Background
Evidence of xenobiotic metal toxicity
Metals play an important role in human biology. Iron is critical for oxygen transport in hemoglobin. Zinc is a critical part of the angiotensin converting enzyme metalloprotein. But there are many metals that have no beneficial role in human biology. These metals have been referred to as heavy metals, or toxic metals. The terms are imprecise because these definitions all relate either to specific chemical properties or atomic weight, or functional aspects in human biology. We will use the term xenobiotic metal to refer to those metals without any specific role in the human body.
The epidemiologic evidence that many xenobiotic metals are toxic is robust. For example, arsenic, cadmium, lead, and mercury are ranked among the top 10 on the current Agency for Toxic Substances and Disease Registry Priority List of Hazardous Substances. Arsenic, lead, and mercury are ranked as the top 3 hazardous substances (1).
Within the cardiovascular system, xenobiotic metals have been linked to worsening hypertension, atherosclerosis, dyslipidemia, coronary artery disease and peripheral artery disease (PAD). We highlight below two metals, lead and cadmium, with demonstrated hazardous effects on human health that could at least partially explain the beneficial effects of chelation therapy. EDTA chelates both of these metals. We emphasize, however, that other mechanisms may be at play, and that extensive work will have to be carried out to fully understand the mechanism of benefit of the TACT infusions.
Lead
Lead is a toxic environmental pollutant, considered one of the most abundant xenobiotic metals. The main sources of exposure are contaminated water, air, food or soil. Lead, is often acquired through occupational exposure, soldered joints in water pipes, airborne emissions, lead-containing food products and the well-known lead-based paints (2).
Once absorbed, more than 90% of lead is accumulated in the bone. The lead stored in bone can remain for many years and be released years later into the blood stream, long after exposure. Blood lead levels are the usual method of testing for toxicity, but they only reflect recent exposure. In fact, the levels of blood lead have been falling steadily since leaded gasoline for road-driven vehicles was eliminated. However, environmental exposure continues, and the cardiovascular consequences are not restricted to high, recent exposure levels.
Epidemiological studies have found associations between elevated lead levels and the prevalence of cardiovascular disease (3–9). In 4 large population-based studies published from the National Health and Nutrition Examination Survey (NHANES), higher blood lead levels were found to be associated with a more than 25% increased risk of all cause mortality, 55% increased risk of cardiovascular mortality and a 2 fold higher risk of mortality from stroke (5–8). Other important associations of high blood lead levels include peripheral artery disease, dyslipidemia and hypertension (3,4,6,7,9). These complications have been associated with the upper limit of what is considered safe blood lead levels for adults (5), suggesting that there may be no “safe” levels for this toxic substance.
The mechanisms of lead toxicity are not fully understood. Lead may cause endothelial dysfunction by binding and inhibiting endothelial nitric oxide synthase and decreasing nitric oxide production (10) Lead also creates reactive oxygen species (ROS) which damage cell structures including DNA and cell membranes. Lead can replace other metal cofactors during enzymatic reactions therefore altering or compromising the end product. In the mitochondria, lead can replace zinc cofactor halting the formation of hemoglobin and accumulating toxic levels of aminolevulinic acid.
Cadmium
Cadmium is an extremely toxic metal that does not have any biological role in humans. Rechargeable batteries account for the majority of cadmium industrial use presently. And their safe disposal remains a challenge. Some plants, fruits and grains, can avidly absorb cadmium from the soil and/or water, creating another source for human exposure and toxicity (2). Tobacco plants, for example, avidly take up cadmium in the leaf. Hence smoking is the most important single source of cadmium exposure in the general population (11,12).
There is robust literature on the association of cadmium with cardiovascular disease (5,6,8,13–15) For example, Tellez-Plaza et al recently reported a study of 3,348 patients with measurement of urinary cadmium. The patients were participants in the Strong Heart Study, a large cohort of the Native American population. Elevated levels or urinary cadmium were found to increase the incidence of all-cause, cardiovascular, and coronary heart disease mortality. These associations were found to be stronger in patients with known diabetes (14), a finding that presaged the TACT diabetes results. A systematic review of 12 studies of cadmium exposure and clinical cardiovascular disease reported a positive association between cadmium levels and coronary heart disease, stroke and peripheral vascular disease. These associations were significant after adjusting for smoking status (15). Similarly, Navas-Acien et al, studied the association between blood cadmium levels and PAD (6). The investigators reported a 2.9 fold increase in the prevalence of PAD when comparing the highest and lowest quartiles of blood cadmium levels.
The toxic mechanism of cadmium is thought to be related to the inhibition of endothelial and calcium-calmodulin constitutive nitric oxide synthesis. Cadmium is also known to increase oxidative stress by being a catalyst in the formation of reactive oxygen species, increasing lipid peroxidation, and depleting glutathione and protein-bound sulfhydryl groups (6).
Other xenobiotic metals
In 2011, Agarwal and colleagues retrospectively studied the association of metals with cardiovascular and cerebrovascular disease (16). Multivariable analyses found independent associations between the combined end point and increased levels of antimony, cadmium, cobalt and tungsten. This study is important, not only because it highlights the effect of cadmium on cardiovascular disease, but also because of the wide net it casts over other metals, implicating then in cardiovascular disease as well. And more to the point of this review, EDTA chelates these metals. EDTA does not, however chelate all metals associated with cardiovascular disease. It is a weak chelating agent for arsenic and does not effectively chelate mercury.
