Abstract
Because DSM-IV cocaine dependence (CD) is heterogeneous, it is not an optimal phenotype to identify genetic variation contributing to risk for cocaine use and related behaviors (CRBs). We used a cluster analytic method to differentiate homogeneous, highly heritable subtypes of CRBs and to compare their utility with that of the DSM-IV CD as traits for genetic association analysis. Clinical features of CRBs and co-occurring disorders were obtained via a poly-diagnostic interview administered to 9,965 participants in genetic studies of substance dependence. A subsample of subjects (N = 3,443) were genotyped for 1,350 single nucleotide polymorphisms (SNPs) selected from 130 candidate genes related to addiction. Cluster analysis of clinical features of the sample yielded five subgroups, two of which were characterized by heavy cocaine use and high heritability: a heavy cocaine use, infrequent intravenous injection group and an earlyonset, heavy cocaine use, high comorbidity group. The utility of these traits was compared with the CD diagnosis through association testing of 2,320 affected subjects and 480 cocaine-exposed controls. Analyses examined both single SNP (main) and SNP–SNP interaction (epistatic) effects, separately for African-Americans and European-Americans. The two derived subtypes showed more significant P values for 6 of 8 main effects and 7 of 8 epistatic effects. Variants in the CLOCK gene were significantly associated with the heavy cocaine use, infrequent intravenous injection group, but not with the DSM-IV diagnosis of CD. These results support the utility of subtypes based on CRBs to detect risk variants for cocaine addiction.
Keywords: cocaine, cocaine dependence, candidate genes, subtyping, subtype analysis
INTRODUCTION
Cocaine use is common in the United States. The National Survey on Drug Use and Health reported that in 2010 an estimated 0.6% of Americans aged 12 or older (i.e., one million people) used cocaine within the prior month (Substance Abuse and Mental Health Services Administration, 2011]. Cocaine dependence (CD) is associated with serious social, legal, medical, and psychiatric problems and thus represents a major public health problem in the United States.
Results from adoption, twin, and family studies demonstrate a substantial genetic contribution to the risk for CD, including factors both specific to CD and common to different forms of substance dependence [Tsuang et al., 1996, 1998; Kendler and Prescott, 1998; Gelernter et al., 2005a]. The heritability in a female twin population was estimated to be 0.39 for cocaine use and 0.65 for CD symptoms [Kendler and Prescott, 1998]. However, the genetics of CD are complex and there is substantial phenotypic variance in CD populations [Uhl et al., 1995; Gelernter et al., 2005b; Kranzler et al., 2008]. Thus, insights into the genetic etiology of CD are limited by both genetic heterogeneity and the complex clinical manifestations of the disorder [Kranzler et al., 2008; Roncero et al., 2012]. This suggests that the trait defined by the DSM-IV diagnosis of CD [American Psychiatric Association, 1994], which is based on a syndromal model of the disorder, may not be well suited to the identification of specific genetic variants contributing to CD risk.
Empirical subtyping methods are based on theories that emphasize the multifaceted nature of substance dependence and related behaviors [Basu et al., 2004]. Multivariate cluster analysis has been used commonly to subtype substance dependence [Cardoso et al., 2006; Gelernter et al., 2006; Kuo et al., 2008; Chan et al., 2011], including CD [Kranzler et al., 2008]. However, these approaches have focused exclusively on the cluster homogeneity of the clinical features, rather than aiming to optimize the heritability of the subtypes and enhance the potential to identify specific genes that contribute to subgroup membership.
In this study, we differentiated homogeneous, highly heritable clinical subtypes of CD to identify subtype-specific genetic variants. To accomplish this, we designed a multivariate clustering method using clinical features and symptoms of CD to assign cocaine-using subjects to subgroups. Using traits derived from the CD subgroups, we examined a panel of genetic variants for association, including both main effects and pairwise epistatic interactions.
MATERIALS AND METHODS
Subjects
A total of 9,965 identically evaluated subjects were aggregated from family-based and case–control genetic studies of DSM-IV CD, opioid dependence (OD), and alcohol dependence (AD). Subjects were recruited at five sites: Yale University School of Medicine (N = 4,450, 44.7%), University of Connecticut Health Center (N = 3,698, 37.1%), University of Pennsylvania Perelman School of Medicine (N = 968,9.7%), Medical University of South Carolina (N = 596, 6.0%), and McLean Hospital (N = 253, 2.5%). Subjects with a clinical diagnosis of schizophrenia, bipolar disorder, or gross cognitive impairment were excluded. The institutional review board at each site approved the study protocol and informed consent forms. The National Institute on Drug Abuse and the National Institute on Alcohol Abuse and Alcoholism each provided a Certificate of Confidentiality to protect participants. Subjects were paid for their participation.
The sample included 2,379 subjects from 1,009 small nuclear families (SNFs) and 7,586 unrelated individuals. Of the families, 747 (74%) had ≥2 members with a lifetime DSM-IV diagnosis of CD. In addition to the proband in each of the SNFs, there were 1,160 siblings, 169 parents, and 41 other family members. Additionally, 4,575 subjects with CD and/or OD and 3,011 controls (screened to exclude those with a lifetime substance use disorder) from case-control studies were included in the analysis. Pedigree information was obtained for all families in the study.
The self-reported population distribution of the sample was 38.7% European-American (EA), 45.1% African-American (AA), 8% Hispanic, and 8.2% Native American, Pacific Islander or members of other minority groups. The majority of the sample (56.4%) was never married; 25.6% was widowed, separated, or divorced; and 18.0% was married. Few subjects (4.5%) completed grade school only; 28.8% had some high school, but no diploma; 25.5% completed high school only; and 41.2% received education beyond high school.
Assessments
Phenotypic information was obtained through administration of the Semi-Structured Assessment for Drug Dependence and Alcoholism (SSADDA), a computer-assisted interview comprised of 26 sections (including one for cocaine use and related behaviors) that yields lifetime DSM-IV diagnoses of substance use and Axis I psychiatric disorders, as well as antisocial personality disorder [Pierucci-Lagha et al., 2005, 2007]. The test–retest and inter-rater reliabilities of the SSADDA DSM-IV diagnosis of CD were excellent, with κ = 0.92 and 0.83, respectively [Pierucci-Lagha et al., 2005].
The four most common lifetime DSM-IV diagnoses in the sample were CD (60.6%; 3,540 men and 2,500 women), nicotine dependence (54%), AD (47.5%), and OD (32.4%). Major depressive episode (MDE) was the most common psychiatric disorder (15.5%), followed by posttraumatic stress disorder (PTSD) (13.3%), antisocial personality disorder (ASPD) (12.3%), and pathological gambling (7.9%).
