Abstract
The concept of stereotactic radiosurgery (SRS) was first described by Lars Leksell in 1951. It was proposed as a noninvasive alternative to open neurosurgical approaches to manage a variety of conditions. In the following decades, SRS emerged as a unique discipline involving a collegial partnership among neurosurgeons, radiation oncologists, and medical physicists. SRS relies on the precisely guided delivery of high-dose ionizing radiation to an intracranial target. The focused convergence of multiple beams yields a potent therapeutic effect on the target and a steep dose fall-off to surrounding structures, thereby minimizing the risk of collateral damage. SRS is typically administered in a single session but can be given in as many as five sessions or fractions. By providing an ablative effect noninvasively, SRS has altered the treatment paradigms for benign and malignant intracranial tumors, functional disorders, and vascular malformations. Literature on extensive intracranial radiosurgery has unequivocally demonstrated the favorable benefit-to-risk profile that SRS affords for appropriately selected patients. In a departure from conventional radiotherapeutic strategies, radiosurgical principles have recently been extended to extracranial indications such as lung, spine, and liver tumors. The paradigm shift resulting from radiosurgery continues to alter the landscape of related fields.
INTRODUCTION
In the early era of neurosurgery, conventional microsurgical tools were insufficient for complex intracranial pathology. For certain patients, operative morbidity and/or mortality were high and the complete resection of an intracranial lesion was not always feasible. Harvey Cushing, the founder of modern-day neurosurgery, resorted to the radium bomb to treat early brain tumors.1 Walter Dandy borrowed a ureteral endoscope from Howard Kelly, a colleague in gynecology, and its use ushered endoscopy to neurosurgery procedures.2
Paradigm shifts in medicine often arise when substantial limits of existing approaches and a need for significant improvement to meet a clinical need are recognized. Elements comprising a paradigm shift are usually drawn from other arenas and are combined in a way not previously conceived, so that the resulting approach is a radical departure from the field and seems strangely foreign, even though the individual elements themselves may not be so. Hence, initial resistance on the part of some is the norm and not the exception. However, over time, the approach is embraced for its virtues and its limitations are appropriately recognized.
Stereotactic radiosurgery (SRS) is a classic example of a paradigm-shifting approach. It was devised out of necessity and blended distinct fields of neurosurgery, radiation oncology, and medical physics. Although the concept of radiosurgery has been present for more than six decades, radiosurgery continues to be refined and expanded. Thus, the paradigm shift is not yet complete. Herein, we describe radiosurgery's origins and current practice and speculate its future direction.
HISTORY
The field of SRS developed in the last century. Clarke and Horsley developed the initial stereotactic system, but this system was used for research.3 Spiegel and Weeks4 were the first to apply the stereotactic method for neurosurgery clinically. This approach allowed intracranial structures to be localized by their spatial relationship to a Cartesian coordinate system related to a ring rigidly secured to the skull.
A prerequisite to Lars Leksell's development of radiosurgery was frame-based stereotactic neurosurgery. Leksell wanted to devise a method to destroy localized structures situated deep within the brain but to do so without the same degree of morbidity associated with the open neurosurgical procedures of that era. His resulting idea was to converge multiple beams of ionizing radiation at one defined point. While an inconsequential dose is delivered along the path of each beam, the point where the beams intersect receives a dose proportional to the sum total of the individual beams and would have a potent effect on the target tissue. The delivery device would be designed to ensure sharp fall off of delivered radiation at the edge of the intersection point. This would allow a precise effect at the targeted lesion and a minimized effect to the surrounding tissue. In 1951, Leksell defined this concept as stereotactic radiosurgery.5
Different types of ionizing radiation were tried. Leksell first used an orthovoltage x-ray tube coupled to a rigid stereotactic frame, which he used to treat several patients with trigeminal neuralgia or obsessive compulsive disorder.5 Later, Leksell used a cyclotron as an accelerated proton source and treated various intracranial pathologies.6,7 Rather than using the effects on the Bragg peak, they relied on the flat portion of the proton depth-dose profile that precedes the Bragg peak, called the plateau. Leksell named it “stralkiven,” or ray knives.8 The cyclotron, however, proved too cumbersome for intracranial radiosurgery, and a cyclotron's expense seemed to preclude its widespread application. Leksell and his team evaluated a first-generation linear accelerator for radiosurgery but, at that time, they found it to lack the inherent precision necessary for this approach. Stationary sources of high-energy photons emitting 60-Cobalt on to a fixed stereotactic target met the requirements of precision and compactness that Leksell had in mind for an intracranial radiosurgery system. Leksell and physicist Larsson oversaw the building of the first Gamma Knife unit between 1965 and 1968.
Around the time Leksell was developing the Gamma Knife, Raymond Kjellberg and Jacob Fabrikant were conducting innovative work in the field of radiosurgery using heavy particles from cyclotrons. And in 1983, in a hospital in Buenos Aires, Argentina, Betti and Derechinsky developed the concept of a modified linear accelerator for SRS.9,10 Their system relied on a 10-MV linear accelerator and used a chair for the patient that was based on the Talairach stereotactic frame.11 Winston and Lutz in Boston, MA; Hartman and Sturm in Heidelberg, Germany; Barcia-Salorio in Valencia, Spain; Colombo in Vincenza, Italy; and Podgorsak in Canada came up with other innovative developments in linear accelerator–based SRS devices shortly thereafter.9,10,12–15
Using a single high dose of ionizing beams to treat intracranial disorders was a novel and creative concept. Its application changed the direction of many fields such as neurosurgery and radiation oncology. Significant contributions have been made by numerous neurosurgeons, radiation oncologists, and physicists to advance the field of SRS. However, despite all the changes in SRS over the decades, the fundamental concepts have not changed.
THE DEFINITION, DEVICES, AND MULTIDISCIPLINARY APPROACH FOR SRS
Stereotactic radiosurgery was traditionally delivered in a single session, using specialized delivery devices. For the first couple of decades, SRS was delivered with regularity at only a handful of centers throughout the world. Over time, the successes of radiosurgery became evident to many. More clinicians were sufficiently trained and the technology became practical enough for diverse health systems to acquire. However, as the technology and indications evolved, the clinical volume of radiosurgery cases increased too. This in part prompted the need for a formal definition of radiosurgery. In March 2006, representatives of the American Association of Neurological Surgeons, Congress of Neurological Surgeons, and the American Society for Radiation Oncology met and formally defined radiosurgery.16 Radiosurgery is the use of image-guided ionizing radiation to make inactive or eradicate a specific target within the brain or spine. Targeting is accomplished via high-resolution imaging and by using stereotactic principles. Radiosurgery is delivered through a rigid stereotactic guiding device, immobilization system, or image-guidance system. The ionizing radiation is delivered in one to five sessions. Although this definition is not universally sanctioned, the definition is the one most frequently applied for SRS.
From its inception, radiosurgery has been multidisciplinary. The definition sanctioned by the American Association of Neurological Surgeons, Congress of Neurological Surgeons, and the American Society for Radiation Oncology calls for the radiosurgery to be performed by a team consisting of a neurosurgeon, a radiation oncologist, and a medical physicist.16 The American College of Radiology recommended a similar multidisciplinary approach to ensure quality of care and went so far as to specify responsibilities for the individual members of the multidisciplinary team during the SRS process.17
Radiosurgery can currently be delivered via a number of devices. Contemporary devices include the Gamma Knife Perfexion (Elekta AB, Stockholm, Sweden), Cyberknife (Accuray, Sunnyvale, CA), TrueBeam STx (Varian, Palo Alto, CA), and Novalis (Brainlab, Feldkirchen, Germany). Although it is more frequently used for fractionated radiation therapy, proton-beam systems at selected centers such as Massachusetts General Hospital (Boston, MA) are also used for radiosurgery.
CONTEMPORARY STEREOTACTIC RADIOSURGERY
Radiosurgery was the first method of dose escalation for late-responding tissues. Thus, radiosurgery has a preferred biologic effect on functioning pituitary adenomas, arteriovenous malformations, and some other intracranial malignancies. The radiobiologic effect of radiosurgery is partly because of a vascular effect and not purely a cytotoxic effect.18
SRS is currently performed on a diverse set of intracranial pathologies. Though a comprehensive review of all such practices goes beyond the scope of this article, we focus on three representative indications: pituitary adenomas, arteriovenous malformations, and brain metastases.
Stereotactic Radiosurgery for Pituitary Adenomas
SRS is used to treat a number of so-called benign intracranial tumors, and pituitary adenomas represent one such example. Pituitary adenomas are quite common among the general population; they comprise 10% to 20% of all intracranial tumors.19,20 They are classified by size (microadenomas are < 1 cm in size; macroadenomas are ≥ 1 cm in size) and by hormonal secretory status (functioning lesions have hormone hypersecretion; nonfunctioning lesions have no abnormal hormone production).
In Table 1, we detail the major radiosurgical series of patients with nonfunctioning adenomas since 2002.21–45 Single-session radiosurgery resulted in tumor-control rates of 83% to 100%, with a mean of 95.2%; new-onset hypopituitarism following radiosurgery was observed in 0% to 40% of patients, with a mean of 8.8% (Table 1).21–45 At the University of Virginia, we studied 140 patients with nonfunctioning pituitary adenomas, and we previously reported an approximately 90% tumor control and delayed hypopituitarism in 30% of patients.42 New or worsening cranial nerve deficits were observed in 14% of patients. In a recent multicenter trial evaluating SRS for 512 patients with nonfunctioning pituitary adenomas (median follow-up, 36 months; range, 1 to 223 months), an overall tumor-control rate of 93% was reported.45 SRS-related hypopituitarism was observed in 21% of patients.45 Patients older than 50 years, those with tumor volumes less than 5 cm3, and those without prior radiation therapy had more favorable outcomes of tumor control and neurologic preservation.45
Table 1.