Metals and diabetes mellitus
Diabetes mellitus is a well-known risk factor for developing early cardiovascular disease and mortality (17). The evidence that xenobiotic metals are involved in the development of vascular complications of diabetes has two sources. Firstly, epidemiological evidence, recently reported, suggests that the vascular complications of arsenic and cadmium are more prominent in patients with diabetes (14,18).
Secondly, metal-catalyzed oxygen chemistry is necessary for the non-enzymatic production of reactive oxygen species and formation of most advanced glycation end products (AGEs), advanced lipoxygenation end products (ALEs), and protein oxidation products. This is quite important, since the formation of these oxidation products, particularly AGEs, appears to be a plausible explanation for the development of complications of diabetes (19,20). Other transitional xenobiotic metals have also been found to be capable of catalyzing AGEs, and AGEs byproducts (21,22). During AGEs metabolism, the metals are neither discarded nor consumed. They continue to exist in cationic form, able to participate in subsequent reactions.
The third line of evidence implicating metal chelation in the prevention of complications of diabetes stems from the little known finding that some medications commonly recognized as reducing complications of diabetes have been found to inhibit the Maillard reaction and the formation of AGEs via metal chelation (23). Thus, in light of this basic and epidemiologic work, it is not surprising that metal chelation should show additional benefit in patients with diabetes.
Chelation
When a molecule, either organic or inorganic, has the potential to form two or more stable bonds with a single metal atom, it is called a chelator, chelating agent or chelating ligand. This new complex is usually a non-ionic molecule, more stable, soluble, and resists dissociation. The word chelation comes directly from the Greek root khele, which means “claw”. This term accurately describes the claw like molecular structure and the enhanced metal affinity exhibited by chelating ligands when compared to other non-chelating molecules. A good example of an organic chelator is the porphyrin ring, which in turn, chelates the iron molecule and forms a chelate complex called the heme molecule. There are many organic and synthetic chelators.
Many chelating ligands were synthetized specifically for binding certain metals. These compounds show enhanced chelating properties towards their targets. Important examples of synthetic chelators currently in use are dimercaprol (BAL), dimercaptosuccinic acid (DMSA), deferoxamine and various salts of ethylenediamine tetraacetic acid (EDTA). EDTA is a synthesized amino acid with strong, non-specific chelating properties for valences of +2 to +6. The EDTA-metal chelate is water soluble, and typically excreted in the urine. It can form strong covalent bonds with some xenobiotic metals, and increase the urinary excretion of calcium, zinc, cadmium and lead (24). Its remarkable affinity to calcium led to the early hypothesis that EDTA might decalcify atherosclerotic plaque.
Application of EDTA to treat atherosclerosis
In the 1950s, Clarke et al tested the effect of EDTA in a group of patients with severe angina, and found remarkable symptomatic and/or electrocardiographic improvement after repeated EDTA infusions in 17 out of 20 patients (25). Following these observations, and despite the lack of well-powered clinical trials to evaluate its efficacy, the use of EDTA to treat angina and other forms of atherosclerotic disease continued to increase in the following 50 years after Clarke's initial report. This uncontrolled use led to many case reports and case series, some of them extensive, supporting EDTA chelation for cardiovascular disease. Unfortunately these case reports could, at best, be interpreted only as hypothesis generating.
Three small, randomized studies, 2 in claudication (26,27) and one in angina (28) encompassing 268 patients in aggregate tested the role of chelation therapy for atherosclerosis disease. Thus, these studies were probably underpowered to detect small to moderate clinical benefits of chelation therapy on the surrogate endpoint of exercise capacity, and none evaluated meaningful clinical endpoints such as cardiac events or mortality.
These findings however, were interpreted by some authors as evidence against the efficacy of chelation therapy (29). Yet the absence of evidence of efficacy did not constitute evidence of absence of chelation's efficacy. A small or moderate benefit of EDTA chelation could not be excluded (30).
In fact, a 2002 Cochrane systematic review on the role of chelation therapy in coronary heart disease concluded that the existing body of work on chelation therapy and coronary heart disease was insufficient to recommend for or against its use (31). Nevertheless, in 2007 more than 100,000 adults in United States were using this form of therapy (32), which could potentially cause severe hypocalcemia and death when misused (33). It was in this context of uncertainty and in large, to respond to the public health question posed by EDTA chelation therapy, that the Trial to Assess Chelation Therapy (TACT) was born (34). Thus, after nearly 50 years of empirical use of chelation therapy for atherosclerosis, TACT was the first randomized trial designed to evaluate the effects of an EDTA-based chelation regimen on clinical outcomes in patients with coronary disease.
Overview of the TACT protocol
The TACT investigators were tasked with testing the most prevalent chelation treatment as used in the community. Thus, a 10-component solution (Table 1) was now the standard. In addition, the EDTA-based chelation solution was also typically administered with high doses of oral multivitamins and multiminerals (MVM). In order to prevent confounding, and understand the interaction of the chelation solution with oral supplements, the trial was designed as a 2 × 2 factorial (34). Patients were randomly assigned to 4 groups:
Table I. Chelation Infusion Contents.
The dose of EDTA changes on estimated GFR; the maximum dose is 3 grams.
| Additive |
|---|
| 2 grams of magnesium chloride |
| 100 mg of procaine HCL |
| 2500 units of heparin |
| 7 grams of ascorbate |
| 2 mEq KCl |
| 840 mg sodium bicarbonate |
| 250mg pantothenic acid |
| 100mg of thiamine |
| 100mg of pyridoxine |
|
|
| QS with sterile water to 500ml |
Adapted with permission from:
American Heart Journal
Lamas GA, Goertz C, Boineau R, Mark DB, Rozema T, Nahin RL, et al. Design of the Trial to Assess Chelation Therapy (TACT). Am Heart J. Mosby, Inc.; 2012 Jan;163(1):7–12.
