Abstract
Advances in radiotherapy planning and delivery have been used to treat patients with limited metastatic disease. With these techniques, high rates of treated metastasis control and low toxicity have been reported. Some patients have long disease-free intervals after radiotherapy similar to those seen after surgical resection. Ongoing studies will determine the benefit of these irradiation techniques to treat limited metastases, identify appropriate candidates, and assist in integrating these treatments into management strategies for specific diseases.
INTRODUCTION
To render patients with metastatic disease permanently free of cancer is an eminently worthwhile but often elusive goal. Advances in systemic therapies have improved long-term survival and cured some patients with pediatric, germ cell, and hematologic malignancies. With the development of therapies targeted to specific molecular mutations, some long-term remissions are now seen for a variety of nonhematologic malignancies. However, for adult solid tumors, rare is the patient cured with systemic therapy alone.
Systemic therapy remains standard as the initial treatment for most patients with metastatic cancer, allowing for improved survival and quality of life compared with best supportive care. The utility of systemic therapy is traditionally predicated on two fundamental principles.1 First, the only way to deliver effective treatment to multiple metastases in many anatomic locations throughout the body is via the circulatory system, and second, microscopic metastases are present in nearly all patients with metastases, and systemic therapy is required to eradicate these deposits.
However, an alternative theory of the natural history of cancer, called the spectrum hypothesis,2 may explain vastly different outcomes among metastastic patients. An intermediate clinical state between widespread metastases and locoregionally confined malignancy, referred to as oligometastases, was proposed by Hellman and Weichselbaum3 based on the clinical behaviors of patients with metastatic cancer. In this intermediate state, it was hypothesized that treatment of all known macroscopic metastases may improve disease-free intervals and potentially survival, in addition to standard systemic therapy.
A growing body of evidence suggests that radical ablation with surgery or advanced radiotherapy is associated with improved outcomes for select patients with limited metastases. Although selectively using radiotherapy to eradicate metastases was described as early as the 1960s,4 this approach was historically not successful, given less effective systemic therapy, supportive care, and technology. In recent years, advances in imaging, patient immobilization, and radiation treatment planning and delivery have allowed for improved targeting precision and accuracy, expanding the indications for radiotherapy. The high rates of tumor control in patients treated with ablative radiotherapy for limited metastases have prompted widespread implementation of stereotactic body radiation therapy (SBRT) for patients with limited metastatic disease of the lung, liver, adrenal gland, and spine.5
Much work remains to determine the benefit of radical irradiation (and surgery) in oligometastatic patients. How to appropriately select patients, although critical to successful long-term outcomes, is unclear at the moment. Furthermore, the integration of newer radiotherapy techniques with cytotoxins and targeted systemic therapies remains a work in progress, as does the best way to leverage the nonoverlapping toxicities of radiotherapy and surgery to best benefit patients. Finally, continuous refinement of radiotherapy techniques is an ongoing process, potentially allowing for treatment of more patients with limited metastatic disease.
Do Patients With Limited Metastases Exist?
Although discussions of theories underlying the natural history of metastatic cancer are largely academic, a pertinent clinical question is, “Do patients with limited metastases exist?” with the corollary, “If so, how many of these patients have metastases limited in number and distribution with a slower natural history (ie, true oligometastatic disease)?” Among patients initially diagnosed with metastatic disease as well as those who experience progression from locoregionally confined disease with metastases after initial treatment, subsets with limited involvement have been observed. In a study of patients with newly diagnosed stage IV non–small-cell lung cancer (NSCLC) from the University of Chicago, 74% presented with metastases confined to one to two organs, and 50% had ≤ three metastatic sites in addition to the lung primary tumor.6 Long-term follow-up of more than 1,700 surgically managed patients with early-stage NSCLC found that of those experiencing progression with metastases, 33% had solitary metastases, with an additional 19% limited to two to three metastases.7 Similarly, in men with biochemical recurrence after locoregional therapy for prostate cancer who experienced progression to metastatic disease, 40.5% presented with ≤ five metastases on annual imaging.8 Limited metastatic presentation is also common in women in breast cancer therapeutic clinical trials. Recently reported phase II and III studies of modern systemic therapies including more than 2,500 patients with breast cancer showed that 43% to 77% had disease limited to ≤ two metastases.9–14 Similarly, landmark series of patients with colorectal cancer (CRC) demonstrated that 38% had disease limited to one metastatic site,15 and 55% to 85% had disease limited to two metastatic sites.16,17 Therefore, a significant proportion of patients present with limited metastatic disease in the most commonly seen cancers.
Do Patients With Limited Metastatic Disease Behave Differently Than Those With More Widespread Metastases?
For the oligometastatic state to be clinically relevant, an appreciable percentage of patients presenting with few sites of metastases should have a different clinical behavior than those with polymetastases. This has been shown for many different tumor types. Not only do patients with prostate cancer with ≤ five metastases have survival similar to those without metastases (73% and 36% at 5 and 10 years v 75% and 45% at 5 and 10 years, respectively), they have significantly better survival than those who develop more than five metastases (45% and 18% at 5 and 10 years, respectively; P = .02).8 Furthermore, patients with early-stage breast cancer who experience progression to less than five metastases have improved 5-year (59.6% v 11.6%) and median survival (107.7 v 22 months; P = .001) after progression compared with those with more than five.18 Finally, longer median survival is seen in patients with early-stage NSCLC with oligometastases compared with those with diffuse metastases (13 v 7 months; hazard ratio, 0.53; 95% CI, 0.41 to 0.69; P < .001).7 Those with limited metastases are often likely to experience progression in known sites of cancer rather than in new sites, as shown in two studies of patients with metastatic NSCLC.6,19 Furthermore, if progression does happen in new sites, those sites are often limited in number.20–22
It is possible that some measure of the improved survivorship of patients with oligometastatic disease is related to lead-time bias, whereby patients are being seen at an earlier point in the natural history of their cancer dissemination. Arguing against this explanation as the sole reason for higher rates of overall survival in patients with limited metastatic disease are preclinical and clinical observations of the multistep nature of the molecular process of cancer evolution within a host from initial to subsequently more and more aggressive genotypes.23–26 Therefore, these data suggest that patients with fewer metastases tend to behave less aggressively than those with more metastases at diagnosis, resulting in improved survival. When progression occurs, it often does so in known metastases or in few new metastases.
Has the Aggressive Treatment of Metastases Already Improved Outcomes?
Intensifying the treatment of metastatic disease (although not strictly oligometastatic disease) has improved patient outcomes in some randomized phase III studies. For example, patients with metastatic spinal cord compression undergoing immediate direct circumferential decompression of the spinal cord in addition to irradiation had not only improved ambulation and continence but also had improved survival compared with those receiving radiotherapy alone (126 v 100 days; P = .033).27 Furthermore, the use of surgery28 and radiosurgery29 in addition to whole-brain radiotherapy has been shown to improve survival for patients with limited brain metastases. Finally, irradiation of all known metastases in some pediatric malignancies has improved outcomes, such as whole-lung radiotherapy in Ewing's sarcoma, which improved 4-year event-free survival from 40% to 19% (P < .05).30
For adult patients with metastases confined in number and extent, it remains unknown to what extent resection and/or ablation of metastases have an impact on overall or progression-free survival. Aggressive resection of pulmonary,31 hepatic,32 adrenal,33 and intracranial34 metastases have been associated with long-term disease control for select patients. Additionally, analyses restricted to patients with breast cancer,35,36 CRC,37 and melanoma38 have shown improved outcomes for those who underwent metastasectomy versus those who did not.
Radical Irradiation for Oligometastases
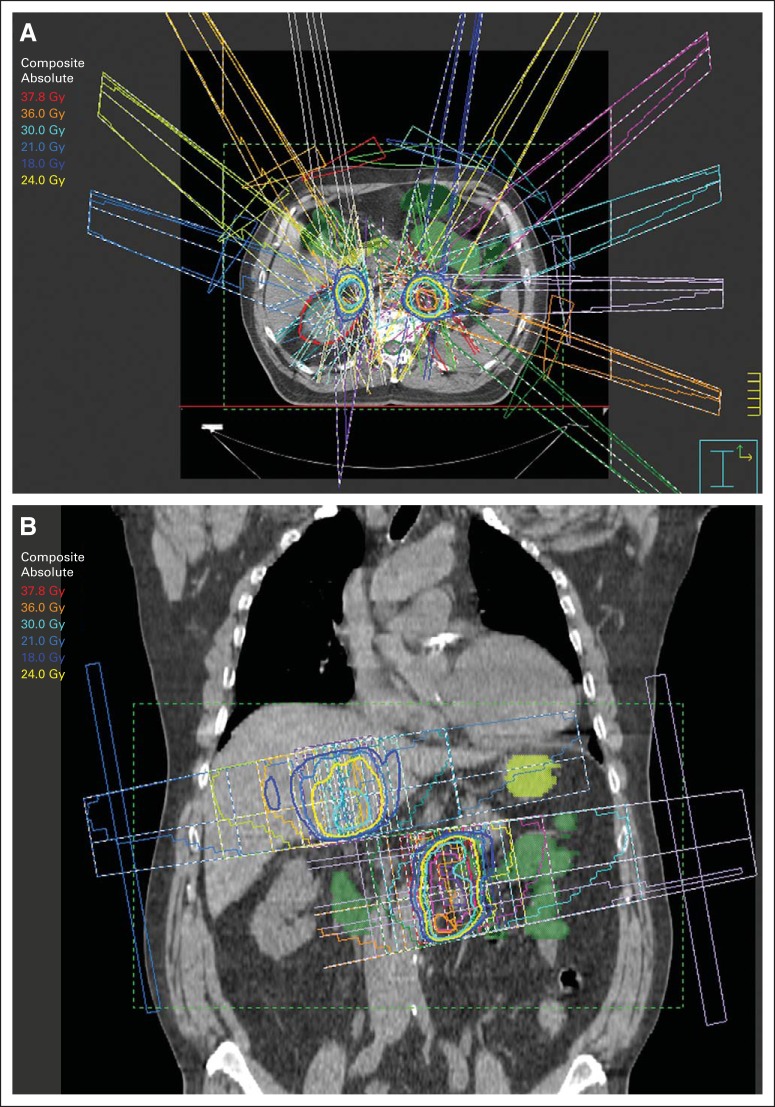
A single or few focal, precise, high doses of radiation have been associated with high rates of irradiated tumor control. The use of stereotactic technologies, developed decades ago as ablative treatment for intracranial targets, has been extrapolated to body radiotherapy. These techniques, termed SBRT or stereotactic ablative radiation (SABR), but perhaps best described as hypofractionated image-guided radiotherapy (HIGRT), have become widely studied for the treatment of limited metastases. The unifying principles of these treatments are precise patient positioning and immobilization, accurate targeting, respiratory motion analysis and management, high dose per treatment, and steep dose gradients between tumors and surrounding normal tissues (Fig 1).39 Various commercial systems are available that facilitate SBRT/SABR/HIGRT delivery. Although most outcomes reports involve the use of fractionation schedules of ≤ five treatments and apply the terms SBRT or SABR to describe the approach, many studies have reported highly favorable outcomes after slightly longer schedules of six to 10 fractions, still completing the entire course in a much shorter timeline than a conventional 6- to 8-week schedule of treatment.40,41
Fig 1.
