Abstract
Purpose
Although patient-reported outcomes (PROs) have become a key component of clinical oncology trials, many challenges exist regarding their optimal application. The goal of this article is to methodically review these barriers and suggest strategies to overcome them. This review will primarily focus on radiation oncology examples, will address issues regarding the “why, how, and what” of PROs, and will provide strategies for difficult problems such as methods for reducing missing data. This review will also address cancer survivorship because it closely relates to PROs.
Methods
Key articles focusing on PROs, quality of life, and survivorship issues in oncology trials are highlighted, with an emphasis on radiation oncology clinical trials. Publications and Web sites of various governmental and regulatory agencies are also reviewed.
Results
The study of PROs in clinical oncology trials has become well established. There are guidelines provided by organizations such as the US Food and Drug Administration that clearly indicate the importance of and methodology for studying PROs. Clinical trials in oncology have repeatedly demonstrated the value of studying PROs and suggested ways to overcome some of the key challenges. The Radiation Therapy Oncology Group (RTOG) has led some of these efforts, and their contributions are highlighted. The current state of cancer survivorship guidelines is also discussed.
Conclusion
The study of PROs presents significant benefits in understanding and treating toxicities and enhancing quality of life; however, challenges remain. Strategies are presented to overcome these hurdles, which will ultimately improve cancer survivorship.
INTRODUCTION
Patient-reported outcomes (PROs) have become more widely included in clinical oncology trials over the last decade. PROs have been defined by the US Food and Drug Administration (FDA) as “any report of the status of a patient's health condition that comes directly from the patient, without interpretation of the patient's response by a clinician or anyone else.”1 This is a broad term that includes within its purview health-related quality of life (HRQOL), experiences with treatment, and patient satisfaction.
Yet many challenges remain regarding the optimal application of PROs in oncology, not only within clinical trials, but even more importantly, in the clinic setting itself. The goal of this review article is to methodically review these barriers and suggest strategies to overcome them. For example, considering the additional resources, time, and effort required, why should PROs be used? Once one decides to include PROs in a particular setting, how does one select the optimal instruments, time points, and end points? Then, after the PRO data are finally collected, what does it actually mean? Perhaps the greatest challenge relates to missing data, which plagues many quality-of-life (QOL) studies. Although this article is not meant to review all aspects of PROs in oncology, it will primarily focus on radiation oncology examples and tackle the key questions of “why, how, and what,” and will provide practical solutions for difficult problems, such as strategies to reduce missing data. This review will also address survivorship concerns, because they are often best measured by PROs. Ultimately, when considering PROs, how do we overcome the cons?
ON A FUNDAMENTAL LEVEL, WHY SHOULD PROs BE MEASURED?
Benefits for Physicians and Medical Research
Measuring PROs provides key added value for physicians and medical research. Perhaps the most important example is that pretreatment QOL has been shown in many oncology trials to be a strong predictor of outcome, including local control and survival. Table 1 lists several examples from the radiation oncology literature. PROs can also help identify problems or symptoms that are likely to be missed during routine clinical queries. For example, depressive symptoms in patients with breast cancer were associated with a higher risk of recurrence and early death.10 PROs also help in understanding the impact of cancer and its treatments in late sequelae and long-term toxicities when these patients are following up with their oncologists.11,12 An understanding of the impact of treatment on long-term QOL and survivorship may help physicians guide patients when deciding between two equally efficacious treatments.
Table 1.
Radiation Therapy Trials in Which QOL Instruments Were Prognostic for Outcome
| Reference | No. of Patients | Cancer Type | Treatment | PRO-Related Conclusions |
|---|---|---|---|---|
| De Boer et al2 | 133 | H&N | Sx, RT | Factors predicting for better LRC and OS were high level of perceived self-efficacy, low score on uncertainty handling illness, more psychosocial complaints |
| de Graeff et al3 | 208 | H&N | Sx, RT | Patients with cognitive score of 100 on EORTC QLQ-C30 had greater OS and LRC than those with a score of < 100 |
| Fang et al4 | 102 | H&N | RT | High pretreatment baseline fatigue score predicted for significantly poorer 2-year OS. A 10-point increase in score resulted in 17% reduction in likelihood of survival |
| Kaasa et al5 | 102 | NSCLC | CT, RT | General symptoms and psychosocial well being predicted for OS |
| Langendijk et al6 | 198 | NSCLC | RT | Baseline global QOL scores significantly predicted for OS in patients with pathologically involved lymph nodes |
| Montazeri et al7 | 129 | Lung | Pretreatment global QOL was a significant predictor of survival | |
| Siddiqui et al8 | 1,093 | H&N | RT, RT + CT | FACT H&N and the functional well-being component of FACT-G predicted significantly for LRC |
| Movsas et al9 | 239 | NSCLC | RT + CT | Baseline global QOL predicted for OS. A 10-point increase in baseline score decreased hazard of death by 10% |
Abbreviations: CT, chemotherapy; EORTC QLQ-C30, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire C30; FACT-G, Functional Assessment of Cancer Therapy–General; FACT-H&N, FACT–Head and Neck; H&N, head and neck; LRC, locoregional control; NSCLC, non–small-cell lung cancer; OS, overall survival; QOL, quality of life; RT, radiation therapy; Sx, surgery.
Benefits for Regulatory Agencies and Society
Various government and regulatory agencies have embraced the study of PRO measures and have used PROs to guide policy and health care delivery. The FDA published PRO guidelines for industry and drug manufacturers in 2009.1 This document provides recommendations on the use of PRO as an end point, the instruments to use, clinical trial design, data analysis, and statistics. The FDA has allowed QOL data as a basis for seeking approval for drugs since the 1980s.13 Indications for approved drugs have been extended to include conditions under which they help relieve or minimize symptoms.14
PROs are also proving useful in health care decision making. Recommendations have recently been published for incorporating PROs into comparative effectiveness research (CER).15,16 The Institute of Medicine (IOM) defines CER as “the generation and synthesis of evidence that compares the benefits and harms of alternative methods to prevent, diagnose, treat, and monitor a clinical condition or to improve the delivery of care. The purpose of CER is to assist consumers, clinicians, purchasers, and policy makers to make informed decisions that will improve health care at both the individual and population levels.”17 One economic measure incorporating PROs is quality-adjusted life years (QALY). By using this approach, a person's state of health is assigned a utility which runs on a continuum from 1 (perfect health) to 0 (death). A QALY is defined as 1 year of life adjusted for the health state. Thus, 1.0 QALY equates to 1 year spent in perfect health. A cost of $50,000 per QALY or less is typically considered economically efficient for health care delivery.18 Such analyses have been conducted in radiation oncology trials for comparing two treatment regimens.19–21 QALY analyses have also been used when assessing novel radiation technologies, such as intensity-modulated radiation therapy and proton therapy.22,23 Sloan et al24 recently conducted simulation studies to assess the impact of survival differences, toxicity rates, and utility values on QALY.