Active intravenous (IV) chelation infusions + active oral MVM
Active IV chelation infusions + placebo oral MVM
Placebo IV chelation infusions + active oral MVM
Placebo IV chelation infusions + placebo oral MVM
Chelation-based infusions or placebo were administered 40 times: 30 weekly infusions, followed by 10 maintenance infusions 2 to 8 weeks apart. Oral high-dose MVM or oral placebo was administered throughout the course of the trial.
Eligible patients were at least 50 years old, had experienced a myocardial infarction (MI) 6 weeks or more prior to enrollment, and had a serum creatinine of 2.0 mg/dL or less. The primary endpoint was a composite of death from any cause, reinfarction, stroke, coronary revascularization, or hospitalization for angina. The composite of cardiovascular death, reinfarction, or stroke was a prespecified secondary end point, as was quality of life. Treatment comparisons were performed according to the intention-to-treat principle and included all patients in the group to which they were randomized.
The statistical plan originally called for 2372 patients to have 85% power to detect a 25% difference in the primary endpoint. During the course of enrollment it became clear that 1700 patients followed for a longer period was a more realistic goal. Thus, 1708 patients followed a median of 55 months maintained the same statistical power.
Results of TACT – overall population
The first patient was enrolled in September of 2003 and TACT was unblinded in August of 2012. 1708 patients were enrolled and randomized (839 patients to chelation, and 869 patients to placebo) at 134 sites, of which 81 (60%) were sites in which chelation therapy was already practiced. Patients received a total of 55,222 infusions. The median number of infusions received was 40 (IQR, 30-40); 76% of patients completed at least 30 infusions and 65% completed all 40 infusions. The median duration of follow-up was 55 (IQR, 26-60) months overall. Active treatment patients were followed up for 56 (IQR, 28-60) months and placebo patients were followed up for 53 (IQR, 24-60) months.
TACT population
The median age was 65 (IQR, 59-72) years, 18% were women, 9% were minority, and the median body mass index was 30. The qualifying MI had occurred a median of 4.6 (IQR, 1.6-9.2) years prior to enrollment. The study population had a high prevalence of self-declared diabetes (31%), prior coronary revascularizations (83%), and high use of guideline-recommended medications such as aspirin (84%), P-blockers (72%), and statins (73%). Patients had a median fasting glucose level of 102 (IQR, 92-121) mg/dL and a low-density lipoprotein cholesterol (LDL-C) level of 89 (IQR, 67-115) mg/dL.
Overview of publications
The initial TACT publication compared EDTA-based chelation with placebo infusions. Follow-up publications have reported on the oral MVM comparison, analysis of the 4 factorial groups, and an analysis of the subgroup of patients with diabetes (Table 2).
Table II.
TACT summary of results.
| Population | Endpoint | Treatment Comparison | HR | 95% CI | P | 5-yr NNT |
|---|---|---|---|---|---|---|
| Overall | Primary | EDTA vs. Placebo | 0.82 | 0.69-0.99 | 0.035 | 18 |
| Overall | Primary | EDTA + oral MVM vs. Placebo + placebo | 0.74 | 0.57-0.95 | 0.016 | 12 |
| Diabetes | Primary | EDTA vs. Placebo | 0.59 | 0.44-0.79 | 0.0002 | 6.5 |
| Diabetes | Death | EDTA vs. Placebo | 0.57 | 0.36-0.88 | 0.011 | 12 |
| Diabetes | Primary | EDTA + oral MVM vs. Placebo + placebo | 0.49 | 0.33-0.75 | <0.001 | 5.5 |
MVM= multivitamins and multiminerals; NNT= number needed to treat to prevent an event Primary endpoint = death, MI, stroke, coronary revascularization, hospitalization for angina
Comparison of chelation with placebo infusions
The primary end point occurred in 222 (26%) of the chelation group and 261 (30%) of the placebo group (35). The Kaplan-Meier 5-year estimates for the primary end point were 32.8% (95% CI, 29.1% to 36.5%) in the chelation group and 38.5% (95% CI, 34.6% to 42.3%) in the placebo group (HR, 0.82; 95% CI, 0.69 to 0.99; P = .035) (Figure 1). The 5-year number needed to treat (NNT) to prevent an event was 18. The statistical goal of the trial had been met. The effect of EDTA chelation on the components of the primary end point other than death was of similar magnitude as its overall effect. Finally, the event curves continued to separate after the infusions ended at about 14 months, suggesting the continued effect of chelating a metal toxin that might have taken a lifetime to accumulate. Chelation therapy reduced cardiovascular outcomes in a secondary prevention population.
Figure 1.
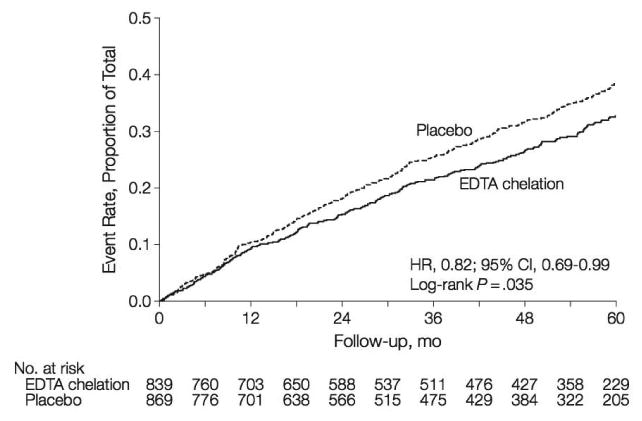
Kaplan-Meier Estimates of the Primary Composite End Point, EDTA Chelation Therapy vs placebo.
