Abstract
Site-2 protease (S2P) is a membrane-embedded protease that site-specifically cleaves intramembrane transcription factors, a necessary step for their maturation. S2P is well known to regulate cholesterol biosynthesis and endoplasmic reticulum stress in mammalian cells. In this study, we hypothesized that S2P could be responsible for the regulation of cellular oxidative injury under oxidative stress. Wild type Chinese hamster ovary (WT CHO) cells and their mutant M19 cells with defective S2P gene were exposed to different oxidative stress conditions. Results showed that oxidative stress significantly up-regulated S2P expression in WT CHO cells. Notably, M19 cells had remarkably higher level of superoxide and elevated rates of cell death than WT CHO cells. The vulnerability to oxidative stress was reversed by the transfection of S2P gene but not rescued by exogenous supplement of cholesterol, oleate, and mevalonate, indicating that lack of S2P gene leads cells to be more vulnerable to oxidative stress. Furthermore, compared with WT CHO cells, M19 cells had higher nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity and lower paraoxonase-2 expression. Taken together, these results suggest that S2P can be a protease responding to oxidative stress and has the function of regulating cellular oxidative injury.
Sequential cleavage, a process designated as regulated intramembrane proteolysis, is required for maturation of intramembrane transcription factors1,2,3. It includes in tandem cleavages at specific sites of substrates by two membrane embedded proteases accordingly: site-1 protease (S1P) and site-2 protease (S2P)1,2,3. As a hydrophobic integral membrane protease, S2P is essential for cholesterol biosynthesis in mammalian cells owing to its activation of the sterol regulatory element binding proteins (SREBPs), a group of critical transcription factors regulating cholesterol uptake and synthesis4,5,6. SREBPs bind to sterol regulatory element of DNA and trigger transcription of dozens genes controlling lipid metabolism. Normally, SREBPs are inactive precursors at endoplasmic reticulum (ER). In response to low level of cellular cholesterol, SREBPs can be transported from ER membrane to Golgi and sequentially cleaved by S1P and S2P. The resulting released N-terminal domain is then translocated to nucleus, starting transcription of genes encoding key enzymes involved in the uptake and synthesis of cholesterol and fatty acid, including 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) synthase, HMG-CoA reductase and so on6,7,8,9. The resultant increase of cholesterol feedback inhibits SREBPs transport and cleavage10,11. Through the S2P cascade, the cholesterol feedback pathway is stringently regulated. Meantime, S2P plays crucial roles in regulating ER stress through sequential cleavage in response to unfolded proteins and ER stress signaling by using the transcription factors as substrates including activating transcription factor 6 (ATF6)12,13, cAMP response element binding protein homolog14 and old astrocyte specifically-induced substance15,16,17. However, whether S2P has other biological functions is unknown yet.
After S2P gene was cloned in 1997, scientists started to explore the roles of this intramembrane protease in different pathological states. Dysfunction of intramembrane proteases is linked to diverse signaling pathways2,18 and several diseases such as familial Alzheimer's disease19,20, Parkinson's disease21 and diabetes22. Occurrence and progression of these diseases are associated with free radicals and oxidative stress23. Recent evidence indicates that intramembrane proteases have the potentials to modulate oxidative stress, which may impact the process of those diseases. For example, paraoxonase-2 (PON-2) is a ubiquitous expressed cellular anti-oxidative enzyme that reduces reactive oxygen species (ROS) mediated cellular injury24,25 and PON-2 is a target gene of SREBP-226. Sre-1, the yeast ortholog of SREBPs, functions as an oxygen sensor and stimulates transcription of genes for hypoxia adaptation in fission yeast27. Therefore, how S2P regulates oxygen sensor SREBPs cleavage and oxidative stress is an interesting question. In this study, we designed a series of experiments to test the hypothesis that S2P could regulate oxidative stress through promoting SREBP-2 cleavage and PON-2 expression, and inhibiting nicotinamide adenine dinucleotide phosphate (NADPH) oxidase.
Results
Oxidative stress induced S2P mRNA expression in WT CHO cells and brain microvascular endothelial cells
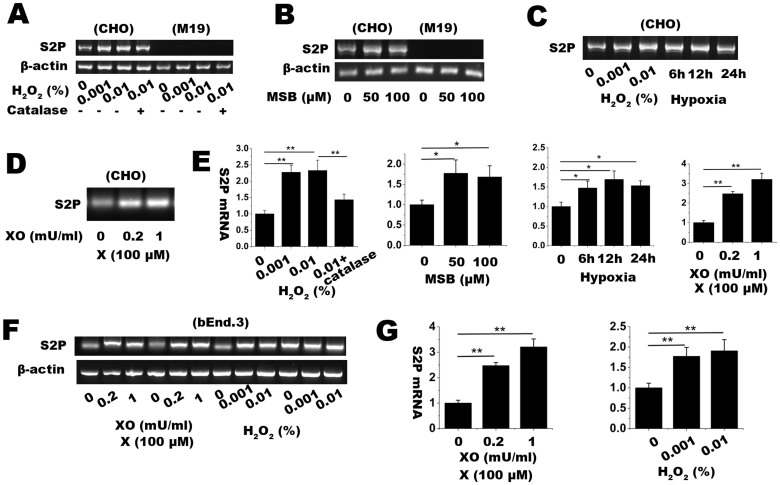
We first challenged WT CHO cells with different oxidative stress conditions, including xanthine/xanthine oxidase (X/XO), menadione sodium bisulfate (MSB), H2O2 and hypoxia. As a classic free radical generating system, xanthine oxidase catalyzes the substrate xanthine to produce superoxide (O2·−). Cells were treated with 100 μM xanthine plus different units (0.2, 0.5, 1 mU) of xanthine oxidase for 2 h; Menadione (2-methyl-1,4-naphthoquinone or vitamin K3), a polycyclic aromatic ketone, can generate O2·− through redox cycling and glutathione depletion. As water soluble form of menadione, (MSB) commonly works as endogenous oxidative stress reagent. Cells were incubated with 20, 50, 100 μM of MSB; Hydroxyl peroxide (H2O2) and hypoxia are commonly used as extraneous and endogenous oxidative stress models respectively. As showed in Figure 1A–E, treatments of H2O2, MSB and X/XO up-regulated the expression of S2P mRNA in WT CHO cells. Meanwhile, hypoxic treatment also induced the expression of S2P mRNA in WT CHO cells. To elucidate whether other cells have similar responses of S2P gene to oxidative stress, mouse brain microvascular endothelial bEnd.3 cells was applied in the study. Similarly, both X/XO and H2O2 dose-dependently induced the increased expression of S2P mRNA in bEnd.3 cells (Figure 1F–G). Those results suggest that S2P could be a protease being sensitive to oxidative stress.
