Summary
Populations of bone marrow stromal cells (BMSCs, also known as bone marrow-derived “mesenchymal stem cells”) contain a a subset of cells that are able to recapitulate the formation of a bone/marrow organ (skeletal stem cells, SSCs). The biological properties of BMSC cultures are assessed by a variety of assays, both in vitro and in vivo. Application of these assays in an appropriate fashion provide a great deal of information on the role of BMSCs, and the subset of SSCs, in health and in disease.
Keywords: bone marrow, colony forming unit-fibroblast, bone, cartilage, stroma, marrow adipocytes, in vitro assays, in vivo transplantation
1. Introduction
It has long been recognized, based on the work by Friedenstein and Owen, that bone marrow contains an adherent, non-hematopoietic cell that is a component of the bone marrow stroma [reviewed in (1–3)]. In a series of experiments, starting with non-clonal populations of these bone marrow stromal cells (BMSCs, also known as bone marrow-derived “mesenchymal stem cells”), and subsequently with clonal populations that arise from individual Colony Forming Unit-Fibroblasts (CFU-Fs), it was demonstrated that a subset of BMSCs is multipotent. When clonal strains were transplanted in vivo, some of the clonal strains formed bone and cartilage in closed systems (diffusion chambers). When transplanted in an open system (with access to the circulation), some of the clonal strains formed bone, stroma that supports hematopoiesis, and marrow adipocytes, all of donor origin, and blood of recipient origin (4). These experiments firmly established the multipotent nature of a subset of BMSCs, suggesting the existence of a stem cell able to differentiate into skeletal cell phenotypes [a skeletal stem cell, SSC, (3,5)]. More recently, it has been determined that these multipotent cells arise from specialized clonogenic BMSCs that are found on the abluminal side of bone marrow sinusoids(6). Very importantly, their ability to self-renew was established by passaging and subsequent serial transplantation of phenotype-defined clonogenic cells in vivo (6). Based on these findings, it is clear that bone marrow stroma contains a stem cell by the most rigorous criteria: the ability of the progeny of a single cell to reform and support a complete organ (the bone/marrow organ), and the ability to self renew.
The experimental proof of the existence of the SSC was based on a number of assays that required both ex vivo expansion of clonally derived cells, and in vivo transplantation, which is the gold standard by which to evaluate the differentiation capacity of the cell population. Nonetheless, several in vitro differentiation assays are widely used for determination of osteogenic and adipogenic differentiation, but are prone to artifact as described below. While cartilage formation was first demonstrated by in vivo transplantation of BMSCs in diffussion chambers, more recent assays rely on the formation of high density cell pellets in vitro (7). Lastly, expression of markers representative of a particular cell phenotype has also been employed as a means of determining differentiation. However, expression of several markers does not faithfully predict the differentiation capacity of cells, but assessment of the pattern of expression of markers is a useful tool when studying different stages of differentiation, and when used in conjuction with in vivo assays.
BMSCs can most likely be isolated from any species in which bone marrow exists, although culture conditions often vary from one species to another. As an example, methods of isolation and characterization of murine and human BMSCs do vary. They are the main focus of this chapter due to the fact that the methods highlight differences between establishing cultures from these two different species. Furthermore, murine and human BMSCs are the most frequently used, based on the wealth of transgenic and knockout animal models that exhibit skeletal disorders, and from humans, both normal and with diseases.
What follows below is a description of current in vitro and in vivo assays for the assessment of BMSCs, and the subset of SSCs within the population, that can be applied to normal and pathological bone and marrow from mice and from humans.
2. Materials
2.1. Solutions
Unless specified, reagents can be obtained from many vendors.
Marrow collection medium (MCM): α-MEM (with 100 U/ml sodium heparin for human bone marrow aspirates).
Serum-containing medium (SM): αMEM, 2mM glutamine or glutamax, 100 U/ml penicillin, 100 µg/ml streptomycin sulfate, and 20% lot-selected fetal bovine serum, NON-heat inactivated (see Note 1).
Hanks balanced salt solution (HBSS).
100% methanol.
Enzymatic digestions: Trypsin/EDTA (0.05% Trypsin with 0.53 mM EDTA in HBSS), or collagenase (1 mg/ml Collagenase IV in α-MEM).
Osteogenic medium (OM): αMEM, 2mM glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin sulfate and 20% lot-selected fetal bovine serum, NON-heat inactivated (see Note 1), supplemented with 10−8 M dexamethasone, 10−4 M L-ascorbic acid-2-phosphate, and 2–5 mM β-glycerophosphate.
Adipogenic medium (AM): αMEM supplemented with: 1) 0.5 µM isobutylmethylxanthine, 0.5 µM hydrocortisone, 60 µM indomethacin, or 2) αMEM with 10−4 M L-ascorbic acid 2-phosphate and 10−8–10−7 M dexamethasone, or 3) αMEM containing glutamine and penicillin/streptomycin, with 20% lot-selected rabbit serum, 10−4 L-ascorbic acid 2-phosphate and 10−8 M dexamethasone, or 4) αMEM with the 0.1–10 µM of the PPARγ ligand, rosiglitizone.
Chondrogenic medium (CM): Coon’s modified Ham’s F12 medium supplemented with 10−6 M bovine insulin, 8 × 10−8 M human apo-transferrin, 8 × 10−8 M bovine serum albumin, 4 × 10−6 M linoleic acid, 10−3 M sodium pyruvate, 10 ng/ml rhTGFβ1 (Austral Biologics), 10−8 M dexamethasone and 2.5 × 10−4 M L- ascorbic acid-2 phosphate.
