Abstract
The balance between pro- and anti-inflammatory signalling is critical to maintain the immune homeostasis under physiological conditions as well as for the control of inflammation in different pathological settings. Recent progress in the signalling pathways that control this balance has led to the development of novel therapeutic agents for diseases characterized by alterations in the activation/suppression of the immune response. Different molecules have a key role in the regulation of the immune system, including the receptors PD-1 (Programmed cell Death 1), CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4) and galectins; or the intracellular enzyme IDO (indoleamine 2,3-dioxygenase). In addition, other molecules as CD69, AhR (Aryl hydrocarbon Receptor), and GADD45 (Growth Arrest and DNA Damage-inducible 45) family members, have emerged as potential targets for the regulation of the activation/suppression balance of immune cells. This review offers a perspective on well-characterized as well as emergent negative immune regulatory molecules in the context of autoimmune inflammatory diseases.
Keywords: Immunoregulatory molecules, Tolerance, Treg, Inflammation, Autoimmune diseases
1. Introduction
The magnitude of the immune response is determined by the balance between positive and negative regulatory signals. Thus, different immunosuppressive mechanisms are essential to maintain an effective level of immune response in different pathological conditions without causing tissue damage, and also to preserve the homeostasis under physiological conditions. Central tolerance occurs in the thymus and is established by the deletion of auto-reactive lymphocytes through the presentation of high affinity self-antigens [1]. However, a small proportion of auto-reactive T lymphocytes are not deleted by the thymic negative selection and these cells are able to reach the periphery. Since the immune system is constantly exposed to self-antigens, several immune strategies have been developed to avoid the generation of deleterious autoimmune responses, including the activation-induced cell death, clonal anergy and immunosuppression mediated by regulatory T cells (Treg) [2]. During the last years, numerous efforts have focused in understanding the mechanisms that regulate the immune response. Novel pathways and new therapeutic agents are currently under investigation for those pathological conditions in which the normal balance between activation and suppression of the immune response has failed [3]. Cell surface receptors, soluble proteins and signalling molecules provide critical signals to limit the immune response. Some of these molecules display a well-characterized negative regulatory function of the inflammatory response. Some examples are the cytokines TGF-beta and IL-10 which, in addition to their direct effects on immune cells, also control Treg cell differentiation and function [4,5].
Cell surface receptors such as members of the B7:CD28 family also have key roles in the regulation of T cell activation and tolerance. These B7:CD28 pathways are not only essential second signals that promote T cell responses, but they also originate critical negative second signals that limit T cell activation [6].
In addition to cell receptors and cytokines, a number of intracellular molecules are also key components of tolerance mechanisms. Some examples are the cytosolic enzyme indoleamine 2,3-dioxygenase (IDO) and SOCS signalling proteins, which are molecular switches that provide negative regulation of cytokine signalling. Recent studies in humans and animal models have revealed new molecules involved in regulation on the immune response. Some examples are galectins and CD69 as well as selected members of the GADD45 family. These molecules are part of complex signalling networks in which deregulation of one of these molecules often alters the expression and/or function of others, tilting the scales against tolerance.
We review herein the characteristics of an apparent heterogeneous group of molecules, which have in common that exert an important role in the regulation of the inflammatory phenomenon and the immune response.
2. Leukocyte membrane receptor molecules: CTLA-4, PD-1, ICOS, CD69 and galectins
2.1. The familiy of B7/CD28 immunoregulatory molecules
The CD28 family of receptors comprise at least four members (CD28, CTLA-4, ICOS, and PD-1), which upon interaction with their corresponding ligands are able to generate potent costimulatory or inhibitory signals in T lymphocytes.
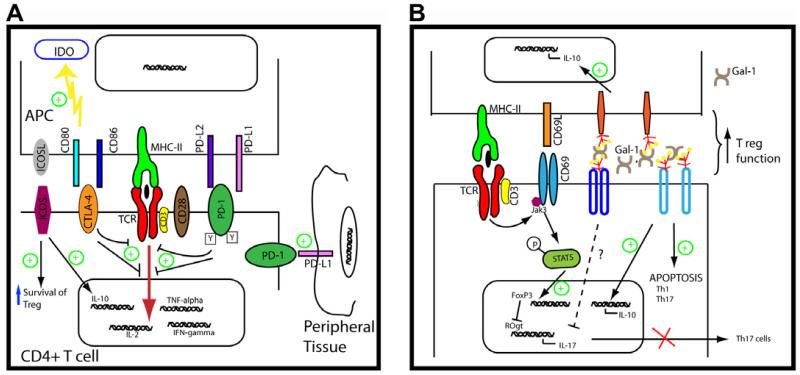
The CD80/CD86 (B7):CD28/CTLA-4 pathways are the best characterized T cell co-stimulatory signals. It is a complex functional axis due to the dual specificity of CD80 and CD86 for the stimulatory receptor CD28 as well as the inhibitory receptor CTLA-4. CD28/B7 interactions mediate co-stimulation and significantly enhance peripheral T-cell responses. In contrast, CTLA-4 activation decreases T-lymphocyte activity and limits the immune response. Similarly, PD-1 receptor interactions with its ligands PD-L1 and PD-L2 down-regulate T-cell immune responses. Despite these similarities, the regulatory roles of the CTLA-4 and PD-1 pathways are different. This may be due, at least in part, to the differential temporal and spatial expression patterns of their ligands. CTLA-4 signalling seems to be required early in the lymph node during initiation of an immune response, while PD-1 pathway acts late in the periphery to limit T-cell activity locally [6]. Inducible T cell Costimulator (ICOS) has overlapping functions with CD28 in early T-cell activation, and has emerged as an important receptor in the immune system to regulate T-cell effector functions. In addition, ICOS plays a critical role in controlling IL-10 producing Tregs [7].
2.2. CTLA-4
The function of CTLA-4 in the control of T cell responses is exerted by synergistic actions, on effector T cells and Tregs. CD28 is constitutively expressed on T cells and CTLA-4 is up-regulated upon TCR activation. Such expression pattern supports that the intrinsic role of CTLA-4 is to regulate T-cell responses after cells have received co-stimulatory signals. The higher affinity and avidity of CTLA-4 for both B7 ligands compared to CD28 seems to be critical in maintaining immune tolerance. CTLA-4 binding to B7 proteins antagonizes early T-cell activation, leading to decreased IL-2 production, inhibition of cell cycle progression, decreased cyclin expression, and negative modulation of TCR signalling [8] (Fig. 1A).
Fig. 1. Negative regulatory molecules on the cellular membrane surface.
(A) B7 family members: CTLA-4, ICOS, ICOSL, PD-1, PD-L-1 and PD-L2. CTLA-4 competes with CD28 for binding to their counter-receptors CD80 and CD86 and attenuates TCR signalling induced during Ag recognition. In addition, the engagement of CTLA-4 induces IDO expression on APC, reinforcing its immunoregulatory effect. PD-1 signalling induced by its ligands (PD-L1 and PD-L2) expressed on APC also counteracts TCR activation, inhibiting early proximal signalling events. Also, PD-1 can interact with PD-L1 expressed in peripheral tissues contributing to peripheral tolerance. ICOS/ICOSL pathway regulates T-cell tolerance through the control of IL-10-producing Tregs. (B) Signalling pathways influenced by CD69 and Gal-1. CD69 is early induced following TCR activation. The engagement of CD69 by as yet an unknown ligand activates Jak3-STAT5 pathway promoting Treg differentiation and Th17 inhibition. Gal-1 can be found on the membrane surface linked to glycoreceptors inducing apoptosis of Th1 and Th17 cells and promoting IL-10 production. Both molecules facilitate Treg function. Green plus symbols indicate tolerogenic signals.
Mice deficient for CTLA-4 develop a lympho-proliferative disease and die within 3-4 weeks of age [9]. This is a strong evidence of the critical role of CTLA-4 in the inhibition of T-cell responses and immune homeostasis. Importantly, some polymorphisms in the CTLA-4 gene have been associated with the susceptibility to different autoimmune diseases, including type 1 diabetes (T1D), multiple sclerosis (MS), rheumatoid arthritis (RA), Graves’ disease, colitis and systemic lupus erythematosus [10].
Tregs cells constitutively express high levels of CTLA-4, suggesting that this molecule participates in their suppressive activity [11]. However, normal numbers of CD4+CD25+Foxp3+Tregs cells are found in CTLA-4-null mice [12], indicating that this molecule is dispensable for the development, survival, and homeostasis of Tregs lymphocytes. CTLA-4 blockade specifically affected the suppression capacity of Tregs in vitro and in an experimental model of colitis [13,14]. In addition, CTLA-4-deficient Tregs inefficiently controlled T effector responses in an adoptive transfer model of diabetes [15].
