Abstract
Background and Aims:
Perioperative shivering, in geriatric patients undergoing urological surgery under central neuraxial blockade is a common complication. Prophylactic measures to reduce shivering are quintessential to decrease the morbidity and mortality. Believing that oral formulation will bring down the cost of treatment, we decided to compare the efficacy of oral clonidine and tramadol, as premedication, in prevention of shivering in patients undergoing transurethral resection of prostate (TURP) under spinal anesthesia in a prospective and double-blind manner.
Materials and Methods:
The patients were randomly allocated into three groups (40 patients each). Group I received oral clonidine 150 μg, Group II received oral tramadol 50 mg, while Group III received a placebo. Number of patients having shivering, their grades and duration, hemodynamic changes, and side-effects in the form of sedation were recorded. Data were analyzed using analysis of variance, Student's t-test, Z test as and when appropriate.
Results:
In group I and II, 38 patients (95%) and 37 patients (92.5%) did not shiver, respectively. Although in the group III, 24 patients (60%) exhibited no grade of shivering, the shivering was of significantly severe intensity and lasted for a longer duration. No, clinically significant collateral effects were observed in patients who were administered clonidine or tramadol.
Conclusions:
Oral clonidine and tramadol were comparable in respect to their effect in decreasing the incidence, intensity, and duration of shivering when used prophylactically in patients who underwent TURP under subarachnoid blockade.
Keywords: Clonidine, postanesthetic shivering, spinal anesthesia, tramadol, transurethral resection of prostate
Introduction
Shivering in transurethral resection of prostate (TURP) may be due to decreased core temperature secondary to peripheral vasodilatation from sympathetic blockade and/or cold irrigating fluids.[1] Elderly patients are not able to initiate autonomic protective responses.[2] Thus, the reported increased morbidity after TURP could be due to rapid decrease in body temperature leading to increased perioperative cardiac stress.
Clonidine, a centrally acting selective partial alpha-2 agonist, has been previously used to obtund shivering[3] in patients undergoing urological surgery. Tramadol, a cyclohexanol derivative, has μ agonist activity as well as acts as an inhibitor of serotonin and norepinephrine uptake. Mainly used as an analgesic, its role as an agent to reduce perioperative shivering is well-established. Oral formulations of both these drugs are easily available. Oral tramadol Rs. 6.80/tablet and clonidine Re. 1/tablet) are economical when compared to their intravenous formulation (tramadol is for Rs. 17/injection and clonidine costs Rs. 43 for a 150 μg/ml injection). This study was intended to compare the prophylactic effectiveness of enteral formulations of clonidine and tramadol for prevention of perioperative shivering. Proving of their efficacy might integrate the use of these drugs as premedication in future.
Materials and Methods
After obtaining approval of Hospital Ethical Committee along with written informed consent, 120 geriatric male patients (American Society of Anesthesiologists Grades I to III), scheduled for elective TURP under subarachnoid blockade (SAB) were enrolled in this prospective, randomized double-blind study. Patients who were allergic to tramadol or clonidine, obese (body mass index >35%), febrile had a history of ischemic heart disease, thyroid disease, cerebrovascular disease, severe diabetic autonomic neuropathy, contraindication to regional anesthesia, or patients who had an infection of the urinary tract or external auditory meatus were excluded from the study. Vasodilator/vasoconstrictor medications likely to cause alteration in thermoregulation if taken by the patients also precluded us from considering them to be a part of the study.
Patients were randomly allocated to three groups by computer generated numbers, each receiving a sealed envelope of an oral formulation 90 min prior to the surgery. Group I received oral clonidine 150 μg, Group II received oral tramadol 50 mg, while the control Group III received starch tablet. Anesthesiology Department technicians who were not involved in the study prepared these trial preparations. They recorded the group randomization separately, such that the anesthesiologist recording the data and caring for the patient was unaware of what the preparation contained or which group the patient belonged to.
All patients were examined and investigated a day prior to surgery, and were advised fasting for 6 h prior to surgery.
All operations were performed in the same operation theater, which was maintained at constant ambient temperature and humidity. The operating room was not equipped to provide laminar flow. In the operation theater electrocardiogram, pulse oximetry and noninvasive blood pressure were attached and monitoring was initiated. SAB was achieved with bupivacaine (0.5% hyperbaric) administered under all aseptic precautions in a sitting position. Patients were made supine following the block and a level up to T9–T10 was achieved. Warmed (to body temperature) intravenous and irrigating fluids were used. Patients wore cotton surgical drapes and no means of active warming were used until deemed essential.
