Abstract
Nanotechnology, the manipulation of matter on atomic and molecular scales, is a relatively new branch of science. It has already made a significant impact on clinical medicine, especially in oncology. Nanomaterial has several characteristics that are ideal for oncology applications, including preferential accumulation in tumors, low distribution in normal tissues, biodistribution, pharmacokinetics, and clearance, that differ from those of small molecules. Because these properties are also well suited for applications in radiation oncology, nanomaterials have been used in many different areas of radiation oncology for imaging and treatment planning, as well as for radiosensitization to improve the therapeutic ratio. In this article, we review the unique properties of nanomaterials that are favorable for oncology applications and examine the various applications of nanotechnology in radiation oncology. We also discuss the future directions of nanotechnology within the context of radiation oncology.
INTRODUCTION
Nanotechnology, the manipulation of matter on atomic and molecular scales, is a relatively new branch of science. The idea was first discussed by world-renowned physicist and Nobel laureate Richard Feynman in 1959.1 In a lecture titled “There's Plenty of Room at the Bottom,” he discussed the possibility of engineering materials on a nanometer scale and the potential applications of such technology. Although impossible at the time, advances in physics, chemistry, and materials science eventually made nanomaterials possible in the 1970s.2 The term “nanotechnology,” however, did not become popularized until the 1980s.3 Despite being only four decades old, nanotechnology has made rapid progress and is evident in a wide range of applications, from industrial manufacturing to consumer cosmetics.4 Today, it is estimated that there are more than 1,600 nanotechnology-based consumer products, with new ones entering the market at a rapid pace.5
One of the key applications of nanotechnology is in medicine, especially for the treatment of cancer.6,7 Engineering matter on a nanoscale can give nanoparticles (NPs; nanomaterials that are < 100 nm in size) unique properties that small molecules and bulk materials do not possess. These properties have been used for the development of novel medical diagnostics and therapeutics. For example, iron oxide NPs possess superparamagnetic properties that are not present in other iron oxide materials.8 In the presence of an external magnetic field, iron oxide NPs can provide strong paramagnetic signals at low doses, which make them excellent contrast agents in magnetic resonance imaging (MRI).
Nanomaterials also have several characteristics that are ideally suited for oncology applications. These include the enhanced permeability and retention (EPR) effect, distinct biodistribution and pharmacokinetics, and controlled release.9,10 The leaky vasculature and inefficient lymphatics of tumors allow NPs to extravasate into tumors but prevent their exit back into circulation.11,12 This results in the preferential accumulation of NPs in tumors, which is called the EPR effect.11,13 Such preferential accumulation is advantageous for both diagnostic and therapeutic applications. NPs also have a unique biodistribution when compared with small molecules. Because of their large size compared with small molecules, NPs are unable to penetrate normal vasculature and capillaries. This leads to lower accumulation in organs such as skin, lung, and heart when compared with small molecules.12 Moreover, unlike small molecules that are cleared via several different routes of excretion from the body, NPs are mainly removed from the circulation through the mononuclear phagocytic system and hepatic excretion.14 This clearance pattern provides NPs with different pharmacokinetic properties than small molecules and makes NPs well suited for the delivery of chemotherapeutics. Finally, many NPs can be engineered to release their content in a slow and controlled fashion. This controlled release can be beneficial for therapeutic applications because it increases the exposure of tumor cells to the therapeutic cargo in NPs.
Although much of the research and translation efforts on nanotechnology in oncology have been focused on the development of diagnostics and chemotherapy delivery,15 there has been strong interest in applying nanotechnology to improve radiation oncology. This review discusses the impact that nanotechnology has had on radiation oncology and will have on potential future applications.
NP PLATFORMS
NPs have been engineered from a wide range of materials that can be divided into inorganic and organic NPs.6,16 Inorganic NPs include carbon-based NPs, quantum dots, and metal NPs. The ability to combine materials with different chemical and physical properties in inorganic NPs has generated materials with novel properties. One example is iron oxide NPs, already mentioned. Another is quantum dots (made of semiconductor materials) that are small enough to display quantum mechanical properties.17 In medical applications, quantum dots can be engineered to have specific fluorescent emission properties with minimal photobleaching. Because of these unique properties, inorganic NPs have generally been used for diagnostics.
Organic NPs are usually formulated with biocompatible materials such as lipids, biocompatible polymers, nucleic acids, and peptides. Well-established organic NP platforms include liposomes and other lipid-based micelles, polymeric micelles, dendrimers, and DNA NPs. Because of their biocompatibility, organic nanoplatforms have primarily been used for therapeutic applications. Liposomes are composed of a lipid bilayer, and lipid-based NPs are excellent carriers for hydrophilic molecules such as water-solution chemotherapeutics and nucleic acids. Polymeric micelles are generally composed of a hydrophilic surface and a hydrophobic core.18 The hydrophobic core allows the delivery of hydrophobic chemotherapeutics such as paclitaxel. Dendrimers (repetitively branched molecules) and DNA NPs can be covalently modified with therapeutic agents.
NANOTECHNOLOGY IN IMAGING AND DIAGNOSTICS
Nanotechnology has made a strong impact on medical imaging and diagnostics. In imaging, advances in nanotechnology have resulted in the clinical translation of iron oxide NPs as MRI contrast agents. The most extensively studied agent is the lymphotrophic superparamagnetic iron oxide NP ferumoxtran-10, which can be used to detect subcentimeter lymph node metastases.19 The seminal study on ferumoxtran was conducted in prostate cancer by Harisinghani et al20 on the detection of clinically occult lymph node metastases. Eighty-eight patients with resectable prostate cancer underwent MRI imaging with and without lymphotrophic superparamagnetic NPs followed by lymph node resection or biopsy. The imaging findings were compared with and correlated with histopathologic findings. The investigators found that MRI scans with lymphotrophic superparamagnetic NPs had significantly higher sensitivity than conventional MRI scans (90.5% v 35.4%) and had high specificity because they correctly predicted all patients with lymph node metastases.
