Abstract
Stereotactic body radiation therapy (SBRT) is generally a tumor-ablative radiation modality using essential technologies capable of accurately and precisely damaging the target with a high dose while geometrically sparing innocent normal tissues. The intent, conduct, and tissue biology are all dramatically distinct from conventionally fractionated radiotherapy such that new understanding is required for its optimization. It is most practical, tolerable, and tumoricidal in its most potent form treating tumors in the lung and liver. However, it is increasingly being used for tumors adjacent to bowels and nervous tissue, albeit with somewhat less ablative potency. Its strengths include high rates of tumor eradication via a noninvasive, convenient outpatient treatment. Its weakness relates to the possibility of causing difficult-to-manage toxicity (eg, ulceration, stenosis, fibrosis, and even necrosis) that may occur considerably later after treatment, particularly in the vicinity of the body's many tubular structures (eg, organ hila, bowel). However, clinical trials in a variety of organs and sites have shown SBRT to result in good outcomes in properly selected patients. Given its short course, lack of need for recovery, and favorable overall toxicity profile, there is great hope that SBRT will find a prominent place in the treatment of metastatic cancer as a consolidative partner with systemic therapy. With considerable published experience, available required technologies and training, and many patients in need of local therapy, SBRT has found a place in the routine cancer-fighting arsenal.
INTRODUCTION
Stereotactic irradiation, first introduced in the context of intracranial stereotactic radiosurgery, is now an established treatment approach for a large variety of cancer presentations throughout the body. Although most extracranial tissues are considerably more apt to repair or tolerate radiation damage compared with CNS tissues, stereotactic radiation in the body was delayed in implementation as a result of difficulties in both physiologic motion and confidence in targeting. With better solutions to these problems,1 treatments in the body have expanded as an indication for achieving local control.
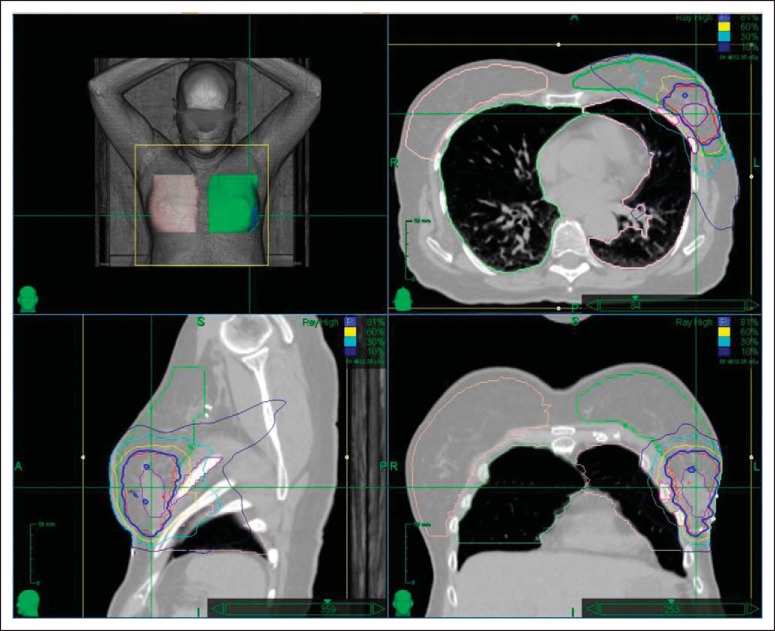
Initially the treatments were called extracranial stereotactic radioablation2 and later stereotactic body radiation therapy (SBRT).3 More recently, the descriptive term stereotactic ablative radiotherapy4 has come into common use. Although SBRT constitutes a potpourri of technologies and techniques, including three-dimensional conformal, intensity modulation, image guidance, motion control, and stereotactic targeting, the hallmark of SBRT is delivery of a potent, ablative or nearly ablative dose in oligofractions (ie, five or fewer fractions). Although the technologies and techniques used are interesting in their own right, their use in SBRT is primarily to create a compact dose delivered accurately to the intended target with steep gradients in all directions (geometric avoidance).1 Thus, unlike conventional radiotherapy where differential radiation repair between tumor and normal tissue is exploited for a therapeutic advantage, SBRT basically attempts to hit the tumor while ideally altogether avoiding the normal tissue. This is a dramatically different approach than conventional radiotherapy, where large volumes of normal tissues are typically included, even in the high-dose region, as shown in Figure 1.
Fig 1.
Comparison of stereotactic body radiation therapy (SBRT) plan in the left panel versus historical postage stamp anterior/posterior-directed field arrangements shown in the right panel. The SBRT plan uses advanced imaging and guidance to reduce the necessary margin around the tumor. In addition, it spares the high-dose (60 Gy, yellow) and intermediate-dose (30 Gy, green) volumes in exchange for a considerably larger low-dose (10 Gy, orange) volume.
Biologically, the current application of SBRT has not yet reached a point of optimized refinement. However, this simple approach of geometric avoidance was first successful in the brain, one of the least radiotolerant structures in the body, and is proving to be even more successful in the body. Furthermore, the coupling of oligofractionation along with sophisticated geometric avoidance is serving as a model for hypofractionation in general. Less radical forms of hypofractionation (eg, between six and 20 fractions) also performed with improved image guidance, motion control, and compact dosimetry can be used as an adjuvant (ie, prophylactic) therapy and may eventually commandeer indications from conventional fractionation for many common radiotherapy indications. Hence, there is a hypofractionationed revolution under way within the field of radiotherapy, and SBRT is the poster child of its possibilities for success.
Many review articles on the history of and clinical experience with SBRT have been written.5 In this article, we will discuss the essence of the treatment, how it has been exploited, and how it may be further exploited in the future.