Major Radiosurgery Series for Nonfunctioning Pituitary Adenomas
| Study | Publication Year | No. of Patients | Mean or Median Follow-Up (months) | Mean or Median Margin Dose (Gy) | Radiographic Tumor Control (%) |
|---|---|---|---|---|---|
| Feigl et al19 | 2002 | 61 | 55.2 | 15 | 94 |
| Sheehan et al28 | 2002 | 42 | 31.2 | 16 | 97.6 |
| Wowra et al29 | 2002 | 30 | 57.7 | 16 | 93.3 |
| Petrovich et al26 | 2003 | 52 | 34 | 15 | 100 |
| Losa et al23 | 2004 | 54 | 41.1 | 16.6 | 96.3 |
| Muacevic et al25 | 2004 | 51 | 21.7 | 16.5 | 95 |
| Kajiwara et al22 | 2005 | 14 | 32.1 | 12.6 | 92.9 |
| Picozzi et al27 | 2005 | 51 | 40.6 | 16.5 | 96.1 |
| Iwai et al21 | 2005 | 28 | 36.4 | 12.3 | 93 |
| Mingione et al24 | 2006 | 100 | 46.4 | 18.5 | 92.2 |
| Voges et al41 | 2006 | 37 | 56.6 | 13.4 | 100 |
| Liscak et al36 | 2007 | 140 | 60 | 20 | 100 |
| Pollock et al38 | 2008 | 62 | 64 | 16 | 96.8 |
| Hoybye et al20 | 2009 | 23 | 78 | 20 | 100 |
| Kobayashi et al35 | 2009 | 71 | 50.2 | NR | 96.7 |
| Castro et al30 | 2010 | 14 | 42 | 12.5 | 100 |
| Hayashi et al33 | 2010 | 43 | 36 | 18.2 | 100 |
| Gopalan et al32 | 2011 | 48 | 95 | 18.4 | 83 |
| Iwata et al34 | 2011 | 100 | 33 | 21 Gy/3 fr, 25 Gy/5 fr | 98 |
| Park et al37 | 2011 | 125 | 62 | 13 | 90 |
| El-Shehaby et al31 | 2012 | 21 | 44 | 12 | 85 |
| Runge et al39 | 2012 | 65 | 83 | 13 | 98.3 |
| Starke et al40 | 2012 | 140 | 50.4 | 18 | 90 |
| Wilson et al42 | 2012 | 51 | 50 | 14 | 100 |
| Sheehan et al43 | 2013 | 512 | 36 | 16 | 93 |
Abbreviations: fr, fraction; NR, not reported.
The primary radiosurgical goal for functioning adenomas, unlike other benign intracranial tumors, is both endocrine remission and radiologic control. Radiologic control usually accompanies endocrine remission, but some adenomas exhibit radiologic control yet fail to achieve complete endocrine remission. Radiosurgery plays an important role in the treatment of persistent Cushing's disease and acromegaly refractory to surgical and/or medical management. Table 2 lists recent major radiosurgical series for Cushing's disease.21,22,24,28,35,37,43,46–64 Endocrine remission was typically defined as a normal 24-hour urinary-free cortisol or serum cortisol. Most radiosurgical series for Cushing's disease show endocrine remission in the majority of patients after radiosurgery; the mean remission rate across major series is 51% (Table 2). The mean time interval after radiosurgery to endocrine remission in successfully treated patients is 12 months.57 The most likely explanation for this finding is that radiosurgical doses typically required to attain endocrine remission in Cushing's disease are higher than those used to control the growth of nonfunctioning adenomas. Delayed endocrine recurrence after radiosurgery-induced remission can occur. For example, in a radiosurgical series of 90 patients with Cushing's disease, after a mean follow-up period of 45 months, Cushing's disease recurred in 10 patients at a mean time of 27 months after initial remission.57
Table 2.
Major Radiosurgical Series for Cushing's Disease
| Study | Publication Year | No. of Patients | Mean or Median Follow-Up (months) | Mean or Median Margin Dose (Gy) | Endocrine Remission (%) |
|---|---|---|---|---|---|
| Izawa et al45 | 2000 | 12 | 26.4 | 23.8 | 16.7 |
| Sheehan et al49 | 2000 | 43 | 39.1 | 20 | 63 |
| Shin et al50 | 2000 | 6 | 88.2 | 32.3 | 50 |
| Hoybye et al22 | 2001 | 18 | 16.8 | NR | 44 |
| Feigl et al19 | 2002 | 4 | 55.2 | 15 | 60 |
| Kobayashi et al46 | 2002 | 20 | 64 | 28.7 | 23.3 |
| Laws et al47 | 2002 | 40 | NR | 20 | 74 |
| Pollock et al48 | 2002 | 9 | 42.4 | 20 | 78 |
| Choi et al44 | 2003 | 7 | 42.5 | 28.5 | 55.6 |
| Petrovich et al26 | 2003 | 4 | 34 | 15 | NR |
| Witt et al52 | 2003 | 8 | 24 | 24 | 0 |
| Wong et al53 | 2003 | 5 | 38 | NR | 100 |
| Devin et al54 | 2004 | 35 | 42 | 14.7 | 49 |
| Kajiwara et al22 | 2005 | 2 | 38.5 | 26 | 50 |
| Voges et al41 | 2006 | 17 | 58.7 | 16.4 | 52.9 |
| Castinetti et al76 | 2007 | 40 | 54.7 | 29.5 | 42.5 |
| Jagannathan et al55 | 2007 | 90 | 45 | 23 | 54 |
| Petit et al56 | 2008 | 33 | 62 | 20 | 52 |
| Pollock et al57 | 2008 | 8 | 73 | 20 | 87 |
| Tinnel et al58 | 2008 | 12 | 37 | 25 | 50 |
| Castinetti et al51 | 2009 | 18 | 94 | 28 | 50 |
| Kobayashi et al35 | 2009 | 30 | 64.1 | 28.7 | 35 |
| Wan et al61 | 2009 | 68 | 67.3 | 23 | 27.9 |
| Hayashi et al33 | 2010 | 13 | 36 | 25.2 | 38 |
| Sheehan et al60 | 2011 | 82 | 31 | 24 | 54 |
| Wein et al62 | 2012 | 17 | 23 | 18 | 58.8 |
| Grant et al59 | 2013 | 15 | 40.2 | 35 | 73 |
Abbreviation: NR, not reported.
For acromegaly, Table 3 lists recent major radiosurgical series.21,24,28,35,40,43,46,47,50,52–54,60–63,65–84 Endocrine remission varied widely across series (range, 0% to 82%), but the mean remission rate for acromegalic patients after radiosurgery was 44.7%. Patients with a functioning adenoma volume of less than 3 cm3 at the time of radiosurgery have been noted to have a significantly higher chance of endocrine remission.62 Thus, a strong case can be made for maximum safe surgical resection before radiosurgery to increase the chance of endocrine remission. At the University of Virginia, the mean time to endocrine remission after radiosurgery for patients with acromegaly was 24 months, and this was longer than for comparable patients with Cushing's disease.62 Although the data are drawn from retrospective studies, there seems to be compelling evidence to temporarily halt pituitary suppressive medications for patients with acromegaly around the time of radiosurgery; this approach seems to result in a greater rate of endocrine remission after SRS.85
Table 3.
Major Radiosurgical Series for Acromegaly
| Study | Publication Year | No. of Patients | Mean or Median Follow-Up (months) | Mean or Median Margin Dose (Gy) | Endocrine Remission (%) |
|---|---|---|---|---|---|
| Izawa et al45 | 2000 | 29 | 26.4 | 23.8 | 41.4 |
| Shin et al50 | 2000 | 6 | 42.7 | 34.4 | 66.7 |
| Zhang et al79 | 2000 | 68 | 34 | 31.3 | 36.8 |
| Fukuoka et al80 | 2001 | 9 | 42 | 20 | 50 |
| Ikeda et al81 | 2001 | 17 | 55.8 | 25 | 82 |
| Feigl et al19 | 2002 | 9 | 55.2 | 15 | 60 |
| Pollock et al48 | 2002 | 26 | 42.4 | 20 | 42 |
| Attanasio et al69 | 2003 | 30 | 46 | 20 | 23 |
| Choi et al44 | 2003 | 9 | 42.5 | 28.5 | 50 |
| Muramatsu et al78 | 2003 | 4 | 30 | 27.5 | 50 |
| Petrovich et al26 | 2003 | 5 | 34 | 15 | NR |
| Witt et al52 | 2003 | 4 | 24 | 24 | 25 |
| Castinetti et al51 | 2005 | 82 | 49.5 | 25 | 17 |
| Gutt et al77 | 2005 | 44 | 22.8 | 18 | 47.7 |
| Kajiwara et al22 | 2005 | 2 | 53.5 | 13.5 | 0 |
| Koybayashi et al75 | 2005 | 67 | 63.3 | 18.9 | 4.8 |
| Jezkova et al71 | 2006 | 96 | 53.7 | 35 | 50 |
| Voges et al41 | 2006 | 64 | 54.3 | 16.5 | 37.5 |
| Pollock et al72 | 2007 | 46 | 63 | 20 | 50 |
| Roberts et al73 | 2007 | 9 | 25.4 | 21 | 44.4 |
| Vik-Mo et al74 | 2007 | 61 | 66 | 26.5 | 17 |
| Jagannathan et al70 | 2008 | 95 | 57 | 22 | 53 |
| Losa et al68 | 2008 | 83 | 69 | 21.5 | 60.2 |
| Pollock et al57 | 2008 | 27 | 46.9 | 20 | 67 |
| Tinnel et al58 | 2008 | 9 | 35 | 25 | 44.4 |
| Castinetti et al76 | 2009 | 43 | 102 | 24 | 42 |
| Ronchi et al69 | 2009 | 35 | 120 | 20 | 46.0 |
| Wan et al61 | 2009 | 103 | 67.3 | 21.4 | 36.9 |
| Hayashi et al33 | 2010 | 25 | 36 | 25.2 | 40.0 |
| Iwai et al67 | 2010 | 26 | 84 | 20 | 38.0 |
| Poon et al66 | 2010 | 40 | 73.8 | 20-35 | 75 |
| Sheehan et al60 | 2011 | 130 | 31 | 24 | 53 |
| Franzin et al65 | 2012 | 103 | 71 | 22.5 | 60.7 |
| Liu et al64 | 2012 | 40 | 72 | 21 | 47.5 |
| Grant et al59 | 2013 | 13 | 40.2 | 35 | 61 |
Abbreviation: NR, not reported.
