Abstract
The development of molecular targeted therapeutics in oncology builds on many years of scientific investigation into the cellular mechanics of malignant transformation and progression. The past two decades have brought an accelerating pace to the clinical investigation of new molecular targeted agents, particularly in the setting of metastatic disease. The integration of molecular targeted agents into phase III clinical trial design has lagged in the curative treatment setting, particularly in combination with established therapeutic modalities such as radiation. In this review, we discuss the interaction of radiation and molecular targeted therapeutics. The dynamics of cellular and tumor response to radiation offer unique opportunities for beneficial interplay with molecular targeted agents that may go unrecognized with conventional screening and monotherapy clinical testing of novel agents. By using epidermal growth factor receptor (EGFR) as a primary example, we discuss recent clinical studies that illustrate the potential synergy of molecular targeted agents with radiation and highlight the clinical value of such interactions. For various molecular targeted agents, their greatest clinical impact may rest in combination with radiation, and efforts to facilitate systematic investigation of this approach appear highly warranted.
INTRODUCTION
Approximately 60% of all patients with cancer receive radiation therapy at some point during their treatment course. Radiation has been used as a cancer treatment modality for more than 100 years and continues to be a central component of curative and palliative treatment regimens. With the advancement of cytotoxic chemotherapy over the past 50 years, various approaches to combining radiation and chemotherapy have been explored. The curative potential of such combinations is highlighted by improvements in tumor response rates and survival with the use of combined chemoradiotherapy therapy across a range of malignancies.1
The interaction of radiation and chemotherapy was prominently described in the 1970s by George Steel, who postulated four mechanisms by which combined modality treatment could improve clinical outcomes.2 The theme of independent toxicities was particularly critical to Steel's conceptualization because combined treatments with incompletely overlapping adverse effects allowed for improved disease control without prohibitive toxicity and thereby a greater therapeutic window than single modality dose escalation. Despite clear successes, the reality of chemoradiotherapy in many clinical contexts is a modest improvement in clinical outcome accompanied by an increased toxicity profile. The limited specificity of most conventional chemotherapy agents commonly results in not only enhanced tumor response but also increased normal tissue toxicity when combined with radiation.
In various cancer settings, improvement in conformal targeting of radiation along with dose escalation holds potential for improving local disease control. Studies of stereotactic ablative body radiotherapy, in particular, suggest improved clinical outcomes compared with those previously seen with the combination of conventional radiation and chemotherapy.3 Nevertheless, the physical targeting and shaping of radiation with modern techniques is approaching a technological plateau in many instances, limited by unavoidable anatomic constraints. Although ablative stereotactic treatments may continue to gain traction in early-stage and oligometastatic disease, in which targets are generally smaller and often better defined, it is unlikely that further advances in physical targeting and fractionation alone will result in marked improvements in survival among patients with locally advanced disease. Here, the tumor-bearing targets are generally larger and include adjacent regions of gross or occult lymph node spread, obliging exposure of larger volumes of normal tissue to high-dose radiation. Furthermore, the risk of progression to metastatic disease is heightened in these settings, raising logical consideration for combinations of locoregional and systemic treatments. The development of molecular targeted therapeutics presents a renewed opportunity to exploit the beneficial cooperative effects of combined modality treatment.
Among the most prominent advances in oncology over the last 20 years has been the development of molecular targeted therapeutics. Although initially defined by the US Food and Drug Administration (FDA) as an agent approved together with a prerequisite diagnostic molecular test, molecular targeted therapies are more broadly defined by their specificity to aberrant cellular processes or molecular characteristics of the tumors they are designed to treat. In this context, molecular targeted agents include antibodies and small molecules that are intended to target a well-defined molecule or pathway resulting in tumor inhibition or destruction. Although cytotoxic chemotherapeutics do commonly target important molecules (for example interfering with some aspect of DNA function), molecular targeted agents modify specific molecular and cellular functions critical to tumor cell progression rather than the generic processes of cell division. Because of this specificity, molecular targeted agents may cause a toxicity profile independent from that of radiotherapy and may therefore facilitate cooperative effects without undue toxicity. In this way, these agents may better fulfill the promise of the Steel hypothesis for expansion of the therapeutic window through combined modality treatment.
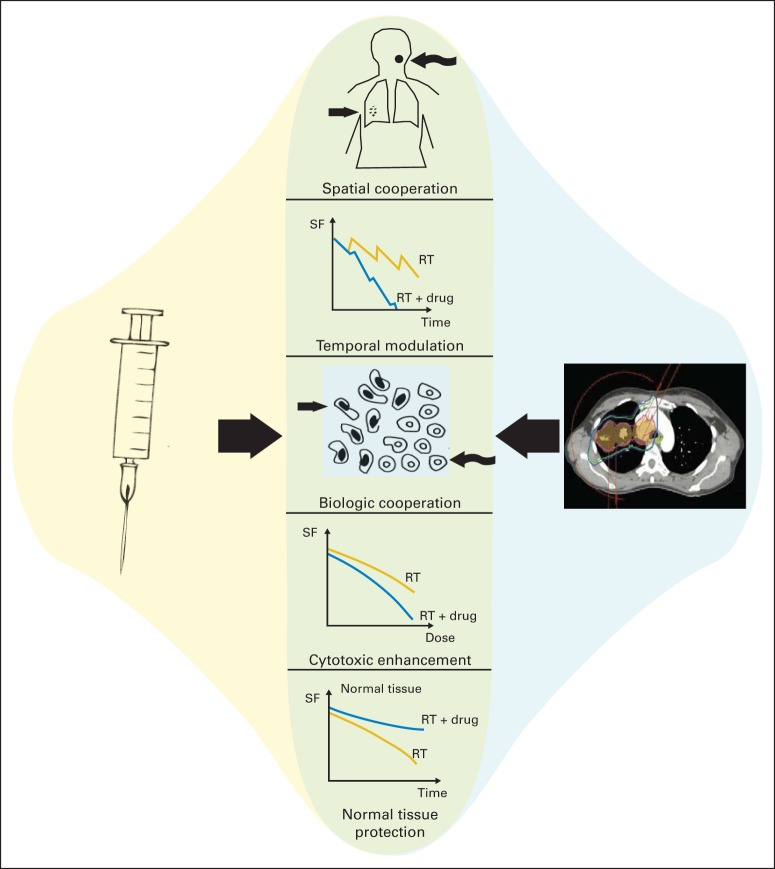
The specificity and diversity of contemporary molecular targeted drugs was not fully imagined in the 1970s, and a modernization of the Steel hypothesis has been proposed to afford continued utility in describing the exploitable interactions of radiation and cancer drugs.4 Under this revised framework, radiation and molecular targeted agents may interact to improve clinical outcomes by five distinct mechanisms: (1) spatial cooperation, (2) temporal modulation, (3) biologic cooperation, (4) cytotoxic enhancement, and (5) normal tissue protection (Fig 1).