LATE EFFECTS
Clinically observable tissue changes, both desirable and undesirable, occur in two distinct time frames after radiation exposure, coined early and late. Immediate effects of DNA damage in proliferating tissues manifest early, affording tumor response and mucosal/marrow injury after both conventional and hypofractionated radiation delivery. Slowly or nonproliferating tissues like blood vessels, nerves, and connective tissue demonstrate injury clinically later, often months or years after radiation exposure. It has long been recognized that hypofractionated radiation delivery is considerably more likely to promote late radiation injury, also called late effects, than conventionally fractionated radiotherapy.
Late effects in normal tissues have generally been more dreaded than early effects primarily because they have been more difficult to manage. Late vascular injury can cause tissues to become less viable with poor blood supply, poor wound healing, and less functional capacity. Late connective tissue injury leads to fibrosis and contracture often associated with pain and dysfunction. Such tissues may simply undergo necrosis, resulting in catastrophic problems like fistula and ulceration, which can be debilitating and even deadly. In the 1980s and 1990s, observations of these untoward late effects and the established knowledge that, for the same given total dose, they are more likely to occur after hypofractionation than conventional fractionation prompted several notable leaders in radiation oncology to reject the use of hypofractionation for cancer therapy except in patients with a very limited life span who were unlikely to live long enough to experience the delayed onset.6–8
However, before altogether rejecting hypofractionation as a curative cancer therapy, two important caveats should be considered. First, there is no clinical concern if late effects, even severe ones, occur within gross tumor targets. Indeed, such a circumstance might even be ideal, allowing tumor kill by both classic DNA damage as well as via vascular injury.9 Second, although no existing radiotherapy delivery technology is capable of totally limiting potent dose to just the tumor and not to the surrounding normal tissues, it must be acknowledged that nearly all normal tissues have capacity to repair injury, even severe injury, so long as the scope of injury is limited. The entire field of surgery, which injures normal tissues by definition in its conduct, is based on this observation. What surgeons have come to understand, however, is that even severe normal tissue injury can ultimately heal if limited and after providing the opportune circumstances for healing.
The condemning circumstances associated with late effects after hypofractionation mentioned earlier were observed in an era when technologic shortcomings resulted in large-volume radiation exposure to full therapeutic dose. Although unintended, this large-volume dose delivery was only tolerable by delivering numerous small daily doses, allowing differential repair between tumor and normal tissue. Radiation oncologists might have rationalized this practice by arguing for an added value of covering both tumor infiltration by direct extension and possible involvement of immediately adjacent lymph nodes (regional infiltration). Subsequently, the regional treatments became even more extensive despite little clinical evidence of improvement in outcome by such broad treatment.10 In the end, the technologically limited dose distributions of the one- and two-dimensional eras led to a pervasive locoregional treatment strategy coupled with championing of the less potent but practically essential protracted fractionation radiotherapy schedules. Even when dramatic technologic advancements capable of geometric avoidance occurred, such as the harnessing of charged particles (eg, protons), classically trained radiation oncologists continued to use conventional (protracted) fractionation and traditional (ie, locoregional) target-volume definitions.
Technology intensive, focally targeted, oligofractionated SBRT has been used in clinical practice since the mid-1990s, and reports on long-term outcome are available.11,12 High or intolerable rates of severe late effects have infrequently been observed with prudent use. Phase I studies exploring the boundaries of effective and tolerable therapy (ie, escalating until severe toxicity is observed) have shown the ability to deliver potent dose far in excess of most predictions. However, SBRT is clearly associated with toxicity.13 But the essence of the therapy to deliver a biologically potent, tumoricidal, hypofractionated dose to the target while using a variety of modern technologies and techniques to achieve rapid falloff limiting dose to innocent, adjacent normal tissues is confirmed. In this context, normal tissue injury after therapy absolutely occurs in all cases. However, as with surgery, the injury is limited, allowing host factors to facilitate effective repair.
SBRT DOSE SELECTION
The technologies used for geometric avoidance in SBRT could be used to deliver conventionally fractionated radiotherapy similar to what has typically been done with proton beam therapy. Although a reasonably effective adjuvant therapy, apart from its utilization for selected radiosensitive hematopoietic and germ cell malignancies, even high-dose conventionally fractionated radiotherapy is associated with unimpressive gross tumor control rates for the most common deadly solid tumors. Instead, early adopters of SBRT chose ablative hypofractionation (eg, few fractions with dose > 8 Gy per fraction) with the hopes of more dramatically impacting cancer outcome.
Because ablative hypofractionation was not used historically as a result of intolerance, dose-finding studies were in order. The most formal and valid dose-finding studies, phase I dose-escalation studies, were carried out by several groups early on. Others groups, less optimally, selected treatment dose levels by expert consensus or mathematical modeling. With multiple permutations of total dose, fractionation, tumor size selection, tumor type, and so on, the latter method would require a great deal of luck to actually determine the optimal dose balancing efficacy and toxicity. In a phase I study, patient selection, tumor type, stage, and other determinants of outcome are controlled by the methodology of the study. The only variable, dose, is controlled by the study itself and, importantly, not by the treating physician who would otherwise allow bias to influence dose selection. In the end, a variety of dose levels are tested and evaluated for outcome. Importantly, the investigators are not committed to any of the dose levels and can simply choose the best combination of efficacy and toxicity without bias or emotion.
In this way, several phase I studies of SBRT were carried out across the world, resulting in data-informed dose selection.2,14–26 Most studies enrolled between only 25 and 100 patients, allowing initial assessment of both toxicity and early efficacy. Although limited in number, considerable information was gleaned with careful follow-up of each patient. In contrast, dose selection for conventional radiotherapy delivery has been optimized over eras of treatment typically covering decades. Although both approaches have their merits, the phase I testing approach is clearly a more efficient dose-finding technique that defines effective treatment boundaries in a shorter period while exposing fewer patients to either too low or too high dose levels.
