Abstract
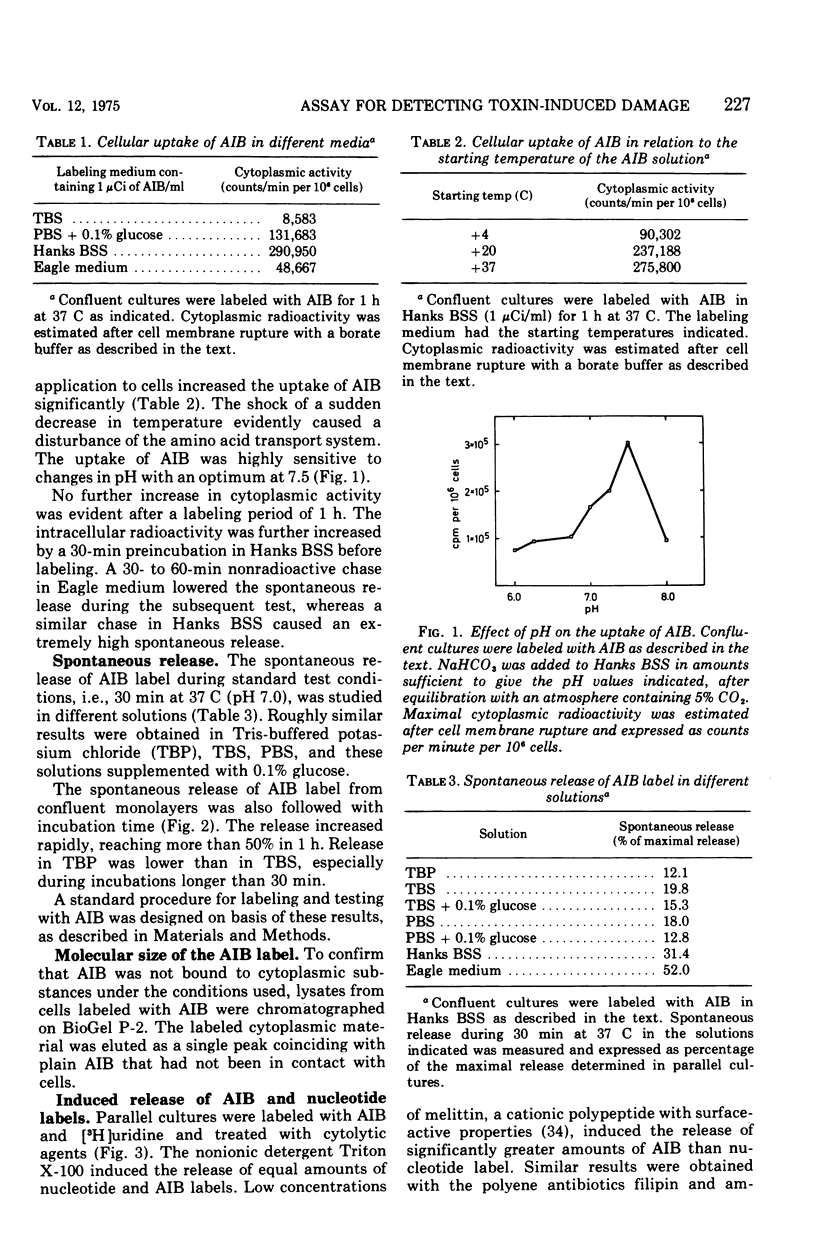
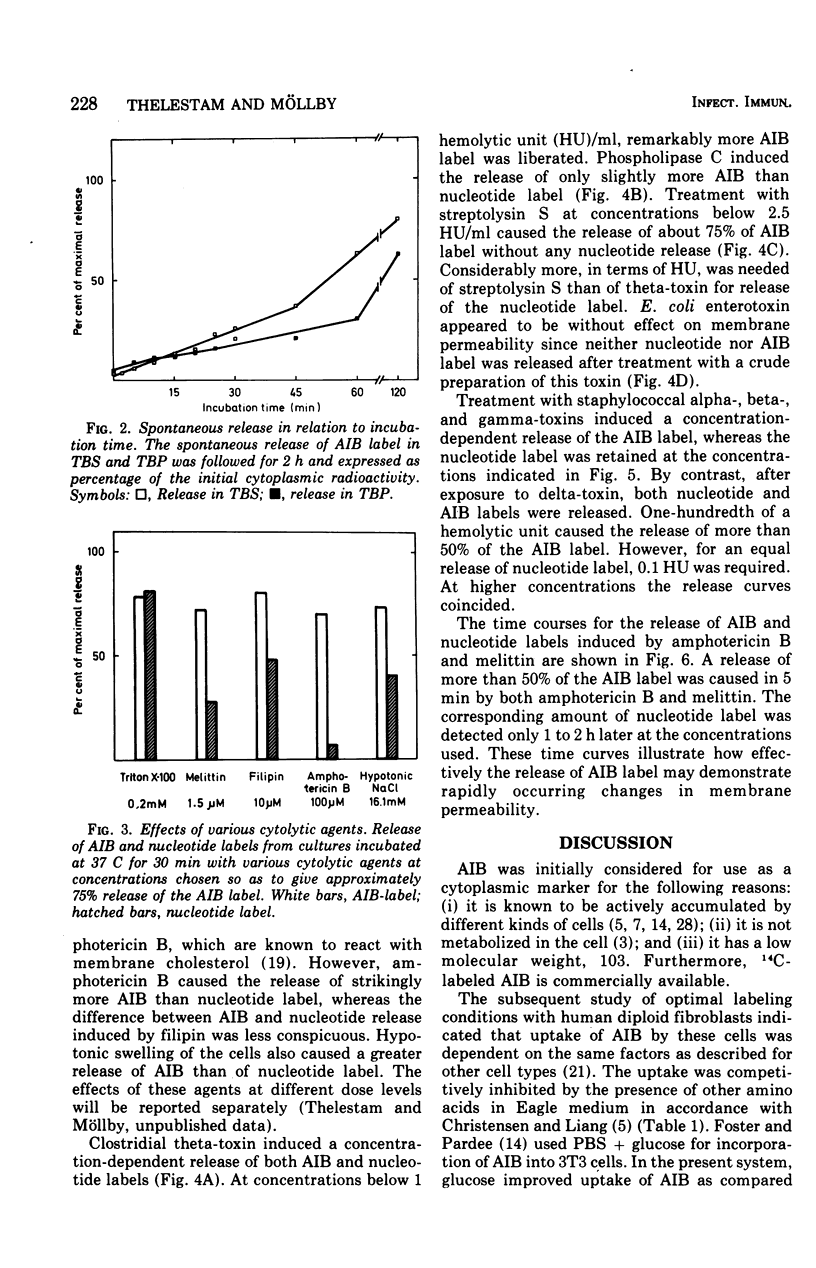
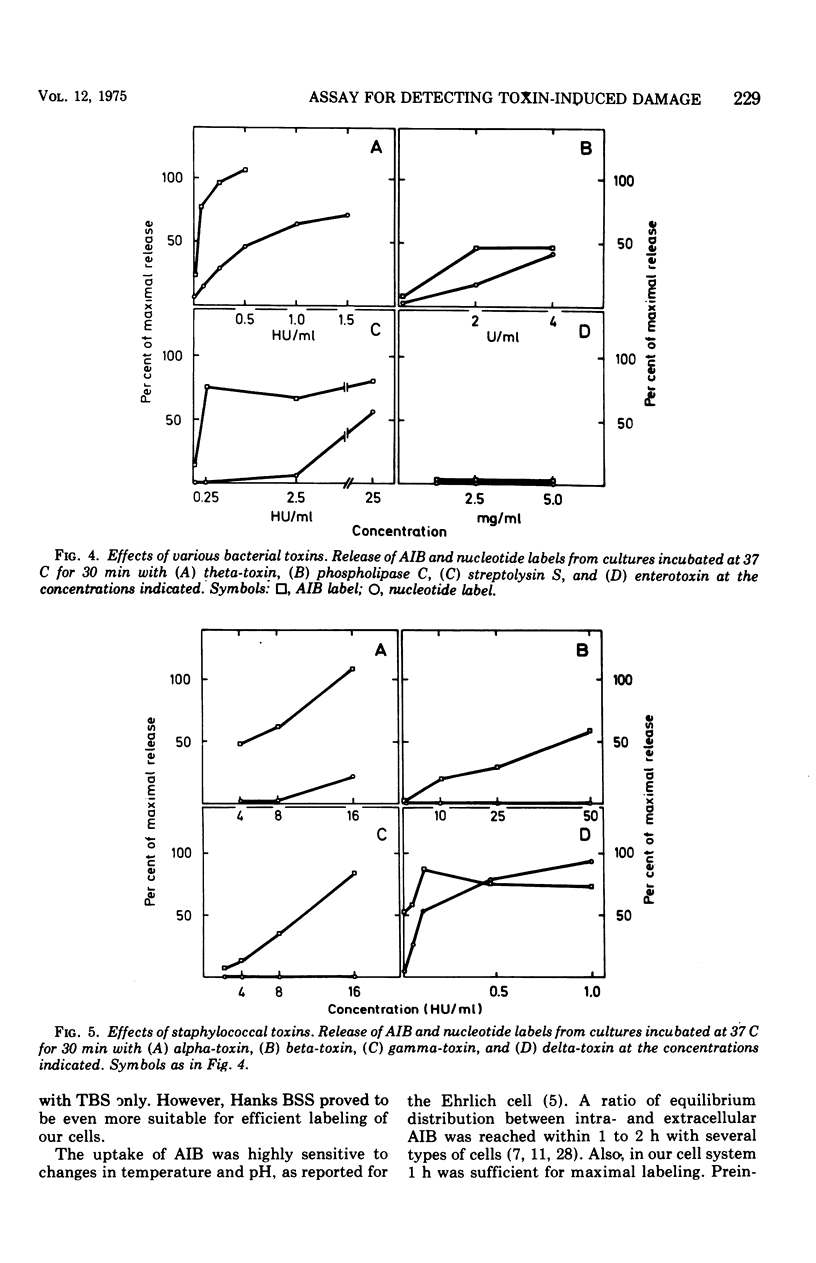
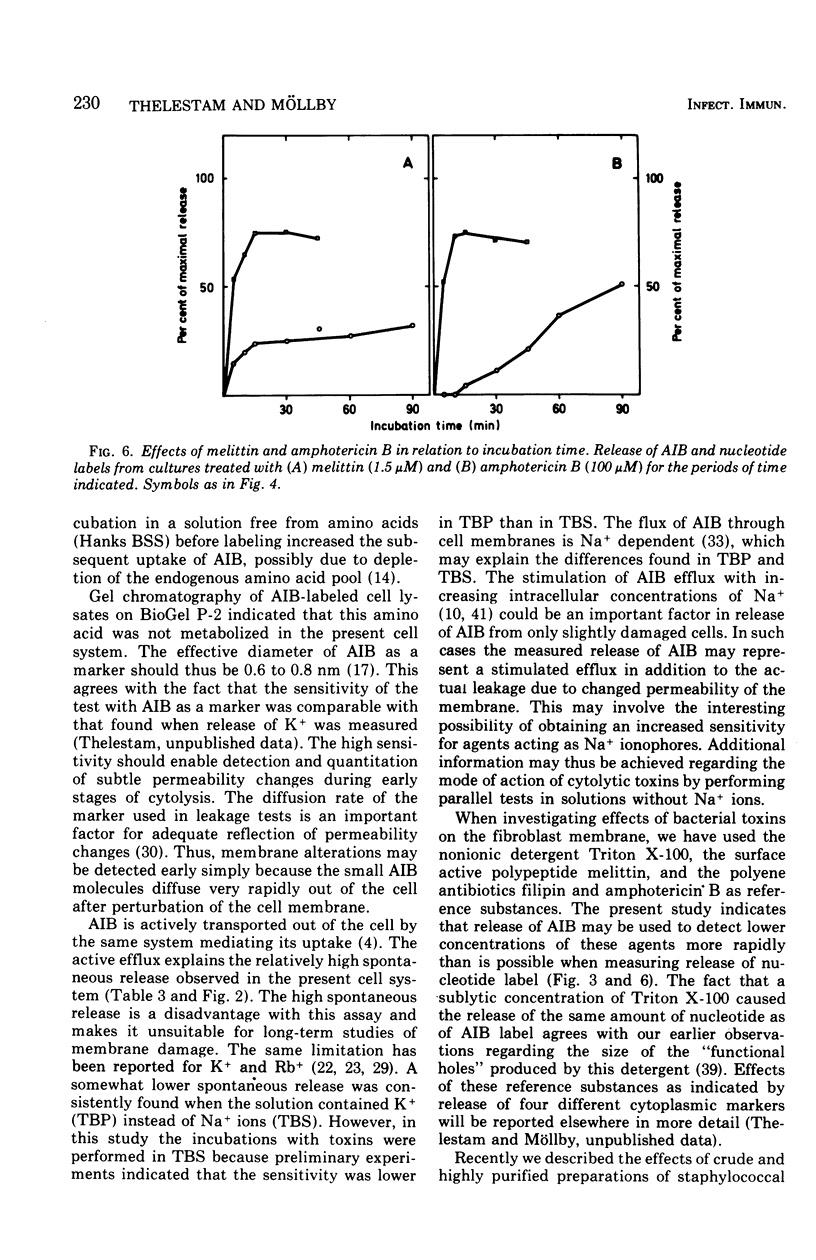
A sensitive assay was developed for detection and quantitation of subtle permeability changes in the cytoplasmic membrane of human diploid fibroblasts. Release of the non-metabolizable amino acid [1-14C]alpha-aminoisobutyric acid (AIB; molecular weight (103) from the cytoplasm of prelabeled cells was used as an indicator of toxin-induced membrane damage. An optimal procedure for labeling these cells was designed after varying the conditions with regard to pH, temperature, concentration of AIB, composition of medium, and incubation time. Toxin-induced release of AIB was compared with release of a previously described nucleotide label, [3H]uridine. Melittin from bee venom and the polyene antibiotics filipin and amphotericin B in low concentrations induced a strikingly greater release of AIB than of nucleotide label. The sensitivity of this assay was furthermore demonstrated by treatment with the following bacterial cytolysins: phospholipase C and theta-toxin from Clostridium perfringens, alpha-, beta-, delta-, and gamma-toxins from Staphylococcus aureus, and streptolysin S from Streptococcus pyogenes. In spite of their different modes of action, all these membrane-active toxins at low concentrations induced a significant release of AIB label. For an equal release of nucleotide label, several times higher concentrations were required.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHRISTENSEN H. N., ASPEN A. J., RICE E. G. Metabolism in the rat of three amino acids lacking alpha-hydrogen. J Biol Chem. 1956 May;220(1):287–294. [PubMed] [Google Scholar]