Additionally, CTLA-4 signalling influences CD4+ T-cell differentiation. CTLA-4-deficient mice display T cells strongly skewed towards a Th2 phenotype, even in the absence of the Th2 lineage transcription factor signal transducer and activator of transcription-6 (STAT6) [16]. Consistently, enhanced Th2 cell differentiation after anti-CTLA-4 mAb injections in BALB/c mice resulted in more severe airway inflammation, bronchial eosinophilia and IL-4 and IL-5 levels in broncho-alveolar lavage following repeated allergen inhalations. Importantly, a significant reduction of TGF-beta synthesis was detected in animals in which CTLA-4 signalling was blocked, suggesting a potential mechanism for the regulation of Th2 sensitization [17]. CTLA-4 also influences Th17 responses; anti-CTLA-4 monoclonal antibodies increased Th17 differentiation and IL-17 production both in vitro and in vivo [18]. In addition, several studies indicate that CTLA-4 regulates the severity of peptide-induced experimental autoimmune encephalomyelitis (EAE), in mouse strains that are inherently susceptible to the disease [19]. CTLA-4 engagement also controls disease susceptibility in BALB/c mice, a strain considered to be resistant to EAE [20]. More-over, CTLA-4 blockade enhances initial and ongoing experimental autoimmune neuritis in B6 mice [21]. Thus, the CTLA-4/B7 inhibitory pathway plays a critical role in the control of auto-reactivity and the breakdown of tolerance to auto-antigens. Given its negative regulatory role, CTLA-4 is a potential therapeutic target to foster the immune response in certain pathological settings. Indeed, CTLA-4 blockade with the monoclonal antibody ipilimumab has a significant therapeutic effect in patients with advanced or metastatic melanoma, and two randomized phase III studies demonstrated that CTLA-4 blockade prolonged overall survival in these patients, leading to its approval by the FDA [22].
2.3. PD-1
PD-1 is induced on peripheral CD4+ and CD8+ T cells, B cells and monocytes upon activation. PD-1 ligands have distinct patterns of expression: PD-L1 is constitutively expressed in myeloid and lymphoid cells and is up-regulated upon their activation, it is also expressed in non-hematopoietic cells and in non-lymphoid organs. In contrast, expression of PD-L2 is restricted to macrophages and DCs in response to cytokines; PD-L2 is also expressed on bone marrow-derived mast cells and B cells. PD-1 ligation inhibits PI3K activation and alters membrane-proximal signalling events in T cells (Fig. 1A). An additional tolerance mechanism of PD-1 is the promotion of Treg development and function [23].
The role of PD-1 in immune tolerance was indicated by the development of autoimmunity in PD-1 knock-out (KO) mice. PD-1KO mice on a C57BL/6 background displayed arthritis and glomerulonephritis [24], while the disruption of the PD-1 gene in BALB/c mice causes dilated cardiomyopathy. High-titers of autoantibodies against cardiac troponin I (cTnI) are detected on these animals, which clearly indicate that PD-1 controls autoimmunity [25]. In addition, PD-1 is critical for the maintenance of peripheral tolerance and the prevention of several autoimmune diseases, including type 1 diabetes (T1D) and experimental autoimmune encephalitis (EAE) [26,27]. Accordingly, the induction of expression of PD-L1 prevents lupus nephritis in BXSB mice [28], strongly suggesting that engagement of the immuno-inhibitory receptor PD-1 on activated lymphocytes is able to preclude unwanted T and B cell activation.
Human genomic studies have confirmed the immunoregulatory role of PD-1. Thus, the single nucleotide polymorphism (SNP) in PD-1 termed PD-1.3 is significantly linked to development of Systemic Lupus Erythematosus (SLE) [29], and lupus nephritis [30]. Moreover, the same SNP has also been associated with increased risk of T1D [31] and with the development of seronegative RA [32]. This SNP is located in an enhancer of PD-1 gene within its fourth intron. This SNP affects the binding of the runtrelated transcription factor (RUNX-1) diminishing the expression of this receptor [29]. In this regard, lower PD-1 receptor expression is found in SLE patients and their relatives, with a significant correlation with the PD-1.3A allele [33]. In addition, this PD-1 polymorphism is associated with disease progression in MS. Interestingly, PD-1-mediated inhibition of T-cell cytokine secretion (IFN-gamma) is impaired in patients carrying this PD-1 polymorphism [34].
Other finding that supports the role of PD-1/PD-L1 in RA is the presence of autoantibodies against PD-L1 in sera of patients with RA, correlating with active disease [35]. The presence of autoantibodies against PD-L1 could favour the presence of defective T cell responses in RA patients. In addition, soluble forms of PD-1 and PD-L1 have been detected in sera and synovial fluid (SF) of RA patients. The levels of soluble PD-1 significantly correlated with those of TNF-alpha in SF. Characterization of soluble PD-1 revealed that it corresponded to an alternative splice variant (PD-1Deltaex3) that could affect the regulatory function of membrane-bound PD-1 on T cell activation [36].
Up-regulation of PD-L2, CD40 and CD86 has been detected in patients with MS under therapy with IFN-beta. These data suggest that modulation of positive and negative co-stimulatory signals is part of the mechanism of action of this cytokine in MS [37]. Other authors have reported upregulation of PD-1 and PD-L1 that results in a higher production of IL-10 and a higher rate of apoptosis during the quiescent phases of the MS, However, in this study, successful therapy with IFN-beta resulted in a down-regulation of PD-L1-expressing cells [38]. Recent studies suggest that defects in PD-1 and its ligands participate in the immunopathogenesis of MS. Although PD-L1 is significantly expressed at higher levels in MS brain lesions compared to controls, most CD8+ T lymphocytes found in MS lesions are negative for PD-1. Moreover, while blood vessels in normal brain tissues are PD-L2 positive, only about half of them express PD-L2 in MS lesions. The absence of PD-1 could render CD8+ T cells refractory to the inhibitory signal from PD-L1 expressing CNS cells [39].
2.4. ICOS/ICOSL
ICOS is expressed on T cells following activation, while ICOS ligand (ICOSL) is detected on APC and non-hematopoietic cells. ICOS/ICOSL pathway plays a critical role in the induction of effector T-cells responses and in the generation of follicular helper T cells, an important T-cell subset that promotes humoral immunity. In this regard, blockade of ICOS/ICOSL pathways ameliorates inflammation in several models of autoimmune diseases, such as CIA and lupus nephritis [7]. However, recent evidence supports the role of ICOS/ICOSL pathway in immune tolerance (Fig. 1A). Thus, it has been described that ICOS regulates the survival of both effectormemory T cells and FoxP3+ Treg cells during homeostasis and during Ag-specific immune response [40]. Indeed, ICOS deficiency or its blockade results in an exacerbated forms of EAE [41,42] and T1D [43]. In addition, ICOS/ICOSL pathway has a critical role in the induction of tolerance to allergens, by promoting the development and function of IL-10 producing Treg cells [44]. In this regard, it has been reported that ICOS expression identifies a subset of highly suppressive CD4+Foxp3+ Treg cells that display a suppressive potential in a model of contact hypersensitivity [45], as well as a subpopulation of intra-islet Treg lymphocytes in a T1D model [46]. Moreover, deficiency of ICOS abrogated Treg-mediated functions and exacerbated the disease in this model [46]. Recent data show that Langerhans cells (LCs) are able to induce cutaneous immune tolerance by a mechanism that involves the activation of ICOS+CD4+Foxp3+Treg cells [47]. The role of ICOS in immune tolerance is also supported by studies with CD4+ T cells from ICOS deficient patients, where it has been demonstrated that the absence of ICOS inhibits the induction of T cell anergy and differentiation into suppressive T cells by tolerogenic DCs [48]. Beside the role of IL-10 in the function of ICOS+ Treg cells, a recent report shows that these cells are able to suppress IL-17 production through the synthesis of IL-35 [49].
2.5. CD69
CD69 is a type II transmembrane protein and a member of the C-type lectin-like receptor family that is expressed in leukocytes upon stimulation [50]. It is mainly detected in the cell infiltrates of several chronic inflammatory diseases such as RA [51]; however, no apparent association has been detected between polymorphisms of CD69 and enhanced risk for this condition [52].
CD69 acts as a signal transducer in inflammatory processes. Recent studies using models of inflammatory disease point to an immunoregulatory role for CD69 during the immune response. Mice lacking CD69 develop exacerbated forms of CIA [53]. Accordingly, in a hapten-induced cutaneous contact hypersensitivity rodent model, CD69 deletion or the administration of blocking anti-CD69 mAb increased inflammation. CD69 deficiency also induced enhanced inflammatory responses in animal models of allergic asthma [54], autoimmune experimental myocarditis model [55] and colitis [56]. These findings clearly establish the negative regulatory role of CD69 in the differentiation of Th17 lymphocytes, since these cells are major mediators of these conditions. Molecular studies identified that CD69 promotes activation the Jak3-STAT5 signalling pathway, thereby inhibiting Th17 cell differentiation [57] (Fig. 1B). Taken together all these studies establish that CD69 is an intrinsic negative modulator of T cell responses [58]. In addition, this molecule may also have an important role in the generation and activity of T reg cells.
Several in vivo studies also suggest a possible role for CD69 in the regulation of immune cell migration. Constitutive CD69 expression in transgenic mice identified a potential role for CD69 in the control of thymocyte export [59]. A subsequent report has shown that CD69 can form a complex with the sphingosin-1 phosphate S1P receptor in T lymphocytes, promoting its down-regulation and inducing lymphocyte retention in lymphoid organs [60]. Likewise, DCs migration from the periphery towards lymph nodes is significantly increased in CD69-deficient mice, indicating its role as a negative regulator of chemoattractant-directed migration [61].
Although CD69 was generally regarded as one of the markers of Treg cells; recent studies suggest that this molecule may exert a significant role in their immunosupressive activity. In this regard, in patients with systemic sclerosis has been described a diminished function of Treg cells which is associated with a diminished expression of CD69 and a defective synthesis of TGF-beta [62]. In addition, a new subset of CD69(+)CD4(+)CD25(−) Treg cells, has been recently identified in mice bearing tumors. These cells do neither express Foxp3 expression nor synthesize IL-10, but show high expression of CD69 and membrane-bound TGF-beta1 and help tumors to scape from immune surveillance [63]. These regulatory T cells increase dramatically along tumor progression, and mediate suppression of T cell proliferation through TGF-beta1. Furthermore, engagement of CD69 maintains high expression of membrane-bound TGF-beta1 on CD69(+)CD4(+)CD25(−) T cells via ERK activation. These novel regulatory cells have been identified in patients; their occurrence is associated to reduced risk of acute graft-versus-host disease after allotransplants in humans [64]. Most recently, tumor-derived CD69(+) T cells have been found to induce an increase of IDO expression in monocytes. This constitutes a potential mechanism of negative crosstalk between innate and adaptative immune cells, that may contribute to immune surveillance evasion by tumor cells [65].