Measures
The cocaine use and related behaviors section of the SSADDA contains 25 questions on age of onset, frequency, and intensity of cocaine use; route of cocaine administration; occurrence of psychosocial and medical consequences of cocaine use; attempts to quit cocaine use; and cocaine abuse treatment sought and received, resulting in 160 variables. In a previous study, in which we used a subset of the present sample, we identified 68 key questions (see Supplementary Tables SI and SII) in this section that performed well for subtyping cocaine use and related behaviors [Kranzler et al., 2008]. Those features were based on their clinical utility as discriminators of cocaine use behavior subtypes and were used here to generate subtypes of cocaine use and related behaviors. Demographics and other substance use and psychiatric variables obtained from the SSADDA interview and estimated heritability served to test the validity of the clusters.
The majority (53) of the 68 key variables were categorical, with four possible response categories: “yes,” “no,” “obligate no,” and “missing.” The SSADDA skipped out of the cocaine section when the respondent reported never having used cocaine more than 10 times (lifetime), and subsequent categorical items in the cocaine section were scored as “obligate no” and subsequent continuous items as “obligate missing;” these form their own categories in our analysis. About 1.1% of the data on the 68 key variables were missing for the 9,965 subjects. Thus, there were 312 possible categories for the 68 key variables.
Genotyping
DNA was extracted from immortalized cell lines, blood, or saliva. A total of 1,350 single nucleotide polymorphisms (SNPs) selected from 130 candidate genes and 186 ancestry informative markers (AIMs) were genotyped using the Illumina GoldenGate Assay platform (Illumina, Inc., San Diego, CA) for 3,443 subjects, a subset of the aggregate sample used in the phenotypic analysis. The AIMs were used to assign ancestry coefficients and thereby identify each subject’s primary ancestry. As described previously, the 130 candidate genes were selected on the basis of their roles in functional domains important in the addictions and in the commonly co-occurring phenotypes of anxiety and depressive disorders [Hodgkinson et al., 2008].
Phenotypic Cluster Analysis
Our phenotypic analysis consisted of three consecutive components: data reduction, cluster analysis, and heritability estimation. As described in detail in Supplementary Materials, data on the 68 key clinical variables from 9,965 subjects were analyzed to derive subtypes of cocaine use and related behaviors. Briefly, we used multiple correspondence analysis (MCA) [Abdi and Valentin, 2007; Murtagh, 2007; LeRoux and Rouanet, 2009], a non-parametric method, to reduce the large number of variables to a limited number of dimensions. Cluster analysis was used to group similar subjects together based on the retained dimensions to create clusters of subjects. To estimate the heritability of each of the clusters, logistic regression was used to compute the likelihood of each subject’s membership in the cluster. Together with pedigree information, the log likelihood values for 9,436 EAs and AAs, including 2,268 individuals from 957 multi-member families, were analyzed using the Sequential Oligogenic Linkage Analysis Routines (SOLAR) program [Almasy and Blangero, 1998] to estimate the heritability of the cluster-derived trait, with sex, age and race as covariates.
We identified five mutually exclusive clusters. As shown in Table I, the groups differed significantly on age, sex, race, education, and marital status. Specifically, Groups 2–5 (the cocaine use clusters) included significantly more men than women and were less educated than Group 1 (the “non-cocaine use group”). Group 5 had the lowest level of education and the fewest married members and included a significantly higher proportion of EAs than Groups 2–4. Groups 3 and 4 included a significantly higher proportion of AAs than the other three groups.
TABLE I.
Demographic Characteristics by Group [N (%)]
| Characteristic | Group 1, 3,370 (33.8) |
Group 2, 1,241 (12.5) |
Group 3, 180 (1.81) |
Group 4, 3,258 (32.7) |
Group 5, 1,916 (19.2) |
χ2 (df) |
|---|---|---|---|---|---|---|
| Age | 39.5 (14.2) | 3971 (10.0) | 52.39 (6.4) | 40.89 (8.1) | 39.20 (9.5) | 591.8 (4) |
| Sex [N (%)] | 262.7 (4) | |||||
| Men | 1,466 (43.5) | 750 (60.4) | 104 (57.8) | 1,816 (55.7) | 1,226 (64.0) | |
| Women | 1,904 (56.5) | 491 (39.6) | 76 (42.2) | 1,442 (44.3) | 690 (36.0) | |
| Race [N (%)] | 638.7 (12) | |||||
| AA | 1,303 (38.7) | 549 (44.2) | 94 (52.2) | 1,969 (60.4) | 580 (30.3) | |
| EA | 1,612 (47.8) | 490 (39.5) | 66 (36.7) | 793 (24.3) | 897 (46.8) | |
| Hispanic | 201 (6.0) | 107 (8.6) | 7 (3.9) | 243 (7.5) | 240 (12.5) | |
| Other | 253 (7.5) | 94 (7.6) | 13 (7.2) | 253 (7.8) | 199 (10.4) | |
| Education [N (%)] | 1,495.4 (12) | |||||
| No HS | 60 (1.8) | 73 (5.9) | 5 (2.8) | 138 (4.2) | 168 (8.8) | |
| Some HS | 382 (11.3) | 432 (34.8) | 51 (28.3) | 1,172 (36.0) | 832 (43.4) | |
| HS graduate | 656 (19.5) | 397 (32.0) | 63 (35.0) | 950 (29.2) | 472 (24.6) | |
| Beyond HS | 2,271 (67.4) | 338 (27.2) | 61 (33.9) | 997 (30.6) | 442 (23.1) | |
| Marital status [N (%)] | 634.5 (8) | |||||
| Never married | 1,731 (51.4) | 789 (63.6) | 47 (26.1) | 1,920 (58.9) | 1,135 (59.2) | |
| Married | 1,025 (30.4) | 144 (11.6) | 20 (11.1) | 398 (12.2) | 210 (11.0) | |
| Div/Sep/Wid | 614 (18.2) | 308 (24.9) | 113 (62.8) | 940 (28.9) | 571 (29.8) |
Based on the clusters’ lifetime prevalence of substance use and psychiatric disorders (Table II) and cocaine-related features (Table III), we named Group 2 the “moderate cocaine use group,” Group 3 the “late-onset heavy cocaine use group,” Group 4 the “heavy cocaine use, infrequent intravenous injection group,” and Group 5 the “early-onset, heavy cocaine use, high comorbidity group.”
TABLE II.