Anesthesia: Combine 225 µl Ketamine, 69 µl of Xylazine, 75 µl of Acepromazine (Sigma), and 231 µl of H2O (total volume = 600 µl), use 100 µl/mouse (25 g), or 2–5% isoflurane).
Betadine
70% ethanol
Standard histological stains: Saturated methyl violet, hematoxylin and eosin (H&E), Alizarin Red S, von Kossa, Oil Red O, Toluidine Blue.
Antibodies: For cell surface analysis and immunohistochemistry (numerous vendors).
2.2. Equipment and Supplies
Hemocytometer: For use in cell enumeration.
Sterile labware: Tissue culture dishes and flasks of various sizes, pippetes, centrifuge tubes (not vendor specific), cell strainers (70µ pore size, Becton Dickinson), Scienceware® cloning cylinders (Sigma), sterilized vacuum grease.
Surgical equipment: Sterile scalpels, small scissors, forceps, small spatula and autoclips (not vendor specific).
Scaffolds for in vivo transplantation: Ceramic particles (hydroxyapatite/tricalcium phosphate, or variations there of, from a variety of sources) for human and mouse transplants; collagen sponges (from a variety of sources) for mouse transplants (see Note 2).
CO2 incubators: Set to 37° and 5% CO2 (not vendor specific). In some instances, incubators are made hypoxic (2–5% O2) (see Note 3).
Microscopes: Standard inverted phase contrast, dissecting microscopes, bright and dark field microscopes (not vendor specific).
Standard FACS equipment and supplies (not vendor specific).
Standard PCR equipment, supplies and primers (not vendor specific).
2.3. Source of Bone Marrow
Murine: Typically femora, tibiae, and humeri, collected from any strain of mice.
Human: Bone fragments collected as surgical waste and bone marrow aspirates from normal volunteers and patients with skeletal diseases under Internal Review Board approved protocols for the use of human subjects in research.
Guinea pigs: Guinea pigs (Hartley Davis, Charles River Laboratories) are used to create irradiated bone marrow feeder cells for certain types of murine cultures.
2.4. Recipients for In Vivo Transplantation Assays
Autologous transplantation: For larger animals (e.g., sheep, non-human primates, etc.), bone marrow is aspirated, and after ex vivo expansion, BMSCs are transplanted back into the original donor with an appropriate scaffold.
Syngeneic transplantation: Any inbred strain of mice, rats, rabbits, guinea pig, etc.
Xenogeneic transplantation: Female immunocompromised mice of various strains such as Bg Nu/Nu-Xid, NOD-SCID, etc. from a variety of animal vendors (see Note 4).
3. Methods
There have been a number of modifications of the original procedure developed by Friedenstein, which relies on the rapid adherence of BMSCs to tissue culture plastic (4,8,9). These include subfractionation of bone marrow single cell suspensions by density gradient centrifugation. However, this often results in a marked loss of BMSCs. More recently, prospective isolation of BMSCs using sets of cell surface markers and FACS have been employed (5,6). However, to date, there is no standard in vivo assay in which UNCULTURED SORTED cells can be transplanted directly. Hence, all sorted fractions of stromal cells must be cultured prior to in vivo assays. For this reason, establishing primary cultures at clonal density (CFU-F cultures) is a practical good surrogate for purification of the whole population of clonogenic stromal progenitors (6).
3.1. Collection and Preparation of Single Cell Suspensions of Bone Marrow
Euthanize mice by CO2 inhalation or terminal anesthesia in compliance with institutionally approved protocols for the use of animals in research, collect femora, tibiae, and humeri aseptically, clean muscle from bone, cut the epiphyses and flush the entire bone marrow content of medullary cavities with MCM and combine; for human surgical specimens, scrape trabecular bone fragments with a steel blade into MCM; for human bone marrow aspirates, collect 0.5 mls, mix with 5 ml of ice-cold MCM containing 100 U/ml sodium heparin, for both types of human preparations, centrifuge at 135 × g for 10 minutes, and resuspend in fresh MCM.
Pipet up and down several times, pass through needles of decreasing diameter (gauges 16 and 20) to break up aggregates, filter through a cell strainer (see Note 5), and count mononuclear cells with a hemocytometer.
Prepare guinea pig bone marrow suspensions in a similar fashion; irradiate them (feeder cells for murine single colony forming cultures described below), with 6000 cGy to prevent proliferation of adherent guinea pig cells.
3.2. Colony Forming Efficiency (CFE) – enumeration of CFU-Fs
The concentration of CFU-Fs in bone marrow, as determined by the CFE assay, is a rough estimation of the number of the SSCs in the BMSC population (10), expressed as the colony forming efficiency (CFE, number of BMSC colonies per 1×105 marrow nucleated cells in the original marrow cell suspension (see Note 6).
Plate murine cells (6–15×105 nucleated cells), or human cells (1–6×105 nucleated cells) into 25 cm2 plastic culture flasks in 5 mls of SM, in either triplicate or quadruplicate. These cell densities have been chosen, based on previously established colony forming efficiency (CFE) values, so that discrete BMSC colonies are formed in numbers sufficient for statistical analysis.
Remove unattached cells after 2–3 hours and wash vigorously three times with SM.