Heart rate, noninvasive blood pressure, respiratory rate, arterial oxygen saturation (SpO2); skin temperatures (axillary and forehead temperature, measured using temperature probes of GE Medical,Datex-Ohmeda Excel SE 210 Anesthesia Machine), core temperatures (tympanic membrane temperature, monitored with Braun Thermoscan®) were noted every 5 min for the 1st h and then every 10 min for the next half an hour. Baseline value was when the patient was made supine after subarachnoid bupivacaine was administered. The grades of shivering were recorded at a period of 0, 1, 5, 10, 15, 30, 45, 60, and 90 min from the baseline as per grades similar to those used by Wrench et al.[4] [Table 1].
Table 1.
Grading of shivering as per wrench

No measures were taken to forestall shivering, but warming blankets were available if it became necessary. Perioperatively if shivering occurred; it was treated in the same manner in all the groups with reassurance, warming blanket, or meperidine. The sedative side-effects were assessed with a four-point scale (sedation score) as per Filos et al.[5] [Table 2].
Table 2.
The sedation score assessed with a four-point scale as per filos

Statistical analysis
After completion of the study, observations obtained were tabulated and analyzed using Statistical Package for Social Sciences (SPSS version 16.0; Chicago, IL). To calculate the sample size, we hypothesized that keeping Type I (alpha) error at 5% and Type II (beta) error at 20%, the study drugs would be able to reduce the incidence of shivering by 15% and using the variability of data as a standard deviation (SD), 40 patients per study group was arrived. Unless otherwise stated, results are expressed as mean ± SD. Student t-test was used to compare different groups among themselves and analysis of variance (ANOVA) for repetitive observations. For determining the significance of difference between different groups ANOVA was applied. Post-hoc Tukey multiple comparison test was applied for pair-wise comparison. A P < 0.05 was considered to be statistically significant.
Results
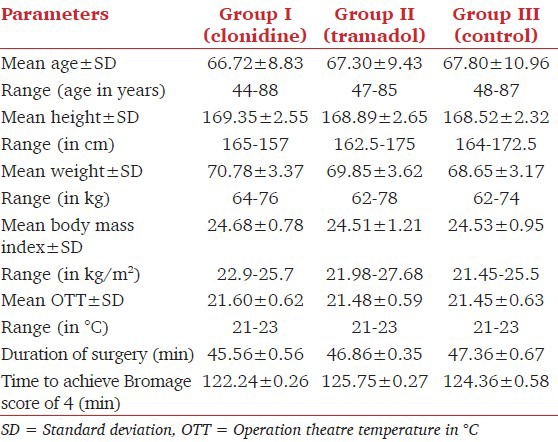
The demographic data, duration of the surgery and time required to achieve a Bromage score of 4 were similar amongst all the three groups [Table 3].
Table 3.
Demographic profile and characteristic of surgery and anesthesia
Shivering
In Group I, 38 patients (95%) did not shiver while 2 patients (5%) did. Both patients experienced shivering of Grade 2, while one of them showed progression from Grade 1 to 2. None had Grades 3 or 4 shivering. In Group II, around 37 patients (92.5%) did not shiver, whereas three patients (7.5%) did. All three patients experienced Grades 1-3 shivering, during the observation period. None of the patient experienced Grade 4 shivering. In the group III, 24 patients (60%) did not experience shivering, but 16 patients (40%) experienced different grades of shivering. On comparison of incidence of shivering [Figure 1] among group I versus group III and Group II versus Group III, a statistically significant variation was seen (P < 0.01), whereas group I versus group II had statistically insignificant variation.
Figure 1.