Although much is yet to be learned about dose effects, there have already been several surprises. For example, both melanoma and renal cancer histologies have historically been deemed radioresistant based on decades of understanding from conventional radiotherapy experiences. Yet, with large dose per fraction, both of these histologies emerge as more sensitive, even compared with squamous or adenocarcinoma histologies.27 In contrast, it is increasingly recognized that colorectal cancer poses a more radioresistant challenge to SBRT compared with other primary sites, with several reports showing poor control at modest dose levels.28 Surprises have also emerged with regard to normal tissue response. Liver tumors were often considered off limits for conventional radiotherapy due to radiation-induced liver disease, yet this complication has rarely been observed in the SBRT experience even at high-dose levels able to eradicate liver tumors.29 Some investigations that limited SBRT dose escalation according to lessons learned from conventional radiotherapy experience and perspective in the end lost the opportunity to achieve the high rates of tumor control observed in other more traditional phase I study designs.30
EARLY CLINICAL MODELS
Treatment of liver and lung tumors, both primary and metastatic, defined the majority of the initial clinical experience. These organs have both been categorized as parallel-functioning organs, meaning they constitute parenchymal tissues of which subdivisions are performing a similar and independent function (in parallel).31 The right lung is performing the same function as the left lung; hence, it may constitute a reserve of function, allowing removal or destruction of part of the organ without clinically manifest toxicity. This contrasts with so-called serial-functioning tissues, or organs where function depends on a cascade of events occurring along a pathway (eg, the esophagus delivering a food bolus from the pharynx to the stomach). If any position along the esophagus is removed or destroyed, the entire function ceases, resulting in profound, clinically significant injury. Because toxicity is usually less in parallel tissues, it made sense to perform initial evaluations in liver and lung.
Among these early experiences, early-stage lung cancer in patients unable to tolerate the rigors of standard surgery became the predominant clinical model for testing SBRT. These patients were motivated to try new therapies because existing options were lacking, with poor outcomes from conventional radiotherapy, heroic surgeries, and especially observation. Furthermore, patients with medically inoperable lung cancer often had relatively small, solitary targets, making for more reasonable dosimetry in normal lung. These patients were not necessarily at immediate end of life, allowing the opportunity to measure both early and late toxicity as well as treatment efficacy. Investigators in many countries saw this population as ideal and carried out prospective trials.
Results from across the world in medically inoperable lung cancer were both remarkably similar and unexpectedly positive. Collectively, primary tumor control rates with SBRT were in the 80% to more than 90% range,17,32–34 nearly double historical control rates with conventional radiotherapy.35–37 Yet rates of severe toxicity were fairly low (15% to 20%), even in frail patients.34 Indeed, this population of poor performers with multiple medical problems has typically been purposefully excluded from cancer therapy trials for fear of poor tolerance. Yet they both tolerated the therapy and survived considerably longer than expected.
Randomized trials comparing SBRT with conventional radiotherapy in medically inoperable populations with early lung cancer have been proposed and are enrolling patients. Even if these trials show no survival advantage, the convenience and good tolerance of SBRT will make a strong case for widespread acceptance. For operable patients, however, the case for SBRT is more difficult. Importantly, operable patients already have a good and proven treatment option with lobectomy. Patient in this group are aware of this distinction and continue to appreciate the role of standard surgery, making a less attractive case for SBRT. Randomized trials in the operable population have all suffered from poor accrual, primarily because patients struggle to accept a random assignment to an invasive but clearly established therapy versus a noninvasive but unproven therapy. In instances where it is too difficult for a patient to process the possibility of a random assignment to one of two such disparate alternatives, the technique of so-called prerandomization may be used, where a patient's treatment assignment is presented to him or her after the random assignment is performed, so that the patient only has to consider trial participation with a known expected therapy. The best known application of the prerandomization method in a major US cooperative group study is likely the National Surgical Adjuvant Breast and Bowel Project B-06 trial comparing mastectomy versus lumpectomy and radiotherapy.38 This type of trial design is under consideration for a possible surgery versus SBRT trial for operable early lung cancer.
Phase II trials using SBRT in patients with lung and liver metastases have also shown considerable promise.22,24,26,28–30,39–43 Randomized trials in this population will likely be even more difficult given the broad variability of the clinical presentations and relatively infrequent use of local therapies in patients with metastatic cancer.
CURRENT STATUS
At the time of this writing, published clinical results on SBRT exist for the following indications: early-stage lung cancer in medically inoperable patients or those refusing surgery, lung metastases from a large variety of primary cancers, primary liver cancer in medically inoperable patients, liver metastases (as with lung metastases), pancreas cancer, adrenal metastases, primary kidney cancer in medically inoperable patients, organ-confined prostate cancer, selected intrathoracic and intra-abdominal lymph node metastases, recurrent and primary head and neck cancers, spinal tumors, and vertebral bone metastases.