2.6. Galectins
Galectins are a family of highly conserved glycan-binding proteins. Although, galectins were initially described to mediate developmental processes, it is now clear that some members of this family of proteins have an essential role in the development of innate and adaptive immune responses. Galectins are expressed both intracellular and extracellularly; once secreted galectins can be found on the membrane surface because their binding to glycoreceptors [66]. Three galectins in particular have been extensively studied, due to their ability to modulate adaptive immune responses: galectin (Gal)-1, Gal-3 and Gal-9.
Galectin-1 (gal-1) acts as a negative regulator of the inflammatory response. Different in vitro functions mediated by gal-1 such as the promotion of apoptosis of Th1 cells, induction of IL-10 and downregulation of pro-inflammatory cytokines result in limiting the immune response [66,67] (Fig. 1B). Administration of exogenous gal-1 induces immunosuppression and counters inflammation in various experimental models of inflammation and autoimmunity such as inflammatory bowel disease (IBD), autoimmune retinal disease, autoimmune diabetes or CIA [66]. In a rodent model of CIA, overexpression of gal-1 induces higher levels of antigen-induced T-cell death in lymph nodes from arthritic rats and significantly reduces the joint index scores [68]. In addition, gal-1 deficiency exacerbated Th1 and Th17 responses and enhanced susceptibility to autoimmune neuroinflammation [66]. Gal-9 downregulates Th1 and Th17 responses in models of skin inflammation through its interaction with TIM-3, a Th-1 specific cell surface molecule [69]. Different models of inflammatory diseases, such as immune complex-induced arthritis or diabetes, support the anti-inflammatory properties of gal-9 [70,71].
Data regarding expression or function of galectins in human autoimmune diseases is scarce. Circulating autoantibodies against gal-1 have been found in SLE patients [72]. Although the pathological significance of this finding is unclear, it is conceivable that antibodies against gal-1 could block its regulatory functions exacerbating inflammation in this pathology. A very recent study reports a clear down-regulation of gal-1 in Langerhans and dermal DCs in both lesional and uninvolved skin from psoriasis patients as well as on myeloid peripheral DCs [73]. Skin mRNA levels of gal-1 correlated with IL-17 and IL-10 mRNA levels [73]. Based on these data, a model can be proposed in which gal-1 expression and ligand engagement limit skin inflammatory responses, suggesting that gal-1 downregulation in human DCs may contribute to the exacerbation of the inflammatory response observed in psoriasis.
3. Signalling molecules: SOCS and GADD45 family members
3.1. SOCS
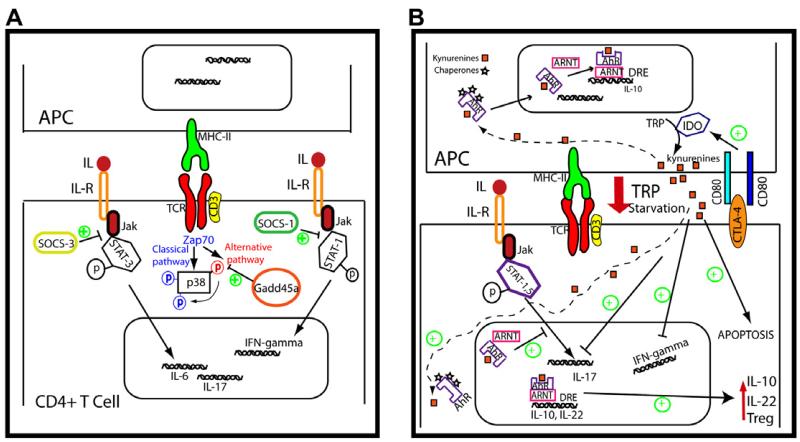
Cytokines signal through receptor oligomerization, which initiates activation of associated Janus kinases (JAKs) [74]. Activated JAKs phosphorylate the cytoplasmic domains of the triggering receptors, creating docking sites for SH2-containing signalling proteins. This result in the phosphorylation and dimerization of STATs, which then translocate to the nucleus and initiate gene transcription. SOCS proteins are important negative regulators of cytokine signalling (Fig. 2A). SOCS proteins have a central SH2 domain; through this domain, they block STAT recruitment to the cytokine receptor; SOCS also target receptors and JAKs for their degradation by the proteasome. In addition, SOCS-1 and SOCS-3 can inhibit JAK tyrosine kinase activity through their kinase inhibitory region. SOCS protein transcription is induced by several cytokines, but also by other soluble mediators not specific for lymphocytes. The general biology of SOCS has been reviewed elsewhere [75]. SOCS-1 and SOCS-3 are the most important members of this family in the regulation of innate and adaptive immune responses.
Fig. 2. Intracellular regulatory molecules.
(A) SOCS proteins interfere with Jak-STAT pathways induced by the interleukin receptors. SOCS-3 blocks STAT3 recruitment and reduces IL-6 and IL-17 expression. SOCS-1 is a negative regulator of Th1 response through the inhibition of STAT-1 activation. Signalling pathway of p38 is involved in proinflammatory cytokines expression. Beside the classical pathway of p38 activation mediated by MAPK, T-cells show an alternative route that is specifically inhibited by Gadd45a. (B) IDO is expressed mainly on DCs and macrophages. Its expression is regulated by several signals, including the engagement of B7 molecules by CTLA-4. Tryptophan (TRP) catabolism by IDO activity counteracts T cell activation by two mechanisms: TRP-induced starvation and by the production of proapoptotic metabolites, named kynurenines. After binding to its ligands (i.e. kynurenines) AhR is translocated to the nucleus where forms a complex with ARNT which induces its binding to dioxin response elements (DRE) promoting the expression of IL-10 and IL-22. In addition, AhR activation blocks STAT-1 and STAT-5 and downregulates Th17 differentiation. AhR activation also induces IL-10 expression on APC. Green plus symbols indicate tolerogenic signals.
SOCS-3 is heavily phosphorylated in the synovial tissue of RA patients compared to tissues from patients with a non-inflammatory, degenerative form of arthritis. Forced expression of SOCS3 in RA-derived synoviocytes inhibits synovial fibroblast proliferation and IL-6 production [76]. Studies in rodent models of arthritis have highlighted the role of SOCS3 in the regulation of inflammatory response in this pathology. Local injection of adenovirus encoding SOCS-3 in mice susceptible to antigen-induced arthritis prevents development of the disease; in a CIA model, local expression of SOCS-3 after establishment of the disease blocks its progression [76]. Mice lacking SOCS-3 in the hematopoietic and endothelial compartments develop severe acute inflammatory arthritis characterized by elevated infiltration of neutrophils in the synovium, bone marrow, peripheral blood and spleen. In this model, SOCS-3 negatively regulates CD4+ T lymphocyte activation including production of IL-17 and osteoclast generation [77].
Also, SOCS1 has emerged as an important negative regulator of Th1 cells as revealed in T cell-specific SOCS-1 conditional KO mice [78] and Th1 hyperactivation promoted by SOCS1-deficient DCs [79]. However, SOCS1 could have a more limited role in joint inflammation compared with SOCS3. In the model of acute inflammatory arthritis SOCS1 −/− develop more severe arthritis with an increased number of macrophages in the synovium. These animals also exhibit a small increase in synovial neutrophils numbers and osteoclast-mediated bone destruction, whereas the opposite was observed in SOCS3 KO mice [77]. Also, SOCS1 polymorphisms have been associated with adult asthma [80] and MS [81]. The possible role of SOCS1 in the development of asthma has been also highlighted using mouse models of allergic airway disease [82]. SOCS1 inactivation induced airway hyperresponsiveness after IL-13 treatment, and in an OVA-induced model induction of SOCS1 in the airways attenuated allergen-induced responses [82]. Interestingly, this study also showed that airway muscle cells from individuals with asthma had an impaired up-regulation of SOCS-1 after IL-13 stimulation [82]. Both SOCS-1 and SOCS-3 have also been implicated in the regulation of inflammation of CNS (reviewed in [83]).