- Christensen H. N., Handlogten M. E. Modes of mediated exodus of amino acids from the Ehrlich ascites tumor cell. J Biol Chem. 1968 Oct 25;243(20):5428–5438. [PubMed] [Google Scholar]
- Christensen H. N., Liang M. On the nature of the "non-saturable" migration of amino acids into Ehrlich cells and into rat jejunum. Bibl Laeger. 1966 Mar 14;112(3):524–531. doi: 10.1016/0926-6585(66)90255-x. [DOI] [PubMed] [Google Scholar]
- Dickson J. A. The uptake of non-metabolizable amino acids as an index of cell viability in vitro. Exp Cell Res. 1970 Aug;61(2):235–245. doi: 10.1016/0014-4827(70)90443-x. [DOI] [PubMed] [Google Scholar]
- Duncan J. L. Characteristics of streptolysin O hemolysis: kinetics of hemoglobin and 86rubidium release. Infect Immun. 1974 Jun;9(6):1022–1027. doi: 10.1128/iai.9.6.1022-1027.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Eddy A. A. The effects of varying the cellular and extracellular concentrations of sodium and potassium ions on the uptake of glycine by mouse ascites-tumour cells in the presence and absence of sodium cyanide. Biochem J. 1968 Jul;108(3):489–498. doi: 10.1042/bj1080489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsas L. J., Rosenberg L. E. Inhibition of amino Acid transport in rat kidney cortex by puromycin. Proc Natl Acad Sci U S A. 1967 Feb;57(2):371–378. doi: 10.1073/pnas.57.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FORBES I. J. Studies of cytotoxicity using P-32. Aust J Exp Biol Med Sci. 1963 Jun;41:255–264. doi: 10.1038/icb.1963.25. [DOI] [PubMed] [Google Scholar]
