Abstract
The purpose of this study was to examine the antiobesity effects of Monascus pilosus-fermented black soybean (F-BS) in C57BL/6 mice with high-fat diet (HFD)-induced obesity. F-BS (oral, 0.5 and 1.0 g/kg per body weight, twice per day) ameliorated obesity by reducing body and liver weight increases, and regulating blood glucose and cholesterol levels in C57BL/6 mice fed a control or HFD with oral administration of F-BS for 12 weeks. F-BS suppressed the growth of epididymal, retroperitoneal, and perirenal fat pads by preventing increases in the adipocyte size. Moreover, the levels of blood glucose, total cholesterol, and leptin were significantly lowered by F-BS administration in a dose-dependent manner. These results indicated that F-BS is a beneficial food supplement for preventing obesity, controlling blood glucose, and lowering cholesterol. Future research strategies should address the mechanisms that selectively regulate obesity, including hyperglycemia and hypercholesterolemia.
Key Words: : anti-obesity effect, fermented black soybean, high-fat diet-induced obesity, hyperglycemia, Glycine maxL. Merrill, Monascus pilosus
Introduction
Soybean and its modified products are known to contain bioactive components such as proteins, oils, carbohydrates, minerals, vitamins, isoflavones, phytosterols, saponins, and ferritins.1–5 For several years, rigorous scientific and clinical research has revealed that most of the components of soybeans exhibit beneficial effects, showing preventive potential for several diseases. Many functional components of soybeans have been characterized, nutritionally and physiologically.5,6
The beneficial effects of soybean have been scientifically validated through in vitro and in vivo studies with animal models and human clinical trials. Soybean and its products has been reported to ameliorate acute or chronic diseases such as inflammation,7 hepatic disorders,4,8 cardiovascular disease,9,10 osteoporosis,11,12 arthritis,13 diabetes,14 kidney,15 neuronal disorder,16 obesity,17 and cancer.18,19
Fermentation is a process of anaerobic digestion that generates energy (such as adenosine triphosphate, ATP) by the oxidation of certain organic compounds such as carbohydrates. Fermentation uses an endogenous, organic electron acceptor.20 Especially, the Monascus species have been used as medicinal foods for over 1000 years in Asia. It has been called “Hon-Chi,” “Anka,” “red koji,” “red Chinese rice,” or “red mold rice” in several countries.21 Fermentation with Monascus spp. produces several bioactive metabolites such as color pigments,22 isoflavones, monacolins, and γ-aminobutyric acid.23,24 Monascus fermentation metabolites have been reported to be beneficial for cholesterol management,25 blood glucose management,26 blood pressure management,27,28 cancer,29 Alzheimer's disease in animal and cell models,30,31 and obesity.32,33
Obesity and diabetes are closely associated with insulin resistance, a condition in which the level of insulin required to achieve a normal metabolic response is higher than usual.34,35 Particularly, obesity is accompanied by systemic inflammatory responses, characterized by abnormal cytokine production and the activation of inflammatory signaling pathways such as tumor necrosis factor-α, interleukin 6, and monocyte chemoattractant protein-1.36,37 Therefore, regulation of dietary energy and body weight is very important for maintenance of health.
The black bean and its bioactive compounds has been used as a natural medicine for various health disorders such as diabetes, atherosclerosis, carcinogenesis, inflammation, and high-fat diet-induced obesity.17,38–40 However, the effects of fermented black soybean (F-BS) on high-fat diet (HFD)-induced obesity and hyperglycemia are not known. In the present study, we examined the effects of F-BS on HFD-induced obesity and hyperglycemia in C57BL/6 mice for 12 weeks and analyzed the changes in related parameters.
Materials and Methods
Cell strain and reagents
The yeast strain of Monascus pilosus (KCCM 60084) was purchased from the Korea Culture Center of Microorganisms (KCCM). All chemical reagents were obtained from Sigma-Aldrich. Serological and leptin assay kits were obtained from Asan Pharmaceutical and R&D systems, respectively.