Example of patient with limited metastatic disease in abdomen treated with stereotactic body radiation therapy to both metastases simultaneously. (A) Coronal and (B) axial slices show how radiotherapy planning needs to account for contributions of entrance and exit doses from one metastasis to another.
Appropriate treatment planning for radical irradiation of metastases usually includes minimal margin for microscopic spread beyond known radiographic or metabolic metastatic borders, although this may not apply to spinal metastases42 or some cases of lymph node involvement.43 The amount of respiratory-induced motion should be determined at time of radiation treatment planning. Additionally, techniques such as breath hold, respiratory gating, tumor tracking, or fiducial markers should be used to minimize margin for respiratory motion, and known organ tolerances should be respected.44,45
The optimal radiation fraction size and fraction number for oligometastatic tumor control and normal tissue tolerance are unknown for any given clinical situation and are likely affected by innumerable variables. When using a three-fraction regimen, doses > 54 Gy are associated with higher rates of tumor control for lung and liver metastases.46 However, when larger metastases are irradiated, a lower dose per fraction and higher number of fractions are likely warranted.47
Early SBRT reports focused on treating primary and metastatic tumors in specific organs as a means of implementing and refining treatment techniques. High treated metastasis control (TMC) rates (as opposed to local control, which inherently refers to primary tumors) were reported after SBRT for adrenal,48–54 hepatic,55–59 pulmonary,60–63 spinal,64–66 and abdominal metastases43,67–73 (Table 1). Most toxicity was low grade, although rare significant toxicity was seen in patients with hepatic failure79 or with tumors in close proximity to the spinal cord.80 Although often reported in these studies, heterogeneous patient populations, presence of untreated metastases, and different radiation dose or fractionation schedules made interpretation of survival data difficult.
Table 1.
Selected Studies of SBRT Used to Treat Metastases in Many Different Organs
| Study | Year | No. of Patients | Median Follow-Up (months) | Total Dose (Gy) | No. of Doses | TMC (%) | OS (%) |
|---|---|---|---|---|---|---|---|
| Pulmonary metastases | |||||||
| Chinese Academy of Medical Sciences (Beijing, China)63 | 2011 | 71 | 24.7 | 30-60 | 2-12 | 3 years: 75.4 | 3 years: 40.8 |
| University Hospital S. Giovanni Battista di Torino (Torino, Italy)62 | 2012 | 61 | 20.4 | 26-45 | 1-4 | 2 years: 89 | 2 years: 66.5 |
| Kyoto University Graduate School of Medicine (Kyoto, Japan)60 | 2008 | 34 | 27 | 48-60 | 4-5 | 2 years: 90 | 2 years: 84.3 |
| Multi-institutional (United States)74 | 2009 | 38 | 15.4 | 48-60 | 3 | 2 years: 96 | 2 years: 39 |
| University of Rochester (Rochester, NY)61 | 2006 | 30 | 18.7 | 50-55 | 10 | 3 years: 91 | 2 years: 38 |
| University of Heidelberg (Heidelberg, Germany)74a | 2002 | 61 | 14 | 12-30 | 1 | 1 year: 59 | 1 year: 78 |
| Hepatic metastases | |||||||
| University of Rochester (Rochester, NY)56 | 2007 | 69 | 50 | 10 | 1 year: 76 | 14 months: 50 | |
| University of Toronto Princess Margaret Hospital (Toronto, Ontario, Canada)57 | 2009 | 68 | 27.7-60* | 6 | 1 year: 71 | 18 months: 47 | |
| Multi-institutional phase I/II study (United States)58 | 2009 | 47 | 36-60 | 3 | 2 years: 92 | 2 years: 30 | |
| Multi-institutional pooled analysis (United States)55 | 2011 | 65 | 14.4 | 22-60; median, 42 | 1-6 | 1 year: 67 | 1 year: 72 |
| Multi-institutional pooled analysis (United States)59 | 2013 | 153 | Mean, 25.2 | Variable | Variable | 1 year: 62 | 1 year: 52 |
| Adrenal metastases | |||||||
| University of Rochester (Rochester, NY)53 | 2009 | 30 (14 with definitive RT) | 9.8 | 40 | 10 | 1 year: 55 | 1 year: 44 |
| Hokkaido University (Sapporo, Japan)51 | 2008 | 9 | 16 | 48 | 8 | 1 year: 100 | 1 year: 78 |
| University of Florence (Florence, Italy)50 | 2011 | 48 | 16 | 36 | 3 | 1 year: 90 | 1 year: 40 |
| University of Pittsburgh (Pittsburgh, PA)49 | 2011 | 7 | 14 | 16, one RT fraction; 27, three RT fractions | 1 or 3 | 1 year: 63 | NS |
| RWTH Aachen University (Aachen, Germany)52 | 2011 | 13 | 21 | 20-40 | 5 | 1 year: 77 | Median, 23 months |
| Humanitas Cancer Center (Milan, Italy)/University of Turin (Turin, Italy)54 | 2012 | 34 | 41 | 32 | 4 | 1 year: 66 | 2 years: 53 |
| University of Chicago (Chicago, IL)48 | 2013 | 10 | 15 | 24-50 | 3-10 | 1 year: 73 | 1 year: 90 |
| Spinal metastases | |||||||
| MD Anderson Cancer Center (Houston, TX)65 | 2012 | 149 | 16 | 27-30 | 3 | 1 year: 80.5 | 1 year: 72 |
| Memorial Sloan-Kettering Cancer Center (New York, NY)66 | 2008 | 93 | 18-24 | 1 | 1 year: 90 | ||
| University of Pittsburgh (Pittsburgh, PA)75 | 2007 | 393 | 21 | 12.5-25 | 1 | Crude, 88-90 | NS |
| University of California San Francisco (San Francisco, CA)76 | 2007 | 38 | 8.5 | 7-30 | 1-5 | 1 year: 85 | Median, 18 months |
| Henry Ford Hospital (Detroit, MI)77 | 2003 | 10 | 6 | 6-8* | 1 | NS | NS |
| Stanford University (Stanford, CA)78 | 2007 | 72 | 9 | 16-25 | 1-5 | NS | 1 year: 46 |
Abbreviations: NS, not stated; OS, overall survival; RT, radiotherapy; RWTH, Rheinisch-Westfaelische Technische Hochschule; SBRT, stereotactic body radiation therapy; TMC, treated metastasis control.
All treatments delivered after prior radiation therapy of 25 Gy in 10 fractions.
The next generation of studies restricted inclusion to limited metastases1–6 and required irradiation of all metastases20,41,81 (Table 2). Despite slightly different inclusion criteria and radiation doses, these studies had many important unifying findings. First, SBRT could be delivered simultaneously to multiple organs, with acceptable toxicity and promising TMC. Second, in each of these studies, there was a subset of patients rendered alive and free of disease (18% to 44%) with longer than 18-month follow-up. Those with fewer metastases and/or lower bulk of disease burden had better outcomes compared with those with more metastases. Finally, patterns of progression of these patients were most commonly in only a few locations. In fact, many patients (approximately 25% to 30%) were able to receive a second course of SBRT or other metastasis-directed therapy.21
Table 2.
Selected Studies of Oligometastatic Patients Treated With Irradiation of All Known Metastases
| Study | No. of Patients | No. of Metastases per Patient |
Dose (Gy) |
Follow-Up (months) |
Metastasis Control (%) | OS (%) | Toxicity Grade ≥ 3 (%) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Median | Range | Total | No. of Fractions | Median | Range | |||||
| Mt Sinai (New York, NY)82 | 21 | 1 | 1-5 | 40-60 | 10 | 10 | 2-18 | 1 year: 85 | 1 year: 75 | NA |
| University of Rochester (Rochester, NY)41 | 121 | 2 | 1-5 | 50 | 10 | 85 | 55-125* | 2 years: 67 | 4 years: 28 | 1† |
| University of Chicago (Chicago, IL)20 | 61 | 2 | 1-5 | 24-48 | 3 | 21 | 3-61 | 2 years: 53 | 2 years: 57 | 10† |
| Vrije University (Brussels, Belgium)83 | 309 | 2 | 1-5 | 40-50 | 10 | 12 | 1-84 | 2 years: 33 | 3 years: 32 | NS |
Abbreviations: NA, not applicable; NS, not stated; OS, overall survival.
Surviving patients with breast cancer.