3.2. GADD45
Members of the GADD45 (growth arrest and DNA damageinducible genes) family proteins are involved in many key biological processes such as cell growth, differentiation and apoptosis. Three members of this family have been identified in mammalian cells: Gadd45a, Gadd45b, and Gadd45g. Mounting evidence indicates that Gadd45 family members have important functions in T cells. Gadd45b deficiency in CD4+ T cells impairs their responses to TCR stimulation or inflammatory cytokines [84]. Moreover, Gadd45b KO dendritic cells (DCs) show reduced IL-12 and IL-6 production, and Gadd45b-deficient mice show an impaired Th1 response [84]. Gadd45-b also antagonizes TNF-alpha cytotoxicity by suppressing TNF induced c-Jun N-terminal kinase activation. Although neither Gadd45b nor Gadd45g are expressed in naïve T cells, both are up-regulated in response to T cell activation or stimulation with IL-12. Also, there is evidence that Gadd45b is involved in IL-12 and IL-18-induced IFN-gamma production [85]. Gadd45 g expression is higher in Th1 cells than in Th2 cells. Th1 cells from Gadd45g KO mice are severely compromised in their ability to activate p38 and JNK in response to TCR signalling; and produce decreased levels of IFN-gamma upon re-stimulation [86]. In contrast to the other two family members, Gadd45a is expressed in resting T cells as well as many other tissues. In the immune context, Gadd45a has been described as a negative regulator of activation-induced T cell proliferation. p38 is a well-characterized mediator of signal stress, including pro-inflammatory molecules such as TNF-a and IL-1. Importantly, p38 is also activated in response to TCR signalling and participates in Th1 differentiation [87]. The mechanism of p38 activation involves the phosphorylation cascade of classic MAPK pathway. In T cells, p38 is also activated by an alternative mechanism in response to TCR signalling [88] that is negatively modulated by Gadd45a (Fig. 2A) [89]. Gadd45a KO mice spontaneously develop an autoimmune disease remarkably similar to human systemic lupus erythematosus [90]. Activation of the alternative pathway of p38 triggering has been recently found in patients with active RA compared with patients in remission. However, no significant differences in Gadd45a expression were found between patients with active RA or in remission [91]. This study establishes the importance of the alternative p38 pathway in T cell activation in RA patients and points out the possible inhibition of this pathway as a therapeutic target to down-regulate p38 activity. Further studies of the different Gadd45 family members warrant research to elucidate their role in the immunopathogenesis of autoimmune diseases.
4. Intracellular molecules: IDO and AhR
4.1. IDO
IDO is a cytosolic enzyme that catalyzes the rate-limiting step in the catabolism of the essential amino acid tryptophan (TRP). Tryptophan sequestration by up-regulation of IDO is one of the mechanisms involved in the suppression of the immune response via T cell starvation [92] (Fig. 2B). IDO activation induces the production of proapoptotic tryptophan metabolites known as kynurenines (Fig. 2B). IDO expression and its enzymatic activity are tightly controlled; IDO is induced by IFNs released in response to inflammation in several cell types, such as DCs, macrophages, eosinophils or endothelial cells. In addition, other molecules with negative immunoregulatory functions such as CTL4-A or 4-1BB (CD137) control IDO expression (Fig. 2B). The immunoregulatory effects of IDO are mainly mediated by DCs and involve not only tryptophan deprivation but also production of kynurenines. Although some evidence supports the possible involvement of IDO in regulating Th2 responses, the majority of data indicate that the immunosuppressive effect of IDO is mainly exerted on Th1 cell-mediated immune responses [92].
An early study suggested that IDO participates in controlling fetal allograft rejection in mice [93]. Since then, a number of studies support the importance of IDO as a negative costimulatory molecule. Use of pharmacologic inhibitors of IDO and tryptophan metabolites or its derivatives have revealed the key role of IDO in several autoimmune inflammatory diseases. In vitro, IDO is induced in microglia upon treatment with IFN-gamma. During the course of EAE, both IDO expression and its activity are up-regulated. Furthermore, inhibition of IDO activity by the specific inhibitor 1 methyl tryptophan exacerbates the disease [94]. As stated above, kynunerines are tryptophan metabolites that participate in immunosuppresion induced by IDO. Treatment with synthetic tryptophan metabolites inhibits proliferation of myelin-specific T cells and production of Th1 cytokines and reverses paralysis in mice with EAE [95]. Recently, IDO deficiency has been shown to promote T cell responses, down-regulate Treg responses, and enhance EAE severity [96]. In contrast, tryptophan metabolite 3-hydroxyanthranillic acid (3-HAA) promoted Treg function in vitro, and administration of 3-HAA in mice reduced the severity of EAE [96]. We conclude that local expression of IDO during brain inflammation may constitute a self-protection mechanism that limits antigen-specific immune responses in the CNS.
The role of IDO as a negative regulator of the inflammatory response has also been observed in other animal models of autoimmune disease. In CIA, which is primarily mediated by a Th1-like immune response, IDO activity is increased and its inhibition results in an exacerbated Th1 response [97]. Furthermore, IDO-deficient mice show an exacerbated CIA severity that correlates with an increased production of IFN-gamma and IL-17 by lymph node T cells, and increased infiltration of Th1 and Th17 cells in the affected joints [98]. Recently, bone marrow-derived DCs transduced with IDO and the exosomes they produce were assayed for their immunosuppressive ability in CIA. Both DCs and exosomes derived from IDO+ DCs displayed anti-inflammatory properties [99]. In addition, intra-articular delivery of IDO gene ameliorated arthritis of CIA rats by induction of CD4+ T cells apoptosis and reduction of IL-17 production [100].
Although toll-like receptors (TLR)s were initially considered as stimulatory molecules capable of activating defence mechanisms against invading pathogens, emerging evidence suggests that they can also exert a regulatory function. Administration of TLR ligands induces high levels of IDO, and TLR9 ligand-induced pulmonary IDO activity inhibits Th2-driven experimental asthma [101]. IDO activity expressed by resident lung cells, rather than pulmonary DCs, suppressed lung inflammation and airway hyperreactivity[101]. The function of the TLR/IDO axis has been observed in two additional models of autoimmune diseases, experimental autoimmune diabetes [102] and experimental colitis [103].
Tolerance to self-antigens present in apoptotic cells is critical to maintain immune-homeostasis and prevent systemic autoimmunity. A recent study reports that systemic administration of apoptotic cells to mice induces IDO expression. Also, chronic exposure of IDO-deficient mice to apoptotic cells induced a lupus-like disease. In this scenario, IDO inhibition increased T cell response to apoptotic cell-associated antigens and accelerated disease progression in lupus-prone MRL mice [104].
Regarding human autoimmune diseases, increased TRP degradation has been observed in the blood of RA patients, and the decrease in the TRP level correlates with the severity of the disease[105]. However, decreased levels of IDO-expressing peripheral blood mononuclear cells from RA patients has been recently described [106]. DCs derived from joint synovial fluid from RA patients express IDO that is fully functional and thus able to catabolize tryptophan. However, synovial auto-reactive T cells from these patients are resistant to IDO-mediated inhibition. Enhanced expression of tryptophanyl-tRNA-sythetase (TTS) in T cells was responsible for their resistance to IDO-mediated tryptophan deprivation [107]. TTS is a constitutively expressed cytoplasmic enzyme able to bind tryptophan with its specific tRNA [108]. The tryptophan/tRNA complex constitutes a reservoir of tryptophan for protein synthesis that can antagonize IDO-mediated immunosuppression by tryptophan deprivation.
Data regarding the induction of IDO and the subsequent synthesis of kynurenines in MS patients is lacking. Early studies reported the tryptophan degradation and the activation of the kynurenine pathway in MS in the plasma and cerebrospinal fluid (CSF) of MS patients [109]. Additional studies confirmed these data, describing low concentrations of TRP in serum and CSF from patients with chronic MS [110]. In primary biliary cirrhosis patients, defects in IDO expression have also been observed, which correlated with an increased frequency of a gain-of-function SNP within the TGF-beta promoter region, a molecule known to suppress IDO transcription [111]. However, increased IDO activity is associated with the severity in other diseases such as SLE [112]. Likewise, lesional skin from psoriasis patients expresses higher levels of IDO mRNA compared with non-lesional skin [113]. Myeloid DCs (CD11c+, CD68−) are major vehicles of IDO expression in psoriasis.[114] However, IDO up-regulation in psoriasis is not enough to surpass the inflammatory response in these patients. Therefore, it would be interesting to test the activity of the enzyme in these patients and the susceptibility of inflammatory cells to IDO activation.
4.2. AhR
Aryl hydrocarbon receptor (AhR) is a ligand-activated transcription factor. AhR is an important transcriptional regulator of drug metabolizing enzymes, best known for mediating the toxicity of dioxin. It is well established that dioxin and other related compounds cause immunosuppression and thymic atrophy. AhR also has endogenous functions that include controlling cell cycle, immune responses and cell differentiation. In the absence of ligand, AhR is located in the cytoplasm and forms a complex with several proteins including the chaperone protein hsp90. Ligand binding induces a conformational change in AhR, which facilitates its translocation to the nucleus, dissociation from the chaperone proteins and heterodimerization with ARNT (aryl hydrocarbon receptor nuclear translocator). The resulting complex binds to regulatory sequences termed dioxin responsive element (DRE) that eventually results in transcription of target genes [115] (Fig. 2B). In addition to environmental chemicals or xenobiotics as dioxin, AhR can be activated by endogenous ligands such as 6-formylindolo[3,2-b]carbazole (FICZ), bilirubin, or the eicosanoid Lipoxin A4 [115]. Interestingly, kynurenine (the downstream metabolite of IDO activity) is an AhR agonist [116] (Fig. 2B). Moreover, IDO is induced by DCs in response to dioxin [117] and AhR deficient LCs do not express IDO [118].
AhR is differentially expressed in different lymphocyte subsets. Th17 cells and DCs express high levels of AhR. Some of the effects of AhR activation in the immune system are: modulation of the Th1/Th2 balance [119], generation of regulatory T cells [120] and regulation of Treg and Th17 cell differentiation [121].
Studies in animal models have revealed the regulatory role of AhR in inflammatory diseases. Activation of AhR by FICZ, a high-affinity ligand for AhR during EAE induction exacerbated the disease by promoting Th17 cell differentiation [121,122]. In contrast, administration of dioxin increases Treg activity and proliferation, and suppresses EAE. It is therefore conceivable that, depending on the ligand, AhR is capable of regulating both Treg and Th17 cell differentiation [121]. On the other hand, induction of CIA in AhR deficient mice results in reduced levels of pro-inflammatory cytokines and generation of Th17 cells. This effect depends on the presence of AhR in T cells because lack of AhR specifically in T cells significantly suppressed the development of CIA, whereas AhR deficiency in macrophages had no effect [123]. Regarding to Th17 regulation, it has been reported that AhR interacts with both STAT-1 and STAT-5, and regulates the activation of STAT1 during Th17 polarization conditions [124], which negatively regulates Th17 differentiation (Fig. 2B).