Stereotactic Radiosurgery for Arteriovenous Malformations
After Roentgen discovered x-rays, there was substantial interest in using ionizing radiation to treat arteriovenous malformations (AVMs) and other vascular malformations (eg, aneurysms) through the period up to the mid-20th century. A long-term angiographic follow-up, albeit in a small series of AVMs treated with fractionated radiation therapy by Johnson in the 1950s,86 revealed that 45% of AVMs were obliterated. However, other series treating AVMs with radiation had discouraging results, and therefore radiotherapy was often considered ineffective and was used as a last resort.87,88
With the introduction of Leksell's Gamma Knife, the therapeutic value of ionizing radiation for vascular malformations was re-evaluated. In Apri1 1970, the first radiosurgical treatment for an AVM was performed by Steiner et al89 at the Karolinska Institute. Through a combination of intelligence and serendipity plus a bit of audacity, Steiner and his colleagues chose to deliver a near optimal radiosurgical dose of 25 Gy to the fistulous point of a sizeable AVM, and the AVM obliterated shortly thereafter. Unlike some other radiosurgical indications, AVM obliteration seems best accomplished using a single session (rather than multisession radiosurgery) and a generally high-margin dose (eg, 18 to 25 Gy). In an institutional series of 1,012 AVM patients treated with SRS at the University of Virginia, an overall obliteration rate of 69% was achieved; permanent deficits from SRS were observed in 2.2% of patients. Factors that led to the favorable outcome included no prior hemorrhage, AVM in a noneloquent location, and AVMs with a volume of less than 4 cm3.90 In another large series at the University of Pittsburgh, 906 patients with AVMs underwent radiosurgery and were eligible for 3 years of follow-up. Complete nidus obliteration was achieved in 78% of patients.91 Adverse radiation effects occurred in 2.6% of patients. Major SRS series for AVMs are listed in Table 4.91–98
Table 4.
Major Radiosurgical Series for Arteriovenous Malformations
Embolization has been used to reduce an AVM nidus to a volume more suitable for radiosurgery. It is also frequently used to occlude arteriovenous fistulae, which are considered relatively radioresistant, and perinidal aneurysm, which is an accompanying vascular feature that is highly prone to rupture. Although a prospective study has not been undertaken, retrospective studies evaluating the effect of embolization before radiosurgery have generally demonstrated a reduction in obliteration compared with equivalent AVMs without prior embolization.
Stereotactic Radiosurgery for Brain Metastases
Though radiosurgery was used on AVMs and pituitary adenomas before malignant tumors, brain metastases have come to represent the single largest indication for SRS. The treatment of brain metastases historically has included whole-brain radiation therapy (WBRT), a therapeutic approach first reported in early 1950s.99,100 The Radiation Therapy Oncology Group (RTOG) conducted numerous trials from 1971 to 1993 to investigate various doses and fractionation schemes for WBRT.101–106 However, though neurologic symptoms and signs improved in the majority of patients, local control rates were low and neurologic death still occurred in 25% to 54% of patients with brain metastases.101 Radiosurgery has come to represent an important approach for patients with brain metastases, either as a stand-alone treatment or used in conjunction with WBRT or resection.107–113 Radiosurgery has been shown to offer a high rate of local tumor control and a low risk of adverse effects, including neurocognitive decline (Table 5; Fig 1).109,110,114
Table 5.
Significant Series Evaluating Radiation Therapy and Radiosurgery for Brain Metastasis
| Study and Design | No. of Patients | No. of Lesions | Margin Dose (Gy) | Local Tumor Control at 1 Year (%) | Recurrence at 1 Year (%) | Median Overall Survival (months) |
|---|---|---|---|---|---|---|
| Patchell, 1990114 | ||||||
| WBRT | 23 | 1 | NA | 48 | NA | 3.75 |
| OP + WBRT | 25 | 1 | NA | 80 | NA | 10.0 |
| Noordijk, 1994113 | ||||||
| WBRT | 63* | 1 | 40 Gy/wk | NA | NA | 6 |
| OP + WBRT | 1 | 40 Gy/wk | NA | NA | 10 | |
| Auchter, 1996107 | ||||||
| SRS + WBRT | 122 | 1 | 10-27 Gy (SRS) + 25-40 Gy (WBRT) | 86 | NA | 14 |
| Sanghavi, 2001106 | ||||||
| WBRT | 502 | NA | 12-58 Gy | NA | NA | 16.1 |
| WBRT + SRS | 12-58 Gy + NA | NA | NA | 7.1 | ||
| Sneed, 2002103 | ||||||
| SRS | 268 | No limit | NA | NA | NA | 8.2 (RPA1), 8.6 (RPA2) |
| SRS + WBRT | 301 | NA + 30-50.4 Gy | NA | NA | 14.0 (RPA1), 15.2 (RPA2) | |
| Andrew, 2004111 | ||||||
| WBRT | 164 | 1-3 | 37.5 Gy/3 wk | 71 | 30 | 6.5 |
| WBRT + SRS | 167 | 1-3 | 37.5 Gy/3 wk + 15-24 Gy | 82 | 25 | 4.9 |
| Shehata, 2004108 | ||||||
| SRS | 228 | NA | 7-30 Gy | 87 (7 m FU) | NA | NA |
| SRS + WBRT | 240 | 7-30 Gy + 6.75-50.4 Gy | 97 (7 m FU) | NA | NA | |
| Aoyama, 2006120 | ||||||
| SRS | 67 | 1-4 | 18-25 Gy | 72.5 | 76.4 | 8 |
| SRS + WBRT | 65 | 1-4 | 18-25 Gy + 30 Gy | 88.7 | 46.8 | 7.5 |
| Muacevic, 2008110 | ||||||
| OP + WBRT | 33 | 1 | 40 Gy/4 wk | 82 | NA | 9.5 |
| SRS | 31 | 1 | 14-27 Gy | 96.8 | NA | 10.3 |
| Chang, 2009121 | ||||||
| SRS | 30 | 1-3 | 15-20 Gy | 67 | 27 | 15.2 |
| SRS + WBRT | 28 | 1-3 | 15-20 Gy + 30 Gy/12 fr | 100 | 73 | 5.7 |
| Serizawa, 2010123 | ||||||
| SRS | 778 | 1-10 | 13.5-30 Gy | 98.4 (tiny) to 92.3 (small) to 77.9 (medium) | 45.7 | 26.4 (RPA1), 8.4 (RPA2), 3.6 (RPA3) |
| Kocher, 2011109 | ||||||
| SRS + WBRT | 99 | 1-3 | 20 Gy (SRS) | 81 | 48 | 10.9 (WBRT) |
| SRS alone | 100 | 1-3 | 30 Gy/10 fr (WBRT) | 69 | 33 | 10.7 (no WBRT) |
| OP + WBRT | 81 | 1-3 | 73 | 42 | ||
| OP alone | 79 | 1-3 | 41 | 23 | ||
| Lal, 2012122 | ||||||
| SRS alone | 31 | 1-3 | 15-20 Gy | NA | 71 | 15.2 |
| SRS + WBRT | 27 | 1-3 | 15-20 Gy + 30 Gy/12 fr | NA | 15 | 5.7 |
| Yamamoto, 2013124 | ||||||
| SRS | 548 | 1-4 | 10-32 Gy | 91.5† | 30.3† | 7.9 |
| SRS | 548 | > 5 | 96.1† | 29.0† | 7.0 |
Abbreviations: fr, fraction; FU, follow-up; GH, growth hormone; m, month; NA, not available; OP, operation; RPA, recursive partitioning analysis; SRS, stereotactic radiosurgery; WBRT, whole-brain radiation therapy; wk, weeks.
Total No. of patients in this series.
FU period was not mentioned.
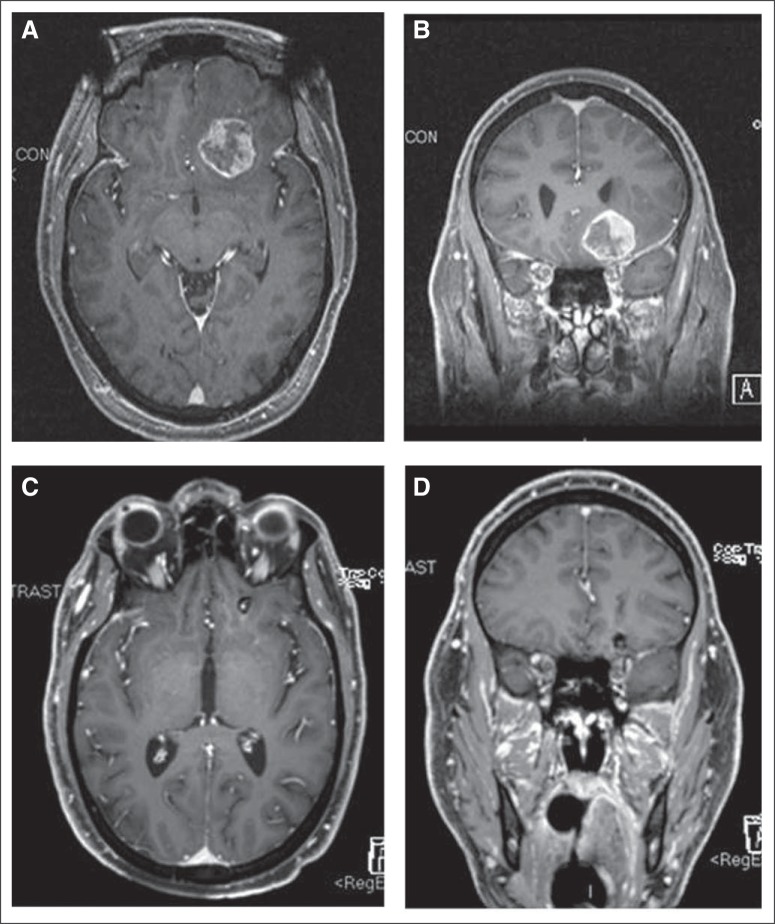
Fig 1.