Crude rate.
Many issues regarding appropriate dose-fractionation schedules for irradiation of multiple oligometastases are still unresolved, particularly for tumors abutting critical normal tissues (ie, centrally located thoracic structures, GI visceral organs, and/or spinal cord). Although reports of SBRT for head and neck reirradiation are emerging,84–87 there are few data about the use of SBRT for metastases to the head and neck region.20,88 Care should be taken, given the potential to cause brachial plexopathy.89 Although some radiotherapy schedules commonly used to treat oligometastases are safe to treat the mediastinum and central lung tumors,40,90 others are not.91 Volume design and dose selection for abdominal targets must take sensitivity of the stomach, small intestine, and liver into consideration.20,79,92,93 Finally, when using single fractions to treat spinal metastases, doses should be kept ≤ 20 Gy to avoid treatment-related vertebral body compression fracture.94
These challenges are magnified when metastases in are in close proximity to one another such that the radiation-dose contribution from multiple different targets results in a relatively large volume of high dose to the surrounding normal tissues. Multi-institutional cooperative group studies are using different approaches to satisfy these goals. In the ongoing SABR Comet study, a risk-adapted schema is being used.95 NRG BR001, however, will treat patients with two to four breast, NSCLC, or prostate metastases, with accepted doses determined by metastatic location. If toxicity occurs, doses will be de-escalated.
How Has Radical Irradiation Been Integrated Into the Treatment of Specific Diseases?
The next evolution of oligometastasis irradiation studies are those reporting the outcomes of patients with specific malignancies radically irradiated at all known metastases. Prospective phase II studies of patients with NSCLC96 and CRC93 have been completed, with other disease-specific investigations ongoing or planned. The remaining data from retrospective single-institution studies show similar trends, with high rates of TMC and patients rendered free of disease long after the completion of radiotherapy (Table 3). They also show differences in survival and disease-free survival based on varying inclusion criteria. Some have questioned if the promising outcomes are the result of underlying tumor biology that would have been present regardless of intervention in oligometastatic patients.111 The studies described here, as well as ongoing studies, will help to elucidate the utility of radical metastasis-directed intervention for these patients.
Table 3.
Selected Studies of Oligometastatic Patients With Specific Diseases Treated at All Known Metastases
| Study | No. of Patients | No. of Metastases per Patient |
Dose (Gy) |
Follow-Up (months) |
Metastasis Control (%) | OS (%) | Toxicity Grade ≥ 3 (%) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Median | Range | Total | No. of Fractions | Median | Range | |||||
| Colorectal cancer | ||||||||||
| Aarhus University (Aarhus, Denmark)93 | 64 | 2 | 1-6 | 45 | 3 | 52 | 2-76 | 2 years: 86 | 4 years: 13 | Crude, 55 |
| Erasmus University (Rotterdam, the Netherlands)97 | 20 | 1 | 1-3 | 37.5-45 | 3 | 26 | 6-57 | 2 years: 74 | 2 years: 83 | Crude, 10 |
| Multi-institution pooled analysis (United States/Canada)55 | 65 | 1 | 1-4 | 22-60 | 1-6 | 14 | 4-62 | 1 year: 67 | 1 year: 72 | Crude, 6 |
| Korea Cancer Center Hospital (Seoul, Korea)98 | 13 | 1 | 1-3 | 39-51 | 3 | 28 | 15-57 | 3 years: 53 | 3 years: 65 | 0 |
| NSCLC | ||||||||||
| University of Rochester (Rochester, NY)99 | 38 | 1-8 | 50-60 | 5-10 | 13.5 | 1-87 | NR | 5 years: 14 | NR | |
| University Medical Centre Maastricht (Maastricht, the Netherlands)96 | 1-5 | 3 years: 17.5 | ||||||||
| University of Chicago (Chicago, IL)100 | 25 | 2 | 1-5 | 24-50 | 3-10 | 14 | 1.5 years: 71 | 1.5 years: 53 | NR | |
| Rush University (Chicago, IL)101 | 1-2 | Variable | 17 | NR | 2 years: 40 | Crude, 17 | ||||
| Breast cancer | ||||||||||
| University of Rochester (Rochester, NY)102 | 40 | 2 | 1-4 | 40-60 | 10 | NR | 4 years: 89 | 4 years: 59 | NR | |
| Prostate cancer | ||||||||||
| Ludwig-Maximilian University (Munich, Germany)103 | 44 | 1 | 1-2 | 20 | 1 | 14 | 3-48 | 1 year: 96 | 1.5 years: 75 | 0 |
| University of Milan (Milan, Italy)104 | 19 | 1 | 1 | 33-36 | 3 | 17 | 3-35 | 100 | NR | 8 |
| University of Firenze (Firenze, Italy)105 | 25 | NS | 30 | 3 | 29 | 14-48 | 3 years: 90 | 3 years: 92 | 0 | |
| RCC | ||||||||||
| University of Chicago (Chicago, IL)106 | 18 | 2 | 1-7 | 24-48 and 50 | 3 and 10 | 21 | 2 years: 91 | 2 years: 85 | 0 | |
| University of Colorado (Aurora, CO)107 | 13 | 2 | 1-3 | 40-50 and 42-60 | 5 and 3 | 28 | 4-68 | 1.5 years: 88* | 1.5 year: 60† | Crude, 7 |
| Methodist Hospital (Houston, TX)108 | 14 | NS | 24-40 | 3-6 | 9 | Crude, 87 | NR | 0 | ||
| Karolinska Institutet (Karolinska, Sweden)109 | 50 | 1-4 | 32-45 | 4-5 | 37 | 7-80 | Crude, 90 | 2 years: 60† | Crude, 33† | |
| Melanoma | ||||||||||
| University of Colorado (Aurora, CO)107 | 17 | 2 | 1-3 | 40-50 and 42-60 | 5 and 3 | 28 | 4-68 | 1.5 years: 88* | 1.5 years: 60† | Crude, 7 |
| Sarcoma | ||||||||||
| University of Rochester (Rochester, NY)110 | 14 | 4 | 1-16 | 50 | 10 | 11 | 4-88 | 3 years: 82 | 2 years: 45† | 0 |
Abbreviations: NR, not reported; NSCLC, non–small-cell lung cancer; OS, overall survival; RCC, renal cell carcinoma.
Melanoma and RCC.
Approximate.
NSCLC
Reported series of patients with oligometastatic NSCLC continue to describe a proportion of patients who are disease free long after the completion of SBRT to all known metastases. Median survival in these series is either on par or only slightly worse than that of stage III NSCLC at 14 to 28 months, and 2-year survival in these series ranges from 14% to 38%.96,99,100,112 A recent systematic review of oligometastatic NSCLC including approximately 1,300 patients with a controlled primary tumor found that median progression-free survival was 12 months, and overall survival was approximately 19 months.113 Two randomized studies have been attempted, one trying to determine the role of SBRT integrated into a standard chemotherapy regimen (NCT00887315) and another testing the role of consolidative SBRT to remaining sites after chemotherapy (NCT00776100). Both of these studies had difficulties accruing and were closed. Given increasing interest, additional studies are ongoing (NCT01185639, NCT01725165) or being planned.
A developing role for radical irradiation is integrated into the course of therapy for patients with epidermal growth factor receptor (EGFR) +, anaplastic lymphoma kinase (ALK) +, and other oncogene-driven diseases. Recent studies have shown that 49% of patients treated with EGFR or ALK inhibitors experience progression in ≤ four metastases, characteristic of oligoprogressive disease, wherein patients receiving systemic therapy are maintaining clinical benefit in all but a few sites that manifest resistant clones. In this setting, ablation of all metastases via SBRT or other aggressive local therapy can eradicate metastases resistant to systemic therapy, allowing for continued delivery of a systemic agent providing clinical benefit elsewhere. In patients with primarily ALK-positive oligoprogressive NSCLC, the addition of ablative therapy to all metastases resulted in continued crizotinib administration for a median duration of 28 months compared with 10.8 months in those not receiving ablative metastasis-directed therapy.114,115 The single-arm phase II ATOM study (NCT01941654) is investigating if locally ablative therapy will improve 1-year progression-free survival in patients with a partial response to EGFR therapy and ≤ four positron emission tomography (PET) –avid sites of residual disease.
Extensive-Stage Small-Cell Lung Cancer
The irradiation of subclinical brain metastases, prophylactic cranial irradiation, improves survival for patients with extensive-stage small-cell lung cancer (ESCLC) responding to systemic therapy.116 Additionally, irradiation to the chest in patients with ESCLC with a complete response to initial chemotherapy improved 5-year survival (9%) versus those who received only further chemotherapy (3.7%; P = .041).117 For patients with ESCLC with more extensive metastases, a phase I/II study exploring the integration of hemi-body irradiation with standard systemic therapy had long-term survivors and promising 5-year overall survival of 16%,118 although this came at the price of significant toxicity. On the basis of these results, the Radiation Therapy Oncology Group (RTOG) is conducting a randomized trial (RTOG 0937) investigating if prophylactic cranial irradiation (25 Gy in 2.5-Gy fractions), thoracic radiotherapy, and metastasis-directed radiotherapy (45 Gy in 3-Gy fractions) can improve 1-year survival compared with standard of care.119
Metastatic CRC
Promising outcomes with high rates of treated tumor control and survival are seen after irradiation of CRC metastastases,93,98,120 which are similar to those seen fter surgical resection of hepatic,121–126 pulmonary,127 and other metastases.128 The largest prospective study to date was a phase II study of 64 patients with one to six CRC metastases treated with 45 Gy in three fractions.93 The vast majority of patients (94%) had metastases to one organ, most (69%) with liver metastases. Median survival was 19 months, and 2-year overall survival was 38%. Other large analyses have reported similar 5-year survival of 28% to 29%,129,130 despite including predominately nonhepatic, mostly lymph node metastases.
