Abstract
In the current health care system, high costs without proportional improvements in quality or outcome have prompted widespread calls for change in how we deliver and pay for care. Value-based health care delivery models have been proposed. Multiple impediments exist to achieving value, including misaligned patient and provider incentives, information asymmetries, convoluted and opaque cost structures, and cultural attitudes toward cancer treatment. Radiation oncology as a specialty has recently become a focus of the value discussion. Escalating costs secondary to rapidly evolving technologies, safety breaches, and variable, nonstandardized structures and processes of delivering care have garnered attention. In response, we present a framework for the value discussion in radiation oncology and identify approaches for attaining value, including economic and structural models, process improvements, outcome measurement, and cost assessment.
“Nowadays people know the price of everything and the value of nothing.”
Lord Henry Wotton, from The Picture of Dorian Gray, Oscar Wilde
INTRODUCTION
In recent years, the continued and unsustainable rise in health care spending in the United States (fast approaching 20% of GDP)1 without a proportional rise in quality or improvement in outcomes has raised critical questions about the inevitable need for change in our current health care delivery system. Patients, health care providers, and payers all desire improvement in health outcomes with simultaneous control of rising costs. Transformation to a value-based health care delivery model is proposed to address the challenge.2,3 The value proposition in health care represents an elusive yet highly sought-after goal for our health care system and for the specialty of radiation oncology. Herein, we discuss the meaning of value, the current economic landscape and impediments to achieving value, and some considerations for achieving a value-based health care delivery model in the future.
VALUE DEFINED
To address the question of how to achieve value in health care delivery, we must first understand what value is. In the world outside of medicine, a so-called good value is a desirable product or service that can be purchased for a fair price. The definition of value will vary depending on several factors, including the social identity and the social context of the person purchasing the product or service. For instance, value in automobile purchasing will mean different things to a consumer who prefers luxury cars than it will to one who prefers an off-road vehicle or an economy car. The desirable product or service as well as the fair price is in the eye of the beholder. This concept rings true in defining value in health care as well. Michael Porter, a leading proponent of the value proposition in health care, defines value as health outcomes divided by costs. He points out that “value should always be defined around the customer.”4(p2477) In this vein, we would argue in line with the classic Donabedian model, that health care structure and process, along with outcomes, are highly relevant considerations in the value proposition (Table 1).5 Structure refers to the context in which care is delivered, including facilities, organizational characteristics, and, importantly for radiation oncology, equipment and technology. Process encompasses all components of health care delivery, including interactions between patient and physician and the technical delivery of care. Outcomes refer to the effects of health care on patients and include both objective (eg, survival) and subjective outcome measures (eg, quality of life). Intuitively, structure, process, and outcome fundamentally affect the patient's experience when interfacing with the health care system and inherently matter to an individual's value equation.
Table 1.
Current Challenges to Achieving Value
| Structure | Process | Outcomes | Costs | Global |
|---|---|---|---|---|
| System: lack of cross-specialty integration of care | Inadequate emphasis on accessibility, timeliness, and coordination of care | Inadequate systems for longitudinal discrete data capture (eg, registries) | Not well measured for disease process, nor for cycles of care | Information asymmetries between patients and providers |
| Setting: lack of meaningful practice standardization/accreditation | Provider centered, not patient centered | Lack of standardized instruments for patient-reported outcomes | Financial incentives toward overutilization | Overutilization driven by moral hazard and provider-induced demand |
| Provider: lack of meaningful practice standardization/certification | Insufficient evidence-based guidelines/best practices and lack of incentivized adherence | Inadequate subjective and objective outcome data for quality assessment | Not transparent and not commensurate with quality | Difficulties in risk stratification between patient groups hinder comparison |
CURRENT ECONOMIC LANDSCAPE AND CHALLENGES TO ACHIEVING VALUE
Describing the Cost Conundrum
Remarkably, inability to assess cost poses the greatest challenge in determining value in health care. Providers and administrators do not have clarity on the various components of care that need to be measured or what the actual costs of these components are. Although providers often know what they charge and may know many of the individual direct costs within a radiation oncology clinic, they nevertheless are unable to calculate the total cost of a treatment administered at the patient level. In this regard, because costs are borne by providers and repaid by insurers and patients, providers approximate costs by using billing charges or reimbursement, not the calculated total cost of providing care. From a system point of view, the situation is further complicated by the fact that charges for the same procedure can vary widely among different providers. There is no good justification beyond the notion of free market for this practice. The inability to consistently and reproducibly measure costs is a manifest impediment to overcome if we are to address the value proposition in radiation oncology.