(A) T1 axial and (B) coronal T1 postcontrast magnetic resonance image (MRI) of a patient with a left inferior frontal brain metastasis obtained at the time of stereotactic radiosurgery. (C and D) The comparable follow-up MRIs of the brain 20 months later show substantial tumor regression.
SRS WITH WBRT
Since the 1990s, trials investigated the use of SRS with WBRT in recurrent or newly diagnosed brain metastases, working under the hypothesis that the combination would better control brain metastases locally. Sanghavi et al109 analyzed the outcome of 502 patients with brain metastases who were treated by SRS with WBRT in a large retrospective study from 10 institutions. Patients' median survival rate was 10.7 months, with favorable factors of higher Karnofsky performance score and lower recursive partitioning analysis (RPA) class. Adding SRS improved patients' median survival rates (16, 10, and 9 months for RPA classes 1, 2, and 3, respectively) versus rates for comparable patients who received WBRT alone (7, 4, and 2 months for RPA classes 1, 2, and 3, respectively).
RTOG 9508 is a level 1 study of patients with brain metastases treated with SRS plus WBRT versus WBRT alone.114 This randomized clinical trial comprised 333 patients from 55 participating RTOG institutions. Patients had one to three newly diagnosed brain metastases on magnetic resonance image; each metastasis was ≤ 4 cm in diameter. Univariate analysis showed a survival advantage in the WBRT plus SRS group for patients with a single brain metastasis (median survival, 6.5 months v 4.9 months; P = .04). Patients in the SRS plus WBRT group were also more likely to have a stable or improved Karnofsky performance score at their 6 months' follow-up than were patients assigned to WBRT alone (43% v 27%; P = .03). The risk of developing a local recurrence was 43% greater in the WBRT-alone group (P = .002). According to these results, Andrews et al114 concluded that WBRT and SRS boost treatment improved functional status for all patients and survival rates for patients with a single unresectable brain metastatic lesion.
Aoyama et al115 also conducted a randomized trial of SRS versus SRS plus WBRT (Japanese Radiation Oncology Study Group trial 99-1). They attempted to determine whether WBRT combined with SRS versus SRS alone improved survival, brain tumor control, functional preservation rate, and frequency of neurologic death. Between 1999 and 2003, they enrolled 132 patients who had one to four brain metastases (each less than 3 cm in diameter) onto the study and randomly assigned the patients to receive SRS alone versus SRS plus WBRT. The median survival time and the 1-year actuarial survival rates were 7.5 months and 38.5% in the WBRT plus SRS group and 8.0 months and 28.5% for SRS alone (P = .42). Aoyama et al115 concluded that the use of WBRT plus SRS versus SRS alone did not improve survival rates for patients with one to four brain metastases, but intracranial relapse occurred considerably more frequently in those patients who did not receive WBRT. In their follow-up article, Aoyama et al116 also mentioned that the most important factor to influence stabilization of neurocognitive function was brain tumor control.
In a study by Chang et al,117 patients with one to three brain metastases were randomly assigned to SRS plus WBRT or SRS alone. The trial was stopped by the data monitoring committee secondary to an increased probability of neurocognitive decline in the patients treated with WBRT. This decline was observed in learning and memory function indices 4 months after treatment. An accompanying cost-effectiveness analysis demonstrated a higher effectiveness for SRS and observation in patients with one to three brain metastases.118
In a trial by the European Organisation for Research and Treatment of Cancer, Kocher et al112 demonstrated the other view of adjuvant WBRT after SRS; 359 patients with one to three brain metastases were treated with complete surgery or radiosurgery and were then randomly assigned to adjuvant WBRT (30 Gy in 10 fractions) or observation. Kocher et al set the primary end point as time to WHO performance status deterioration to more than 2. They found that the median time to WHO performance status of more than 2 was 10.0 months after observation and 9.5 months after WBRT. Overall survival was similar in the WBRT and observation arms. However, WBRT reduced the 2-year relapse rate both at the initial sites and new sites, and salvage therapies were used more frequently after observation than after WBRT. They concluded that adjuvant WBRT reduced intracranial relapses and neurologic deaths but failed to improve the duration of functional independence and overall survival.
Tsao et al119 summarized these randomized clinical trials (RCTs) in 2013. In their meta-analysis report, two RCTs reported on the WBRT and SRS boost versus WBRT alone in patients with two to four brain metastases and found no difference in overall survival, but local tumor control was significantly favored by the WBRT plus SRS boost. Three RCTs reported on SRS alone versus the WBRT plus SRS boost for patients with one to four brain metastases and found there was no difference in overall survival, but local tumor control was also significantly favored in the WBRT plus SRS boost group. Conclusively, for a limited number of brain metastases, there are no survival benefits for WBRT plus SRS boost compared with SRS alone. Although additional WBRT improves local and distant brain metastases control, SRS alone should be considered a routine treatment owing to better neurocognitive outcomes and a lower risk of late adverse effects.
SRS AS A SOLE TREATMENT APPROACH
For radioresistant histologies such as melanoma, SRS alone has long been a popular approach. Treating radioresistant histologies with WBRT has disadvantages120 and adverse effects, particularly in terms of neurocognition, that make WBRT less appealing to selected patients and their treating physicians. Nevertheless, when considering the adverse effects on quality of life and neurologic function of long-term survivors,116,117 the use of SRS alone is becoming more and more popular. Regarding tumor control, the rationale against using WBRT with SRS is that the benefit of WBRT on nonvisualized brain metastases is mitigated by the fact that once WBRT ends, any new metastases are now untreated. Hence, recurrent brain metastases are best treated expectantly by delayed WBRT or repeat SRS.
Sneed et al106 collected clinical data from 10 institutions and compared the survival rates of patients with newly diagnosed brain metastases. For all RPA classes, the median survival times for patients were comparable between the two treatment groups (RPA class I, 14 months v 15 months; RPA class 2, 8 months v 7 months; RPA class III, 5 months v 6 months). The report concluded that the upfront WBRT did not improve survival compared with SRS alone.
The Japanese Leksell Gamma Knife Society undertook a prospective study (JLGK 0901 study) of SRS without WBRT to establish evidence that such a treatment strategy is feasible for five to 10 brain metastases. A preliminary report showed that the overall survival rate for patients with five to 10 brain metastases was almost the same as that of patients with two to four brain lesions.121 More recently, Yamamoto et al122 demonstrated a post-SRS median survival difference of 0.9 months between the two groups, suggesting noninferiority when using SRS alone for patients with five or more brain metastases.
The role of radiosurgery for brain metastasis has expanded significantly over the past decade. It can be used as either an upfront treatment or after prior WBRT. Radiosurgery affords a high rate of local tumor control, even in radioresistant histologies. Although radiosurgery seems to avoid collateral damage that can translate to neurocognitive decline, it does so at the risk of greater distant intracranial disease progression over time and may require salvage treatment such as repeat SRS, WBRT, or resection.
FUTURE DIRECTIONS FOR STEREOTACTIC RADIOSURGERY
Radiosurgery has shown substantial growth. Beginning in 2003 in the United States, SRS was more frequently performed than craniotomy for nonmeningioma tumors.123 There has also been a dramatic increase in access to radiosurgical services throughout much of the world, and a premium is being placed on radiosurgical education and training.123,124 It is clear that radiosurgery has altered practice patterns and will have a lasting presence in the treatment of patients with intracranial disorders.
Radiosurgery indications are also likely to expand. An initial multicenter prospective trial examining the use stereotactic radiosurgery for mesial temporal lobe epilepsy was completed and showed reasonable success.125 This prompted funding for the Radiosurgery or Open Surgery for Epilepsy trial by the National Institutes for Health. Radiosurgery for functional and psychiatric indications have also shown renewed interest as of late.126,127
Traditional radiosurgery was delivered in a single session. However, with the advent of relocatable immobilization systems and reliable intrafraction image guidance systems, multisession radiosurgery has also led to broader indications for this discipline.128,129 A multisession approach capitalizes on the four R's of radiation therapy and the greater ease of radiosurgical delivery even when the target abuts a radiation-sensitive critical structure.130
The principles of radiosurgery have also extended to extracranial sites. In 1996, Hamilton et al131 reported on radiosurgery for spinal lesions. Since then, spinal radiosurgery has been added to the treatment armamentarium for patients with metastases, arteriovenous malformations, and benign spinal tumors such as neurofibromas.132–134 This and other extracranial applications in the burgeoning field of stereotactic body radiation therapy are discussed in more detail in other articles in this issue.
CONCLUSION
In the more than six decades since its conception, stereotactic radiosurgery has disrupted old approaches and led to improved treatment of patients with intracranial disorders. The principles of Leksell have stood the test of time and continue to shape the fields of neurosurgery and radiation oncology. The ripples of radiosurgery are even being felt beyond the traditional intracranial realm and are affecting the management of patients with spinal and thoracic pathologies. Technologic refinements, appropriate education and training, and preserving the multidisciplinary approach for SRS will likely lead to further benefits for patients and further growth for the field.