Of special interest is the current use of SBRT in prostate cancer given how commonly this cancer is diagnosed. Unlike the deadly-at-any-stage clinical models like primary lung cancer, the gamut of survival is broad with prostate cancer. Indeed, the lower risk forms are most common, where patients enjoy high cure rates with a variety of well-tolerated existing therapies. In this context, especially for low-risk patients, the introduction of high-potency SBRT could constitute a threat to good outcome in a disease where improvements in both disease-free survival and tolerance are unlikely. Yet existing treatments are far from perfect. For example, conventionally fractionated high-dose intensity-modulated radiation therapy is both expensive for payers and inconvenient for patients. Numerous reports with limited follow-up show that 5-fraction SBRT for low-risk prostate cancer positively addresses both complaints. Using dose regimens that are less potent than those used for tumors in other sites, prostate SBRT results in excellent clinical outcomes compared with other standard treatments44–46 in a convenient and cost-effective manner.47,48
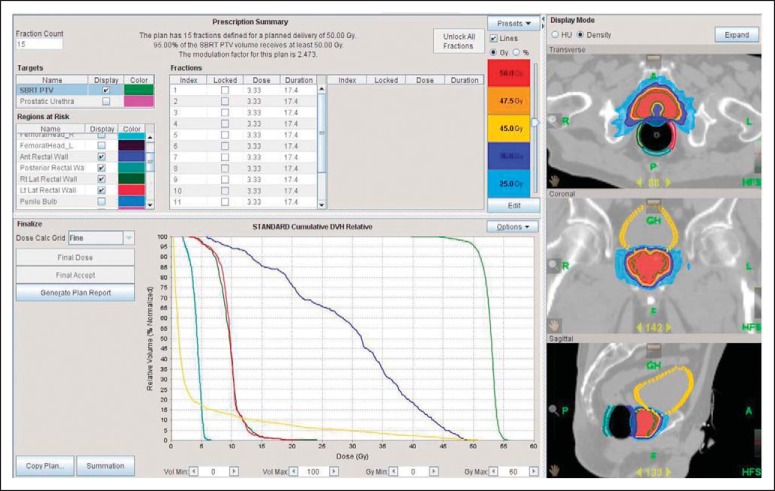
Attempts to improve on the outcomes achieved for intermediate- and high-risk prostate cancer via SBRT dose escalation will face challenges from normal tissue tolerance. Maximally aggressive SBRT is ablative and can render the targeted tissues incapable of sustaining cell division and incapable of further function, the definition of radioablation. In the classic treatment of organ-confined prostate cancer, the target is made up of the entire gland (ie, mostly normal tissue), including notable serial functioning tissues like the urethra and the anterior wall of the rectum, as depicted in Figure 2. Furthermore, although imaging to identify gross nests of prostate cancer is increasingly available, the entire gland remains at risk for multifocal microscopic tumor involvement. In all likelihood, optimizing outcomes for intermediate- and high-risk prostate cancer will require exploration of higher doses for finding the ideal therapeutic ratio,49 perhaps approaching toxicity limits for the adjacent rectum or intervening urethra.
Fig 2.
Dose-volume histogram of a prostate stereotactic body radiation therapy (SBRT) plan illustrating 50 Gy delivered on protocol over five treatments. The patient was immobilized in a custom-designed setup including a rectal balloon to limit the collateral dose. Note the intensity-modulated radiation therapy–facilitated sparing of specifically the rectal wall (at the expense of the rectal lumen) to avoid circumferential damage to rectal mucosa clonagens.
Another special case of SBRT utilization is in treating pancreatic cancer. The prognosis of pancreatic cancer, regardless of stage, is extremely poor, with few patients surviving past 5 years. Although primary and nodal tumor progression can result in pain and obstruction, the poor survival is mostly because of a high rate of metastases that occur early in the course of the disease. Justifiably, efforts aimed at improving outcomes focus generally on improving systemic therapies. For patients with locally advanced, unresectable pancreatic cancer, the typical patient survives approximately 12 months from diagnosis,50,51 during which conventional radiotherapy (concurrent with chemotherapy) takes up approximately 10% of their remaining time. Conventional radiotherapy fields have historically been fairly large because of uncertainties in tumor location and position within the respiratory cycle. Improved tumor imaging and motion control associated with SBRT inherently addresses some of these problems. More importantly, an entire course of SBRT can be administered in less than 1 to 2 weeks. Using SBRT as the local therapy may facilitate integration of more aggressive systemic chemotherapies such as folic acid, fluorouracil, irinotecan, and oxaliplatin (FOLFIRINOX).52 As a clinical example, a multi-institutional consortium (The Johns Hopkins Hospital, Stanford University Hospital, and Memorial Sloan Kettering Cancer Center) performed a prospective trial enrolling 49 patients with unresectable pancreatic cancer and Karnofsky performance score more than 70. Patients received 33 Gy in five 6.6-Gy fractions of SBRT between cycles of gemcitabine chemotherapy. Median survival was 13.9 months, and 1- and 2-year overall survival rates were 61% and 18%, respectively. Rates of acute and late grade ≥ 2 gastritis, fistula, enteritis, or ulcer toxicities were 2% and 11%, respectively. Importantly, in this clinical example, both acute and late toxicity were low, whereas global quality-of-life scores did not change appreciably with treatment (Herman et al, submitted for publication). Similar investigations at single institutions using three to five fractions of 5 to 12 Gy of radiation have been recently reported with good local tumor control (Fig 3) and similar survival and toxicity profiles.53–55 Future studies will evaluate further dose escalation of SBRT and integration with more aggressive chemotherapy in patients with localized pancreatic cancer.56
Fig 3.
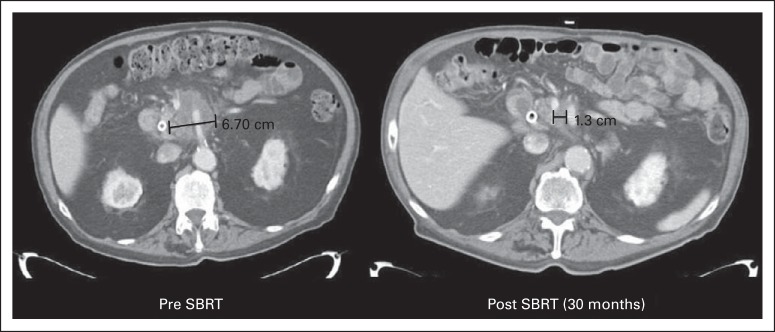
Pre– and post–stereotactic body radiation therapy (SBRT) imaging of an unresectable pancreatic head cancer showing a favorable treatment response.
