1016/j.ophtha.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 25.Mendrinos E, Dang-Burgener NP, Stangos AN, Sommerhalder J, Pournaras CJ. Primary vitrectomy without scleral buckling for pseudophakic rhegmatogenous retinal detachment. Am J Ophthalmol. 2008;28:1063–70. doi: 10.1016/j.ajo.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 26.Lai MM, Ruby AJ, Sarrafizadeh R, Urban KE, Hassan TS, Drenser KA, et al. Repair of primary rhegmatogenous retinal detachment using 25-gauge transconjunctival sutureless vitrectomy. Retina. 2008;28:729–34. doi: 10.1097/IAE.0b013e318162b01c. [DOI] [PubMed] [Google Scholar]
- 27.Heimann H, Zou X, Jandeck C, Kellner U, Bechrakis NE, Kreusel KM, et al. Primary vitrectomy for rhegmatogenous retinal detachment: An analysis of 512 cases. Graefes Arch Clin Exp Ophthalmol. 2006;244:69–78. doi: 10.1007/s00417-005-0026-3. [DOI] [PubMed] [Google Scholar]
- 28.Dugas B, Lafontaine PO, Guillaubey A, Berrod JP, Hubert I, Bron AM, et al. The learning curve for primary vitrectomy without scleral buckling for pseudophakic retinal detachment. Graefes Arch Clin Exp Ophthalmol. 2009;247:319–24. doi: 10.1007/s00417-008-0997-y. [DOI] [PubMed] [Google Scholar]
- 29.Hakin KN, Lavin MJ, Leaver PK. Primary vitrectomy for rhegmatogenous retinal detachment. Graefes Arch Clin Exp Ophthalmol. 1993;231:344–6. doi: 10.1007/BF00919031. [DOI] [PubMed] [Google Scholar]
- 30.Escoffery RF, Olk RJ, Grand MG, Boniuk I. Vitrectomy without scleral buckling for primary rhegmatogenous retinal detachment. Am J Ophthalmol. 1985;99:275–81. doi: 10.1016/0002-9394(85)90356-3. [DOI] [PubMed] [Google Scholar]
- 31.Azad RV, Chanana B, Sharma YR, Vohra R. Primary vitrectomy versus conventional retinal detachment surgery in phakic rhegmatogenous retinal detachment. Acta Ophthalmol Scand. 2007;85:540–5. doi: 10.1111/j.1600-0420.2007.00888.x. [DOI] [PubMed] [Google Scholar]
- 32.Miki D, Hida T, Hotta K, Shinoda K, Hirakata A. Comparison of scleral buckling and vitrectomy for retinal detachment resulting from flap tears in superior quadrants. Jpn J Ophthalmol. 2001;45:187–91. doi: 10.1016/s0021-5155(00)00377-4. [DOI] [PubMed] [Google Scholar]
- 33.Chang CJ, Lai WW, Edward DP, Tso MO. Apoptotic photoreceptor cell death after traumatic retinal detachment in humans. Arch Ophthalmol. 1995;113:880–6. doi: 10.1001/archopht.1995.01100070054025. [DOI] [PubMed] [Google Scholar]
- 34.Arroyo JG, Yang L, Bula D, Chen DF. Photoreceptor apoptosis in human retinal detachment. Am J Ophthalmol. 2005;139:605–10. doi: 10.1016/j.ajo.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 35.Berglin L, Algvere PV, Seregard S. Photoreceptor decay over time and apoptosis in experimental retinal detachment. Graefes Arch Clin Exp Ophthalmol. 1997;235:306–12. doi: 10.1007/BF01739640. [DOI] [PubMed] [Google Scholar]
- 36.Maruko I, Iida T, Sekiryu T, Saito M. Morphologic changes in the outer layer of the detached retina in rhegmatogenous retinal detachment and central serous chorioretinopathy. Am J Ophthalmol. 2009;147:489–94. doi: 10.1016/j.ajo.2008.09.030. [DOI] [PubMed] [Google Scholar]
- 37.Ross WH, Kozy DW. Visual recovery in macula-off rhegmatogenous retinal detachments. Ophthalmology. 1998;105:2149–53. doi: 10.1016/S0161-6420(98)91142-3. [DOI] [PubMed] [Google Scholar]
- 38.Speicher MA, Fu AD, Martin JP, von Fricken MA. Primary vitrectomy alone for repair of retinal dtachments following cataract surgery. Retina. 2000;20:459–64. doi: 10.1097/00006982-200009000-00005. [DOI] [PubMed] [Google Scholar]
- 39.Bourla DH, Bor E, Axer-Siegel R, Mimouni K, Weinberger D. Outcomes and complications of rhegmatogenous retinal detachment repair with selective sutureless 25-gauge pars plana vitrectomy. Am J Ophthalmol. 2010;149:630–4. doi: 10.1016/j.ajo.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Chong DY, Fuller DG. The declining use of scleral buckling with vitrectomy for primary retinal detachments. Arch Ophthalmol. 2010;128:1206–7. doi: 10.1001/archophthalmol.2010.190. [DOI] [PubMed] [Google Scholar]