Fig 1.
Schematic illustration of a modernized Steel hypothesis. The interaction of radiation and molecular targeted therapeutics can take several forms and may be exploited to improve clinical outcomes in the treatment of malignancy. Originally described by Steel in the 1970s, the growing complexity of such interactions prompts revision of this original framework. The potentially exploitable interactions of radiation and molecularly targeted therapeutics include spatial cooperation, temporal modulation, biologic cooperation, cytotoxic enhancement, and normal tissue protection. RT, radiation therapy; SF, surviving fraction of cells. Adapted with permission.4
Because radiation is a locoregional therapy, concurrent or sequential administration with systemic agents may elicit spatial cooperation by separately addressing the distinct risks of locoregional and distant disease. To limit normal tissue toxicities, radiation is typically administered in a fractionated regimen. Between each fraction of radiation therapy, cells may undergo DNA damage repair, repopulation, reoxygenation, and cell cycle redistribution. Various molecular targeted agents may interfere with these processes and alter the relationship of tumor cell killing and dose fractionation, thereby eliciting temporal modulation. Conversely, molecular targeted agents may elicit biologic cooperation with radiation by effectively killing a population of cells that would otherwise survive radiation. Such may be the case for drugs that target angiogenesis and modulate the hypoxic microenvironment that might otherwise confer relative resistance to radiation. Chemotherapeutics may also interact with radiation by modulating the induction, repair, or response to DNA damage in a tissue-specific manner and thereby confer either cytotoxic enhancement of tumor cell killing or normal tissue protection. Importantly, a given molecular targeted agent may simultaneously interact with radiation through more than one of these mechanisms.
The revised Steel hypothesis provides an essential framework for conceptualizing the interaction of radiation and molecularly targeted therapeutics. In this article, we review this interaction through a discussion of illustrative preclinical and clinical studies that investigate the combination of radiation and molecular targeted agents. For the purpose of this review, we focus on antibody and small molecule therapeutic platforms, with hormonal and drug-conjugated agents being beyond the scope of consideration.
FROM BENCH TO BEDSIDE: COMBINATION OF MOLECULAR TARGETED AGENTS AND RADIOTHERAPY
During the early twentieth century, Paul Ehrlich conceptualized molecular targeted therapeutics when he postulated the existence of selective receptors on microorganisms that could be targeted by organic molecules for therapeutic effect. A half century later, the earliest broad-spectrum cytotoxic chemotherapies, including nitrogen mustard and aminopterin, were pursued with the intent of targeting molecules such as DNA or the pathway of folic acid synthesis.5 However, early examples of what would now be considered molecular targeted therapeutics were not formally developed until the introduction of monoclonal antibodies as a therapeutic platform by Levy et al in 1981.6 This targeting strategy rapidly expanded with the development of antibodies targeting cell surface receptors critical to signal transduction pathways such as EGFR and human epidermal growth factor receptor 2 (HER2). A further platform of targeted therapeutics was established in the 1990s with the development of small molecule inhibitors of specific or multiple kinases, tumor-specific fusion proteins, and various other proteins critical to tumor cell survival. During the last 20 years, the number of promising molecular targets and molecular targeted agents has rapidly expanded, reflecting a tremendous public and private investment in the advancement of cancer research.
The current era of molecular targeting in oncology has followed from the early clinical success of the anti-HER2 antibody trastuzumab and the small molecule inhibitor of the BCR-ABL fusion protein imatinib. Subsequent clinical studies have demonstrated therapeutic efficacy for several additional agents, including antibodies targeting EGFR-family receptors, vascular endothelial growth factor, markers of immune cell lineages, and receptors regulating immune cell activation. Small molecule inhibitors of histone deacetylases, proteasomes, specific kinase domains, and other distinct signaling pathways have also entered clinical practice. In many cases, preclinical evidence suggests promising clinical opportunity for the combination of these agents with radiotherapy. This promise is perhaps best illustrated by the combination of radiation and antibodies targeting EGFR.
EGFR was first identified in the early 1980s as a viable molecular target for functional inhibition with a monoclonal antibody by Sato and Mendelsohn.7 After extensive preclinical validation, early-phase clinical studies demonstrated safety and disease response from inhibition of EGFR with the human-mouse chimeric anti-EGFR antibody cetuximab. Following phase III study, this agent was approved for clinical use in advanced colorectal cancer in 2004.8 Yet preclinical studies from the late 1990s also suggested the potential for strong therapeutic efficacy through the combination of cetuximab and radiation.9–11 These studies indicated that inhibition of EGFR signaling could modulate cellular sensitivity to radiation and enhance tumor cell response to radiation in vitro and in animal model systems. The mechanisms underlying this cooperation appeared to involve effects on cell cycle distribution, attenuated DNA damage response, inhibition of accelerated repopulation, and enhancement of radiation-induced apoptosis. These preclinical studies established a foundation for the formal clinical investigation of the combination of radiation and cetuximab, culminating in the 2006 FDA approval of cetuximab for treatment of head and neck squamous cell cancer (HNSCC) in combination with radiation.