Studies assessing the role of SBRT in the overall management of oligometastatic CRC are ongoing. Most are investigating the integration of SBRT into the treatment of CRC hepatic metastases. These include the phase III RAS01 (Radiofrequency Ablation Versus Sterotactic Radiotherapy in Colorectal Liver Metastases) trial (NCT01233544), randomly assigning patients with one to four CRC liver metastases to either radiofrequency ablation or SBRT in the hope of determining which modality has the best local progression-free survival. Furthermore, in patients with limited hepatic CRC metastases, the integration of SBRT with systemic therapies including bevacizumab (NCT01569984) and low-dose irinotecan (NCT01847495) is ongoing.
The ORCHESTRA (A Randomized Multicenter Clinical Trial for Patients With Multi-Organ, Colorectal Cancer Metastases Comparing the Combination of Chemotherapy and Maximal Tumor Debulking Versus Chemotherapy Alone) study (NCT01792934) is looking to answer a more global question: Can ablative therapy to the majority of metastases improve overall survival in patients with CRC with multiorgan metastases. Patients with ≥ two CRC metastases and either more than three extrahepatic metastases, or ≥ one hepatic metastasis and positive para-aortal or celiac lymph nodes, or more than five hepatic metastases not limited to one lobe are being randomly assigned to maximal tumor debulking to at least 80% of known metastases with either SBRT, transarterial chemoembolization, surgery, radiofrequency ablation, or standard-of-care systemic therapy or systemic therapy alone.
Breast Cancer
The long natural history of some metastatic breast cancers, particularly those with bone-only metastases and those with hormonal responsive disease, as well as the radiosensitivity of breast cancer, may provide the ideal setting to demonstrate the utility of SBRT to prolong progression-free and overall survival when used to treat all known metastases. Many patients with oligometastatic breast cancer have been included in studies of irradiation of all known disease. When prospectively observed patients were analyzed, 4-year overall survival, progression-free survival, and TMC were 59%, 38%, and 89%, respectively. Single metastases (v two to five), smaller tumor volume, bone-only disease, and stable or regressing lesions before SBRT were associated with more favorable outcome.102
On the basis of these data, as well as promising data after surgical resection of all known breast cancer metastases,35 the NRG (National Surgical Adjuvant Breast and Bowel Project, RTOG, and Gynecologic Oncology Group) is developing a phase II/III study (NRG BR-002) randomly assigning patients with one to two breast cancer metastases to ablative therapy (either surgery or irradiation) plus standard-of-care systemic therapy or standard-of-care systemic therapy alone (with radiotherapy reserved for palliation). The primary end point for the phase II study is improved progression-free survival, which, if seen, will roll over into the phase III trial with survival as its primary end point.
Renal Cell Cancer, Melanoma, and Sarcoma
Although renal cell cancer (RCC), sarcoma, and melanoma are often considered radioresistant, outcomes after SBRT in these patients are similar to those in patients with traditionally radiosensitive histologies.107,110,131 This is likely because of the fact that these ablative doses of radiation act via different mechanisms than conventional radiotherapy, including endothelial cell damage132,133 and immune mediation.134 In patients with metastatic melanoma, recent studies have demonstrated that the cytotoxic T-lymphocyte antigen 4 (CTLA4) inhibitor ipilumumab and protein PD-1 inhibitors improve survival compared with standard chemotherapy.135,136 Studies combining radical irradiation of metastases with ipilumimab (NCT01565837,137 NCT01557114,138 NCT01497808,139 NCT01970527, and NCT01973608) and interleukin-2 (IL-2) are ongoing and will determine if any immune-mediated synergy exists regarding treated metastases.
Can Irradiation of Metastases Enhance Immunotherapy?
There is marked interest in combining SBRT and systemic immune modulators. The effects of ablative-dose radiotherapy have been shown to be mediated via CD8+ T cells57 as well as type I interferon-dependent innate and adaptive immunities.140 The potential for enhanced responses with exposure to immunomodulatory therapies has been seen with SBRT followed by high-dose IL-2 in patients with metastatic RCC and melanoma, which resulted in higher-than-expected response rates (71% v 16% with IL-2 monotherapy; P = .05).141 Studies are ongoing in melanoma (NCT01416831) and RCC (NCT01896271) assessing the utility of SBRT combined with IL-2 and ipilumumab (NCT01497808).
Reports of radiation-induced abscopal effects (ie, immune-mediated effects on nonirradiated metastases) after administration of chemotherapy or ipilumumab have generated much interest.142–145 Although preclinical work suggested that fractionated radiotherapy146 would better induce abscopal effects, they have also been seen clinically after single-dose radiotherapy.147 Response of untreated metastases after irradiation of melanoma metastases and administration of ipilumumab has been associated with antibodies to melanoma-associated antigen A3, PAS domain–containing protein 1, and the central portion and c-terminus of cancer/testis antigen 1 (also known as autoimmunogenic cancer/testis antigen NY-ESO-1).147 These immunologic responses might be attributable to enhanced antigen presentation within the tumor or stroma from radiotherapy.148
How Do We Combine Radical Irradiation of Extracranial Oligometastases With Systemic Therapy?
As metastasis-directed therapies are offered more often, practitioners wonder how to integrate them with standard systemic therapy regimens. It is unknown if these treatments should be delivered before systemic therapy, concurrently with systemic therapy, immediately after systemic therapy, or at time of progression if metastases are limited in number. It is likely that these decisions will have to be guided based on studies specific to the underlying disease. However, for nonselected oligometastatic patients, sunitinib 37.5 mg daily can be safely combined with 50 Gy in 10 fractions in patients with one to five metastases.81
How Commonly Is SBRT Being Used for the Treatment of Extracranial Oligometastses?
A recent international survey suggests that more than 60% of the more than 1,000 radiation oncologists who responded offer radical irradiation to their patients with limited metastatic disease.149 The most common reason for offering these treatments was durable TMC in nonsurgical patients, considered to be on par with intensity-modulated radiation therapy, another recent technologic improvement in radiotherapy planning and delivery. These treatments were commonly offered in both academic and community settings, 98% of the time at centers also offering surgical resection of metastases. Furthermore, the use of SBRT for oligometastases is going to increase; most of those already offering this treatment indicated plans to increase their treatment volume in the near future, and among those not offering the treatment, 59% planned to start in the next 3 years.
How Do We Observe Patients Treated With Radical Irradiation of Oligometastases?
For oligometastatic patients treated with SBRT to all known metastases, there is no accepted follow-up schedule. Although short follow-up for acute toxicity determination is routine, it is unclear when and how to image patients. Studies completed to date have varied, with some performing routine whole-body metabolic imaging and others performing only axial imaging of treated locations. Given the propensity of metastases to progress in limited number and the possibility to treat newly detected metastases with another course of SBRT, combined anatomic and metabolic imaging (PET–computed tomography [CT]) may be the best option. In addition, PET-CT has the ability to determine response in osseous metastases not typically considered measureable on CT imaging.150 Care should be taken when interpreting PET-CT; in one study, 38% of responses that initially appeared to be partial responses to initial therapy later proved to be complete responses.150 Liver lesions take approximately 5 months to reach lowest maximum standardized uptake value and may fluctuate upward before lowering again.151 Some have proposed a maximum standardized uptake value of 6 to determine hepatic treated metastasis progression.
How Should Patients Be Selected for Radical Irradiation of Oligometastases?
Patients with few metastases should be considered for aggressive radiotherapy if they have good performance status and all metastases can be safely targeted with radiotherapy. Those with one to two metastases have had more favorable outcomes with HIGRT,20 although those with more metastases can be considered for ablative radiotherapy. All patient cases should be discussed with a multidisciplinary team. In addition to number of metastases, primary tumor histology should be taken into consideration, because small-cell lung cancer and Ewing's tumors,20 nonadenocarcinomas,83 and lung, ovarian, and noncolorectal GI cancers58 have all been associated with worse outcomes after SBRT. Additionally, the timing of systemic therapy should be considered, because prior treatment with systemic therapy before SBRT has been associated with worse overall survival.74 However, upfront treatment with SBRT may make reliable measure of radiographic response to systemic therapy difficult.
Some have attempted to build prognostic models to aid in patient selection. In one of the largest analyses published to date, male sex, nonadenocarcinoma histology, presence of intracranial metastases, and synchronous presentation of metastases were all associated with poorer outcomes. Patients with zero to two risk factors had median survival > 23 months compared with those with three (9 months) or four risk factors (4 months).83 These factors likely contribute to underlying biologic processes that some have suggested are associated with specific microRNAs.152,153 All patients should be enrolled onto a clinical trial if they qualify.
Future Considerations
The promising early data on radiotherapeutic treatment of oligometastatic patients need to be validated in ongoing and planned randomized studies to determine the true benefit (if any) that radiotherapy offers and which subsets of patients are most likely to derive this benefit. So long as equipoise exists, there is an opportunity to develop and conduct meaningful studies. These studies will be challenging to develop, because the technology and prescription of radiotherapy (ie, dose fractionation and definition of target volumes) differ across institutions, and they will be challenging to conduct, because vigilant quality assurance is required with such complex radiotherapy techniques. Translational end points will be critical to identify prognostic and predictive markers of clinical outcome.