In radiation oncology, lymphotrophic superparamagnetic NPs have been applied to lymph node mapping. Radiation therapy to regional lymphatics is an important component in the curative treatment of many cancers, including head and neck, breast, and anal cancers.21–23 Therefore, the delineation (mapping) of at-risk lymph nodes can be critical in planning radiation treatment.22,24 Lymph node mapping techniques generally rely on computed tomography (CT) imaging scans and anatomic surrogates for lymphatics, such as bony landmarks. The locations of lymph nodes at risk are generally not well defined, and there is large variation among radiation oncologists in identifying lymph node treatment volumes. Because lymphotrophic superparamagnetic NPs can accurately identify lymph nodes on MRI scans, several studies have used the technique in radiation lymph node mapping. Shih et al25 studied 18 lymph node–positive patients with prostate cancer who were part of a larger clinical trial that evaluated lymphotrophic nanoparticle–enhanced MRI (LN-MRI) in prostate cancer. The suspected nodal disease on MRI imaging was biopsied by using CT guidance and was confirmed pathologically. In total, there were 69 positive nodes, and these nodes were mapped to a common template on the basis of their relation to skeletal or vascular anatomy. The investigators found that nodal metastases are largely localized near vasculature rather than bony anatomy. By using these pathologically positive nodes, they created a general guide on how to define lymph node treatment volume for prostate cancer radiotherapy (Fig 1). In a similar study, MacDonald et al examined LN-MRI in breast cancer radiation planning. The investigators found that conventional radiotherapy fields do not always adequately cover the lymph nodes at risk, with the 50-Gy isodose line encompassing only 60% of nodes identified by LN-MRI. On the basis of these data, they recommended a margin when defining lymph node treatment volumes by using CT imaging. Further clinical translation of iron oxide NPs in radiation oncology has been hindered by the lack of clinical adoption of these agents as MRI contrast agents.
Fig 1.
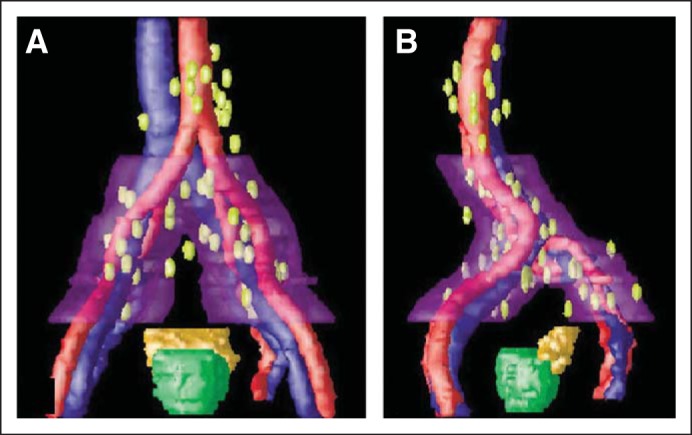
Metastatic nodes referenced to pelvic vessels with a 2-cm clinical target volume expansion to the region along pelvic vessels at greatest risk. (A) Anterior-posterior view. (B) Left lateral view. Image adapted.1
Nanotechnology has also been applied in x-ray generation. Carbon nanotubes (CNTs) have been used as electron emitters in x-ray imaging.26 Unlike conventional x-ray imaging in which this is a single electron emitter, the CNT x-ray imager uses an array of CNTs with each CNT functioning as an electron emitter. The advantages of CNT-based x-ray imaging over conventional imaging include higher resolution, lower radiation dose, and smaller sized equipment. These approaches have been developed for breast tomosynthesis diagnostic studies, and the machines are in clinical trial. Because of these characteristics, CNT has been applied in microradiotherapy systems for preclinical research.27 Because of its ability to generate x-ray beams on the micrometer scale, CNT has also been used in the development of a microbeam radiation therapy system.28 Such machines may be useful in understanding the biologic effects of radiotherapy at the single-cell level.
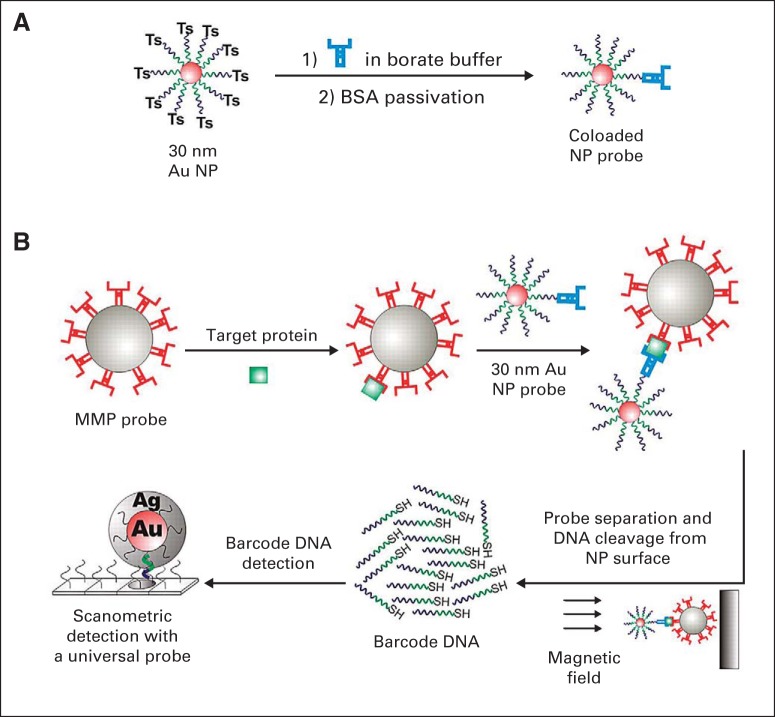
Nanotechnology-based medical diagnostics have been translated clinically, with several assays already in clinical use.29,30 The enthusiasm for nano-diagnostics is a result of their ability to detect analytes at femtomolar (10−15 M) concentrations, several hundred times more sensitive than conventional diagnostics. Some of these diagnostics can be directly applied in radiation oncology. For example, Thaxton et al31 developed a novel bio-barcode assay to detect prostate-specific antigen (PSA) by using gold NPs (Fig 2). This assay was evaluated in a pilot study with 18 men who had undergone radical prostatectomy and were deemed to have undetectable PSA by conventional PSA tests. The new barcode assay was able to detect PSA as low as 330 fg/mL. Despite the initially undetectable levels (< 0.1 mg/mL) of PSA in these men, the investigators found that men who had rapidly rising PSA detected by using the barcode assay had worse clinical outcomes. They suggested that such ultrasensitive PSA tests may be helpful in better identifying men who need salvage radiotherapy after surgery.
