Abstract
Aneurysmal subarachnoid hemorrhage is associated with high mortality. Understanding of the underlying pathophysiology is important as early intervention can improve outcome. Increasing age, altered sensorium and poor Hunt and Hess grade are independent predictors of adverse outcome. Early operative interventions imposes an onus on anesthesiologists to provide brain relaxation. Coiling and clipping are the two treatment options with increasing trends toward coiling. Intraoperatively, tight control of blood pressure and adequate brain relaxation is desirable, so that accidental aneurysm rupture can be averted. Patients with poor grades tolerate higher blood pressures, but are prone to ischemia whereas patients with lower grades tolerate lower blood pressure, but are prone to aneurysm rupture if blood pressure increases. Patients with Hunt and Hess Grade I or II with uneventful intraoperative course are extubated in operation theater, whereas, higher grades are kept electively ventilated. Postoperative management includes attention toward fluid status and early management of vasospasm.
Keywords: Aneurysm, neuroanesthesia, sub-arachnoid hemorrhage
Introduction
Aneurysmal subarachnoid hemorrhage (aSAH) is a mortifying disease affecting multiple organs besides brain.[1,2] The prognosis remains poor, with 1-month case fatality as high as 35% even in developed countries.[3] Of those who survive, one-third need life-long care, with a further third having residual cognitive impairment that affects their functional status and quality of life.[3] Thus, prompt diagnosis, early and appropriate treatment of SAH are essential[4] and may improve outcome.[5] The anesthetist is an integral part of the team managing these patients at all the stages during the course of illness. This review aims to provide knowledge regarding optimal care that can be provided at various stages in patients with aSAH, which can translate into improved neurological outcome.
Epidemiology
Approximately 85% of cases of SAH are related to spontaneous rupture of an intracranial aneurysm in the basal cerebral arteries. This accounts for an aSAH incidence of 2-22.5/1,00,000 population.[6] At least 60% of aneurysm rupture occurs between ages of 40 and 60 years.[7] A slight sexual preponderance is noted, with female to male ratio of approximately 3:2. The vast majority of aneurysms (80-90%) are located in the anterior (carotid) circulation, the anterior and posterior communicating, and the middle cerebral artery. The remaining 10-20% are located in the posterior (vertebrobasilar) circulation.
Etiology
The intracranial aneurysms are thought to be primarily acquired vascular lesions secondary to degenerative changes in the muscular and elastic components of vessel walls. Combination of possibly genetic, hemodynamic, nicotine, cocaine, and alcohol induced structural defects and chronic hemodynamically induced shear stress often associated with hypertension can cause aneurysmal out-pouchings in subarachnoid space at the base of the brain.[8] Infection, trauma, diseases associated with hypertension like coarctation of aorta, polycystic kidney disease and deficiency of type III collagen have been seen to be associated with cerebral aneurysm and SAH. Unruptured aneurysms are often silent with patients usually presenting after their rupture.
Clinical Presentation and Diagnosis
The classical history of SAH is the sudden onset of severe headache, often described as the “worst imaginable.” Other symptoms include neck stiffness, vomiting, epileptic seizures and neurological injury varying between unconsciousness, depressed consciousness, focal neurological deficits and isolated cranial nerve palsies.
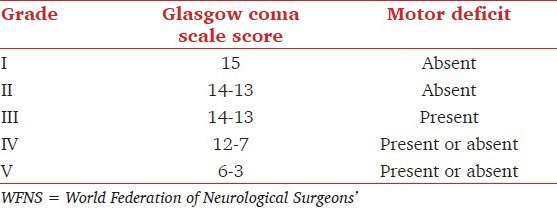
For the standardization of surgical risk assessment and to estimate the prognosis, clinical grading scales such as one by Hunt and Hess [Table 1][9] and other by the World Federation of Neurological Surgeons (WFNS) [Table 2][10] had been proposed. While the WFNS scale based on the Glasgow coma scale is the most important as it correlates with the preoperative level of consciousness, modified Hunt and Hess grading scale is still the most commonly used, because of both familiarity and ease of application.
Table 1.
Modified Hunt and Hess clinical grades for patients with subarachnoid hemorrhage*

Table 2.
WFNS grades for patients with subarachnoid hemorrhage
The higher the clinical grade, the more likely are cerebral vasospasm, elevated intracranial pressure (ICP), impaired cerebral autoregulation, and impaired vascular CO2 reactivity. The worse clinical grade is also associated with higher incidence of cardiac arrhythmias and myocardial dysfunction[11] and hypovolemia and hyponatremia.[12,13]
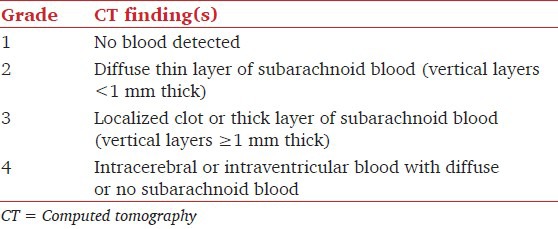
Unenhanced cranial computed tomography (CT) is the initial diagnostic tool of choice in all cases of suspected SAH.[4] CT findings are graded according to Fisher's four point scale [Table 3].[14] It is probably the best method to describe the CT findings with regard to the clot burden and the risk of vasospasm in aneurysmal SAH. In case of a normal scan, particularly early after ictus, in the presence of high suspicion, lumbar puncture should be performed. Confirmation of the presence of red blood cells or their metabolites in cerebrospinal fluid (CSF) identifies patients who subsequently have aneurysm detected in angiography.
Table 3.
Fisher grades for CT findings in subarachnoid hemorrhage
Computed tomography angiography (CTA) is the investigation of choice once SAH is confirmed by unenhanced CT or lumbar puncture so as to identify the anatomical territory of aneurysm. Additional imaging in form of formal four vessel digital subtraction angiography, magnetic resonance imaging and spinal catheter angiography can be done for treatment planning.
Major Complications of Subarachnoid Hemorrhage
The major complications of SAH include rebleeding, cerebral vasospasm, seizures, and hydrocephalus.
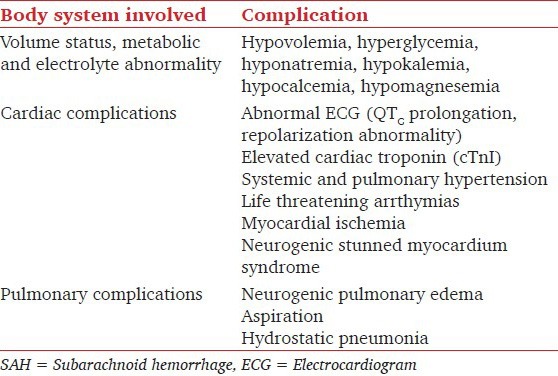
Various nonneurological complications are common after SAH and are independent factors associated with poor prognosis [Table 4].[15]
Table 4.