Economic Incentives, Utility, and Cost
The lack of understanding about cost and the other components of value creates difficulties for all stakeholders in health care. For example, the patient's lack of clarity regarding value creates an incongruity that can lead to increased cost, over- and underutilization, and decreased quality. Economists believe that consumers are utility maximizers—that is, they will choose to have the highest utility for the lowest dollar. Utility is defined in economic terms as usefulness (ie, ability of something to satisfy needs or wants). However, in health care, there is no reliable or consistent correlation between cost and utility. Radiation oncology clinics can be paid the same for a given service, but without an equal outcome or quality of the service provided. At the same time, charges widely vary among cancer treatment facilities. Medical device pricing is equally opaque; when surveyed, most physicians cannot correctly estimate the price of medical devices they routinely use.6,7 If this were the case for products outside of health care, the market would naturally drive the price of the costlier item toward that of cheaper substitutes, because consumers would not pay a higher price for a nonsuperior product. So why does the opposite often happen in health care? The answer lies in deficiency of information; consumers do not know the real costs or quality offered by different providers. Moreover, patients often assume equality in these factors. In economics terms, there is an information asymmetry between the providers and consumers of health care.8 If patients do not know how a provider ranks in terms of key factors that inform utility, they cannot truly be utility maximizers. Therefore, a situation exists in our current encounter-based, frequency-rewarding, fee-for-service health care payment system where providers do not understand their own true costs and cannot rationally or rigorously relate their charges to actual cost or quality. At the same time, the patient/consumer also lacks needed practical information to make informed decisions regarding purchase of health care. From a value perspective, our health care market is remarkably inefficient, where both sides of the transaction have high levels of unreliable critical information. In response, payers have proposed and in some cases have attempted to link aspects of payment to various quality indicators. To date, there is a dearth of validated quality indicators that can be used as quality measures linked to payment in radiation oncology. Those that do exist are said to not have a demonstrable effect of lowering cost or improving quality. Consequently, in our current circumstance, the predominant market response of payers is to grind on unit price whenever possible. This maneuver is performed without valid consideration of the true cost of care or an understanding of the value to the patient or to society of the care supplied. In some market situations, technical services for radiation oncology are contracted at what seem to be marginal rates, without consideration of the actual cost of delivering the care or maintaining its quality and safety. The situation is further confounded in certain submarkets where issues of payer mix create inadequate access for some patients while other patients receive unneeded treatment.9–13
As costs continue to rise at troubling rates, patient, consumers, payers, providers, and society are all beginning to ask: Are the costs of medical services commensurate with the utility provided? Where do high costs come from? A basic tenet of economics is that incentives are major determinants of people's behaviors and choices. Many experts attribute high costs to misaligned incentives among the various stakeholders in health care—that is, the goals of patients, providers, and payers are not always aligned. Specifically, health policy analysts are fast coming to the conclusion that the fee-for-service payment system, which reimburses providers based on volume of service and not on quality or value, encourages overuse and overprescription, leading to higher overall costs.14 In addition, the simplistic payer response to the cost spiral—that of merely lowering reimbursement for provider services—instead results in a volume behavior effect, which further exacerbates the situation.15 In this milieu, information asymmetries, together with misaligned financial incentives, create the so-called principal-agent problem, which may in turn lead to supplier-induced demand. Patients (ie, principals) assume that their physicians (ie, agents), who have more information about medical treatments, will act in the principals' best interest. However, when the agent has an incentive to recommend one treatment over another, the principal-agent problem emerges. Striking examples of supplier-induced demand for radiation therapy services were recently described in a New England Journal of Medicine article noting urologists' increased use of intensity-modulated radiation therapy (IMRT) in prostate cancer when they had an economic stake in the radiation therapy treatment equipment. The study demonstrated that when urologists had ownership in radiotherapy practices in the setting of limited specialty groups (< four specialties), their patients were significantly more likely to receive IMRT for prostate cancer as opposed to other less expensive treatment options. This phenomenon even extended to their patients who were age > 80 years.9 The agent—in this case, a self-referring physician—acts in his own economic best interest rather than in the principal's medical interest. In a similar study conducted by the US Government Accountability Office, however, when the ownership interest was dispersed within multispecialty groups with > 20 specialties, the principal-agent problem notably disappeared.16
Patient Incentives to Choose High-Value Care
What incentives do patients have to choose high-value health care? Although providers may have incentives to create supplier-induced demand, patients have their own misaligned incentives that prevent the attainment of value. For example, moral hazard arises when patients who are insured by a third party (eg, employer, government) have access to comprehensive health care plans for which they pay only a small fraction of the total cost. Various studies have shown that patients use more medical services when they have more generous health plans that are less restricted or require less cost sharing.17–19 To address moral hazard, health plans have increased cost sharing by using copayments and higher deductibles. However, this practice may be counterproductive in achieving value; it runs the risk of preventing patients from pursuing necessary, high cost–sharing medical treatments.20–22 By attempting to curb overutilization of medical services in this way, insurers may actually be worsening patients' health and increasing ultimate associated costs of their covered populations.