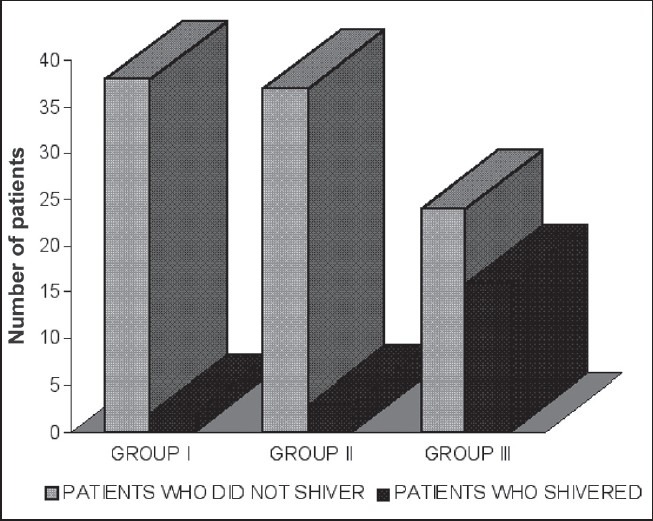
Incidence of shivering in the three groups
The variation in shivering was markedly significant at 30, 45, and 60 min among group I and group III (P < 0.01) [Table 4], while at 5, 10, 15, and 90 min the variation in shivering was statistically insignificant (P < 0.05). Similarly, the comparison of group II with group III showed a statistically significant difference (P < 0.01 at 5 and 60 min and P < 0.05 at 10, and 90 min). It was seen that shivering started earlier (within 5 min) and persisted for a longer duration in the group III (range = 5-90 min). On the contrary the onset of shivering was delayed (15 min) and the duration was also significantly less (range = 15-60 min) among group I and group II patients. All the patients who experienced Grades 3 and 4 of shivering had tachycardia, increased blood pressure recording and decrease in arterial oxygen saturation. Three patients in group III who experienced Grade 4 of shivering had premature ventricular complexes, which were successfully treated with intravenous preservative free lignocaine 2% (1.5 mg/kg). Tympanic membrane temperature showed a statistically insignificant variation between groups I and II, while there was a significant variance especially in the latter part of the study (P < 0.01) when comparing these to patients in group III [Figure 2]. Axillary and forehead temperature showed no significant variation among the three groups [Figures 3 and 4].
Table 4.
Incidence of shivering amongst various groups
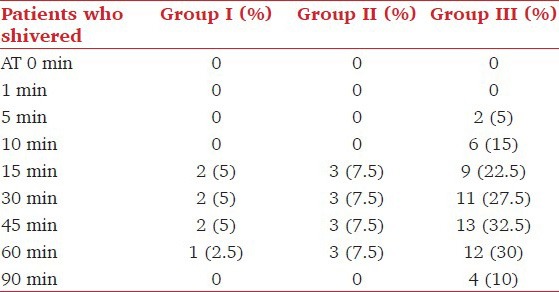
Figure 2.
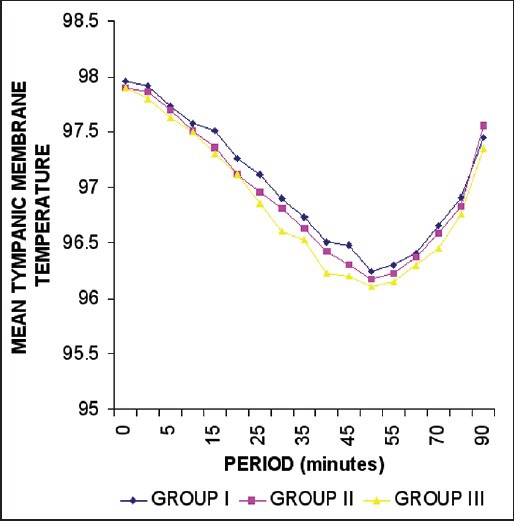
Trends in the tympanic membrane temperature (in Fahrenheit)
Figure 3.
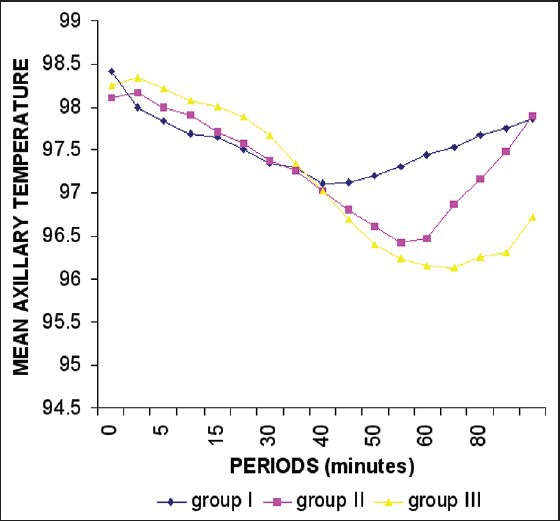
Trends in axillary temperature (in Fahrenheit)
Figure 4.