Add 5 mls of SM; for monoclonal murine cultures, add irradiated guinea pig feeder cells (1.0–1.5×107 nucleated cells per flask).
Incubate at 37° in a humidified atmosphere of 5% CO2 with air; on day 10–14, wash with HBSS, fix with methanol, and stain with an aqueous solution of saturated methyl violet.
Count colonies containing 50 or more cells using a dissecting microscope and determine colony forming efficiency (number of colonies per 1×105 nucleated cells plated). If cultures are harvested earlier than 10 days, colonies smaller than 50 cells can be counted; however, for the 10–14 day harvesting time, 50 cells is a reasonable cut off that discriminates colonies that are actually growing from smaller “clusters” of cells that ceased proliferation.
3.3. Establishment of Single Colony-Derived Strains of BMSCs
A number of studies have focused on the characterization of single colony-derived strains, prepared as described below. It is by clonal analysis and appropriate differentiation assays that the multipotent nature of the subset of BMSCs that are SSCs is established.
Plate murine cells (6–15×105 nucleated cells), plate cells from human surgical specimens, (0.007–3.5×103 nucleated cells/cm2) or from aspirates (0.14–14.0×103 nucleated cells/cm2) into 150 mm diameter Petri dishes for preparation of single colony-derived strains; add 30–50 mls of SM; or plate by limiting dilution into 96 well microtiter plates. The low cell densities employed in this assay have been chosen to allow discrete BMSC colonies to be formed at a distance from each other, so that the colonies can grow significantly, without approaching each other, before being isolated.
Wash vigorously with HBSS after 2–3 hrs, add irradiated guinea pig cells to mouse cultures as described above.
After 14–16 days, visually inspect and identify well-separated colonies of perfectly round shape for cloning, wash with HBSS, and surround each colony with a cloning cylinder attached to the dish with sterilized high vacuum grease.
Treat cells inside the cylinder with two consecutive aliquots of Trypsin/EDTA for 5–10 min each at room temperature, add cold FBS into each fraction as it is collected (final concentration 3%) to inhibit Trypsin, combine fractions and transfer to individual wells of 6-well plates containing SM.
Passage before cells reach confluence, usually 5 to 10 days later, transfer consecutively to a 25 cm2 flask (2nd passage) and to a 75 cm2 flask (3rd passage).
3.4. Preparation of Multi-Colony Derived Strains of BMSCs
For many studies, multi-colony derived strains are sufficient, and necessary for biochemical analysis of BMSCs undergoing differentiation into various phenotypes, and changes as the result of genetic manipulation, either naturally occuring, or induced. However, multi-colony derived strains cannot be soley used to determine the nature of SSCs (in particular, their multipotency).
For murine cultures (see Note 7), plate approximately 6–8×107 nucleated cells per 75 cm2 flask; for human surgical specimens, plate at 5×106 to 5×107 nucleated cells, and from aspirates, plate at 5× 106 to 20×107 nucleated cells into 75 cm2 flasks or 150 mm diameter dishes containing 30–50 mls of SM. The cell densities used for generation of BMSC multi-colony derived strains are based on our data of many years and are chosen to ensure vigorous BMSC growth starting with hundreds of colonies in each flask. When chosing these densities, multiple factors were considered, including, for mouse cultures, the stimulating effect of hematopoietic cells on BMSC proliferation, and, for human aspirates, a highly variable degree of contamination with peripheral blood in bone marrow aspirates.
Culture at 37° in a humidified atmosphere of 5% CO2 with air, replace medium on day 1 for human aspirates, and at day 7 for all other cultures; passage generally is performed on day 12 to 14.
Passage cultures by washing twice with HBSS, two treatments with Trypsin/EDTA for 25–30 min (for murine) or 10–15 min (for human) at room temperature, followed by a wash with SM. If murine cultures develop significant amounts of extracellular matrix, treatment with collagenase (1 mg/ml Collagenase IV in α-MEM) may be needed prior to trypsin/EDTA.
Add cold FBS into each fraction as it is collected (final concentration 3%) to inhibit enzymatic activity; combine fractions, pipet to break up cell aggregates, centrifuge at 135 × g for 10 min, resuspend cell pellet in fresh SM; plate murine cells at 2–10×106 cells per 75 cm2, plate human cells at 2×106 cells per 75 cm2 flask or 150 mm diameter dish, and passage again when approximately 70% confluency. These cell densities ensure a fast growth of BMSCs so that in 3 to 5 days, maximum BMSC numbers can be collected; for mouse cultures, they also take into consideration a highly variable concentration of macrophages among BMSCs.
3.5. FACS Analysis and Sorting
There are a number of cell surface markers that have been utilized to prospectively isolate BMSCs from other cell types (5). Prospective isolation will only acquire major significance once strategies for transplanting unclutured cells will be available. In culture, BMSCs are negative for hematopoietic and endothelial markers, and positive for a number of markers that are commonly expressed by many connective tissue cell types. In vivo, human BMSCs are identified by expression of ALP, CD146, CD105, CD90, [reviewed in (5)], and CD271 (11). Combinations of these markers, along with STRO-1 (12), can be used to enrich clonogenic stromal cells to near purity. There may be important differences in the phenotype of murine and human cells. Prospective isolation experiments are crucial to define the correlation between ex vivo observed properties and in vivo identity of stromal cells (see Note 8).