Special Case of the Patient With Cancer
The patient with cancer presents a special case for payment reform. There is a heretofore prevailing attitude that cancer is, to some extent, a so-called sacred cow when it comes to reining in cost—even the cost associated with futile care. One study reported that inflation-adjusted direct medical spending on cancer care exhibited a 50% higher growth rate compared with the rest of health care over a 20-year period.23 Informing a particular dilemma for patients with cancer, recent studies have highlighted the effect that out-of-pocket (OOP) costs have on patients' ability to access medical care and ability to pay for other, nonmedical, life necessities.24–27 Medicare patients with cancer pay substantially more OOP than patients without cancer, and adjuvant radiotherapy and systemic chemotherapies contribute to the discrepancy.28 As a partial solution to this problem, it is advocated that clinicians have frank discussions with patients about incremental benefit and the costs of prescribed treatments and procedures.22,29 To understand why OOP costs affect patients' access to health care, we can look to the basic economic concept of supply and demand. The demand for health care, as with any product, depends on five main factors: OOP costs, income, prices of other complementary or substitute goods and services, tastes or utility, and expectations. The cost of health care, in one form or another, involves all five of the factors that affect demand. Economists once believed the demand curve for health care was relatively inelastic—that is, a rise in price of health care minimally influenced the demand for health care. But this theory probably applies only to well-insured patients. The manifest risk is that as OOP health costs rise and incomes remain flat throughout the country, the demand curve may begin to look more elastic—that is, patients will demand less routine medical care and only seek medical attention when they are exceptionally ill. Patients may also demand less medical care as the prices of complementary goods (eg, medications) rise or price of substitutes (eg, housing, education, food) rise. We now know that costs clearly matter to patients—even patients with cancer. Therefore, patient understanding of the incremental benefit of a particular treatment or procedure is one aspect of achieving high value. This understanding can alter the patient demand equation, both by decreasing the cost component and by changing patient utility and expectations of medical care.
ACHIEVING VALUE IN RADIATION ONCOLOGY
Radiation oncology is an arcane, technology-based, tertiary specialty. It is rooted in costly technology infrastructure that is ever evolving, and proper treatment delivery is critically dependent on a team effort requiring not only physicians and nurses but also specially trained physicists, dosmetrists, and radiation therapists. As an obscure specialty, until recently, radiation oncology was rarely mentioned in discussions of health care reform. However, notable recent events have shifted the spotlight onto the specialty. The Centers for Medicare and Medicaid Services identified the billing code associated with IMRT as one of the top 10 codes across all specialties contributing to Medicare spending growth from 2004 to 2010.30 The New York Times ran a series of articles highlighting injuries sustained by overdoses of therapeutic radiation,31–33 and numerous articles have questioned the value of high-cost proton-beam therapy.34–36 These and other high-profile references to radiation oncology highlighting cost and potential safety concerns have incited a newfound health policy interest in radiation oncology.
As a framework for discussing value in radiation oncology, we will modify the Porter value equation (value = outcomes/cost) by expanding the numerator to include structure and process along with outcomes consistent with the Donabedian model for quality.5 Hence, expanding the Porter equation to include structure and process essentially transforms the value equation as follows: value = quality/cost (Fig. 1).
Fig 1.
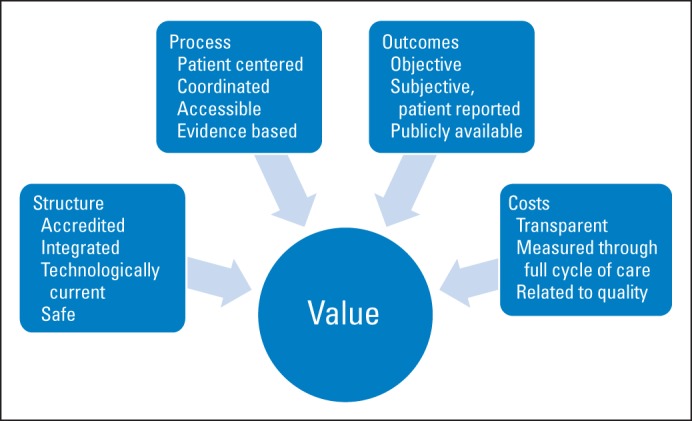
Key components of value.
Structure
Structural components of medical care include “the adequacy of facilities and equipment; the qualifications of medical staff and their organization; the administrative structure and operations of programs and institutions providing care; fiscal organization and the like.”5(p695) In recent years, radiation therapy has become significantly more sophisticated, with enhanced complexity in all of its treatment modalities, including brachytherapy, IMRT, and stereotactic radiation. To address rising concerns about ensuring proper structure for the delivery of radiation oncology care, several initiatives attempting to standardize the structural components required to advance high-value care have emerged. In 2012, a consortium of 12 radiation oncology societies authored “Safety Is No Accident,” which set forth specific requirements for radiation oncology facilities with regard to structure, personnel, and technical processes to ensure a safe environment for the delivery of radiation therapy.37 In 2013, the American Society for Radiation Oncology (ASTRO) put forth detailed draft standards for practice accreditation, which included structure and a few process elements.38 The ASTRO Accreditation Program for Excellence (APEX) will provide third-party, impartial peer review of personnel, equipment, treatment planning, medical records, patient-safety policies, and quality control/quality assessment activities. As with previous radiation oncology practice accreditation programs, the draft standards stress structural elements, but an emerging new emphasis on process should be noted. The American Society for Clinical Oncology (ASCO) has created its own quality assessment and improvement programs for the medical oncology community.39 In addition, ASCO certifies oncology practices that have surpassed certain quality thresholds via its Quality Certification Program, akin to the ASTRO APEX. In theory, achieving practice accreditation would indicate a threshold for quality and in effect impart a seal of approval for referring physicians, prospective patients, peers, regulatory agencies, and payers. However, implementation of these efforts leads to the following question: Do these parties know or care about accreditation or what the process certifies? Currently, payers do not mandate accreditation, nor are there meaningful economic incentives to seek or maintain accreditation. Such a mandate would facilitate the setting of a quality threshold and would address one of the major criticisms and detractors of value for the specialty: widespread variation in structure not otherwise linked to quality.