Nonneurological complications of SAH
All these complications occurring in patients with aSAH can adversely affect outcome, thus, there is a growing trend toward early operation so that the risk of re-bleeding is eliminated, vasospasm and other mentioned complications more aggressively treated.[16,17]
Re-bleeding
The greatest risk of re-bleeding in patients with aSAH occurs within the first 24 h (4%) and is highest in those with poorest grade. It then levels off at 1.5%/day for up to 2 weeks after ictus.[17] Overall the incidence of re-bleeding is 11%. Patients who suffer from re-bleed have a very high mortality rate between 64% and 90%.[17]
This is the reason that there is a shift in the trend toward early securing of ruptured aneurysm. Early clipping allows to eliminate the risk of re-bleed and also allows effective treatment of vasospasm. Recently, a meta-analysis has proved that short term use of antifibrinolytics should only be considered in those who are at high risk of re-bleeding in whom definitive treatment of aneurysm is delayed.[18] The use of antifibrinolytics though reduces the risk of re-bleeding, has not been associated with improved outcome, possibly because it can lead to microthrombosis and hence cerebral ischemia.[19]
Seizures
Blood can irritate the cerebral cortex resulting in seizures. However, only about 5% of patients with SAH go on to have epilepsy. Although seizures can worsen the outcome of a patient in the intensive care unit (ICU), the risks of side-effects from common anti-epileptics may outweigh the benefits unless the patient is noted to have a seizure. In this case, the patient may be continued on a medication for 6-12 months after the hemorrhage.
Hydrocephalus
Hydrocephalus following subarachnoid hemorrhage can progress acutely (0-3 days), sub-acutely (4-13 days) or chronically (after 13 days). The incidence of hydrocephalus is around 28%. Patients usually present with worsening of neurological status and diagnosis is confirmed on CT scan. Preexisting diabetes, higher Fisher grade and intraventricular hemorrhage are associated with increased chances of development of hydrocephalus.[20] The treatment involves placement of ventriculoperitoneal shunt.
Vasospasm
Cerebral vasospasm remains an important cause of morbidity and mortality (incidence: 13.5%).[16] Cerebral vasospasm usually develops 3-12 days after SAH and lasts on an average for 2 weeks. The incidence and severity of delayed cerebral vasospasm has been shown to correlate with the amount and location of blood in the basal cisterns.[21] The sub-arachnoid blood causes formation of oxyhemoglobin, free radicals, lipid peroxidation products, calcium, and prostaglandins which have been implicated in causing contraction of the smooth muscles to the thickening of the vessel wall, thus, contributing to the cerebral vasospasm.[22]
Though the frequency of angiographic detected vasospasm is estimated to be far more in SAH patients (up to 70%), symptomatic and clinically significant vasospasm develops less often (20-30%). Clinically, patients developing vasospasm following SAH present with signs of delayed cerebral ischemia, which manifest as decreased level of consciousness, new onset focal neurological signs and mutism.
The diagnosis is usually confirmed by CTA, however, DSA remains the gold standard diagnostic tool. Transcranial Doppler (TCD) is a noninvasive technology, which is helpful in earlier diagnosis and to titrate the therapy to vasospasm. TCD being a bed side tool, allows repeated evaluation of patients with vasospasm without resorting to frequent angiographic investigations.[23] Jugular bulb oximetry, xenon enhanced CT, laser Doppler flowmetry, thermal dilution, brain tissue PO2, and cerebral microdialysis are some of the other monitoring techniques for estimation of cerebral blood flow (CBF), which may be helpful. Unlike cerebral angiography and TCD, these techniques measure regional perfusion, not merely diameter of blood vessels or flow velocities.[24] Hence, these techniques predict clinical outcome better, but the drawback is that at present, these are not routinely available.
Preventive and Treatment Strategies of Cerebral Vasospasm
General preventive measures of cerebral vasospasm include administration of nimodipine, positive fluid balance, mild sedation, avoidance of hypotensive episodes, and hyponatremia.
Symptomatic treatment of cerebral vasospasm consists of triple-H therapy, balloon angioplasty and intra-arterial papaverine.
Nimodipine
Nimodipine, a specific antagonist of L-type voltage gated calcium channels, is one of the most extensively studied drugs with promising results. This is given in the dosages of 60 mg orally or by nasogastric tube every 4 hourly for 21 days.[25] The beneficial effects are thought to occur due to its action either on the distal vessel site or at the cellular level. However, nimodipine causes vasodilation, so attention must be paid to hydration status and careful titration of anesthetic drugs during induction of anesthesia to prevent hypotension. The need for intraoperative and postoperative vasopressors is also increased in patients taking nimodipine preoperatively.
New Pharmacological Therapies
Magnesium sulfate is a cerebral vasodilator and N-methyl-D aspartate antagonist that has been reported to be efficacious in decreasing vasospasm in animal models. However, recent studies have shown no beneficial effects and worse clinical outcomes with induced hypermagnesemia.[26,27]
Statins through their anti-inflammatory and modulating effect on cerebral vasculature have been used to prevent and treat ischemia. However, no significant effect in improving neurological outcome and mortality has been seen in recent studies.[28] Thus, current evidence suggest their use should be continued in patients already on statins. Introduction of acute statin therapy should be considered in carefully selected patients who are at high risk of delayed cerebral ischemia.[29]
Tiralazad: A nonglucocorticoid 21 amino-steroid free radical scavenger showed initial beneficial results. The drug was well-tolerated with few adverse effects. However, later studies could not demonstrate clinical benefit.[30]
TAK-044: An endothelin A/B (ETA/B receptor antagonist) was attempted on the premise that endothelins mediate vasoconstriction in the setting of cerebral vasospasm. However, there was no clinically significant benefit.[30]
Clazosentan-a selective ETA antagonist showed some promise based on its pharmacological profile. However, despite angiographic evidence of decreased spasm, clinical outcome was not improved.[30]
Nitric oxide is a free radical which mediates vasodilation in cerebral vessels. Nitric oxide donors like intraventicular administration of sodium nitroprusside and Transdermal application of nitroglycerin has been attempted without much success.[30]
As of now, the available evidence does not support use of any of the above pharmacological interventions except nimodipine and triple-H therapy.
Triple-H Therapy
Hypervolemia, hypertension and hemodulution is the most widely used and consistently effective regimen for prevention and treatment of ischemic neurological deficits due to cerebral vasospasm. The use of triple H therapy is based on the fact that due to increased resistance to blood flow from cerebral vasospasm and in the absence of effective autoregulation, CBF becomes pressure dependent.[31]
The therapeutic goal, for this purpose is to increase systolic arterial pressure to approximately 120-150 mm Hg in unclipped and 160-200 mm Hg in clipped aneurysms (using intravenous (I.V.) fluids and vasopressors). Central venous pressure (CVP) is maintained at 8-12 mm Hg (or pulmonary artery wedge pressure at 15-18 mm Hg) and hematocrit is decreased to approximately 30-33%.