Harvest and wash the cells with PBS, then adjust the cell suspension to a concentration of 1–5×106 cells/ml in ice cold PBS, 10% FCS or BSA, 1% sodium azide (omit for viable cell sorting).
Add the primary labeled antibody (0.1–10 µg/ml) and incubate for ~30 min at room temperature or 4°C.
Wash the cells 3× by centrifugation at 400 g for 5 minutes, then re-suspend in 500 µl to 1 ml of ice cold PBS, 10% FBS or BSA, 1% sodium azide (omit for viable cell sorting).
Keep the cells in the dark on ice or at 4°C until analyzed.
Analyze using appropriate instrument settings (for the FACS analyzer or sorter) and data acquisition software.
3.6. In Vitro Differentiation Assays
In vitro differentiation assays do not probe the inherent, native differentiation potential of cells, but only their response to chemical cues. They are prone to artifact. Dystrophic calcification cannot be distinguished from matrix mineralization by histochemical stains (13). In some cases, cells adsorb lipids from the serum rather that synthesize them de novo (14). While cartilage formation was first demonstrated by in vivo transplantation of BMSCs in diffussion chambers, more recent assays rely on the formation of high density cell pellets in vitro (7), which appears to provide the appropriate 3D configuration to support cartilage formation. To date, it has been difficult to form cartilage with BMSCs in vivo in open systems due to the lack of appropriate scaffolds that inhibit vascular invasion, but yet maintain nutrient exchange.
3.6.1. In Vitro Osteogenic Differentiation Assay
Plate BMSCs at a density of 1.5×103 cells/cm2 in SM, switch to OM when cells reach confluency (see Note 9).
Incubate cultures for up to six weeks with medium changes every three days.
Once calcification is visually apparent (mineral is phase bright), fix and stain with either Alizarin Red S or von Kossa.
3.6.2 In Vitro Adipogenic Differentiation Assay
Plate BMSCs at a density of 4×103 cells/cm2 in SM and then switch to one of the AM formulations indicated above once they reach confluency.
Incubate cultures for up to 4 weeks with medium changes every three days.
Once fat accumulation is visually apparent, fix and stain with Oil Red O.
3.6.3. In Vitro Chondrogenic Differentiation Assay
Centrifuge BMSCs (2.5×105) at 500 g in 15 ml polypropylene conical tubes in 5 mls of chondrogenic medium (see Note 10).
Incubate with caps partially unscrewed for 3 weeks at 37° in 5% CO2, with a medium change at 2–3 day intervals.
Harvest by washing with PBS, fix in 4% neutral buffered formalin for 2 hours, brief demineralization with 10% EDTA in PBS, embed in paraffin, section at 5 micrometers, and perform histological analysis by staining with toluidine blue (cartilage matrix is stained purple) (see Note 11).
3.6.3. Analysis of Gene Expression
There are numerous methods for the analysis of gene expression of cultured cells (and from cells transplanted in vivo as described below) using RT-PCR, quantitative RT-PCR, and microarry profiling, too lengthy to list here. There is a very characteristic pattern of gene expression as cells undergo differentiation. However, measurement of expression of these markers is not a guarentee of true differentiation: expression of markers must be matched with evidence of true differentiation as based on appropriate assays (in vivo transplantation as described below for osteogenic and adipogenic differentiation, the cartilage pellet assay as described above).
For osteogenesis: Runx2, Alkaline Phosphatase, Osterix, Osteopontin, Bone Sialoprotein, Osteocalcin.
For adipogenesis: CEBPα, PPARγ, Lipoprotein Lipase, Perilipin.
For chondrogenesis: Sox9, Type II collagen, Aggrecan.
3.7. In Vivo Differentiation Assay – Formation of an Ectopic Bone/Marrow Organ (Ossicle)
In vivo transplantation of BMSCs has become the gold standard by which to measure their multipotential nature. Both murine and human BMSCs have the ability to form bone, myelosupportive stroma and adipocytes when transplanted subcutaneously along with an appropriate carrier (an ectopic ossicle). However, human BMSCs form ectopic ossicle only with ceramic particles, whereas murine BMSCs can do so on both ceramic-based scaffolds and in collagen sponges (15).
When murine and human clonal strains, derived from a single CFU-F, are interrogated by in vivo transplantation, ~10–20% were found to be multipotent (formed a complete bone/marrow organ), ~50% form bone only, and the remainder form fibrous tissue (16). Thus, not all BMSCs, not even all CFU-Fs, are multipotent. The in vivo transplantation assay is the only assay that descrimates between cells that are multipotent, and cells that are not (5,10).
3.7.1. Ceramic Carrier Constructs (Human and Mouse BMSCs)
Sterilize ceramic particles by heating at 220° C overnight, then aliquot 40 mg aseptically into sterile round bottomed 1 ml cryotubes.
Pellet BMSCs at 135 × g for 10 min and resuspend in SM to the volume in mls equal to the number of transplants to be prepared.
Wash ceramic particles twice with SM.
Transfer BMSCs (1–2×106 cells in 1 ml of SM) into tubes with particles, mix and incubate at 37°C for 70–100 min with slow rotation.
Centrifuge particles with adherent BMSCs (135 × g for 1 min) and remove the supernatant.
Transplant as described below.
3.7.2. Collagen Sponge Constructs (Murine BMSCs)
Sterilize sponges if necessary, cut collagen sponges into cubes of the desired size or into any other shape, place into SM and squeeze with forceps to remove air bubbles.