CLINICAL INVESTIGATION OF MOLECULAR TARGETED AGENTS IN COMBINATION WITH RADIATION THERAPY
Combination of Radiation and Molecular Agents Targeting EGFR-Family Receptors
The clinical development of cetuximab illustrates the potential benefits of combining radiation and a molecular targeted agent and demonstrates that such combinations may offer an absolute survival advantage to patients treated with curative intent. The initial FDA approval of cetuximab followed demonstration of improved median survival in patients with refractory metastatic colorectal cancer whose tumors expressed EGFR.8,12 Concurrent with these studies, early-phase clinical trials were initiated to explore the combination of radiation and cetuximab in patients with HNSCC.13 High rates of complete response in these early trials together with strong preclinical data prompted the design of a phase III study to evaluate the efficacy of combining radiation and cetuximab.14 Between 1999 and 2002, that study enrolled 424 patients with locally advanced HNSCC who were randomly assigned to curative-intent radiation or radiation plus weekly cetuximab. This trial demonstrated a near doubling of median survival and, most importantly, a durable 9.2% improvement in overall survival.15 The absolute survival benefit of cetuximab in this study may reflect its interaction with radiation, a finding that critically underscores the potential value to investigate other molecular targeted agents combined with radiation for clinical benefit.
Several interesting preliminary findings emerge from subset analyses of the radiation with or without cetuximab HNSCC trial that warrant comment. Of course, these represent post hoc, unplanned subset analyses and thus must be considered accordingly. Three radiation fractionation regimens were allowed in the trial (once daily, twice daily, and concomitant boost treatment schedules), and the benefit of cetuximab was most significant in patients receiving the concomitant boost fractionation schedule (56% of study patients). Radiotherapy fractionation schedules were highly institution-specific in this trial and may reflect an additional confounding variable. It remains unknown whether radiation fractionation may reflect a true biologic interaction with EGFR signaling or simply a subset finding. There are several other interesting subsets that showed improved outcome favoring the cetuximab arm including patients with oropharynx cancer as opposed to larynx and hypopharynx cancer, patients who developed grade 2 to 4 cetuximab rash, patients with higher Karnofsky performance scores of 90 to 100, male sex, younger age, US location for treatment, and so on. Although there has been considerable speculation that this favorable profile may reflect the demographic of patients likely associated with the human papillomavirus (HPV), data for p16 staining from archived specimens are only now being analyzed with results anticipated for presentation at the 50th Annual Meeting of the American Society of Clinical Oncology in 2014. What can be said at present is that this trial confirmed an absolute survival benefit for patients with HNSCC receiving radiation plus cetuximab better than that achieved with radiation alone.
Following the demonstration of a durable overall survival benefit from the combination of cetuximab and radiation, several additional studies have been advanced to further define the role of cetuximab in the treatment of patients with locally advanced HNSCC. The phase III Radiation Therapy Oncology Group (RTOG) 0522 study (Radiation Therapy and Cisplatin With or Without Cetuximab in Treating Patients With Stage III or Stage IV Head and Neck Cancer) evaluated the potential benefit of adding cetuximab to concurrent cisplatin chemoradiotherapy and demonstrated no improvement in progression-free or overall survival.16 This result suggests that, although the addition of cetuximab to radiation improves outcome in HNSCC, the addition of cetuximab to concurrent chemoradiotherapy with cisplatin does not provide additional benefit. The ongoing phase III RTOG 1016 trial (Radiation Therapy With Cisplatin or Cetuximab in Treating Patients With Oropharyngeal Cancer), with more than 800 patients enrolled to date, compares the use of radiation with either concurrent cetuximab or cisplatin in HPV-positive patients with locally advanced HNSCC. This important trial will provide new information about whether a molecular targeting agent can safely and effectively replace a cytotoxic agent in combination with radiation in the treatment of HPV-positive HNSCC. In the high-risk postoperative setting, the phase II/III RTOG 0920 trial (Radiation Therapy With or Without Cetuximab in Treating Patients Who Have Undergone Surgery for Locally Advanced Head and Neck Cancer) is evaluating adjuvant radiotherapy with or without cetuximab in patients following resection of HNSCC. The recently reported phase II RTOG 0234 study (Adjuvant Cetuximab and Chemoradiotherapy Using Either Cisplatin or Docetaxel in Treating Patients With Resected Stage III or Stage IV Squamous Cell Carcinoma or Lymphoepithelioma of the Head and Neck) demonstrates safety and feasibility for combining cetuximab with either cisplatin or docetaxel in the high-risk postoperative setting (Harari et al, manuscript submitted for publication). This study provides rationale for the recently initiated RTOG 1216 phase III trial (Radiation Therapy With Cisplatin, Docetaxel, or Cetuximab After Surgery in Treating Patients With Stage III-IV Squamous Cell Head and Neck Cancer), which evaluates the efficacy of adjuvant chemoradiotherapy with cisplatin, docetaxel, or docetaxel and cetuximab in the high-risk postoperative setting. Although phase III clinical trials illustrate the potential for improved survival with a combination of cetuximab and radiation in patients with HNSCC, clinical investigations combining radiation and various molecular targeted agents continue to emerge for other disease sites.