HR indicates hazard ratio. The primary end point was a composite of death from any cause, reinfarction, stroke, coronary revascularization, or hospitalization for angina.
Adapted with permission from
The Journal of the American Medical Association:
“Gervasio A. Lamas, MD Christine Goertz, DC, PhD Robin Boineau, MD, MA Daniel B. Mark, MD, MPH Theodore Rozema, MD Richard L. Nahin, PhD, MPH Lauren Lindblad, MS Eldrin F. Lew is, MD, MPH Jeanne Drislco M, Kerry L. Lee P for the TI. Effect of Disodium EDTA Chelation Regimen on Cardiovascular Events in Patients With Previous Myocardial Infarction: The TACT Randomized Trial. JAMA. 2013;309:1241”
Analysis of the 4 factorial groups
The analysis of the 4 factorial groups is particularly important because it evaluates chelation therapy as it is practiced in the community, and allows one to tease out the individual components (chelation, MVM, both, or neither) of benefit, if present. Overall, there was a stepwise reduction with the addition to double placebo of oral MVM, or chelation, or both. The 5-year Kaplan-Meier event rate estimate for the primary endpoint in the chelation + high-dose vitamin group was 31.9%, in the chelation + placebo vitamin group 33.7%, in the placebo infusion + active vitamin group 36.6%, and in the placebo infusions + placebo vitamin group 40.2 % (Figure 2) (36). The primary endpoint by treatment group occurred in 139 (32%) of the placebo infusion + placebo vitamin group, and 108 (26%) of patients in the chelation + high-dose vitamin group. The comparison of active infusion + active vitamins with placebo infusions + placebo vitamins was significant (HR 0.74, 95% CI (0.57 to 0.95; p=0.016, Figure 2). The absolute difference in 5-year Kaplan-Meier estimated event rates between placebo-placebo and active-active groups was 8.3% and the number needed to treat (NNT) to prevent 1 event over 5 years was 12 (Table 2). Thus, EDTA chelation plus MVM on a background of optimal medical therapy offered important benefits when compared to optimal medical therapy, including statins, alone.
Figure 2.
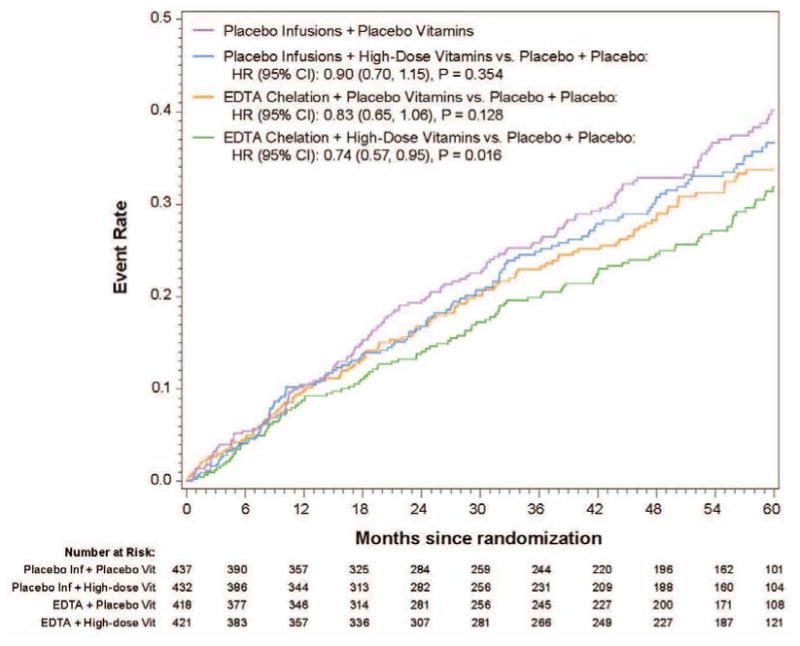
5-year Kaplan-Meier Event Rate Estimates for the Primary Composite Endpoint for the Four Factorial Groups.
Adapted with permission from
American Heart Journal:
“Lamas GA, Boineau R, Goertz C, Mark DB, Rosenberg Y, Stylianou M, et al. EDTA chelation therapy alone and in combination with oral high-dose multivitamins and minerals for coronary disease: The factorial group results of the Trial to Assess Chelation Therapy. Am Heart J [Internet]. 2014 Apr [cited 2014 Jun 5]; Available from: http://dx.doi.org/10.1016/j.ahj.2014.02.012
Analysis of patients with diabetes
Diabetes mellitus was prespecified as a subgroup for analysis in TACT. A total of 633 (37%) patients enrolled in the study met the definition of diabetes mellitus. Treatment with EDTA infusions reduced the primary end point (EDTA chelation vs. placebo: 25% vs. 38%; HR 0.59; 95% CI 0.44 – 0.79; p<0.0002) (37). The Kaplan-Meier curves again continued to diverge after the infusions stopped, indirectly supporting the metal-chelation hypothesis. There was a 15% absolute decrease in the 5-year Kaplan Meier primary event rate (Figure 3)_and a 41% relative reduction in risk. The 5 year NNT was 6.5 (95% CI, 4.4 – 12.7) (Table 2). There were important and consistent reductions in the components of the primary endpoint as well. Rates of the principal secondary endpoint, cardiovascular death, MI, or stroke were also lower for diabetic patients randomized to EDTA chelation (HR, 0.60; 95% CI, 0.39–0.91; P=0.017), with a 5.1% absolute decrease in the 5-year Kaplan–Meier event rate and a relative reduction of 40%. Patients with diabetes mellitus randomized to EDTA chelation had a significant reduction in recurrent MI (HR, 0.48; 95% CI, 0.26–0.88; P=0.015), in all-cause mortality (HR, 0.57; 95% CI, 0.36–0.88; P=0.011; Figure 4; 5-year NNT= 12; Table 2), and in coronary revascularizations (HR, 0.68; 95% CI, 0.47–0.99; P=0.042). Furthermore, when the factorial analyses comparing chelation + active vitamins with placebo infusions + placebo oral vitamins, the primary endpoint occurred in 56 (38%) of the placebo infusion + placebo vitamin group, and 36 (23%) of patients in the chelation + high-dose vitamin group (HR 0.49, 95% CI (0.33, 0.75) p<0.001, Figure 5), with a 5-year NNT=5.5 (Table 2) (36).