Transfer BMSCs (1–2×106 cells/1 ml of SM) into individual 1 ml Eppendorf tube, pellet at 135 × g for 10 min, and discard all but 50–150 µl of the supernate (depending on the size of the sponge), and resuspend the pellet.
Blot sponges between two sheets of sterile filter paper and immediately place into freshly resuspended cells in the Eppendorf tube where the sponge expands, absorbing the cells.
Transplant as described below.
3.8. Surgery to Create Ectopic Ossicles
This procedure describes the use of mice as recipients. Similar procedures can be used when using other species of recipients.
Anesthetize the mouse, shave or use a depilatory if necessary, clean the skin with betadine and 70% ethanol, and make a single 3 cm longitudinal incision with a sterile scalpel in the skin along the dorsal surface.
Use the tip of sterile round-tipped scissors to make a pocket for the transplant by inserting the scissor subcutaneously, and then by opening the scissors by approximately 1 cm, use a sterile spatula to insert ceramic transplants, sterile forceps to insert collagen sponge transplants, (usually 4 transplants/mouse), and close the incision with several autoclips.
Harvest at various time points, fix with 4% neutral buffered formaldehyde overnight, decalcify and embedd in paraffin for standard histological analyses. If histochemical staining of the paraffin sections is intended, it is better to perform demineralization with 10% EDTA in PBS. Its duration depends on the size of the transplants, on the amount of bone and/or of hydroxyapatite in the transplants, on the temperature (much longer at 4°C than at room temperature). Decalcification is shorter if the solution is replaced often and if shaking is performed. The most gentle demineralization can take up to 6 weeks. To be sure that demineralization is completed and no more calcified structures are left, X-rays of the transplants may be performed.
Determine donor origin in transplants by donor specific in situ hybrization probes or antibodies, or by use of reporters introduced into the donor cells.
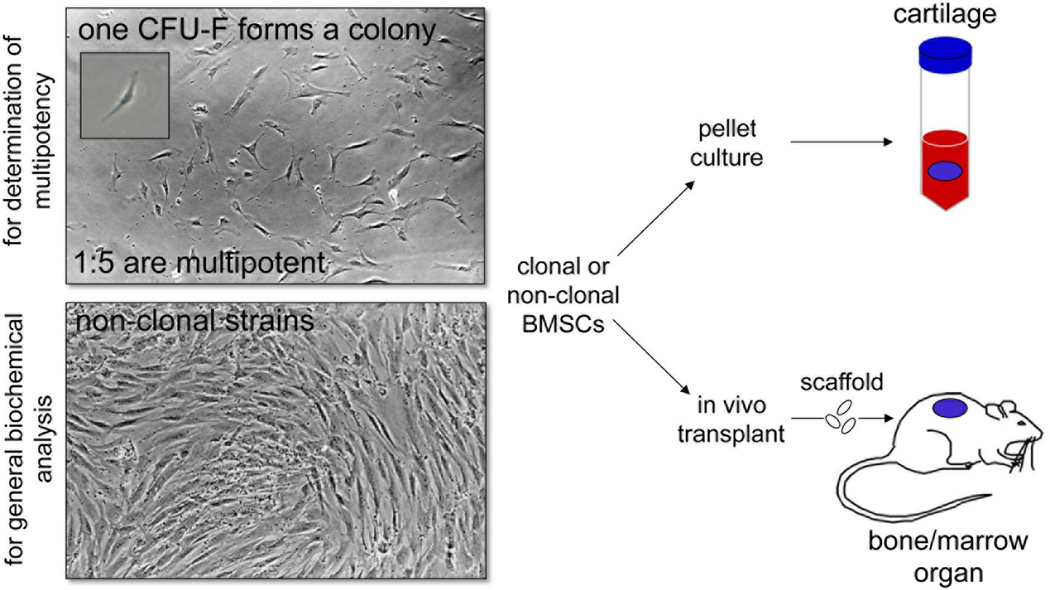
Fig. 1.
Establishment of clonal and non-clonal cultures of BMSCs. Clonal cultures are essential in order to determine the multipotency of the SSC subset of cells within the population. Single cells of bone marrow are plated at clonal density, and a single CFU-F adheres, and proliferates to form a colony. When bone marrow cells are plated at high density, non-clonal BMSC cultures are generated that can be used for general biochemical analysis. When both types of cultures near confluency, they are assessed for cartilage formation by pellet cultures, or for the ability to support the formation of a bone/marrow organ upon in vivo transplantation with appropriate scaffolds.