FUTURE CLINICAL PRACTICE
Unfortunately, cancer is common. Although obvious, it should be noted that truly important cancer therapies are used commonly at the point where patients actually receive care. Extraordinarily complex treatments, those that require tremendous skill or expertise, or those with tremendously high start-up costs may be valuable to those treated, but lose importance if few patients with the condition have the opportunity to receive the treatments. SBRT has credibility because it has been initiated and accepted by the general radiation oncology community, both academic and private practice. Indeed, the use of SBRT in the community is becoming widespread because of availability of technologies, knowledge- and practice-based training, and acceptance of clinical results.57,58
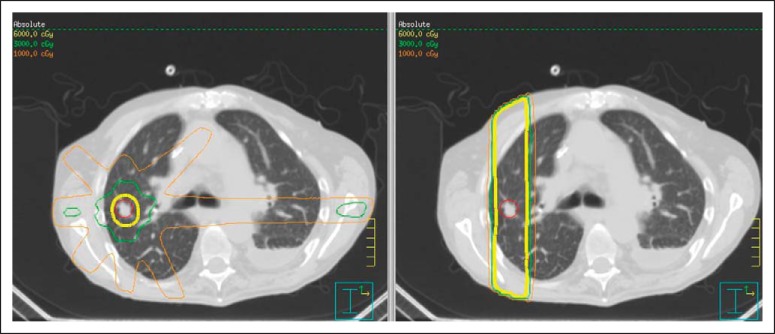
To date, SBRT has been used primarily in patients unable to tolerate standard surgeries, particularly in lung and liver. More recently, it has been used in secretory or glandular tissues like prostate and breast cancer.59 For bread-and-butter radiotherapy indications like prostate and breast cancer to become SBRT mainstream indications, long-term reports must show that severe late effects are uncommon in the context of a mostly adjuvant treatment (Fig 4). If clinical results are maintained or improved, it is likely that the mix of patients will become increasingly healthy (ie, operable). This will happen slowly, probably over decades, unless a randomized study is performed and provides the dramatic testimony of high-level evidence suggesting SBRT can compete with surgery. After all, transitions in standard treatments move dramatically faster after the collection, presentation, and acceptance of high-level evidence.
Fig 4.
Three-dimensional, axial, sagittal, and coronal views of dosimetry for stereotactic body radiation therapy (SBRT) for adjuvant therapy of breast cancer after lumpectomy in a patient treated on protocol. The tumor bed delineated by the heavy blue line constitutes normal breast tissue (possibly admixed with residual cancer cells). In this setting, radioablation is not advisable for this target because the higher therapeutic dose includes considerable late reacting normal tissue. However, the mechanics of SBRT still allow reduction of low and intermediate doses to adjacent breast, lung, and heart, facilitating hypofractionation.
The true frontier for SBRT, however, is unlikely to be in treating primary cancers. Metastatic cancer is currently and appropriately treated with systemic therapies like chemotherapy or targeted agents. A large majority of patients so treated, even if the initial response was favorable, will experience progression most commonly in sites of existing tumor burden at the start of systemic treatment rather than new sites of progression.60 This would imply a rationale for adding local therapies along with the systemic treatment in patients with metastatic disease. At a minimum, controlling original sites of tumor burden could delay progression. More optimistically, controlling original sites in conjunction with an effective systemic therapy could even prolong overall survival.
Surgeons have lamented over what they consider an underutilization of their craft in treating patients with metastatic disease.61 They point to circumstances where they even apparently cure patients independent of systemic therapy so long as all gross tumor burden is resected.62 Ideal local treatments used in treating metastatic cancer would have the following characteristics: minimal invasiveness or noninvasiveness; efficient completion, including treatment and recovery, in days to avoid delays in systemic treatment; capacity to facilitate a more prolonged use of the current line of systemic therapy by reducing isolated failure (saving other lines of potentially effective drugs for later); avoidance of overlapping toxicity with systemic therapy; and high rate of tumor control (comparable to surgery).63 Although several local therapies could compete as optimal contenders based on these criteria, SBRT has documented early strength fitting the bill.
Local treatment of metastatic disease with SBRT would effectively be a new indication for radiotherapy, resulting in potentially dramatic growth in the average radiotherapy practice. Interestingly, the rationale becomes even stronger with the discovery of more effective systemic therapies. In previous eras when systemic therapies seldom even controlled microscopic disease long term, local treatment for bulky sites of disease was often futile. Many recall patients with presumed oligometastatic disease who underwent metastasectomy and difficult recoveries only to quickly develop new sites of metastases, making the endeavor seem pointless. Better systemic therapies will still likely struggle to control areas of larger tumor burden at presentation, providing an even stronger rationale for minimally invasive and effective local therapies. This topic is discussed further elsewhere in this issue.
It remains to be seen whether there will be expanded utilization of particle therapy in the context of SBRT treatments for oligometastases and other indications. To date, the majority of experiences using proton and carbon ion therapy have been used according to the protracted fractionation paradigm. However, these tools are capable of significant geometric sparing and would potentially be ideal for delivering SBRT.
Finally, the future likely holds a considerable opportunity to significantly decrease the intensity of SBRT doses without compromising tumor control. Tumor control, especially for deadly cancers, demands a potent assault. However, the current form of therapy is not yet optimized from a biologic perspective. Research in hypofractionation will be forthcoming now that hypofractionation is back in the clinic. It is well known that radiation effects both in normal tissues and tumors follow a sigmoid dose response beginning with a threshold activation dose. Research will eventually exploit variable threshold effects, develop drugs that synergistically sensitize SBRT, and, importantly, identify specific, personalized assessments of a patient's individual tumor vulnerability. Collectively, these improvements in the realm of biology and pharmacology will afford appropriate dose reduction of SBRT, making it safer for patients and less intimidating for treating physicians.
In conclusion, stereotactic irradiation in the body has risen as a common treatment option in a surprisingly short time. Published results from well-conducted clinical experiments have strengthened the case for a broad scope of indications, particularly in eradicating gross primary disease. SBRT is complementary to surgery given its ability to be used in inoperable patients. SBRT struggles with toxicity next to the body's tubes and wires, the so-called serially functioning tissues. As such, surgery, which is not limited in this realm, is complementary to SBRT. Given its oligofractionation, SBRT integrates nearly seamlessly with systemic therapies. It follows that the largest future expansion of SBRT might be in the realm of treating the numerous patients with metastatic disease hoping to improve progression-free or overall survival by eliminating or consolidating gross disease. In any event, the widespread acceptance and application of SBRT indicate it is already an important cancer therapy.