- Foster D. O., Pardee A. B. Transport of amino acids by confluent and nonconfluent 3T3 and polyoma virus-transformed 3T3 cells growing on glass cover slips. J Biol Chem. 1969 May 25;244(10):2675–2681. [PubMed] [Google Scholar]
- Freer J. H., Arbuthnott J. P., Bernheimer A. W. Interaction of staphylococcal alpha-toxin with artificial and natural membranes. J Bacteriol. 1968 Mar;95(3):1153–1168. doi: 10.1128/jb.95.3.1153-1168.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN H., FLEISCHER R. A., BARROW P., GOLDBERG B. The cytotoxic action of immune gamma globulin and complement on Krebs ascites tumor cells. II. Chemical studies. J Exp Med. 1959 May 1;109(5):511–521. doi: 10.1084/jem.109.5.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale E. F. The release of potassium ions from Candida albicans in the presence of polyene antibiotics. J Gen Microbiol. 1974 Feb;80(2):451–465. doi: 10.1099/00221287-80-2-451. [DOI] [PubMed] [Google Scholar]
- Hamilton-Miller J. M. Fungal sterols and the mode of action of the polyene antibiotics. Adv Appl Microbiol. 1974;17(0):109–134. doi: 10.1016/s0065-2164(08)70556-2. [DOI] [PubMed] [Google Scholar]
- Hammond S. M., Lambert P. A., Kliger B. N. The mode of action of polyene antibiotics; induced potassium leakage in Candida albicans. J Gen Microbiol. 1974 Apr;81(2):325–330. doi: 10.1099/00221287-81-2-325. [DOI] [PubMed] [Google Scholar]
- Henney C. S. Studies on the mechanism of lymphocyte-mediated cytolysis. II. The use of various target cell markers to study cytolytic events. J Immunol. 1973 Jan;110(1):73–84. [PubMed] [Google Scholar]