Fig 2.
Schematic of the gold (Au) nanoparticle (NP) barcode assay. (A) Barcode DNA-functionalized Au-NPs (30 nm) are conjugated to PSA-specific antibodies through barcode terminal tosyl (Ts) modification to generate the co-loaded PSA Au-NP probes. In a second step, the PSA Au-NP probes are passivated with BSA. (B) The bio-barcode assay is a sandwich immunoassay. First, MMPs surface-functionalized with monoclonal antibodies to PSA are mixed with the PSA target protein. Next, PSA Au-NP probes are added to sandwich the MMP-bound PSA. PSA-specific DNA barcodes are then released into solution and detected using the scanometric assay, which takes advantage of Au-NP catalyzed silver enhancement. Approximately half of the barcode DNA sequence (green) is complementary to the “universal“ scanometric Au-NP probe DNA, and the other half (purple) is complementary to a chip-surface immobilized DNA sequence that is responsible for sorting and binding barcodes complementary to the PSA barcode sequence. Ag, silver, BSA, bovine serum albumin; MMP, magnetic microparticle probe; PSA, prostate-specific antigen; SH, sulfur and hydrogen; Ts, tosyl (blue symbol) PSA-specific antibodies. Image adapted.28
Other NP-based diagnostics hold potential in improving early detection of cancer and monitoring disease progression as well as disease recurrence.32 Shao et al33 reported on a novel device that is capable of detecting circulating microvesicles that are secreted by glioblastoma tumor cells. By using the detected microvesicles, the investigators were able to monitor and predict treatment response to two chemotherapeutics in a mouse model. Nanotechnology has also been incorporated into devices to capture circulating tumor cells (CTCs).34 CTC devices also allow early detection of cancer and real-time monitoring of disease. The significance of CTCs is being explored for use in radiation oncology.35 Collectively, these NP-based diagnostic assays can provide real-time information on radiotherapy treatment response. They may also increase the curative management of cancers that involve radiotherapy.
INORGANIC NPS AS RADIOSENSITIZERS
There has been long-standing interest in the development of radiosensitizers, agents that can sensitize tumor cells to radiotherapy, to improve the therapeutic ratio of radiotherapy.36 Current efforts have generally focused on chemotherapeutics and targeted agents. One unique strategy is to increase the radiation dose within tumor tissue by using material with high atomic numbers (Z). This is because the dose absorbed by any tissue is related to the Z2 of the material.37 If an agent can increase the overall effective Z of tumor without affecting the Z of nearby tissue, it can lead to increased radiotherapy dose to tumors and higher therapeutic efficacy.
By using this strategy, Maggiorella et al38 studied hafnium oxide NPs as radiosensitizers. The investigators engineered hafnium oxide NPs (NBTXR3) that are approximately 50 nm in size. NBTXR3 was evaluated by using tumor xenograft models with two sarcoma cell lines and a colorectal cancer cell line. The agent was given through intratumoral injection. The investigators found that NBTXR3 demonstrated radiosensitizing effects on tumor cells, and the addition of NBTXR3 led to increased delay in tumor growth. They did not observe significant toxicity in mice. NBTXR3 is currently being evaluated clinically as a radiosensitizer in two phase I trials: one in soft tissue sarcoma of the extremity (NCT01433068) and the other in locally advanced squamous cell carcinoma of the oral cavity or oropharynx (NCT01946867).
Many groups have also studied gold NPs as radiosensitizers.39 Like hafnium, gold is a high Z material and an excellent absorber of x-rays. Gold has a good safety profile because it is inert (elemental gold) and it has been used in medical therapies.40 Several studies have demonstrated the radiosensitization effects of gold NPs in vitro and in vivo.41–43 However, these agents have not been evaluated clinically. The main barrier to clinical translation for high Z inorganic NPs is the lack of a clearance pathway for these NPs.14,44 Without clearance, it is impractical to administer these agents intravenously as radiosensitizers. Thus, the main route of administration is intratumoral injection, which has limited utility in clinical radiation oncology at present.
NANOTHERAPEUTICS TO IMPROVE CHEMORADIOTHERAPY
Chemoradiotherapy, the concurrent administration of chemotherapy and radiotherapy, has been an important treatment paradigm in oncology.45 Although it has improved survival and disease control, chemoradiotherapy also has increased toxicity when compared with sequential treatment or either treatment alone.45 Therefore, there has been strong interest in improving the therapeutic ratio of chemoradiotherapy. One approach is to improve the delivery of chemotherapy to the tumors while reducing drug dose to normal tissue (Fig 3). However, previous efforts using traditional drug delivery techniques have been unsuccessful.46
Fig 3.