Figure 3.
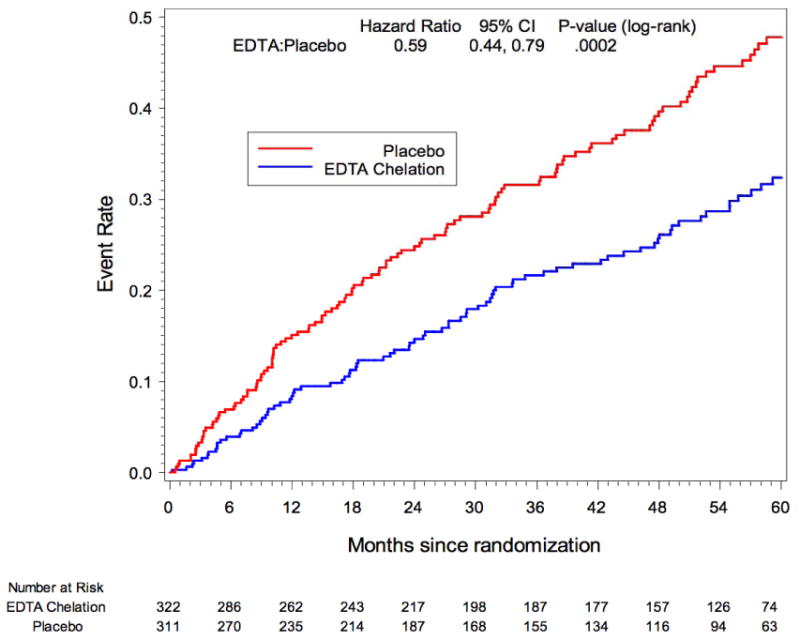
TACT Kaplan-Meier Estimates of the Primary Composite Endpoint EDTA Chelation Therapy vs. Placebo Subset of Patients with Diabetes: Hx, Med Use or Baseline Glucose>=126
Adapted with permission from
Circulation: Cardiovascular Quality Outcomes:
“Escolar E, Lamas G a, Mark DB, Boineau R, Goertz C, Rosenberg Y, et al. The effect of an EDTA-based chelation regimen on patients with diabetes mellitus and prior myocardial infarction in the Trial to Assess Chelation Therapy (TACT). Circ Cardiovasc Qual Outcomes. 2014 Jan;7(1):15–24.”
Figure 4.
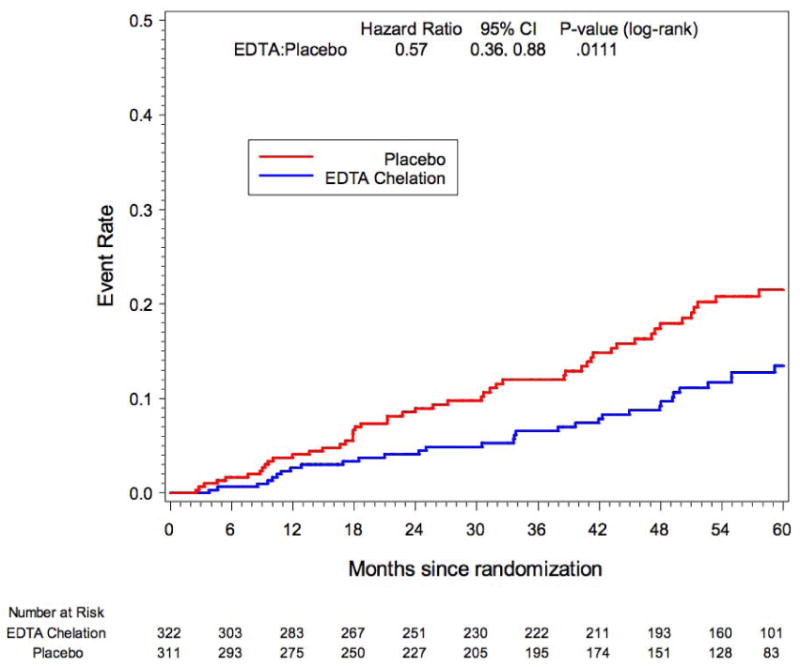
TACT Kaplan-Meier Estimates of Death EDTA Chelation Therapy vs. Placebo Subset of Patients with Diabetes: Hx, Med Use or Basefine Glucose>=126
Adapted with permission from
Circulation: Cardiovascular Quality Outcomes:
“Escolar E, Lamas G a, Mark DB, Boineau R, Goertz C, Rosenberg Y, et al. The effect of an EDTA-based chelation regimen on patients with diabetes mellitus and prior myocardial infarction in the Trial to Assess Chelation Therapy (TACT). Circ Cardiovasc Qual Outcomes. 2014 Jan;7(1):15–24.”