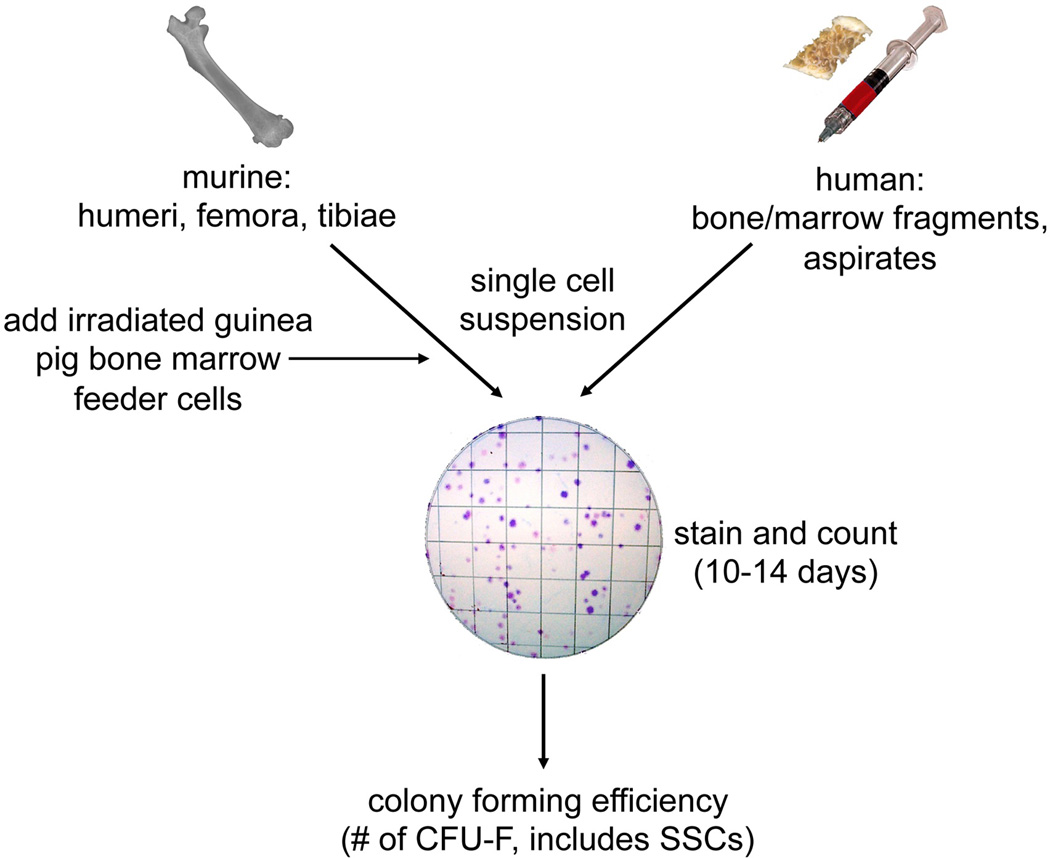
Fig. 2.
Colony forming efficiency assay (CFE). Bone marrow is collected from the long bones of mice, and from bone fragments with marrow or aspirates from humans. Single cell suspensions are plated at clonal density. For murine cultures, irradiated guinea pig bone marrow cells are added as feeders to optimize colony forming efficiency. After 10–14 days, colonies with greater than 50 cells (see page 11) are counted and the (CFE is determined as the number of colonies/100,000 bone marrow nucleated cells. To date, the CFE is the closest approximation to the number of SSCs in the BMSC population.
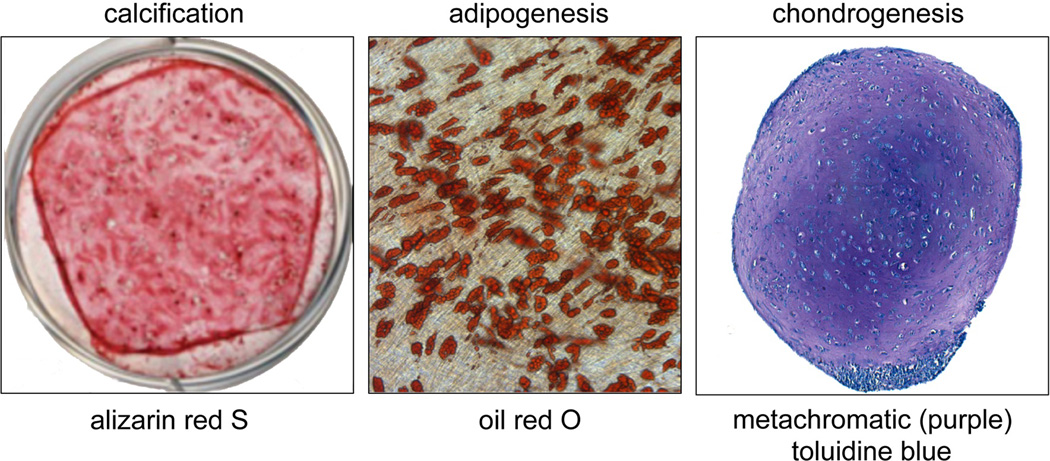
Fig. 3.
In vitro differentiaton assays. The osteogenic and adipogenic in vitro differentiation assays are highly prone to artifact, but are often used. Cells plated in SM, and then switched to OM prior to confluence will begin to calcify, as shown by alizarin red S staining. Cells plated in SM and switched to AM prior to confluence will form multilocular fat droplets within their cytoplasm as shown by staining with oil red O. On the other hand, chondrogenic differentiation is best done in vitro, by forming a high density pellet culture. If successful, chondrocytes will be seen lying in lacunae, surround by a matrix that stains purple with toluidine blue.