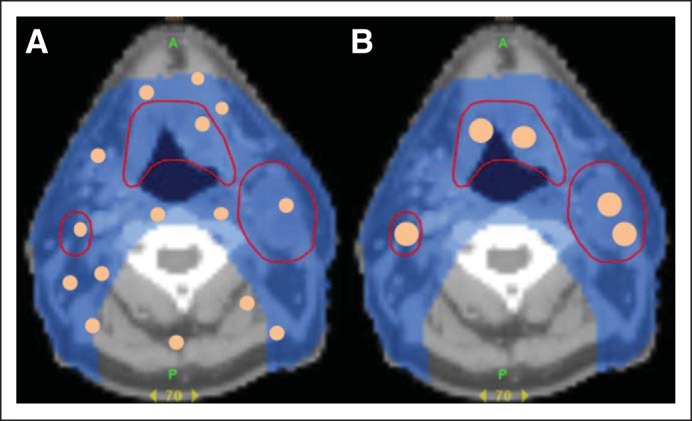
Small molecule (A) versus nanoparticle therapeutics (B) in chemoradiotherapy for head and neck squamous cell carcinoma. Axial computed tomography images of a patient with head and neck squamous cell carcinoma. Areas outlined in red are tumors that should receive high-dose radiation. (A) Small molecule chemotherapeutics (depicted by small orange dots) distributes throughout tissue resulting in large volume of normal tissue receiving radiation and drug. (B) In contrast, nanoparticle drugs (large orange dots) accumulate in tumors, which limits drug dose to normal tissue.
As mentioned previously, NPs possess several important characteristics that are well-suited for the delivery of chemotherapeutics in chemoradiotherapy, including preferential accumulation in tumors, inability of NPs to penetrate normal vasculature leading to lower drug dose to normal tissues and reduced toxicity,12 and slow and controlled drug delivery. Therefore, NP delivery of chemotherapeutics has the potential to significantly improve the therapeutic ratio of chemoradiotherapy.10
Liposomal formulations of doxorubicin were the first NP therapeutics that were developed for the clinical treatment of cancer.9 Clinically, liposomal doxorubicin has been evaluated in several early-phase clinical trials of chemoradiotherapy.10 Koukourakis et al47 conducted most of these studies. His group first reported a phase I trial on the use of liposomal doxorubicin (Caelyx) with conventionally fractionated radiotherapy for locally advanced non–small-cell lung cancer (NSCLC) and head and neck cancer. As expected, they found the dose-limiting toxicities were mucositis for treatment of head and neck cancer and esophagitis for treatment of NSCLC. In a follow-up phase I/II study, patients with inoperable (stage IIIb; T3,4-N2,3-M0) NSCLC were enrolled to receive docetaxel, doxorubicin, and radiotherapy.48 Grade 3 and higher esophagitis, which was the dose-limiting toxicity, developed in nine (36%) of 25 patients. The response rates were 40% complete response (CR) and 47% partial response. Liposomal formulations of doxorubicin have also been investigated in chemoradiotherapy for cervical cancer, recurrent breast cancer, and bladder cancer. Despite promising results from these small clinical trials, liposomal doxorubicin was not adopted into chemoradiotherapy treatment regimens. The main reason is that small molecule doxorubicin has not been used in chemoradiotherapy. Moreover, although the clinical results from the liposomal doxorubicin were promising, they were not clearly superior to standard chemoradiotherapy regimens.
Liposomal formulations of cisplatin, although not as extensively studied as doxorubicin, have also been investigated in early-phase clinical trials. In 20 patients with head and neck cancer treated at the University of Pennsylvania, Rosenthal et al49 examined liposomal cisplatin as a radiosensitizer concurrent with conventionally fractionated radiotherapy. Grade 3 skin and mucosal toxicities within the radiation field were minimal, occurring in only one and six patients, respectively. Furthermore, 11 (55%) of the 20 patients had an initial CR at the primary tumor site after completing treatment. In a study of 12 patients with locally advanced gastric cancer who received liposomal cisplatin, fluorouracil, and concurrent radiation therapy, high CR rates and minor toxicities were also observed.50 CR rates improved from 33% in patients treated with four cycles to 80% in patients treated with five cycles of combined chemoradiotherapy with liposomal cisplatin. The toxicity profile was comparable with that of conventional cisplatin. These results on liposomal cisplatin were encouraging and await validation in larger clinical trials.
Polymer-drug conjugates can be considered NPs since they are nanometers in size, although they do not possess some of the key properties of NPs, such as controlled drug release. Several preclinical studies have examined polymer-drug conjugates in chemoradiotherapy, including polymer-gemcitabine and polymer-paclitaxel (PG-TXL).51–53 Preclinical data from PG-TXL have led to two clinical studies evaluating it as a radiosensitizer. The first study was a phase I trial using PG-TXL with concurrent radiotherapy in esophageal and gastric cancer.54 The other trial studied PG-TXL with temozolomide and radiotherapy in high-grade glioma.55 Unfortunately, combining temozolomide and PG-TXL led to high rates of and prolonged (5 months) hematologic toxicity.
NP albumin-bound (nab) paclitaxel, the most recently approved NP chemotherapeutic, has been evaluated preclinically in chemoradiotherapy.56 Mice bearing ovarian or mammary carcinomas were treated with nab-paclitaxel, radiotherapy, or both. Nab-paclitaxel improved radiosensitization, lowering the dose to achieve 50% tumor cure from 54.3 Gy to 35.2 Gy. The greatest treatment effect occurred when radiation was given 2 to 3 days after nab-paclitaxel was administered. Currently, several phase III chemoradiotherapy clinical trials in lung, esophageal, head and neck, endometrial, and cervical cancer are evaluating the concurrent administration of nab-paclitaxel with radiotherapy.