Figure 5.
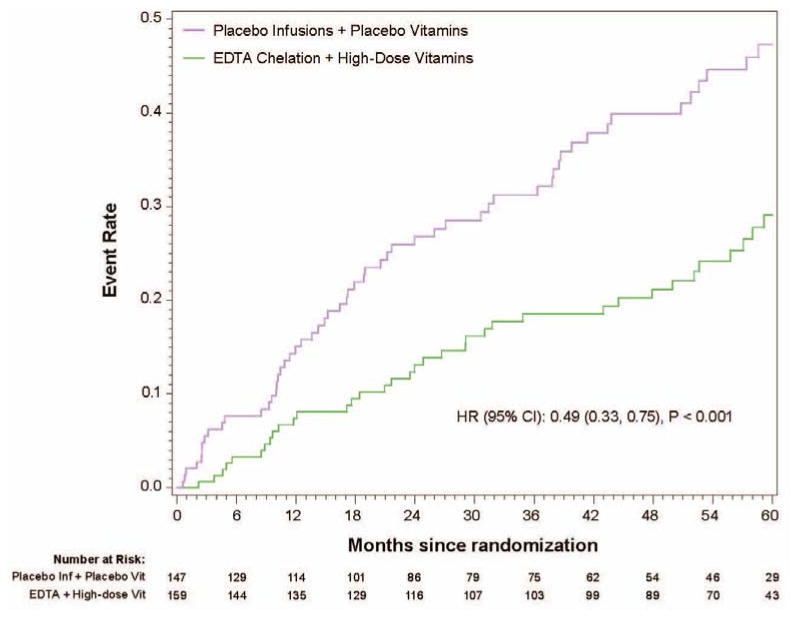
5-year Kaplan-Meier Event Rate Estimates for the Primary Composite Endpoint in Diabetic Patients. Active-active vs. Placebo-placebo
Adapted with permission from
American Heart Journal:
“Lamas GA., Boineau R, Goertz C, Mark DB, Rosenberg Y, Stylianou M, et al. EDTA chelation therapy alone and in combination with oral high-dose multivitamins and minerals for coronary disease: The factorial group results of the Trial to Assess Chelation Therapy. Am Heart J [Internet]. Elsevier B.V.; 2014 Apr [cited 2014 Jun 5]; Available from: http://dx.doi.org/10.1016/j.ahj.2014.02.012
Safety of chelation therapy
TACT also showed that, as used in the trial, treatment with EDTA was safe. EDTA was associated with few side effects and there were no differences in serious adverse events compared with placebo. Hypocalcemia, defined as a calcium level less than 8.5 mg/dL prior to an infusion, was reported in 52 chelation patients (6.2%) and 30 placebo patients (3.5%) (P=0.008). One patient had symptomatic hypocalcemia associated with muscle cramping that led to an emergency department visit.
TACT in context
TACT is a unique trial that crossed rigid lines of scientific thought. Its scientific basis is decades deep. The association of metals with cardiovascular disease is not new. And neither is the participation of metal-catalyzed oxygen chemistry in the production of AGEs. But this knowledge has been held in silos - in environmental health, epidemiology, and diabetes basic science– silos the cardiologist does not often visit. Thus, TACT served as a logical clinical test of these lines of knowledge. As such, the results have unveiled an exciting area of new cardiovascular research with the underlying concept that xenobiotic metals may be a modifiable risk factor for cardiovascular disease. Despite the epidemiological association between xenobiotic metals and cardiovascular disease, and the presumed effect of EDTA removing these toxins; the exact mechanism of how chelation reduced major cardiovascular events, is still unknown. Indeed, the positive effect of high doses of vitamin C on vascular endothelium should not be neglected as a potential contributing agent.
The TACT results, also, must be placed in context. In a 2006 review of statin therapy (38), the 5.1 year NNT for major cardiovascular events in a secondary prevention population, an endpoint similar to TACT, was 16. For chelation + oral vitamins it was 12. Even more striking is the effect on patients with diabetes. The 5.1 year NNT for major coronary events for statins was 17, for the TACT infusion treatment alone, 6.5 (5-year NNT).
Conclusions
Among patients with a prior MI, a regimen of 40 infusions of disodium EDTA-based infusions safely reduced cardiovascular events in a post-MI population, suggesting that xenobiotic metal pollutants are cardiovascular risk factors. The effect of the chelation infusions was enhanced with high doses of oral multivitamins and multiminerals. The therapeutic benefit was particularly striking in diabetic patients. These results were observed against a background of modern evidence-based post-MI therapy.
Future research
At present, the unique nature of these findings demands followup research to replicate findings and understand mechanisms. Such research should take place in patients with diabetes and macrovascular disease, including peripheral artery disease. The xenobiotic metal hypothesis that we have described should be tested, as should the development of AGEs and RAGE activation. Additionally, pragmatic, patient oriented outcome trials, comparing traditional treatment of severe PAD with standard care plus chelation could be carried out. Because of the generic nature of this intervention, there is absolutely no pharmaceutical company interest in this therapy, so all of us await NIH initiatives. Still, the magnitude of benefit was such that it suggests urgency in replication and implementation, which should, due the excellent safety record, occur simultaneously.