Lifetime Prevalence of Substance Use and Psychiatric Disorders by Group
| Disorders | Group 1, 3,370 (33.8) |
Group 2, 1,241 (12.5) |
Group 3, 180 (1.81) |
Group 4, 3,258 (32.7) |
Group 5, 1,916 (19.2) |
χ2 (df = 4) |
|---|---|---|---|---|---|---|
| Substance use disorders | ||||||
| Cocaine dependence | 0 (0.0) | 770 (62.1) | 156 (86.7) | 3,207 (98.4) | 1,907 (99.5) | 788.4 |
| Nicotine dependence | 663 (19.7) | 707 (57.0) | 112 (62.2) | 2,331 (71.6) | 1,568 (81.8) | 2,164.8 |
| Alcohol dependence | 765 (22.7) | 537 (43.3) | 105 (58.3) | 2,042 (62.7) | 1,288 (67.2) | 1,361.6 |
| Opioid dependence | 274 (8.1) | 559 (45.0) | 65 (36.1) | 946 (29.0) | 1,389 (72.5) | 1,723.1 |
| Sedative dependence | 32 (1.0) | 64 (5.2) | 12 (6.7) | 190 (5.8) | 301 (15.7) | 313.5 |
| Stimulant dependence | 15 (0.5) | 43 (3.5) | 13 (7.2) | 167 (5.1) | 216 (11.3) | 203.5 |
| Other substance dependence | 66 (2.0) | 160 (12.9) | 18 (10.0) | 340 (10.4) | 747 (39.0) | 1,019.6 |
| Psychiatric disorders | ||||||
| ASPD | 177 (5.3) | 135 (10.9) | 9 (5.0) | 484 (14.9) | 423 (22.1) | 316.9 |
| MDE | 400 (11.9) | 148 (11.9) | 37 (20.6) | 560 (17.2) | 395 (20.6) | 92.2 |
| PTSD | 195 (5.8) | 115 (9.3) | 30 (16.7) | 576 (17.7) | 413 (21.6) | 323.0 |
| OCD | 35 (1.0) | 18 (1.5) | 3 (1.7) | 93 (2.9) | 74 (3.9) | 49.5 |
| Social phobia | 89 (2.6) | 42 (3.4) | 12 (6.7) | 148 (4.5) | 130 (6.8) | 54.9 |
| Agoraphobia | 87 (2.6) | 41 (3.3) | 15 (8.3) | 195 (6.0) | 161 (8.4) | 97.7 |
| Panic disorder | 86 (2.6) | 45 (3.6) | 16 (8.9) | 183 (5.6) | 201 (10.5) | 148.4 |
| Compulsive gambling | 95 (2.8) | 72 (5.8) | 19 (10.6) | 364 (11.2) | 236 (12.3) | 198.2 |
TABLE III.
Cocaine Use Characteristics, Cocaine-Related Effects, and Cocaine Treatment History by Group [N (%)]
| Behaviors | Group 2 1,241 (12.5) |
Group 3 180 (1.81) |
Group 4 3,258 (32.7) |
Group 5 1,916 (19.2) |
χ2 (df=3) |
|---|---|---|---|---|---|
| Mean [SD] age of first cocaine use in year | 21.1 (5.7) | 36.5 (11.8) | 21.4 (5.2) | 17.9 (4.3) | 1,057.8 |
| Mean [SD] age of onset of heaviest cocaine use in year | 27.2 (8.2) | 45.1 (5.0) | 29.3 (7.4) | 25.8 (8.4) | 2,158.0 |
| Used cocaine daily or almost daily | 861 (69.4) | 157 (87.2) | 3,026 (92.9) | 1,857 (96.9) | 540.8 |
| Injected cocaine intravenously | 388 (31.3) | 44 (24.4) | 129 (3.9) | 1,417 (74.0) | 1,804.4 |
| Stayed high from cocaine for a whole day or more | 594 (47.9) | 131 (72.8) | 2,628 (80.7) | 1,623 (84.7) | 576.2 |
| Strong desire for cocaine made it hard to think of anything else | 404 (32.6) | 118 (65.6) | 2,532 (77.7) | 1,619 (84.5) | 933.9 |
| Cocaine interfered with work, school, or home life | 318 (25.6) | 111 (617) | 2,475 (76.0) | 1,650 (86.1) | 1,132.4 |
| Family members, friends, doctor, clergy, boss, Or people at work or school objected to cocaine use |
475 (38.3) | 108 (60.0) | 2,683 (82.4) | 1,664 (86.9) | 961.2 |
| Been arrested or had trouble with the police because Of cocaine use |
310 (25.0) | 61 (33.9) | 1,776 (54.5) | 1,233 (64.4) | 465.6 |
| Gave up or greatly reduced important activities due to cocaine use |
419 (33.8) | 131 (72.8) | 2,853 (87.6) | 1,775 (92.6) | 1,418.3 |
| Ever treated for a cocaine-related problem | 432 (34.8) | 109 (60.6) | 2,372 (72.8) | 1,421 (74.2) | 585.6 |
| Ever attended self-help group for cocaine use | 363 (29.3) | 90 (50.0) | 2,186 (67.1) | 1,369 (71.5) | 601.2 |
Group 1 was the largest, consisting of 3,370 subjects (56.5% women), none of whom had a diagnosis of CD. Group 1 had a mean of 3.8 (SD = 3.1) reported lifetime episodes of cocaine use and the lowest prevalence of all other substance dependence and psychiatric disorders (except ASPD, which was slightly lower in Group 3). The estimated heritability of the non-cocaine use group was 0.41 (SE = 0.06). Group 2, comprised of 1,241 subjects, had a significantly lower rate of CD (62.1%) and was less likely to have comorbid dependence on nicotine, alcohol, sedatives, or stimulants and psychiatric disorders (except ASPD) than groups 3–5. Of the cocaine-use groups, Group 2 had the lowest percentage of individuals reporting daily or almost daily cocaine use, negative effects due to cocaine use, and a history of cocaine treatment, but a higher rate of intravenous cocaine injection (31.3%) than Groups 3 (24.4%) or 4 (3.9%). Group 2 had the highest estimated heritability (0.69, SE = 0.05).
Groups 3–5 were heavy cocaine use groups. Of these, Group 3, the late-onset group, was the smallest (N = 180) and had the lowest rate of CD (86.7%), percentage of subjects with negative cocaine-related effects, and likelihood of cocaine treatment. This group had a significantly older mean age of first cocaine use (36.5 years, SD = 11.8) and heaviest cocaine use (45.1 years, SD = 5) than Groups 4 and 5. The heritability of Group 3 was only 0.07 (SE = 0.06), likely in part due to the small sample size. Group 4 was the largest cocaine use group, comprising 3,258 subjects, 98.4% of whom were diagnosed with CD. This group had a rate of intravenous cocaine injection that was much lower than all of the other cocaine use groups. The percentages of subjects in Group 4 that experienced negative effects due to cocaine use and that received treatment were intermediate between Groups 3 and 5. The heritability of Group 4 was 0.66 (SE = 0.05). The 1,916 subjects in Group 5, the early-onset, heavy cocaine use, high comorbidity group, had the highest prevalence of substance dependence and psychiatric disorders. This group reported the earliest onset of cocaine use (17.9 years, SD = 4.3) and of heaviest use (25.8 years, SD = 8.4), and endorsed a high rate of adverse effects of cocaine use (Table III). The heritability of Group 5 was 0.64 (SE = 0.05). Because of the high prevalence of CD, Groups 4 and 5 were chosen as the most clinically informative subtypes of CD and were compared with DSM-IV CD as traits for association analysis.