Current clinical and preclinical research efforts on nanotherapeutics have focused on polymeric NP platforms. Several polymeric NPs have been evaluated clinically, and one polymeric micelle formulation (Genexol-PM) has been approved for clinical use in Korea.9 Although none of these polymeric NPs have been studied clinically in chemoradiotherapy, there are several preclinical studies. Our group has evaluated Genexol-PM with external beam radiotherapy in a mouse model of NSCLC.57 Genexol-PM was compared with paclitaxel at equivalent doses of paclitaxel. We found that chemoradiotherapy with Genexol-PM was more effective than with paclitaxel in vivo with potentially lower toxicity since there is less paclitaxel dose in the normal lung with Genexol-PM than paclitaxel at 6 hours after administration. Jung et al58 also studied polymeric NP formulations of paclitaxel and docetaxel with radiotherapy in mouse models of NSCLC. They observed enhanced synergistic effect with reduced survival fraction of NSCLC cells in vitro and enhanced tumor growth delay in vivo in xenograft mice.
In addition to established chemotherapeutics, NPs can also deliver potent radiosensitizers, such as DNA double-strand repair inhibitors, that are too toxic to administer as small molecules. We have demonstrated proof of principle of this approach by engineering an NP formulation of wortmannin.59 Wortmannin is a potent radiosensitizer that inhibits DNA repair. However, it has high hematologic and hepatic toxicity. We demonstrated that NP wortmannin has a significantly lower toxicity than wortmannin, with the maximum-tolerated dose of NP wortmannin being three to five times higher than that of wortmannin. More importantly, NP wortmannin is more effective than wortmannin as a radiosensitizer and has high therapeutic efficacy even at low doses. Our results highlight the potential of novel NP radiosensitizers in chemoradiotherapy. Clinical translation efforts on polymeric NP therapeutics in chemoradiotherapy are ongoing. We have recently initiated a phase IB/II trial evaluating CRLX101, an NP formulation of camptothecin with flurouracil and radiotherapy, in the neoadjuvant treatment of locally advanced rectal cancer (NCT02010567).
RADIATION-GUIDED DRUG DELIVERY USING NPS
Radiotherapy can be used to improve the delivery of NP therapeutics to tumors. Ionizing radiation can increase the vascular permeability of tumors, further enhancing the EPR effect. This can directly translate into higher intratumoral nanotherapeutic concentrations after radiotherapy. Indeed, Lammers et al51 found that radiotherapy can increase the tumor accumulation of polymer-drug conjugates when examining polymer-gemcitabine conjugates in chemoradiotherapy in a rat prostate carcinoma tumor model. This phenomenon was also observed in a separate study by Giustini et al60 using iron oxide NPs. They found that radiotherapy at 15 Gy significantly increased the tumor accumulation of iron oxide NPs when they used a syngeneic mouse breast cancer model. This preferential accumulation effect can be further enhanced by triggered drug release, such as hyperthermia-induced drug release, which has been used in conjunction with radiotherapy.61
In addition to passively improving the distribution of NP therapeutics to tumors, radiotherapy can also be used to actively guide NP drug delivery. Hallahan et al62 identified a peptide that can bind to integrins on the irradiated tumor microvasculature. By functionalizing the surfaces of NPs with this unique peptide, the investigators were able to preferentially deliver liposomes and albumin-based NPs to tumors. In subsequent preclinical studies, they demonstrated that the peptide can also be used to target iron platinum NPs,63 nab-paclitaxel,64 and liposomal doxorubicin.65 Each study demonstrated that radiotherapy enhanced tumor-targeted NP delivery, which resulted in higher therapeutic efficacy. The data collectively suggest that radiation-guided drug delivery can be a novel strategy in cancer treatment.
FUTURE DIRECTIONS
Innovation and progress in radiation oncology have traditionally been driven by advances in technology. For example, a key improvement in radiation oncology was the development of intensity-modulated radiation therapy, which was made possible by progress in physics and computer science.66 It is entirely possible that the next major driving force of progress in radiation oncology will be nanotechnology.
In imaging and diagnostics, NP-based agents can improve radiation treatment planning beyond simply producing guidelines on how to contour lymph node volumes. Radiotherapy can be planned with the concurrent administration of an NP-based imaging agent for accurate identification of the tumor volume as well as lymph node metastases. Such personalized treatment planning may improve therapeutic efficacy as well as reduce toxicity. NP-based assays can also be used to monitor radiotherapy treatment response in real time, which may further improve personalized cancer treatment.
There are strong preclinical data demonstrating the potential of NP-based therapeutics in improving the therapeutic index of radiotherapy. However, there is a lack of clinical data examining these agents in conjunction with radiotherapy. Thus, future efforts should be focused on conducting clinical trials with selected nanotherapeutics as they enter clinical evaluation. NPs also offer the opportunity to use clinically potent radiosensitizers that are too toxic to be used in their molecular form. More preclinical research is needed to determine how to engineer NPs that are best suited to deliver these radiosensitizers. Finally, NPs can facilitate the clinical translation of novel biologic therapies, such as small interfering RNA.67 Such therapeutics may provide new therapeutic regimens in radiation oncology as well as in other areas of medicine.
In less than four decades, nanotechnology has made an impact on radiation oncology. However, we are still in the early stages of nanotechnology's clinical development. Today, there are many nanotechnology-based diagnostics and therapeutics under clinical development. By taking advantage of these novel agents as well as the new technical capabilities offered by nanotechnology, we can further improve radiotherapy's therapeutic efficacy, reduce its toxicity, and enable more personalized treatment.