The combination of radiation and cetuximab has been actively investigated in non–small-cell lung cancer (NSCLC), and several phase I/II trials demonstrate the safety of combined cetuximab and thoracic radiation or chemoradiotherapy.17 These studies provide a basis for evaluating the addition of cetuximab to chemoradiotherapy in locally advanced NSCLC in the ongoing phase III RTOG 0617 study (High-Dose or Standard-Dose Radiation Therapy and Chemotherapy With or Without Cetuximab in Treating Patients With Newly Diagnosed Stage III Non-Small Cell Lung Cancer That Cannot Be Removed by Surgery). Additional antibodies targeting EGFR are also incorporated in early-phase clinical studies in combination with radiation, including the fully human anti-EGFR antibody panitumumab and the humanized immunoglobulin G1 anti-EGFR antibody nimotuzumab. In addition, small molecule inhibitors of EGFR tyrosine kinase activity are the subject of early-phase clinical investigation in combination with thoracic radiation, yet these studies do not consistently suggest efficacy and raise concern for increased rates of severe pneumonitis.18,19
In esophageal cancer, early-phase studies demonstrated safety with the combination of radiation and cetuximab thus leading to the phase III RTOG 0436 trial (Paclitaxel, Cisplatin, and Radiation Therapy With or Without Cetuximab in Treating Patients With Locally Advanced Esophageal Cancer), which randomly assigned patients to neoadjuvant cisplatin, paclitaxel, and radiation with or without cetuximab. At interim analysis in 2012, this study failed to meet a prespecified end point of improved clinical disease response and was therefore closed to further enrollment.20 The ongoing phase III SCOPE trial (Cisplatin, Capecitabine, and Radiation Therapy With or Without Cetuximab in Treating Patients With Esophageal Cancer) in the United Kingdom similarly evaluates the potential benefit of adding cetuximab to neoadjuvant chemoradiotherapy in patients with locally advanced esophageal cancer. Enthusiasm for further investigation of EGFR inhibitors in esophageal cancer has been tempered, however, by results of the REAL 3 study (REAL 3: A Randomised Open-labelled Multicentre Trial of the Efficacy of Epirubicin, Oxaliplatin and Capecitabine [EOX] With or Without Panitumumab in Previously Untreated Advanced Oesophago-gastric Cancer), which suggested inferior survival for patients with metastatic esophageal cancer receiving panitumumab when combined with epirubicin, oxaliplatin, and capecitabine.21 In rectal cancer, the phase II EXPERT-C trial (Oxaliplatin, Capecitabine, and Radiation Therapy With or Without Cetuximab in Treating Patients Undergoing Surgery for High-Risk Rectal Cancer [EXPERT-C]) examined the effect of adding cetuximab to induction chemotherapy and concurrent chemoradiotherapy with capecitabine and oxaliplatin. In patients with wild-type KRAS/BRAF, this study indicated that cetuximab improves rates of radiographic response and overall survival, although it did not meet its primary end point of improvement in rates of complete response.22 EGFR inhibitors have also been studied with radiation in phase I/II trials for patients with high-grade glioma, and those studies have generally demonstrated safety but thus far have not clearly shown improved efficacy.23
Combinations of radiation and inhibitors of other EGFR-family receptors have been investigated in various clinical settings. Despite widespread use of both trastuzumab and radiation in HER2-positive breast cancer, the combination of these two has undergone only limited study in the context of clinical trials. Early phase II data from a multicenter French study suggested the potential for cardiac toxicity with concurrent administration of trastuzumab and radiation,24 although a subsequent phase II study did not reproduce such toxicity and indicated potential for radiosensitization.25 The phase III National Surgical Adjuvant Breast and Bowel Project B-43 (NSABP-B-43) study (Radiation Therapy With or Without Trastuzumab in Treating Women With Ductal Carcinoma In Situ Who Have Undergone Lumpectomy) is currently enrolling patients with HER2-positive ductal carcinoma in situ to examine the effect of adding trastuzumab to adjuvant radiation. In the setting of esophageal cancer, trastuzumab has also been evaluated in early-phase clinical trials that demonstrated safety and promising efficacy.20 The ongoing phase III investigation RTOG 1010 (Radiation Therapy, Paclitaxel, and Carboplatin With or Without Trastuzumab in Treating Patients With Esophageal Cancer) evaluates the potential benefit of adding trastuzumab to carboplatin and paclitaxel in concurrent neoadjuvant radiation for locally advanced HER2-positive esophageal or gastroesophageal junction tumors. In addition, the recently reported RTOG 0524 study (Paclitaxel and Radiation Therapy With or Without Trastuzumab in Treating Patients Who Have Undergone Surgery for Bladder Cancer) showed encouraging response rates in patients with HER2-positive muscle-invasive bladder cancer who were treated with radiation, paclitaxel, and trastuzumab but also demonstrated increases in certain toxicities including marrow suppression.25a Clinical investigations of radiation together with additional agents targeting EGFR-family receptors are underway but are generally limited to early-phase studies.