This therapy is most successful when administered at early stages before the onset of infarction. However, due to the risk of re-bleeding when administered before aneurysmal clipping, it should be instituted only after clipping of aneurysm. The therapy also carries risks of other potentially life-threatening complications such as re-bleeding (19%), pulmonary edema (17%), myocardial ischemia (2%), dilutional hyponatremia (3%),congestive cardiac failure, coagulopathy (3%), intracranial hypertension, worsening of cerebral edema, hemorrhagic transformation of cerebral infarct, and hypertensive encephalopathy.[30]
Thus, the recent consensus recommends that euvolemia rather than hypervolemia should be the target for both prophylaxis and treatment and hemodilution should not be used.[32] The pressure should be increased in a stepwise fashion with maintenance of ABP at supranormal level under guided assessment of neurological function and TCD monitoring.[33,34]
Endovascular Treatment
Transluminal balloon angioplasty has been widely used to reverse vasospasm in major basal as well as distal arteries with or without concomitant intra-arterial administration of papaverine.[35,36] This is indicated in patients with persistent new neurological deficit that is unresponsive to medical therapy. However, timing of intervention is crucial as there is evidence to suggest that early angioplasty (within 2 h from onset of symptoms) is associated with sustained clinical improvement.[35]
Intra-arterial administration of vasodilators such as papaverine, verapamil, milrinone, nimodipine in the distal vessels through super selective intra-arterial infusion have been shown to be effective.
Management of subarachnoid hemorrhage
Most of the patients with aneurysm are operated as soon as possible. Management of SAH includes securing the aneurysm, so that further bleeding is prevented and management of any complications resulting thereof. The advancements in minimally invasive neurosurgery have led to options of either clipping the aneurysms surgically or occluding them via the endovascular route.
Clipping Versus Coiling
Coiling works by placing extremely coiled material inside the aneurysm, the material of which is extremely thrombogenic which allows aneurysm to be occluded completely.[37] Coiling of an unruptured aneurysm has been reported to result in 7.4% reduction in mortality risk as compared to clipping.[38] This has prompted many surgeons to favor coiling over clipping.
However, coiling itself carries many risks. Firstly, the aneurysm may rupture with the angiographic manipulation, secondly part of the coil could embolise out of the aneurysm into a more distal artery and lastly, the thrombus formation may extend out of the aneurysm and cause thrombus formation in the feeding vessels.[39] However, the controversy continues which one — clipping or coiling has a better outcome. The usual practice is that patients are first evaluated by an intervention specialist. If coiling can be safely performed, then this is tried first. If coiling fails or coiling is considered difficult then clipping is performed. This decision is based on the expertise and experience of the interventional specialist and the available facilities.[40,41]
Preoperative assessment of a patient posted for aneurysm clipping:[42]
The main steps in preoperative evaluation are as follows:
Assessment of the patient's neurologic condition and clinical grading of the SAH.
A review of the intracranial pathologic condition by evaluating CT for evidence of raised ICP.
Evaluation of other systemic functions, premorbid as well as current condition, with emphasis on systems known to be affected by SAH.
Communication with the neurosurgeon regarding positioning and special monitoring requirements.
Optimization of the patient's condition by correcting any existing electrolyte disturbances which are quite common in aneurysm patients.
Premedication
Premedications such as barbiturates and narcotics may cause respiratory depression with resultant increase in PaCO2, leading to an increase in CBF and cerebral blood volume.[43] So premedication is often avoided. Anxious patients with good Hunt and Hess grades may receive smaller doses of oral benzodiazepines. Patients should continue to receive their regular doses of anticonvulsants, nimodipine and dexamethasone. Drugs to reduce gastric acidity and increase gastric emptying should be prescribed as part of premedication or given I.V. before induction of anesthesia.
Intraoperative Considerations
Any sudden rise in blood pressure during tracheal intubation can result in rupture of aneurysm. Therefore, the goal during induction of anesthesia is to reduce the risk of aneurysm rupture by minimizing the transmural pressure (TMP) while simultaneously maintaining an adequate cerebral perfusion pressure (CPP). As illustrated in Figure 1, both TMP and CPP are determined by the same equation, mean arterial blood pressure (MAP) minus ICP (MAP — ICP). Thus these goals represent opposite objectives.[42]
Figure 1.
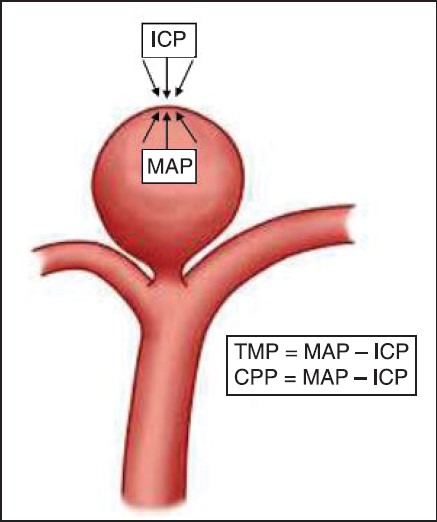
Transmural pressure = Mean arterial blood pressure (MAP) — intracranial pressure (ICP); cerebral perfusion pressure = MAP — ICP
Blood pressure management should be done taking into account patient's clinical grade and baseline blood pressure values. Patients who have been normotensive or those with SAH Grades 0, I, and II generally have normal ICP and are not experiencing acute ischemia.[8] These patients, therefore, tolerate a bigger transient decrease in blood pressure (30-35%). In contrast, patients with poor clinical grades frequently have increased ICP, low CPP, and ischemia.[44] The elevated ICP decreases the TMP and partially protects the aneurysm from re-rupture. These patients may not tolerate transient hypotension as well, and the duration and magnitude of blood pressure decrease should be moderated. As a general principle, the patient's blood pressure should be reduced to 20-25% below the baseline value, and prophylaxis to blunt the hypertensive response to laryngoscopy and intubation should be instituted before tracheal intubation is attempted.