Footnotes
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: None Research Funding: Robert D. Timmerman, Varian Medical Systems Expert Testimony: None Patents, Royalties, and Licenses: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Robert D. Timmerman
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Papiez L, Timmerman R, DesRosiers C, et al. Extracranial stereotactic radioablation: Physical principles. Acta Oncol. 2003;42:882–894. doi: 10.1080/02841860310013490. [DOI] [PubMed] [Google Scholar]
- 2.Timmerman R, Papiez L, McGarry R, et al. Extracranial stereotactic radioablation: Results of a phase I study in medically inoperable stage I non-small cell lung cancer. Chest. 2003;124:1946–1955. doi: 10.1378/chest.124.5.1946. [DOI] [PubMed] [Google Scholar]
- 3.Potters L, Steinberg M, Rose C, et al. American Society for Therapeutic Radiology and Oncology and American College of Radiology practice guideline for the performance of stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2004;60:1026–1032. doi: 10.1016/j.ijrobp.2004.07.701. [DOI] [PubMed] [Google Scholar]
- 4.Loo BW, Jr, Chang JY, Dawson LA, et al. Stereotactic ablative radiotherapy: What's in a name? Pract Radiat Oncol. 2011;1:38–39. doi: 10.1016/j.prro.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Timmerman RD, Kavanagh BD, Cho LC, et al. Stereotactic body radiation therapy in multiple organ sites. J Clin Oncol. 2007;25:947–952. doi: 10.1200/JCO.2006.09.7469. [DOI] [PubMed] [Google Scholar]
- 6.Fletcher GH. Hypofractionation: Lessons from complications. Radiother Oncol. 1991;20:10–15. doi: 10.1016/0167-8140(91)90106-q. [DOI] [PubMed] [Google Scholar]
- 7.Rosenthal DI, Glatstein E. We've got a treatment, but what's the disease? Or a brief history of hypofractionation and its relationship to stereotactic radiosurgery. Oncologist. 1996;1:1–7. [PubMed] [Google Scholar]
- 8.Cox JD. Large-dose fractionation (hypofractionation) Cancer. 1985;55(suppl 9):2105–2111. doi: 10.1002/1097-0142(19850501)55:9+<2105::aid-cncr2820551412>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 9.Kolesnick R, Fuks Z. Radiation and ceramide-induced apoptosis. Oncogene. 2003;22:5897–5906. doi: 10.1038/sj.onc.1206702. [DOI] [PubMed] [Google Scholar]
- 10.Yuan S, Sun X, Li M, et al. A randomized study of involved-field irradiation versus elective nodal irradiation in combination with concurrent chemotherapy for inoperable stage III nonsmall cell lung cancer. Am J Clin Oncol. 2007;30:239–244. doi: 10.1097/01.coc.0000256691.27796.24. [DOI] [PubMed] [Google Scholar]
- 11.Lagerwaard FJ, Verstegen NE, Haasbeek CJ, et al. Outcomes of stereotactic ablative radiotherapy in patients with potentially operable stage I non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2012;83:348–353. doi: 10.1016/j.ijrobp.2011.06.2003. [DOI] [PubMed] [Google Scholar]
- 12.Fakiris AJ, McGarry RC, Yiannoutsos CT, et al. Stereotactic body radiation therapy for early-stage non-small-cell lung carcinoma: Four-year results of a prospective phase II study. Int J Radiat Oncol Biol Phys. 2009;75:677–682. doi: 10.1016/j.ijrobp.2008.11.042. [DOI] [PubMed] [Google Scholar]
- 13.Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006;24:4833–4839. doi: 10.1200/JCO.2006.07.5937. [DOI] [PubMed] [Google Scholar]
- 14.Hof H, Herfarth KK, Münter M, et al. Stereotactic single-dose radiotherapy of stage I non-small-cell lung cancer (NSCLC) Int J Radiat Oncol Biol Phys. 2003;56:335–341. doi: 10.1016/s0360-3016(02)04504-2. [DOI] [PubMed] [Google Scholar]
- 15.Whyte RI, Crownover R, Murphy MJ, et al. Stereotactic radiosurgery for lung tumors: Preliminary report of a phase I trial. Ann Thorac Surg. 2003;75:1097–1101. doi: 10.1016/s0003-4975(02)04681-7. [DOI] [PubMed] [Google Scholar]
- 16.McGarry RC, Papiez L, Williams M, et al. Stereotactic body radiation therapy of early-stage non-small-cell lung carcinoma: Phase I study. Int J Radiat Oncol Biol Phys. 2005;63:1010–1015. doi: 10.1016/j.ijrobp.2005.03.073. [DOI] [PubMed] [Google Scholar]
- 17.Nagata Y, Takayama K, Matsuo Y, et al. Clinical outcomes of a phase I/II study of 48 Gy of stereotactic body radiotherapy in 4 fractions for primary lung cancer using a stereotactic body frame. Int J Radiat Oncol Biol Phys. 2005;63:1427–1431. doi: 10.1016/j.ijrobp.2005.05.034. [DOI] [PubMed] [Google Scholar]
- 18.Le QT, Loo BW, Ho A, et al. Results of a phase I dose-escalation study using single-fraction stereotactic radiotherapy for lung tumors. J Thorac Oncol. 2006;1:802–809. [PubMed] [Google Scholar]
- 19.Rusthoven KE, Kavanagh BD, Burri SH, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for lung metastases. J Clin Oncol. 2009;27:1579–1584. doi: 10.1200/JCO.2008.19.6386. [DOI] [PubMed] [Google Scholar]
- 20.Kavanagh BD, Schefter TE, Cardenes HR, et al. Interim analysis of a prospective phase I/II trial of SBRT for liver metastases. Acta Oncol. 2006;45:848–855. doi: 10.1080/02841860600904870. [DOI] [PubMed] [Google Scholar]