New Economic and Structural Incentives to Decrease Cost and Improve Value
Newer coverage models that incentivize patients to choose high-value providers by allowing a credit or discount on their copay if they choose high-value hospitals and providers (those that report their outcomes and costs) are emerging. Some large employers have started to pay for employees' elective cardiac and orthopedic surgical care, travel, lodging, and food costs if they have their procedures performed at what benefit managers consider to be high-value institutions. These so-called high-value or preferred providers presumably practice using established standards characterized by optimal patient selection, treatment delivery, and outcomes. For radiation therapy, the notion of preferred providers for certain high-stakes procedures such as brachytherapy or stereotactic radiation could potentially encourage patients to select high-value providers who meet certain standards of quality and cost. Incentives would be actively aligned; patients spend less (via premium credit or discount for OOP costs) for consistent care, and insurers spend a predetermined amount for selected high-value providers. Once again, with the deficit of clear quality indicators, difficulties with risk stratification, and ongoing inconsistency and disagreement regarding patient selection and the process of treatment delivery, there exists a risk that lowest cost would primarily drive the selection of providers.
A related example of a market-based pricing arrangement, directed at patient health care purchasing behavior and intended to decrease cost and improve value, is the so-called narrow or tiered network offered by health plans. According to the health plans, narrow provider networks are chosen based on quality and cost. However, given the current relative absence of quality indicators in radiation oncology, involvement by providers in these tiered networks is typically based on price or provider relationships with a larger market-dominant entity. Nevertheless, payment reform has caused acceleration of these narrow network products, at times as components of accountable care organizations (ACOs). To maximize value and provide sustainable affordability for consumers, ACOs devise new payment mechanisms, including performance-based contracts, bundles of care and episodes of care payments, shared risk between provider and payer, and capitated payment.40,41 In some markets, clinically integrated networks are intended to combine broad market coverage with global risk-sharing payment methodologies to address the cost and quality imperatives. In this regard, some have predicted that the health care market is likely to shift from traditional open-access preferred provider organizations to these new ACO models. The intent of these coverage vehicles is to focus on quality and cost. It is proposed that these new health plan offerings will be less about being narrow networks and more about a care delivery model that provides the highest efficiency and best value for the consumer. Although the form and execution of these evolving concepts of payment reform are not yet known, and their effects on radiation oncology care are uncertain, they pose clear incentives to integrate episodes of cancer care around treatment directives, clear patient-centered processes of care, and validated outcome metrics.
In this regard, integration of medical practice across the continuum of care has been identified as a high-value organizational structure for delivery of health care, particularly in the management of complex diseases such as cancer.3 In the specific case of integrated practice units (IPUs), teams of clinical and nonclinical personnel provide care through the entire continuum of the disease process.2 Critical features of the IPU model include the shared development and use of care pathways and treatment directives following evidence-based guidelines, as well as feedback mechanisms for continual refinement and improvement of care. This model also delineates utilization of resources through each step of the process of care, with an eye toward economic efficiency and patient convenience. Through the IPU approach, overuse of tests and procedures is reduced. Value is not only achieved through cost savings enabled by this care delivery structure, but is further enhanced by the increased use of the patient-centered shared treatment decision-making paradigm. Radiation oncologists are aware that misleading or incorrect information delivered upstream to the patient's radiation oncology encounter in the traditional referral-based process of care can obfuscate the decision-making process for the patient. The IPU model obviates that issue for the patient, thereby further improving value.42
The concept of integration of care is not new, but until recently, it was slow to catch on. New initiatives in integration of care, such as medical and surgical homes as well as cross-specialty integration of cancer care, have recently gained traction.3,41,43 Patient-centered integration of care requires reassessment of the traditional interactions between subspecialists and ancillary staff, as well as the siloed use of physical space. The quest for value in health reform enables new reimbursement models that can transform the ways providers deliver care.40 As payers and providers realize that fragmented care is inefficient, costs too much, and is not patient centric, the market is beginning to appreciate value-bundled payments for entire episodes of care. IPUs create a care delivery structure that enhances value because the patient is followed through an entire continuum of care for a given condition, making the measurement of all components of care possible. The IPU structure can inform the process and cost components of value as well as create value.