Sample preparation
Black soybean (Glycine max L. Merrill) obtained from the National Agriculture Cooperative Federation (NACF) was thoroughly washed with water and homogenized with a Waring blender (HMF-1710; Hanil) to 2–4 mm particle size. A mixture of homogenates [450 g, moisture content: ∼50% (w/w)] and 50 g of wheat bran as solid media was autoclaved at 121°C for 90 min, then cooled under aseptic conditions at room temperature. For fermentation of the sample, precultured M. pilosus suspension (7 days at 28°C) was inoculated into 2% rice powder −3% glucose −2% peptone −0.8% KH2PO4 −0.05% MgSO4·7H2O −0.2% CH3COOK −0.1% NaCl containing solid media to 10% (w/w) and further cultured for 8 days at 28°C. The freeze-dried fermented sample (moisture content: <5%) was homogenized and passed through 100 mesh nylon filters. The powder was kept in a −80°C freezer and used for further study.
Animal experiments
Male, 5-week-old specific pathogen-free C57BL/6J mice (18–23 g) were purchased from Orient Bio. The experiments were performed in accordance with the principles and with the approval of the Ethics Committee of the Wonkwang University, Iksan, Korea (Approval No. WKU11-001). All animals were maintained in a temperature-controlled room (temperature 22°C±2°C, humidity 50%±5%) with a 12-h light/12-h dark cycle and acclimatized to the laboratory environment while housed in individual cages for 1 week before the experiment. Obesity was induced by a HFD (60% energy from fat), and mice were randomly divided into three groups after the first 6 weeks on the high-fat diet (AIN-93G, cat., #101556; Research Diet, Inc.) as follows: the high-fat diet group (HFD, control group), HFD+0.5 g/kg F-BS (HFD+F-BS 0.5), and HFD+1.0 g/kg F-BS (HFD+F-BS 1.0). The control group (normal diet) ate regular diet during the same period. For oral administration, a placebo (water) or two doses of F-BS were administered twice daily for 12 weeks. At the end of the experiment, 4-h-fasted mice were anesthetized using a mixture of xylazine/ketamine (1:3, v/v), and blood samples were collected and stored at −80°C until their analyses.
Measurements of glucose, triglyceride, cholesterol, and leptin concentrations
After 12 weeks of treatment with and without F-BS, blood samples were collected and centrifuged at 2500 g for 15 min at 4°C, and then levels of serum glucose, triglyceride (TG), total cholesterol (T-CHO), high-density lipoprotein-cholesterol (HDL-C), low-density lipoprotein-cholesterol (LDL-C), and leptin were measured using commercial kits according to the manufacturer's instruction.
Measurements of body, organ, and fat weight
Body weight was measured once a week during the feeding period. Internal organs were dissected and weighed. Fat tissue samples also were stored at −80°C until they were analyzed.
Histological analysis
Histological analysis was conducted following routine methods. Briefly, the dissected epididymal adipose tissues were fixed in 10% neutral buffered formalin for histological analysis and embedded in paraffin. The paraffin-embedded sections were cut to a thickness of 4 μm and stained with hematoxylin and eosin. Adipocyte sizes were measured in randomly chosen microscopic areas from independent animals using a Nikon microscope system (TE-2000), and the average adipocyte size was calculated by dividing each chosen microscopic area by total adipocyte cell number in the area.
Statistical analyses
All data are expressed as mean±SEM, and the differences between groups were analyzed using one-way ANOVA and Duncan's multiple-range test. All analyses were performed using SPSS 10.0 (SPSS, Inc.). Each value was the mean of at least three separate experiments in each group, and data with different superscript letters are significantly different when P-value is less than .05.
Results
Effects of F-BS on food and water intake
We measured the changes in food and water intake of the mice once a week after 6 weeks of receiving a normal diet or HFD with or without F-BS administration. Compared to HFD mice, significant changes in food intake were not observed among groups (Fig. 1A). In the normal diet group, food consumption was transiently increased during 12 weeks, but maintained a similar pattern with HFD, HFD+F-BS 0.5, and HFD+F-BS 1.0, and the water intake showed a similar pattern (Fig. 1B).
FIG. 1.