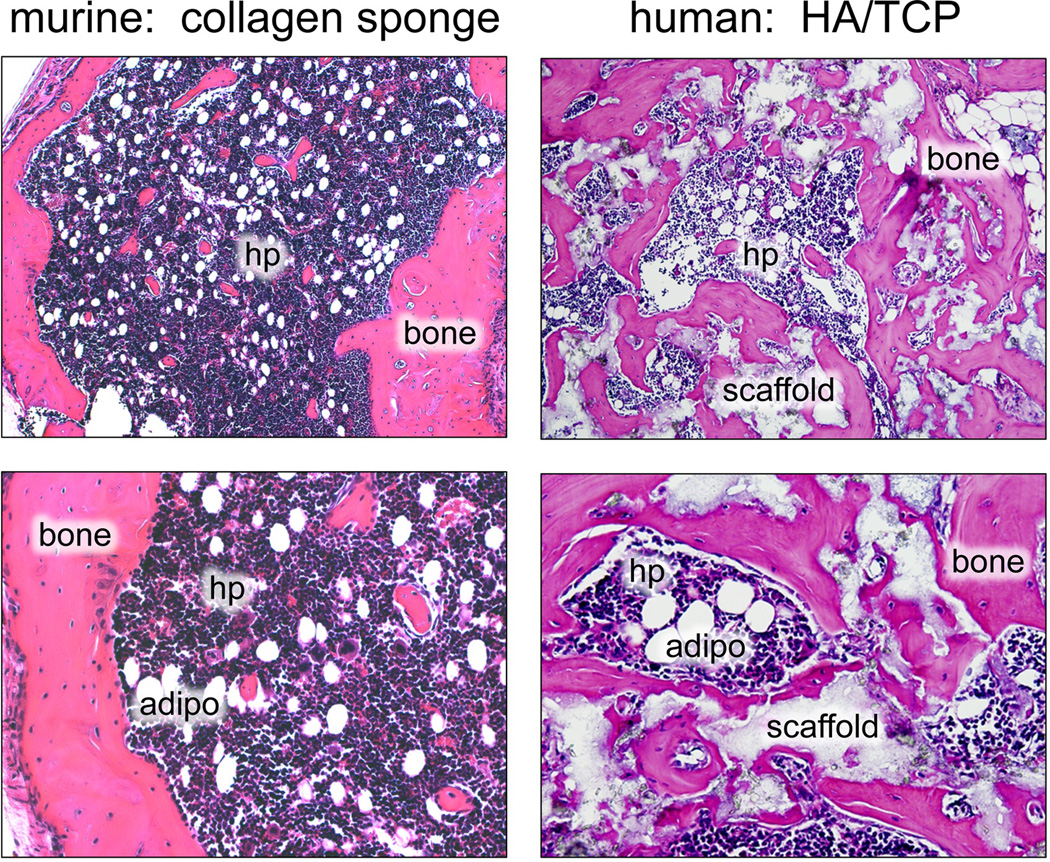
Fig. 4.
In vivo differentiation assays. Murine BMSCs will form a bone/marrow organ (bone, hematopoiesis supportive stroma, marrow adipocytes (adipo) of donor origin, with hematopoiesis (hp) of recipient origin) when transplanted in conjunction with collagen sponges (A,B), and with hydroxyapatite/tricalcium phosphate (HA/TCP, s - scaffold) (data not shown). On the other hand, human BMSCs will only form a bone/marrow organ with HA/TCP (C, D).
Footnotes
It is not well recognized that culture conditions vary from one species to another (9), and that fetal bovine serum must be tested extensively to select lots that are suitable for one animal species or another. The specific lot of fetal bovine serum used is critical for determination of CFE (17). Furthermore, it has been determined that heat inactivation can substantially reduce the ability of FBS to support colony formation and growth (17).
Identification of a scaffold that is able to maintain the biological activities of BMSCs/SSCs is critical. Unfortunately, many of the commercially available scaffolds, both ceramic or otherwise have not been found to support even bone formation very well. Generally speaking hydroxyapatite/tricalcium phosphate (60%/40%) have been useful (e.g., MASTERGRAFT™, Medtronic, Inc.), and other scaffolds currently under development may be even better. For murine cells, Gelfoam™, Pfizer, Inc. has shown the most consistent results to date.
It has been reported that the colony forming efficiency and proliferation of murine BMSCs (and possibly human BMSCs as well) is increased by growth in hypoxic (2–5% O2) conditions. However, it is not yet clear that the full biological activity of BMSCs is maintained under these growth conditions.
Generally, recipient immunocompromised mice are female due to the propensity of male mice to fight. In addition, using female recipients provides the possibility of using the male Y chromosome as a marker for cells of donor origin in the in vivo transplants of male cells. More generally speaking, when human cells are implanted into immunocompromised mouse, donor cells can be identified by either anti-human antibodies (such as antibodies against human mitochondria) or anti-human DNA sequences (such as alu). If, however, mouse cells are implanted, donor cells of male origin can be identified in a female recipient by a FISH probe against mouse Y chromosome, produced by many companies.
Excessive pressure, both positive and negative, should be avoided while passing cell suspensions through the needles. Murine cells, in particular, are very sensitive to rapid changes in pressure.
Not all BMSCs are clonogenic. The cell concentrations indicated will result in densities that will allow for density independent growth of BMSCs from a single CFU-F. By clonal analysis, ~1:5 of the colonies are multipotent based on the in vivo transplantation assay (16). Thus the colony forming efficiency is a rough estimate of the number of SSCs.
Rodent bone marrow stromal cells are often highly contaminated with hematopoietic cells, primarily macrophages (which can take on a BMSC-like appearance to the untrained eye). Passaging significantly reduces their presence, but does not eliminate them. Magnetic bead sorting or FACS sorting strategies have been used to eliminate the hematopoietic cells from murine BMSC cultures (18).