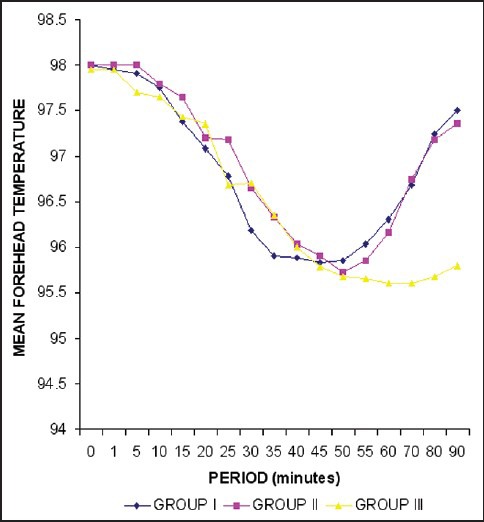
Trends in forehead temperature (in Fahrenheit)
Hemodynamic and other parameters
There was statistically significant variation in pulse rate between group I versus group II and group I versus group III (P < 0.01), throughout the study period. Not much variance was seen between group II versus group III. There was statistically insignificant intragroup variation, with respect to blood pressure, respiratory rate, and SpO2.
Discussion
In our study, we found that oral clonidine and tramadol were significantly effective in providing a prophylaxis against shivering in TURP under SAB. This is comparable to study conducted by Mao et al.[6] who observed that oral clonidine 150 μg when given prophylactically prevents postspinal shivering. It was found to lower the incidence of shivering to 4% in the study group compared to 44% in the control group. This is also in concordance with various studies, which showed that tramadol reduces the incidence, severity and duration of perioperative shivering.[7,8,9,10]
We observed that patients who were given clonidine or tramadol had mild to moderate shivering. In our study, it was seen that shivering started earlier (in 5 min) and persisted for a longer duration in the control group (range = 5-90 min), while in clonidine and tramadol treated patients the onset was delayed (15 min) and the duration was also significantly less (range = 15-60 min). This is in concordance with study by Yang et al.,[11] wherein shivering was observed after 16.8 ± 9 min, (range = 5-30 min) in the control group, while it occurred at 18 (range/mean and SD) minute in patient's who received intravenous clonidine 150 μg 20 min before epidural anesthesia was administered. Our study also corresponds to the results of study conducted by Vanderstappen et al.[12] who found that clonidine decreased the total incidence (P = 0.024), the severity (P = 0.005) and duration (P = 0.01) of postoperative shivering.
The significant fall in the tympanic membrane temperature in group III patients as compared with group I and II patients (P < 0.001, <0.0001, <0.001, respectively) is in variance to the study conducted by Mao et al. who did not observe any fall in the tympanic membrane temperature, perhaps as they recorded the temperature at 30 min from baseline. However we observed lowest mean tympanic temperature at 50 min, 45 min, and 50 min in group I, II and III, respectively. Though there was no significant intergroup difference in the decrease in forehead and axillary temperatures, the fall of these within each group was significant.
Hypothermia causes bradycardia, reduced cardiac output and peripheral vasoconstriction. There are additional adverse effects on every other body system.[4] Previous studies have reported a reduction in core temperature during transurethral prostatectomy.[6,11,13,14,15] The cardiovascular system is stressed as a result of increased afterload, effects on myocardial excitability and conduction and the metabolic demands of rewarming.[4] The metabolic cost of shivering is an increase in oxygen consumption from 300% to 800%, respectively.[16] Clonidine and tramadol would be effective in preventing shivering (and its adverse effects) but not hypothermia. Despite premedication with these drugs it would still be important to adopt measures to maintain normothermia. Hence maintaining strict normothermia alongwith premedication with either clonidine or tramodol can prevent shivering during regional anesthesia.