Figure 1. RT-PCR assay for determining relative mRNA expression level of S2P gene in CHO and M19 cells treated with several oxidative stress conditions.
The conditions included (A) 0.001 and 0.01% H2O2 with or without 100 U/ml catalase for 1 h, (B) 50 and 100 μM MSB for 2 h, (C) hypoxia (1% O2 and 5% CO2 balanced with N2) for 6, 12, 24 h, and (D) 100 μM xanthine plus 0.2 or 1 mU/ml xanthine oxidase (X/XO) for 2 h. β-actin was used as internal loading control. (E) S2P mRNA level in oxidative stress conditions were quantified with ImageJ software and shown in bar graph. (F) RT-PCR assay for determining relative mRNA expression level of S2P gene in bEnd.3 cells, a mouse brain microvascular cell line. β-actin was used as internal loading control. (G) S2P expression in bEnd.3 cells were quantified and shown in bar graph. Full length images are presented in supplementary information (Supplementary Figure S1 and S2). The relative fold changes were expressed as mean ± SD, n = 3. * p < 0.05, ** p < 0.01. The results showed that oxidative stress induced S2P mRNA expression in CHO and bEnd.3 cells.
S2P deficiency increased cellular vulnerability to oxidative stress
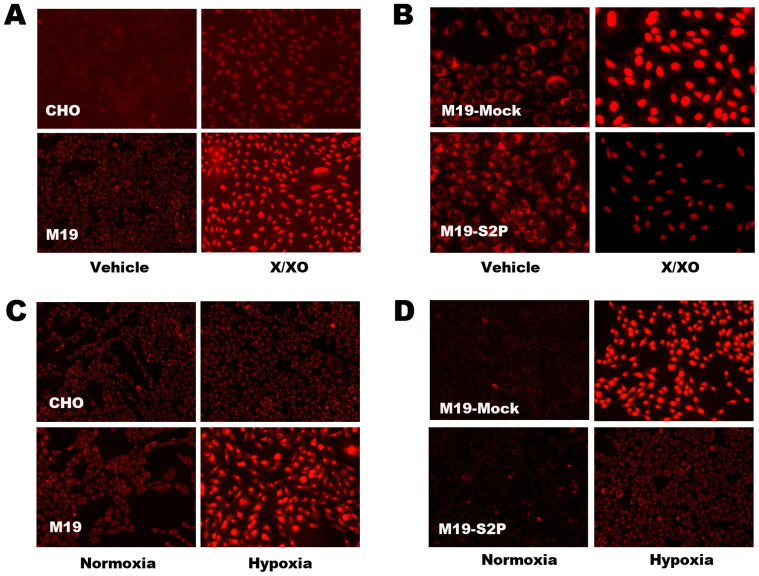
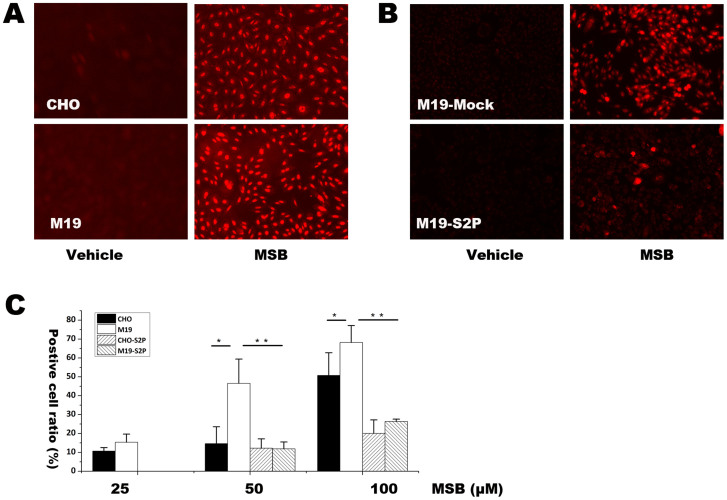
We next investigated the function of S2P in regulating cellular oxidative injury. M19 cells, which are specifically defective in S2P gene28,29,30, were used in the study. Hydroethidine (HEt)-staining, fluorescence microscopy and flow cytometery were used to detect superoxide production. After exposed to 100 μM xanthine plus 0.2 mU xanthine oxidase (Figure 2A), 15 h hypoxia (Figure 2C) or 20 μM to 100 μM MSB (Figure 3A, 3C), M19 cells had remarkably higher HEt-staining fluorescence than WT CHO cells. In agreement with the results, transfection of S2P gene into M19 cells abolished HEt-staining fluorescence under different oxidative stress conditions including X/XO (Figure 2B), hypoxia (Figure 2D) and MSB (Figure 3B, 3C). Those results indicate that deficiency of S2P could induce relatively high level of superoxide production in cells.
Figure 2. Hydroethidine (HEt) staining and observation with fluorescent microscopy for detecting superoxide in CHO and M19 cells treated with xanthine plus xanthine oxide (X/XO) and hypoxia.
(A) The intracellular superoxide detection in CHO and M19 cells treated with xanthine (X, 100 μM) plus xanthine oxide (XO, 0.2 mU) for 2 h. (B) Superoxide detection in M19 cells transfected with empty vector (Mock) or S2P expressive vector (S2P) followed by treatment with X/XO (X: 100 μM; XO: 0.2 mU) for 2 h. (C) CHO and M19 cells were exposed at normoxic condition (atmospheric O2 concentration in the incubator containing 5% CO2) or hypoxic condition (1% O2) for 15 h followed by HEt staining and fluorescent microscopic observation. (D) M19 cells transfected with Mock and S2P vector followed 15 h hypoxic treatment. The results showed that S2P could reduce superoxide production induced by X/XO and hypoxia.
Figure 3. HEt staining followed by fluorescence microscopic observation and flow cytometery for determining superoxide level after treatment with MSB in CHO, M19, M19 cells transfected with Mock and S2P vector.
(A) Fluorescent picture of HEt staining in CHO and M19 cells treated with 20 μM MSB for 2 h. (B) Fluorescent picture of HEt staining under treatment of 20 μM MSB for 2 h in M19 cells transfected with Mock or S2P vector. (C) Percentages of HEt positive cells were determined by flow cytometery in CHO and M19 cells with or without transfection of S2P gene. The percentages of positive staining cells were expressed as mean ± SD, n = 3. Statistical analysis was done by ANOVA. * p < 0.05, ** p < 0.01. The results showed that S2P reduced superoxide production induced by MSB.