Footnotes
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Fisher B. Laboratory and clinical research in breast cancer: A personal adventure—The David A. Karnofsky memorial lecture. Cancer Res. 1980;40:3863–3874. [PubMed] [Google Scholar]
- 2.Hellman S. Karnofsky memorial lecture: Natural history of small breast cancers. J Clin Oncol. 1994;12:2229–2234. doi: 10.1200/JCO.1994.12.10.2229. [DOI] [PubMed] [Google Scholar]
- 3.Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol. 1995;13:8–10. doi: 10.1200/JCO.1995.13.1.8. [DOI] [PubMed] [Google Scholar]
- 4.Rubin P, Green J. Solitary Metastases. Springfield, IL: C.C. Thomas; 1968. [Google Scholar]
- 5.Alongi F, Arcangeli S, Filippi AR, et al. Review and uses of stereotactic body radiation therapy for oligometastases. Oncologist. 2012;17:1100–1107. doi: 10.1634/theoncologist.2012-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehta N, Mauer AM, Hellman S, et al. Analysis of further disease progression in metastatic non-small cell lung cancer: Implications for locoregional treatment. Int J Oncol. 2004;25:1677–1683. [PubMed] [Google Scholar]
- 7.Torok J, Kelsey CR, Salama JK. Patterns of distant metastases in surgically managed early stage non-small cell lung cancer. Presented at the 55th Annual Meeting of the American Society for Radiation Oncology; September 22-25, 2013; Atlanta, GA. [Google Scholar]
- 8.Singh D, Yi WS, Brasacchio RA, et al. Is there a favorable subset of patients with prostate cancer who develop oligometastases? Int J Radiat Oncol Biol Phys. 2004;58:3–10. doi: 10.1016/s0360-3016(03)01442-1. [DOI] [PubMed] [Google Scholar]
- 9.Albain KS, Nag SM, Calderillo-Ruiz G, et al. Gemcitabine plus paclitaxel versus paclitaxel monotherapy in patients with metastatic breast cancer and prior anthracycline treatment. J Clin Oncol. 2008;26:3950–3957. doi: 10.1200/JCO.2007.11.9362. [DOI] [PubMed] [Google Scholar]
- 10.Bergh J, Bondarenko IM, Lichinitser MR, et al. First-line treatment of advanced breast cancer with sunitinib in combination with docetaxel versus docetaxel alone: Results of a prospective, randomized phase III study. J Clin Oncol. 2012;30:921–929. doi: 10.1200/JCO.2011.35.7376. [DOI] [PubMed] [Google Scholar]
- 11.Tawfik H, Rostom Y, Elghazaly H. All-oral combination of vinorelbine and capecitabine as first-line treatment in HER2/Neu-negative metastatic breast cancer. Cancer Chemother Pharmacol. 2013;71:913–919. doi: 10.1007/s00280-013-2082-4. [DOI] [PubMed] [Google Scholar]
- 12.Hurvitz SA, Dirix L, Kocsis J, et al. Phase II randomized study of trastuzumab emtansine versus trastuzumab plus docetaxel in patients with human epidermal growth factor receptor 2–positive metastatic breast cancer. J Clin Oncol. 2013;31:1157–1163. doi: 10.1200/JCO.2012.44.9694. [DOI] [PubMed] [Google Scholar]
- 13.Gianni L, Romieu GH, Lichinitser M, et al. AVEREL: A randomized phase III Trial evaluating bevacizumab in combination with docetaxel and trastuzumab as first-line therapy for HER2-positive locally recurrent/metastatic breast cancer. J Clin Oncol. 2013;31:1719–1725. doi: 10.1200/JCO.2012.44.7912. [DOI] [PubMed] [Google Scholar]
- 14.Sledge GW, Neuberg D, Bernardo P, et al. Phase III trial of doxorubicin, paclitaxel, and the combination of doxorubicin and paclitaxel as front-line chemotherapy for metastatic breast cancer: An intergroup trial (E1193) J Clin Oncol. 2003;21:588–592. doi: 10.1200/JCO.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 15.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 16.Van Cutsem E, Köhne CH, Láng I, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: Updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29:2011–2019. doi: 10.1200/JCO.2010.33.5091. [DOI] [PubMed] [Google Scholar]
- 17.Douillard JY, Siena S, Cassidy J, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: The PRIME study. J Clin Oncol. 2010;28:4697–4705. doi: 10.1200/JCO.2009.27.4860. [DOI] [PubMed] [Google Scholar]
- 18.Dorn PL, Meriwether A, LeMieux M, et al. Patterns of distant failure and progression in breast cancer: Implications for the treatment of oligometastatic disease. Int J Radiat Oncol Biol Phys. 2011;81:S643–S643. [Google Scholar]
- 19.Rusthoven KE, Hammerman SF, Kavanagh BD, et al. Is there a role for consolidative stereotactic body radiation therapy following first-line systemic therapy for metastatic lung cancer? A patterns-of-failure analysis. Acta Oncol. 2009;48:578–583. doi: 10.1080/02841860802662722. [DOI] [PubMed] [Google Scholar]
- 20.Salama JK, Hasselle MD, Chmura SJ, et al. Stereotactic body radiotherapy for multisite extracranial oligometastases: Final report of a dose escalation trial in patients with 1 to 5 sites of metastatic disease. Cancer. 2012;118:2962–2970. doi: 10.1002/cncr.26611. [DOI] [PubMed] [Google Scholar]
- 21.Milano MT, Philip A, Okunieff P. Analysis of patients with oligometastases undergoing two or more curative-intent stereotactic radiotherapy courses. Int J Radiat Oncol Biol Phys. 2009;73:832–837. doi: 10.1016/j.ijrobp.2008.04.073. [DOI] [PubMed] [Google Scholar]
- 22.Milano MT, Katz AW, Okunieff P. Patterns of recurrence after curative-intent radiation for oligometastases confined to one organ. Am J Clin Oncol. 2010;33:157–163. doi: 10.1097/COC.0b013e3181979238. [DOI] [PubMed] [Google Scholar]
- 23.Yachida S, Jones S, Bozic I, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010;467:1114–1117. doi: 10.1038/nature09515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campbell PJ, Yachida S, Mudie LJ, et al. The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature. 2010;467:1109–1113. doi: 10.1038/nature09460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weichselbaum RR, Hellman S. Oligometastases revisited. Nat Rev Clin Oncol. 2011;8:378–382. doi: 10.1038/nrclinonc.2011.44. [DOI] [PubMed] [Google Scholar]
- 26.Gupta GP, Massagué J. Cancer metastasis: Building a framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: A randomised trial. Lancet. 2005;366:643–648. doi: 10.1016/S0140-6736(05)66954-1. [DOI] [PubMed] [Google Scholar]
- 28.Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: A randomized trial. JAMA. 1998;280:1485–1489. doi: 10.1001/jama.280.17.1485. [DOI] [PubMed] [Google Scholar]
- 29.Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: Phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363:1665–1672. doi: 10.1016/S0140-6736(04)16250-8. [DOI] [PubMed] [Google Scholar]
- 30.Paulussen M, Ahrens S, Burdach S, et al. Primary metastatic (stage IV) Ewing tumor: Survival analysis of 171 patients from the EICESS studies—European Intergroup Cooperative Ewing sarcoma studies. Ann Oncol. 1998;9:275–281. doi: 10.1023/a:1008208511815. [DOI] [PubMed] [Google Scholar]
- 31.Long-term results of lung metastasectomy. Prognostic analyses based on 5206 cases—The International Registry of Lung Metastases. J Thorac Cardiovasc Surg. 1997;113:37–49. doi: 10.1016/s0022-5223(97)70397-0. [DOI] [PubMed] [Google Scholar]
- 32.Fong Y, Cohen AM, Fortner JG, et al. Liver resection for colorectal metastases. J Clin Oncol. 1997;15:938–946. doi: 10.1200/JCO.1997.15.3.938. [DOI] [PubMed] [Google Scholar]
- 33.Tanvetyanon T, Robinson LA, Schell MJ, et al. Outcomes of adrenalectomy for isolated synchronous versus metachronous adrenal metastases in non–small-cell lung cancer: A systematic review and pooled analysis. J Clin Oncol. 2008;26:1142–1147. doi: 10.1200/JCO.2007.14.2091. [DOI] [PubMed] [Google Scholar]
- 34.Wroński M, Arbit E, Burt M, et al. Survival after surgical treatment of brain metastases from lung cancer: A follow-up study of 231 patients treated between 1976 and 1991. J Neurosurg. 1995;83:605–616. doi: 10.3171/jns.1995.83.4.0605. [DOI] [PubMed] [Google Scholar]
- 35.Pockaj BA, Wasif N, Dueck AC, et al. Metastasectomy and surgical resection of the primary tumor in patients with stage IV breast cancer: Time for a second look? Ann Surg Oncol. 2010;17:2419–2426. doi: 10.1245/s10434-010-1016-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yhim HY, Han SW, Oh DY, et al. Prognostic factors for recurrent breast cancer patients with an isolated, limited number of lung metastases and implications for pulmonary metastasectomy. Cancer. 2010;116:2890–2901. doi: 10.1002/cncr.25054. [DOI] [PubMed] [Google Scholar]
- 37.Ahmed S, Leis A, Fields A, et al. Survival impact of surgical resection of primary tumor in patients with stage IV colorectal cancer: Results from a large population-based cohort study. Cancer. 2014;120:683–691. doi: 10.1002/cncr.28464. [DOI] [PubMed] [Google Scholar]
- 38.Howard JH, Thompson JF, Mozzillo N, et al. Metastasectomy for distant metastatic melanoma: Analysis of data from the first Multicenter Selective Lymphadenectomy Trial (MSLT-I) Ann Surg Oncol. 2012;19:2547–2555. doi: 10.1245/s10434-012-2398-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kirkpatrick JP, Kelsey CR, Palta M, et al. Stereotactic body radiotherapy: A critical review for non-radiation oncologists. Cancer. 2014;120:942–954. doi: 10.1002/cncr.28515. [DOI] [PubMed] [Google Scholar]
- 40.Haasbeek CJ, Lagerwaard FJ, Slotman BJ, et al. Outcomes of stereotactic ablative radiotherapy for centrally located early-stage lung cancer. J Thorac Oncol. 2011;6:2036–2043. doi: 10.1097/JTO.0b013e31822e71d8. [DOI] [PubMed] [Google Scholar]
- 41.Milano MT, Katz AW, Zhang H, et al. Oligometastases treated with stereotactic body radiotherapy: Long-term follow-up of prospective study. Int J Radiat Oncol Biol Phys. 2012;83:878–886. doi: 10.1016/j.ijrobp.2011.08.036. [DOI] [PubMed] [Google Scholar]
- 42.Chawla S, Schell MC, Milano MT. Stereotactic body radiation for the spine: A review. Am J Clin Oncol. 2013;36:630–636. doi: 10.1097/COC.0b013e31822dfd71. [DOI] [PubMed] [Google Scholar]