Similarly, discretion should be used whether hyperventilation will be beneficial or harmful. Patients with a good clinical grade should not be hyperventilated, because the reduction in CBF will lead to a reduction in ICP and consequently, an increase in TMP. Conversely, patients with poor clinical grades should be managed with moderate hyperventilation to improve cerebral perfusion. Sudden changes in MAP should be avoided to reduce the risk of aneurysm rupture and ischemia.[45]
Monitoring
Standard monitoring usually includes 5-lead electrocardiogram, continuous intra-arterial pressure, pulse oximetry, capnography, urinary output, body temperature, and neuromuscular block. It is preferable to initiate invasive blood pressure monitoring prior to induction. Many neuroanesthetists routinely insert a central venous catheter for guidance of intravascular volume, for the injection of potent cardiovascular drugs in the case of severe cardiovascular instability, and for the administration of mannitol (which may cause local inflammation when administered through a smaller peripheral vein.[46] Cardiac compromised patients or those with severe vasopspasm benefit from invasive cardiac output and pulmonary capillary wedge pressure monitoring, so Swan-Ganz catheter should be considered in these patients. Monitoring for adequacy of cerebral circulation may be performed by Jugular oximetry, noninvasive cerebral oximetry, TCD, brain stem auditory, somatosensory, and motor evoked potentials depending on the location of aneurysm. Jugular oximetry reflects the balance between cerebral metabolic supply and demand. This approach is analogous to monitoring of mixed venous oxygen saturation as an index of the balance between systemic metabolic requirement and cardiac output. The use of jugular venous oximetry in the intensive care of head-injured patients is relatively well-established. Its use in the intraoperative management of patients undergoing cerebral aneurysm surgery; however, remains investigative rather than routine.[42]
Regional cerebral oximetry uses optical spectroscopy to measure brain vascular hemoglobin saturation in a noninvasive manner to provide similar information. Recently, microscope integrated intraoperative near-infrared indocyanine green angiography has been used to access circulation in the operative field.[47] It should be noted that data from these monitoring techniques should be analyzed in conjunction with each other and each of these monitoring systems have their own drawbacks.
Induction
Intravenous induction is preferred and any of the short acting induction agents, propofol, thiopentone sodium, or etomidate can be used provided these agents are used in carefully titrated doses avoiding sudden changes in blood pressure.[48] Short acting opioids like fentanyl, alfentanil or sufentanil are included in induction. There is concern of increased in ICP when using opiods especially sufentanil. Nevertheless, concurrent use of induction agents and mild hyperventilation should mitigate any increase in ICP.
Agents used to blunt laryngoscopic response to intubation include use of high-dose narcotics (e.g., fentanyl, 5-10 μg/kg, or sufentanil, 0.5-1.0 μg/kg), β-adrenergic antagonists (e.g., esmolol, 0.5 mg/kg), labetalol (10-20 mg), I.V. or topical lidocaine (1.5-2.0 mg/kg), a second dose of propofol (0.5-1 mg/kg) or thiopental (1-2 mg/kg), or a deep level of an inhalation anesthetic such as isoflurane or sevoflurane. We routinely administer I.V. lidocaine, 1.5 mg/kg 2-3 min before intubation.[49]
Muscle relaxants
The choice of muscle relaxant depends on the anesthesiologist's preference. There is concern of using succinylcholine which has been reported to increase ICP; however, it has been used successfully in many aneurysm patients with no known sequelae.[50] Moreover, this increase in ICP is not seen when the patients are deeply anesthetized or when succinylcholine is preceded by a defasciculating dose of a nondepolarizing agent.[51] However, in view of these concerns many neuroanesthesiologists prefer to use a nondepolarizing agent for intubation also. Our practice is to use a succinylcholine only for anticipated difficult airway or patients with a full stomach. Rocuronium is a nondepolarizing agent which has no effect on CBF or ICP and is a suitable alternative for rapid sequence induction in a dose of 0.6-1.2 mg/kg.
Positioning
Patients should be carefully positioned avoiding injuries to eyes, nose, and pressure points. Care should be taken to avoid excessive neck flexion or lateral rotation as this can obstruct venous drainage and increase ICP. In particular, care should be taken to avoid increase in heart rate and blood pressure on inserting head pins. This is done by giving additional dose of fentanyl bolus prior to pin insertion or by deepening the plane of anesthesia using IV anesthetic agents or volatile agents.
Maintenance of anesthesia
The goals during maintenance of anesthesia are to:[42]
Provide a relaxed or “slack” brain that will allow minimal retraction pressure.
Maintain perfusion to the brain.
Reduce TMP if necessary during dissection of the aneurysm and final clipping and
Allow prompt awakening and assessment of patients with good SAH grades.
With the trend toward early surgery, greater number of cases can be expected to have tight brain with difficult operating conditions in which maximal brain “relaxation” therapy is required. The choice of anesthetic agents should take into account the brain condition based on preoperative radiologic investigations and Hunt and Hess grading. Either an I.V. or inhalation anesthetic or a combination of both can be used to provide such conditions. Propofol has cerebral vasoconstrictive actions, thus it causes decrease in CBF, cerebral metabolic rate and thus decreases ICP.[52,53] Isoflurane, sevoflurane and desflurane may also be used in concentrations <1 minimum alveolar concentration (MAC) beyond which these cause increase in ICP. There is however, some evidence to suggest that sevoflurane may be used in concentration up to 1.5 MAC since it tends to preserve autoregulation even up to 1.5 MAC, beyond which brain oxygen consumption is increased by 25%.[54,55] Sudden increases in inspired concentration of desflurane tends to increase ICP due to its sympathoadrenal effect which may be mitigated by avoiding rapid increases in dial settings of desflurane.
Intraoperative analgesia is provided either by intermittent boluses or infusions of opioids. Periods of intense stimulation like pin insertion, skin incision or elevation of periostium and incision of duramater should be covered by administering additional doses of opioids and deepening the planes of anesthesia to prevent hypertension.[42]
Brain relaxation
Optimal brain relaxation and reduction in brain bulk helps surgical exposure, reduce the forces required for brain retraction, and facilitates clipping of the aneurysm. Following agents/maneuvers have been used in combination:[45,56]
Positioning
15-30° head up position is the optimal position which decreases ICP yet maintains CPP. Excessive neck flexion or rotation should be avoided. Endotracheal tube should be taped instead of tying it around the neck. If it has to be tied across the neck, it should be ensured that the tie is not too tight and there is no pressure on neck veins.
Mannitol
Mannitol is usually the drug of choice to decrease brain water content. 20% mannitol (0.5-2 g/kg) is usually given over 30 min. The usual dose is 1 g/kg; an additional dose is given when indicated by the brain conditions. A total dose of 2 g/kg is frequently given when temporary artery occlusion is planned.
Frusemide
Frusemide reduces CSF formation and water and ion movement across the blood–brain barrier. The prolonged diuresis after the administration of frusemide can potentiate the effect of mannitol by sustaining elevated serum osmolality.[57] Frusemide is used in a dose of 0.25-1 g/kg.
Hypertonic saline
Hypertonic saline (3%) is a equi-efficacious to 20% mannitol in the extent of brain relaxation.[58] However, mannitol continues to remains the drug of choice for intraoperative brain relaxation.