Process
Process encompasses all components of health care delivery, including interactions between patient and physician and the technical delivery of care. Some components of process can be readily observed and appreciated by patients, whereas others, which may be of critical import in a technologically advanced specialty like radiation oncology, are less readily appreciated. Patients may value care delivery in an esthetically pleasing environment, service with a smile, shared decision making, and timely coordinated care. However, just as critical to achieving true quality are less tangible components, including physician expertise and medical physics quality assurance oversight for treatment planning and delivery. With regard to physician expertise, radiation oncology has much in common with surgery. It is a locoregional treatment strategy, procedural in nature, reliant on technologic advances, and ever evolving. The concept of the learning curve is well known in the surgical literature, as is establishing proficiency through procedure-specific training, proctoring, and outcome review.44–50 Several studies have also highlighted the importance of a learning curve for radiation oncology techniques, including brachytherapy procedures, IMRT volume delineation, and technical IMRT delivery.51–54 Repeated performance of best practices and standardization of care where possible are clearly associated with improved outcomes in both the operating room and intensive care unit settings.55–57
As regards radiation oncology, Radiation Therapy Oncology Group (RTOG) cooperative group clinical trials are an example of effective practice standardization. These trials specify treatment-planning specifics, including contouring guidelines, dose constraints, planning techniques, and trial compliance criteria. Centralized review processes, including rapid review of initial protocol cases and timely review of subsequent cases, ensure proficiency and ultimately quality. Studies examining the impact of compliance with treatment-planning specifics in cooperative group trials suggest that protocol deviations are associated with inferior outcomes.54,58,59
In recent years, the emphasis of the specialty has been directed toward defining indications for radiation therapy by the promulgation of so-called evidence-based guidelines. Significantly less emphasis has been placed on defining process-focused optimal treatment delivery. As a result, there remains considerable variation in practice and a lack of standardization of technical delivery of care. Defining excellence in technical delivery of radiation therapy is necessary to allow for proper assessment and accountability. The budding best practices initiative of ASTRO, based on the RAND/University of California Los Angeles appropriateness criteria,60 serves to address this important deficit in refining the process of care in radiation oncology.
Outcomes
Outcomes comprise multiple measures that can be categorized on a spectrum spanning from the finitely objective to the qualitatively subjective. Objective outcomes are the ones that providers and payers have customarily measured: health status, such as disease control and survival; functional physical status and pain level; time to recovery; disease recurrence; complications of treatment; and rate of disease prevention. Subjective outcomes are considered by some observers to have so-called softer end points, with methodologies for their measurement that may not necessarily comport with traditionally accepted scientific methods. They are, nevertheless, of equal importance in the value discussion. These subjective measures include patient-reported outcomes, psychosocial ramifications of the disease or treatment, ability to maintain employment status, and measurement of the patient's understanding of his or her medical condition. Of the proposed components of value, the medical community is best at measuring outcomes on the objective side of the spectrum. In this regard, there is general agreement within the specialty of radiation oncology that the scope and detail of its objective outcome metrics should be enhanced. However, development of desired high-quality and high-level medical evidence is both labor-intensive and costly. In addition, important issues such as risk stratification pose a challenge in comparing outcomes between providers and various treatment modalities and regimens. Specialty-based and cross-disciplinary integrated real-time observational registries are emerging as a method to help address deficits of information about treatment outcomes.61,62 Likewise, patient-reported outcomes in radiation oncology could not only inform translational and comparative effectiveness research, health care technology assessment, and quality assurance, but may also be used as a basis to quantify value from a patient perspective and potentially be used as an aspect of reimbursement.63 With regard to the processes and outcomes of care, those aspects of care that are apparent and matter to patients are best measured by directly asking patients about their experience with their health care. Radiation oncology–specific instruments for patient-reported outcomes are currently in development.64
MOVING TOWARD A VALUE-BASED PARADIGM FOR RADIATION ONCOLOGY CARE
Development of a value-based paradigm for radiation oncology care is a formative process. We are at its beginning. The components of value—structure, process, outcome, and cost—are not theoretic or esoteric (Table 2). However, the core of this value transformation for radiation oncology involves the arduous task of establishing and standardizing structures and processes that reduce variation in care and ensure high quality, while simultaneously rigorously measuring process, outcomes, and costs. In addition, the measurement of health outcomes must now transcend current convention and address elements that matter to the patient and embrace the patient's sense of value. In this regard, measuring the cost of care, for a field in which high cost is an overarching problem, is nothing short of critical. Finally, reorganizing care though patient-centered integration of practice is foundational to creating value, because it facilitates the measurement of outcomes and cost through the entire continuum, and it puts patients first.
Table 2.