Footnotes
Disclosures: There are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Agency for Toxic Substances and Disease Registry (ATSDR) The priority list of hazardous substances. Atlanta (GA): U. S. Department of Health of Human Services, Public Health Service; 2011. [Google Scholar]
- 2.Nordberg GF, Nogawa K, Nordberg MFL. Cadmium. In: Nordberg GF, Fowler BA, Nordberg MFL, editors. Handbook on the Toxicology of Metals. Third. Elsevier; 2007. pp. 445–86. [Google Scholar]
- 3.Pirkle JL, Schwartz JE, Landis JR, Harlan WR. The relationship between blood lead levels and blood pressure and its cardiovascular risk implications. Am J Epidemiol. 1985;121:246–58. doi: 10.1093/oxfordjournals.aje.a113995. [DOI] [PubMed] [Google Scholar]
- 4.Nawrot T, Thijs L, Den Hond E, Roels H, Staessen J. An epidemiological reappraisal of the association between blood pressure and blood lead: a metaanalysis. J Hum Hypertens. 2002;16:123–31. doi: 10.1038/sj.jhh.1001300. [DOI] [PubMed] [Google Scholar]
- 5.Menke A, Muntner P, Batuman V, Silbergeld EK, Guallar E. Blood lead below 0.48 micromol/L (10 microg/dL) and mortality among US adults. Circulation. 2006 Sep 26;114(13):1388–94. doi: 10.1161/CIRCULATIONAHA.106.628321. [DOI] [PubMed] [Google Scholar]
- 6.Navas-Acien A, Selvin E, Sharrett AR, Calderon-Aranda E, Silbergeld E, Guallar E. Lead, cadmium, smoking, and increased risk of peripheral arterial disease. Circulation. 2004 Jun 29;109(25):3196–201. doi: 10.1161/01.CIR.0000130848.18636.B2. [DOI] [PubMed] [Google Scholar]
- 7.Lustberg M, Silbergeld E. Blood lead levels and mortality. Arch Intern Med. 2002;162:2443–9. doi: 10.1001/archinte.162.21.2443. [DOI] [PubMed] [Google Scholar]
- 8.Tellez-Plaza M, Navas-Acien A, Crainiceanu CM, Guallar E. Cadmium exposure and hypertension in the 1999-2004 National Health and Nutrition Examination Survey (NHANES) Environ Health Perspect. 2008;116:51–6. doi: 10.1289/ehp.10764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stohs SJ, Bagchi D. Oxidative mechanisms in the toxicity of metal ions. Free Radical Biology and Medicine. 1995:321–36. doi: 10.1016/0891-5849(94)00159-h. [DOI] [PubMed] [Google Scholar]
- 10.Vaziri ND, Ding Y, Ni Z. Nitric oxide synthase expression in the course of lead-induced hypertension. Hypertension. 1999;34:558–62. doi: 10.1161/01.hyp.34.4.558. [DOI] [PubMed] [Google Scholar]
- 11.Wagner GJ, Yeargan R. Variation in cadmium accumulation potential and tissue distribution of cadmium in tobacco. Plant Physiol. 1986;82:274–9. doi: 10.1104/pp.82.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Järup L, Berglund M, Elinder CG, Nordberg G, Vahter M. Health effects of cadmium exposure--a review of the literature and a risk estimate. Scand J Work Environ Health. 1998;24(Suppl 1):1–51. [PubMed] [Google Scholar]
- 13.Tellez-Plaza M, Navas-Acien A, Menke A, Crainiceanu CM, Pastor-Barriuso R, Guallar E. Cadmium Exposure and All-Cause and Cardiovascular Mortality in the U. S. General Population. Environ Health Perspect. 2012 Jul;120(7):1017–22. doi: 10.1289/ehp.1104352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tellez-Plaza M, Guallar E, Howard BV, Umans JG, Francesconi Ka, Goessler W, et al. Cadmium exposure and incident cardiovascular disease. Epidemiology. 2013;24:421–9. doi: 10.1097/EDE.0b013e31828b0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tellez-Plaza M, Jones MR, Dominguez-Lucas A, Guallar E, Navas-Acien A. Cadmium exposure and clinical cardiovascular disease: a systematic review. Curr Atheroscler Rep. 2013;15:356. doi: 10.1007/s11883-013-0356-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agarwal S, Zaman T, Tuzcu EM, Kapadia SR. Heavy metals and cardiovascular disease: results from the National Health and Nutrition Examination Survey (NHANES) 1999-2006. Angiology. 2011 Jul;62(5):422–9. doi: 10.1177/0003319710395562. [DOI] [PubMed] [Google Scholar]
- 17.The Emerging Risk Factors Collaboration. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–22. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moon KA, Guallar E, Umans JG, Devereux RB, Best LG, Francesconi KA, et al. Association between exposure to low to moderate arsenic levels and incident cardiovascular disease. A prospective cohort study. Ann Intern Med. 2013;159:649–59. doi: 10.7326/0003-4819-159-10-201311190-00719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamagishi S, Maeda S, Matsui T, Ueda S, Fukami K, Okuda S. Role of advanced glycation end products (AGEs) and oxidative stress in vascular complications in diabetes. Biochim Biophys Acta Elsevier BV. 2012 May;1820(5):663–71. doi: 10.1016/j.bbagen.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 20.Manigrasso MB, Juranek J, Ramasamy R, Schmidt AM. Unlocking the biology of RAGE in diabetic microvascular complications. Trends in Endocrinology and Metabolism. 2014:15–22. doi: 10.1016/j.tem.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suhartono E, Triawanti T, Leksono AS, Djati MS. The Role of Cadmium in Proteins Glycation by Glucose: Formation of Methylglyoxal and Hydrogen Peroxide in Vitro. J Med Bioeng. 2014;3(1):59–62. [Google Scholar]