Cerebrospinal fluid drainage
Decreasing the volume of CSF using a lumbar subarachnoid or ventriculostomy catheter is an effective means of reducing brain bulk and may become necessary to achieve satisfactory brain relaxation. Extreme caution should however, be exercised during insertion of the drain to minimize CSF loss and a sudden decrease in ICP, so as to avoid an abrupt increase in TMP and a re-bleed. Due to the risk of brainstem herniation, lumbar drainage of CSF is contraindicated in patients with intracerebral hematoma. In theory, free drainage should be allowed only after the dura is open to minimize the risk of re-bleeding; in practice; however, 20-30 mL of CSF is usually drained just before dural opening to facilitate dural incision. The drain is usually left open during the procedure, until the aneurysm is clipped or until the beginning of dural closure.
Hyperventilation
Controlling CO2 levels can be used therapeutically to lower ICP. However, excessive hyperventilation carries the risk of inducing ischemia especially in poor grade patients. Thus, use of hyperventilation should be individualized according to the operating conditions. A reasonable approach is to institute mild hypocapnia (30-35 mm Hg) before the dura is open, moderate hypocapnia (25-30 mm Hg) after the dura is open, and relative normocapnia during induced hypotension and after the aneurysm is clipped.[42]
Fluids and electrolytes
Fluids should be planned to ensure normovolemia and be guided by clinical judgment and CVP. In general, glucose containing fluids should not be administered. Ringer's lactate being hypoosmolar as compared to plasma should also be avoided. Normal saline and balanced crystalloids can be administered. After aneurysm is clipped, relative hypervolemia (CVP 10-12 cm H2O) should be targeted.
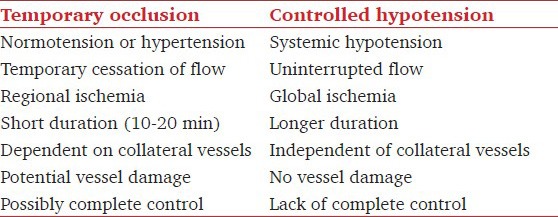
Controlled hypotension versus temporary clipping
In the past, during dissection around the aneurysm, deliberate hypotension was attempted in order to reduce wall tension to prevent aneurysm from rupturing. Hypotension also reduces bleeding, allowing better visualization of the anatomy of the aneurysm and the perforating vessels. However, nowadays temporary clips are applied on the major feeding vessel.[59] This effectively stops further blood supply into that vessel so that dissection can be safely performed around the aneurysm. The differences between hypotension and temporary clipping are given in Table 5. In contrast to controlled hypotension, blood pressure is slightly increased during temporary clipping so as to increase blood flow through the collaterals.
Table 5.
Temporary arterial occlusion versus controlled hypotension during cerebral aneurysm surgery
Cerebral protection during temporary clipping
High-dose mannitol (2 g/kg) has been used.[60]
Sendai cocktail: It includes a combination of mannitol (500 mL of a 20% solution, or 100 g), vitamin E (500 mg), and dexamethasone (50 mg).[61] The regimen is based on the fact that neuronal damage may be mediated by the production of free radicals and this combination has anti-oxidant properties. Up to 60 min of temporary arterial occlusion has been obtained with use of this regimen without apparent postoperative neurologic deficits. In general, however, 15-20 min is considered to be the upper limit of safety.[62]
Metabolic suppression using propofol, thiopentone or etomidate. The theoretical basis for their use is that cerebral metabolic suppression reduces the energy consumed by the brain cells, ischemia thus is better tolerated.[63] Ideally, burst suppression using electroencephalography should be employed when using these agents. Etomidate appears less effective than others.
Hypothermia: Hypothermia has important cerebroprotective effects. It causes a reduction in cerebral metabolic requirement for oxygen beyond what can be achieved with other neuroprotective agents.[64] However, hypothermia is associated with increased incidence of coagulopathy, cardiac dysrhythmias, wound infection, and need for postoperative ventilation.[65] Even mild levels of hypothermia are not without potential risk. Passive rewarming is associated with peripheral vasoconstriction, shivering, and subsequent increases in oxygen consumption and myocardial work.[42] Drug metabolism is decreased, prolonging the effect of even short acting anesthetic drugs. A conservative recommendation would be mild body temperature reduction (34-35°C) until closure is imminent, followed by active rewarming.[42] However, the evidence for benefits of this approach is lacking as studies evaluating mild hypothermia (33.5°C) in aneurysm surgery found no effect on neurologic outcome in patients with favorable clinical grade aneurysms.[66] Careful temperature monitoring should continue throughout the perioperative period.
Magnesium has shown some promise for neuroprotection, but the results are conflicting.[67]
Emergence and recovery
In general, patients with Grade I or II with uneventful surgery may be extubated in operating room itself. Grade IV and V patients are often kept electively intubated and ventilated. Decision in Grade III patients should be tailored often in coordination with the surgical team. Patients who have experienced intraoperative aneurysm rupture or those with vertebrobasilar aneurysms must be considered individually irrespective of their preoperative clinical SAH grade. I.V. lidocaine, 1.5 mg/kg, may be used during extubation to prevent coughing. Excessive increases in blood pressures may be treated with esmolol (0.1-0.5 mg/kg) or labetalol (5-10 mg) boluses.
Postoperative management
Patients are usually kept in ICU postoperatively where close monitoring is continued and HHH therapy mentioned above is initiated. Attention should be given to patient's neurological status, hemodynamic parameters and fluid status. In patients with multiple aneurysms, systemic blood pressure must continue under strict control (within 20% of their normal blood pressure) to prevent rupture of unclipped aneurysms during emergence and recovery from anesthesia.