- 21.Baumann P, Nyman J, Hoyer M, et al. Stereotactic body radiotherapy for medically inoperable patients with stage I non-small cell lung cancer: A first report of toxicity related to COPD/CVD in a non-randomized prospective phase II study. Radiother Oncol. 2008;88:359–367. doi: 10.1016/j.radonc.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 22.Rule W, Timmerman R, Tong L, et al. Phase I dose-escalation study of stereotactic body radiotherapy in patients with hepatic metastases. Ann Surg Oncol. 2011;18:1081–1087. doi: 10.1245/s10434-010-1405-5. [DOI] [PubMed] [Google Scholar]
- 23.Palma DA, Haasbeek CJ, Rodrigues GB, et al. Stereotactic ablative radiotherapy for comprehensive treatment of oligometastatic tumors (SABR-COMET): Study protocol for a randomized phase II trial. BMC Cancer. 2012;12:305. doi: 10.1186/1471-2407-12-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herfarth KK, Debus J, Lohr F, et al. Stereotactic single-dose radiation therapy of liver tumors: Results of a phase I/II trial. J Clin Oncol. 2001;19:164–170. doi: 10.1200/JCO.2001.19.1.164. [DOI] [PubMed] [Google Scholar]
- 25.Koong AC, Le QT, Ho A, et al. Phase I study of stereotactic radiosurgery in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2004;58:1017–1021. doi: 10.1016/j.ijrobp.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Goodman KA, Wiegner EA, Maturen KE, et al. Dose-escalation study of single-fraction stereotactic body radiotherapy for liver malignancies. Int J Radiat Oncol Biol Phys. 2010;78:486–493. doi: 10.1016/j.ijrobp.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 27.Stinauer MA, Kavanagh BD, Schefter TE, et al. Stereotactic body radiation therapy for melanoma and renal cell carcinoma: Impact of single fraction equivalent dose on local control. Radiat Oncol. 2011;6:34. doi: 10.1186/1748-717X-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herfarth KK, Debus J, Wannenmacher M. Stereotactic radiation therapy of liver metastases: Update of the initial phase-I/II trial. Front Radiat Ther Oncol. 2004;38:100–105. doi: 10.1159/000078271. [DOI] [PubMed] [Google Scholar]
- 29.Rusthoven KE, Kavanagh BD, Cardenes H, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol. 2009;27:1572–1578. doi: 10.1200/JCO.2008.19.6329. [DOI] [PubMed] [Google Scholar]
- 30.Lee MT, Kim JJ, Dinniwell R, et al. Phase I study of individualized stereotactic body radiotherapy of liver metastases. J Clin Oncol. 2009;27:1585–1591. doi: 10.1200/JCO.2008.20.0600. [DOI] [PubMed] [Google Scholar]
- 31.Wolbarst AB, Chin LM, Svensson GK. Optimization of radiation therapy: Integral-response of a model biological system. Int J Radiat Oncol Biol Phys. 1982;8:1761–1769. doi: 10.1016/0360-3016(82)90299-1. [DOI] [PubMed] [Google Scholar]
- 32.Baumann P, Nyman J, Hoyer M, et al. Outcome in a prospective phase II trial of medically inoperable stage I non-small-cell lung cancer patients treated with stereotactic body radiotherapy. J Clin Oncol. 2009;27:3290–3296. doi: 10.1200/JCO.2008.21.5681. [DOI] [PubMed] [Google Scholar]
- 33.Lagerwaard FJ, Haasbeek CJ, Smit EF, et al. Outcomes of risk-adapted fractionated stereotactic radiotherapy for stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2008;70:685–692. doi: 10.1016/j.ijrobp.2007.10.053. [DOI] [PubMed] [Google Scholar]
- 34.Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303:1070–1076. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Armstrong JG, Minsky BD. Radiation therapy for medically inoperable stage I and II non-small cell lung cancer. Cancer Treat Rev. 1989;16:247–255. doi: 10.1016/0305-7372(89)90044-3. [DOI] [PubMed] [Google Scholar]
- 36.Kaskowitz L, Graham MV, Emami B, et al. Radiation therapy alone for stage I non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 1993;27:517–523. doi: 10.1016/0360-3016(93)90374-5. [DOI] [PubMed] [Google Scholar]
- 37.Dosoretz DE, Katin MJ, Blitzer PH, et al. Medically inoperable lung carcinoma: The role of radiation therapy. Semin Radiat Oncol. 1996;6:98–104. doi: 10.1053/SRAO00600098. [DOI] [PubMed] [Google Scholar]
- 38.Fisher B, Bauer M, Margolese R, et al. Five-year results of a randomized clinical trial comparing total mastectomy and segmental mastectomy with or without radiation in the treatment of breast cancer. N Engl J Med. 1985;312:665–673. doi: 10.1056/NEJM198503143121101. [DOI] [PubMed] [Google Scholar]
- 39.Chang DT, Swaminath A, Kozak M, et al. Stereotactic body radiotherapy for colorectal liver metastases: A pooled analysis. Cancer. 2011;117:4060–4069. doi: 10.1002/cncr.25997. [DOI] [PubMed] [Google Scholar]
- 40.Katz AW, Carey-Sampson M, Muhs AG, et al. Hypofractionated stereotactic body radiation therapy (SBRT) for limited hepatic metastases. Int J Radiat Oncol Biol Phys. 2007;67:793–798. doi: 10.1016/j.ijrobp.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 41.Hoyer M, Roed H, Traberg Hansen A, et al. Phase II study on stereotactic body radiotherapy of colorectal metastases. Acta Oncol. 2006;45:823–830. doi: 10.1080/02841860600904854. [DOI] [PubMed] [Google Scholar]
- 42.Schefter TE, Kavanagh BD, Timmerman RD, et al. A phase I trial of stereotactic body radiation therapy (SBRT) for liver metastases. Int J Radiat Oncol Biol Phys. 2005;62:1371–1378. doi: 10.1016/j.ijrobp.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 43.Blomgren H, Lax I, Näslund I, et al. Stereotactic high dose fraction radiation therapy of extracranial tumors using an accelerator: Clinical experience of the first thirty-one patients. Acta Oncol. 1995;34:861–870. doi: 10.3109/02841869509127197. [DOI] [PubMed] [Google Scholar]
