Abstract
Rubinstein-Taybi syndrome (RTS) is a multisystem involvement disease. These children may present for various surgeries of different systems. Due to multisystem involvement, perioperative management of such patients poses peculiar challenges for the anesthesiologists. We report the successful anesthetic management of a patient with RTS with tonsillar hypertrophy grade III scheduled for ovarian cystectomy.
Keywords: Anesthesia, broad thumb and hallux syndrome, ovarian cystectomy, RTS
Introduction
Rubinstein-Taybi syndrome (RTS), alternatively known as the “Broad Thumb and Hallux” syndrome, is a rare autosomal dominant chromosomal disorder, that occurs due to microdeletion of chromosome 16p13.[1,2] It is characterized by mental retardation, short stature, broad thumbs and toes, psychomotor retardation, delayed bone age, congenital heart disease, recurrent respiratory infections, craniofacial anomalies, breathing and swallowing difficulties, malformations of the kidneys, urogenital system, and skeletal system.[3,4] All these features can have an impact in the management of anesthesia in a patient of RTS scheduled for surgeries like correction of craniofacial, orthopedic, orthodontic, ophthalmic, or cardiac lesions apart from incidental surgical conditions. We report the successful anesthetic management of a patient with RTS with tonsillar hypertrophy scheduled for ovarian cystectomy.
Case Report
A 14-year-old female weighing 41 kg, a known case of RTS since birth, was scheduled for exploratory laparotomy for ovarian cystectomy. She had history of mental retardation, delayed developmental milestones, and atrial septal defect with patent ductus arteriosus diagnosed at one month of age. She had a learning difficulty but was able to communicate with her parents. There was no history suggestive of gastroesophageal reflux and her effort tolerance was good. The parent gave history of excessive snoring during sleep. On examination, she has antimongoloid slanting eyes, beaked nose, thoracic kyphoscoliosis, broad thumbs and toes. Airway examination showed modified Mallampati class III, high-arched palate, tonsillar hypertrophy (grade III), restricted neck extension, small mandible, and protruding upper incisors. Routine hematological and biochemical investigations were within normal limits. Presently, echocardiography showed no obvious cardiac anomaly. The indirect laryngoscopy examination showed only grade-III tonsillar hypertrophy with no adenoids. However, the vocal cord could not be visualized as the patient was uncooperative.
The patient received oral ranitidine 150 mg as premedication in the night before and on day of surgery. Preoperative sedation was avoided. In the operation room, all equipment necessary to manage a difficult airway, including fiberoptic bronchoscope were arranged. As patient was uncooperative, sedation could not be administered in view of absence of intravenous line. Moreover, due to history of excessive snoring, we allowed patient's mother to escort her to the operation room. After shifting the patient to operation room, standard monitors like pulse oximeter, noninvasive blood pressure and electrocardiography were attached. General anesthesia was induced with incremental sevoflurane in oxygen, started with 1% concentration and increased up to 8%. After induction, an intravenous line was secured and 40 mcg fentanyl was administered. A check laryngoscopy was performed to ascertain the potential difficulty of intubation and insertion of proseal laryngeal mask airway (PLMA). Both the tonsils were enlarged and were almost touching the midline. The epiglottis was also larger than usual, with a vocal cord view of Cormack and Lehane Grade IIb even with optimal external laryngeal manipulation (OELM). Assisted check bag and mask ventilation was effective. Intravenous atracurium 0.5 mg/kg was then administered and the trachea was intubated using cuffed endotracheal tube of size 7 mmID and a stylet with maintenance of OELM. Anesthesia was maintained with desflurane in oxygen and air (50:50) (MAC 0.8). Prior to start of surgery, under aseptic precautions, intrathecal morphine 0.1 mg was administered at L4-L5 interspace using 25 G spinal needle. The surgery lasted about around 2 h and blood loss was minimal. At the end of surgery, residual neuromuscular block was reversed with neostigmine and glycopyrrolate and the trachea was extubated when the patient was fully awake and the Train of Four (TOF) count was more than 0.9. The patient was then shifted to the recovery room for observation. The patient was pain free and was shifted to the ward after 2 h. In the ward, the patient received intravenous paracetamol 1 g every 8 hourly and remained pain free. She was discharged uneventfully on 3rd postoperative day.
Discussion
RTS was first reported by Rubinstein and Taybi in 1963.[5] Multisystem involvement like airway, cardiac, musculoskeletal, respiratory, and urogenital system poses challenge to anesthesiologist for managing such cases [Table 1].[1,2,3,4,5,6,7]
Table 1.