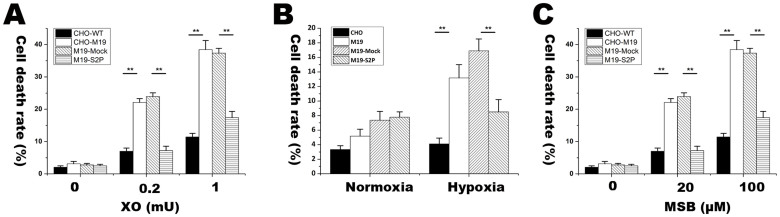
We then detected the release of LDH into cultured medium as an index of cell death. After exposed to X/XO, MSB and hypoxia, M19 cells had much higher LDH level in cultured medium than WT CHO cells (Figure 4A–C). We then compared S2P-transfected M19 cells (M19-S2P) and Mock vector transfected M19 cells (M19-Mock) in the release of LDH into medium under oxidative stress. Notably, the M19-S2P cells displayed remarkably lower level of LDH release than the M19-Mock cells under oxidative stress (Figure 4A–C). These results indicate that lacking S2P could increase cellular vulnerability to oxidative stress.
Figure 4. Lactate dehydrogenase (LDH) release assay for determining the rate of cell death in CHO and M19 cells treated with X/XO, hypoxia and MSB.
(A) is the rate of cell death under the treatment of 100 μM X plus 0.2 and 1 mU XO for 2 h in CHO, M19, M19 cells transfected with Mock and S2P vector. (B) is the rate of cell death under 15 h hypoxia in CHO, M19, M19 cells transfected with Mock and S2P vector. (C) is the rate of cell death under the treatment of 20 and 100 μM MSB for 2 h in CHO, M19, M19 cells transfected with Mock and S2P vector. The percentages of cell death were expressed as mean ± S.D. n = 3. Statistical analysis was determined by ANOVA. * p < 0.05. ** p < 0.01. The results showed that S2P reduced the cell death induced by X/XO, hypoxia and MSB.
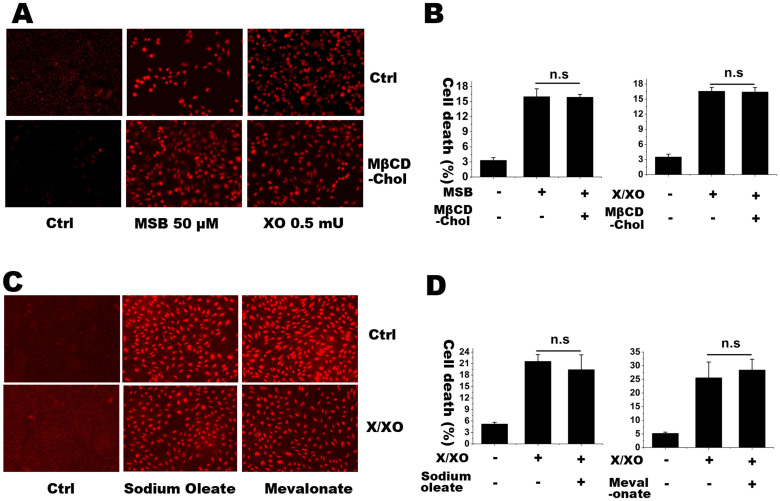
M19 cells, selected for resistance to amphotericin B, have low level of intracellular cholesterol and unsaturated fatty acid due to the deletion of the gene encoding the S2P protease that cuts the SREBP at site 230. One may argue that low intracellular level of cholesterol or unsaturated fatty acid, instead of S2P gene itself, might contribute to the enhanced superoxide production and vulnerability to oxidative stress. To address this question, the cells were incubated with 50 μM methyl-β-cyclodextrin cholesterol (MβCD-chol), or sodium oleate, or mevalonate (to provide nonsterol isoprenoids) for 2 h. The increased intracellular free cholesterol and cholesterol esters were confirmed with a microenzymatic fluorescence assay as our previous report31. Incorporation of cholesterol with these lipids had no effect on superoxide production and cell death in M19 cells after challenged with MSB or X/XO (Figure 5A–D). Those results suggest that S2P deficiency itself, instead of lipid deficiency, contributes to the enhanced cellular oxidative injury in M19 cells under oxidative stress.
Figure 5. HEt staining and LDH release assay for detecting superoxide level and the rates of cell death, respectively, in M19 cells with or without water soluble cholesterol MβCD-chol, sodium oleate and mevalonate.
(A) is the superoxide detection in M19 cells treated with MSB (50 μM) or X/XO (X, 100 μM; XO, 0.5 mU) with or without 50 μM MβCD-chol for 2 h. (B) is the rate of cell death with the same treatments. (C) is superoxide detection in M19 cells treated with X/XO (X, 100 μM; XO, 0.5 mU) with or without 1 mM sodium oleate and 100 μM mevalonate for 2 h. (D) is the rate of cell death with the same treatments. The percentages of cell death were expressed as mean ± S.D. n = 3. Statistical analysis was done by ANOVA. n.s.: no significance. The results showed that lipids incorporation did not affect cellular superoxide level and cell death induced by MSB and X/XO.