- 44.Madsen BL, Hsi RA, Pham HT, et al. Stereotactic hypofractionated accurate radiotherapy of the prostate (SHARP), 33.5 Gy in five fractions for localized disease: First clinical trial results. Int J Radiat Oncol Biol Phys. 2007;67:1099–1105. doi: 10.1016/j.ijrobp.2006.10.050. [DOI] [PubMed] [Google Scholar]
- 45.King CR, Freeman D, Kaplan I, et al. Stereotactic body radiotherapy for localized prostate cancer: Pooled analysis from a multi-institutional consortium of prospective phase II trials. Radiother Oncol. 2013;109:217–221. doi: 10.1016/j.radonc.2013.08.030. [DOI] [PubMed] [Google Scholar]
- 46.Katz AJ, Santoro M, Diblasio F, et al. Stereotactic body radiotherapy for localized prostate cancer: Disease control and quality of life at 6 years. Radiat Oncol. 2013;8:118. doi: 10.1186/1748-717X-8-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hodges JC, Lotan Y, Boike TP, et al. Cost-effectiveness analysis of stereotactic body radiation therapy versus intensity-modulated radiation therapy: An emerging initial radiation treatment option for organ-confined prostate cancer. J Oncol Pract. 2012;8(suppl 3):e31s–e37s. doi: 10.1200/JOP.2012.000548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parthan A, Pruttivarasin N, Davies D, et al. Comparative cost-effectiveness of stereotactic body radiation therapy versus intensity-modulated and proton radiation therapy for localized prostate cancer. Front Oncol. 2012;2:81. doi: 10.3389/fonc.2012.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boike TP, Lotan Y, Cho LC, et al. Phase I dose-escalation study of stereotactic body radiation therapy for low- and intermediate-risk prostate cancer. J Clin Oncol. 2011;29:2020–2026. doi: 10.1200/JCO.2010.31.4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huguet F, Andre T, Hammel P, et al. Impact of chemoradiotherapy after disease control with chemotherapy in locally advanced pancreatic adenocarcinoma in GERCOR phase II and III studies. J Clin Oncol. 2007;25:326–331. doi: 10.1200/JCO.2006.07.5663. [DOI] [PubMed] [Google Scholar]
- 51.Loehrer PJ, Sr, Feng Y, Cardenes H, et al. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: An Eastern Cooperative Oncology Group trial. J Clin Oncol. 2011;29:4105–4112. doi: 10.1200/JCO.2011.34.8904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Conroy T, Gavoille C, Samalin E, et al. The role of the FOLFIRINOX regimen for advanced pancreatic cancer. Curr Oncol Rep. 2013;15:182–189. doi: 10.1007/s11912-012-0290-4. [DOI] [PubMed] [Google Scholar]
- 53.Gurka MK, Collins SP, Slack R, et al. Stereotactic body radiation therapy with concurrent full-dose gemcitabine for locally advanced pancreatic cancer: A pilot trial demonstrating safety. Radiat Oncol. 2013;8:44. doi: 10.1186/1748-717X-8-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mahadevan A, Jain S, Goldstein M, et al. Stereotactic body radiotherapy and gemcitabine for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2010;78:735–742. doi: 10.1016/j.ijrobp.2009.08.046. [DOI] [PubMed] [Google Scholar]
- 55.Tozzi A, Comito T, Alongi F, et al. SBRT in unresectable advanced pancreatic cancer: Preliminary results of a mono-institutional experience. Radiat Oncol. 2013;8:148. doi: 10.1186/1748-717X-8-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Von Hoff DD, Ramanathan RK, Borad MJ, et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: A phase I/II trial. J Clin Oncol. 2011;29:4548–4554. doi: 10.1200/JCO.2011.36.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pan H, Simpson DR, Mell LK, et al. A survey of stereotactic body radiotherapy use in the United States. Cancer. 2011;117:4566–4572. doi: 10.1002/cncr.26067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shikama N, Tsujino K, Nakamura K, et al. Survey of advanced radiation technologies used at designated cancer care hospitals in Japan. Jpn J Clin Oncol. 2014;44:72–77. doi: 10.1093/jjco/hyt161. [DOI] [PubMed] [Google Scholar]
- 59.Bondiau PY, Bahadoran P, Lallement M, et al. Robotic stereotactic radioablation concomitant with neo-adjuvant chemotherapy for breast tumors. Int J Radiat Oncol Biol Phys. 2009;75:1041–1047. doi: 10.1016/j.ijrobp.2008.12.037. [DOI] [PubMed] [Google Scholar]
- 60.Rusthoven KE, Hammerman SF, Kavanagh BD, et al. Is there a role for consolidative stereotactic body radiation therapy following first-line systemic therapy for metastatic lung cancer? A patterns-of-failure analysis. Acta Oncol. 2009;48:578–583. doi: 10.1080/02841860802662722. [DOI] [PubMed] [Google Scholar]
- 61.Ollila DW, Caudle AS. Surgical management of distant metastases. Surg Oncol Clin N Am. 2006;15:385–398. doi: 10.1016/j.soc.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 62.Rees M, Tekkis PP, Welsh FK, et al. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: A multifactorial model of 929 patients. Ann Surg. 2008;247:125–135. doi: 10.1097/SLA.0b013e31815aa2c2. [DOI] [PubMed] [Google Scholar]
- 63.Timmerman RD, Bizekis CS, Pass HI, et al. Local surgical, ablative, and radiation treatment of metastases. CA Cancer J Clin. 2009;59:145–170. doi: 10.3322/caac.20013. [DOI] [PubMed] [Google Scholar]