Footnotes
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Jason P. Sheehan, Jay S. Loeffler
Collection and assembly of data: Chun-Po Yen, Cheng-Chia Lee
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Seymour ZA, Cohen-Gadol AA. Cushing's lost cases of “radium bomb” brachytherapy for gliomas. J Neurosurg. 2010;113:141–148. doi: 10.3171/2009.11.JNS091393. [DOI] [PubMed] [Google Scholar]
- 2.Hsu W, Li KW, Bookland M, et al. Keyhole to the brain: Walter Dandy and neuroendoscopy. J Neurosurg Pediatr. 2009;3:439–442. doi: 10.3171/2009.1.PEDS08342. [DOI] [PubMed] [Google Scholar]
- 3.Clarke RH, Horsley V. The classic: On a method of investigating the deep ganglia and tracts of the central nervous system (cerebellum)—Br Med J 1906:1799-1800. Clin Orthop Relat Res. 2007;463:3–6. doi: 10.1097/BLO.0b013e31814d4d99. [DOI] [PubMed] [Google Scholar]
- 4.Spiegel EA, Wycis HT, Marks M, et al. Stereotaxic apparatus for operations on the human brain. Science. 1947;106:349–350. doi: 10.1126/science.106.2754.349. [DOI] [PubMed] [Google Scholar]
- 5.Leksell L. The stereotaxic method and radiosurgery of the brain. Acta Chir Scand. 1951;102:316–319. [PubMed] [Google Scholar]
- 6.Leksell L, Larsson B, Andersson B, et al. Lesions in the depth of the brain produced by a beam of high energy protons. Acta radiol. 1960;54:251–264. doi: 10.3109/00016926009172547. [DOI] [PubMed] [Google Scholar]
- 7.Larsson B, Leksell L, Rexed B. The use of high energy protons for cerebral surgery in man. Acta Chir Scand. 1963 [Google Scholar]
- 8.Bussiere MR, Loeffler JS, Chapman PH, et al. Youmans Neurological Surgery: Techniques of Radiosurgery. Philadelphia, PA: Elsevier; 2011. [Google Scholar]
- 9.Betti OO. History of adiosurgery [in French] Cancer Radiother. 1998;2:101–104. doi: 10.1016/s1278-3218(98)89080-x. [DOI] [PubMed] [Google Scholar]
- 10.Betti O, Derechinsky V. Multiple-beam stereotaxic irradiation [in French] Neurochirurgie. 1983;29:295–298. [PubMed] [Google Scholar]
- 11.Mehta MP. The physical, biologic, and clinical basis of radiosurgery. Curr Probl Cancer. 1995;19:265–329. [PubMed] [Google Scholar]
- 12.Colombo F, Benedetti A, Pozza F, et al. External stereotactic irradiation by linear accelerator. Neurosurgery. 1985;16:154–160. doi: 10.1227/00006123-198502000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Podgorsak EB, Olivier A, Pla M, et al. Physical aspects of dynamic stereotactic radiosurgery. Appl Neurophysiol. 1987;50:263–268. doi: 10.1159/000100723. [DOI] [PubMed] [Google Scholar]
- 14.Hartmann GH, Schlegel W, Sturm V, et al. Cerebral radiation surgery using moving field irradiation at a linear accelerator facility. Int J Radiat Oncol Biol Phys. 1985;11:1185–1192. doi: 10.1016/0360-3016(85)90068-9. [DOI] [PubMed] [Google Scholar]
- 15.Lutz W, Winston KR, Maleki N. A system for stereotactic radiosurgery with a linear accelerator. Int J Radiat Oncol Biol Phys. 1988;14:373–381. doi: 10.1016/0360-3016(88)90446-4. [DOI] [PubMed] [Google Scholar]
- 16.Barnett GH, Linskey ME, Adler JR, et al. Stereotactic radiosurgery: An organized neurosurgery-sanctioned definition. J Neurosurg. 2007;106:1–5. doi: 10.3171/jns.2007.106.1.1. [DOI] [PubMed] [Google Scholar]
- 17.Seung SK, Larson DA, Galvin JM, et al. American College of Radiology (ACR) and American Society for Radiation Oncology (ASTRO) practice guideline for the performance of stereotactic radiosurgery (SRS) Am J Clin Oncol. 2006;36:310–315. doi: 10.1097/COC.0b013e31826e053d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia-Barros M, Paris F, Cordon-Cardo C, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300:1155–1159. doi: 10.1126/science.1082504. [DOI] [PubMed] [Google Scholar]
- 19.Vance ML. Treatment of patients with a pituitary adenoma: One clinician's experience. Neurosurg Focus. 2004;16:E1. doi: 10.3171/foc.2004.16.4.2. [DOI] [PubMed] [Google Scholar]
- 20.Dekkers OM, Pereira AM, Romijn JA. Treatment and follow-up of clinically nonfunctioning pituitary macroadenomas. J Clin Endocrinol Metab. 2008;93:3717–3726. doi: 10.1210/jc.2008-0643. [DOI] [PubMed] [Google Scholar]
- 21.Feigl GC, Bonelli CM, Berghold A, et al. Effects of gamma knife radiosurgery of pituitary adenomas on pituitary function. J Neurosurg. 2002;97:415–421. doi: 10.3171/jns.2002.97.supplement. [DOI] [PubMed] [Google Scholar]
- 22.Höybye C, Rähn T. Adjuvant Gamma Knife radiosurgery in non-functioning pituitary adenomas; low risk of long-term complications in selected patients. Pituitary. 2009;12:211–216. doi: 10.1007/s11102-008-0163-x. [DOI] [PubMed] [Google Scholar]
- 23.Iwai Y, Yamanaka K, Yoshioka K. Radiosurgery for nonfunctioning pituitary adenomas. Neurosurgery. 2005;56:699–705. doi: 10.1227/01.neu.0000156836.42945.28. [DOI] [PubMed] [Google Scholar]
- 24.Kajiwara K, Saito K, Yoshikawa K, et al. Image-guided stereotactic radiosurgery with the CyberKnife for pituitary adenomas. Minim Invasive Neurosurg. 2005;48:91–96. doi: 10.1055/s-2004-830261. [DOI] [PubMed] [Google Scholar]
- 25.Losa M, Valle M, Mortini P, et al. Gamma knife surgery for treatment of residual nonfunctioning pituitary adenomas after surgical debulking. J Neurosurg. 2004;100:438–444. doi: 10.3171/jns.2004.100.3.0438. [DOI] [PubMed] [Google Scholar]
- 26.Mingione V, Yen CP, Vance ML, et al. Gamma surgery in the treatment of nonsecretory pituitary macroadenoma. J Neurosurg. 2006;104:876–883. doi: 10.3171/jns.2006.104.6.876. [DOI] [PubMed] [Google Scholar]
- 27.Muacevic A, Uhl E, Wowra B. Gamma knife radiosurgery for nonfunctioning pituitary adenomas. Acta Neurochir Suppl. 2004;91:51–54. doi: 10.1007/978-3-7091-0583-2_5. [DOI] [PubMed] [Google Scholar]
- 28.Petrovich Z, Yu C, Giannotta SL, et al. Gamma knife radiosurgery for pituitary adenoma: Early results. Neurosurgery. 2003;53:51–59. doi: 10.1227/01.neu.0000068702.00330.47. [DOI] [PubMed] [Google Scholar]
- 29.Picozzi P, Losa M, Mortini P, et al. Radiosurgery and the prevention of regrowth of incompletely removed nonfunctioning pituitary adenomas. J Neurosurg. 2005;102(suppl):71–74. doi: 10.3171/jns.2005.102.s_supplement.0071. [DOI] [PubMed] [Google Scholar]
- 30.Sheehan JP, Kondziolka D, Flickinger J, et al. Radiosurgery for residual or recurrent nonfunctioning pituitary adenoma. J Neurosurg. 2002;97(suppl 5):408–414. doi: 10.3171/jns.2002.97.supplement. [DOI] [PubMed] [Google Scholar]
- 31.Wowra B, Stummer W. Efficacy of gamma knife radiosurgery for nonfunctioning pituitary adenomas: A quantitative follow up with magnetic resonance imaging-based volumetric analysis. J Neurosurg. 2002;97:429–432. doi: 10.3171/jns.2002.97.supplement. [DOI] [PubMed] [Google Scholar]
- 32.Castro DG, Cecilio SA, Canteras MM. Radiosurgery for pituitary adenomas: Evaluation of its efficacy and safety. Radiat Oncol. 2010;5:109. doi: 10.1186/1748-717X-5-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.El-Shehaby AM, Reda WA, Tawadros SR, et al. Low-dose Gamma Knife surgery for nonfunctioning pituitary adenomas. J Neurosurg. 2012;117(suppl):84–88. doi: 10.3171/2012.6.GKS12986. [DOI] [PubMed] [Google Scholar]
- 34.Gopalan R, Schlesinger D, Vance ML, et al. Long-term outcomes after Gamma Knife radiosurgery for patients with a nonfunctioning pituitary adenoma. Neurosurgery. 2011;69:284–293. doi: 10.1227/NEU.0b013e31821bc44e. [DOI] [PubMed] [Google Scholar]
- 35.Hayashi M, Chernov M, Tamura N, et al. Gamma Knife robotic microradiosurgery of pituitary adenomas invading the cavernous sinus: Treatment concept and results in 89 cases. J Neurooncol. 2010;98:185–194. doi: 10.1007/s11060-010-0172-2. [DOI] [PubMed] [Google Scholar]
- 36.Iwata H, Sato K, Tatewaki K, et al. Hypofractionated stereotactic radiotherapy with CyberKnife for nonfunctioning pituitary adenoma: High local control with low toxicity. Neuro Oncol. 2011;13:916–922. doi: 10.1093/neuonc/nor055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kobayashi T. Long-term results of stereotactic gamma knife radiosurgery for pituitary adenomas: Specific strategies for different types of adenoma. Prog Neurol Surg. 2009;22:77–95. doi: 10.1159/000163384. [DOI] [PubMed] [Google Scholar]