- 43.Hasselle MD, Salama JK, Tye K, et al. Patterns of progression following hypofractionated image-guided radiotherapy (HIGRT) to abdominal lymph nodes in oligometastatic (OM) patients. Presented at the 52nd Annual Meeting of the American Society for Radiation Oncology; October 31-November 4, 2010; San Diego, CA. [Google Scholar]
- 44.Salama JK, Kirkpatrick JP, Yin FF. Stereotactic body radiotherapy treatment of extracranial metastases. Nat Rev Clin Oncol. 2012;9:654–665. doi: 10.1038/nrclinonc.2012.166. [DOI] [PubMed] [Google Scholar]
- 45.Milano MT, Constine LS, Okunieff P. Normal tissue toxicity after small field hypofractionated stereotactic body radiation. Radiat Oncol. 2008;3:36. doi: 10.1186/1748-717X-3-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McCammon R, Schefter TE, Gaspar LE, et al. Observation of a dose-control relationship for lung and liver tumors after stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2009;73:112–118. doi: 10.1016/j.ijrobp.2008.03.062. [DOI] [PubMed] [Google Scholar]
- 47.Corbin KS, Ranck MC, Hasselle MD, et al. Feasibility and toxicity of hypofractionated image-guided radiotherapy for large volume limited metastatic disease. Pract Radiat Oncol. 2013;3:316–322. doi: 10.1016/j.prro.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 48.Rudra S, Malik R, Ranck MC, et al. Stereotactic body radiation therapy for curative treatment of adrenal metastases. Technol Cancer Res Treat. 2013;12:217–224. doi: 10.7785/tcrt.2012.500320. [DOI] [PubMed] [Google Scholar]
- 49.Torok J, Wegner RE, Burton SA, et al. Stereotactic body radiation therapy for adrenal metastases: A retrospective review of a noninvasive therapeutic strategy. Future Oncol. 2011;7:145–151. doi: 10.2217/fon.10.165. [DOI] [PubMed] [Google Scholar]
- 50.Casamassima F, Livi L, Masciullo S, et al. Stereotactic radiotherapy for adrenal gland metastases: University of Florence experience. Int J Radiat Oncol Biol Phys. 2012;82:919–923. doi: 10.1016/j.ijrobp.2010.11.060. [DOI] [PubMed] [Google Scholar]
- 51.Katoh N, Onimaru R, Sakuhara Y, et al. Real-time tumor-tracking radiotherapy for adrenal tumors. Radiother Oncol. 2008;87:418–424. doi: 10.1016/j.radonc.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 52.Holy R, Piroth M, Pinkawa M, et al. Stereotactic body radiation therapy (SBRT) for treatment of adrenal gland metastases from non-small cell lung cancer. Strahlenther Onkol. 2011;187:245–251. doi: 10.1007/s00066-011-2192-z. [DOI] [PubMed] [Google Scholar]
- 53.Chawla S, Chen Y, Katz AW, et al. Stereotactic body radiotherapy for treatment of adrenal metastases. Int J Radiat Oncol Biol Phys. 2009;75:71–75. doi: 10.1016/j.ijrobp.2008.10.079. [DOI] [PubMed] [Google Scholar]
- 54.Scorsetti M, Alongi F, Filippi AR, et al. Long-term local control achieved after hypofractionated stereotactic body radiotherapy for adrenal gland metastases: A retrospective analysis of 34 patients. Acta Oncol. 2012;51:618–623. doi: 10.3109/0284186X.2011.652738. [DOI] [PubMed] [Google Scholar]
- 55.Chang DT, Swaminath A, Kozak M, et al. Stereotactic body radiotherapy for colorectal liver metastases: A pooled analysis. Cancer. 2011;117:4060–4069. doi: 10.1002/cncr.25997. [DOI] [PubMed] [Google Scholar]
- 56.Katz AW, Carey-Sampson M, Muhs AG, et al. Hypofractionated stereotactic body radiation therapy (SBRT) for limited hepatic metastases. Int J Radiat Oncol Biol Phys. 2007;67:793–798. doi: 10.1016/j.ijrobp.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 57.Lee MT, Kim JJ, Dinniwell R, et al. Phase I study of individualized stereotactic body radiotherapy of liver metastases. J Clin Oncol. 2009;27:1585–1591. doi: 10.1200/JCO.2008.20.0600. [DOI] [PubMed] [Google Scholar]
- 58.Rusthoven KE, Kavanagh BD, Cardenes H, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol. 2009;27:1572–1578. doi: 10.1200/JCO.2008.19.6329. [DOI] [PubMed] [Google Scholar]
- 59.Berber B, Ibarra R, Snyder L, et al. Multicentre results of stereotactic body radiotherapy for secondary liver tumours. HPB (Oxford) doi: 10.1111/hpb.12044. [epub ahead of print on January 14, 2013] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Norihisa Y, Nagata Y, Takayama K, et al. Stereotactic body radiotherapy for oligometastatic lung tumors. Int J Radiat Oncol Biol Phys. 2008;72:398–403. doi: 10.1016/j.ijrobp.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 61.Okunieff P, Petersen AL, Philip A, et al. Stereotactic body radiation therapy (SBRT) for lung metastases. Acta Oncol. 2006;45:808–817. doi: 10.1080/02841860600908954. [DOI] [PubMed] [Google Scholar]
- 62.Ricardi U, Filippi AR, Guarneri A, et al. Stereotactic body radiation therapy for lung metastases. Lung Cancer. 2012;75:77–81. doi: 10.1016/j.lungcan.2011.04.021. [DOI] [PubMed] [Google Scholar]
- 63.Zhang Y, Xiao JP, Zhang HZ, et al. Stereotactic body radiation therapy favors long-term overall survival in patients with lung metastases: Five-year experience of a single-institution. Chin Med J (Engl) 2011;124:4132–4137. [PubMed] [Google Scholar]
- 64.Nelson JW, Yoo DS, Sampson JH, et al. Stereotactic body radiotherapy for lesions of the spine and paraspinal regions. Int J Radiat Oncol Biol Phys. 2009;73:1369–1375. doi: 10.1016/j.ijrobp.2008.06.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang XS, Rhines LD, Shiu AS, et al. Stereotactic body radiation therapy for management of spinal metastases in patients without spinal cord compression: A phase 1-2 trial. Lancet Oncol. 2012;13:395–402. doi: 10.1016/S1470-2045(11)70384-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yamada Y, Bilsky MH, Lovelock DM, et al. High-dose, single-fraction image-guided intensity-modulated radiotherapy for metastatic spinal lesions. Int J Radiat Oncol Biol Phys. 2008;71:484–490. doi: 10.1016/j.ijrobp.2007.11.046. [DOI] [PubMed] [Google Scholar]
- 67.Almaghrabi MY, Supiot S, Paris F, et al. Stereotactic body radiation therapy for abdominal oligometastases: A biological and clinical review. Radiat Oncol. 2012;7:126. doi: 10.1186/1748-717X-7-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bae SH, Kim MS, Cho CK, et al. High dose stereotactic body radiotherapy using three fractions for colorectal oligometastases. J Surg Oncol. 2012;106:138–143. doi: 10.1002/jso.23058. [DOI] [PubMed] [Google Scholar]
- 69.Jereczek-Fossa BA, Fariselli L, Beltramo G, et al. Linac-based or robotic image-guided stereotactic radiotherapy for isolated lymph node recurrent prostate cancer. Radiother Oncol. 2009;93:14–17. doi: 10.1016/j.radonc.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 70.Bignardi M, Navarria P, Mancosu P, et al. Clinical outcome of hypofractionated stereotactic radiotherapy for abdominal lymph node metastases. Int J Radiat Oncol Biol Phys. 2011;81:831–838. doi: 10.1016/j.ijrobp.2010.05.032. [DOI] [PubMed] [Google Scholar]
- 71.Choi CW, Cho CK, Yoo SY, et al. Image-guided stereotactic body radiation therapy in patients with isolated para-aortic lymph node metastases from uterine cervical and corpus cancer. Int J Radiat Oncol Biol Phys. 2009;74:147–153. doi: 10.1016/j.ijrobp.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 72.Kim MS, Yoo SY, Cho CK, et al. Stereotactic body radiotherapy for isolated para-aortic lymph node recurrence after curative resection in gastric cancer. J Korean Med Sci. 2009;24:488–492. doi: 10.3346/jkms.2009.24.3.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim MS, Cho CK, Yang KM, et al. Stereotactic body radiotherapy for isolated paraaortic lymph node recurrence from colorectal cancer. World J Gastroenterol. 2009;15:6091–6095. doi: 10.3748/wjg.15.6091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rusthoven KE, Kavanagh BD, Burri SH, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for lung metastases. J Clin Oncol. 2009;27:1579–1584. doi: 10.1200/JCO.2008.19.6386. [DOI] [PubMed] [Google Scholar]
- 74a.Hof H, Hoess A, Oetzel D, et al. Stereotactic single-dose radiotherapy of lung metastases. Strahlenther Onkol. 2007;183:673–678. doi: 10.1007/s00066-007-1724-z. [DOI] [PubMed] [Google Scholar]
- 75.Gerszten PC, Burton SA, Ozhasoglu C, et al. Radiosurgery for spinal metastases: Clinical experience in 500 cases from a single institution. Spine (Phila Pa 1976) 2007;32:193–199. doi: 10.1097/01.brs.0000251863.76595.a2. [DOI] [PubMed] [Google Scholar]
- 76.Sahgal A, Larson DA, Chang EL. Stereotactic body radiosurgery for spinal metastases: A critical review. Int J Radiat Oncol Biol Phys. 2008;71:652–665. doi: 10.1016/j.ijrobp.2008.02.060. [DOI] [PubMed] [Google Scholar]
- 77.Ryu S, Fang Yin F, Rock J, et al. Image-guided and intensity-modulated radiosurgery for patients with spinal metastasis. Cancer. 2003;97:2013–2018. doi: 10.1002/cncr.11296. [DOI] [PubMed] [Google Scholar]
- 78.Gibbs IC, Kamnerdsupaphon P, Ryu MR, et al. Image-guided robotic radiosurgery for spinal metastases. Radiother Oncol. 2007;82:185–190. doi: 10.1016/j.radonc.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 79.Blomgren H, Lax I, Näslund I, et al. Stereotactic high dose fraction radiation therapy of extracranial tumors using an accelerator: Clinical experience of the first thirty-one patients. Acta Oncol. 1995;34:861–870. doi: 10.3109/02841869509127197. [DOI] [PubMed] [Google Scholar]
- 80.Sahgal A, Weinberg V, Ma L, et al. Probabilities of radiation myelopathy specific to stereotactic body radiation therapy to guide safe practice. Int J Radiat Oncol Biol Phys. 2013;85:341–347. doi: 10.1016/j.ijrobp.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 81.Tong CC, Ko EC, Sung MW, et al. Phase II trial of concurrent sunitinib and image-guided radiotherapy for oligometastases. PLoS One. 2012;7:e36979. doi: 10.1371/journal.pone.0036979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kao J, Packer S, Vu HL, et al. Phase 1 study of concurrent sunitinib and image-guided radiotherapy followed by maintenance sunitinib for patients with oligometastases: Acute toxicity and preliminary response. Cancer. 2009;115:3571–3580. doi: 10.1002/cncr.24412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.de Vin T, Engels B, Gevaert T, et al. Stereotactic radiotherapy for oligometastatic cancer: A prognostic model for survival. Ann Oncol. 2014;25:467–471. doi: 10.1093/annonc/mdt537. [DOI] [PubMed] [Google Scholar]