S2P deficiency had no effect on the expression of ATF6 and SOD during oxidative stress
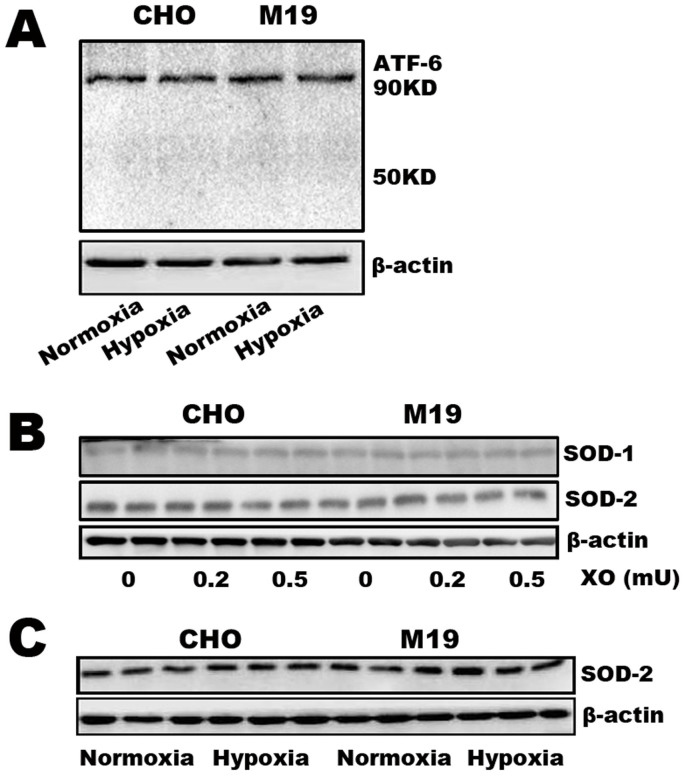
We then addressed whether ER stress associated proteins and antioxidant enzymes contribute to the enhanced vulnerability to oxidative stress in S2P null M19 cells. ATF-6 is a substrate of SREBP-2 and transcription factor in response to unfolded protein under ER stress and may involved in the regulation of oxidative stress. As an ER-resident protein, ATF-6 binds to the ER chaperone binding immunoglobulin protein (BiP) under normal physiological conditions32. During ER stress, BiP binds to unfolded proteins releasing ATF6 which translocates to the Golgi apparatus and is cleaved by S1P and S2P to release the domain with transcriptional activity12,13. The increased ATF6 expression was found in various oxidative stress conditions including hypoxia and ischemia/reperfusion33,34,35. In the present study, compared with WT cells, M19 cells had no significant difference in the expression of ATF-6 (90 KD) under normoxic and hypoxic conditions (Figure 6A). Meanwhile, hypoxia could not induce a detectable ATF-6 cleavage (~50 KD) in WT and M19 cells (Figure 6A). Furthermore, after exposed to X/XO and hypoxia, M19 cells had no significant difference in the expressions of SOD-1 and SOD-2 in comparison with WT CHO cells (Figure 6B–C). These data indicate that S2P deficiency has no influence on the expression of ER stress associated proteins and antioxidant enzymes during oxidative stress.
Figure 6. Western blot assay to detect protein expressions of ATF6, SOD-1 and SOD-2 in CHO and M19 cells treated with X/XO and hypoxia.
(A) Protein expression level of ATF-6 in CHO-WT and M19 cells exposed on normoxia and 15 h hypoxia. (B–C) Protein level of SOD-1 and SOD-2 in CHO and M19 cells treated with 100 μM plus 0.2 and 0.5 μM XO or 15 h hypoxia. Full length blots are presented in supplementary information (Supplementary Figure S3). The results showed that hypoxia did not induce significant changes of ATF-6 and SOD between CHO and M19 cells.
SREBP-2/PON-2 pathway was involved in the vulnerability of CHO cells with deficiency of S2P
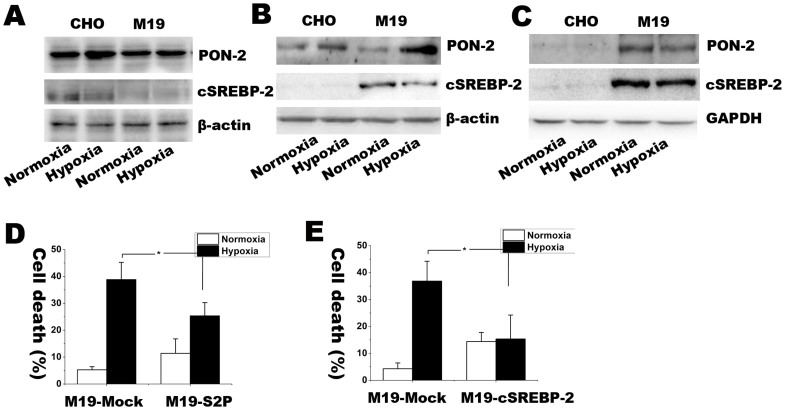
PON-2, a target gene of SREBP-2, is a ubiquitously expressed anti-oxidative enzyme24,25. PON-2 is response to ROS, mediates the reduced susceptibility to oxidative stress36 and counteracts lipid peroxidation at the plasma membrane37. To study whether PON-2 is involved in anti-oxidative regulation of S2P, we detected the expression of PON-2 in WT CHO and M19 cells under hypoxic condition. As showed in Figure 7A, after exposed to 15 h hypoxia, WT CHO cells instead of M19 cells had remarkably up-regulated the expression of PON-2, suggesting that S2P might be essential for oxidative stress mediated PON-2 activation. To further confirm this phenomenon, we transfectedM19 cells with S2P, active form of SREBP-2 (cleaved SREBP-2, cSREBP-2) or Mock vector, then compared the expression of PON-2 and the rate of cell death. Consistently, S2P and cSREBP-2 transfection rescued the expression of PON-2 in response to hypoxic treatment. Furthermore, S2P and cSREBP-2 overexpression in M19 cells significantly reduced the rate of cell death under hypoxia. Those results suggest that S2P might play essential roles in regulating antioxidant activities in response to oxidative stress through regulating SREBP-2/PON-2 pathway.
Figure 7. Western blot assay for determining SREBP-2 and PON-2 protein expressions under 15 h normoxia or hypoxia in CHO, M19, M19 cells transfected with S2P, cSREBP-2 or Mock vectors.
(A–C) is the Western blot picture of SREBP-2 and PON-2. (D–E) is the rate of cell death with the same treatments. The percentages of cell death were expressed as mean ± S.D. n = 3. Statistical analysis was done by ANOVA. * p < 0.05, n.s.: no significance. Full length blots are presented in supplementary information (Supplementary Figure S4). The results showed that S2P was required for the PON-2 elevation in response to hypoxia.
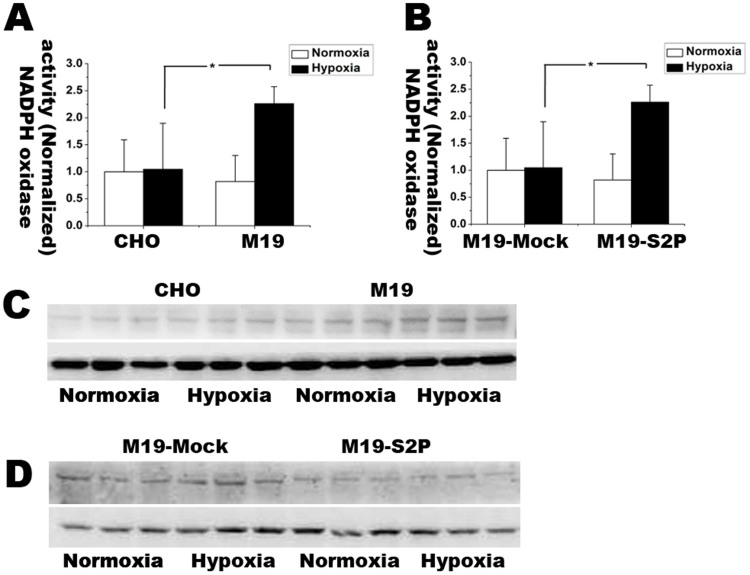
M19 cells had higher NADPH oxidase activity and more elevated expression of subunit gp91-phox than in WT CHO cells under hypoxic condition
To explore whether anti-oxidative activity of S2P is related to regulate NADPH oxidase, we detected NADPH oxidase activity by using lucigenin-enhanced chemiluminescence assay and investigated the expressions of NADPH oxidase subunits including gp91-phox, p47-phox, p67-phox and p22-phox with Western blot analysis. The results were shown in Figure 8A–D. Compared with normoxia, hypoxic treatment resulted in more than 2 folds increase in the expression of NADPH oxidase in M19 cells rather than WT CHO cells. S2P transfection remarkably abolished the induction of NADPH oxidase in the M19 cells. Moreover, after exposed to 15 h hypoxia, M19 cells had the up-regulated expression of gp91-phox, the catalytic subunit of NADPH oxidase, whereas S2P transfection into M19 cells restored gp91-phox to normal level. There was no significant change in expressions of p47-phox, p67-phox and p22-phox (data not shown). Therefore, S2P might have inhibitory effects on NADPH oxidase activity through down-regulating gp91-Phox subunit under hypoxic condition.