AhR could have an important role in the maintenance of immune barriers. AhR deficient mice display impaired skin gamma/delta T cell proliferation, which are essential to the LCs maturation in the skin [118,125]. Recent observations also shown that AhR regulates gut immunity by promoting the function of innate lymphoid cells as well as IL-22 production [126]. Interestingly, intestine tissue from patients with inflammatory bowel diseases express significantly less AhR compared to control cells [127]. Although more studies are necessary to elucidate the importance of AhR in IBD, these data indicate that this intracellular receptor may play a key role the immunopathogenesis of gut inflammation.
5. Concluding remarks
Whereas the immunological mechanisms that promote inflammation during autoimmune inflammatory diseases are relatively well characterized, the specific mechanisms that down-modulate inflammatory responses are poorly understood.
For many years the therapy of autoimmune inflammatory diseases was based in the use of anti-inflammatory compounds (mainly glucocorticoids) and non-selective cytotoxic immunosuppressive drugs (cyclophosphamide, azathioprine, etc.). In the recent years, the identification of different receptors and intracellular molecules that exert a key role in the activation of immune cells has allowed the development of more selective immunosuppressive drugs (tacrolimus, sirolimus) as well as the generation of different biological agents that are able to eliminate immune cells (e.g., anti-CD20 agents) or to interfere with the action of proinflammatory cytokines (e.g., TNF-α blocking agents). However, in some cases these approaches are not exempt of risk.
In the last years the study of molecules with negative co-stimulatory activity has revealed the importance of tolerance signals in the pathogenesis and progression of these diseases and new targets are now under intensive research. It is evident that additional therapeutic agents are necessary for the treatment of autoimmune/inflammatory conditions. In this regard, the recent advances in the knowledge of the different immunoregulatory molecules reviewed herein, will allow the identification of novel therapeutic targets, with the subsequent development of additional immunosuppressive and highly selective agents. Furthermore, the characterization of the receptors and intracellular molecules involved in the generation and activation of Treg cells will be very useful for the design of novel strategies of immunosuppression. These molecules not only dampen pro-inflammatory signals but also promote both generation and function of Treg cells. Further studies are necessary to unravel strategies to potentiate or improve their function.
Acknowledgments
We thank M. Vicente-Manzanares, M. Gómez Gutiérrez and R. Gonzalez-Amaro for critical reading of the manuscript. This work is supported by grants SAF2011-25834 and ERC-2011-AdG294340-GENTRIS. The Centro Nacional de Investigaciones Cardiovasculares (CNIC) is supported by Spanish Ministry of the Economy and the Pro-CNIC Foundation.
References
- [1].Kyewski B, Klein L. A central role for central tolerance. Annu. Rev. Immunol. 2006;24:571–606. doi: 10.1146/annurev.immunol.23.021704.115601. [DOI] [PubMed] [Google Scholar]
- [2].Mueller DL. Mechanisms maintaining peripheral tolerance. Nat. Immunol. 2010;11:21–27. doi: 10.1038/ni.1817. [DOI] [PubMed] [Google Scholar]
- [3].Pentcheva-Hoang T, Corse E, Allison JP. Negative regulators of T-cell activation: potential targets for therapeutic intervention in cancer, autoimmune disease, and persistent infections. Immunol. Rev. 2009;229:67–87. doi: 10.1111/j.1600-065X.2009.00763.x. [DOI] [PubMed] [Google Scholar]
- [4].Yang L, Pang Y, Moses HL. TGF-beta and immune cells: an important regulatory axis in the tumor microenvironment and progression. Trends Immunol. 2010;31:220–227. doi: 10.1016/j.it.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu. Rev. Immunol. 2011;29:71–109. doi: 10.1146/annurev-immunol-031210-101312. [DOI] [PubMed] [Google Scholar]
- [6].Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu. Rev. Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- [7].Nurieva RI, Liu X, Dong C. Yin-Yang of costimulation: crucial controls of immune tolerance and function. Immunol. Rev. 2009;229:88–100. doi: 10.1111/j.1600-065X.2009.00769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Fife BT, Bluestone JA. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol. Rev. 2008;224:166–182. doi: 10.1111/j.1600-065X.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- [9].Waterhouse P, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- [10].Gough SC, Walker LS, Sansom DM. CTLA4 gene polymorphism and autoimmunity. Immunol. Rev. 2005;204:102–115. doi: 10.1111/j.0105-2896.2005.00249.x. [DOI] [PubMed] [Google Scholar]
- [11].Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, Mak TW, Sakaguchi S. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J. Exp. Med. 2000;192:303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- [13].Tang Q, Boden EK, Henriksen KJ, Bour-Jordan H, Bi M, Bluestone JA. Distinct roles of CTLA-4 and TGF-beta in CD4+CD25+ regulatory T cell function. Eur. J. Immunol. 2004;34:2996–3005. doi: 10.1002/eji.200425143. [DOI] [PubMed] [Google Scholar]
- [14].Read S, Greenwald R, Izcue A, Robinson N, Mandelbrot D, Francisco L, Sharpe AH, Powrie F. Blockade of CTLA-4 on CD4+CD25+ regulatory T cells abrogates their function in vivo. J. Immunol. 2006;177:4376–4383. doi: 10.4049/jimmunol.177.7.4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Schmidt EM, et al. Ctla-4 controls regulatory T cell peripheral homeostasis and is required for suppression of pancreatic islet autoimmunity. J. Immunol. 2009;182:274–282. doi: 10.4049/jimmunol.182.1.274. [DOI] [PubMed] [Google Scholar]
- [16].Bour-Jordan H, Grogan JL, Tang Q, Auger JA, Locksley RM, Bluestone JA. CTLA-4 regulates the requirement for cytokine-induced signals in T(H)2 lineage commitment. Nat. Immunol. 2003;4:182–188. doi: 10.1038/ni884. [DOI] [PubMed] [Google Scholar]
- [17].Hellings PW, Vandenberghe P, Kasran A, Coorevits L, Lutgart O, Mathieu C, Ceuppens JL. Blockade of CTLA-4 enhances allergic sensitization and eosinophilic airway inflammation in genetically predisposed mice. Eur. J. Immunol. 2002;32:585–594. doi: 10.1002/1521-4141(200202)32:2<585::AID-IMMU585>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- [18].Ying H, Yang L, Qiao G, Li Z, Zhang L, Yin F, Xie D, Zhang J. Cutting edge: CTLA-4-B7 interaction suppresses Th17 cell differentiation. J. Immunol. 2010;185:1375–1378. doi: 10.4049/jimmunol.0903369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Karandikar NJ, Eagar TN, Vanderlugt CL, Bluestone JA, Miller SD. CTLA-4 downregulates epitope spreading and mediates remission in relapsing experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2000;109:173–180. doi: 10.1016/s0165-5728(00)00322-2. [DOI] [PubMed] [Google Scholar]
- [20].Hurwitz AA, Sullivan TJ, Sobel RA, Allison JP. Cytotoxic T lymphocyte antigen-4 (CTLA-4) limits the expansion of encephalitogenic T cells in experimental autoimmune encephalomyelitis (EAE)-resistant BALB/c mice. Proc. Natl. Acad. Sci. U S A. 2002;99:3013–3017. doi: 10.1073/pnas.042684699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zhu J, et al. Cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) blockade enhances incidence and severity of experimental autoimmune neuritis in resistant mice. J. Neuroimmunol. 2001;115:111–117. doi: 10.1016/s0165-5728(01)00255-7. [DOI] [PubMed] [Google Scholar]
- [22].Davar D, Tarhini AA, Kirkwood JM. Adjuvant therapy for melanoma. Cancer J. 2012;18:192–202. doi: 10.1097/PPO.0b013e31824f118b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol. Rev. 2010;236:219–242. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–151. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- [25].Nishimura H, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291:319–322. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- [26].Ansari MJ, et al. The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J. Exp. Med. 2003;198:63–69. doi: 10.1084/jem.20022125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Salama AD, et al. Critical role of the programmed death-1 (PD-1) pathway in regulation of experimental autoimmune encephalomyelitis. J. Exp. Med. 2003;198:71–78. doi: 10.1084/jem.20022119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ding H, Wu X, Wu J, Yagita H, He Y, Zhang J, Ren J, Gao W. Delivering PD-1 inhibitory signal concomitant with blocking ICOS co-stimulation suppresses lupus-like syndrome in autoimmune BXSB mice. Clin. Immunol. 2006;118:258–267. doi: 10.1016/j.clim.2005.10.017. [DOI] [PubMed] [Google Scholar]