- Hingson D. J., Massengill R. K., Mayer M. M. The kinetics of release of 86rubidium and hemoglobin from erythrocytes damaged by antibody and complement. Immunochemistry. 1969 Mar;6(2):295–307. doi: 10.1016/0019-2791(69)90166-9. [DOI] [PubMed] [Google Scholar]
- Kantor H. S., Tao P., Wisdom C. Action of Escherichia coli enterotoxin: adenylate cyclase behavior of intestinal epithelial cells in culture. Infect Immun. 1974 Jun;9(6):1003–1010. doi: 10.1128/iai.9.6.1003-1010.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MADOFF M. A., COOPER L. Z., WEINSTEIN L. HEMOLYSIS OF RABBIT ERYTHROCYTES BY PURIFIED STAPHYLOCOCCAL ALPHA-TOXIN. III. POTASSIUM RELEASE. J Bacteriol. 1964 Jan;87:145–149. doi: 10.1128/jb.87.1.145-149.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor R. D., 2nd, Tobias C. A. Molecular sieving of red cell membranes during gradual osmotic hemolysis. J Membr Biol. 1972 Dec 29;10(3):345–356. doi: 10.1007/BF01867865. [DOI] [PubMed] [Google Scholar]
- Mahoney M. J., Rosenberg L. E. Uptake of alpha-aminoisobutyric acid by cultured human fibroblasts. Biochim Biophys Acta. 1970 Dec 1;219(2):500–502. doi: 10.1016/0005-2736(70)90232-4. [DOI] [PubMed] [Google Scholar]
- Martz E., Burakoff S. J., Benacerraf B. Interruption of the sequential release of small and large molecules from tumor cells by low temperature during cytolysis mediated by immune T-cells or complement. Proc Natl Acad Sci U S A. 1974 Jan;71(1):177–181. doi: 10.1073/pnas.71.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. G., Dunkley M. Quantitative analysis of the 51Cr release cytotoxicity assay for cytotoxic lymphocytes. Cell Immunol. 1974 Nov;14(2):284–302. doi: 10.1016/0008-8749(74)90212-3. [DOI] [PubMed] [Google Scholar]