Figure 8. Lucigenin enhanced chemiluminescence and Western blot assay for detecting NADPH oxidase activity and expression level of gp91-phox respectively.
(A) NADPH oxidase activity in CHO and M19 under normoxia and 15 h hypoxia. The activity was normalized with the one in CHO cells. (B) NADPH oxidase activity in M19 cells transfected with empty or S2P vector under normoxia and 15 h hypoxia. The activity of each group was normalized with the one in M19-Mock. (C–D) Protein expression levels of NADPH oxidase subunit gp91-phox in each group under normoxia and 15 h hypoxia. β-actin was used as loading control. The relative fold changes of NADPH oxidase activity were expressed as mean ± S.D. n = 3. Statistical analysis was done by ANOVA. * p < 0.05. Full length blots are presented in supplementary information (Supplementary Figure S5). The results showed NADPH oxidase increased in M19 cells under hypoxia.
Discussion
S2P is an intramembrane protease participating in regulating lipid metabolism and ER stress but other physiological functions are largely unknown. In present study, we are the first to report a novel function of S2P serving as antioxidant regulating protein in response to oxidative stress. S2P protects mammalian cells from oxidative injury through up-regulating SREBP-2/PON-2 pathway and inhibiting NADPH oxidase.
S2P is an important signaling mechanism conserved from bacteria to human beings in regulated intramembrane proteolysis3,38. The critical step of this process is the site-specific cleavage of transmembrane transcription factors by S2P within the lipid bilayer. Although the S2P-mediated proteolysis was intensively investigated in last decades, little is known about the roles of S2P in regulating oxidative stress. Previous study reported an interesting phenomenon that Sre-1, the substrate of yeast S2P, was cleaved and activated, subsequently mediating transcription of genes encoding oxygen-depending enzymes in response to lower oxygen in fission yeast27. In present study, we stepped forward to report that S2P might be an antioxidant regulating proteins in mammalian cells in response to oxidative stress. This conclusion was derived from the systematic experiments with different mammalian cells and their mutant cells with the deficiency and transfection of S2P under both endogenous and extraneous oxidative stress models. Hypoxia was used as endogenous oxidative stress model and treatments of X/XO, MSB and H2O2 applied for extraneous oxidative stress. The increased S2P mRNA was consistently found in both WT CHO cells and mouse brain microvascular endothelial bEnd.3 cells under different oxidative stress conditions, indicating that S2P is sensitive to oxidative stress.
To elucidate the functions of S2P in regulating oxidative stress, we applied WT CHO cells and M19 cells, which were derived from CHO cells with specifically defective in S2P gene. Under oxidative stress conditions, M19 cells revealed remarkably higher superoxide level and higher rate of cell death than WT CHO cells. Furthermore, transfection of S2P vector into the M19 cells restored the antioxidant ability. Those results appear to support that conclusion that S2P could regulate oxidative stress. However, mounting evidence indicates the relationship between cellular cholesterol level and oxidative stress39,40,41. S2P plays essential roles in cholesterol biosynthesis by cutting the SREBP at site 2. As M19 cells have low level of intracellular cholesterol and unsaturated lipids due to the deletion of S2P protease. We should eliminate the potential affects that low lipid level, rather than S2P gene itself, might contribute to the enhanced superoxide production and vulnerability to oxidative stress. Thus we conducted lipid incorporation experiments. The results revealed that incorporation of cholesterol and unsaturated lipids had no effect on superoxide production and cell death in M19 cells. Thus, we conclude that S2P is a player in regulating oxidative stress induced cellular injury.
We then addressed potential mechanisms of S2P in regulating oxidative stress. S2P is a player for regulating ER stress through the sequential cleavage in response to unfolded protein and ER stress signaling by using the transcription factors as substrates including ATF612,13. ATF-6 is a substrate of SREBP-2 and transcription factor in response to unfolded protein under ER stress and may involve in the oxidative stress regulation. We logically investigated the expression of ATF6. The results revealed that M19 cells had similar expression level of ATP-6 to WT CHO cells under both normoxia and hypoxia, indicating the increased vulnerability to oxidative stress might not be related to ER stress. As a target gene of SREBP-2, PON-2 was reported to regulate the susceptibility to oxidative stress in different experimental systems24,25,26. We subsequently investigated the roles of PON-2 in our experiments. Under hypoxic condition, gain and loss of S2P and cSREBP-2 in the CHO cells remarkably altered PON-2 expression, indicating that S2P enzymatic activity might regulate PON-2 expression and oxidative injury. In addition, we studied the expression and activities of NADPH oxidase, an important source of ROS, in the experiments. Compared with WT CHO cells, M19 cells had enhanced expression level of gp91-phox, the catalytic subunit of NADPH oxidase42, and increased NADPH oxidase activity after exposed to hypoxic condition, indicating that deficiency of S2P might inhibit SREBP-2/PON-2 signaling and promote NADPH oxidase activity. Those results, when taken together, provide strong evidence to support the function of S2P as an antioxidant protein under oxidative stress.
In conclusion, S2P could function as an antioxidant protein for regulating oxidative injury. The anti-oxidative role of S2P could be related to the regulation of PON-2 expression and NADPH oxidase activity. This study provides a new cue for understanding cellular defense mechanisms against oxidative injury. Its physiological functions and implications in different physiological and pathological conditions remain to be further studied.