- 22.Husna aH, Ramadhani Ea, ED T, Yulita aF, Suhartono E. The Role Formation of Methylglyoxal, Carbonyl Compound, Hydrogen Peroxide and Advance Oxidation Protein Product Induced Cadmium in Ovarian Rat. Int J Chem Eng Appl. 2014 Aug;5(4):319–23. [Google Scholar]
- 23.Nagai R, Murray DB, Metz TO, Baynes JW. Chelation: A Fundamental Mechanism of Action of AGE Inhibitors, AGE Breakers, and Other Inhibitors of Diabetes Complications. Diabetes. 2012:549–59. doi: 10.2337/db11-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waters RS, Bryden Na, Patterson KY, Veillon C, Anderson RA. EDTA chelation effects on urinary losses of cadmium, calcium, chromium, cobalt, copper, lead, magnesium, and zinc. Biol Trace Elem Res. 2001 Dec;83(3):207–21. doi: 10.1385/BTER:83:3:207. [DOI] [PubMed] [Google Scholar]
- 25.Clarke CN, Clarke NE. Treatment of angina pectoris with disodium ethylene diamine tetraacetic acid. Am J Med Sci. 1956;6(232):654–66. doi: 10.1097/00000441-195612000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Guldager B, Jelnes R, Jørgensen SJ, Nielsen JS, Klaerke A, Mogensen K, et al. EDTA treatment of intermittent claudication--a double-blind, placebo-controlled study. Journal of internal medicine. 1992 Mar;:261–7. doi: 10.1111/j.1365-2796.1992.tb00533.x. [DOI] [PubMed] [Google Scholar]
- 27.Van Rij AM, Solomon C, Packer SG, Hopkins WG. Chelation therapy for intermittent claudication. A double-blind, randomized, controlled trial. Circulation. 1994:1194–9. doi: 10.1161/01.cir.90.3.1194. [DOI] [PubMed] [Google Scholar]
- 28.Knudtson ML, Wyse DG, Galbraith PD, Brant R, Hildebrand K, Paterson D, et al. Chelation therapy for ischemic heart disease: a randomized controlled trial. JAMA. 2002;287:481–6. doi: 10.1001/jama.287.4.481. [DOI] [PubMed] [Google Scholar]
- 29.Ernst E. Chelation therapy for coronary heart disease: An overview of all clinical investigations. Am Heart J. 2000;140:139–41. doi: 10.1067/mhj.2000.107548. [DOI] [PubMed] [Google Scholar]
- 30.Lamas Ga, Ackermann A. Clinical evaluation of chelation therapy: is there any wheat amidst the chaff? Am Heart J. 2000 Jul;140(1):4–5. doi: 10.1067/mhj.2000.107549. [DOI] [PubMed] [Google Scholar]
- 31.Villarruz MV, Dans A, Tan F. Chelation therapy for atherosclerotic cardiovascular disease. Cochrane Database Syst Rev. 2002:CD002785. doi: 10.1002/14651858.CD002785. [DOI] [PubMed] [Google Scholar]
- 32.Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report. 2008:1–23. [PubMed] [Google Scholar]
- 33.Deaths associated with hypocalcemia from chelation therapy--Texas, Pennsylvania, and Oregon, 2003-2005. MMWR Morb Mortal Wkly Rep. 2006 Mar 3;55(8):204–7. [PubMed] [Google Scholar]
- 34.Lamas GA, Goertz C, Boineau R, Mark DB, Rozema T, Nahin RL, et al. Design of the Trial to Assess Chelation Therapy (TACT) Am Heart J Mosby, Inc. 2012 Jan;163(1):7–12. doi: 10.1016/j.ahj.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lamas Gervasio A, MD, Goertz Christine, DC, PhD, Boineau Robin, MD, MA, Mark Daniel B, MD, MPH, Rozema Theodore, MD, Nahin Richard L, PhD, MPH, et al. for the TI. Effect of Disodium EDTA Chelation Regimen on Cardiovascular Events in Patients With Previous Myocardial Infarction: The TACT Randomized Trial. JAMA. 2013;309:1241. doi: 10.1001/jama.2013.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lamas GA, Boineau R, Goertz C, Mark DB, Rosenberg Y, Stylianou M, et al. EDTA chelation therapy alone and in combination with oral high-dose multivitamins and minerals for coronary disease: The factorial group results of the Trial to Assess Chelation Therapy. Am Heart J. 2014 Apr; doi: 10.1016/j.ahj.2014.02.012. Internet. [cited 2014 Jun 5]; Available from: http://dx.doi.org/10.1016/j.ahj.2014.02.012. [DOI] [PMC free article] [PubMed]
- 37.Escolar E, Lamas Ga, Mark DB, Boineau R, Goertz C, Rosenberg Y, et al. The effect of an EDTA-based chelation regimen on patients with diabetes mellitus and prior myocardial infarction in the Trial to Assess Chelation Therapy (TACT) Circ Cardiovasc Qual Outcomes. 2014 Jan;7(1):15–24. doi: 10.1161/CIRCOUTCOMES.113.000663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Costa J, Borges M, David C, Vaz Carneiro A. Efficacy of lipid lowering drug treatment for diabetic and non-diabetic patients: meta-analysis of randomised controlled trials. BMJ. 2006;332:1115–24. doi: 10.1136/bmj.38793.468449.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]