Genetic Association Analysis
Of the 3,443 subjects genotyped, 612 were never exposed to cocaine. These individuals were classified as “unknown” rather than “unaffected” and were removed from subsequent analyses. To correct possibly unreliable self-reported sex, we calculated the chromosome X heterozygosity for each subject and removed 31 subjects: 14 self-reported males with heterozygosity of more than 20% and 17 self-reported females with heterozygosity of less than 20%. Race was classified using STRUCTURE v2.3 [Pritchard et al., 2000] and AIMs, which stratified the remaining 2,800 subjects into two population groups: AAs and EAs. Of the 1,478 AAs, 93.78% had AA as their self-reported race. Of the 1,322 EAs, 89.71% had EA as their self-reported race. The sample included 253 self-reported Hispanics, among whom 82 reported AA origin (43 were in the African ancestry and 39 in the European ancestry clusters) and 171 reported EA origin (168 were in the European ancestry and 3 in the African ancestry clusters). We excluded other population groups from the association analysis. Association tests for AA and EA population groups were performed separately. Principal components analysis (PCA) was performed on the 186 AIMs for each population. The first PCA dimension was used in the subsequent association tests as a covariate to correct for the residual population structure. SNPs for which data were available for less than 95% of the subjects, or for which the P value for Hardy–Weinberg equilibrium was less than 10−7, were excluded from further analysis. The minor allele frequency (MAF) of each SNP was calculated within each population in each association test for different traits. SNPs with MAF < 5% in a population were removed from the association tests for the trait in that population.
Association testing was performed for three traits: DSM-IV CD and the two heavy cocaine-use subtypes to identify SNPs that exerted a significant main effect or that, in pair-wise combinations, exerted a joint (epistatic) effect on the traits. GEE logistic regression was used in main effects tests to correct for correlations among related individuals in families. All SNP pairs were tested for association in epistatic analyses. We compared the findings of pair-wise joint effects detected using three methods: GEE logistic regression [SAS Institute, Inc., 2008], regular logistic regression [Purcell et al., 2007] and a bioinformatics method called BOOST [Wan et al., 2010]. For each SNP pair, BOOST estimates two logistic models: one uses the two SNPs as covariates; the other uses the two SNPs and their cross-product as covariates. An upper bound is calculated to approximate the ratio of the two logistic models, which aims to assess the additional effect that the cross-product contributes. The bound has been shown to be tight, and most nonsignificant interactions can be pruned. In all three models, age, sex and the first PCA dimension calculated from the AIMs were included as covariates. Permutation tests were performed to identify empirical thresholds to correct the P-value for multiple tests of main effects. Parallelized permutation tests were developed and conducted using a high performance computing facility with 120 processors to identify empirical thresholds and avoid inflating the P-value for epistatic effects.
RESULTS
Removing the controls never exposed to cocaine from the genotyped subsample left 480 controls (187 AAs and 293 EAs). After data cleaning, there were 2,320 cases with a lifetime DSM-IV diagnosis of CD (1,291 AAs and 1,029 EAs). Of these, 779 AAs and 390 EAs were in Group 4 and 259 AAs and 436 EAs were in Group 5. Cases with data on all three phenotypes (DSM-IV CD, Groups 4 or 5) were tested separately for association against the same control samples, separately for AAs and EAs. SNPs were processed using the quality control steps for each individual case-control comparison, resulting in different numbers tested for association. Table IV describes the subjects and SNPs used in the association analyses.
TABLE IV.
Summary of the Subjects and Single Nucleotide Polymorphisms (SNPs) Used in the Association Tests
| Cases |
Controls |
No. of SNPs |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Phenotype | Total no. | Male (%) | Average age |
Total no. | Male (%) | Average age |
Missinga | MAFb | Remained in the analysis |
| AA | |||||||||
| CD diagnosis | 1,291 | 49.6 | 41.1 | 212 | 1,106 | ||||
| Group 4 | 779 | 45.7 | 40.4 | 187 | 56.7 | 42.4 | 32 | 215 | 1,103 |
| Group 5 | 259 | 61.4 | 42.0 | 210 | 1,108 | ||||
| EA | |||||||||
| CD diagnosis | 1,029 | 58.0 | 37.5 | 346 | 971 | ||||
| Group 4 | 390 | 52.7 | 37.7 | 293 | 62.1 | 36.3 | 33 | 350 | 967 |
| Group 5 | 436 | 63.1 | 36.9 | 352 | 965 | ||||
SNPs were removed because of missing values on > 5% of subjects.
SNPs were removed because of minor allele frequency [MAF] < 5%.
Main Effects
Table V lists the SNPs that were associated with any of the three traits at P<5E–04. Two statistically significant variants were identified in AAs. HTR2C (rs5988072) was associated with both DSM-IV CD (Padj = 8.89E–03, Bonferroni corrected) and the early-onset, heavy cocaine use, high comorbidity group (Group 5) (Padj = 4.99E–02, corrected by 1,000 permutation tests). A variant in CLOCK (rs11939815, Padj = 4.27E–02) was significantly associated with the heavy cocaine use, infrequent intravenous injection subtype (Group 4). Several genes contained nominally significant variants (P < 5E–04), but none were significant using permutation to adjust for multiple comparisons. In AAs, these included ALDH1A1 (rslll43429, P=3.35E–04), which was associated with DSM-IV CD; CLOCK (3 SNPs:rs3805155, P = 1.11 E–04; rsl3116194, P=2.11E–04; rs6850524, P=2.01E–04) and GLRA1 (rs991738, P=3.02E–04), which were associated with Group 4. In EAs, there was also a nominal association of a variant in SLC18A2 (rs363256, P = 2.27E–04) with this subtype. A variant in OXT (rs3761248, P = 3.04E–04) was nominally associated with Group 5 in AAs. Except for the findings related to HTR2C and ALDH1A1, the P values for all other variants listed in Table V were smaller for the derived subtypes than for DSM-IV CD.
TABLE V.