- Möllby R., Thelestam M., Wadström T. Effect of Clostridium perfringens phospholipase C(alpha-toxin) on the human diploid fibroblast membrane. J Membr Biol. 1974;16(4):313–330. doi: 10.1007/BF01872421. [DOI] [PubMed] [Google Scholar]
- Möllby R., Wadström T. Purification of phospholipase C (alpha-toxin) from Clostridium perfringens. Biochim Biophys Acta. 1973 Oct 10;321(2):569–584. doi: 10.1016/0005-2744(73)90200-3. [DOI] [PubMed] [Google Scholar]
- SIEGEL I., COHEN S. ACTION OF STAPHYLOCOCCAL TOXIN ON HUMAN PLATELETS. J Infect Dis. 1964 Dec;114:488–502. doi: 10.1093/infdis/114.5.488. [DOI] [PubMed] [Google Scholar]
- Schultz S. G., Curran P. F. Coupled transport of sodium and organic solutes. Physiol Rev. 1970 Oct;50(4):637–718. doi: 10.1152/physrev.1970.50.4.637. [DOI] [PubMed] [Google Scholar]
- Sessa G., Freer J. H., Colacicco G., Weissmann G. Interaction of alytic polypeptide, melittin, with lipid membrane systems. J Biol Chem. 1969 Jul 10;244(13):3575–3582. [PubMed] [Google Scholar]
- Smyth C. J. The identification and purification of multiple forms of theta-haemolysin (theta-toxin) of Clostridium perfringens type A. J Gen Microbiol. 1975 Apr;87(2):219–238. doi: 10.1099/00221287-87-2-219. [DOI] [PubMed] [Google Scholar]
- Strandberg K., Möllby R., Wadström T. Histamine release from mast cells by highly purified phospholipase C (alpha-toxin) and theta-toxin from Clostridium perfringens. Toxicon. 1974 Mar;12(2):199–208. doi: 10.1016/0041-0101(74)90246-3. [DOI] [PubMed] [Google Scholar]
- Stulting R. D., Berke G. The use of 51Cr release as a measure of lymphocyte-mediated cytolysis in vitro. Cell Immunol. 1973 Dec;9(3):474–476. doi: 10.1016/0008-8749(73)90062-2. [DOI] [PubMed] [Google Scholar]