Combination of Radiation and Molecular Agents Targeting Angiogenesis
The concept of targeting angiogenesis for therapeutic effect in cancer was initially conceived as a means of depriving tumors of oxygen and nutrients. However, subsequent preclinical studies demonstrated that inhibition of angiogenesis may result in normalization of tumor vasculature and enhanced perfusion in certain contexts.26 The potential role of antiangiogenic agents in enhancing tumor oxygenation makes them attractive candidates for combination with radiotherapy. The most mature antiangiogenesis agent, the anti–vascular endothelial growth factor antibody bevacizumab, received FDA approval in 2004 as a first-line treatment for metastatic colorectal cancer.27 Subsequent early-phase studies examined the potential for enhanced tumor response with the combination of radiation and bevacizumab in rectal, esophageal, head and neck, prostate, lung, and pancreatic cancers as well as high-grade glioma and soft tissue sarcoma.28 Early-phase studies examining the neoadjuvant treatment of rectal cancer with radiation and bevacizumab differed with respect to safety and efficacy.29 Reports on the use of bevacizumab and radiation in treatment of NSCLC also raised concern for increased esophageal toxicity,30,31 although no increase in toxicity was observed from a phase II study of bevacizumab plus chemoradiotherapy for locally advanced esophageal cancer.32 In the setting of high-risk prostate cancer treatment with radiation, long-term androgen suppression and bevacizumab resulted in heightened late toxicities compared with historical controls, although acute toxicity was not markedly affected.33 Conversely, treatment of locally advanced head and neck cancer with bevacizumab and twice daily radiation demonstrated poor efficacy in an early-phase study,34 although use with conventional chemoradiotherapy in a phase II study suggested promising safety and efficacy.35,36 In pancreatic cancer, an initial evaluation of bevacizumab plus radiation raised concern for potential duodenal toxicity,37 but a subsequent study did not report excess toxicity.38 A phase II trial of the combination of bevacizumab and cisplatin-based definitive chemoradiotherapy in locally advanced cervical cancer also indicated feasibility and safety.39 Finally, in high-grade glioma, multiple phase II studies similarly demonstrated safety and feasibility with the addition of bevacizumab to definitive radiation, although the phase III AVAglio (Phase 3 Trial of Bevacizumab Plus Temozolomide and Radiotherapy in Newly Diagnosed Glioblastoma Multiforme) and RTOG 0825 (Temozolomide and Radiation Therapy With or Without Bevacizumab in Treating Patients With Newly Diagnosed Glioblastoma) studies showed improvement in progression-free but not overall survival with such regimens.40,41
Combination of Radiation and Other Molecular Targeted Agents in Clinical Trials
Beyond the inhibitors of EGFR-family receptors and angiogenesis, relatively few molecular targeted agents have advanced to phase III clinical trials in combination with radiation (Table 1), although many such agents have been investigated in this capacity in early-phase studies. Notable among these is the histone deacetylase inhibitor vorinostat, which enhances radiosensitivity in preclinical models.42 Early-phase studies show safety for this agent together with radiation in head and neck and rectal cancers as well as high-grade glioma, and vorinostat is now under phase III study in combination with bevacizumab for pediatric high-grade glioma.42 In addition, early-phase trials have investigated the multikinase inhibitors sorafenib and sunitinib in various tumor types together with radiation, and the combination of sorafenib and stereotactic ablative radiation therapy is now in phase III study in hepatocellular carcinoma.43 Multiple additional early-phase trials were completed or are underway to examine the combination of radiation with mammalian target of rapamycin inhibitors, including temsirolimus and everolimus, the proteasome inhibitor bortezomib, and the B-rapidly accelerated fibrosarcoma (BRAF) inhibitor vemurafenib. Available reports from these studies have raised concerns for excess toxicity when combining radiation with temsirolimus or vemurafenib.44,45 Of note, preclinical studies suggest strong potential for synergy of radiation and immunotherapy, with early-phase clinical trials underway to evaluate the combination of radiation with molecular inhibitors of the coregulatory T-cell checkpoint receptors as well as other immunologic targets.46
Table 1.
Molecular Targeted Agents Currently Under Investigation in Phase III Studies in Combination With Radiation Therapy
| Drug | Target | Class of Inhibitor | Site | Notes |
|---|---|---|---|---|
| Bevacizumab | VEGF | Antibody | Newly diagnosed and recurrent high-grade gliomas | Two open trials, two closed to enrollment |
| Cetuximab | EGFR | Antibody | HNSCC, NSCLC, esophageal/gastroesophageal junction | Twenty-one trials in varied phases of enrollment, 17 in HNSCC |
| Endostatin | Angiogenesis | Peptide | Nasopharynx | With induction chemotherapy |
| Gefitinib | EGFR | Small molecule | NSCLC | One study of maintenance gefitinib after definitive chemoradiation, two involving whole-brain radiation |
| Lapatinib | EGFR and HER2 | Small molecule | HNSCC | Single trial in the high-risk adjuvant setting |
| Rituximab | CD20 | Antibody | Lymphoma | Twelve studies, three involving total body radiation |
| Sorafenib | Multiple kinases | Small molecule | Liver | In combination with stereotactic ablative body radiation |
| Trastuzumab | HER2 | Antibody | Breast, esophageal/gastroesophageal junction | Seven studies in varied phases of enrollment, six for breast cancer |
| Vorinostat | Histone deacetylase | Small molecule | Pediatric high-grade glioma | Single study, also incorporates bevacizumab |
| Monoclonal antibody 17-1A | Epcam | Antibody | Rectal | Study terminated, no results available |
Abbreviations: EGFR, epidermal growth factor receptor; HER2, human epidermal growth factor receptor 2; HNSCC, head and neck squamous cell cancer; VEGF, vascular endothelial growth factor.
FUTURE DIRECTIONS IN COMBINING RADIATION AND MOLECULAR TARGETED THERAPEUTICS
Although radiation is a critical treatment modality for a substantial proportion patients with cancer, there is a relative dearth of clinical trials that formally explore combinations of radiation and molecular targeted therapeutics. Through 2009, only 36 phase III studies had examined the use of radiation together with such agents.47 Most of these trials were conducted at single institutions and were initiated only after FDA approval of the molecular targeted agent. A search of currently registered clinical trials in the United States during the preparation of this review suggests that this disparity persists. Among current phase III trials for cancer, a total of 1,415 (28.1%) investigate an intervention with radiotherapy and 850 (16.9%) evaluate a molecular targeted therapeutic, yet only 46 (0.9%) examine a combination of these two interventions (Fig 2). This may reflect the predominant approach to contemporary cancer drug testing that emphasizes demonstration of efficacy in the metastatic setting before evaluation with standard treatments in the curative setting. Such a paradigm unintentionally deflects the investigation of molecular agents that may hold potential for clinical benefit in combined modality treatment regimens with radiation.
Fig 2.