Methods
Cell culture
Wild type Chinese hamster ovary (WT CHO) cells were obtained originally from ATCC (Manassas, VA, USA). M19 cells, the mutant CHO cell line which has defective in S2P gene, were developed and provided by Professor Ta Yuan Chang's laboratory at Dartmouth Medical School, USA. WT CHO cells and M19 cells were grown as a monolayer in Ham's F-12 (Invitrogen, Carlsbad, CA, USA) supplemented with 10% (v/v) fetal bovine serum (FBS, Invitrogen) and 10 μg/ml antibiotics (penicillin and streptomycin, Invitrogen). After cell density reached to about 70% confluence, the cells were pre-incubated in serum-free medium over-night. Meanwhile, mouse brain microvascular endothelial bEnd.3 cells were obtained from ATCC and cultured in DMEM supplemented with FBS and antibiotics. The rest protocol for bEnd.3 cells is the same as that of CHO cells.
Oxidative stress and lipids incorporation
Cells were cultured in the medium without serum and then exposed to four different oxidative stress conditions: (1) Menadione treatment: Menadione (2-methyl-1,4-naphthoquinone or vitamin K3), a polycyclic aromatic ketone, is commonly used as oxidative stress reagent that can generate superoxide anion through redox cycling43,44,45. Cells were incubated with 20, 50, 100 μM of menadione sodium bisulfate (MSB, Sigma, St. Louis, MO, USA), a water soluble form of menadione, for 2 h at 37°C; (2) Xanthine plus xanthine oxidase (X/XO) treatment: To induce superoxide anions, cells were treated with 100 μM xanthine (Sigma) plus different units (0.2, 0.5, 1 mU) of xanthine oxidase (Sigma) for 2 h; (3) H2O2 treatment: Cells were incubated with H2O2 solution (Merck, Darmstadt, Germany) at the concentrations of 0.001% and 0.01% for 1 h with or without 100 U/ml catalase (Sigma). (4) Hypoxia treatment: Cells were incubated in hypoxia chamber (Billups-Rothenberg, Del Mar, CA, USA) perfused with the mixture gas containing 95% N2 and 5% CO2. O2 concentration was monitored with PA-10A paramagnetic O2 analyzer (Sable Systems International, Las Vegas, NV, USA). After O2 concentration reached to 1%, cells were continuously exposed to the hypoxic environment for 6, 12 and 24 h.
In a parallel study, lipids incorporation experiments were conducted in M19 cells. Water soluble cholesterol (50 μM, cholesterol-methyl-β-cyclodextrin, MβCD-Chol, Sigma), sodium oleate (1 mM, Aladdin Reagents, Shanghai, China) and mevalonate (100 μM, Aladdin Reagents) were added into the culture medium prior to the treatments of oxidative stress. RNA or proteins were extracted. Reverse transcription-PCR and Western blot analysis were performed to detect the expressions of specific mRNA and protein respectively.
S2P and cSREBP-2 gene transfection with Lipofectamine 2000
Before undergoing the transfection procedure, M19 cells were seeded on 6-well plates with antibiotics and serum-free medium. The full-length S2P gene was inserted into expression vector pCMV-HSV or with specific restriction endonucleases. The vector containing S2P, cSREBP-2 or empty vector (Mock) and Lipofectamine 2000 were diluted in Opti-MEM (Invitrogen). Then DNA and Lipofectamine 2000 were mixed and incubated for 20 min at room temperature to form DNA-Lipofectamine 2000 complex and incubated in culture medium with the cells for 5 h followed by replacement of medium with serum and antibiotics. After 24 h transfection, cells were prepared for treatment and observation.
RNA extraction and RT-PCR
Total RNA was extracted by TRIzol® reagent (Invitrogen) according to product manual. RNA was quantified and underwent the SuperScript first-stand synthesis system for RT-PCR (Invitrogen). S2P transcript was amplified with forward (5′-GTT GGG GTG CTC ATC ACT GAA-3′) and backward (5′-CAT TAC CGT GCT GTA ACC ATC CAG-3′) primers, yielding a PCR product of 790 bases. Housekeeping gene β-actin (internal control) was amplified with forward, 5′-CTA CAA TGA GCT GCG TGT GGC-3′, and backward, 5′-CAG GTC CAG ACG CAG GAT GGC-3′, primers, producing a PCR product of 270 bases. The annealing temperature was 51°C for S2P and 55°C for β-actin amplification. 40 and 30 cycles were used for S2P and β-actin respectively.
Western blot analysis
Cell lysates were prepared with RIPA lysis buffer (Sigma) plus protease inhibitors cocktail (Merck) and denatured prior to resolved on 10% to 12% SDS-PAGE. Proteins were then transferred to polyvinylidene difluoride membranes (Millipore, Billerica, MA, USA). After blocking, membranes were incubated with primary antibodies overnight at 4°C followed by washing and secondary antibody incubation. Antibodies included SOD-1 (Cu-Zn SOD, 1:1000, Santa Cruz, CA, USA), SOD-2 (Mn-SOD, 1:1000, Santa Cruz), β-actin (1:5000, Santa Cruz), PON-2 (1:500, Abcam, Cambridge, UK), P22-phox (1:500, Santa Cruz), P47-phox (1:500, Millipore), P67-phox (1:1000, Millipore), gp91-phox (1:1000, Millipore), SREBP-2 (1:1000, Abcam) and ATF-6 (1:1000, Santa Cruz). Chemiluminescence detection was performed using ECL reagents (GE healthcare, Little Chalfont, Buckinghamshire, UK).
Superoxide detection with fluorescent microscopy
Hydroethidine (HEt), a cell permeable fluorescence probe, was used to detect the production of superoxide anions in cells. HEt can be oxidized by superoxide to form the fluorescent 2-hydroxy-ethidium (2-OH-E+)46. In the experiments, after exposed to oxidative stress treatments, cells were incubated with 10 μM HEt for 15 min at room temperature. After washing with PBS, cells were kept in phenol red-free medium for observation under fluorescent microscope.
Superoxide detection with flow cytometery
Intracellular superoxide level was quantified with flow cytometery. After cells were treated with MSB and incubated with HEt, the cell pellets were collected and suspended in 500 μl PBS for measurement. The HEt-positive staining cells were counted by flow cytometer (EPICS XC, Beckman Coulter, Kraemer Boulevard Brea, CA, USA) with a laser emitting excitation light at 488 nm and the emission 575 nm.