Proposed Steps for Creating Value in Radiation Oncology
| Structure | Process | Outcomes | Costs |
|---|---|---|---|
| Engage integrated practice unit models for delivery of care | Optimize accessibility, timeliness, and care coordination | Measure both objective and subjective outcomes | Measure all costs involved with providing care for given episode of care |
| Promote or mandate accreditation to reduce variation among practices | Establish process standards that are patient centered and safety focused | Create easy-to-use national registries | Move beyond billed charges and know true total cost for cycle of care |
| Encourage patients to select providers who meet high-value standards | Facilitate patient access to information about process aspects of their care | Report outcomes, guideline adherence, and best practice adherence among providers | Drive value via payment reform strategies |
Footnotes
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Collection and assembly of data: Sewit Teckie, Michael L. Steinberg
Data analysis and interpretation: Sewit Teckie, Michael L. Steinberg
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Fuchs VR. The gross domestic product and health care spending. N Engl J Med. 2013;369:107–109. doi: 10.1056/NEJMp1305298. [DOI] [PubMed] [Google Scholar]
- 2.Porter ME. A strategy for health care reform: Toward a value-based system. N Engl J Med. 2009;361:109–112. doi: 10.1056/NEJMp0904131. [DOI] [PubMed] [Google Scholar]
- 3.Porter ME, Lee TH. The strategy that will fix health care. Harvard Business Review. 2013 Oct [Google Scholar]
- 4.Porter ME. What is value in health care? N Engl J Med. 2010;363:2477–2481. doi: 10.1056/NEJMp1011024. [DOI] [PubMed] [Google Scholar]
- 5.Donabedian A. Evaluating the quality of medical care: 1966. Milbank Q. 2005;83:691–729. doi: 10.1111/j.1468-0009.2005.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pauly MV, Burns LR. Price transparency for medical devices. Health Aff (Millwood) 2008;27:1544–1553. doi: 10.1377/hlthaff.27.6.1544. [DOI] [PubMed] [Google Scholar]
- 7.Okike K, O'Toole RV, Pollack AN, et al. Survey finds few orthopedic surgeons know the costs of the devices they implant. Health Aff (Millwood) 2014;33:103–109. doi: 10.1377/hlthaff.2013.0453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steinberg ML. Introduction: Health policy and health care economics observed. Semin Radiat Oncol. 2008;18:149–151. doi: 10.1016/j.semradonc.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell JM. Urologists' use of intensity-modulated radiation therapy for prostate cancer. N Engl J Med. 2013;369:1629–1637. doi: 10.1056/NEJMsa1201141. [DOI] [PubMed] [Google Scholar]
- 10.Mitchell JM. Urologists' self-referral for pathology of biopsy specimens linked to increased use and lower prostate cancer detection. Health Aff (Millwood) 2012;31:741–749. doi: 10.1377/hlthaff.2011.1372. [DOI] [PubMed] [Google Scholar]
- 11.Hershman DL, Unger JM, Barlow WE, et al. Treatment quality and outcomes of African American versus white breast cancer patients: Retrospective analysis of Southwest Oncology studies S8814/S8897. J Clin Oncol. 2009;27:2157–2162. doi: 10.1200/JCO.2008.19.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gwyn K, Bondy ML, Cohen DS, et al. Racial differences in diagnosis, treatment, and clinical delays in a population-based study of patients with newly diagnosed breast carcinoma. Cancer. 2004;100:1595–1604. doi: 10.1002/cncr.20169. [DOI] [PubMed] [Google Scholar]
- 13.Bickell NA, Wang JJ, Oluwole S, et al. Missed opportunities: Racial disparities in adjuvant breast cancer treatment. J Clin Oncol. 2006;24:1357–1362. doi: 10.1200/JCO.2005.04.5799. [DOI] [PubMed] [Google Scholar]
- 14.Davidson SM. Open questions concerning influences on clinical decision making. J Ambul Care Manage. 2013;36:88–107. doi: 10.1097/JAC.0b013e31828596de. [DOI] [PubMed] [Google Scholar]
- 15.He D, Mellor JM. Hospital volume responses to Medicare's Outpatient Prospective Payment System: Evidence from Florida. J Health Econ. 2012;31:730–743. doi: 10.1016/j.jhealeco.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 16.US Government Accountability Office. Higher Use of Costly Prostate Cancer Treatment by Providers Who Self-Refer Warrants Scrutiny. http://www.gao.gov/assets/660/656026.pdf.
- 17.Lohr KN, Brook RH, Kamberg CJ, et al. Use of medical care in the RAND Health Insurance Experiment: Diagnosis- and service-specific analyses in a randomized controlled trial. Med Care. 1986;24(suppl):S1–S87. [PubMed] [Google Scholar]
- 18.Brook RH, Ware JE, Rogers WH, et al. Santa Monica, CA: RAND Corporation; 1984. The Effect of Coinsurance on the Health of Adults: Results from the RAND Health Insurance Experiment. [Google Scholar]