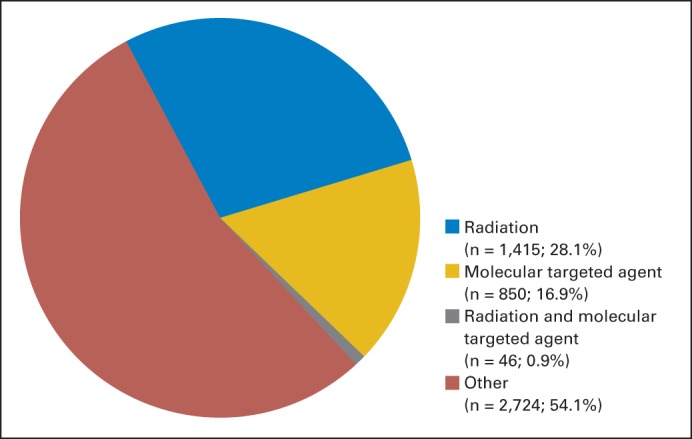
Distribution of current phase III clinical trials in oncology. A search of ClinicalTrials.gov for phase III clinical trials returned 5,035 trials for condition = “cancer.” When intervention = “radiation” was added to this search, 1,461 studies were identified. When the 5,035 phase III cancer trials were sorted by intervention, 896 studies involved a molecular targeted agent as defined in this review. Of these, only 46 studies examined a combination of a molecular targeted agent and radiation. Nine studies involving total-body irradiation were excluded from that category.
A powerful societal desire with respect to cancer is the development of treatments that offer potential for cure. By screening new drugs on the basis of monotherapy efficacy, we may fail to identify agents that ultimately provide their strongest clinical impact by exploiting basic principles of radiobiology (repair, repopulation, redistribution, reoxygenation). Consequently, contemporary clinical investigation of molecular targeted therapeutics may overlook agents that work most effectively in combination with radiation. Given the wide ranging utility of radiation, this approach could limit advances not only in the treatment of localized but also oligometastatic disease, in which radiation may assume an increasing role in combination with systemic therapies. Early evaluation of new molecular agents in combination with radiation may therefore prove valuable and serve the greater societal interest for the identification of cancer treatment approaches that improve overall survival.
Next-generation clinical trials investigating combinations of radiation with molecular targeted therapeutics should incorporate not only novel agents but also new disease sites. Radiation is a critical component of curative-intent treatments for various tumor types, including prostate, cervix, anorectal, bladder, head and neck, breast, lung, brain, pancreas, skin, and soft tissue sarcomas. The potential to improve survival by augmenting existing treatments for these disease sites represents an opportunity for clinical innovation with combinations of molecular targeted therapeutics and radiation. Challenges to such studies will include appropriate patient selection and definition of clinical end points as well as critical evaluation of cost-effectiveness. Rigorous assessment of toxicities will also be important, and these may be both unpredictable and predictive.15 Effective collaboration between academic and industry investigators will prove valuable in these efforts, as will the attraction of federal funding, which has historically lagged in radiation oncology.48 Ultimately, the success of personalized medicine will rely on demonstrable advances in cancer cure and palliation. In this context, there may be as yet unrecognized opportunities for significant advances from the combination of radiation with molecular targeted therapeutics.
Footnotes
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Administrative support: Paul M. Harari
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Kimple RJ. Strategizing the clone wars: Pharmacological control of cellular sensitivity to radiation. Mol Interv. 2010;10:341–353. doi: 10.1124/mi.10.6.3. [DOI] [PubMed] [Google Scholar]
- 2.Steel GG. Terminology in the description of drug-radiation interactions. Int J Radiat Oncol Biol Phys. 1979;5:1145–1150. doi: 10.1016/0360-3016(79)90634-5. [DOI] [PubMed] [Google Scholar]
- 3.Iyengar P, Timmerman RD. Stereotactic ablative radiotherapy for non-small cell lung cancer: Rationale and outcomes. J Natl Compr Canc Netw. 2012;10:1514–1520. doi: 10.6004/jnccn.2012.0157. [DOI] [PubMed] [Google Scholar]
- 4.Bentzen SM, Harari PM, Bernier J. Exploitable mechanisms for combining drugs with radiation: Concepts, achievements and future directions. Nat Clin Pract Oncol. 2007;4:172–180. doi: 10.1038/ncponc0744. [DOI] [PubMed] [Google Scholar]
- 5.Mendelsohn J. Personalizing oncology: Perspectives and prospects. J Clin Oncol. 2013;31:1904–1911. doi: 10.1200/JCO.2012.45.3605. [DOI] [PubMed] [Google Scholar]
- 6.Miller RA, Maloney DG, Warnke R, et al. Treatment of B-cell lymphoma with monoclonal anti-idiotype antibody. N Engl J Med. 1982;306:517–522. doi: 10.1056/NEJM198203043060906. [DOI] [PubMed] [Google Scholar]
- 7.Kawamoto T, Sato JD, Le A, et al. Growth stimulation of A431 cells by epidermal growth factor: Identification of high-affinity receptors for epidermal growth factor by an anti-receptor monoclonal antibody. Proc Natl Acad Sci U S A. 1983;80:1337–1341. doi: 10.1073/pnas.80.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 9.Huang SM, Bock JM, Harari PM. Epidermal growth factor receptor blockade with C225 modulates proliferation, apoptosis, and radiosensitivity in squamous cell carcinomas of the head and neck. Cancer Res. 1999;59:1935–1940. [PubMed] [Google Scholar]
- 10.Huang SM, Harari PM. Modulation of radiation response after epidermal growth factor receptor blockade in squamous cell carcinomas: Inhibition of damage repair, cell cycle kinetics, and tumor angiogenesis. Clin Cancer Res. 2000;6:2166–2174. [PubMed] [Google Scholar]