Apoptosis detection with flow cytometery
Double staining with fluorescenin isothiocyanate conjugated with annexin V and propidium iodide were used to detect apoptotic cell death. After treatment, cells were collected and resuspended in 1 × Annexin V binding buffer. After incubation with 5× Annexin V-PE for 15 min, positive staining rates were counted with flow cytometer using a single laser-emitting excitation light (488 nm) and the emission signal (575 nm) generated by Annexin V-PE.
Lactate dehydrogenase (LDH) release assay
LDH release assay was used to detect oxidative stress-mediated cell injury. Briefly, culture medium (50 μl) was collected and incubated with 50 μl of the reaction mixture from the Cytotoxicity Detection Kit (Invitrogen) for 30 min at room temperature (24°C). The optical density of the solution was measured at 490 nm on a 3350 microplate reader (Bio-Rad, Hercules, CA, USA). Medium collected in the well without cells was used as negative control counting as 0%. Triton-X 100-treated cell medium was used as 100% cell death control. The rates of cell death were calculated by using the formula: Cell death rate (%) = (Experimental absorbance value - culture medium absorbance value)/(Triton-X 100-treated absorbance value - culture medium absorbance value) × 100%.
NADPH oxidase activity
NADPH oxidase activities were assayed with lucigenin enhanced chemiluminescence method as previous described47,48,49. Briefly, cells were suspended and lysed in NADPH lysis buffer (50 mM phosphate buffer, pH 7.0, 1 mM EGTA, 150 mM sucrose, and protease inhibitors). Total cell lysate containing 100 μg proteins were incubated with 10 μmol diethyldithiocarbamic acid (Sigma) at 37°C for 30 min. Then mixtures were treated with 5 μmol lucigenin (Sigma) and 0.1 μmol NADPH (Sigma) balanced with NADPH lysis buffer. After incubation for 10 min at 37°C in dark, the samples were detected with luminometer. Reactions were terminated with 10 μmol tiron (Sigma). The enzyme activity was expressed as relative light units/μg protein and relative fold changes were used to indicate the activity changes.
Statistic analysis
Data were expressed as Means ± S.D. For multiple groups designed experiments, comparisons were made by one-way analysis of variance (ANOVA) and followed by Dunnett test for two group comparison within the multiple groups. For two groups designed experiments, comparisons were determined using unpaired Student's t-test. Statistic analysis was performed in the SPSS 16.0 statistical program (SPSS, Chicago, IL, USA). p < 0.05 was considered to be statistically significant in the compared groups.
Author Contributions
J.S. obtained the funding and initiate the experiments. J.S. and Y.G. conceived and designed the experiments. Y.G., W.L. and J.S. performed the experiments and analyzed the data. J.S. and Y.G. wrote the manuscript and all authors reviewed the manuscript.
Supplementary Material
Supplementary information
Acknowledgments
We thank Prof. Ta Yuan Chang for his kindly providing us the M19 cell line. The work was supported by General Research Fund (GRF), University Grants Committee, Hong Kong SAR (No. 777611M and No. 776512M), Seed Funding Programme for Basic Research, the University of Hong Kong (No. 201211159042, No. 201111159021), and Grant of Natural Science Foundation of China (No. 31270902).
References
- Brown M. S., Ye J., Rawson R. B. & Goldstein J. L. Regulated intramembrane proteolysis: a control mechanism conserved from bacteria to humans. Cell 100, 391–8 (2000). [DOI] [PubMed] [Google Scholar]
- Urban S. & Freeman M. Intramembrane proteolysis controls diverse signalling pathways throughout evolution. Curr Opin Genet Dev 12, 512–8 (2002). [DOI] [PubMed] [Google Scholar]
- Wolfe M. S. & Kopan R. Intramembrane proteolysis: theme and variations. Science 305, 1119–23 (2004). [DOI] [PubMed] [Google Scholar]
- Brown M. S. & Goldstein J. L. A proteolytic pathway that controls the cholesterol content of membranes, cells, and blood. Proc Natl Acad Sci U S A 96, 11041–8 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai J. et al. Sterol-regulated release of SREBP-2 from cell membranes requires two sequential cleavages, one within a transmembrane segment. Cell 85, 1037–46 (1996). [DOI] [PubMed] [Google Scholar]
- Brown M. S. & Goldstein J. L. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell 89, 331–40 (1997). [DOI] [PubMed] [Google Scholar]
- Goldstein J. L. & Brown M. S. Regulation of the mevalonate pathway. Nature 343, 425–30 (1990). [DOI] [PubMed] [Google Scholar]
- Ericsson J., Jackson S. M., Lee B. C. & Edwards P. A. Sterol regulatory element binding protein binds to a cis element in the promoter of the farnesyl diphosphate synthase gene. Proc Natl Acad Sci U S A 93, 945–50 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawson R. B. The site-2 protease. Biochim Biophys Acta (2013). [DOI] [PubMed] [Google Scholar]
- Matsuda M. et al. SREBP cleavage-activating protein (SCAP) is required for increased lipid synthesis in liver induced by cholesterol deprivation and insulin elevation. Genes Dev 15, 1206–16 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G. & Zhang X. New insights into S2P signaling cascades: regulation, variation, and conservation. Protein Sci 19, 2015–30 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. et al. IRE1-mediated unconventional mRNA splicing and S2P-mediated ATF6 cleavage merge to regulate XBP1 in signaling the unfolded protein response. Genes Dev 16, 452–66 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J. et al. ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol Cell 6, 1355–64 (2000). [DOI] [PubMed] [Google Scholar]
- Zhang K. et al. Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic inflammatory response. Cell 124, 587–99 (2006). [DOI] [PubMed] [Google Scholar]
- Kondo S. et al. OASIS, a CREB/ATF-family member, modulates UPR signalling in astrocytes. Nat Cell Biol 7, 186–94 (2005). [DOI] [PubMed] [Google Scholar]
- Murakami T. et al. Cleavage of the membrane-bound transcription factor OASIS in response to endoplasmic reticulum stress. J Neurochem 96, 1090–100 (2006). [DOI] [PubMed] [Google Scholar]
- Denard B. et al. The membrane-bound transcription factor CREB3L1 is activated in response to virus infection to inhibit proliferation of virus-infected cells. Cell Host Microbe 10, 65–74 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. R., Urban S., Garvey C. F. & Freeman M. Regulated intracellular ligand transport and proteolysis control EGF signal activation in Drosophila. Cell 107, 161–71 (2001). [DOI] [PubMed] [Google Scholar]