Benefits for Patients
By definition, PROs are reported directly by the patients themselves without any interference by health care professionals. This provides a significant advantage because it has been documented that there is a disconnect between the perspective of providers and that of patients.25–28 PRO data can help patients choose between two therapies as opposed to being influenced by physician bias.29,30 Perhaps the most important reason for studying PROs is simply that patients want their physicians to do so.31 Randomized trials reported by Detmar et al32 and Velikova et al33 have shown that physician-patient interaction and communication improve when queries are made regarding PROs. This, in turn, improves patient QOL.
ONCE IT HAS BEEN DECIDED TO INCORPORATE PROs INTO A PARTICULAR SETTING, HOW SHOULD THEY BE MEASURED?
The most important criteria for choosing a particular PRO instrument in a clinical trial is the formulation of a well-thought-out hypothesis. The question being asked must be addressed by a validated PRO instrument, one that has been found to be reliable, valid, sensitive, and responsive to change.34 Gotay et al35 suggested incorporating QOL end points into randomized trials when comparing palliative regimens, when the treatments are likely to be equally effective, or when one treatment may be superior to the other but at the expense of greater toxicity.
The following factors should considered regarding the QOL instrument to be used, some of which have also been described by Gelber and Gelber.36 What is the objective of the study? Is the QOL instrument validated for the cancer being studied? Is it validated for the specific patient population with regard to demographic factors such as sex, race, and age? Are validated translations available for non-English speakers? How many instruments need to be used? One or many? How many items are there in the instrument? Is there an abbreviated version that may provide the same information? What is the instrument's clinical relevance? How often will the PRO information be collected? Are there any copyright clearances or permissions to obtain and is there a cost associated with the use of the instrument?
There are hundreds of validated PRO instruments available with a multitude of choices, depending on the clinical question that needs to be answered. HRQOL instruments are broadly categorized as either generic instruments or disease-specific instruments.
Generic Instruments
Generic instruments cover broader aspects of diseases and can be applied in various situations to assess the general health status of individuals. Two examples of such instruments are the Functional Assessment of Cancer Therapy–General (FACT-G)37 and the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire C30 (EORTC QLQ-C30),38 which have been translated and validated in many languages. Another widely used instrument is the Medical Outcomes Study (MOS) questionnaire, which contains a core set of 116 items intended to measure physical health, mental health, and general health.39 Shorter versions of this instrument are also available, and they are known as the 36-item Short Form Health Survey (SF-36) and SF-12.40–42
Disease-Specific or Cancer-Specific Instruments
The aforementioned general instruments can be combined with cancer-specific instruments that include additional items querying the cancer being studied. Examples of such instruments are the FACT-B for breast cancer,43 FACT-L for lung cancer,44 and EORTC-BN20 for brain tumors,45 among many others.
A detailed description of these instruments is beyond the scope of this article. Guidance regarding which instrument to use can be obtained from various sources, including the Patient Reported Outcomes Measurement Information System (PROMIS),46 PROQOLID,47 and the Radiation Therapy Oncology Group (RTOG) QOL/PRO library.48 The National Institutes of Health (NIH) has funded two major initiatives related to PROs to facilitate better standardized and better validated metrics. The first is PROMIS, an initiative launched in 2004.49,50 The aim of this program is to “provide clinicians and researchers access to efficient, precise, valid, and responsive adult- and child-reported measures of health and well-being.” The second major initiative is the Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE).51 The PRO-CTCAE has developed an electronic-based system for patient self-reporting of symptom adverse events listed in the CTCAE. The aim is to improve the accuracy and precision of grading of this class of adverse events.
The National Cancer Institute's (NCI's) Symptom Management and Health-Related Quality of Life Committee recently convened a clinical trials planning meeting to provide recommendations for a core set of patient-reported symptoms to be considered for inclusion across all cancer clinical trials and for three common solid tumors: head and neck, prostate, and ovarian. Using a common core set of PROs will enable comparisons within and among trials and meta-analyses to better understand prevalence, duration, and severity of cancer and cancer treatment–related symptoms.
The optimal timing of using a PRO instrument varies based on the hypothesis being tested, the natural course of the disease, and the anticipated adverse effects. It is critical to limit the PRO time points as much as possible to reduce patient burden and resources.
It is also important to be aware of and follow the recent CONSORT (Consolidated Standards of Reporting Trials) PRO guidelines by Calvert et al.52,53 The aim of these guidelines is to “improve the reporting of PROs in trials to facilitate the use of results to inform clinical practice and health policy.”
AFTER THE PRO DATA ARE COLLECTED, WHAT DO THEY MEAN?
A major ongoing challenge in this field is to understand and analyze the clinical meaningfulness of PRO data. Although clinicians understand the relevance of a high-temperature recording, what does a certain change in a particular QOL end point signify? Fortunately, clinically meaningful changes in QOL have been analyzed and are defined as “a difference in score that is large enough to have an implication for the patient's treatment or care.”54 This leads to the concept of minimal clinically important difference, which has been defined as “the smallest difference in the score in the domain of interest that patients perceive as important, either beneficial or harmful, and which would lead the clinician to consider a change in the patient's management.”55 For example, Osoba et al56 used the EORTC QLQ C-30 instrument and a subjective significance questionnaire for patients receiving chemotherapy for breast and small-cell lung cancer. They noted that if the change in scores on the EORTC QLQ-C30 was between 5 and 10 points, patients perceived their condition as “a little” better or worse. For a change of 10 to 20 points, the perception was “moderately” better or worse, denoting a clinically meaningful change. Of note, Sarna et al25 reported that in RTOG-9801 (A Phase III Study of Amifostine Mucosal Protection For Patients With Favorable Prognosis Inoperable Stage II-IIIA/B Non-Small Cell Lung Cancer [NSCLC] Receiving Sequential Induction and Concurrent Hyperfractionated Radiotherapy With Paclitaxel and Carboplatin) that 10 points lower on the baseline EORTC QLQ-LC13 lung instrument corresponded to a 10% increase in mortality 5 years later, indeed a clinically meaningful finding.