- 38.Liscak R, Vladyka V, Marek J, et al. Gamma knife radiosurgery for endocrine-inactive pituitary adenomas. Acta Neurochir (Wien) 2007;149:999–1006. doi: 10.1007/s00701-007-1253-7. [DOI] [PubMed] [Google Scholar]
- 39.Park KJ, Kano H, Parry PV, et al. Long-term outcomes after gamma knife stereotactic radiosurgery for nonfunctional pituitary adenomas. Neurosurgery. 2011;69:1188–1199. doi: 10.1227/NEU.0b013e318222afed. [DOI] [PubMed] [Google Scholar]
- 40.Pollock BE, Cochran J, Natt N, et al. Gamma knife radiosurgery for patients with nonfunctioning pituitary adenomas: Results from a 15-year experience. Int J Radiat Oncol Biol Phys. 2008;70:1325–1329. doi: 10.1016/j.ijrobp.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 41.Runge MJ, Maarouf M, Hunsche S, et al. LINAC-radiosurgery for nonsecreting pituitary adenomas: Long-term results. Strahlenther Onkol. 2012;188:319–325. doi: 10.1007/s00066-011-0052-5. [DOI] [PubMed] [Google Scholar]
- 42.Starke RM, Williams BJ, Jane JA, Jr, et al. Gamma Knife surgery for patients with nonfunctioning pituitary macroadenomas: Predictors of tumor control, neurological deficits, and hypopituitarism. J Neurosurg. 2012;117:129–135. doi: 10.3171/2012.4.JNS112250. [DOI] [PubMed] [Google Scholar]
- 43.Voges J, Kocher M, Runge M, et al. Linear accelerator radiosurgery for pituitary macroadenomas: A 7-year follow-up study. Cancer. 2006;107:1355–1364. doi: 10.1002/cncr.22128. [DOI] [PubMed] [Google Scholar]
- 44.Wilson PJ, De-Loyde KJ, Williams JR, et al. A single centre's experience of stereotactic radiosurgery and radiotherapy for non-functioning pituitary adenomas with the Linear Accelerator (Linac) J Clin Neurosci. 2012;19:370–374. doi: 10.1016/j.jocn.2011.07.025. [DOI] [PubMed] [Google Scholar]
- 45.Sheehan JP, Starke RM, Mathieu D, et al. Gamma Knife radiosurgery for the management of nonfunctioning pituitary adenomas: A multicenter study. J Neurosurg. 2013;119:446–456. doi: 10.3171/2013.3.JNS12766. [DOI] [PubMed] [Google Scholar]
- 46.Choi JY, Chang JH, Chang JW, et al. Radiological and hormonal responses of functioning pituitary adenomas after gamma knife radiosurgery. Yonsei Med J. 2003;44:602–607. doi: 10.3349/ymj.2003.44.4.602. [DOI] [PubMed] [Google Scholar]
- 47.Izawa M, Hayashi M, Nakaya K, et al. Gamma knife radiosurgery for pituitary adenomas. J Neurosurg. 2000;93(suppl 3):19–22. doi: 10.3171/jns.2000.93.supplement. [DOI] [PubMed] [Google Scholar]
- 48.Kobayashi T, Kida Y, Mori Y. Gamma knife radiosurgery in the treatment of Cushing disease: Long-term results. J Neurosurg. 2002;97:422–428. doi: 10.3171/jns.2002.97.supplement. [DOI] [PubMed] [Google Scholar]
- 49.Laws ER, Reitmeyer M, Thapar K, et al. Cushing's disease resulting from pituitary corticotrophic microadenoma: Treatment results from transsphenoidal microsurgery and gamma knife radiosurgery. Neurochirurgie. 2002;48:294–299. [PubMed] [Google Scholar]
- 50.Pollock BE, Nippoldt TB, Stafford SL, et al. Results of stereotactic radiosurgery in patients with hormone-producing pituitary adenomas: Factors associated with endocrine normalization. J Neurosurg. 2002;97:525–530. doi: 10.3171/jns.2002.97.3.0525. [DOI] [PubMed] [Google Scholar]
- 51.Sheehan JM, Vance ML, Sheehan JP, et al. Radiosurgery for Cushing's disease after failed transsphenoidal surgery. J Neurosurg. 2000;93:738–742. doi: 10.3171/jns.2000.93.5.0738. [DOI] [PubMed] [Google Scholar]
- 52.Shin M, Kurita H, Sasaki T, et al. Stereotactic radiosurgery for pituitary adenoma invading the cavernous sinus. J Neurosurg. 2000;93(suppl 3):2–5. doi: 10.3171/jns.2000.93.supplement. [DOI] [PubMed] [Google Scholar]
- 53.Castinetti F, Nagai M, Morange I, et al. Long-term results of stereotactic radiosurgery in secretory pituitary adenomas. J Clin Endocrinol Metab. 2009;94:3400–3407. doi: 10.1210/jc.2008-2772. [DOI] [PubMed] [Google Scholar]
- 54.Witt TC. Stereotactic radiosurgery for pituitary tumors. Neurosurg Focus. 2003;14:e10. doi: 10.3171/foc.2003.14.5.11. [DOI] [PubMed] [Google Scholar]
- 55.Wong GK, Leung CH, Chiu KW, et al. LINAC radiosurgery in recurrent Cushing's disease after transsphenoidal surgery: A series of 5 cases. Minim Invasive Neurosurg. 2003;46:327–330. doi: 10.1055/s-2003-812497. [DOI] [PubMed] [Google Scholar]
- 56.Devin JK, Allen GS, Cmelak AJ, et al. The efficacy of linear accelerator radiosurgery in the management of patients with Cushing's disease. Stereotact Funct Neurosurg. 2004;82:254–262. doi: 10.1159/000083476. [DOI] [PubMed] [Google Scholar]
- 57.Jagannathan J, Sheehan JP, Pouratian N, et al. Gamma Knife surgery for Cushing's disease. J Neurosurg. 2007;106:980–987. doi: 10.3171/jns.2007.106.6.980. [DOI] [PubMed] [Google Scholar]
- 58.Petit JH, Biller BM, Yock TI, et al. Proton stereotactic radiotherapy for persistent adrenocorticotropin-producing adenomas. J Clin Endocrinol Metab. 2008;93:393–399. doi: 10.1210/jc.2007-1220. [DOI] [PubMed] [Google Scholar]
- 59.Pollock BE, Brown PD, Nippoldt TB, et al. Pituitary tumor type affects the chance of biochemical remission after radiosurgery of hormone-secreting pituitary adenomas. Neurosurgery. 2008;62:1271–1276. doi: 10.1227/01.neu.0000333298.49436.0e. [DOI] [PubMed] [Google Scholar]
- 60.Tinnel BA, Henderson MA, Witt TC, et al. Endocrine response after gamma knife-based stereotactic radiosurgery for secretory pituitary adenoma. Stereotact Funct Neurosurg. 2008;86:292–296. doi: 10.1159/000151717. [DOI] [PubMed] [Google Scholar]
- 61.Grant RA, Whicker M, Lleva R, et al. Efficacy and safety of higher dose stereotactic radiosurgery for functional pituitary adenomas: A preliminary report. World Neurosurg. doi: 10.1016/j.wneu.2013.01.127. doi: 10.1016/j.wneu.2013.01.127 [epub ahead of print on February 4, 2013] [DOI] [PubMed] [Google Scholar]
- 62.Sheehan JP, Pouratian N, Steiner L, et al. Gamma Knife surgery for pituitary adenomas: Factors related to radiological and endocrine outcomes. J Neurosurg. 2011;114:303–309. doi: 10.3171/2010.5.JNS091635. [DOI] [PubMed] [Google Scholar]
- 63.Wan H, Chihiro O, Yuan S. MASEP gamma knife radiosurgery for secretory pituitary adenomas: Experience in 347 consecutive cases. J Exp Clin Cancer Res. 2009;28:36. doi: 10.1186/1756-9966-28-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wein L, Dally M, Bach LA. Stereotactic radiosurgery for treatment of Cushing disease: An Australian experience. Intern Med J. 2012;42:1153–1156. doi: 10.1111/j.1445-5994.2012.02903.x. [DOI] [PubMed] [Google Scholar]
- 65.Liu X, Kano H, Kondziolka D, et al. Gamma knife radiosurgery for clinically persistent acromegaly. J Neurooncol. 2012;109:71–79. doi: 10.1007/s11060-012-0862-z. [DOI] [PubMed] [Google Scholar]
- 66.Liu X, Kano H, Kondziolka D, et al. Gamma knife stereotactic radiosurgery for drug resistant or intolerant invasive prolactinomas. Pituitary. 2013;16:68–75. doi: 10.1007/s11102-012-0376-x. [DOI] [PubMed] [Google Scholar]
- 67.Franzin A, Spatola G, Losa M, et al. Results of gamma knife radiosurgery in acromegaly. Int J Endocrinol. 2012;2012:342034. doi: 10.1155/2012/342034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Poon TL, Leung SC, Poon CY, et al. Predictors of outcome following Gamma Knife surgery for acromegaly. J Neurosurg. 2010;113(suppl):149–152. [PubMed] [Google Scholar]
- 69.Iwai Y, Yamanaka K, Yoshimura M, et al. Gamma knife radiosurgery for growth hormone-producing adenomas. J Clin Neurosci. 2010;17:299–304. doi: 10.1016/j.jocn.2009.05.040. [DOI] [PubMed] [Google Scholar]
- 70.Losa M, Gioia L, Picozzi P, et al. The role of stereotactic radiotherapy in patients with growth hormone-secreting pituitary adenoma. J Clin Endocrinol Metab. 2008;93:2546–2552. doi: 10.1210/jc.2008-0135. [DOI] [PubMed] [Google Scholar]