Summary
Patients presenting with aneurysmal SAH should be operated as soon as possible. Better understanding of altered physiology and central nervous system effects of available drugs allows adequate brain relaxation, thereby ensuring good outcomes.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Pong RP, Lam AM. Anaesthetic management of cerebral aneurysm surgery. In: Cotrell JE, Young WL, editors. Neuroanaesthesia. 5th ed. Missouri: Mosby Elsevier; 2010. pp. 218–46. [Google Scholar]
- 2.Macmillan CS, Grant IS, Andrews PJ. Pulmonary and cardiac sequelae of subarachnoid haemorrhage: Time for active management? Intensive Care Med. 2002;28:1012–23. doi: 10.1007/s00134-002-1382-7. [DOI] [PubMed] [Google Scholar]
- 3.Passier PE, Visser-Meily JM, van Zandvoort MJ, Post MW, Rinkel GJ, van Heugten C. Prevalence and determinants of cognitive complaints after aneurysmal subarachnoid hemorrhage. Cerebrovasc Dis. 2010;29:557–63. doi: 10.1159/000306642. [DOI] [PubMed] [Google Scholar]
- 4.Al-Shahi R, White PM, Davenport RJ, Lindsay KW. Subarachnoid haemorrhage. BMJ. 2006;333:235–40. doi: 10.1136/bmj.333.7561.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Gans K, Nieuwkamp DJ, Rinkel GJ, Algra A. Timing of aneurysm surgery in subarachnoid hemorrhage: A systematic review of the literature. Neurosurgery. 2002;50:336–40. doi: 10.1097/00006123-200202000-00018. [DOI] [PubMed] [Google Scholar]
- 6.Feigin VL, Lawes CM, Bennett DA, Barker-Collo SL, Parag V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: A systematic review. Lancet Neurol. 2009;8:355–69. doi: 10.1016/S1474-4422(09)70025-0. [DOI] [PubMed] [Google Scholar]
- 7.Kotapka MJ, Flamm ES. Cerebral aneurysms: Surgical considerations. In: Cotrell JE, Smith DS, editors. Anaesthesia and Neurosurgery. 4th ed. St.Louis: Mosby; 2001. pp. 353–65. [Google Scholar]
- 8.Feigin VL, Rinkel GJ, Lawes CM, Algra A, Bennett DA, van Gijn J, et al. Risk factors for subarachnoid hemorrhage: An updated systematic review of epidemiological studies. Stroke. 2005;36:2773–80. doi: 10.1161/01.STR.0000190838.02954.e8. [DOI] [PubMed] [Google Scholar]
- 9.Hunt WE, Hess RM. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg. 1968;28:14–20. doi: 10.3171/jns.1968.28.1.0014. [DOI] [PubMed] [Google Scholar]
- 10.Report of World Federation of Neurological Surgeons Committee on a Universal Subarachnoid Hemorrhage Grading Scale. J Neurosurg. 1988;68:985–6. doi: 10.3171/jns.1988.68.6.0985. [DOI] [PubMed] [Google Scholar]
- 11.Kothavale A, Banki NM, Kopelnik A, Yarlagadda S, Lawton MT, Ko N, et al. Predictors of left ventricular regional wall motion abnormalities after subarachnoid hemorrhage. Neurocrit Care. 2006;4:199–205. doi: 10.1385/NCC:4:3:199. [DOI] [PubMed] [Google Scholar]
- 12.Diringer MN, Lim JS, Kirsch JR, Hanley DF. Suprasellar and intraventricular blood predict elevated plasma atrial natriuretic factor in subarachnoid hemorrhage. Stroke. 1991;22:577–81. doi: 10.1161/01.str.22.5.577. [DOI] [PubMed] [Google Scholar]
- 13.Nelson RJ, Roberts J, Rubin C, Walker V, Ackery DM, Pickard JD. Association of hypovolemia after subarachnoid hemorrhage with computed tomographic scan evidence of raised intracranial pressure. Neurosurgery. 1991;29:178–82. doi: 10.1097/00006123-199108000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Fisher CM, Kistler JP, Davis JM. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery. 1980;6:1–9. doi: 10.1227/00006123-198001000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Macrea LM, Tramèr MR, Walder B. Spontaneous subarachnoid hemorrhage and serious cardiopulmonary dysfunction: A systematic review. Resuscitation. 2005;65:139–48. doi: 10.1016/j.resuscitation.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 16.Kassell NF, Torner JC, Haley EC, Jr, Jane JA, Adams HP, Kongable GL. The International Cooperative Study on the Timing of Aneurysm Surgery. Part 1: Overall management results. J Neurosurg. 1990;73:18–36. doi: 10.3171/jns.1990.73.1.0018. [DOI] [PubMed] [Google Scholar]
- 17.Haley EC, Jr, Kassell NF, Torner JC. The International Cooperative Study on the Timing of Aneurysm Surgery. The North American experience. Stroke. 1992;23:205–14. doi: 10.1161/01.str.23.2.205. [DOI] [PubMed] [Google Scholar]
- 18.Gaberel T, Magheru C, Emery E, Derlon JM. Antifibrinolytic therapy in the management of aneurismal subarachnoid hemorrhage revisited. A meta-analysis. Acta Neurochir (Wien) 2012;154:1–9. doi: 10.1007/s00701-011-1179-y. [DOI] [PubMed] [Google Scholar]
- 19.Roos YB, Rinkel GJ, Vermeulen M, Algra A, van Gijn J. Antifibrinolytic therapy for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev. 2003;2:CD001245. doi: 10.1002/14651858.CD001245. [DOI] [PubMed] [Google Scholar]
- 20.Demirgil BT, Tugcu B, Postalci L, Guclu G, Dalgic A, Oral Z. Factors leading to hydrocephalus after aneurysmal subarachnoid hemorrhage. Minim Invasive Neurosurg. 2003;46:344–8. doi: 10.1055/s-2003-812500. [DOI] [PubMed] [Google Scholar]
- 21.Harrod CG, Bendok BR, Batjer HH. Prediction of cerebral vasospasm in patients presenting with aneurysmal subarachnoid hemorrhage: A review. Neurosurgery. 2005;56:633–54. doi: 10.1227/01.neu.0000156644.45384.92. [DOI] [PubMed] [Google Scholar]
- 22.Zetterling M, Hallberg L, Hillered L, Karlsson T, Enblad P, Ronne Engström E. Brain energy metabolism in patients with spontaneous subarachnoid hemorrhage and global cerebral edema. Neurosurgery. 2010;66:1102–10. doi: 10.1227/01.NEU.0000370893.04586.73. [DOI] [PubMed] [Google Scholar]