Two approaches for data interpretation, called anchor-based and distribution-based methods, were introduced by Lydick and Epstein57 and further explained by Wyrwich et al.54 In the anchor-based method, the scores on the QOL instrument are correlated with another independent measure (anchor). In the distribution-based method, the data are interpreted in terms of the relation between the magnitude of effect and some measure of variability in results.58,59 Cohen has suggested that a standard deviation of 0.5 represents a moderate change.60
CHALLENGE OF MISSING PRO DATA
Another major issue that has plagued studies examining PROs has been missing data. For example, some QOL studies in cancer are performed when comparing palliative treatment regimens. In such studies, long-term patient follow-up may be compromised resulting in either missing items and/or missing entire forms, which is more serious.61 Curran et al62 present possible reasons for missing forms: (1) research staff or administrative staff failed to distribute the questionnaire, (2) patients considered the questionnaire a violation of privacy, (3) patients felt that the questionnaire was time consuming, (4) patients withdrew or refused to complete the questionnaire, (5) patients felt too ill, or (6) patients' disease progressed. Loss of data may lead to a bias in the study as well as a loss of power. Statistical methods for dealing with missing data have been described.63 One of the more common methods used is termed “imputing,” such that missing values are substituted by a mean value, provided that a minimum percentage of questions within a subscale have been answered.
Every effort must be made to minimize missing data in PRO studies. Selection of abbreviated validated forms with a smaller number of questions to answer the hypothesis question should be chosen to minimize patient burden. PROs should be included as an integral part of the study and not as an added-on component. Quality control checks should be in place to ensure that questionnaires are administered to the study participants at appropriate times, collected within a defined time frame, checked to make sure that items are complete, and entered into electronic databases expeditiously. Real-time data entry and tracking software are now available that send e-mail reminders to patients, enable them to complete questionnaires online, prompt when items or forms are incomplete, and populate centralized databases in real time. Such efforts have been piloted by the RTOG, and the experience has recently been reported by Movsas et al.64 RTOG-0828 (Pilot Project to Reduce Missing RTOG Quality of Life Data Via Electronic Web-Based Form Collection: A Companion Study for RTOG 0415) was a prospective companion study of RTOG-0415 (A Phase III Randomized Study of Hypofractionated 3DCRT/IMRT Versus Conventionally Fractionated 3DCRT/IMRT in Patients Treated for Favorable-Risk Prostate Cancer), a randomized study of conventional versus hypofractionated radiation therapy for favorable-risk patients with prostate cancer. In RTOG-0415, which used paper forms, the 6-month QOL completion rate was 52%. By using Web-based tools, the 6-month completion rate increased to 90% (P < .001). Moreover, the 1-year QOL completion rate using the Web-based technology was 82% compared with 36% using paper forms.
ROLE OF RTOG IN RADIATION ONCOLOGY PRO STUDIES
The RTOG was established in 1968 with the intent of conducting radiation oncology and multidisciplinary collaborative trials for patients with cancer. Recently, it merged with the National Surgical Adjuvant Breast and Bowel Project (NSABP) and Gynecologic Oncology Group (GOG) to form the NRG Oncology Group under the auspices of the NCI.
The growing importance of PRO evaluation and studies was recognized by the RTOG more than two decades ago with the establishment of the RTOG QOL Subcommittee in 1991. The commitment of this subcommittee was to incorporate PRO end points into clinical trials to assess potential differences between treatment arms and study the impact of cancer therapy on patient PROs.65 RTOG developed a model that includes a comprehensive set of outcomes to guide phase III clinical trial end point development including clinical, economic, physical, biologic, and humanistic end points (Fig 1). PROs (QOL, utilities, symptoms) and neurocognitive and behavioral studies contribute to the humanistic end points. This model helps investigators consider all relevant end points and to posit associations between and among the end points. For example, RTOG-0534 (A Phase III Trial of Short-Term Androgen Deprivation With Pelvic Lymph Node or Prostate Bed Only Radiotherapy [SPPORT] in Prostate Cancer Patients With a Rising PSA After Radical Prostatectomy) is a phase III trial of short-term androgen-deprivation therapy with pelvic lymph node or prostate bed only radiotherapy in patients with a rising prostate-specific antigen scores after radical prostatectomy. The study will randomly assign more than 1,700 patients and will assess the outcomes depicted in Figure 1.
Fig 1.
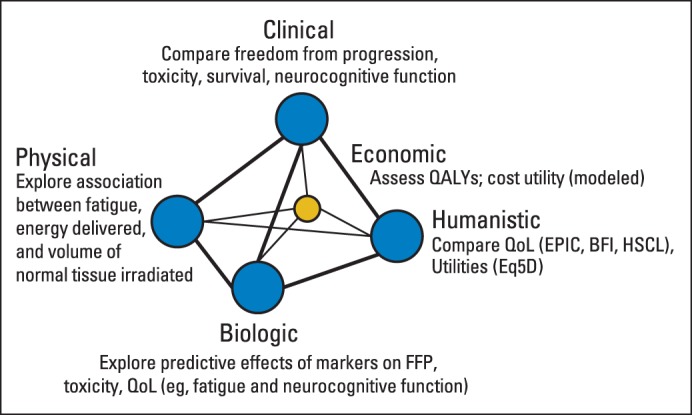
The Radiation Therapy Oncology Group outcomes model. BFI, Brief Pain Inventory; EPIC, Expanded Prostate Cancer Index Composite; EQ5D, EuroQOL Five Dimensions Questionnaire; FFP, freedom from progression; HSCL, Hopkins Symptom Checklist; QALY, quality-adjusted life year; QOL, quality of life.
RTOG studies focusing on PROs have helped to change clinical guidelines. RTOG-9714 (Randomized Trial of Palliative Radiation Therapy for Osseous Metastases: A Study of Palliation of Symptoms and Quality of Life), a phase III trial that compared 8 Gy in one fraction to 30 Gy in 10 fractions, found both regimens were equivalent in terms of patient-reported pain and objective narcotic relief at 3 months,66 and there were no QOL differences between arms. Moreover, despite a higher re-treatment rate, 8 Gy was found to be cost-effective.67 Data from that trial was used in the Palliative Radiotherapy for Bone Metastases: An ASTRO Evidence-Based Guideline,68 which is the most frequently cited article in the International Journal of Radiation Oncology, Biology, Physics from 2011. That study provided rationale for the currently active phase III RTOG-0631 (Phase II/III Study of Image-Guided Radiosurgery/SBRT for Localized Spine Metastasis) trial assessing pain relief from a novel radiation technology, radiosurgery (16 to 18 Gy in a single fraction), versus the 8 Gy using conventional techniques. This study also incorporates QOL (FACT-G) and the EuroQOL Five Dimensions Questionnaire (EQ-5D) for health utilities.