Top Findings From Main Effect Tests
| CD diagnosis |
Group 4 |
Group 5 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chr | Position (bp) |
SNPID | Gene | RAF | Coef. | P-Value | RAF | Coef | P-Value | RAF | Coef. | P-Value |
| AA | ||||||||||||
| X | 113,890,903 | rs5988072 | HTR2C | 0.883 | −0.569 | 8.04E–06a,b | 0.868 | −0.491 | 2.42E–04 | 0.869 | −0.714 | 5.80E–05c |
| 9 | 75,607,884 | rslll43429 | ALDH1A1 | 0.802 | −0.461 | 3.35E–04b | 0.795 | −0.440 | 2.34E–03 | 0.783 | −0.567 | 1.53E–03 |
| 4 | 56,358,283 | rsll939815 | CLOCK | 0.372 | 0.404 | 4.00E–04 | 0.363 | 0.503 | 3.87E–05a,b | 0.420 | 0.308 | 2.52E–02 |
| 5 | 151,298,449 | rs991738 | GLRA1 | 0.555 | −0.391 | 5.72E–04 | 0.553 | −0.421 | 3.02E–04b | 0.518 | −0.302 | 3.59E–02 |
| 4 | 56,364,009 | rs3805155 | CLOCK | 0.799 | −0.438 | 6.05E–04 | 0.801 | −0.520 | 1.11E–04b | 0.767 | −0.335 | 3.17E–02 |
| 4 | 56,397,317 | rsl3116194 | CLOCK | 0.792 | −0.411 | 7.57E–04 | 0.793 | −0.472 | 2.11E–04b | 0.760 | −0.325 | 3.18E–02 |
| 4 | 56,381,997 | rs6850524 | CLOCK | 0.641 | −0.372 | 1.37E–03 | 0.649 | −0.460 | 2.01E–04b | 0.599 | −0.290 | 3.42E–02 |
| 20 | 3,050,393 | rs3761248 | OXT | 0.908 | 0.717 | 3.27E–03 | 0.913 | 0.709 | 5.19E–03 | 0.909 | 1.010 | 3.04E–04b |
| EA | ||||||||||||
| 10 | 119,024,225 | rs363256 | SLC18A2 | 0.896 | −0.391 | 7.68E–03 | 0.899 | −0.646 | 2.27E–04b | 0.872 | −0.364 | 2.86E–02 |
RAF, reference allele frequency.
Significant at P< 0.05 after Bonferroni correction for multiple tests.
The smallest P-value obtained among the three traits.
Significant at P< 0.05 after correction for multiple tests by permutation (empirical threshold P< 5.9E–05].
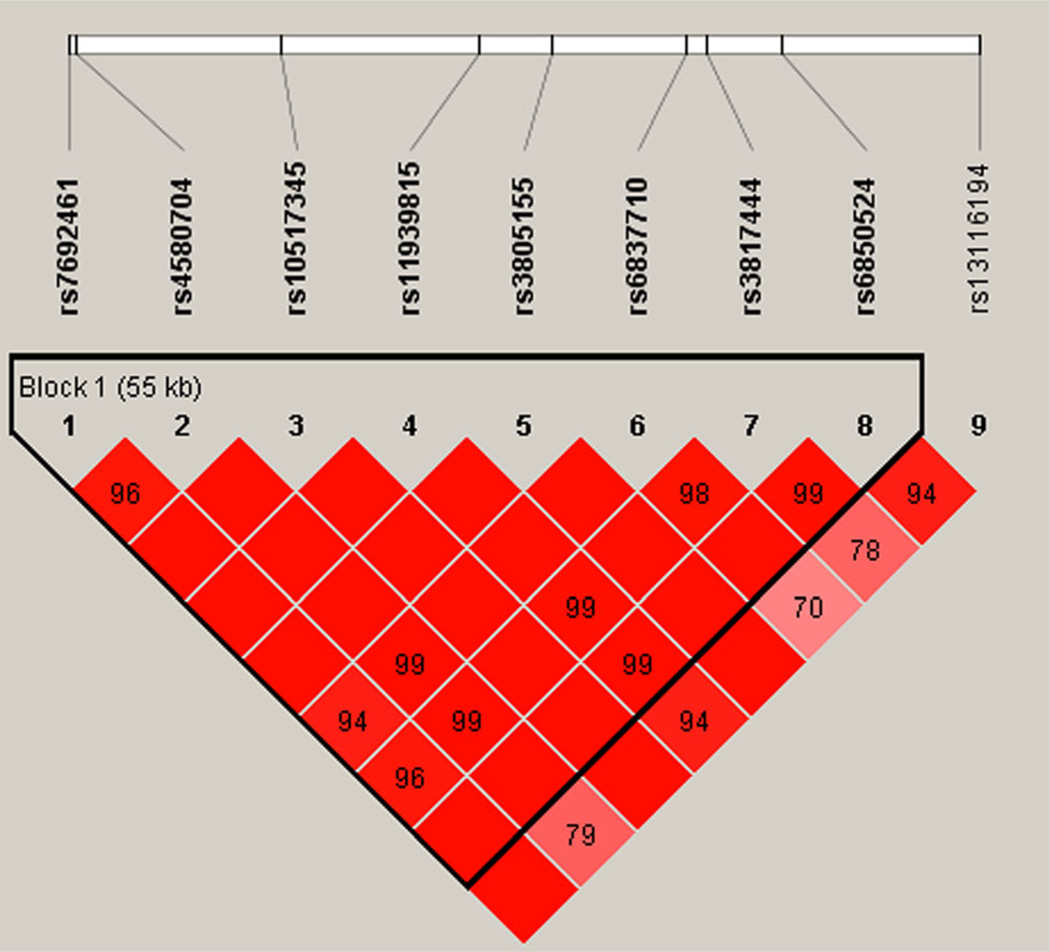
Based on the association in AAs of one of the SNPs in the CLOCK gene with membership in Group 4, we examined the linkage disequilibrium (LD) structure of the 9 genotyped CLOCK SNPs in the 1,478 AAs for which genotypes were available. These SNPs spanned 71,151 bp on chromosome 4. Figure 1 shows the LD relationships of the nine SNPs analyzed using Haploview 4.1 software. Rs119398115, the SNP that was significantly associated with membership in Group 4, had very high D′ (normalized LD) with respect to two of the nominally significant SNPs from the CLOCK gene: rs6850524 and rs13116194, which were also in high LD with one another.
FIG. 1.
LD structure of nine SNPs spanning the CLOCK gene in the AA genotyped sample. Shades of pink/red represent D′ < 1 and L0D ≥ 2 with the value of D′ written in the boxes, bright red represents D′ = 1 and L0D ≥ 2.
Pairwise Epistatic Effects
As shown in Table VI, after Bonferroni correction, no SNP pair was significantly associated with any of the traits in either AAs or EAs. However, this conservative correction does not account for the correlation between SNP pairs, and likely resulted in an inflation of type II error. In permutation tests to determine an empirical significance threshold, >106 pairs were tested for each randomly permuted phenotype based on the case–control ratios for the three traits: DSM-IV CD, Groups 4 and 5. We parallelized the standard permutation approach based on logistic regression (i.e., using PLINK) and distributed the process to 120 processors to identify empirical thresholds for significance. Table VI lists the SNP pairs with Pgee < 1E–05 (GEE), Plr < 1E–05 (logistic regression using PLINK [Purcell, 2009]), or Pboost< 1E–04 (BOOST) for at least one of the traits, which identifies candidates for possible subsequent validation. Five SNP pairs were identified in AAs, including one that was strongly associated with DSM-IV CD and Group 4 (heavy cocaine use with infrequent intravenous injection), one associated only with Group 4 and three that were associated with Group 5 (early-onset, heavy cocaine use, high comorbidity). The association of a SNP pair comprised of one variant each from GRM1 and OPRM1 with Group 5 was nominally significant (P=7.6E–06), but exceeded the empirical threshold (P < 7.6E–07). Three pairs showed nominal significance in EAs as well, including two with Group 4 and one with Group 5. No SNP pairs were nominally associated with the DSM-IV diagnosis of CD. A SNP pair with variants from CRHR2 and BDNF was nominally significant for Group 4 after multiple test correction (P=3.5E–06), but it also exceeded the empirical threshold (P< 1.6E–6). Despite the fact that none of the results reached statistical significance when corrected for multiple comparisons, the findings in Table VI show that the two derived subtypes provide more statistical power for association analysis than the DSM-IV diagnosis of CD.