Footnotes
Supported by Grants No. U54-CA151652 from the National Institutes of Health Centers for Nanotechnology Excellence and No. R01CA178748-01 (A.Z.W.) from the National Institutes of Health, National Cancer Institute.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: Andrew Z. Wang, Coordination Therapeutics (co-founder) Honoraria: None Research Funding: None Expert Testimony: None Patents, Royalties, and Licenses: Andrew Z. Wang, None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Andrew Z. Wang, Joel E. Tepper
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Appenzeller T. The man who dared to think small. Science. 1991;254:1300. doi: 10.1126/science.254.5036.1300. [DOI] [PubMed] [Google Scholar]
- 2.Gregoriadis G, Ryman BE. Lysosomal localization of enzyme-containing liposomes injected into rats. Biochem J. 1972;128:142P–143P. doi: 10.1042/bj1280142pb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amato I. The apostle of nanotechnology. Science. 1991;254:1310–1311. doi: 10.1126/science.254.5036.1310. [DOI] [PubMed] [Google Scholar]
- 4.Hassan MH. Nanotechnology: Small things and big changes in the developing world. Science. 2005;309:65–66. doi: 10.1126/science.1111138. [DOI] [PubMed] [Google Scholar]
- 5.PEN, the Project on Emerging Nanotechnologies. Consumer Products Inventory: An inventory of nanotechnology-based consumer products introduced on the market. http://www.nanotechproject.org/cpi/
- 6.Kim BY, Rutka JT, Chan WC. Nanomedicine. N Engl J Med. 2010;363:2434–2443. doi: 10.1056/NEJMra0912273. [DOI] [PubMed] [Google Scholar]
- 7.Service RF. Materials and biology: Nanotechnology takes aim at cancer. Science. 2005;310:1132–1134. doi: 10.1126/science.310.5751.1132. [DOI] [PubMed] [Google Scholar]
- 8.Laurent S, Forge D, Port M, et al. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem Rev. 2008;108:2064–2110. doi: 10.1021/cr068445e. [DOI] [PubMed] [Google Scholar]
- 9.Wang AZ, Langer R, Farokhzad OC. Nanoparticle delivery of cancer drugs. Annu Rev Med. 2012;63:185–198. doi: 10.1146/annurev-med-040210-162544. [DOI] [PubMed] [Google Scholar]
- 10.Miller SM, Wang AZ. Nanomedicine in chemoradiation. Ther Deliv. 2013;4:239–250. doi: 10.4155/tde.12.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jain RK, Stylianopoulos T. Delivering nanomedicine to solid tumors. Nat Rev Clin Oncol. 2010;7:653–664. doi: 10.1038/nrclinonc.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maeda H, Wu J, Sawa T, et al. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J Control Release. 2000;65:271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 13.Hobbs SK, Monsky WL, Yuan F, et al. Regulation of transport pathways in tumor vessels: Role of tumor type and microenvironment. Proc Natl Acad Sci U S A. 1998;95:4607–4612. doi: 10.1073/pnas.95.8.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Longmire M, Choyke PL, Kobayashi H. Clearance properties of nano-sized particles and molecules as imaging agents: Considerations and caveats. Nanomedicine (Lond) 2008;3:703–717. doi: 10.2217/17435889.3.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zamboni WC, Torchilin V, Patri AK, et al. Best practices in cancer nanotechnology: Perspective from NCI nanotechnology alliance. Clin Cancer Res. 2012;18:3229–3241. doi: 10.1158/1078-0432.CCR-11-2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doll TA, Raman S, Dey R, et al. Nanoscale assemblies and their biomedical applications. J R Soc Interface. 2013;10:20120740. doi: 10.1098/rsif.2012.0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yue Z, Lisdat F, Parak WJ, et al. Quantum-dot-based photoelectrochemical sensors for chemical and biological detection. ACS Appl Mater Interfaces. 2013;5:2800–2814. doi: 10.1021/am3028662. [DOI] [PubMed] [Google Scholar]
- 18.Devulapally R, Paulmurugan R. Polymer nanoparticles for drug and small silencing RNA delivery to treat cancers of different phenotypes. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2014;6:40–60. doi: 10.1002/wnan.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saksena MA, Saokar A, Harisinghani MG. Lymphotropic nanoparticle enhanced MR imaging (LNMRI) technique for lymph node imaging. Eur J Radiol. 2006;58:367–374. doi: 10.1016/j.ejrad.2005.12.041. [DOI] [PubMed] [Google Scholar]
- 20.Harisinghani MG, Barentsz J, Hahn PF, et al. Noninvasive detection of clinically occult lymph-node metastases in prostate cancer. N Engl J Med. 2003;348:2491–2499. doi: 10.1056/NEJMoa022749. [DOI] [PubMed] [Google Scholar]
- 21.Grégoire V, Ang K, Budach W, et al. Delineation of the neck node levels for head and neck tumors: A 2013 update—DAHANCA, EORTC, HKNPCSG, NCIC CTG, NCRI, RTOG, TROG consensus guidelines. Radiother Oncol. doi: 10.1016/j.radonc.2013.10.010. [epub ahead of print on October 31, 2013] [DOI] [PubMed] [Google Scholar]
- 22.Myerson RJ, Garofalo MC, El Naqa I, et al. Elective clinical target volumes for conformal therapy in anorectal cancer: A Radiation Therapy Oncology Group consensus panel contouring atlas. Int J Radiat Oncol Biol Phys. 2009;74:824–830. doi: 10.1016/j.ijrobp.2008.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ragaz J, Olivotto IA, Spinelli JJ, et al. Locoregional radiation therapy in patients with high-risk breast cancer receiving adjuvant chemotherapy: 20-year results of the British Columbia randomized trial. J Natl Cancer Inst. 2005;97:116–126. doi: 10.1093/jnci/djh297. [DOI] [PubMed] [Google Scholar]