- [29].Prokunina L, et al. A regulatory polymorphism in PDCD1 is associated with susceptibility to systemic lupus erythematosus in humans. Nat. Genet. 2002;32:666–669. doi: 10.1038/ng1020. [DOI] [PubMed] [Google Scholar]
- [30].Prokunina L, et al. The systemic lupus erythematosus-associated PDCD1 polymorphism PD1.3A in lupus nephritis. Arthritis Rheum. 2004;50:327–328. doi: 10.1002/art.11442. [DOI] [PubMed] [Google Scholar]
- [31].Nielsen C, Hansen D, Husby S, Jacobsen BB, Lillevang ST. Association of a putative regulatory polymorphism in the PD-1 gene with susceptibility to type 1 diabetes. Tissue Antigens. 2003;62:492–497. doi: 10.1046/j.1399-0039.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- [32].Prokunina L, et al. Association of the PD-1.3A allele of the PDCD1 gene in patients with rheumatoid arthritis negative for rheumatoid factor and the shared epitope. Arthritis Rheum. 2004;50:1770–1773. doi: 10.1002/art.20280. [DOI] [PubMed] [Google Scholar]
- [33].Kristjansdottir H, Steinsson K, Gunnarsson I, Grondal G, Erlendsson K, Alarcon-Riquelme ME. Lower expression levels of the programmed death 1 receptor on CD4+CD25+ T cells and correlation with the PD-1.3A genotype in patients with systemic lupus erythematosus. Arthritis Rheum. 2010;62:1702–1711. doi: 10.1002/art.27417. [DOI] [PubMed] [Google Scholar]
- [34].Kroner A, Mehling M, Hemmer B, Rieckmann P, Toyka KV, Maurer M, Wiendl H. A PD-1 polymorphism is associated with disease progression in multiple sclerosis. Ann. Neurol. 2005;58:50–57. doi: 10.1002/ana.20514. [DOI] [PubMed] [Google Scholar]
- [35].Dong H, et al. Costimulating aberrant T cell responses by B7-H1 autoantibodies in rheumatoid arthritis. J. Clin. Invest. 2003;111:363–370. doi: 10.1172/JCI16015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wan B, et al. Aberrant regulation of synovial T cell activation by soluble costimulatory molecules in rheumatoid arthritis. J. Immunol. 2006;177:8844–8850. doi: 10.4049/jimmunol.177.12.8844. [DOI] [PubMed] [Google Scholar]
- [37].Wiesemann E, Deb M, Trebst C, Hemmer B, Stangel M, Windhagen A. Effects of interferon-beta on co-signaling molecules: upregulation of CD40, CD86 and PD-L2 on monocytes in relation to clinical response to interferon-beta treatment in patients with multiple sclerosis. Mult. Scler. 2008;14:166–176. doi: 10.1177/1352458507081342. [DOI] [PubMed] [Google Scholar]
- [38].Trabattoni D, et al. Costimulatory pathways in multiple sclerosis: distinctive expression of PD-1 and PD-L1 in patients with different patterns of disease. J. Immunol. 2009;183:4984–4993. doi: 10.4049/jimmunol.0901038. [DOI] [PubMed] [Google Scholar]
- [39].Pittet CL, Newcombe J, Prat A, Arbour N. Human brain endothelial cells endeavor to immunoregulate CD8 T cells via PD-1 ligand expression in multiple sclerosis. J. Neuroinflamm. 2011;8:155. doi: 10.1186/1742-2094-8-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Burmeister Y, Lischke T, Dahler AC, Mages HW, Lam KP, Coyle AJ, Kroczek RA, Hutloff A. ICOS controls the pool size of effector-memory and regulatory T cells. J. Immunol. 2008;180:774–782. doi: 10.4049/jimmunol.180.2.774. [DOI] [PubMed] [Google Scholar]
- [41].Dong C, Juedes AE, Temann UA, Shresta S, Allison JP, Ruddle NH, Flavell RA. ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature. 2001;409:97–101. doi: 10.1038/35051100. [DOI] [PubMed] [Google Scholar]
- [42].Rottman JB, et al. The costimulatory molecule ICOS plays an important role in the immunopathogenesis of EAE. Nat. Immunol. 2001;2:605–611. doi: 10.1038/89750. [DOI] [PubMed] [Google Scholar]
- [43].Herman AE, Freeman GJ, Mathis D, Benoist C. CD4+CD25+ T regulatory cells dependent on ICOS promote regulation of effector cells in the prediabetic lesion. J. Exp. Med. 2004;199:1479–1489. doi: 10.1084/jem.20040179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Akbari O, et al. Antigen-specific regulatory T cells develop via the ICOS-ICOS-ligand pathway and inhibit allergen-induced airway hyperreactivity. Nat. Med. 2002;8:1024–1032. doi: 10.1038/nm745. [DOI] [PubMed] [Google Scholar]
- [45].Vocanson M, et al. Inducible costimulator (ICOS) is a marker for highly suppressive antigen-specific T cells sharing features of TH17/TH1 and regulatory T cells. J. Allergy Clin. Immunol. 2010;126:280–289. doi: 10.1016/j.jaci.2010.05.022. [DOI] [PubMed] [Google Scholar]
- [46].Kornete M, Sgouroudis E, Piccirillo CA. ICOS-dependent homeostasis and function of Foxp3+ regulatory T cells in islets of nonobese diabetic mice. J. Immunol. 2012;188:1064–1074. doi: 10.4049/jimmunol.1101303. [DOI] [PubMed] [Google Scholar]
- [47].Gomez de Aguero M, et al. Langerhans cells protect from allergic contact dermatitis in mice by tolerizing CD8(+) T cells and activating Foxp3(+) regulatory T cells. J. Clin. Invest. 2012;122:1700–1711. doi: 10.1172/JCI59725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Tuettenberg A, et al. The role of ICOS in directing T cell responses: ICOS-dependent induction of T cell anergy by tolerogenic dendritic cells. J. Immunol. 2009;182:3349–3356. doi: 10.4049/jimmunol.0802733. [DOI] [PubMed] [Google Scholar]
- [49].Whitehead GS, Wilson RH, Nakano K, Burch LH, Nakano H, Cook DN. IL-35 production by inducible costimulator (ICOS)-positive regulatory T cells reverses established IL-17-dependent allergic airways disease. J. Allergy Clin. Immunol. 2012;129(207-15):e1–e5. doi: 10.1016/j.jaci.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Sancho D, Gomez M, Sanchez-Madrid F. CD69 is an immunoregulatory molecule induced following activation. Trends Immunol. 2005;26:136–140. doi: 10.1016/j.it.2004.12.006. [DOI] [PubMed] [Google Scholar]
- [51].Laffon A, Garcia-Vicuna R, Humbria A, Postigo AA, Corbi AL, de Landazuri MO, Sanchez-Madrid F. Upregulated expression and function of VLA-4 fibronectin receptors on human activated T cells in rheumatoid arthritis. J. Clin. Invest. 1991;88:546–552. doi: 10.1172/JCI115338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Rueda B, Fernandez-Gutierrez B, Balsa A, Pacual-Salcedo D, Lamas JR, Raya E, Gonzalez-Gay MA, Martin J. Investigation of CD69 as a new candidate gene for rheumatoid arthritis. Tissue Antigens. 2008;72:206–210. doi: 10.1111/j.1399-0039.2008.01094.x. [DOI] [PubMed] [Google Scholar]
- [53].Sancho D, et al. CD69 downregulates autoimmune reactivity through active transforming growth factor-beta production in collagen-induced arthritis. J. Clin. Invest. 2003;112:872–882. doi: 10.1172/JCI19112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Martin P, et al. The leukocyte activation antigen CD69 limits allergic asthma and skin contact hypersensitivity. J. Allergy Clin. Immunol. 2010;126:355–365. 365 e1-3. doi: 10.1016/j.jaci.2010.05.010. [DOI] [PubMed] [Google Scholar]
- [55].Cruz-Adalia A, et al. CD69 limits the severity of cardiomyopathy after autoimmune myocarditis. Circulation. 2010;122:1396–1404. doi: 10.1161/CIRCULATIONAHA.110.952820. [DOI] [PubMed] [Google Scholar]
- [56].Radulovic K, Manta C, Rossini V, Holzmann K, Kestler HA, Wegenka UM, Nakayama T, Niess JH. CD69 regulates type I IFN-induced tolerogenic signals to mucosal CD4 T cells that attenuate their colitogenic potential. J. Immunol. 2012;188:2001–2013. doi: 10.4049/jimmunol.1100765. [DOI] [PubMed] [Google Scholar]
- [57].Martin P, Gomez M, Lamana A, Cruz-Adalia A, Ramirez-Huesca M, Ursa MA, Yanez-Mo M, Sanchez-Madrid F. CD69 association with Jak3/Stat5 proteins regulates Th17 cell differentiation. Mol. Cell. Biol. 2010;30:4877–4889. doi: 10.1128/MCB.00456-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Martin P, Sanchez-Madrid F. CD69: an unexpected regulator of TH17 cell-driven inflammatory responses. Sci. Signal. 2011;4:pe14. doi: 10.1126/scisignal.2001825. [DOI] [PubMed] [Google Scholar]
- [59].Feng C, et al. A potential role for CD69 in thymocyte emigration. Int. Immunol. 2002;14:535–544. doi: 10.1093/intimm/dxf020. [DOI] [PubMed] [Google Scholar]