TABLE VI.
Top Findings From Pairwise Epistatic Effect Tests
| SNP1 |
SNP2 |
GEE P-Value |
PLINK P-Value |
BOOST P-Value |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chr | Position (bp) |
SNP ID | Gene | Chr | Position (bp) |
SNP ID | Gene | CD diagnosis |
Group 4 | Group 5 | CD diagnosis |
Group 4 | Group 5 | CD diagnosis |
Group 4 | Group 5 |
| AA | ||||||||||||||||
| 4 | 47,423,595 | rs4591574 | GABRB1 | 15 | 27,008,264 | rs8041610 | GABRB3 | 3.2E–6 | 6.5E–6 | 1.9E–4 | 6.0E–7 | 2.0E–6 | 5.4E–5 | 3.0E–5 | 7.1E–5 | 1.5E–3 |
| 7 | 50,620,275 | rs2329341 | DOC | 15 | 27,514,749 | rs751085 | GABRG3 | 5.8E–5 | 1.4E–6 | 3.6E–2 | 1.8E–4 | 3.7E–6 | 1.6E–3 | 4.5E–3 | 8.4E–5 | 2.2E–2 |
| 8 | 54,140,999 | rs3802280 | 0PRK1 | 11 | 113,803,666 | rs17116138 | HTR3B | 6.8E–4 | 1.3E–2 | 4.7E–6 | 2.3E–4 | 6.3E–4 | 2.5E–5 | 4.8E–3 | 1.6E–2 | 3.5E–5 |
| 8 | 54,140,999 | rs3802280 | 0PRK1 | 11 | 113,801,668 | rs17116121 | HTR3B | 7.9E–4 | 1.3E–2 | 6.6E–6 | 2.8E–4 | 5.7E–4 | 3.3E–5 | 2.3E–3 | 1.1E–2 | 5.5E–5 |
| 6 | 146,446,024 | rs9373486 | GRM1 | 6 | 154,445,215 | rs10485058 | 0PRM1 | 7.2E–4 | 8.5E–3 | 7.5E–6 | 1.8E–4 | 1.3E–4 | 7.6E–6 | 8.4E–4 | 3.4E–2 | 4.9E–6 |
| EA | ||||||||||||||||
| 7 | 30,713,608 | rs2284217 | CRHR2 | 11 | 27,744,859 | rsl2273363 | BDNF | 2.2E–5 | 2.8E–6 | 5.0E–3 | 1.5E–5 | 3.5E–6 | 1.4E–3 | 6.7E–4 | 9.9E–5 | 3.2E–2 |
| 4 | 47,051,185 | rs4315750 | GABRB1 | 15 | 27,048,768 | rs4S177S9 | GABRB3 | 1.4E–4 | 6.6E–6 | 4.2E–2 | 1.4E–4 | 8.7E–6 | 3.5E–4 | 3.1E–3 | 9.8E–5 | 1.0E–2 |
| 2 | 171,682,740 | rs3828275 | GAD1 | 17 | 43,894,798 | rs81189 | CRHR1 | 2.8E–5 | 4.4E–3 | 7.6E–6 | 4.0E–5 | 1.2E–3 | 3.1E–6 | 1.7E–3 | 2.9E–2 | 1.0E–4 |
Bold font numbers are the smallest P values among the three traits for each of the three methods. All numbers were rounded to the first decimal place in the mantissa of the scientific number notation.
DISCUSSION
We compared the utility of three traits—a DSM-IV CD diagnosis and two highly heritable subtypes based on cocaine use and related behaviors—for association analysis of 130 candidate genes related to addictions in two American populations. We obtained the most significant findings in the AA population, where two SNPs exceeded the empirical significance threshold for a main effect after correction for multiple comparisons. Rs5988072 (in HTR2C) was significantly associated with both the CD diagnosis and the CD subtype of early-onset and heavy cocaine use, with high comorbidity. Rs11939815 (in CLOCK) was associated only with the CD subtype of heavy cocaine use with infrequent intravenous injection.
Because cocaine can increase the level of serotonin in the synapse by blocking reuptake via the serotonin transporter [Cami and Farre, 2003], genetic variants involved in the serotonergic system are candidates for CD risk [Saxon et al., 2005]. The 5-HT2C receptor, encoded by HTR2C, modulates the discriminative stimulus effects of cocaine [Callahan and Cunningham, 1995; Frankel and Cunningham, 2004]. Our finding of an association of HTR2C with both DSM-IV CD and one of the two subtypes we examined is, to our knowledge, the first report of such an association. The finding is consistent with the pharmacology of cocaine and, if replicated, points to differences in the pathophysiology of CD and AD, as studies of HTR2C have failed to show an association with AD [Herman and Balogh, 2012].
In addition, we found that CLOCK variation was associated with the CD subtype characterized by heavy cocaine use with infrequent intravenous injection. Because the finding was limited to that CD subtype, if replicated, it confirms the important role of phenotype refinement through multivariate cluster analysis in the identification of genetic factors for CD [Gelernter et al., 2005b; Kranzler et al., 2008; Sun et al., 2012]. The phenotypic heterogeneity in CD may partially explain why there has been no significant replicated association implicating circadian system regulation of cocaine sensitization and the reward system in animal models [Andretic et al., 1999; Abarca et al., 2002; Yuferov et al., 2005; Perreau-Lenz et al., 2007]. Of specific interest in this regard is the finding of increased cocaine reward and excitability of midbrain dopamine neurons in mice lacking a functional CLOCK gene [McClung et al., 2005]. In addition to the SNP in the CLOCK gene that reached significance (Table V), three other CLOCK variants were strongly associated with the CD subtype of heavy cocaine use with infrequent intravenous injection (Table V), consistent with a contribution of CLOCK variation to CD risk. Our previous association study of a CLOCK variant with DSM-IV CD in a smaller sample was negative [Malison et al., 2006].
As a complex disorder, CD, like other forms of substance dependence, is the product of both environmental and genetic risk factors. Its genetic determinants likely include interactions among multiple neurochemical systems, including those involving dopamine, GABA, endogenous opioid, endogenous cannabinoid, and serotonin [Saxon et al., 2005]. Variability in these interactions due to genetic effects is a possible source of vulnerability to CD. We found several pairs of variants that approached significance for an association with CD and the two subtypes. An interaction between the genes encoding the beta 1 and beta 3 subunits of the GABA-A receptor was present in both AAs and EAs. Because GABA interneurons synapse on dopamine neurons to inhibit dopamine release, variation in the expression or function of GABA-A receptor subunits could alter the rewarding effects of cocaine [Spanagel and Weiss, 1999]. In AAs, the interaction of variants in DDC and GABRG3 also approached significance for an association with the CD subtype of heavy cocaine use with infrequent intravenous injection, which could affect the dopamine-mediated reward produced by cocaine, both directly (through dopamine metabolism) and indirectly (through GABA interneurons).