In another series of studies, RTOG has carefully evaluated the effect of prophylactic cranial irradiation (PCI) on neurocognition and QOL, which has similarly shaped clinical practice. Although PCI significantly reduced the risk of brain metastases in stage III non–small-cell lung cancer, RTOG-0214 (A Phase III Comparison of Prophylactic Cranial Irradiation Versus Observation in Patients With Locally Advanced Non–Small-Cell Lung Cancer) also demonstrated a significant decline in memory (Hopkins Verbal Learning Test) at 1 year, such that PCI is not considered a standard approach in this setting. Importantly, the clinical results69 and neurocognitive and QOL outcomes70 of that randomized study were published back-to-back in the Journal of Clinical Oncology to provide the whole story to the reader. Moreover, by planning the use of similar instruments and time points across studies, RTOG was able to perform a pooled analysis of RTOG randomized trials such as RTOG-0212 (A Phase II/III Randomized Trial of Two Dose Schedules for Delivering Prophylactic Cranial Irradiation for Patients With Limited Disease Small-Cell Lung Cancer) and RTOG-0214, which demonstrated that PCI is associated with a decline in Hopkins Verbal Learning Test score and self-reported cognitive functioning.71 This has led to an attempt to mitigate these effects of brain radiation by developing novel strategies such as memantine, a medication for Alzheimer's disease (RTOG-0614 [A Randomized, Phase III, Double-Blind, Placebo-Controlled Trial of Memantine for Prevention of Cognitive Dysfunction in Patients Receiving Whole-Brain Radiotherapy]) and hippocampal avoidance (RTOG-0933 [A Phase II Trial of Hippocampal Avoidance During Whole-Brain Radiotherapy for Brain Metastases]). Ultimately, phase III randomized trials should focus on PROs with clear hypotheses that can lead to clinically meaningful interventions.
RTOG has ongoing trials in which PRO end points are not only an integral part of the study, but are in fact the primary objective. For example, RTOG-0938 (A Randomized Phase II Trial of Hypofractionated Radiotherapy for Favorable Risk Prostate Cancer) is a randomized phase II trial comparing two hypofractionated radiation regimens. The bladder and bowel domains of the Expanded Prostate Cancer Index Composite (EPIC) instrument are being used to measure HRQOL as the primary end point of that study.
SURVIVORSHIP ISSUES IN RADIATION ONCOLOGY
PROs and QOL are especially important facets of cancer survivorship. The National Coalition for Cancer Survivorship defines an individual as a cancer survivor from the time of diagnosis. In 2012, there were nearly 14 million cancer survivors in the United States.72 Late effects of the tumor and related treatments affect many of these survivors. Given this significant burden, the American Society of Clinical Oncology (ASCO) has recognized the importance of survivorship care, the limitations of our existing care model, and opportunities for the future through education, research, and advocacy.73
There has been significant research on late effects in survivors of childhood cancer. In the United States, the largest body of work comes from the Childhood Cancer Survivor Study (CCSS).74 The CCSS is an NCI-funded study with data from more than 14,000 survivors of childhood cancer. Importantly, 4,000 siblings are also included in the study to provide a comparison cohort. This impressive effort has resulted in more than 200 publications describing the physical, emotional, and psychosocial effects seen in long-term survivors. The cumulative incidence of chronic health conditions in this patient population is more than 70% with more than half of those conditions being categorized as severe or life-threatening.75 There is a correlation between the presence of symptoms related to these late effects and lower QOL.76 However, despite the importance of PROs, there are fewer data regarding QOL issues as self-reported by survivors. These deficits in QOL vary depending on demographics, primary diagnosis, and treatment.77
Although a corresponding adult counterpart to the CCSS with the extensive treatment and survey data does not exist, there are numerous studies of survivors of adult cancer. For example, the National Health Interview Survey provides an opportunity to assess QOL in these survivors. QOL was assessed by using a 10-item general QOL version of PROMIS. In the 2010 survey, 1,822 self-reported cancer survivors were compared with 24,802 adults without a cancer history.78 Poor physical and mental health QOL was reported in 24.5% and 10.1% of the survivors compared with 10.2% and 5.9% in controls. Although there is a high prevalence of low QOL, there appears to be improvement in self-reported QOL over time.79,80
Because of the extensive late effects data in survivors of childhood cancer, the Children's Oncology Group (COG) has developed and published long-term follow-up guidelines81 that are based on the diagnosis and therapy received. As an example, for patients who received 20 Gy or more to the chest, COG recommends annual breast examination, mammogram, and breast magnetic resonance imaging scans starting 8 years after radiation or at age 25, whichever occurs later. Another example is cardiac function screening. COG follow-up recommendations for the frequency of echocardiogram testing depend on the age at the time of treatment, dose of radiation, and dose of anthracycline. However, there are fewer robust data for survivors of adult cancer. As a result, there are fewer follow-up recommendations for these patients. For example, the National Comprehensive Cancer Network (NCCN)82, makes specific recommendations for breast cancer screening for patients with Hodgkin lymphoma who received chest radiation, but this does not extend to other diagnoses. The NCCN does provide recommendations regarding anxiety, depression, cognitive function, exercise, fatigue, immunization, pain, sexual function, and sleep disorders.
Despite the limitations, the importance of survivorship continues to be recognized on a national level. The IOM first reported on this issue in 2005 in its report “From Cancer Patient to Cancer Survivor: Lost in Transition.”83 Guidelines for a Survivorship Care Plan (SCP) were outlined in that report. The two broad components of the SCP are a treatment plan summary and a follow-up care plan. Essential elements of the care plan include survivorship and psychosocial care plans, health promotions, and recommendations for care coordination with primary care. The importance of the SCP is underscored by the accreditation requirement of American College of Surgeons Commission on Cancer for providing an SCP by 2015. Despite the fact that the SCP is felt to be critical of the overall care of cancer survivors, use of the SCP is far from standard. Even in COG institutions, only 68% provide SCPs that require, on average, 2 hours for the initial SCP visit.84 Barriers to implementation include lack of evidence showing a benefit of SCPs, reimbursement, staff workload, insurance coverage, and lack of training.85,86 To help address these SCP barriers, ASCO has convened a working group to re-examine the IOM recommendations and provide some guidance to improve the successful implementation of SCPs. The American Society of Therapeutic Radiation Oncology (ASTRO) is also developing innovative programs to support and recognize cancer survivors, including the Survivor Circle Grants to honor cancer survivors.