- [60].Shiow LR, Rosen DB, Brdickova N, Xu Y, An J, Lanier LL, Cyster JG, Matloubian M. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440:540–544. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- [61].Lamana A, et al. CD69 modulates sphingosine-1-phosphate-induced migration of skin dendritic cells. J. Invest. Dermatol. 2011;131:1503–1512. doi: 10.1038/jid.2011.54. [DOI] [PubMed] [Google Scholar]
- [62].Radstake TR, et al. Increased frequency and compromised function of T regulatory cells in systemic sclerosis (SSc) is related to a diminished CD69 and TGFbeta expression. PLoS One. 2009;4:e5981. doi: 10.1371/journal.pone.0005981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Han Y, Guo Q, Zhang M, Chen Z, Cao X. CD69+ CD4+ CD25-T cells, a new subset of regulatory T cells, suppress T cell proliferation through membrane-bound TGF-beta 1. J. Immunol. 2009;182:111–120. doi: 10.4049/jimmunol.182.1.111. [DOI] [PubMed] [Google Scholar]
- [64].Lu SY, Huang XJ, Liu KY, Liu DH, Xu LP. High frequency of CD4+ CD25-CD69+ T cells is correlated with a low risk of acute graft-versus-host disease in allotransplants. Clin. Transplant. 2012;26:E15–E67. doi: 10.1111/j.1399-0012.2012.01630.x. [DOI] [PubMed] [Google Scholar]
- [65].Zhao Q, Kuang DM, Wu Y, Xiao X, Li XF, Li TJ, Zheng L. Activated CD69+ T cells foster immune privilege by regulating IDO expression in tumor-associated macrophages. J. Immunol. 2012;188:1117–1124. doi: 10.4049/jimmunol.1100164. [DOI] [PubMed] [Google Scholar]
- [66].Rabinovich GA, Toscano MA. Turning ‘sweet’ on immunity: galectin-glycan interactions in immune tolerance and inflammation. Nat. Rev. Immunol. 2009;9:338–352. doi: 10.1038/nri2536. [DOI] [PubMed] [Google Scholar]
- [67].van der Leij J, et al. Dimeric galectin-1 induces IL-10 production in T-lymphocytes: an important tool in the regulation of the immune response. J. Pathol. 2004;204:511–518. doi: 10.1002/path.1671. [DOI] [PubMed] [Google Scholar]
- [68].Wang CR, et al. Intra-articular lentivirus-mediated delivery of galectin-3 shRNA and galectin-1 gene ameliorates collagen-induced arthritis. Gene Ther. 2010;17:1225–1233. doi: 10.1038/gt.2010.78. [DOI] [PubMed] [Google Scholar]
- [69].Niwa H, Satoh T, Matsushima Y, Hosoya K, Saeki K, Niki T, Hirashima M, Yokozeki H. Stable form of galectin-9, a Tim-3 ligand, inhibits contact hypersensitivity and psoriatic reactions: a potent therapeutic tool for Th1- and/or Th17-mediated skin inflammation. Clin. Immunol. 2009;132:184–194. doi: 10.1016/j.clim.2009.04.012. [DOI] [PubMed] [Google Scholar]
- [70].Arikawa T, et al. Galectin-9 ameliorates immune complex-induced arthritis by regulating Fc gamma R expression on macrophages. Clin. Immunol. 2009;133:382–392. doi: 10.1016/j.clim.2009.09.004. [DOI] [PubMed] [Google Scholar]
- [71].Kanzaki M, et al. Galectin-9 and T cell immunoglobulin mucin-3 pathway is a therapeutic target for type 1 diabetes. Endocrinology. 2012;153:612–620. doi: 10.1210/en.2011-1579. [DOI] [PubMed] [Google Scholar]
- [72].Montiel JL, Monsivais-Urenda A, Figueroa-Vega N, Moctezuma JF, Burgos-Vargas R, Gonzalez-Amaro R, Rosenstein Y. Anti-CD43 and anti-galectin-1 autoantibodies in patients with systemic lupus erythematosus. Scand. J. Rheumatol. 2010;39:50–57. doi: 10.3109/03009740903013213. [DOI] [PubMed] [Google Scholar]
- [73].de la Fuente H, et al. Psoriasis in humans is associated with down-regulation of galectins in dendritic cells. J. Pathol. 2012 doi: 10.1002/path.3996. http://dx.doi.org/10.1002/path.3996. [DOI] [PubMed] [Google Scholar]
- [74].O’Shea JJ, Plenge R. JAK and STAT signaling molecules in immunoregulation and immune-mediated disease. Immunity. 2012;36:542–550. doi: 10.1016/j.immuni.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Yoshimura A, Suzuki M, Sakaguchi R, Hanada T, Yasukawa H. SOCS, Inflammation, and Autoimmunity. Front Immunol. 2012;3:20. doi: 10.3389/fimmu.2012.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Shouda T, et al. Induction of the cytokine signal regulator SOCS3/CIS3 as a therapeutic strategy for treating inflammatory arthritis. J. Clin. Invest. 2001;108:1781–1788. doi: 10.1172/JCI13568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Wong PK, et al. SOCS-3 negatively regulates innate and adaptive immune mechanisms in acute IL-1-dependent inflammatory arthritis. J. Clin. Invest. 2006;116:1571–1581. doi: 10.1172/JCI25660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Tanaka K, et al. Loss of suppressor of cytokine signaling 1 in helper T cells leads to defective Th17 differentiation by enhancing antagonistic effects of IFN-gamma on STAT3 and Smads. J. Immunol. 2008;180:3746–3756. doi: 10.4049/jimmunol.180.6.3746. [DOI] [PubMed] [Google Scholar]
- [79].Hanada T, et al. Induction of hyper Th1 cell-type immune responses by dendritic cells lacking the suppressor of cytokine signaling-1 gene. J. Immunol. 2005;174:4325–4332. doi: 10.4049/jimmunol.174.7.4325. [DOI] [PubMed] [Google Scholar]
- [80].Harada M, et al. Functional polymorphism in the suppressor of cytokine signaling 1 gene associated with adult asthma. Am. J. Respir. Cell Mol. Biol. 2007;36:491–496. doi: 10.1165/rcmb.2006-0090OC. [DOI] [PubMed] [Google Scholar]
- [81].Vandenbroeck K, et al. A cytokine gene screen uncovers SOCS1 as genetic risk factor for multiple sclerosis. Genes Immun. 2012;13:21–28. doi: 10.1038/gene.2011.44. [DOI] [PubMed] [Google Scholar]
- [82].Fukuyama S, et al. Pulmonary suppressor of cytokine signaling-1 induced by IL-13 regulates allergic asthma phenotype. Am. J. Respir. Crit. Care Med. 2009;179:992–998. doi: 10.1164/rccm.200806-992OC. [DOI] [PubMed] [Google Scholar]
- [83].Baker BJ, Akhtar LN, Benveniste EN. SOCS1 and SOCS3 in the control of CNS immunity. Trends Immunol. 2009;30:392–400. doi: 10.1016/j.it.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Lu B, Ferrandino AF, Flavell RA. Gadd45beta is important for perpetuating cognate and inflammatory signals in T cells. Nat. Immunol. 2004;5:38–44. doi: 10.1038/ni1020. [DOI] [PubMed] [Google Scholar]
- [85].Yang J, Zhu H, Murphy TL, Ouyang W, Murphy KM. IL-18-stimulated GADD45 beta required in cytokine-induced, but not TCR-induced, IFN-gamma production. Nat. Immunol. 2001;2:157–164. doi: 10.1038/84264. [DOI] [PubMed] [Google Scholar]
- [86].Lu B, Yu H, Chow C, Li B, Zheng W, Davis RJ, Flavell RA. GADD45gamma mediates the activation of the p38 and JNK MAP kinase pathways and cytokine production in effector TH1 cells. Immunity. 2001;14:583–590. doi: 10.1016/s1074-7613(01)00141-8. [DOI] [PubMed] [Google Scholar]
- [87].Rincon M, Pedraza-Alva G. JNK and p38 MAP kinases in CD4+ and CD8+ T cells. Immunol. Rev. 2003;192:131–142. doi: 10.1034/j.1600-065x.2003.00019.x. [DOI] [PubMed] [Google Scholar]
- [88].Salvador JM, Mittelstadt PR, Guszczynski T, Copeland TD, Yamaguchi H, Appella E, Fornace AJ, Jr., Ashwell JD. Alternative p38 activation pathway mediated by T cell receptor-proximal tyrosine kinases. Nat. Immunol. 2005;6:390–395. doi: 10.1038/ni1177. [DOI] [PubMed] [Google Scholar]
- [89].Salvador JM, Mittelstadt PR, Belova GI, Fornace AJ, Jr., Ashwell JD. The autoimmune suppressor Gadd45alpha inhibits the T cell alternative p38 activation pathway. Nat. Immunol. 2005;6:396–402. doi: 10.1038/ni1176. [DOI] [PubMed] [Google Scholar]
- [90].Salvador JM, Hollander MC, Nguyen AT, Kopp JB, Barisoni L, Moore JK, Ashwell JD, Fornace AJ., Jr. Mice lacking the p53-effector gene Gadd45a develop a lupus-like syndrome. Immunity. 2002;16:499–508. doi: 10.1016/s1074-7613(02)00302-3. [DOI] [PubMed] [Google Scholar]
- [91].Lopez-Santalla M, et al. Tyr(3)(2)(3)-dependent p38 activation is associated with rheumatoid arthritis and correlates with disease activity. Arthritis Rheum. 2011;63:1833–1842. doi: 10.1002/art.30375. [DOI] [PubMed] [Google Scholar]
- [92].Mandi Y, Vecsei L. The kynurenine system and immunoregulation. J. Neural Transm. 2012;119:197–209. doi: 10.1007/s00702-011-0681-y. [DOI] [PubMed] [Google Scholar]
- [93].Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, Brown C, Mellor AL. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- [94].Kwidzinski E, Bunse J, Aktas O, Richter D, Mutlu L, Zipp F, Nitsch R, Bechmann I. Indolamine 2,3-dioxygenase is expressed in the CNS and down-regulates autoimmune inflammation. FASEB J. 2005;19:1347–1349. doi: 10.1096/fj.04-3228fje. [DOI] [PubMed] [Google Scholar]