- 71.Ronchi CL, Attanasio R, Verrua E, et al. Efficacy and tolerability of gamma knife radiosurgery in acromegaly: A 10-year follow-up study. Clin Endocrinol (Oxf) 2009;71:846–852. doi: 10.1111/j.1365-2265.2009.03589.x. [DOI] [PubMed] [Google Scholar]
- 72.Jagannathan J, Sheehan JP, Pouratian N, et al. Gamma knife radiosurgery for acromegaly: Outcomes after failed transsphenoidal surgery. Neurosurgery. 2008;62:1262–1269. doi: 10.1227/01.neu.0000333297.41813.3d. [DOI] [PubMed] [Google Scholar]
- 73.Jezkova J, Marek J, Hana V, et al. Gamma knife radiosurgery for acromegaly: Long-term experience. Clin Endocrinol (Oxf) 2006;64:588–595. doi: 10.1111/j.1365-2265.2006.02513.x. [DOI] [PubMed] [Google Scholar]
- 74.Pollock BE, Jacob JT, Brown PD, et al. Radiosurgery of growth hormone-producing pituitary adenomas: Factors associated with biochemical remission. J Neurosurg. 2007;106:833–838. doi: 10.3171/jns.2007.106.5.833. [DOI] [PubMed] [Google Scholar]
- 75.Roberts BK, Ouyang DL, Lad SP, et al. Efficacy and safety of CyberKnife radiosurgery for acromegaly. Pituitary. 2007;10:19–25. doi: 10.1007/s11102-007-0004-3. [DOI] [PubMed] [Google Scholar]
- 76.Vik-Mo EO, Oksnes M, Pedersen PH, et al. Gamma knife stereotactic radiosurgery for acromegaly. Eur J Endocrinol. 2007;157:255–263. doi: 10.1530/EJE-07-0189. [DOI] [PubMed] [Google Scholar]
- 77.Kobayashi T, Mori Y, Uchiyama Y, et al. Long-term results of gamma knife surgery for growth hormone-producing pituitary adenoma: Is the disease difficult to cure? J Neurosurg. 2005;102(suppl):119–123. doi: 10.3171/jns.2005.102.s_supplement.0119. [DOI] [PubMed] [Google Scholar]
- 78.Castinetti F, Taieb D, Kuhn JM, et al. Outcome of gamma knife radiosurgery in 82 patients with acromegaly: Correlation with initial hypersecretion. J Clin Endocrinol Metab. 2005;90:4483–4488. doi: 10.1210/jc.2005-0311. [DOI] [PubMed] [Google Scholar]
- 79.Gutt B, Wowra B, Alexandrov R, et al. Gamma-knife surgery is effective in normalising plasma insulin-like growth factor I in patients with acromegaly. Exp Clin Endocrinol Diabetes. 2005;113:219–224. doi: 10.1055/s-2005-837552. [DOI] [PubMed] [Google Scholar]
- 80.Muramatsu J, Yoshida M, Shioura H, et al. Clinical results of LINAC-based stereotactic radiosurgery for pituitary adenoma [in Japanese] Nihon Igaku Hoshasen Gakkai Zasshi. 2003;63:225–230. [PubMed] [Google Scholar]
- 81.Zhang N, Pan L, Wang EM, et al. Radiosurgery for growth hormone-producing pituitary adenomas. J Neurosurg. 2000;93(suppl 3):6–9. doi: 10.3171/jns.2000.93.supplement. [DOI] [PubMed] [Google Scholar]
- 82.Fukuoka S, Ito T, Takanashi M, et al. Gamma knife radiosurgery for growth hormone-secreting pituitary adenomas invading the cavernous sinus. Stereotact Funct Neurosurg. 2001;76:213–217. doi: 10.1159/000066721. [DOI] [PubMed] [Google Scholar]
- 83.Ikeda H, Jokura H, Yoshimoto T. Transsphenoidal surgery and adjuvant gamma knife treatment for growth hormone-secreting pituitary adenoma. J Neurosurg. 2001;95:285–291. doi: 10.3171/jns.2001.95.2.0285. [DOI] [PubMed] [Google Scholar]
- 84.Attanasio R, Epaminonda P, Motti E, et al. Gamma-knife radiosurgery in acromegaly: A 4-year follow-up study. J Clin Endocrinol Metab. 2003;88:3105–3112. doi: 10.1210/jc.2002-021663. [DOI] [PubMed] [Google Scholar]
- 85.Loeffler JS, Shih HA. Radiation therapy in the management of pituitary adenomas. J Clin Endocrinol Metab. 2011;96:1992–2003. doi: 10.1210/jc.2011-0251. [DOI] [PubMed] [Google Scholar]
- 86.Johnson R. Radiotherapy of Cerebral Angiomas With a Note on Some Problems in Diagnosis. Berlin, Germany: Springer-Verlag; 1975. [Google Scholar]
- 87.French LA, Chou SN, Story JL. Cerebrovascular malformations. Clin Neurosurg. 1964;11:171–182. doi: 10.1093/neurosurgery/11.cn_suppl_1.171. [DOI] [PubMed] [Google Scholar]
- 88.McKissock W, Hankinson J. The surgical treatment of the supratentorial angiomas: Reports and discussions [in French]. The First International Congress of Neurosurgery; July 21-28, 1957; Brussels, Belgium. [Google Scholar]
- 89.Steiner L, Leksell L, Greitz T, et al. Stereotaxic radiosurgery for cerebral arteriovenous malformations: Report of a case. Acta Chir Scand. 1972;138:459–464. [PubMed] [Google Scholar]
- 90.Starke RM, Yen CP, Ding D, et al. A practical grading scale for predicting outcome after radiosurgery for arteriovenous malformations: Analysis of 1012 treated patients. J Neurosurg. 2013;119:981–987. doi: 10.3171/2013.5.JNS1311. [DOI] [PubMed] [Google Scholar]
- 91.Lunsford LD, Niranjan A, Kondziolka D, et al. Arteriovenous malformation radiosurgery: A twenty year perspective. Clin Neurosurg. 2008;55:108–119. [PubMed] [Google Scholar]
- 92.Friedman WA, Bova FJ, Bollampally S, et al. Analysis of factors predictive of success or complications in arteriovenous malformation radiosurgery. Neurosurgery. 2003;52:296–307. doi: 10.1227/01.neu.0000043692.51385.91. [DOI] [PubMed] [Google Scholar]
- 93.Liscák R, Vladyka V, Simonová G, et al. Arteriovenous malformations after Leksell gamma knife radiosurgery: Rate of obliteration and complications. Neurosurgery. 2007;60:1005–1014. doi: 10.1227/01.NEU.0000255474.60505.4A. [DOI] [PubMed] [Google Scholar]
- 94.Lunsford LD, Kondziolka D, Flickinger JC, et al. Stereotactic radiosurgery for arteriovenous malformations of the brain. J Neurosurg. 1991;75:512–524. doi: 10.3171/jns.1991.75.4.0512. [DOI] [PubMed] [Google Scholar]
- 95.Maruyama K, Kawahara N, Shin M, et al. The risk of hemorrhage after radiosurgery for cerebral arteriovenous malformations. N Engl J Med. 2005;352:146–153. doi: 10.1056/NEJMoa040907. [DOI] [PubMed] [Google Scholar]
- 96.Shin M, Maruyama K, Kurita H, et al. Analysis of nidus obliteration rates after gamma knife surgery for arteriovenous malformations based on long-term follow-up data: The University of Tokyo experience. J Neurosurg. 2004;101:18–24. doi: 10.3171/jns.2004.101.1.0018. [DOI] [PubMed] [Google Scholar]
- 97.Steiner L, Lindquist C, Adler JR, et al. Outcome of radiosurgery for cerebral AVM. J Neurosurg. 1992;77:823. doi: 10.3171/jns.1992.77.5.0823. [DOI] [PubMed] [Google Scholar]
- 98.Yen CP, Schlesinger D, Sheehan J. Natural history of cerebral arteriovenous malformations and the risk of hemorrhage after radiosurgery. In: Niranjan A, Lunsford LD, editors. Gamma Knife Radiosurgery for Brain Vascular Malformations. Progress in Neurological Surgery. vol 27. Leipzig, Germany: Karger; 2013. pp. 5–21. [DOI] [PubMed] [Google Scholar]
- 99.Chao JH, Phillips R, Nickson JJ. Roentgen-ray therapy of cerebral metastases. Cancer. 1954;7:682–689. doi: 10.1002/1097-0142(195407)7:4<682::aid-cncr2820070409>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 100.Chu FC, Hilaris BB. Value of radiation theray in the management of intracranial metastases. Cancer. 1961;14:577–581. doi: 10.1002/1097-0142(199005/06)14:3<577::aid-cncr2820140318>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 101.Borgelt B, Gelber R, Kramer S, et al. The palliation of brain metastases: Final results of the first two studies by the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys. 1980;6:1–9. doi: 10.1016/0360-3016(80)90195-9. [DOI] [PubMed] [Google Scholar]
- 102.Komarnicky LT, Phillips TL, Martz K, et al. A randomized phase III protocol for the evaluation of misonidazole combined with radiation in the treatment of patients with brain metastases (RTOG-7916) Int J Radiat Oncol Biol Phys. 1991;20:53–58. doi: 10.1016/0360-3016(91)90137-s. [DOI] [PubMed] [Google Scholar]
- 103.Murray KJ, Scott C, Greenberg HM, et al. A randomized phase III study of accelerated hyperfractionation versus standard in patients with unresected brain metastases: A report of the Radiation Therapy Oncology Group (RTOG) 9104. Int J Radiat Oncol Biol Phys. 1997;39:571–574. doi: 10.1016/s0360-3016(97)00341-6. [DOI] [PubMed] [Google Scholar]