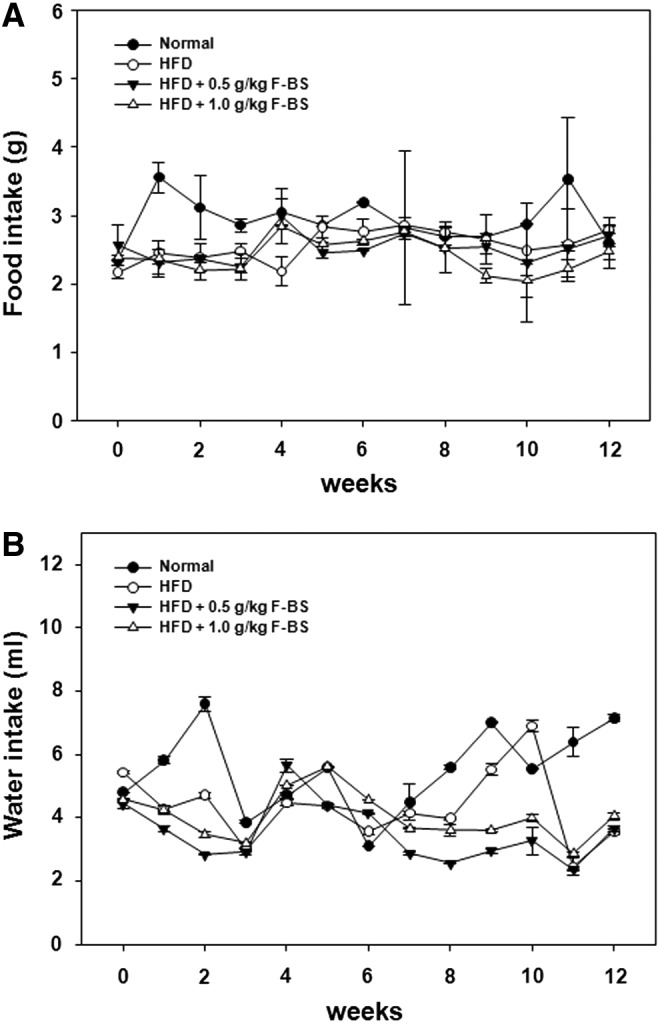
Effects of fermented black soybean (F-BS) on change in food (A) and water (B) intake in a high-fat diet (HFD)-induced mice obesity model. Food and water intakes were measured and analyzed each week. Data are shown as mean±SE (each group n=10).
Effects of F-BS on body weight and blood glucose level
Body weight was significantly increased in all HFD groups after 6 weeks of HFD (Fig. 2A). Administration of F-BS significantly reduced HFD-induced body weight in a dose-dependent manner. At 12 weeks after initiation of the study, the body weight of HFD group was increased up to 144% compared to that of placebo administration, however, the body weight of HFD+F-BS 0.5 and HFD+F-BS 1.0 groups was increased to only 133% and 123%, respectively. Blood glucose in the HFD group was significantly increased (152.38±2.16 mg/dL) compared with the level of normal group (84.38±4.99 mg/dL), but the increase was less in F-BS groups in a dose-dependent manner. Administration of F-BS resulted in lower levels of blood glucose of 131.0±11.40 mg/dL and 117.38±11.61 mg/dL in the F-BS 0.5 and F-BS 1.0 group, respectively. These results suggested that HFD-induced obesity triggered hyperglycemia and that F-BS can ameliorate both health problems.
FIG. 2.
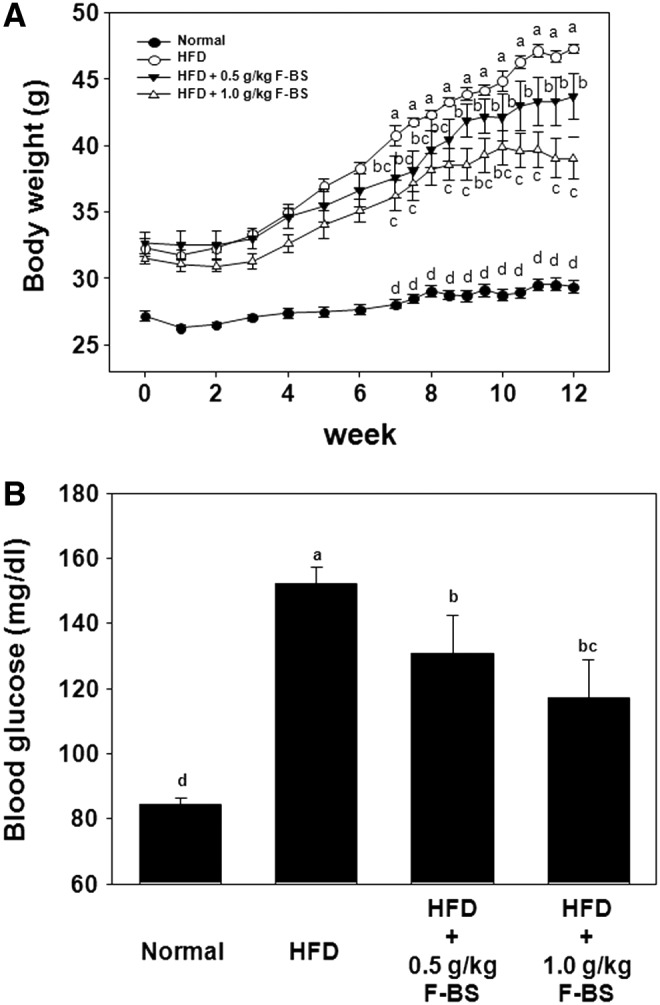
Effects of F-BS on changes in body weight (A) and blood glucose (B) in a HFD-induced mouse obesity model. Body weight was measured every week and blood glucose measured at 12 weeks. Blood was collected from inferior vena cava after sacrifice.abcd Values in the row with different superscript letters are significantly different, P<.05. Data are shown as mean±SEM (each group n=10).