- 24.Krueger EA, Fraass BA, Pierce LJ. Clinical aspects of intensity-modulated radiotherapy in the treatment of breast cancer. Semin Radiat Oncol. 2002;12:250–259. doi: 10.1053/srao.2002.32468. [DOI] [PubMed] [Google Scholar]
- 25.Shih HA, Harisinghani M, Zietman AL, et al. Mapping of nodal disease in locally advanced prostate cancer: Rethinking the clinical target volume for pelvic nodal irradiation based on vascular rather than bony anatomy. Int J Radiat Oncol Biol Phys. 2005;63:1262–1269. doi: 10.1016/j.ijrobp.2005.07.952. [DOI] [PubMed] [Google Scholar]
- 26.Cao G, Lee YZ, Peng R, et al. A dynamic micro-CT scanner based on a carbon nanotube field emission x-ray source. Phys Med Biol. 2009;54:2323–2340. doi: 10.1088/0031-9155/54/8/005. [DOI] [PubMed] [Google Scholar]
- 27.Wang S, Liu Z, Sultana S, et al. A novel high resolution micro-radiotherapy system for small animal irradiation for cancer research. Biofactors. 2007;30:265–270. doi: 10.1002/biof.5520300408. [DOI] [PubMed] [Google Scholar]
- 28.Hadsell M, Zhang J, Laganis P, et al. A first generation compact microbeam radiation therapy system based on carbon nanotube X-ray technology. Appl Phys Lett. 2013;103:183505. doi: 10.1063/1.4826587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu Y, Fine DH, Tasciotti E, et al. Nanodevices in diagnostics. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2011;3:11–32. doi: 10.1002/wnan.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weintraub K. Biomedicine: The new gold standard. Nature. 2013;495:S14–S16. doi: 10.1038/495S14a. [DOI] [PubMed] [Google Scholar]
- 31.Thaxton CS, Elghanian R, Thomas AD, et al. Nanoparticle-based bio-barcode assay redefines “undetectable” PSA and biochemical recurrence after radical prostatectomy. Proc Natl Acad Sci U S A. 2009;106:18437–18442. doi: 10.1073/pnas.0904719106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwong GA, von Maltzahn G, Murugappan G, et al. Mass-encoded synthetic biomarkers for multiplexed urinary monitoring of disease. Nat Biotechnol. 2013;31:63–70. doi: 10.1038/nbt.2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shao H, Chung J, Balaj L, et al. Protein typing of circulating microvesicles allows real-time monitoring of glioblastoma therapy. Nat Med. 2012;18:1835–1840. doi: 10.1038/nm.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hughes AD, King MR. Nanobiotechnology for the capture and manipulation of circulating tumor cells. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2012;4:291–309. doi: 10.1002/wnan.168. [DOI] [PubMed] [Google Scholar]
- 35.Martin OA, Anderson RL, Russell PA, et al. Mobilization of viable tumor cells into the circulation during radiation therapy. Int J Radiat Oncol Biol Phys. 2014;88:395–403. doi: 10.1016/j.ijrobp.2013.10.033. [DOI] [PubMed] [Google Scholar]
- 36.Shewach DS, Lawrence TS. Antimetabolite radiosensitizers. J Clin Oncol. 2007;25:4043–4050. doi: 10.1200/JCO.2007.11.5287. [DOI] [PubMed] [Google Scholar]
- 37.Alkhatib A, Watanabe Y, Broadhurst JH. The local enhancement of radiation dose from photons of MeV energies obtained by introducing materials of high atomic number into the treatment region. Med Phys. 2009;36:3543–3548. doi: 10.1118/1.3168556. [DOI] [PubMed] [Google Scholar]
- 38.Maggiorella L, Barouch G, Devaux C, et al. Nanoscale radiotherapy with hafnium oxide nanoparticles. Future Oncol. 2012;8:1167–1181. doi: 10.2217/fon.12.96. [DOI] [PubMed] [Google Scholar]
- 39.Jeremic B, Aguerri AR, Filipovic N. Radiosensitization by gold nanoparticles. Clin Transl Oncol. 2013;15:593–601. doi: 10.1007/s12094-013-1003-7. [DOI] [PubMed] [Google Scholar]
- 40.Madeira JM, Gibson DL, Kean WF, et al. The biological activity of auranofin: Implications for novel treatment of diseases. Inflammopharmacology. 2012;20:297–306. doi: 10.1007/s10787-012-0149-1. [DOI] [PubMed] [Google Scholar]
- 41.Hainfeld JF, Smilowitz HM, O'Connor MJ, et al. Gold nanoparticle imaging and radiotherapy of brain tumors in mice. Nanomedicine (Lond) 2013;8:1601–1609. doi: 10.2217/nnm.12.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song K, Xu P, Meng Y, et al. Smart gold nanoparticles enhance killing effect on cancer cells. Int J Oncol. 2013;42:597–608. doi: 10.3892/ijo.2012.1721. [DOI] [PubMed] [Google Scholar]
- 43.Ngwa W, Korideck H, Kassis AI, et al. In vitro radiosensitization by gold nanoparticles during continuous low-dose-rate gamma irradiation with I-125 brachytherapy seeds. Nanomedicine. 2013;9:25–27. doi: 10.1016/j.nano.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Choi HS, Liu W, Misra P, et al. Renal clearance of quantum dots. Nat Biotechnol. 2007;25:1165–1170. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vokes EE, Brizel DM, Lawrence TS. Concomitant chemoradiotherapy. J Clin Oncol. 2007;25:4031–4032. [Google Scholar]
- 46.Rasch CR, Hauptmann M, Schornagel J, et al. Intra-arterial versus intravenous chemoradiation for advanced head and neck cancer: Results of a randomized phase 3 trial. Cancer. 2010;116:2159–2165. doi: 10.1002/cncr.24916. [DOI] [PubMed] [Google Scholar]