- 23.Carrera E, Schmidt JM, Oddo M, Fernandez L, Claassen J, Seder D, et al. Transcranial Doppler for predicting delayed cerebral ischemia after subarachnoid hemorrhage. Neurosurgery. 2009;65:316–23. doi: 10.1227/01.NEU.0000349209.69973.88. [DOI] [PubMed] [Google Scholar]
- 24.Dhawan V, DeGeorgia M. Neurointensive care biophysiological monitoring. J Neurointerv Surg. 2012;4:407–13. doi: 10.1136/neurintsurg-2011-010158. [DOI] [PubMed] [Google Scholar]
- 25.Dorhout Mees SM, Rinkel GJ, Feigin VL, Algra A, van den Bergh WM, Vermeulen M, et al. Calcium antagonists for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev. 2007;18:CD000277. doi: 10.1002/14651858.CD000277.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suarez JI. Participants in the International Multidisciplinary Consensus Conference on the Critical Care Management of Subarachnoid Hemorrhage. Magnesium sulfate administration in subarachnoid hemorrhage. Neurocrit Care. 2011;15:302–7. doi: 10.1007/s12028-011-9603-y. [DOI] [PubMed] [Google Scholar]
- 27.Wong GK, Poon WS, Chan MT, Boet R, Gin T, Ng SC, et al. Plasma magnesium concentrations and clinical outcomes in aneurysmal subarachnoid hemorrhage patients: Post hoc analysis of intravenous magnesium sulphate for aneurysmal subarachnoid hemorrhage trial. Stroke. 2010;41:1841–4. doi: 10.1161/STROKEAHA.110.585232. [DOI] [PubMed] [Google Scholar]
- 28.Vergouwen MD, de Haan RJ, Vermeulen M, Roos YB. Effect of statin treatment on vasospasm, delayed cerebral ischemia, and functional outcome in patients with aneurysmal subarachnoid hemorrhage: A systematic review and meta-analysis update. Stroke. 2010;41:e47–52. doi: 10.1161/STROKEAHA.109.556332. [DOI] [PubMed] [Google Scholar]
- 29.Tseng MY. Participants in the International Multidisciplinary Consensus Conference on the Critical Care Management of Subarachnoid Hemorrhage. Summary of evidence on immediate statins therapy following aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2011;15:298–301. doi: 10.1007/s12028-011-9596-6. [DOI] [PubMed] [Google Scholar]
- 30.Rowland MJ, Hadjipavlou G, Kelly M, Westbrook J, Pattinson KT. Delayed cerebral ischaemia after subarachnoid haemorrhage: Looking beyond vasospasm. Br J Anaesth. 2012;109:315–29. doi: 10.1093/bja/aes264. [DOI] [PubMed] [Google Scholar]
- 31.Naval NS, Stevens RD, Mirski MA, Bhardwaj A. Controversies in the management of aneurysmal subarachnoid hemorrhage. Crit Care Med. 2006;34:511–24. doi: 10.1097/01.ccm.0000198331.45998.85. [DOI] [PubMed] [Google Scholar]
- 32.Treggiari MM. Participants in the International Multi-disciplinary Consensus Conference on the Critical Care Management of Subarachnoid Hemorrhage. Hemodynamic management of subarachnoid hemorrhage. Neurocrit Care. 2011;15:329–35. doi: 10.1007/s12028-011-9589-5. [DOI] [PubMed] [Google Scholar]
- 33.Diringer MN, Bleck TP, Claude Hemphill J, 3 rd, Menon D, Shutter L, Vespa P, et al. Critical care management of patients following aneurysmal subarachnoid hemorrhage: Recommendations from the Neurocritical Care Society's Multidisciplinary Consensus Conference. Neurocrit Care. 2011;15:211–40. doi: 10.1007/s12028-011-9605-9. [DOI] [PubMed] [Google Scholar]
- 34.Dankbaar JW, Slooter AJ, Rinkel GJ, Schaaf IC. Effect of different components of triple-H therapy on cerebral perfusion in patients with aneurysmal subarachnoid haemorrhage: A systematic review. Crit Care. 2010;14:R23. doi: 10.1186/cc8886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenwasser RH, Armonda RA, Thomas JE, Benitez RP, Gannon PM, Harrop J. Therapeutic modalities for the management of cerebral vasospasm: Timing of endovascular options. Neurosurgery. 1999;44:975–9. doi: 10.1097/00006123-199905000-00022. [DOI] [PubMed] [Google Scholar]
- 36.Santillan A, Knopman J, Zink W, Patsalides A, Gobin YP. Transluminal balloon angioplasty for symptomatic distal vasospasm refractory to medical therapy in patients with aneurysmal subarachnoid hemorrhage. Neurosurgery. 2011;69:95–101. doi: 10.1227/NEU.0b013e31821424f9. [DOI] [PubMed] [Google Scholar]
- 37.Qureshi AI, Suri MF, Khan J, Kim SH, Fessler RD, Ringer AJ, et al. Endovascular treatment of intracranial aneurysms by using Guglielmi detachable coils in awake patients: Safety and feasibility. J Neurosurg. 2001;94:880–5. doi: 10.3171/jns.2001.94.6.0880. [DOI] [PubMed] [Google Scholar]
- 38.Molyneux AJ, Kerr RS, Yu LM, Clarke M, Sneade M, Yarnold JA, et al. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: A randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2005;366:809–17. doi: 10.1016/S0140-6736(05)67214-5. [DOI] [PubMed] [Google Scholar]
- 39.van Rooij WJ, Sluzewski M, Beute GN, Nijssen PC. Procedural complications of coiling of ruptured intracranial aneurysms: Incidence and risk factors in a consecutive series of 681 patients. AJNR Am J Neuroradiol. 2006;27:1498–501. [PMC free article] [PubMed] [Google Scholar]
- 40.Derdeyn CP, Barr JD, Berenstein A, Connors JJ, Dion JE, Duckwiler GR, et al. The International Subarachnoid Aneurysm Trial (ISAT): A position statement from the Executive Committee of the American Society of Interventional and Therapeutic Neuroradiology and the American Society of Neuroradiology. AJNR Am J Neuroradiol. 2003;24:1404–8. [PMC free article] [PubMed] [Google Scholar]
- 41.Harbaugh RE, Heros RC, Hadley MN. Position statement on the International Subarachnoid Aneurysm Trial (ISAT) [Last accessed on 2006 Dec 30]. Available from: http://www.aans.org/Library/Article.aspx?ArticleId=9703 .