Effect of F-BS on organ weight
The weight of the liver, kidneys, epididymis, and testes of F-BS-treated groups was compared with that of the HFD groups (Fig. 3). As shown in Figure 3, the liver weight of the HFD group was higher compared with the livers of the normal group, and the liver weight of HFD mice treated with F-BS 1.0 was slightly lighter than the weight of the HFD group (Fig. 3. left panel). The weight of other organs (kidney, testis, and epididymis) was not significantly different among the groups.
FIG. 3.
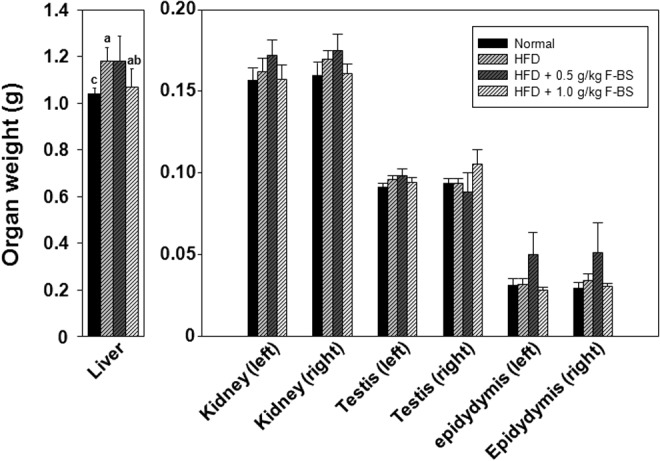
Effects of F-BS on organ weight in a HFD-induced mice obesity model. Weight of collected organs was immediately measured after sacrifice.abc Values in the same row with different superscript letters are significantly different, P<.05. Data are shown as mean±SEM (each group n=10).
Effects of F-BS on absolute fat tissue weight and size of epididymal adipocytes
The absolute fat tissues of epididymal, retroperitoneal, and perirenal sites in the normal and HFD group were collected and fat weighed (Fig. 4). As shown in Figure 4A, the weight of epididymal, retroperitoneal, and perirenal fat tissue in the HFD group was higher compared with the normal group (approximately fivefold), and the fat tissue weight of HFD mice treated with F-BS was significantly lighter than the weight of the HFD group. Furthermore, the diameters of adipocytes from F-BS-administrated HFD animals were lower in a dose-dependent manner (Fig. 4B, C).
FIG. 4.