- 19.Goldman DP, Joyce GF, Zheng Y. Prescription drug cost sharing: Associations with medication and medical utilization and spending and health. JAMA. 2007;298:61–69. doi: 10.1001/jama.298.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keeler EB, Sloss EM, Brook RH, et al. Effects of cost sharing on physiological health, health practices, and worry. Health Serv Res. 1987;22:279–306. [PMC free article] [PubMed] [Google Scholar]
- 21.Chandra A, Gruber J, McKnight R. The impact of patient cost-sharing on low-income populations: Evidence from Massachusetts. J Health Econ. 2014;33:57–66. doi: 10.1016/j.jhealeco.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 22.Moriates C, Shah NT, Arora VM. First, do no (financial) harm. JAMA. 2013;310:577–578. doi: 10.1001/jama.2013.7516. [DOI] [PubMed] [Google Scholar]
- 23.Elkin EB, Bach PB. Cancer's next frontier: Addressing high and increasing costs. JAMA. 2010;303:1086–1087. doi: 10.1001/jama.2010.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Timmons A, Gooberman-Hill R, Sharp L. “It's at a time in your life when you are most vulnerable”: A qualitative exploration of the financial impact of a cancer diagnosis and implications for financial protection in health. PLoS One. 2013;8:e77549. doi: 10.1371/journal.pone.0077549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zafar SY, Peppercorn JM, Schrag D, et al. The financial toxicity of cancer treatment: A pilot study assessing out-of-pocket expenses and the insured cancer patient's experience. Oncologist. 2013;18:381–390. doi: 10.1634/theoncologist.2012-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong YN, Egleston BL, Sachdeva K, et al. Cancer patients' trade-offs among efficacy, toxicity, and out-of-pocket cost in the curative and noncurative setting. Med Care. 2013;51:838–845. doi: 10.1097/MLR.0b013e31829faffd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ito K, Elkin E, Blinder V, et al. Cost-effectiveness of full coverage of aromatase inhibitors for Medicare beneficiaries with early breast cancer. Cancer. 2013;119:2494–2502. doi: 10.1002/cncr.28084. [DOI] [PubMed] [Google Scholar]
- 28.Davidoff AJ, Erten M, Schaffer T, et al. Out-of-pocket health care expenditure burden for Medicare beneficiaries with cancer. Cancer. 2013;119:1257–1265. doi: 10.1002/cncr.27848. [DOI] [PubMed] [Google Scholar]
- 29.Ubel PA, Abernethy AP, Zafar SY. Full disclosure: Out-of-pocket costs as side effects. N Engl J Med. 2013;369:1484–1486. doi: 10.1056/NEJMp1306826. [DOI] [PubMed] [Google Scholar]
- 30.Alhassani A, Chandra A, Chernew ME. The sources of the SGR “hole.”. N Engl J Med. 2012;366:289–291. doi: 10.1056/NEJMp1113059. [DOI] [PubMed] [Google Scholar]
- 31.Bogdanich W. Radiation offers new cures, and ways to do harm. http://www.nytimes.com/2010/01/24/health/24radiation.html?_r=0.
- 32.Bogdanich W. As technology surges, radiation safeguards lag. http://www.nytimes.com/2010/01/27/us/27radiation.html?pagewanted=all.
- 33.Boganich W, Rebelo K. A pinpoint beam strays invisibly, harming instead of healing. http://www.nytimes.com/2010/12/29/health/29radiation.html?pagewanted=all.
- 34.Elnahal SM, Kerstiens J, Helsper RS, et al. Proton beam therapy and accountable care: The challenges ahead. Int J Radiat Oncol Biol Phys. 2013;85:e165–e172. doi: 10.1016/j.ijrobp.2012.10.038. [DOI] [PubMed] [Google Scholar]
- 35.Lievens Y, Pijls-Johannesma M. Health economic controversy and cost-effectiveness of proton therapy. Semin Radiat Oncol. 2013;23:134–141. doi: 10.1016/j.semradonc.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 36.Wallner PE, Steinberg ML, Konski AA. Controversies in the adoption of new healthcare technologies. Front Radiat Ther Oncol. 2011;43:60–78. doi: 10.1159/000322401. [DOI] [PubMed] [Google Scholar]
- 37.American Society for Radiation Oncology. Safety is No Accident: A Framework for Quality Radiation Oncology and Care. https://www.astro.org/uploadedFiles/Main_Site/Clinical_Practice/Patient_Safety/Blue_Book/SafetyisnoAccident.pdf.
- 38.American Society for Radiation Oncology. Apex: Accreditation program for excellence. https://www.astro.org/Practice-Management/Practice-Accreditation/Index.aspx.
- 39.American Society of Clinical Oncology. Quality Oncology Practice Initiative (QOPI) http://qopi.asco.org/
- 40.Klein I, Kolodziej M. Private payers and cancer care: Land of opportunity. J Oncol Pract. 2014;10:15–19. doi: 10.1200/JOP.2013.000897. [DOI] [PubMed] [Google Scholar]
- 41.Enthoven AC, Crosson FJ, Shortell SM. ‘Redefining health care’: Medical homes or archipelagos to navigate? Health Aff (Millwood) 2007;26:1366–1372. doi: 10.1377/hlthaff.26.5.1366. [DOI] [PubMed] [Google Scholar]
- 42.Steinberg M. Presidential address. https://www.astro.org/uploadedFiles/Main_Site/News_and_Media/ASTROnews/Annual_Meeting_Wrap_Up_2012/astronewsAMwrap_up_2012.pdf.