- 11.Saleh MN, Raisch KP, Stackhouse MA, et al. Combined modality therapy of A431 human epidermoid cancer using anti-EGFr antibody C225 and radiation. Cancer Biother Radiopharm. 1999;14:451–463. doi: 10.1089/cbr.1999.14.451. [DOI] [PubMed] [Google Scholar]
- 12.Jonker DJ, O'Callaghan CJ, Karapetis CS, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med. 2007;357:2040–2048. doi: 10.1056/NEJMoa071834. [DOI] [PubMed] [Google Scholar]
- 13.Robert F, Ezekiel MP, Spencer SA, et al. Phase I study of anti-epidermal growth factor receptor antibody cetuximab in combination with radiation therapy in patients with advanced head and neck cancer. J Clin Oncol. 2001;19:3234–3243. doi: 10.1200/JCO.2001.19.13.3234. [DOI] [PubMed] [Google Scholar]
- 14.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 15.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010;11:21–28. doi: 10.1016/S1470-2045(09)70311-0. [DOI] [PubMed] [Google Scholar]
- 16.Ang KK, Zhang QE, Rosenthal DI, et al. A randomized phase III trial (RTOG 0522) of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III-IV head and neck squamous cell carcinomas (HNC) J Clin Oncol. 2011;29(suppl):360s. doi: 10.1200/JCO.2013.53.5633. abstr 5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koh PK, Faivre-Finn C, Blackhall FH, et al. Targeted agents in non-small cell lung cancer (NSCLC): Clinical developments and rationale for the combination with thoracic radiotherapy. Cancer Treat Rev. 2012;38:626–640. doi: 10.1016/j.ctrv.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Okamoto I, Takahashi T, Okamoto H, et al. Single-agent gefitinib with concurrent radiotherapy for locally advanced non-small cell lung cancer harboring mutations of the epidermal growth factor receptor. Lung Cancer. 2011;72:199–204. doi: 10.1016/j.lungcan.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 19.Wan J, Cohen V, Agulnik J, et al. Unexpected high lung toxicity from radiation pneumonitis in a phase I/II trial of concurrent erlotinib with limited field radiation for intermediate prognosis patients with stage III or inoperable stage IIB non-small-cell lung cancer (NSCLC) Int J Radiat Oncol Biol Phys. 2009;75:S110. [Google Scholar]
- 20.Hong TS, Wo JY, Kwak EL. Targeted therapies with chemoradiation in esophageal cancer: Development and future directions. Semin Radiat Oncol. 2013;23:31–37. doi: 10.1016/j.semradonc.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 21.Waddell TS, Chau I, Barbachano Y, et al. A randomized multicenter trial of epirubicin, oxaliplatin, and capecitabine (EOC) plus panitumumab in advanced esophagogastric cancer (REAL3) J Clin Oncol. 2012;30(suppl):239s. abstr LBA4000. [Google Scholar]
- 22.Dewdney A, Cunningham D, Tabernero J, et al. Multicenter randomized phase II clinical trial comparing neoadjuvant oxaliplatin, capecitabine, and preoperative radiotherapy with or without cetuximab followed by total mesorectal excision in patients with high-risk rectal cancer (EXPERT-C) J Clin Oncol. 2012;30:1620–1627. doi: 10.1200/JCO.2011.39.6036. [DOI] [PubMed] [Google Scholar]
- 23.Karpel-Massler G, Schmidt U, Unterberg A, et al. Therapeutic inhibition of the epidermal growth factor receptor in high-grade gliomas: Where do we stand? Mol Cancer Res. 2009;7:1000–1012. doi: 10.1158/1541-7786.MCR-08-0479. [DOI] [PubMed] [Google Scholar]
- 24.Belkacémi Y, Gligorov J, Ozsahin M, et al. Concurrent trastuzumab with adjuvant radiotherapy in HER2-positive breast cancer patients: Acute toxicity analyses from the French multicentric study. Ann Oncol. 2008;19:1110–1116. doi: 10.1093/annonc/mdn029. [DOI] [PubMed] [Google Scholar]
- 25.Horton JK, Halle J, Ferraro M, et al. Radiosensitization of chemotherapy-refractory, locally advanced or locally recurrent breast cancer with trastuzumab: A phase II trial. Int J Radiat Oncol Biol Phys. 2010;76:998–1004. doi: 10.1016/j.ijrobp.2009.03.027. [DOI] [PubMed] [Google Scholar]
- 25a.Michaelson MD, Hu C, Pham HT, et al. The initial report of RTOG 0524: Phase I/II trial of a combination of paclitaxel and trastuzumab with daily irradiation or paclitaxel alone with daily irradiation follwowing transurethral surgery for noncystectomy candidates with muscle-invasive bladder cancer. J Clin Oncol. 2014;(suppl 4):32. doi: 10.1016/j.ijrobp.2016.12.018. abstr LBA287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carmeliet P, Jain RK. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Rev Drug Discov. 2011;10:417–427. doi: 10.1038/nrd3455. [DOI] [PubMed] [Google Scholar]
- 27.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 28.Kleibeuker EA, Griffioen AW, Verheul HM, et al. Combining angiogenesis inhibition and radiotherapy: A double-edged sword. Drug Resist Updat. 2012;15:173–182. doi: 10.1016/j.drup.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 29.Wadlow RC, Ryan DP. The role of targeted agents in preoperative chemoradiation for rectal cancer. Cancer. 2010;116:3537–3548. doi: 10.1002/cncr.25155. [DOI] [PubMed] [Google Scholar]
- 30.Socinski MA, Stinchcombe TE, Moore DT, et al. Incorporating bevacizumab and erlotinib in the combined-modality treatment of stage III non-small-cell lung cancer: Results of a phase I/II trial. J Clin Oncol. 2012;30:3953–3959. doi: 10.1200/JCO.2012.41.9820. [DOI] [PubMed] [Google Scholar]