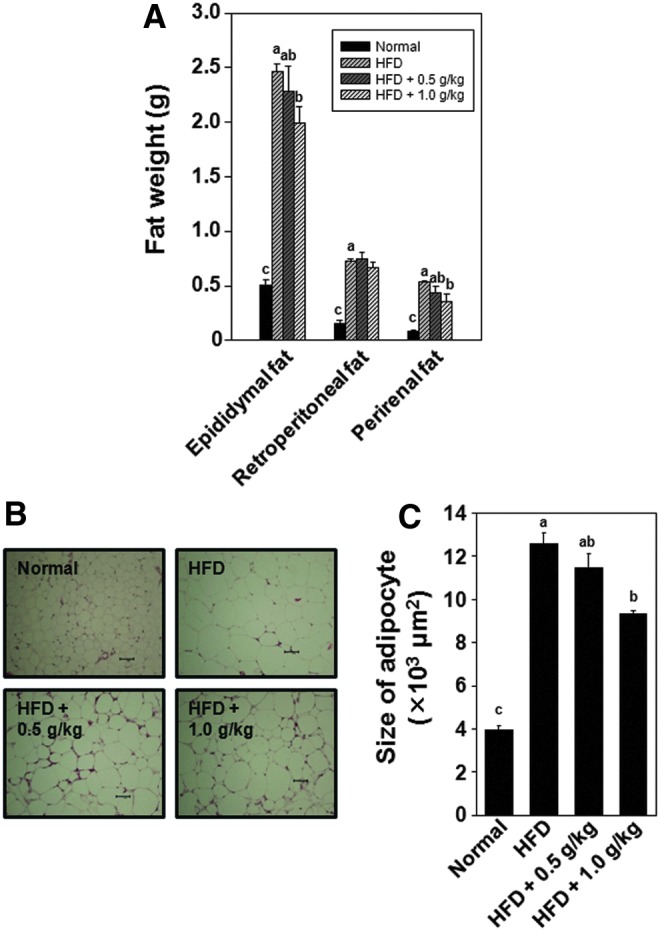
Effects of F-BS on absolute fat weight (A), histological data (B), and statistics of epididymal adipocyte size (C) in a HFD-induced mice obesity model. Weight of epididymal, retroperitoneal, and perirenal fat was measured as described in Materials and Methods.abc Values in the same row with different superscript letters are significantly different, P<.05. Scale bar=100 μm. Data are shown as mean±SEM (each group n=10). Color images available online at www.liebertpub.com/jmf
Effects of F-BS on blood cholesterol and leptin concentration
To determine the ameliorative effects of F-BS on HFD-induced obesity, we measured levels of cholesterol and leptin in serum after 12 weeks (Table 1 and Fig. 5). Administration of F-BS decreased the level of T-CHO in a 1 g/kg F-BS group (Fig. 5A). Leptin levels were also significantly decreased by administration of F-BS in a 1 g/kg F-BS group (Fig. 5B). The HFD feeding substantially increased the levels of TG, LDL-C, and HDL-C compared with the normal diet group, but no significant changes were observed in any F-BS-administered groups (Table 1).
Table 1.
Effects of F-BS on Serum Levels of TG, LDL-C, and HDL-C
| TG (mg/dL) | LDL-C (mg/dL) | HDL-C (mg/dL) | |
|---|---|---|---|
| Normal | 114.11±9.36 | 8.63±0.63 | 96.00±2.75 |
| HFD | 97.50±11.54 | 38.00±1.07a | 187.7500±2.96*** |
| HFD+0.5 | 111.88±7.05 | 34.13±4.40a | 194.13±7.55*** |
| HFD+1.0 | 103.38±8.10 | 34.13±3.82a | 178.6250±9.90*** |
P<.001 versus normal group. Results are expressed as mean±SEM of ten mice per experimental group.
F-BS, fermented black soybean; TG, triglyceride; LDL-C, low-density lipoprotein-cholesterol; HDL-C, high-density lipoprotein-cholesterol.
FIG. 5.
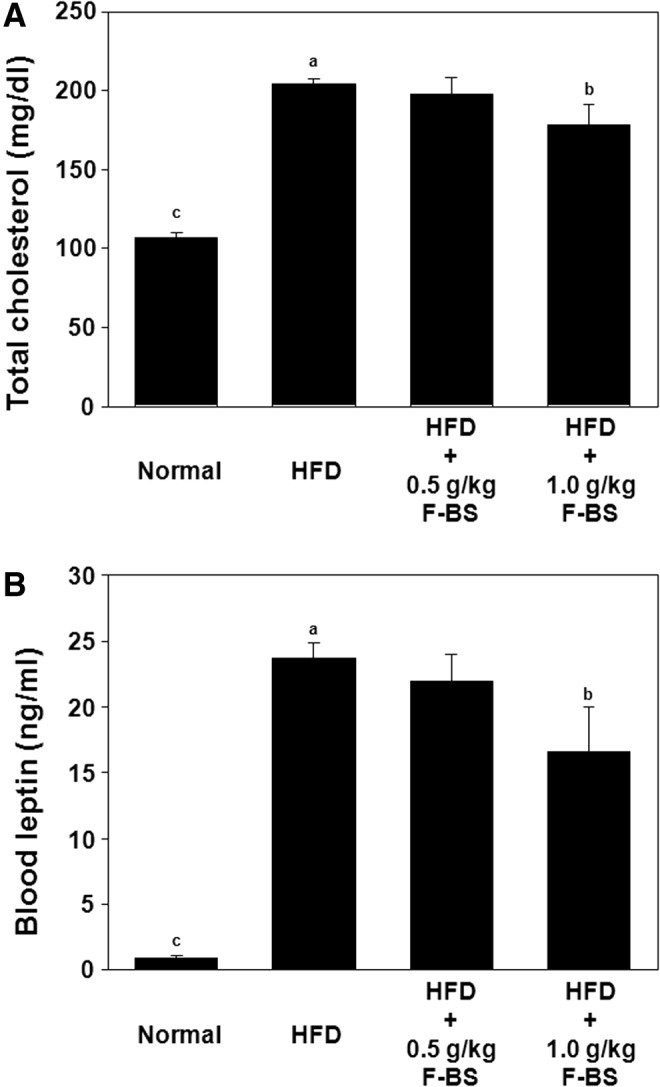
Effects of F-BS on changes in blood total cholesterol and leptin levels in HFD-induced mouse obesity model. Blood was collected from the inferior vena cava after sacrifice and (A) blood total cholesterol and (B) leptin levels were measured as described in Materials and Methods. abc Values in the row with different superscript letters are significantly different, P<.05. Data are shown as mean±SEM (each group n=10).