Interestingly, to date, the number of CFU-Fs that have been enumerated after FACS selection is virtually identical to the number of CFU-Fs that are generated by simple plastic adherence of single cell suspensions plated at clonal density(6)
The in vitro osteogenic assay is highly variable from one animal species to another, from one strain of mice to another, and if from different cell preparations to another. The cell layer has the propensity to roll up if it becomes superconfluent with abundant extracellular matrix and the OM is not added at the right time. Optimization may be required by adding OM at different times before or after reaching confluency, or by reducing the level of serum.
For the chondrogenic assay, it is extremely important to use polypropylene tubes, which prevent cell attachment to the walls of the tube.
In histological evaluation of pellet cultures, staining with toluidine blue is essential to determine if cartilage is formed. Bona fide chondrocytes must be seen lying in lacunae, surrounded by matrix that stains purple with toluidine blue. Although alcian blue or safranin O are often used, alcian blue is not specific enough (osteoid will stain lightly with alcian blue), and safranin O is also used as a nuclear stain.
References
- 1.Owen M, Friedenstein AJ. Stromal stem cells: marrow-derived osteogenic precursors. Ciba Found Symp. 1988;136:42–60. doi: 10.1002/9780470513637.ch4. [DOI] [PubMed] [Google Scholar]
- 2.Bianco P, Riminucci M, Gronthos S, et al. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001;19:180–192. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- 3.Bianco P, Robey PG. Skeletal stem cells. In: Lanza RP, editor. Handbook of Adult and Fetal Stem Cells. San Diego: Academic Press; 2004. pp. 415–424. [Google Scholar]
- 4.Friedenstein AJ. Stromal mechanisms of bone marrow: cloning in vitro and retransplantation in vivo. Hamatol Bluttransfus. 1980;25:19–29. doi: 10.1007/978-3-642-67319-1_3. [DOI] [PubMed] [Google Scholar]
- 5.Bianco P, Kuznetsov S, Riminucci M, et al. Postnatal skeletal stem cells. Meth Enzymol. 2006;419:117–148. doi: 10.1016/S0076-6879(06)19006-0. [DOI] [PubMed] [Google Scholar]
- 6.Sacchetti B, Funari A, Michienzi S, et al. Self-Renewing Osteoprogenitors in Bone Marrow Sinusoids Can Organize a Hematopoietic Microenvironment. Cell. 2007;131:324–336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 7.Johnstone B, Hering TM, Caplan AI, et al. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238:265–272. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- 8.Friedenstein AJ, Latzinik NV, Gorskaya Yu F, et al. Bone marrow stromal colony formation requires stimulation by haemopoietic cells. Bone Miner. 1992;18:199–213. doi: 10.1016/0169-6009(92)90807-p. [DOI] [PubMed] [Google Scholar]
- 9.Kuznetsov SA, Friedenstein AJ, Robey PG. Factors required for bone marrow stromal fibroblast colony formation in vitro. Br J Haematol. 1997;97:561–570. doi: 10.1046/j.1365-2141.1997.902904.x. [DOI] [PubMed] [Google Scholar]
- 10.Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2:313–319. doi: 10.1016/j.stem.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quirici N, Soligo D, Bossolasco P, et al. Isolation of bone marrow mesenchymal stem cells by anti-nerve growth factor receptor antibodies. Exp Hematol. 2002;30:783–791. doi: 10.1016/s0301-472x(02)00812-3. [DOI] [PubMed] [Google Scholar]
- 12.Simmons PJ, Torok-Storb B. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood. 1991;78:55–62. [PubMed] [Google Scholar]
- 13.Bonewald LF, Harris SE, Rosser J, et al. von Kossa staining alone is not sufficient to confirm that mineralization in vitro represents bone formation. Calcif Tissue Int. 2003;72:537–547. doi: 10.1007/s00223-002-1057-y. [DOI] [PubMed] [Google Scholar]
- 14.Diascro DD, Jr, Vogel RL, Johnson TE, et al. High fatty acid content in rabbit serum is responsible for the differentiation of osteoblasts into adipocyte-like cells. J Bone Miner Res. 1998;13:96–106. doi: 10.1359/jbmr.1998.13.1.96. [DOI] [PubMed] [Google Scholar]
- 15.Krebsbach PH, Kuznetsov SA, Satomura K, et al. Bone formation in vivo: comparison of osteogenesis by transplanted mouse and human marrow stromal fibroblasts. Transplantation. 1997;63:1059–1069. doi: 10.1097/00007890-199704270-00003. [DOI] [PubMed] [Google Scholar]
- 16.Kuznetsov SA, Krebsbach PH, Satomura K, et al. Single-colony derived strains of human marrow stromal fibroblasts form bone after transplantation in vivo. J Bone Miner Res. 1997;12:1335–1347. doi: 10.1359/jbmr.1997.12.9.1335. [DOI] [PubMed] [Google Scholar]
- 17.Kuznetsov SA, Mankani MH, Bianco P, et al. Enumeration of the colony-forming units-fibroblast from mouse and human bone marrow in normal and pathological conditions. Stem Cell Res. 2009;2:83–94. doi: 10.1016/j.scr.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chou DB, Sworder B, Bouladoux N, et al. Stromal-derived IL-6 alters the balance of myeloerythroid progenitors during Toxoplasma gondii infection. J Leukoc Biol. 2012;92:123–131. doi: 10.1189/jlb.1011527. [DOI] [PMC free article] [PubMed] [Google Scholar]