Finally, increasing importance is being placed on the patient perspective. The IOM 2001 report “Crossing the Quality Chasm: A New Health System for the 21st Century”87 formally included patient centeredness as one of the six aims for improvement. Patient expectations and perceptions often differ from those of physicians.88 In the realm of survivorship care, patients concerns differ from the expectations of the primary care provider regarding who is responsible for disease follow-up. There is an even more substantial difference between patients and oncologists regarding who is responsible for disease follow-up, screening for new cancers, and general health care. Patients may also be unwilling to discuss QOL issues with their physicians.89 Better understanding and agreement between patients and physicians may further improve survivorship care.90
FUTURE OF PROs AND SURVIVORSHIP
With the realization of the importance of PROs and survivorship in cancer therapy and radiation oncology, these endeavors have moved more into the mainstream. Initiatives by NCI, FDA, RTOG, and other national and international agencies have led to recommendations and standardization for including PROs in clinical trials. Moreover, PROs are now starting to move beyond clinical trials and into the routine clinic setting itself. Recently, electronic PRO systems can link to a patient's electronic medical records and provide real-time e-mail alerts.91 Although implementation of such novel PRO systems into clinical practice will require additional training and resources, the core issues to be addressed are the ones highlighted in this review.
Recently, there have been exciting efforts to study the genetic and molecular pathways associated with changes in patient-reported symptoms.92 For example, RTOG has recently focused on studying fatigue in two large randomized trials on prostate cancer (RTOG-0815 [A Phase III Prospective Randomized Trial of Dose-Escalated Radiotherapy With or Without Short-Term Androgen Deprivation Therapy for Patients With Intermediate-Risk Prostate Cancer] and RTOG-0924 [Androgen Deprivation Therapy and High-Dose Radiotherapy With or Without Whole-Pelvic Radiotherapy in Unfavorable Intermediate or Favorable High-Risk Prostate Cancer: A Phase III Randomized Trial]). These studies, covering intermediate- to high-risk prostate cancer, incorporate the validated seven-item PROMIS fatigue short form at the same time points to create a large database that can be analyzed across these trials. Both studies are also correlating fatigue with circulating proinflammatory cytokines, which have been associated with cancer-related fatigue.93 Ultimately, PROs will play a key role in improving QOL for cancer survivors.
Footnotes
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.US Department of Health and Human Services, Food and Drug Administration. Guidance for Industry: Patient-Reported Outcome Measures–Use in Medical Product Development to Support Labeling Claims. http://www.fda.gov/downloads/Drugs/Guidances/UCM193282.pdf.
- 2.De Boer MF, Van den Borne B, Pruyn JF, et al. Psychosocial and physical correlates of survival and recurrence in patients with head and neck carcinoma: Results of a 6-year longitudinal study. Cancer. 1998;83:2567–2579. [PubMed] [Google Scholar]
- 3.de Graeff A, de Leeuw JR, Ros WJ, et al. Sociodemographic factors and quality of life as prognostic indicators in head and neck cancer. Eur J Cancer. 2001;37:332–339. doi: 10.1016/s0959-8049(00)00385-3. [DOI] [PubMed] [Google Scholar]
- 4.Fang FM, Liu YT, Tang Y, et al. Quality of life as a survival predictor for patients with advanced head and neck carcinoma treated with radiotherapy. Cancer. 2004;100:425–432. doi: 10.1002/cncr.20010. [DOI] [PubMed] [Google Scholar]
- 5.Kaasa S, Mastekaasa A, Lund E. Prognostic factors for patients with inoperable non-small cell lung cancer, limited disease: The importance of patients' subjective experience of disease and psychosocial well-being. Radiother Oncol. 1989;15:235–242. doi: 10.1016/0167-8140(89)90091-1. [DOI] [PubMed] [Google Scholar]
- 6.Langendijk H, Aaronson NK, de Jong JM, et al. The prognostic impact of quality of life assessed with the EORTC QLQ-C30 in inoperable non-small cell lung carcinoma treated with radiotherapy. Radiother Oncol. 2000;55:19–25. doi: 10.1016/s0167-8140(00)00158-4. [DOI] [PubMed] [Google Scholar]
- 7.Montazeri A, Milroy R, Hole D, et al. Quality of life in lung cancer patients: As an important prognostic factor. Lung Cancer. 2001;31:233–240. doi: 10.1016/s0169-5002(00)00179-3. [DOI] [PubMed] [Google Scholar]
- 8.Siddiqui F, Pajak TF, Watkins-Bruner D, et al. Pretreatment quality of life predicts for locoregional control in head and neck cancer patients: A Radiation Therapy Oncology Group analysis. Int J Radiat Oncol Biol Phys. 2008;70:353–360. doi: 10.1016/j.ijrobp.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 9.Movsas B, Moughan J, Sarna L, et al. Quality of life supersedes the classic prognosticators for long-term survival in locally advanced non-small-cell lung cancer: An analysis of RTOG 9801. J Clin Oncol. 2009;27:5816–5822. doi: 10.1200/JCO.2009.23.7420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giese-Davis J, Collie K, Rancourt KM, et al. Decrease in depression symptoms is associated with longer survival in patients with metastatic breast cancer: A secondary analysis. J Clin Oncol. 2011;29:413–420. doi: 10.1200/JCO.2010.28.4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bjordal K, Kaasa S. Psychological distress in head and neck cancer patients 7-11 years after curative treatment. Br J Cancer. 1995;71:592–597. doi: 10.1038/bjc.1995.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Langendijk JA, Doornaert P, Verdonck-de Leeuw IM, et al. Impact of late treatment-related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy. J Clin Oncol. 2008;26:3770–3776. doi: 10.1200/JCO.2007.14.6647. [DOI] [PubMed] [Google Scholar]
- 13.Johnson JR, Temple R. Food and Drug Administration requirements for approval of new anticancer drugs. Cancer Treat Rep. 1985;69:1155–1159. [PubMed] [Google Scholar]
- 14.Johnson JR, Ning YM, Farrell A, et al. Accelerated approval of oncology products: The Food and Drug Administration experience. J Natl Cancer Inst. 2011;103:636–644. doi: 10.1093/jnci/djr062. [DOI] [PubMed] [Google Scholar]
- 15.Ahmed S, Berzon RA, Revicki DA, et al. The use of patient-reported outcomes (PRO) within comparative effectiveness research: Implications for clinical practice and health care policy. Med Care. 2012;50:1060–1070. doi: 10.1097/MLR.0b013e318268aaff. [DOI] [PubMed] [Google Scholar]
- 16.Reeve BB, Wyrwich KW, Wu AW, et al. ISOQOL recommends minimum standards for patient-reported outcome measures used in patient-centered outcomes and comparative effectiveness research. Qual Life Res. 2013;22:1889–1905. doi: 10.1007/s11136-012-0344-y. [DOI] [PubMed] [Google Scholar]