- 47.Koukourakis MI, Koukouraki S, Giatromanolaki A, et al. Liposomal doxorubicin and conventionally fractionated radiotherapy in the treatment of locally advanced non-small-cell lung cancer and head and neck cancer. J Clin Oncol. 1999;17:3512–3521. doi: 10.1200/JCO.1999.17.11.3512. [DOI] [PubMed] [Google Scholar]
- 48.Koukourakis MI, Romanidis K, Froudarakis M, et al. Concurrent administration of docetaxel and Stealth liposomal doxorubicin with radiotherapy in non-small cell lung cancer: Excellent tolerance using subcutaneous amifostine for cytoprotection. Br J Cancer. 2002;87:385–392. doi: 10.1038/sj.bjc.6600486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosenthal DI, Yom SS, Liu L, et al. A phase I study of SPI-077 (Stealth liposomal cisplatin) concurrent with radiation therapy for locally advanced head and neck cancer. Invest New Drugs. 2002;20:343–349. doi: 10.1023/a:1016201732368. [DOI] [PubMed] [Google Scholar]
- 50.Koukourakis MI, Giatromanolaki A, Pitiakoudis M, et al. Concurrent liposomal cisplatin (Lipoplatin), 5-fluorouracil and radiotherapy for the treatment of locally advanced gastric cancer: A phase I/II study. Int J Radiat Oncol Biol Phys. 2010;78:150–155. doi: 10.1016/j.ijrobp.2009.07.1733. [DOI] [PubMed] [Google Scholar]
- 51.Lammers T, Subr V, Peschke P, et al. Image-guided and passively tumour-targeted polymeric nanomedicines for radiochemotherapy. Br J Cancer. 2008;99:900–910. doi: 10.1038/sj.bjc.6604561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lammers T, Peschke P, Kühnlein R, et al. Effect of radiotherapy and hyperthermia on the tumor accumulation of HPMA copolymer-based drug delivery systems. J Control Release. 2007;117:333–341. doi: 10.1016/j.jconrel.2006.10.032. [DOI] [PubMed] [Google Scholar]
- 53.Li C, Ke S, Wu QP, et al. Tumor irradiation enhances the tumor-specific distribution of poly(L-glutamic acid)-conjugated paclitaxel and its antitumor efficacy. Clin Cancer Res. 2000;6:2829–2834. [PubMed] [Google Scholar]
- 54.Dipetrillo T, Milas L, Evans D, et al. Paclitaxel poliglumex (PPX-Xyotax) and concurrent radiation for esophageal and gastric cancer: A phase I study. Am J Clin Oncol. 2006;29:376–379. doi: 10.1097/01.coc.0000224494.07907.4e. [DOI] [PubMed] [Google Scholar]
- 55.Jeyapalan S, Boxerman J, Donahue J, et al. Paclitaxel poliglumex, temozolomide, and radiation for newly diagnosed high-grade glioma: A Brown University Oncology Group Study. Am J Clin Oncol. doi: 10.1097/COC.0b013e31827de92b. [epub ahead of print on February 5, 2013] [DOI] [PubMed] [Google Scholar]
- 56.Wiedenmann N, Valdecanas D, Hunter N, et al. 130-nm albumin-bound paclitaxel enhances tumor radiocurability and therapeutic gain. Clin Cancer Res. 2007;13:1868–1874. doi: 10.1158/1078-0432.CCR-06-2534. [DOI] [PubMed] [Google Scholar]
- 57.Werner ME, Cummings ND, Sethi M, et al. Preclinical evaluation of Genexol-PM, a nanoparticle formulation of paclitaxel, as a novel radiosensitizer for the treatment of non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2013;86:463–468. doi: 10.1016/j.ijrobp.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jung J, Park SJ, Chung HK, et al. Polymeric nanoparticles containing taxanes enhance chemoradiotherapeutic efficacy in non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2012;84:e77–e83. doi: 10.1016/j.ijrobp.2012.02.030. [DOI] [PubMed] [Google Scholar]
- 59.Karve S, Werner ME, Sukumar R, et al. Revival of the abandoned therapeutic wortmannin by nanoparticle drug delivery. Proc Natl Acad Sci U S A. 2012;109:8230–8235. doi: 10.1073/pnas.1120508109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Giustini AJ, Petryk AA, Hoopes PJ. Ionizing radiation increases systemic nanoparticle tumor accumulation. Nanomedicine. 2012;8:818–821. doi: 10.1016/j.nano.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Manzoor AA, Lindner LH, Landon CD, et al. Overcoming limitations in nanoparticle drug delivery: Triggered, intravascular release to improve drug penetration into tumors. Cancer Res. 2012;72:5566–5575. doi: 10.1158/0008-5472.CAN-12-1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hallahan D, Geng L, Qu S, et al. Integrin-mediated targeting of drug delivery to irradiated tumor blood vessels. Cancer Cell. 2003;3:63–74. doi: 10.1016/s1535-6108(02)00238-6. [DOI] [PubMed] [Google Scholar]
- 63.Hariri G, Wellons MS, Morris WH, 3rd, et al. Multifunctional FePt nanoparticles for radiation-guided targeting and imaging of cancer. Ann Biomed Eng. 2011;39:946–952. doi: 10.1007/s10439-010-0219-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hariri G, Yan H, Wang H, et al. Radiation-guided drug delivery to mouse models of lung cancer. Clin Cancer Res. 2010;16:4968–4977. doi: 10.1158/1078-0432.CCR-10-0969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lowery A, Onishko H, Hallahan DE, et al. Tumor-targeted delivery of liposome-encapsulated doxorubicin by use of a peptide that selectively binds to irradiated tumors. J Control Release. 2011;150:117–124. doi: 10.1016/j.jconrel.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guerrero Urbano MT, Nutting CM. Clinical use of intensity-modulated radiotherapy: Part I. Br J Radiol. 2004;77:88–96. doi: 10.1259/bjr/84246820. [DOI] [PubMed] [Google Scholar]
- 67.Kanasty R, Dorkin JR, Vegas A, et al. Delivery materials for siRNA therapeutics. Nat Mater. 2013;12:967–977. doi: 10.1038/nmat3765. [DOI] [PubMed] [Google Scholar]