In the present study, we employed a cluster analytic approach to derive subtypes of cocaine use and related behaviors that were more homogeneous than the group defined by DSM diagnosis in terms of their clinical features, including frequency and intensity of cocaine use, cocaine withdrawal symptoms, cocaine-related adverse effects and treatment history. We hypothesized that the subtypes would be superior to DSM-IV CD as traits for variant detection. Our association analysis provided support for the hypothesis. The association results with the two derived subtypes showed more significant P values for six of eight SNPs in the analysis of main effects (Table V) and seven of eight SNP pairs of epistatic effects (Table VI). Moreover, one variant, rs11939815 in CLOCK, was significantly associated only with Group 4 in AAs. An advantage of the subtyping approach to trait definition was also evident in the pair-wise epistatic effect tests, in which the most significant results were for variants associated with either of the two derived subtypes for all SNP pairs except rs4591574 and rs8041610.
The cluster analysis approach employed in the current study was refined from the one used in Kranzler et al. [2008], from which it differs in three major respects. First, in contrast to the approach used in Kranzler et al., the clustering process in the current analysis consisted of two layers of a cascaded process, as depicted in the supplemental material. This allowed us to identify subtypes that are both homogeneous and highly heritable. Second, in Kranzler et al., k-means cluster analysis was used to generate intermediate clusters. This method is an iterative procedure that is initialized with randomly chosen cluster centers. It is sensitive to outliers, with different initialization known to yield different clusters. In the present study, we used smart k-medoids cluster analysis, which is more robust to variation in the sample distribution. The supplementary material contains additional information about the cluster approach that we used in the present study. Third, the sample used here is much larger than the 1,393 subjects in Kranzler et al. and it yielded larger and more clearly differentiated clusters. Kranzler et al. found 6 clusters, each of which contained 42–350 subjects. The first three were heavy cocaine user groups, but all six clusters (including the low-cocaine-use group) contained subjects with a diagnosis of CD. In contrast, the present analysis yielded a non-cocaine-user group in which no one had a diagnosis of CD. Subjects in Cluster 1 of Kranzler et al. (N = 336) were included in our Cluster 4 (N = 3,258 subjects), which was the largest user group. Subjects in Cluster 2 (N = 303) of Kranzler et al. were included in Cluster 5 in the current study, which contained 1,916 subjects. Cluster 3 in Kranzler et al. was split in to Clusters 3,4, and 5 in the current study. Thus, we believe that the larger sample and more sophisticated methodology in the present study yielded more homogeneous clusters, which are more useful for association analysis.
The study is limited by the lack of suitable samples for independent replication to validate these findings. The scoring functions for each of the two subtypes, which use cocaine use and related behaviors to yield a probabilistic membership score for each subject in each cluster, are available from the first author upon request to allow other investigators to use the Supplementary Material to attempt to replicate our findings for Groups 4 and 5. The permutation tests required to estimate empirically the significance threshold for type I error for the tests of epistatic effects were very computationally intensive. Advances in statistical methods are required to address this problem, which is often encountered in the examination of higher-order interactions.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by NIH grants DA12849, DA12690, AA03510, AA11330, and AA13736 and the VISN 1 and VISN 4 Mental Illness Research, Education, and Clinical Centers (MIRECCs).
Dr. Kranzler has been a consultant or advisory board member for Alkermes, Lilly, Lundbeck, Pfizer, and Roche. He is also a member of the American Society of Clinical Psychopharmacology’s Alcohol Clinical Trials Initiative, which is supported by Lilly, Lundbeck, Abbott, and Pfizer.
Footnotes
Conflict of interests: Dr. Bi, Mr. Sun, and Dr. Gelernter have no disclosures.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of this article at the publisher’s web-site.
REFERENCES
- Abarca C, Albrecht U, Spanagel R. Cocaine sensitization and reward are under the influence of circadian genes and rhythm. Proc Natl Acad Sci USA. 2002;99(13):9026–9030. doi: 10.1073/pnas.142039099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdi H, Valentin D. Multiple correspondence analysis. In: Salkind N, editor. Encyclopedia of measurement and statistics. Thousand Oaks, CA: SAGE; 2007. [Google Scholar]
- Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62(5):1198–1211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Andretic R, Chaney S, Hirsh J. Requirement of circadian genes for cocaine sensitization in Drosophila . Science. 1999;285(5430):1066–1068. doi: 10.1126/science.285.5430.1066. [DOI] [PubMed] [Google Scholar]
- Basu D, Ball SA, Feinn R, Gelernter J, Kranzler HR. Typologies of drug dependence: Comparative validity of a multivariate and four univariate models. Drug Alcohol Depend. 2004;73(3):289–300. doi: 10.1016/j.drugalcdep.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Callahan PM, Cunningham KA. Modulation of the discriminative stimulus properties of cocaine by 5-HT1B and 5-HT2C receptors. J Pharmacol Exp Ther. 1995;274(3):1414–1424. [PubMed] [Google Scholar]
- Cami J, Farre M. Drug addiction. N Engl J Med. 2003;349(10):975–986. doi: 10.1056/NEJMra023160. [DOI] [PubMed] [Google Scholar]
- Cardoso JMN, Barbosa A, Ismail F, Pombo S. NETER alcoholic typology (NAT) Alcohol Alcohol (Oxford, Oxfordshire) 2006;41(2):133–139. doi: 10.1093/alcalc/agh247. [DOI] [PubMed] [Google Scholar]
- Chan G, Gelernter J, Oslin D, Farrer L, Kranzler HR. Empirically derived subtypes of opioid use and related behaviors. Addiction. 2011;106(6):1146–1154. doi: 10.1111/j.1360-0443.2011.03390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel PS, Cunningham KA. m-Chlorophenylpiperazine (mCPP) modulates the discriminative stimulus effects of cocaine through actions at the 5-HT2C receptor. Behav Neurosci. 2004;118(1):157–162. doi: 10.1037/0735-7044.118.1.157. [DOI] [PubMed] [Google Scholar]