Rubinstein-Taybi syndrome: Multisystem involvement and its clinical implications
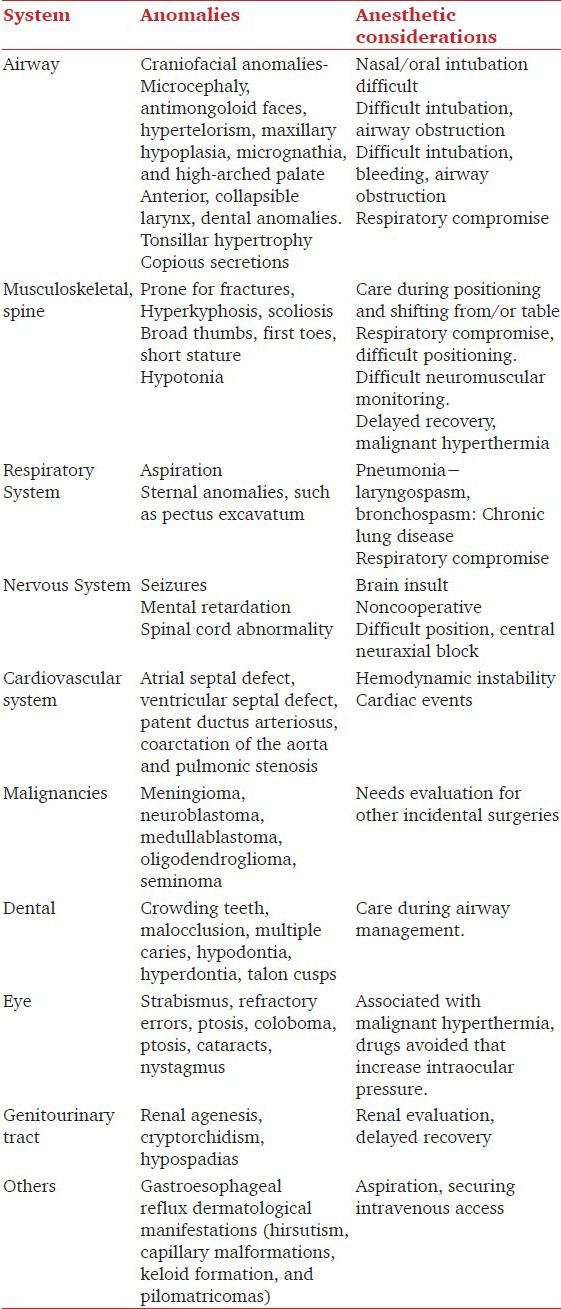
The airway management is challenging in such patients in view of craniofacial abnormalities, laryngotracheal abnormalities, obstructive sleep apnea, gastroesophageal reflux disease, increased risk of aspiration, mental retardation (noncooperation), and increased risk of arrhythmias with succinylcholine.[2,3,4,7] The abnormalities of the head and face include a microcephaly, antimongoloid faces, high-arched palate, widely spaced eyes (hypertelorism), a broad nasal bridge, an abnormally large or “beak-shaped” nose, and an unusually small, hypoplastic lower jaw (micrognathia) with small mouth opening, and bucked upper incisors.[2,4,7] We also observed a large floppy epiglottis which has not been reported earlier in RTS. Aspiration may occur in either awake or sleeping state as well. The presence of recurrent episodes may lead to repeated pneumonias and chronic lung disease. These patients have a tendency of increase secretions which further precipitates lung condition.[4] They have choanal atresia, deviated nasal septum, upper and lower airway narrowing/collapse, postcricoid web, laryngomalacia and tracheoesophageal compression due to vascular ring.[2,3,8] These all features not only poses intubation difficulties but also ventilation difficulties. Probably for these reasons only, self-limiting episodes of desaturations without any provocation have been reported.[4] In our patient, the airway difficulty was obvious but we could not perform an awake fiberoptic-guided tracheal intubation as patient was not cooperative and was not allowing even an intravenous line placement. Though the use of supraglottic airway device may be beneficial to avoid stimuli in view of respiratory infections, but it may be limited due to possibility of airway collapse, anatomical variability, and presence of hypertrophied tonsils as in our patient.[9] So, we avoided PLMA in our patient. Lower airway collapsibility may not be even managed with endotracheal tube as airway collapse distal to the secured area can manifest itself. So, we were ready with difficult airway cart including long hollow tube exchangers.[3] Dental abnormalities have been reported to occur in 67% of individuals with RTS.[10] So, care should be taken during laryngoscopy to prevent damage to dentition which has not been discussed in previous anesthesia literature. We avoided any sedative agent preoperatively as patient was giving a history of excessive snoring.[2] We performed incremental inhalational induction to maintain airway control in our patient. The use of only regional anesthesia is not possible due to presence of mental retardation in these patients and may also be difficult due to spine abnormalities. Therefore, we chose to give general anesthesia which was supplemented with intrathecal morphine to limit the dose of narcotics. In view of all these issues preoperatively, extubation was also a major concern. During emergence from anesthesia one must also attend changes in airway function which can contribute to enhanced risk of airway reactivity and can be associated with bronchospasm and laryngospasm. Delayed recovery after general anesthesia in these patients has been reported in literature.[11]