- 31.Spigel DR, Hainsworth JD, Yardley DA, et al. Tracheoesophageal fistula formation in patients with lung cancer treated with chemoradiation and bevacizumab. J Clin Oncol. 2010;28:43–48. doi: 10.1200/JCO.2009.24.7353. [DOI] [PubMed] [Google Scholar]
- 32.Bendell JC, Meluch A, Peyton J, et al. A phase II trial of preoperative concurrent chemotherapy/radiation therapy plus bevacizumab/erlotinib in the treatment of localized esophageal cancer. Clin Adv Hematol Oncol. 2012;10:430–437. [PubMed] [Google Scholar]
- 33.Vuky J, Pham HT, Warren S, et al. Phase II study of long-term androgen suppression with bevacizumab and intensity-modulated radiation therapy (IMRT) in high-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2012;82:e609–e615. doi: 10.1016/j.ijrobp.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 34.Salama JK, Haraf DJ, Stenson KM, et al. A randomized phase II study of 5-fluorouracil, hydroxyurea, and twice-daily radiotherapy compared with bevacizumab plus 5-fluorouracil, hydroxyurea, and twice-daily radiotherapy for intermediate-stage and T4N0-1 head and neck cancers. Ann Oncol. 2011;22:2304–2309. doi: 10.1093/annonc/mdq736. [DOI] [PubMed] [Google Scholar]
- 35.Fury MG, Lee NY, Sherman E, et al. A phase 2 study of bevacizumab with cisplatin plus intensity-modulated radiation therapy for stage III/IVB head and neck squamous cell cancer. Cancer. 2012;118:5008–5014. doi: 10.1002/cncr.27498. [DOI] [PubMed] [Google Scholar]
- 36.Lee NY, Zhang Q, Pfister DG, et al. Addition of bevacizumab to standard chemoradiation for locoregionally advanced nasopharyngeal carcinoma (RTOG 0615): A phase 2 multi-institutional trial. Lancet Oncol. 2012;13:172–180. doi: 10.1016/S1470-2045(11)70303-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crane CH, Winter K, Regine WF, et al. Phase II study of bevacizumab with concurrent capecitabine and radiation followed by maintenance gemcitabine and bevacizumab for locally advanced pancreatic cancer: Radiation Therapy Oncology Group RTOG 0411. J Clin Oncol. 2009;27:4096–4102. doi: 10.1200/JCO.2009.21.8529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Small W, Jr, Mulcahy MF, Rademaker A, et al. Phase II trial of full-dose gemcitabine and bevacizumab in combination with attenuated three-dimensional conformal radiotherapy in patients with localized pancreatic cancer. Int J Radiat Oncol Biol Phys. 2011;80:476–482. doi: 10.1016/j.ijrobp.2010.02.030. [DOI] [PubMed] [Google Scholar]
- 39.Schefter TE, Winter K, Kwon JS, et al. A phase II study of bevacizumab in combination with definitive radiotherapy and cisplatin chemotherapy in untreated patients with locally advanced cervical carcinoma: Preliminary results of RTOG 0417. Int J Radiat Oncol Biol Phys. 2012;83:1179–1184. doi: 10.1016/j.ijrobp.2011.10.060. [DOI] [PubMed] [Google Scholar]
- 40.Henriksson R, Bottomley A, Mason W, et al. Progression-free survival (PFS) and health-related quality of life (HRQoL) in AVAglio, a phase III study of bevacizumab (Bv), temozolomide (T), and radiotherapy (RT) in newly diagnosed glioblastoma (GBM) J Clin Oncol. 2013;(suppl 15s):31. abstr 2005. [Google Scholar]
- 41.Gilbert MR, Dignam J, Won M, et al. RTOG 0825: Phase III double-blind placebo-controlled trial evaluating bevacizumab (Bev) in patients (Pts) with newly diagnosed glioblastoma (GBM) J Clin Oncol. 2013;(suppl 15s):31. abstr 1. [Google Scholar]
- 42.Shabason JE, Tofilon PJ, Camphausen K. Grand rounds at the National Institutes of Health: HDAC inhibitors as radiation modifiers, from bench to clinic. J Cell Mol Med. 2011;15:2735–2744. doi: 10.1111/j.1582-4934.2011.01296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ibrahim N, Yu Y, Walsh WR, et al. Molecular targeted therapies for cancer: Sorafenib mono-therapy and its combination with other therapies (review) Oncol Rep. 2012;27:1303–1311. doi: 10.3892/or.2012.1675. [DOI] [PubMed] [Google Scholar]
- 44.Sarkaria JN, Galanis E, Wu W, et al. Combination of temsirolimus (CCI-779) with chemoradiation in newly diagnosed glioblastoma multiforme (GBM) (NCCTG trial N027D) is associated with increased infectious risks. Clin Cancer Res. 2010;16:5573–5580. doi: 10.1158/1078-0432.CCR-10-1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Satzger I, Degen A, Asper H, et al. Serious skin toxicity with the combination of BRAF inhibitors and radiotherapy. J Clin Oncol. 2013;31:e220–e222. doi: 10.1200/JCO.2012.44.4265. [DOI] [PubMed] [Google Scholar]
- 46.Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: A paradigm shift. J Natl Cancer Inst. 2013;105:256–265. doi: 10.1093/jnci/djs629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ataman OU, Sambrook SJ, Wilks C, et al. The clinical development of molecularly targeted agents in combination with radiation therapy: A pharmaceutical perspective. Int J Radiat Oncol Biol Phys. 2012;84:e447–e454. doi: 10.1016/j.ijrobp.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 48.Steinberg M, McBride WH, Vlashi E, et al. National Institutes of Health funding in radiation oncology: A snapshot. Int J Radiat Oncol Biol Phys. 2013;86:234–240. doi: 10.1016/j.ijrobp.2013.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]