- Gelernter J, Panhuysen C, Weiss R, Brady K, Hesselbrock V, Rounsaville B, Poling J, Wilcox M, Farrer L, Kranzler HR. Genomewide linkage scan for cocaine dependence and related traits: Significant linkages for a cocaine-related trait and cocaine-induced paranoia. Am J Med Genet Part B. 2005a;136B(1):45. doi: 10.1002/ajmg.b.30189. [DOI] [PubMed] [Google Scholar]
- Gelernter J, Panhuysen C, Weiss R, Brady K, Hesselbrock V, Rounsaville B, Poling J, Wilcox M, Farrer L, Kranzler HR. Genomewide linkage scan for cocaine dependence and related traits: Significant linkages for a cocaine-related trait and cocaine-induced paranoia. Am J Med Genet Part B. 2005b;136B(1):45–52. doi: 10.1002/ajmg.b.30189. [DOI] [PubMed] [Google Scholar]
- Gelernter J, Panhuysen C, Wilcox M, Hesselbrock V, Rounsaville B, Poling J, Weiss R, Sonne S, Zhao H, Farrer L, et al. Genomewide linkage scan for opioid dependence and related traits. Am J Hum Genet. 2006;78(5):759. doi: 10.1086/503631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman AI, Balogh KN. Polymorphisms of the serotonin transporter and receptor genes: Susceptibility to substance abuse. Subst Abuse Rehabil. 2012;3(1):49–157. doi: 10.2147/SAR.S25864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkinson CA, Yuan Q, Xu K, Shen PH, Heinz E, Lobos EA, Binder EB, Cubells J, Ehlers CL, Gelernter J, et al. Addictions biology: Haplotype-based analysis for 130 candidate genes on a single array. Alcohol Alcohol. 2008;43(5):505–515. doi: 10.1093/alcalc/agn032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA. Cocaine use, abuse and dependence in a population-based sample of female twins. Br J Psychiatry. 1998;173:345–350. doi: 10.1192/bjp.173.4.345. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Wilcox M, Weiss RD, Brady K, Hesselbrock V, Rounsaville B, Farrer L, Gelernter J. The validity of cocaine dependence subtypes. Addict Behav. 2008;33(1):41–53. doi: 10.1016/j.addbeh.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo P-H, Aggen SH, Prescott CA, Kendler KS, Neale MC. Using a factor mixture modeling approach in alcohol dependence in a general population sample. Drug Alcohol Depend. 2008;98(1):105–114. doi: 10.1016/j.drugalcdep.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeRoux B, Rouanet H. Multiple correspondence analysis. Los Angeles, CA: Sage; 2009. [Google Scholar]
- Malison RT, Kranzler HR, Yang B-Z, Gelernter J. Human clock, PER1 and PER2 polymorphisms: Lack of association with cocaine dependence susceptibility and cocaine-induced paranoia. Psychiatr Genet. 2006;16(6):245–249. doi: 10.1097/01.ypg.0000242198.59020.ca. [DOI] [PubMed] [Google Scholar]
- McClung CA, Sidiropoulou K, Vitaterna M, Takahashi JS, White FJ, Cooper DC, Nestler EJ. Regulation of dopaminergic transmission and cocaine reward by the Clock gene. Proc Natl Acad Sci USA. 2005;102(26):9377–9381. doi: 10.1073/pnas.0503584102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtagh F. Multiple correspondence analysis and related methods. Psychometrika. 2007;72(2):275–277. [Google Scholar]
- Perreau-Lenz S, Zghoul T, Spanagel R. Clock genes running amok. Clock genes and their role in drug addiction and depression. EMBO Rep. 2007;8:S20–S23. doi: 10.1038/sj.embor.7401016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierucci-Lagha A, Gelernter J, Feinn R, Cubells JF, Pearson D, Pollastri A, Farrer L, Kranzler HR. Diagnostic reliability of the semi-structured assessment for drug dependence and alcoholism (SSADDA) Drug Alcohol Depend. 2005;80(3):303–312. doi: 10.1016/j.drugalcdep.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Pierucci-Lagha A, Gelernter J, Chan G, Arias A, Cubells JF, Farrer L, Kranzler HR. Reliability of DSM-IV diagnostic criteria using the semi-structured assessment for drug dependence and alcoholism (SSADDA) Drug Alcohol Depend. 2007;91(1):85–90. doi: 10.1016/j.drugalcdep.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155(2):945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S. Package: PLINK v1.07. 2009 http://pngumghharvardedu/purcell/plink.
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira M, Bender D, Maller J, Sklar P, de Bakker P, Daly MJ, et al. PLINK: A toolset for whole-genome association and population-based linkage analysis. Am J Hum Genet. 2007;81(3):559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roncero C, Ros-Cucurull E, Daigre C, Casas M. Prevalence and risk factors of psychotic symptoms in cocaine-dependent patients. Actas Esp Psiquiatr. 2012;40(4):187–197. [PubMed] [Google Scholar]
- SAS Institute, Inc. SAS(r) 9.2 enhanced logging facilities. Cary, NC: SAS Institute, Inc; 2008. [Google Scholar]
- Saxon AJ, Oreskovich MR, Brkanac Z. Genetic determinants of addiction to opioids and cocaine. Harv Rev Psychiatry. 2005;13(4):218–232. doi: 10.1080/10673220500243364. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Weiss F. The dopamine hypothesis of reward: Past and current status. Trends Neurosci. 1999;22(11):521–527. doi: 10.1016/s0166-2236(99)01447-2. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. NSDUH Series H-41, HHS Publication No (SMA) 11-4658. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2011. Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings. [Google Scholar]
- Sun J, Bi J, Chan G, Oslin D, Farrer L, Gelernter J, Kranzler HR. Improved methods to identify stable, highly heritable subtypes of opioid use and related behaviors. Addict Behav. 2012;37(10):1138–1144. doi: 10.1016/j.addbeh.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuang MT, Lyons MJ, Eisen SA, Goldberg J, True W, Lin N, Meyer JM, Toomey R, Faraone SV, Eaves L. Genetic influences on DSM-III-R drug abuse and dependence: A study of 3,372 twin pairs. American Journal of Medical Genetics Part B, Neuropsychiatr Genet. 1996;67(5):473–177. doi: 10.1002/(SICI)1096-8628(19960920)67:5<473::AID-AJMG6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Tsuang MT, Lyons MJ, Meyer JM, Doyle T, Eisen SA, Goldberg J, True W, Lin N, Toomey R, Eaves L. Co-occurrence of abuse of different drugs in men: The role of drug-specific and shared vulnerabilities. Arch Gen Psychiatry. 1998;55(11):967–972. doi: 10.1001/archpsyc.55.11.967. [DOI] [PubMed] [Google Scholar]
- Uhl GR, Elmer GI, LaBuda MC, Pickens RW. Genetic influences in drug abuse. New York: Raven Press; 1995. [Google Scholar]
- Wan X, Yang C, Yang Q, Xue H, Fan X, Tang NL, Yu W. BOOST: A fast approach to detecting gene-gene interactions in genome-wide case-control studies. Am J Hum Genet. 2010;87(3):325–340. doi: 10.1016/j.ajhg.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuferov V, Butelman ER, Kreek MJ. Biological clock: Biological clocks may modulate drug addiction. Eur J Hum Genet. 2005;13(10):1101–1103. doi: 10.1038/sj.ejhg.5201483. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.