Conclusions
From our study, we conclude that both drugs have a potential to be used as prophylactic anti-shivering drugs, especially in whom shivering could lead to detrimental consequences. This would lead to reduction in postoperative complications, especially in geriatric patients with already compromised cardiovascular status.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Chow TC, Cho PH. The influence of small dose intrathecal fentanyl on shivering during transurethral resection of prostate under spinal anesthesia. Acta Anaesthesiol Sin. 1994;32:165–70. [PubMed] [Google Scholar]
- 2.Jenkins J, Fox J, Sharwood-Smith G. Changes in body heat during transvesical prostatectomy. A comparison of general and epidural anaesthesia. Anaesthesia. 1983;38:748–53. doi: 10.1111/j.1365-2044.1983.tb12197.x. [DOI] [PubMed] [Google Scholar]
- 3.Tewari A, Katyal S, Singh A, Garg S, Kaul TK, Narula N. Prophylaxis with oral clonidine prevents perioperative shivering in patients undergoing transurethral resection of prostate under subarachnoid blockade. Indian J Urol. 2006;22:208–12. [Google Scholar]
- 4.Wrench IJ, Singh P, Dennis AR, Mahajan RP, Crossley AW. The minimum effective doses of pethidine and doxapram in the treatment of post-anaesthetic shivering. Anaesthesia. 1997;52:32–6. doi: 10.1111/j.1365-2044.1997.006-az006.x. [DOI] [PubMed] [Google Scholar]
- 5.Filos KS, Goudas LC, Patroni O, Polyzou V. Hemodynamic and analgesic profile after intrathecal clonidine in humans. A dose-response study. Anesthesiology. 1994;81:591–601. doi: 10.1097/00000542-199409000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Mao CC, Tsou MY, Chia YY, Chow LH, Chan KH, Lee TY. Pre-anesthetic oral clonidine is effective to prevent post-spinal shivering. Acta Anaesthesiol Sin. 1998;36:137–42. [PubMed] [Google Scholar]
- 7.Mathews S, Al Mulla A, Varghese PK, Radim K, Mumtaz S. Postanaesthetic shivering – A new look at tramadol. Anaesthesia. 2002;57:394–8. doi: 10.1046/j.1365-2044.2002.2457_3.x. [DOI] [PubMed] [Google Scholar]
- 8.Kranke P, Eberhart LH, Roewer N, Tramèr MR. Pharmacological treatment of postoperative shivering: A quantitative systematic review of randomized controlled trials. Anesth Analg. 2002;94:453–60. doi: 10.1097/00000539-200202000-00043. [DOI] [PubMed] [Google Scholar]
- 9.Alfonsi P. Postanaesthetic shivering: Epidemiology, pathophysiology, and approaches to prevention and management. Drugs. 2001;61:2193–205. doi: 10.2165/00003495-200161150-00004. [DOI] [PubMed] [Google Scholar]
- 10.Tsai YC, Chu KS. A comparison of tramadol, amitriptyline, and meperidine for postepidural anesthetic shivering in parturients. Anesth Analg. 2001;93:1288–92. doi: 10.1097/00000539-200111000-00052. [DOI] [PubMed] [Google Scholar]
- 11.Yang CH, Yu CC, Seah YS, Chan HC, Tan PP. Effect of intravenous clonidine on prevention of postepidural shivering. Ma Zui Xue Za Zhi. 1993;31:121–6. [PubMed] [Google Scholar]
- 12.Vanderstappen I, Vandermeersch E, Vanacker B, Mattheussen M, Herijgers P, Van Aken H. The effect of prophylactic clonidine on postoperative shivering. A large prospective double-blind study. Anaesthesia. 1996;51:351–5. doi: 10.1111/j.1365-2044.1996.tb07747.x. [DOI] [PubMed] [Google Scholar]
- 13.Joris J, Banache M, Bonnet F, Sessler DI, Lamy M. Clonidine and ketanserin both are effective treatment for postanesthetic shivering. Anesthesiology. 1993;79:532–9. doi: 10.1097/00000542-199309000-00017. [DOI] [PubMed] [Google Scholar]
- 14.Delaunay L, Bonnet F, Liu N, Beydon L, Catoire P, Sessler DI. Clonidine comparably decreases the thermoregulatory thresholds for vasoconstriction and shivering in humans. Anesthesiology. 1993;79:470–4. doi: 10.1097/00000542-199309000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Reid JL. The Fourth Lilly Prize Lecture, University of Aberdeen, September 1980. The clinical pharmacology of clonidine and related central antihypertensive agents. Br J Clin Pharmacol. 1981;12:295–302. doi: 10.1111/j.1365-2125.1981.tb01217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horvath SM, Spurr GB, Hutt BK, Hamilton LH. Metabolic cost of shivering. J Appl Physiol. 1956;8:595–602. doi: 10.1152/jappl.1956.8.6.595. [DOI] [PubMed] [Google Scholar]