- 17.Institute of Medicine, Committee on Comparative Effectiveness Research Prioritization. Washington, DC: National Academies Press; 2009. Initial National Priorities for Comparative Effectiveness Research. [Google Scholar]
- 18.Grosse SD. Assessing cost-effectiveness in healthcare: History of the $50,000 per QALY threshold. Expert Rev Pharmacoecon Outcomes Res. 2008;8:165–178. doi: 10.1586/14737167.8.2.165. [DOI] [PubMed] [Google Scholar]
- 19.Konski A, Sherman E, Krahn M, et al. Economic analysis of a phase III clinical trial evaluating the addition of total androgen suppression to radiation versus radiation alone for locally advanced prostate cancer (Radiation Therapy Oncology Group protocol 86-10) Int J Radiat Oncol Biol Phys. 2005;63:788–794. doi: 10.1016/j.ijrobp.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 20.Van Den Brink M, Van Den Hout WB, Stiggelbout AM, et al. Cost-utility analysis of preoperative radiotherapy in patients with rectal cancer undergoing total mesorectal excision: A study of the Dutch Colorectal Cancer Group. J Clin Oncol. 2004;22:244–253. doi: 10.1200/JCO.2004.04.198. [DOI] [PubMed] [Google Scholar]
- 21.van den Hout WB, van der Linden YM, Steenland E, et al. Single- versus multiple-fraction radiotherapy in patients with painful bone metastases: Cost-utility analysis based on a randomized trial. J Natl Cancer Inst. 2003;95:222–229. doi: 10.1093/jnci/95.3.222. [DOI] [PubMed] [Google Scholar]
- 22.Kohler RE, Sheets NC, Wheeler SB, et al. Two-year and lifetime cost-effectiveness of intensity modulated radiation therapy versus 3-dimensional conformal radiation therapy for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2013;87:683–689. doi: 10.1016/j.ijrobp.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 23.Mailhot Vega RB, Kim J, Bussière M, et al. Cost effectiveness of proton therapy compared with photon therapy in the management of pediatric medulloblastoma. Cancer. 2013;119:4299–4307. doi: 10.1002/cncr.28322. [DOI] [PubMed] [Google Scholar]
- 24.Sloan JA, Sargent DJ, Novotny PJ, et al. Calibration of quality-adjusted life years for oncology clinical trials. J Pain Symptom Manage. doi: 10.1016/j.jpainsymman.2013.07.016. [epub ahead of print November 15, 2013] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarna L, Swann S, Langer C, et al. Clinically meaningful differences in patient-reported outcomes with amifostine in combination with chemoradiation for locally advanced non-small-cell lung cancer: An analysis of RTOG 9801. Int J Radiat Oncol Biol Phys. 2008;72:1378–1384. doi: 10.1016/j.ijrobp.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Slevin ML, Plant H, Lynch D, et al. Who should measure quality of life, the doctor or the patient? Br J Cancer. 1988;57:109–112. doi: 10.1038/bjc.1988.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watkins-Bruner D, Scott C, Lawton C, et al. RTOG's first quality of life study: RTOG 90-20—A phase II trial of external beam radiation with etanidazole for locally advanced prostate cancer. Int J Radiat Oncol Biol Phys. 1995;33:901–906. doi: 10.1016/0360-3016(95)02002-5. [DOI] [PubMed] [Google Scholar]
- 28.Sonn GA, Sadetsky N, Presti JC, et al. Differing perceptions of quality of life in patients with prostate cancer and their doctors. J Urol. 2013;189:S59–S65. doi: 10.1016/j.juro.2012.11.032. [DOI] [PubMed] [Google Scholar]
- 29.Sommers BD, Beard CJ, D'Amico AV, et al. Predictors of patient preferences and treatment choices for localized prostate cancer. Cancer. 2008;113:2058–2067. doi: 10.1002/cncr.23807. [DOI] [PubMed] [Google Scholar]
- 30.Stiggelbout AM, de Haes JC. Patient preference for cancer therapy: An overview of measurement approaches. J Clin Oncol. 2001;19:220–230. doi: 10.1200/JCO.2001.19.1.220. [DOI] [PubMed] [Google Scholar]
- 31.Detmar SB, Aaronson NK, Wever LD, et al. How are you feeling? Who wants to know? Patients' and oncologists' preferences for discussing health-related quality-of-life issues. J Clin Oncol. 2000;18:3295–3301. doi: 10.1200/JCO.2000.18.18.3295. [DOI] [PubMed] [Google Scholar]
- 32.Detmar SB, Muller MJ, Schornagel JH, et al. Health-related quality-of-life assessments and patient-physician communication: A randomized controlled trial. JAMA. 2002;288:3027–3034. doi: 10.1001/jama.288.23.3027. [DOI] [PubMed] [Google Scholar]
- 33.Velikova G, Booth L, Smith AB, et al. Measuring quality of life in routine oncology practice improves communication and patient well-being: A randomized controlled trial. J Clin Oncol. 2004;22:714–724. doi: 10.1200/JCO.2004.06.078. [DOI] [PubMed] [Google Scholar]
- 34.Testa MA, Simonson DC. Assessment of quality-of-life outcomes. N Engl J Med. 1996;334:835–840. doi: 10.1056/NEJM199603283341306. [DOI] [PubMed] [Google Scholar]
- 35.Gotay CC, Korn EL, McCabe MS, et al. Quality-of-life assessment in cancer treatment protocols: Research issues in protocol development. J Natl Cancer Inst. 1992;84:575–579. doi: 10.1093/jnci/84.8.575. [DOI] [PubMed] [Google Scholar]
- 36.Gelber RD, Gelber S. Quality-of-life assessment in clinical trials. Cancer Treat Res. 1995;75:225–246. doi: 10.1007/978-1-4615-2009-2_11. [DOI] [PubMed] [Google Scholar]
- 37.Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: Development and validation of the general measure. J Clin Oncol. 1993;11:570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 38.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 39.Stewart AL, Ware JE., Jr . Durham, NC: Duke University Press; 1992. Measuring Functioning and Well-Being: The Medical Outcomes Study Approach. [Google Scholar]
- 40.McHorney CA, Ware JE, Jr, Lu JF, et al. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care. 1994;32:40–66. doi: 10.1097/00005650-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 41.McHorney CA, Ware JE, Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 42.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 43.Brady MJ, Cella DF, Mo F, et al. Reliability and validity of the Functional Assessment of Cancer Therapy-Breast quality-of-life instrument. J Clin Oncol. 1997;15:974–986. doi: 10.1200/JCO.1997.15.3.974. [DOI] [PubMed] [Google Scholar]
- 44.Cella DF, Bonomi AE, Lloyd SR, et al. Reliability and validity of the Functional Assessment of Cancer Therapy-Lung (FACT-L) quality of life instrument. Lung Cancer. 1995;12:199–220. doi: 10.1016/0169-5002(95)00450-f. [DOI] [PubMed] [Google Scholar]
- 45.Osoba D, Aaronson NK, Muller M, et al. The development and psychometric validation of a brain cancer quality-of-life questionnaire for use in combination with general cancer-specific questionnaires. Qual Life Res. 1996;5:139–150. doi: 10.1007/BF00435979. [DOI] [PubMed] [Google Scholar]
- 46.Department of Health and Human Services, National Institutes of Health. PROMIS: Dynamic Tools to Measure Health Outcomes From the Patient Perspective. www.nihpromis.org.