- 43.Bosserman LD, Verrilli D, McNatt W. Partnering with a payer to develop a value-based medical home pilot: A West Coast practice's experience. J Oncol Pract. 2012;8(suppl):38s–40s. doi: 10.1200/JOP.2012.000591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128–1137. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 45.Vickers AJ, Bianco FJ, Serio AM, et al. The surgical learning curve for prostate cancer control after radical prostatectomy. J Natl Cancer Inst. 2007;99:1171–1177. doi: 10.1093/jnci/djm060. [DOI] [PubMed] [Google Scholar]
- 46.Yim GW, Kim SW, Nam EJ, et al. Learning curve analysis of robot-assisted radical hysterectomy for cervical cancer: Initial experience at a single institution. J Gynecol Oncol. 2013;24:303–312. doi: 10.3802/jgo.2013.24.4.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barrie J, Jayne DG, Wright J, et al. Attaining surgical competency and its implications in surgical clinical trial design: A systematic review of the learning curve in laparoscopic and robot-assisted laparoscopic colorectal cancer surgery. Ann Surg Oncol. 2014;21:829–840. doi: 10.1245/s10434-013-3348-0. [DOI] [PubMed] [Google Scholar]
- 48.Mirheydar HS, Parsons JK. Diffusion of robotics into clinical practice in the United States: Process, patient safety, learning curves, and the public health. World J Urol. 2013;31:455–461. doi: 10.1007/s00345-012-1015-x. [DOI] [PubMed] [Google Scholar]
- 49.Schreuder HW, Wolswijk R, Zweemer RP, et al. Training and learning robotic surgery, time for a more structured approach: A systematic review. BJOG. 2012;119:137–149. doi: 10.1111/j.1471-0528.2011.03139.x. [DOI] [PubMed] [Google Scholar]
- 50.Zorn KC, Gautam G, Shalhav AL, et al. Training, credentialing, proctoring and medicolegal risks of robotic urological surgery: Recommendations of the society of urologic robotic surgeons. J Urol. 2009;182:1126–1132. doi: 10.1016/j.juro.2009.05.042. [DOI] [PubMed] [Google Scholar]
- 51.Marks LB, Light KL, Hubbs JL, et al. The impact of advanced technologies on treatment deviations in radiation treatment delivery. Int J Radiat Oncol Biol Phys. 2007;69:1579–1586. doi: 10.1016/j.ijrobp.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 52.Mendenhall WM, Amdur RJ, Palta JR. Intensity-modulated radiotherapy in the standard management of head and neck cancer: Promises and pitfalls. J Clin Oncol. 2006;24:2618–2623. doi: 10.1200/JCO.2005.04.7225. [DOI] [PubMed] [Google Scholar]
- 53.Liu HW, Malkoske K, Sasaki D, et al. The dosimetric quality of brachytherapy implants in patients with small prostate volume depends on the experience of the brachytherapy team. Brachytherapy. 2010;9:202–207. doi: 10.1016/j.brachy.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 54.Fairchild A, Straube W, Laurie F, et al. Does quality of radiation therapy predict outcomes of multicenter cooperative group trials? A literature review. Int J Radiat Oncol Biol Phys. 2013;87:246–260. doi: 10.1016/j.ijrobp.2013.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wick EC, Hobson DB, Bennett JL, et al. Implementation of a surgical comprehensive unit-based safety program to reduce surgical site infections. J Am Coll Surg. 2012;215:193–200. doi: 10.1016/j.jamcollsurg.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 56.Arriaga AF, Bader AM, Wong JM, et al. Simulation-based trial of surgical-crisis checklists. N Engl J Med. 2013;368:246–253. doi: 10.1056/NEJMsa1204720. [DOI] [PubMed] [Google Scholar]
- 57.Valentin A. Approaches to decreasing medication and other care errors in the ICU. Curr Opin Crit Care. 2013;19:474–479. doi: 10.1097/MCC.0b013e328364d4f9. [DOI] [PubMed] [Google Scholar]
- 58.Peters LJ, O'Sullivan B, Giralt J, et al. Critical impact of radiotherapy protocol compliance and quality in the treatment of advanced head and neck cancer: Results from TROG 02.02. J Clin Oncol. 2010;28:2996–3001. doi: 10.1200/JCO.2009.27.4498. [DOI] [PubMed] [Google Scholar]
- 59.Weber DC, Tomsej M, Melidis C, et al. QA makes a clinical trial stronger: Evidence-based medicine in radiation therapy. Radiother Oncol. 2012;105:4–8. doi: 10.1016/j.radonc.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 60.Brook RH. The RAND/UCLA Appropriateness Method. Santa Monica, CA: RAND Corporation; 1995. [Google Scholar]
- 61.Efstathiou JA, Nassif DS, McNutt TR, et al. Practice-based evidence to evidence-based practice: Building the National Radiation Oncology Registry. J Oncol Pract. 2013;9:e90–e95. doi: 10.1200/JOP.2013.001003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dodson JA, Reynolds MR, Bao H, et al. Developing a risk model for in-hospital adverse events following ICD implantation: A report from the NCDR® registry. J Am Coll Cardiol. doi: 10.1016/j.jacc.2013.09.079. [epub ahead of print on November 27, 2013] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Basch E, Abernethy AP, Mullins CD, et al. Recommendations for incorporating patient-reported outcomes into clinical comparative effectiveness research in adult oncology. J Clin Oncol. 2012;30:4249–4255. doi: 10.1200/JCO.2012.42.5967. [DOI] [PubMed] [Google Scholar]
- 64.McCloskey SA, Kupelian P, Asch S, et al. Adapting the PRO-CTCAE for patient reporting of toxicity in radiation oncology. Int J Radiat Oncol Biol Phys. 2012;84(suppl):S99–S100. [Google Scholar]