- 84.Cengiz M, Özyiğit G, Yazici G, et al. Salvage reirradiaton with stereotactic body radiotherapy for locally recurrent head-and-neck tumors. Int J Radiat Oncol Biol Phys. 2011;81:104–109. doi: 10.1016/j.ijrobp.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 85.Rwigema JC, Heron DE, Ferris RL, et al. Fractionated stereotactic body radiation therapy in the treatment of previously-irradiated recurrent head and neck carcinoma: Updated report of the University of Pittsburgh experience. Am J Clin Oncol. 2010;33:286–293. doi: 10.1097/COC.0b013e3181aacba5. [DOI] [PubMed] [Google Scholar]
- 86.Roh KW, Jang JS, Kim MS, et al. Fractionated stereotactic radiotherapy as reirradiation for locally recurrent head and neck cancer. Int J Radiat Oncol Biol Phys. 2009;74:1348–1355. doi: 10.1016/j.ijrobp.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 87.Unger KR, Lominska CE, Deeken JF, et al. Fractionated stereotactic radiosurgery for reirradiation of head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2010;77:1411–1419. doi: 10.1016/j.ijrobp.2009.06.070. [DOI] [PubMed] [Google Scholar]
- 88.Siddiqui F, Patel M, Khan M, et al. Stereotactic body radiation therapy for primary, recurrent, and metastatic tumors in the head-and-neck region. Int J Radiat Oncol Biol Phys. 2009;74:1047–1053. doi: 10.1016/j.ijrobp.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 89.Forquer JA, Fakiris AJ, Timmerman RD, et al. Brachial plexopathy from stereotactic body radiotherapy in early-stage NSCLC: Dose-limiting toxicity in apical tumor sites. Radiother Oncol. 2009;93:408–413. doi: 10.1016/j.radonc.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 90.Milano MT, Chen Y, Katz AW, et al. Central thoracic lesions treated with hypofractionated stereotactic body radiotherapy. Radiother Oncol. 2009;91:301–306. doi: 10.1016/j.radonc.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 91.Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006;24:4833–4839. doi: 10.1200/JCO.2006.07.5937. [DOI] [PubMed] [Google Scholar]
- 92.Chang DT, Schellenberg D, Shen J, et al. Stereotactic radiotherapy for unresectable adenocarcinoma of the pancreas. Cancer. 2009;115:665–672. doi: 10.1002/cncr.24059. [DOI] [PubMed] [Google Scholar]
- 93.Hoyer M, Roed H, Traberg Hansen A, et al. Phase II study on stereotactic body radiotherapy of colorectal metastases. Acta Oncol. 2006;45:823–830. doi: 10.1080/02841860600904854. [DOI] [PubMed] [Google Scholar]
- 94.Sahgal A, Atenafu EG, Chao S, et al. Vertebral compression fracture after spine stereotactic body radiotherapy: A multi-institutional analysis with a focus on radiation dose and the spinal instability neoplastic score. J Clin Oncol. 2013;31:3426–3431. doi: 10.1200/JCO.2013.50.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Palma DA, Haasbeek CJ, Rodrigues GB, et al. Stereotactic ablative radiotherapy for comprehensive treatment of oligometastatic tumors (SABR-COMET): Study protocol for a randomized phase II trial. BMC Cancer. 2012;12:305. doi: 10.1186/1471-2407-12-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.De Ruysscher D, Wanders R, van Baardwijk A, et al. Radical treatment of non-small-cell lung cancer patients with synchronous oligometastases: Long-term results of a prospective phase II trial (Nct01282450) J Thorac Oncol. 2012;7:1547–1555. doi: 10.1097/JTO.0b013e318262caf6. [DOI] [PubMed] [Google Scholar]
- 97.van der Pool AE, Méndez Romero A, Wunderink W, et al. Stereotactic body radiation therapy for colorectal liver metastases. Br J Surg. 2010;97:377–382. doi: 10.1002/bjs.6895. [DOI] [PubMed] [Google Scholar]
- 98.Kim MS, Yoo SY, Cho CK, et al. Stereotactic body radiation therapy using three fractions for isolated lung recurrence from colorectal cancer. Oncology. 2009;76:212–219. doi: 10.1159/000201932. [DOI] [PubMed] [Google Scholar]
- 99.Cheruvu P, Metcalfe SK, Metcalfe J, et al. Comparison of outcomes in patients with stage III versus limited stage IV non-small cell lung cancer. Radiat Oncol. 2011;6:80. doi: 10.1186/1748-717X-6-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hasselle MD, Haraf DJ, Rusthoven KE, et al. Hypofractionated image-guided radiation therapy for patients with limited volume metastatic non-small cell lung cancer. J Thorac Oncol. 2012;7:376–381. doi: 10.1097/JTO.0b013e31824166a5. [DOI] [PubMed] [Google Scholar]
- 101.Khan AJ, Mehta PS, Zusag TW, et al. Long term disease-free survival resulting from combined modality management of patients presenting with oligometastatic, non-small cell lung carcinoma (NSCLC) Radiother Oncol. 2006;81:163–167. doi: 10.1016/j.radonc.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 102.Milano MT, Zhang H, Metcalfe SK, et al. Oligometastatic breast cancer treated with curative-intent stereotactic body radiation therapy. Breast Cancer Res Treat. 2009;115:601–608. doi: 10.1007/s10549-008-0157-4. [DOI] [PubMed] [Google Scholar]
- 103.Muacevic A, Kufeld M, Rist C, et al. Safety and feasibility of image-guided robotic radiosurgery for patients with limited bone metastases of prostate cancer. Urol Oncol. 2013;31:455–460. doi: 10.1016/j.urolonc.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 104.Jereczek-Fossa BA, Beltramo G, Fariselli L, et al. Robotic image-guided stereotactic radiotherapy, for isolated recurrent primary, lymph node or metastatic prostate cancer. Int J Radiat Oncol Biol Phys. 2012;82:889–897. doi: 10.1016/j.ijrobp.2010.11.031. [DOI] [PubMed] [Google Scholar]
- 105.Casamassima F, Masi L, Menichelli C, et al. Efficacy of eradicative radiotherapy for limited nodal metastases detected with choline PET scan in prostate cancer patients. Tumori. 2011;97:49–55. doi: 10.1177/030089161109700110. [DOI] [PubMed] [Google Scholar]
- 106.Ranck MC, Golden DW, Corbin KS, et al. Stereotactic body radiotherapy for the treatment of oligometastatic renal cell carcinoma. Am J Clin Oncol. 2013;36:589–595. doi: 10.1097/COC.0b013e31825d52b2. [DOI] [PubMed] [Google Scholar]
- 107.Stinauer MA, Kavanagh BD, Schefter TE, et al. Stereotactic body radiation therapy for melanoma and renal cell carcinoma: Impact of single fraction equivalent dose on local control. Radiat Oncol. 2011;6:34. doi: 10.1186/1748-717X-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Teh B, Bloch C, Galli-Guevara M, et al. The treatment of primary and metastatic renal cell carcinoma (RCC) with image-guided stereotactic body radiation therapy (SBRT) Biomed Imaging Interv J. 2007;3:e6. doi: 10.2349/biij.3.1.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wersäll PJ, Blomgren H, Lax I, et al. Extracranial stereotactic radiotherapy for primary and metastatic renal cell carcinoma. Radiother Oncol. 2005;77:88–95. doi: 10.1016/j.radonc.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 110.Dhakal S, Corbin KS, Milano MT, et al. Stereotactic body radiotherapy for pulmonary metastases from soft-tissue sarcomas: Excellent local lesion control and improved patient survival. Int J Radiat Oncol Biol Phys. 2012;82:940–945. doi: 10.1016/j.ijrobp.2010.11.052. [DOI] [PubMed] [Google Scholar]
- 111.Treasure T. Oligometastatic cancer: An entity, a useful concept, or a therapeutic opportunity? J R Soc Med. 2012;105:242–246. doi: 10.1258/jrsm.2011.110279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Griffioen GH, Toguri D, Dahele M, et al. Radical treatment of synchronous oligometastatic non-small cell lung carcinoma (NSCLC): Patient outcomes and prognostic factors. Lung Cancer. 2013;82:95–102. doi: 10.1016/j.lungcan.2013.07.023. [DOI] [PubMed] [Google Scholar]
- 113.Ashworth A, Rodrigues G, Boldt G, et al. Is there an oligometastatic state in non-small cell lung cancer? A systematic review of the literature. Lung Cancer. 2013;82:197–203. doi: 10.1016/j.lungcan.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 114.Weickhardt AJ, Scheier B, Burke JM, et al. Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene-addicted non-small-cell lung cancer. J Thorac Oncol. 2012;7:1807–1814. doi: 10.1097/JTO.0b013e3182745948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gan GN, Weickhardt AJ, Scheier B, et al. Stereotactic radiation therapy can safely and durably control sites of extra-central nervous system oligoprogressive disease in anaplastic lymphoma kinase-positive lung cancer patients receiving crizotinib. Int J Radiat Oncol Biol Phys. 2014;88:892–898. doi: 10.1016/j.ijrobp.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Slotman B, Faivre-Finn C, Kramer G, et al. Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med. 2007;357:664–672. doi: 10.1056/NEJMoa071780. [DOI] [PubMed] [Google Scholar]
- 117.Jeremic B, Shibamoto Y, Nikolic N, et al. Role of radiation therapy in the combined-modality treatment of patients with extensive-disease small-cell lung cancer: A randomized study. J Clin Oncol. 1999;17:2092–2099. doi: 10.1200/JCO.1999.17.7.2092. [DOI] [PubMed] [Google Scholar]
- 118.Bonner JA, Eagan RT, Liengswangwong V, et al. Long term results of a phase I/II study of aggressive chemotherapy and sequential upper and lower hemibody radiation for patients with extensive stage small cell lung cancer. Cancer. 1995;76:406–412. doi: 10.1002/1097-0142(19950801)76:3<406::aid-cncr2820760310>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 119.Radiation Therapy Oncology Group. Radiation therapy in treating patients with extensive stage small cell lung cancer. http://clinicaltrials.gov/ct2/show/NCT01055197.