- 47.Mapi Research Trust. PROQOLID: Patient-reported outcome and quality of life instruments database. www.proqolid.org.
- 48.National Cancer Institute, American College of Radiology. Radiation Therapy Oncology Group (RTOG) QOL/PRO library. http://www.rtog.org/Researchers/QOLPROMaterials.aspx.
- 49.The Patient Reported Outcomes Measurement Information System (PROMIS) A Walk Through the First Four Years. http://www.nihpromis.org/Documents/PROMIS_The_First_Four_Years.pdf.
- 50.Cella D, Yount S, Rothrock N, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS): Progress of an NIH Roadmap cooperative group during its first two years. Med Care. 2007;45:S3–S11. doi: 10.1097/01.mlr.0000258615.42478.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.National Cancer Institute, Applied Research, Cancer Control and Population Sciences. Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) http://appliedresearch.cancer.gov/tools/pro-ctcae.html. [DOI] [PMC free article] [PubMed]
- 52.Calvert M, Blazeby J, Altman DG, et al. Reporting of patient-reported outcomes in randomized trials: The CONSORT PRO extension. JAMA. 2013;309:814–822. doi: 10.1001/jama.2013.879. [DOI] [PubMed] [Google Scholar]
- 53.Calvert M, Brundage M, Jacobsen PB, et al. The CONSORT Patient-Reported Outcome (PRO) extension: Implications for clinical trials and practice. Health Qual Life Outcomes. 2013;11:184. doi: 10.1186/1477-7525-11-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wyrwich KW, Bullinger M, Aaronson N, et al. Estimating clinically significant differences in quality of life outcomes. Qual Life Res. 2005;14:285–295. doi: 10.1007/s11136-004-0705-2. [DOI] [PubMed] [Google Scholar]
- 55.Guyatt GH, Osoba D, Wu AW, et al. Methods to explain the clinical significance of health status measures. Mayo Clin Proc. 2002;77:371–383. doi: 10.4065/77.4.371. [DOI] [PubMed] [Google Scholar]
- 56.Osoba D, Rodrigues G, Myles J, et al. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16:139–144. doi: 10.1200/JCO.1998.16.1.139. [DOI] [PubMed] [Google Scholar]
- 57.Lydick E, Epstein RS. Interpretation of quality of life changes. Qual Life Res. 1993;2:221–226. doi: 10.1007/BF00435226. [DOI] [PubMed] [Google Scholar]
- 58.Movsas B. Quality of life in oncology trials: A clinical guide. Semin Radiat Oncol. 2003;13:235–247. doi: 10.1016/S1053-4296(03)00029-8. [DOI] [PubMed] [Google Scholar]
- 59.Siddiqui F, Kachnic LA, Movsas B. Quality-of-life outcomes in oncology. Hematol Oncol Clin North Am. 2006;20:165–185. doi: 10.1016/j.hoc.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 60.Cohen J. Statistical Power Analysis for the Behavioral Sciences (ed 2) Hillsdale, NJ: L. Erlbaum Associates; 1988. [Google Scholar]
- 61.Fayers PM, Curran D, Machin D. Incomplete quality of life data in randomized trials: Missing items. Stat Med. 1998;17:679–696. doi: 10.1002/(sici)1097-0258(19980315/15)17:5/7<679::aid-sim814>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 62.Curran D, Molenberghs G, Fayers PM, et al. Incomplete quality of life data in randomized trials: Missing forms. Stat Med. 1998;17:697–709. doi: 10.1002/(sici)1097-0258(19980315/15)17:5/7<697::aid-sim815>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 63.Troxel AB, Fairclough DL, Curran D, et al. Statistical analysis of quality of life with missing data in cancer clinical trials. Stat Med. 1998;17:653–666. doi: 10.1002/(sici)1097-0258(19980315/15)17:5/7<653::aid-sim812>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 64.Movsas B, Hunt D, Watkins-Bruner D, et al. Can electronic web-based technology improve quality of life data collection? Analysis of Radiation Therapy Oncology Group 0828. Pract Radiat Oncol. 2014;4:187–191. doi: 10.1016/j.prro.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Scott CB, Stetz J, Bruner DW, et al. Radiation Therapy Oncology Group quality of life assessment: Design, analysis, and data management issues. Qual Life Res. 1994;3:199–206. doi: 10.1007/BF00435385. [DOI] [PubMed] [Google Scholar]
- 66.Hartsell WF, Scott CB, Bruner DW, et al. Randomized trial of short- versus long-course radiotherapy for palliation of painful bone metastases. J Natl Cancer Inst. 2005;97:798–804. doi: 10.1093/jnci/dji139. [DOI] [PubMed] [Google Scholar]
- 67.Konski AA, Winter K, Cole BF, et al. Quality-adjusted survival analysis of Radiation Therapy Oncology Group (RTOG) 90-03: Phase III randomized study comparing altered fractionation to standard fractionation radiotherapy for locally advanced head and neck squamous cell carcinoma. Head Neck. 2009;31:207–212. doi: 10.1002/hed.20949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lutz S, Berk L, Chang E, et al. Palliative radiotherapy for bone metastases: An ASTRO evidence-based guideline. Int J Radiat Oncol Biol Phys. 2011;79:965–976. doi: 10.1016/j.ijrobp.2010.11.026. [DOI] [PubMed] [Google Scholar]