- 104.Phillips TL, Scott CB, Leibel SA, et al. Results of a randomized comparison of radiotherapy and bromodeoxyuridine with radiotherapy alone for brain metastases: Report of RTOG trial 89-05. Int J Radiat Oncol Biol Phys. 1995;33:339–348. doi: 10.1016/0360-3016(95)00168-X. [DOI] [PubMed] [Google Scholar]
- 105.Sause WT, Scott C, Krisch R, et al. Phase I/II trial of accelerated fractionation in brain metastases RTOG 85-28. Int J Radiat Oncol Biol Phys. 1993;26:653–657. doi: 10.1016/0360-3016(93)90284-3. [DOI] [PubMed] [Google Scholar]
- 106.Sneed PK, Larson DA, Wara WM. Radiotherapy for cerebral metastases. Neurosurg Clin N Am. 1996;7:505–515. [PubMed] [Google Scholar]
- 107.Flickinger JC, Kondziolka D, Lunsford LD, et al. A multi-institutional experience with stereotactic radiosurgery for solitary brain metastasis. Int J Radiat Oncol Biol Phys. 1994;28:797–802. doi: 10.1016/0360-3016(94)90098-1. [DOI] [PubMed] [Google Scholar]
- 108.Fuller BG, Kaplan ID, Adler J, et al. Stereotaxic radiosurgery for brain metastases: The importance of adjuvant whole brain irradiation. Int J Radiat Oncol Biol Phys. 1992;23:413–418. doi: 10.1016/0360-3016(92)90762-7. [DOI] [PubMed] [Google Scholar]
- 109.Sanghavi SN, Miranpuri SS, Chappell R, et al. Radiosurgery for patients with brain metastases: A multi-institutional analysis, stratified by the RTOG recursive partitioning analysis method. Int J Radiat Oncol Biol Phys. 2001;51:426–434. doi: 10.1016/s0360-3016(01)01622-4. [DOI] [PubMed] [Google Scholar]
- 110.Auchter RM, Lamond JP, Alexander E, et al. A multiinstitutional outcome and prognostic factor analysis of radiosurgery for resectable single brain metastasis. Int J Radiat Oncol Biol Phys. 1996;35:27–35. doi: 10.1016/s0360-3016(96)85008-5. [DOI] [PubMed] [Google Scholar]
- 111.Shehata MK, Young B, Reid B, et al. Stereotatic radiosurgery of 468 brain metastases ≤ 2 cm: Implications for SRS dose and whole brain radiation therapy. Int J Radiat Oncol Biol Phys. 2004;59:87–93. doi: 10.1016/j.ijrobp.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 112.Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: Results of the EORTC 22952-26001 study. J Clin Oncol. 2011;29:134–141. doi: 10.1200/JCO.2010.30.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Muacevic A, Wowra B, Siefert A, et al. Microsurgery plus whole brain irradiation versus Gamma Knife surgery alone for treatment of single metastases to the brain: A randomized controlled multicentre phase III trial. J Neurooncol. 2008;87:299–307. doi: 10.1007/s11060-007-9510-4. [DOI] [PubMed] [Google Scholar]
- 114.Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: Phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363:1665–1672. doi: 10.1016/S0140-6736(04)16250-8. [DOI] [PubMed] [Google Scholar]
- 115.Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: A randomized controlled trial. JAMA. 2006;295:2483–2491. doi: 10.1001/jama.295.21.2483. [DOI] [PubMed] [Google Scholar]
- 116.Aoyama H, Tago M, Kato N, et al. Neurocognitive function of patients with brain metastasis who received either whole brain radiotherapy plus stereotactic radiosurgery or radiosurgery alone. Int J Radiat Oncol Biol Phys. 2007;68:1388–1395. doi: 10.1016/j.ijrobp.2007.03.048. [DOI] [PubMed] [Google Scholar]
- 117.Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: A randomised controlled trial. Lancet Oncol. 2009;10:1037–1044. doi: 10.1016/S1470-2045(09)70263-3. [DOI] [PubMed] [Google Scholar]
- 118.Lal LS, Byfield SD, Chang EL, et al. Cost-effectiveness analysis of a randomized study comparing radiosurgery with radiosurgery and whole brain radiation therapy in patients with 1 to 3 brain metastases. Am J Clin Oncol. 2012;35:45–50. doi: 10.1097/COC.0b013e3182005a8f. [DOI] [PubMed] [Google Scholar]
- 119.Tsao M, Xu W, Sahgal A. A meta-analysis evaluating stereotactic radiosurgery, whole-brain radiotherapy, or both for patients presenting with a limited number of brain metastases. Cancer. 2012;118:2486–2493. doi: 10.1002/cncr.26515. [DOI] [PubMed] [Google Scholar]
- 120.Marchan EM, Sheehan J. Stereotactic radiosurgery of brain metastasis from melanoma. Prog Neurol Surg. 2012;25:176–189. doi: 10.1159/000331191. [DOI] [PubMed] [Google Scholar]
- 121.Serizawa T, Yamamoto M, Sato Y, et al. Gamma Knife surgery as sole treatment for multiple brain metastases: 2-center retrospective review of 1508 cases meeting the inclusion criteria of the JLGK0901 multi-institutional prospective study. J Neurosurg. 2010;113(suppl):48–52. doi: 10.3171/2010.8.GKS10838. [DOI] [PubMed] [Google Scholar]
- 122.Yamamoto M, Kawabe T, Sato Y, et al. A case-matched study of stereotactic radiosurgery for patients with multiple brain metastases: Comparing treatment results for 1-4 vs ≥ 5 tumors: Clinical article. J Neurosurg. 2013;118:1258–1268. doi: 10.3171/2013.3.JNS121900. [DOI] [PubMed] [Google Scholar]
- 123.Lunsford LD, Chiang V, Adler JR, et al. A recommendation for training in stereotactic radiosurgery for US neurosurgery residents. J Neurosurg. 2012;117(suppl):2–4. doi: 10.3171/2012.6.GKS12838. [DOI] [PubMed] [Google Scholar]
- 124.Sheehan JP. Resident perceptions of radiosurgical training and the effect of a focused resident training seminar. J Neurosurg. 2010;113:59–63. doi: 10.3171/2010.1.JNS091686. [DOI] [PubMed] [Google Scholar]
- 125.Barbaro NM, Quigg M, Broshek DK, et al. A multicenter, prospective pilot study of gamma knife radiosurgery for mesial temporal lobe epilepsy: Seizure response, adverse events, and verbal memory. Ann Neurol. 2009;65:167–175. doi: 10.1002/ana.21558. [DOI] [PubMed] [Google Scholar]
- 126.Sheehan JP, Patterson G, Schlesinger D, et al. Gamma Knife surgery anterior capsulotomy for severe and refractory obsessive-compulsive disorder. J Neurosurg. 2013;119:1112–1118. doi: 10.3171/2013.5.JNS13201. [DOI] [PubMed] [Google Scholar]
- 127.Young RF, Li F, Vermeulen S, et al. Gamma Knife thalamotomy for treatment of essential tremor: Long-term results. J Neurosurg. 2010;112:1311–1317. doi: 10.3171/2009.10.JNS09332. [DOI] [PubMed] [Google Scholar]
- 128.Sayer FT, Sherman JH, Yen CP, et al. Initial experience with the eXtend System: A relocatable frame system for multiple-session gamma knife radiosurgery. World Neurosurg. 2011;75:665–672. doi: 10.1016/j.wneu.2010.12.051. [DOI] [PubMed] [Google Scholar]
- 129.Schlesinger D, Xu Z, Taylor F, et al. Interfraction and intrafraction performance of the Gamma Knife Extend system for patient positioning and immobilization. J Neurosurg. 2012;117(suppl):217–224. doi: 10.3171/2012.6.GKS12989. [DOI] [PubMed] [Google Scholar]
- 130.Adler JR, Jr, Gibbs IC, Puataweepong P, et al. Visual field preservation after multisession cyberknife radiosurgery for perioptic lesions. Neurosurgery. 2006;59:244–254. doi: 10.1227/01.NEU.0000223512.09115.3E. [DOI] [PubMed] [Google Scholar]
- 131.Hamilton AJ, Lulu BA, Fosmire H, et al. LINAC-based spinal stereotactic radiosurgery. Stereotact Funct Neurosurg. 1996;66:1–9. doi: 10.1159/000099658. [DOI] [PubMed] [Google Scholar]
- 132.Sachdev S, Dodd RL, Chang SD, et al. Stereotactic radiosurgery yields long-term control for benign intradural, extramedullary spinal tumors. Neurosurgery. 2011;69:533–539. doi: 10.1227/NEU.0b013e318218db23. [DOI] [PubMed] [Google Scholar]
- 133.Gerszten PC, Burton SA, Ozhasoglu C, et al. Radiosurgery for spinal metastases: Clinical experience in 500 cases from a single institution. Spine (Phila Pa 1976) 2007;32:193–199. doi: 10.1097/01.brs.0000251863.76595.a2. [DOI] [PubMed] [Google Scholar]
- 134.Sinclair J, Chang SD, Gibbs IC, et al. Multisession CyberKnife radiosurgery for intramedullary spinal cord arteriovenous malformations. Neurosurgery. 2006;58:1081–1089. doi: 10.1227/01.NEU.0000215891.25153.BA. [DOI] [PubMed] [Google Scholar]