- 120.Cheruvu P, Metcalfe SK, Katz AW, et al. Retrospective review of stereotactic body radiotherapy (SBRT) for lung metastases from colorectal cancer (CRC) Int J Radiat Oncol Biol Phys. 2010;78:S316. [Google Scholar]
- 121.Timmerman RD, Bizekis CS, Pass HI, et al. Local surgical, ablative, and radiation treatment of metastases. CA Cancer J Clin. 2009;59:145–170. doi: 10.3322/caac.20013. [DOI] [PubMed] [Google Scholar]
- 122.Kuvshinoff B, Fong Y. Surgical therapy of liver metastases. Semin Oncol. 2007;34:177–185. doi: 10.1053/j.seminoncol.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 123.Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: Analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318. doi: 10.1097/00000658-199909000-00004. discussion 318-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Brown RE, Bower MR, Martin RC. Hepatic resection for colorectal liver metastases. Surg Clin North Am. 2010;90:839–852. doi: 10.1016/j.suc.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 125.Mahmoud N, Bullard Dunn K. Metastasectomy for stage IV colorectal cancer. Dis Colon Rectum. 2010;53:1080–1092. doi: 10.1007/DCR.0b013e3181dcadbc. [DOI] [PubMed] [Google Scholar]
- 126.Al-Asfoor A, Fedorowicz Z, Lodge M. Resection versus no intervention or other surgical interventions for colorectal cancer liver metastases. Cochrane Database Syst Rev. 2008;2:CD006039. doi: 10.1002/14651858.CD006039.pub4. [DOI] [PubMed] [Google Scholar]
- 127.Inoue M, Ohta M, Iuchi K, et al. Benefits of surgery for patients with pulmonary metastases from colorectal carcinoma. Ann Thorac Surg. 2004;78:238–244. doi: 10.1016/j.athoracsur.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 128.Elias D, Liberale G, Vernerey D, et al. Hepatic and extrahepatic colorectal metastases: When resectable, their localization does not matter, but their total number has a prognostic effect. Ann Surg Oncol. 2005;12:900–909. doi: 10.1245/ASO.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 129.Kang JK, Kim MS, Kim JH, et al. Oligometastases confined one organ from colorectal cancer treated by SBRT. Clin Exp Metastasis. 2010;27:273–278. doi: 10.1007/s10585-010-9325-0. [DOI] [PubMed] [Google Scholar]
- 130.Metcalfe SK, Cheruvu P, Katz AW, et al. Prospective trial of stereotactic body radiation therapy for colorectal oligometastases. Presented at the 92nd Annual Meeting of the American Radium Society; May 1-5, 2010; Cancun, Mexico. [Google Scholar]
- 131.Ranck MC, Golden DW, Corbin KS, et al. Stereotactic body radiotherapy for the treatment of oligometastatic renal cell carcinoma. Am J Clin Oncol. 2013;36:589–595. doi: 10.1097/COC.0b013e31825d52b2. [DOI] [PubMed] [Google Scholar]
- 132.Fuks Z, Kolesnick R. Engaging the vascular component of the tumor response. Cancer Cell. 2005;8:89–91. doi: 10.1016/j.ccr.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 133.Garcia-Barros M, Paris F, Cordon-Cardo C, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300:1155–1159. doi: 10.1126/science.1082504. [DOI] [PubMed] [Google Scholar]
- 134.Lee Y, Auh SL, Wang Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: Changing strategies for cancer treatment. Blood. 2009;114:589–595. doi: 10.1182/blood-2009-02-206870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Samlowski W. Concurrent ipilimumab and stereotactic ablative radiation therapy (SART) for oligometastatic but unresectable melanoma. http://clinicaltrials.gov/ct2/show/NCT01565837.
- 138.Gustave Roussy, Cancer Campus. Study of radiotherapy administered in combination with ipilimumab in patients with unresectable stage III or stage IV advanced malignant melanoma (Mel-Ipi-Rx) http://clinicaltrials.gov/ct2/show/NCT01557114.
- 139.Abramson Cancer Center, University of Pennsylvania. RADVAX: A stratified phase I/II dose escalation trial of stereotactic body radiotherapy followed by ipilimumab in metastatic melanoma. http://clinicaltrials.gov/ct2/show/NCT01497808.
- 140.Burnette BC, Liang H, Lee Y, et al. The efficacy of radiotherapy relies upon induction of type i interferon-dependent innate and adaptive immunity. Cancer Res. 2011;71:2488–2496. doi: 10.1158/0008-5472.CAN-10-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Seung SK, Curti BD, Crittenden M, et al. Phase 1 study of stereotactic body radiotherapy and interleukin-2: Tumor and immunological responses. Sci Transl Med. 2012;4:137ra74. doi: 10.1126/scitranslmed.3003649. [DOI] [PubMed] [Google Scholar]
- 142.Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: A paradigm shift. J Natl Cancer Inst. 2013;105:256–265. doi: 10.1093/jnci/djs629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Demaria S, Ng B, Devitt ML, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004;58:862–870. doi: 10.1016/j.ijrobp.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 144.Formenti S, Friedman K, Chao K, et al. Abscopal response in irradiated patients: Results of a proof of priniciple trial. Int J Radiat Oncol Biol Phys. 2008;72:S6–S7. [Google Scholar]
- 145.Golden EB, Demaria S, Schiff PB, et al. An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer Immunol Res. 2013;1:365–372. doi: 10.1158/2326-6066.CIR-13-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dewan MZ, Galloway AE, Kawashima N, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 2009;15:5379–5388. doi: 10.1158/1078-0432.CCR-09-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366:925–931. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhang B, Bowerman NA, Salama JK, et al. Induced sensitization of tumor stroma leads to eradication of established cancer by T cells. J Exp Med. 2007;204:49–55. doi: 10.1084/jem.20062056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lewis S, Porceddu S, Nakamura N, et al. Definitive stereotactic body radiotherapy (SBRT) for extracranial oligometastases: An international survey of > 1000 radiation oncologists. Int J Radiat Oncol Biol Phys. 2013;87(suppl):S605. doi: 10.1097/COC.0000000000000169. [DOI] [PubMed] [Google Scholar]
- 150.Solanki AA, Weichselbaum RR, Appelbaum D, et al. The utility of FDG-PET for assessing outcomes in oligometastatic cancer patients treated with stereotactic body radiotherapy: A cohort study. Radiat Oncol. 2012;7:216. doi: 10.1186/1748-717X-7-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Stinauer MA, Diot Q, Westerly DC, et al. Fluorodeoxyglucose positron emission tomography response and normal tissue regeneration after stereotactic body radiotherapy to liver metastases. Int J Radiat Oncol Biol Phys. 2012;83:e613–e618. doi: 10.1016/j.ijrobp.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 152.Lussier YA, Khodarev NN, Regan K, et al. Oligo- and polymetastatic progression in lung metastasis(es) patients is associated with specific microRNAs. PLoS One. 2012;7:e50141. doi: 10.1371/journal.pone.0050141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lussier YA, Xing HR, Salama JK, et al. MicroRNA expression characterizes oligometastasis(es) PLoS One. 2011;6:e28650. doi: 10.1371/journal.pone.0028650. [DOI] [PMC free article] [PubMed] [Google Scholar]