- 69.Gore EM, Bae K, Wong SJ, et al. Phase III comparison of prophylactic cranial irradiation versus observation in patients with locally advanced non-small-cell lung cancer: Primary analysis of Radiation Therapy Oncology Group study RTOG 0214. J Clin Oncol. 2011;29:272–278. doi: 10.1200/JCO.2010.29.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sun A, Bae K, Gore EM, et al. Phase III trial of prophylactic cranial irradiation compared with observation in patients with locally advanced non-small-cell lung cancer: Neurocognitive and quality-of-life analysis. J Clin Oncol. 2011;29:279–286. doi: 10.1200/JCO.2010.29.6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gondi V, Paulus R, Bruner DW, et al. Decline in tested and self-reported cognitive functioning after prophylactic cranial irradiation for lung cancer: Pooled secondary analysis of Radiation Therapy Oncology Group randomized trials 0212 and 0214. Int J Radiat Oncol Biol Phys. 2013;86:656–664. doi: 10.1016/j.ijrobp.2013.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62:220–241. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 73.McCabe MS, Bhatia S, Oeffinger KC, et al. American Society of Clinical Oncology statement: Achieving high-quality cancer survivorship care. J Clin Oncol. 2013;31:631–640. doi: 10.1200/JCO.2012.46.6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.St. Jude Children's Research Hospital. Childhood Cancer Survivor Study (CCSS) ccss.stjude.org.
- 75.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 76.Huang IC, Brinkman TM, Kenzik K, et al. Association between the prevalence of symptoms and health-related quality of life in adult survivors of childhood cancer: A report from the St Jude Lifetime Cohort study. J Clin Oncol. 2013;31:4242–4251. doi: 10.1200/JCO.2012.47.8867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zeltzer LK, Recklitis C, Buchbinder D, et al. Psychological status in childhood cancer survivors: A report from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2396–2404. doi: 10.1200/JCO.2008.21.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Weaver KE, Forsythe LP, Reeve BB, et al. Mental and physical health-related quality of life among U.S. cancer survivors: Population estimates from the 2010 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev. 2012;21:2108–2117. doi: 10.1158/1055-9965.EPI-12-0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Smith SK, Mayer DK, Zimmerman S, et al. Quality of life among long-term survivors of non-Hodgkin lymphoma: A follow-up study. J Clin Oncol. 2013;31:272–279. doi: 10.1200/JCO.2011.40.6249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hsu T, Ennis M, Hood N, et al. Quality of life in long-term breast cancer survivors. J Clin Oncol. 2013;31:3540–3548. doi: 10.1200/JCO.2012.48.1903. [DOI] [PubMed] [Google Scholar]
- 81.Children's Oncology Group. Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers, Version 3.0. www.survivorshipguidelines.org.
- 82.National Comprehensive Cancer Network. Home page. Network. http://www.nccn.org/professionals/physician_gls/pdf/hodgkins.pdf.
- 83.Institute of Medicine. From Cancer Patient to Cancer Survivor: Lost in Transition, 2005. www.iom.edu/Reports/2005/From-Cancer-Patient-to-Cancer-Survivor-Lost-in-Transition.aspx.
- 84.Eshelman-Kent D, Kinahan KE, Hobbie W, et al. Cancer survivorship practices, services, and delivery: A report from the Children's Oncology Group (COG) nursing discipline, adolescent/young adult, and late effects committees. J Cancer Surviv. 2011;5:345–357. doi: 10.1007/s11764-011-0192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Earle CC, Ganz PA. Cancer survivorship care: Don't let the perfect be the enemy of the good. J Clin Oncol. 2012;30:3764–3768. doi: 10.1200/JCO.2012.41.7667. [DOI] [PubMed] [Google Scholar]
- 86.Virgo KS, Lerro CC, Klabunde CN, et al. Barriers to breast and colorectal cancer survivorship care: Perceptions of primary care physicians and medical oncologists in the United States. J Clin Oncol. 2013;31:2322–2336. doi: 10.1200/JCO.2012.45.6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century, 2001. www.iom.edu/Reports/2001/Crossing-the-Quality-Chasm-A-New-Health-System-for-the-21st-Century.aspx. [PubMed]
- 88.Cheung WY, Neville BA, Cameron DB, et al. Comparisons of patient and physician expectations for cancer survivorship care. J Clin Oncol. 2009;27:2489–2495. doi: 10.1200/JCO.2008.20.3232. [DOI] [PubMed] [Google Scholar]
- 89.Arora NK, Jensen RE, Sulayman N, et al. Patient-physician communication about health-related quality-of-life problems: Are non-Hodgkin lymphoma survivors willing to talk? J Clin Oncol. 2013;31:3964–3970. doi: 10.1200/JCO.2012.47.6705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cheung WY, Neville BA, Earle CC. Associations among cancer survivorship discussions, patient and physician expectations, and receipt of follow-up care. J Clin Oncol. 2010;28:2577–2583. doi: 10.1200/JCO.2009.26.4549. [DOI] [PubMed] [Google Scholar]
- 91.Bennett AV, Jensen RE, Basch E. Electronic patient-reported outcome systems in oncology clinical practice. CA Cancer J Clin. 2012;62:337–347. doi: 10.3322/caac.21150. [DOI] [PubMed] [Google Scholar]
- 92.Sprangers MA, Sloan JA, Veenhoven R, et al. The establishment of the GENEQOL consortium to investigate the genetic disposition of patient-reported quality-of-life outcomes. Twin Res Hum Genet. 2009;12:301–311. doi: 10.1375/twin.12.3.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schubert C, Hong S, Natarajan L, et al. The association between fatigue and inflammatory marker levels in cancer patients: A quantitative review. Brain Behav Immun. 2007;21:413–427. doi: 10.1016/j.bbi.2006.11.004. [DOI] [PubMed] [Google Scholar]