- [95].Platten M, et al. Treatment of autoimmune neuroinflammation with a synthetic tryptophan metabolite. Science. 2005;310:850–855. doi: 10.1126/science.1117634. [DOI] [PubMed] [Google Scholar]
- [96].Yan Y, et al. IDO upregulates regulatory T cells via tryptophan catabolite and suppresses encephalitogenic T cell responses in experimental autoimmune encephalomyelitis. J. Immunol. 2010;185:5953–5961. doi: 10.4049/jimmunol.1001628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Szanto S, Koreny T, Mikecz K, Glant TT, Szekanecz Z, Varga J. Inhibition of indoleamine 2,3-dioxygenase-mediated tryptophan catabolism accelerates collagen-induced arthritis in mice. Arthritis Res. Ther. 2007;9:R50. doi: 10.1186/ar2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Criado G, Simelyte E, Inglis JJ, Essex D, Williams RO. Indoleamine 2,3 dioxygenase-mediated tryptophan catabolism regulates accumulation of Th1/Th17 cells in the joint in collagen-induced arthritis. Arthritis Rheum. 2009;60:1342–1351. doi: 10.1002/art.24446. [DOI] [PubMed] [Google Scholar]
- [99].Bianco NR, Kim SH, Ruffner MA, Robbins PD. Therapeutic effect of exosomes from indoleamine 2,3-dioxygenase-positive dendritic cells in collagen-induced arthritis and delayed-type hypersensitivity disease models. Arthritis Rheum. 2009;60:380–389. doi: 10.1002/art.24229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Chen SY, Shiau AL, Li YT, Lin YS, Lee CH, Wu CL, Wang CR. Suppression of collagen-induced arthritis by intra-articular lentiviral vector-mediated delivery of Toll-like receptor 7 short hairpin RNA gene. Gene Ther. 2011 doi: 10.1038/gt.2011.173. http://dx.doi.org/10.1038/gt.2011.173. [DOI] [PubMed] [Google Scholar]
- [101].Hayashi T, et al. Inhibition of experimental asthma by indoleamine 2,3-dioxygenase. J. Clin. Invest. 2004;114:270–279. doi: 10.1172/JCI21275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Fallarino F, et al. IDO mediates TLR9-driven protection from experimental autoimmune diabetes. J. Immunol. 2009;183:6303–6312. doi: 10.4049/jimmunol.0901577. [DOI] [PubMed] [Google Scholar]
- [103].Ciorba MA, Bettonville EE, McDonald KG, Metz R, Prendergast GC, Newberry RD, Stenson WF. Induction of IDO-1 by immunostimulatory DNA limits severity of experimental colitis. J. Immunol. 2010;184:3907–3916. doi: 10.4049/jimmunol.0900291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Ravishankar B, et al. Tolerance to apoptotic cells is regulated by indoleamine 2,3-dioxygenase. Proc. Natl. Acad. Sci. U S A. 2012;109:3909–3914. doi: 10.1073/pnas.1117736109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Schroecksnadel K, Winkler C, Duftner C, Wirleitner B, Schirmer M, Fuchs D. Tryptophan degradation increases with stage in patients with rheumatoid arthritis. Clin. Rheumatol. 2006;25:334–337. doi: 10.1007/s10067-005-0056-6. [DOI] [PubMed] [Google Scholar]
- [106].Furuzawa-Carballeda J, Lima G, Jakez-Ocampo J, Llorente L. Indoleamine 2,3-dioxygenase-expressing peripheral cells in rheumatoid arthritis and systemic lupus erythematosus: a cross-sectional study. Eur. J. Clin. Invest. 2011;41:1037–1046. doi: 10.1111/j.1365-2362.2011.02491.x. [DOI] [PubMed] [Google Scholar]
- [107].Zhu L, Ji F, Wang Y, Zhang Y, Liu Q, Zhang JZ, Matsushima K, Cao Q. Synovial autoreactive T cells in rheumatoid arthritis resist IDO-mediated inhibition. J. Immunol. 2006;177:8226–8233. doi: 10.4049/jimmunol.177.11.8226. [DOI] [PubMed] [Google Scholar]
- [108].Fleckner J, Martensen PM, Tolstrup AB, Kjeldgaard NO, Justesen J. Differential regulation of the human, interferon inducible tryptophanyl-tRNA synthetase by various cytokines in cell lines. Cytokine. 1995;7:70–77. doi: 10.1006/cyto.1995.1009. [DOI] [PubMed] [Google Scholar]
- [109].Monaco F, Fumero S, Mondino A, Mutani R. Plasma and cerebrospinal fluid tryptophan in multiple sclerosis and degenerative diseases. J. Neurol. Neurosurg. Psychiatr. 1979;42:640–641. doi: 10.1136/jnnp.42.7.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Rudzite V, Berzinsh J, Grivane I, Fuchs D, Baier-Bitterlich G, Wachter H. Serum tryptophan, kynurenine, and neopterin in patients with Guillain-Barre-syndrome (GBS) and multiple sclerosis (MS) Adv. Exp. Med. Biol. 1996;398:183–187. doi: 10.1007/978-1-4613-0381-7_30. [DOI] [PubMed] [Google Scholar]
- [111].Oertelt-Prigione S, et al. Impaired indoleamine 2,3-dioxygenase production contributes to the development of autoimmunity in primary biliary cirrhosis. Autoimmunity. 2008;41:92–99. doi: 10.1080/08916930701619730. [DOI] [PubMed] [Google Scholar]
- [112].Pertovaara M, Hasan T, Raitala A, Oja SS, Yli-Kerttula U, Korpela M, Hurme M. Indoleamine 2,3-dioxygenase activity is increased in patients with systemic lupus erythematosus and predicts disease activation in the sunny season. Clin. Exp. Immunol. 2007;150:274–278. doi: 10.1111/j.1365-2249.2007.03480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Ito M, et al. Gene expression of enzymes for tryptophan degradation pathway is upregulated in the skin lesions of patients with atopic dermatitis or psoriasis. J. Dermatol. Sci. 2004;36:157–164. doi: 10.1016/j.jdermsci.2004.08.012. [DOI] [PubMed] [Google Scholar]
- [114].Scheler M, Wenzel J, Tuting T, Takikawa O, Bieber T, von Bubnoff D. Indoleamine 2,3-dioxygenase (IDO): the antagonist of type I interferon-driven skin inflammation? Am. J. Pathol. 2007;171:1936–1943. doi: 10.2353/ajpath.2007.070281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Esser C, Rannug A, Stockinger B. The aryl hydrocarbon receptor in immunity. Trends Immunol. 2009;30:447–454. doi: 10.1016/j.it.2009.06.005. [DOI] [PubMed] [Google Scholar]
- [116].Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J. Immunol. 2010;185:3190–3198. doi: 10.4049/jimmunol.0903670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Vogel CF, Goth SR, Dong B, Pessah IN, Matsumura F. Aryl hydrocarbon receptor signaling mediates expression of indoleamine 2,3-dioxygenase. Biochem. Biophys. Res. Commun. 2008;375:331–335. doi: 10.1016/j.bbrc.2008.07.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Jux B, Kadow S, Esser C. Langerhans cell maturation and contact hypersensitivity are impaired in aryl hydrocarbon receptor-null mice. J. Immunol. 2009;182:6709–6717. doi: 10.4049/jimmunol.0713344. [DOI] [PubMed] [Google Scholar]
- [119].Negishi T, et al. Effects of aryl hydrocarbon receptor signaling on the modulation of TH1/TH2 balance. J. Immunol. 2005;175:7348–7356. doi: 10.4049/jimmunol.175.11.7348. [DOI] [PubMed] [Google Scholar]
- [120].Funatake CJ, Marshall NB, Steppan LB, Mourich DV, Kerkvliet NI. Cutting edge: activation of the aryl hydrocarbon receptor by 2,3,7,8-tetrachlorodibenzo-p-dioxin generates a population of CD4+ CD25+ cells with characteristics of regulatory T cells. J. Immunol. 2005;175:4184–4188. doi: 10.4049/jimmunol.175.7.4184. [DOI] [PubMed] [Google Scholar]
- [121].Quintana FJ, et al. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- [122].Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC, Stockinger B. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- [123].Nakahama T, Kimura A, Nguyen NT, Chinen I, Hanieh H, Nohara K, Fujii-Kuriyama Y, Kishimoto T. Aryl hydrocarbon receptor deficiency in T cells suppresses the development of collagen-induced arthritis. Proc. Natl. Acad. Sci. U S A. 2011;108:14222–14227. doi: 10.1073/pnas.1111786108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Kimura A, Naka T, Nohara K, Fujii-Kuriyama Y, Kishimoto T. Aryl hydrocarbon receptor regulates Stat1 activation and participates in the development of Th17 cells. Proc. Natl. Acad. Sci. U S A. 2008;105:9721–9726. doi: 10.1073/pnas.0804231105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Kadow S, Jux B, Zahner SP, Wingerath B, Chmill S, Clausen BE, Hengstler J, Esser C. Aryl hydrocarbon receptor is critical for homeostasis of invariant gammadelta T cells in the murine epidermis. J. Immunol. 2011;187:3104–3110. doi: 10.4049/jimmunol.1100912. [DOI] [PubMed] [Google Scholar]
- [126].Qiu J, Heller JJ, Guo X, Chen ZM, Fish K, Fu YX, Zhou L. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity. 2012;36:92–104. doi: 10.1016/j.immuni.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Monteleone I, et al. Aryl hydrocarbon receptor-induced signals up-regulate IL-22 production and inhibit inflammation in the gastrointestinal tract. Gastroenterology. 2011;141:237–248. doi: 10.1053/j.gastro.2011.04.007. [DOI] [PubMed] [Google Scholar]