Such patients are prone for fractures and thus mandates care during shifting and positioning of the patient.[2,5] Due to thumb abnormalities, neuromuscular monitoring is a concern and adductor muscle monitoring may not be feasible mandating use of facial nerve stimulation and observation of orbicular muscle. Cardiac abnormalities like patent ductus arteriosus, atrial septal defect, aortic coarctation, and bicuspid aortic valve are present in 33% of these patients.[2] Though our patient had patent ductus at birth, but repeat echocardiography confirms its spontaneous closure. Antibiotic prophylaxis should be given to those with an underlying cardiac lesion.[2] We should be vigilant in avoiding air bubbles in the intravenous line to prevent paradoxical emboli.[4] These cardiac abnormalities also predispose to arrhythmias with arrhythmogenic drugs like succinylcholine, atropine, and neostigmine.[4] There is a 30% incidence of cardiac dysrhythmias with or without structural changes to the heart.[9] There is increased incidence of seizures, starring spells, and spinal cord abnormalities mandating concerns during central neuraxial blocks.[12] Our patient did not have such neurological issues. Affected individuals have an increased risk of developing malignancies, mainly central nervous and hematological.[2] The presence of ovarian cyst in our case has not been reported earlier.
Conclusion
Patient with RTS needs through preoperative evaluation for the multisystem involvement. They need to be optimized like that of chest infections. Vigilance with regards to possible problems needs to be kept in mind and arrangement for perioperative management made accordingly. Intrathecal morphine provided good perioperative analgesia in such patient without adding any undue concerns.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Petrij F, Dauwerse HG, Blough RI, Giles RH, van der Smagt JJ, Wallerstein R, et al. Diagnostic analysis of the Rubinstein-Taybi syndrome: Five cosmids should be used for microdeletion detection and low number of protein truncating mutations. J Med Genet. 2000;37:168–76. doi: 10.1136/jmg.37.3.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gathuya Z, Bosenberg A. Anaesthesia and Rubinstein-Taybi syndrome. South Afr J Anaesth Analgesia. 2005;0:135–7. [Google Scholar]
- 3.Magillo P, Della Rocca M, Campus R, Bava E, Rossi GA, Dodero P. Congenital tracheal stenosis in a boy with Rubinstein-Taybi syndrome. Can J Anesth. 2005;52:990–1. doi: 10.1007/BF03022064. [DOI] [PubMed] [Google Scholar]
- 4.Agarwal S, Ahmad YH, Talpesh M, Zestos M. Anesthetic management of children with Rubinstein-Taybi syndrome — case reports. Middle East J Anesthesiol. 2011;21:309–12. [PubMed] [Google Scholar]
- 5.Rubinstein JH, Taybi H. Broad thumbs and toes and facial abnormalities. A possible mental retardation syndrome. Am J Dis Child. 1963;105:588–608. doi: 10.1001/archpedi.1963.02080040590010. [DOI] [PubMed] [Google Scholar]
- 6.Levitas AS, Reid CS. Rubinstein Taybi syndrome. Mental Health Aspects Dev Disabil. 2003;6:130–4. [Google Scholar]
- 7.Tatara Y, Kawakami N, Tsuji T, Miyasaka K, Ohara T, Nohara A. Rubinstein-Taybi syndrome with scoliosis. Scoliosis. 2011;6:21. doi: 10.1186/1748-7161-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hennekam RC. Rubinstein-Taybi syndrome. Eur J Hum Genet. 2006;14:981–5. doi: 10.1038/sj.ejhg.5201594. [DOI] [PubMed] [Google Scholar]
- 9.Twigg SJ, Cook TM. Anaesthesia in an adult with Rubinstein-Tyabi syndrome using the ProSeal laryngeal mask airway. Br J Anaesth. 2002;89:786–7. [PubMed] [Google Scholar]
- 10.Munevveroglu AP, Akgol BB. Rubinstein-taybi syndrome: A case report. Case Rep Dent 2012. 2012:483867. doi: 10.1155/2012/483867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dearlove OR, Perkins R. Anaesthesia in an adult with Rubinstein-Taybi syndrome. Br J Anaesth. 2002;90:399. doi: 10.1093/bja/aeg537. [DOI] [PubMed] [Google Scholar]
- 12.Wiley S, Swayne S, Rubinstein JH, Lanphear NE, Stevens CA. Rubinstein-Taybi syndrome medical guidelines. Am J Med Genet A. 2003;119:101–10. doi: 10.1002/ajmg.a.10009. [DOI] [PubMed] [Google Scholar]