- Sherrington R. et al. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer's disease. Nature 375, 754–60 (1995). [DOI] [PubMed] [Google Scholar]
- De Strooper B. et al. Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature 391, 387–90 (1998). [DOI] [PubMed] [Google Scholar]
- Shi G. et al. Functional alteration of PARL contributes to mitochondrial dysregulation in Parkinson's disease. Hum Mol Genet 20, 1966–74 (2011). [DOI] [PubMed] [Google Scholar]
- Walder K. et al. The mitochondrial rhomboid protease PSARL is a new candidate gene for type 2 diabetes. Diabetologia 48, 459–68 (2005). [DOI] [PubMed] [Google Scholar]
- Naviaux R. K. Oxidative shielding or oxidative stress? J Pharmacol Exp Ther 342, 608–18 (2012). [DOI] [PubMed] [Google Scholar]
- Ng C. J. et al. Paraoxonase-2 is a ubiquitously expressed protein with antioxidant properties and is capable of preventing cell-mediated oxidative modification of low density lipoprotein. J Biol Chem 276, 44444–9 (2001). [DOI] [PubMed] [Google Scholar]
- Horke S. et al. Paraoxonase-2 reduces oxidative stress in vascular cells and decreases endoplasmic reticulum stress-induced caspase activation. Circulation 115, 2055–64 (2007). [DOI] [PubMed] [Google Scholar]
- Fuhrman B. et al. Urokinase activates macrophage PON2 gene transcription via the PI3K/ROS/MEK/SREBP-2 signalling cascade mediated by the PDGFR-beta. Cardiovasc Res 84, 145–54 (2009). [DOI] [PubMed] [Google Scholar]
- Hughes A. L., Todd B. L. & Espenshade P. J. SREBP pathway responds to sterols and functions as an oxygen sensor in fission yeast. Cell 120, 831–42 (2005). [DOI] [PubMed] [Google Scholar]
- Rawson R. B. et al. Complementation cloning of S2P, a gene encoding a putative metalloprotease required for intramembrane cleavage of SREBPs. Mol Cell 1, 47–57 (1997). [DOI] [PubMed] [Google Scholar]
- Khan N. et al. Plasma membrane cholesterol: a possible barrier to intracellular oxygen in normal and mutant CHO cells defective in cholesterol metabolism. Biochemistry 42, 23–9 (2003). [DOI] [PubMed] [Google Scholar]
- Hasan M. T., Chang C. C. & Chang T. Y. Somatic cell genetic and biochemical characterization of cell lines resulting from human genomic DNA transfections of Chinese hamster ovary cell mutants defective in sterol-dependent activation of sterol synthesis and LDL receptor expression. Somat Cell Mol Genet 20, 183–94 (1994). [DOI] [PubMed] [Google Scholar]
- Lee W. et al. Free cholesterol accumulation impairs antioxidant activities and aggravates apoptotic cell death in menadione-induced oxidative injury. Arch Biochem Biophys 514, 57–67 (2011). [DOI] [PubMed] [Google Scholar]
- Gardner B. M., Pincus D., Gotthardt K., Gallagher C. M. & Walter P. Endoplasmic reticulum stress sensing in the unfolded protein response. Cold Spring Harb Perspect Biol 5, a013169 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban P. et al. Molecular analysis of endoplasmic reticulum stress response after global forebrain ischemia/reperfusion in rats: effect of neuroprotectant simvastatin. Cell Mol Neurobiol 29, 181–92 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzymski T. et al. The unfolded protein response controls induction and activation of ADAM17/TACE by severe hypoxia and ER stress. Oncogene 31, 3621–34 (2012). [DOI] [PubMed] [Google Scholar]
- Gao J. et al. Hypoxia/oxidative stress alters the pharmacokinetics of CPU86017-RS through mitochondrial dysfunction and NADPH oxidase activation. Acta Pharmacol Sin 34, 1575–84 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano G. et al. Gender differences in brain susceptibility to oxidative stress are mediated by levels of paraoxonase-2 expression. Free Radic Biol Med 58C, 98–108 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann H. et al. Breaking the chain at the membrane: paraoxonase 2 counteracts lipid peroxidation at the plasma membrane. FASEB J 28, 1769–79 (2014). [DOI] [PubMed] [Google Scholar]
- Ehrmann M. & Clausen T. Proteolysis as a regulatory mechanism. Annu Rev Genet 38, 709–24 (2004). [DOI] [PubMed] [Google Scholar]
- Galkina E. & Ley K. Immune and inflammatory mechanisms of atherosclerosis (*). Annu Rev Immunol 27, 165–97 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Radisavljevic Z. M., Siroky M. B. & Azadzoi K. M. Dietary antioxidants improve arteriogenic erectile dysfunction. Int J Androl (2010). [DOI] [PubMed] [Google Scholar]
- Vilhardt F. & van Deurs B. The phagocyte NADPH oxidase depends on cholesterol-enriched membrane microdomains for assembly. EMBO J 23, 739–48 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueckschloss U., Galle J., Holtz J., Zerkowski H. R. & Morawietz H. Induction of NAD(P)H oxidase by oxidized low-density lipoprotein in human endothelial cells: antioxidative potential of hydroxymethylglutaryl coenzyme A reductase inhibitor therapy. Circulation 104, 1767–72 (2001). [DOI] [PubMed] [Google Scholar]
- Thor H. et al. The metabolism of menadione (2-methyl-1,4-naphthoquinone) by isolated hepatocytes. A study of the implications of oxidative stress in intact cells. J Biol Chem 257, 12419–25 (1982). [PubMed] [Google Scholar]
- Criddle D. N. et al. Menadione-induced reactive oxygen species generation via redox cycling promotes apoptosis of murine pancreatic acinar cells. J Biol Chem 281, 40485–92 (2006). [DOI] [PubMed] [Google Scholar]
- Ramamoorthy M. et al. Sporadic Alzheimer disease fibroblasts display an oxidative stress phenotype. Free Radic Biol Med 53, 1371–80 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah A. et al. Thioredoxin-interacting protein mediates high glucose-induced reactive oxygen species generation by mitochondria and the NADPH oxidase, Nox4, in mesangial cells. J Biol Chem 288, 6835–48 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarpey M. M. et al. Chemiluminescent detection of oxidants in vascular tissue. Lucigenin but not coelenterazine enhances superoxide formation. Circ Res 84, 1203–11 (1999). [DOI] [PubMed] [Google Scholar]
- Li L. et al. Endothelin-1 stimulates arterial VCAM-1 expression via NADPH oxidase-derived superoxide in mineralocorticoid hypertension. Hypertension 42, 997–1003 (2003). [DOI] [PubMed] [Google Scholar]
- Abid M. R., Spokes K. C., Shih S. C. & Aird W. C. NADPH oxidase activity selectively modulates vascular endothelial growth factor signaling pathways. J Biol Chem 282, 35373–85 (2007). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information