- Thelestam M., Möllby R. Determination of toxin-induced leakage of different-size nucleotides through the plasma membrane of human diploid fibroblasts. Infect Immun. 1975 Apr;11(4):640–648. doi: 10.1128/iai.11.4.640-648.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelestam M., Möllby R., Wadström T. Effects of staphylococcal alpha-, beta-, delta-, and gamma-hemolysins on human diploid fibroblasts and HeLa cells: evaluation of a new quantitative as say for measuring cell damage. Infect Immun. 1973 Dec;8(6):938–946. doi: 10.1128/iai.8.6.938-946.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidaver G. A., Shepherd S. L. Transport of glycine by hemolyzed and restored pigeon red blood cells. Symmetry properties, trans effects of sodium ion and glycine, and their description by a single rate equation. J Biol Chem. 1968 Dec 10;243(23):6140–6150. [PubMed] [Google Scholar]
- WIGZELL H. QUANTITATIVE TITRATIONS OF MOUSE H-2 ANTIBODIES USING CR-51-LABELLED TARGET CELLS. Transplantation. 1965 May;3:423–431. doi: 10.1097/00007890-196505000-00011. [DOI] [PubMed] [Google Scholar]
- Warren L., Glick M. C. Membranes of animal cells. II. The metabolism and turnover of the surface membrane. J Cell Biol. 1968 Jun;37(3):729–746. doi: 10.1083/jcb.37.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kruijff B., Gerritsen W. J., Oerlemans A., Demel R. A., van Deenen L. L. Polyene antibiotic-sterol interactions in membranes of Acholeplasma laidlawii cells and lecithin liposomes. I. Specificity of the membrane permeability changes induced by the polyene antibiotics. Biochim Biophys Acta. 1974 Feb 26;339(1):30–43. doi: 10.1016/0005-2736(74)90330-7. [DOI] [PubMed] [Google Scholar]