- 42.Pong RP, Lam AM. Anesthetic management of cerebral aneurysm surgery. In: Cottrell JE, Oung WL, editors. Cottrell's and Young's Neuroanesthesia. 5th ed. Philadelphia: Mosby Elsevier; 2010. pp. 218–46. [Google Scholar]
- 43.Dorairaj IL, Hancock SM. Anaesthesia for interventional neuroradiology. Contin Educ Anaesth Crit Care Pain. 2008;8:86–9. [Google Scholar]
- 44.Voldby B, Enevoldsen EM, Jensen FT. Cerebrovascular reactivity in patients with ruptured intracranial aneurysms. J Neurosurg. 1985;62:59–67. doi: 10.3171/jns.1985.62.1.0059. [DOI] [PubMed] [Google Scholar]
- 45.Priebe HJ. Aneurysmal subarachnoid haemorrhage and the anaesthetist. Br J Anaesth. 2007;99:102–18. doi: 10.1093/bja/aem119. [DOI] [PubMed] [Google Scholar]
- 46.Torre-Healy A, Marko NF, Weil RJ. Hyperosmolar therapy for intracranial hypertension. Neurocrit Care. 2012;17:117–30. doi: 10.1007/s12028-011-9649-x. [DOI] [PubMed] [Google Scholar]
- 47.Gruber A, Dorfer C, Standhardt H, Bavinzski G, Knosp E. Prospective comparison of intraoperative vascular monitoring technologies during cerebral aneurysm surgery. Neurosurgery. 2011;68:657–73. doi: 10.1227/NEU.0b013e31820777ee. [DOI] [PubMed] [Google Scholar]
- 48.Saito S, Kadoi Y, Nara T, Sudo M, Obata H, Morita T, et al. The comparative effects of propofol versus thiopental on middle cerebral artery blood flow velocity during electroconvulsive therapy. Anesth Analg. 2000;91:1531–6. doi: 10.1097/00000539-200012000-00043. [DOI] [PubMed] [Google Scholar]
- 49.Woods AW, Allam S. Tracheal intubation without the use of neuromuscular blocking agents. Br J Anaesth. 2005;94:150–8. doi: 10.1093/bja/aei006. [DOI] [PubMed] [Google Scholar]
- 50.Lanier WL, Milde JH, Michenfelder JD. Cerebral stimulation following succinylcholine in dogs. Anesthesiology. 1986;64:551–9. doi: 10.1097/00000542-198605000-00003. [DOI] [PubMed] [Google Scholar]
- 51.Kovarik WD, Mayberg TS, Lam AM, Mathisen TL, Winn HR. Succinylcholine does not change intracranial pressure, cerebral blood flow velocity, or the electroencephalogram in patients with neurologic injury. Anesth Analg. 1994;78:469–73. doi: 10.1213/00000539-199403000-00008. [DOI] [PubMed] [Google Scholar]
- 52.Petersen KD, Landsfeldt U, Cold GE, Petersen CB, Mau S, Hauerberg J, et al. Intracranial pressure and cerebral hemodynamic in patients with cerebral tumors: A randomized prospective study of patients subjected to craniotomy in propofol-fentanyl, isoflurane-fentanyl, or sevoflurane-fentanyl anesthesia. Anesthesiology. 2003;98:329–36. doi: 10.1097/00000542-200302000-00010. [DOI] [PubMed] [Google Scholar]
- 53.Miura Y, Kamiya K, Kanazawa K, Okada M, Nakane M, Kumasaka A, et al. Superior recovery profiles of propofol-based regimen as compared to isoflurane-based regimen in patients undergoing craniotomy for primary brain tumor excision: A retrospective study. J Anesth. 2012;26:721–7. doi: 10.1007/s00540-012-1398-2. [DOI] [PubMed] [Google Scholar]
- 54.Dinsmore J. Anaesthesia for elective neurosurgery. Br J Anaesth. 2007;99:68–74. doi: 10.1093/bja/aem132. [DOI] [PubMed] [Google Scholar]
- 55.Gupta S, Heath K, Matta BF. Effect of incremental doses of sevoflurane on cerebral pressure autoregulation in humans. Br J Anaesth. 1997;79:469–72. doi: 10.1093/bja/79.4.469. [DOI] [PubMed] [Google Scholar]
- 56.Sankhyan N, Vykunta Raju KN, Sharma S, Gulati S. Management of raised intracranial pressure. Indian J Pediatr. 2010;77:1409–16. doi: 10.1007/s12098-010-0190-2. [DOI] [PubMed] [Google Scholar]
- 57.Todd MM, Cutkomp J, Brian JE. Influence of mannitol and furosemide, alone and in combination, on brain water content after fluid percussion injury. Anesthesiology. 2006;105:1176–81. doi: 10.1097/00000542-200612000-00017. [DOI] [PubMed] [Google Scholar]
- 58.Rozet I, Tontisirin N, Muangman S, Vavilala MS, Souter MJ, Lee LA, et al. Effect of equiosmolar solutions of mannitol versus hypertonic saline on intraoperative brain relaxation and electrolyte balance. Anesthesiology. 2007;107:697–704. doi: 10.1097/01.anes.0000286980.92759.94. [DOI] [PubMed] [Google Scholar]
- 59.Choi JW, Kim TS, Joo SP, Lee JK, Kim JH, Kim SH. Effect of temporary clipping on the cerebral infarction in middle cerebral artery aneurysm surgery. Korean J Cerebrovasc Surg. 2006;8:248–53. [Google Scholar]
- 60.Romero Kräuchi O, Verger Bennasar AM. Protective measures against cerebral ischemia following subarachnoid hemorrhage: Part 1. Rev Esp Anestesiol Reanim. 2011;58:230–5. doi: 10.1016/s0034-9356(11)70045-7. [DOI] [PubMed] [Google Scholar]
- 61.Baughman VL. Brain protection during neurosurgery. Anesthesiol Clin North America. 2002;20:315–27. doi: 10.1016/s0889-8537(01)00004-9. vi. [DOI] [PubMed] [Google Scholar]
- 62.Ogilvy CS, Chu D, Kaplan S. Mild hypothermia, hypertension, and mannitol are protective against infarction during experimental intracranial temporary vessel occlusion. Neurosurgery. 1996;38:1202–9. doi: 10.1097/00006123-199606000-00030. [DOI] [PubMed] [Google Scholar]
- 63.McConkey PP, Kien ND. Cerebral protection with thiopentone during combined carotid endarterectomy and clipping of intracranial aneurysm. Anaesth Intensive Care. 2002;30:219–22. doi: 10.1177/0310057X0203000217. [DOI] [PubMed] [Google Scholar]
- 64.El Beheiry H. Protecting the brain during neurosurgical procedures: Strategies that can work. Curr Opin Anaesthesiol. 2012;25:548–55. doi: 10.1097/ACO.0b013e3283579622. [DOI] [PubMed] [Google Scholar]
- 65.Bao L, Xu F. Fundamental research progress of mild hypothermia in cerebral protection. Springerplus. 2013;2:306. doi: 10.1186/2193-1801-2-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nguyen HP, Zaroff JG, Bayman EO, Gelb AW, Todd MM, Hindman BJ, et al. Perioperative hypothermia (33 degrees C) does not increase the occurrence of cardiovascular events in patients undergoing cerebral aneurysm surgery: Findings from the intraoperative hypothermia for aneurysm surgery trial. Anesthesiology. 2010;113:327–42. doi: 10.1097/ALN.0b013e3181dfd4f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhao ZX, Wu C, He M. A systematic review of clinical outcomes, perioperative data and selective adverse events related to mild hypothermia in intracranial aneurysm surgery. Clin Neurol Neurosurg. 2012;114:827–32. doi: 10.1016/j.clineuro.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 68.Chan MT, Boet R, Ng SC, Poon WS, Gin T. Magnesium sulfate for brain protection during temporary cerebral artery occlusion. Acta Neurochir Suppl. 2005;95:107–11. doi: 10.1007/3-211-32318-x_23. [DOI] [PubMed] [Google Scholar]