Discussion
In this study, we provide data to support an ameliorative effect of F-BS on HFD-induced obesity along with various associated pathologies such as hyperglycemia, hypercholesterolemia, and increased organ fat accumulation and enlargement of adipocytes. Treatment of HFD mice with F-BS probably results in alterations in the regulation of fat metabolism and accumulation. Simultaneously, treatment of HFD mice with F-BS resulted in a lower liver weight and serum leptin levels.
Black soybean (Glycine max) has been widely used as a nutritionally rich food in Asia and utilized as a traditional herbal medicine for the prevention of diabetes, aiding liver and kidney functions, and enhancing diuretic actions. Black soybean, different from yellow soybean, is abundant in polyphenols.41,42 F-BS suppressed increases in all adipose tissue weight of epididymal, retroperitoneal, and perirenal fats (Fig. 4) without affecting the food and water intake (Fig. 1). To clarify these phenomena, we focused on the changes in sizes of adipocytes. The critical function of adipose tissue is the maintenance of energy homeostasis in vertebrates.43,44 White adipose tissue stores excess energy as TGs when intake exceeds expenditure, whereas the brown adipose tissue can dissipate energy through thermogenesis. The size of the adipose tissue can be modulated by the formation of new adipocytes from precursor cells and/or increases in the size of adipocytes. In Figure 4C, the size of adipocytes was markedly increased in the HFD group, whereas F-BS reduced the adipocyte size, however, we observed no changes in adipocyte numbers per adipose tissue weight (data not shown). These results closely correlated with blood glucose and T-CHO levels (Figs. 2B and 5A). However, F-BS administration had no significant effect on blood TG, LDL-C, and HDL-C levels (Table 1) and these results are not correlated to lipid value calculation.45 Although the reasons are not clearly understood, it is likely caused by the HFDs used in the experiments. For the investigation of antiobesity effects of F-BS, we measured the weight of adipose tissues such as epididymal fat, retroperitoneal fat, and perirenal fat. However, the mass of the main adipose tissue sites is epididymal, subcutaneous, retroperitoneal, perirenal, mesenteric, gluteal, and interscapular.46–48 Therefore, other adipose tissues need to be examined in our future studies. In addition, we observed transient increases in water intake in the normal group, but there were no significant changes in food intake between both groups (Fig. 1), and the HFD group also showed temporal fluctuation of food intake. These results suggest that increases in body weight in HFD-induced obesity triggered adipocyte hypertrophy and that long-term administration of F-BS (12 weeks) ameliorates these problems.
Sometimes, Monascus-fermented products can be contaminated toxic compounds such as monascidin A, which is toxic to the liver and kidneys, by contamination from unexpected microorganisms such as Penicillium citrinum. Indeed, citrinin, which is a fungus-derived toxic secondary metabolite, has been implicated as a cause of nephropathy and fever in humans.49 In the present study, we used established strains of M. pilosus, then we demonstrated reproducible results and established protocols for a large-scale process; however, these measures could not guarantee absolute biological safety and confidence for industrial purposes. Thus, natural strains of M. pilosus should be explored for the development of traditional food products and commercialization.
The fermented plants such as rice and wheat by M. pilosus are important goods as dietary staple food supplements and have a long tradition in East Asia. Recently, Monascus-fermented foods are considered as medicinal foods and plants to ameliorate obesity, hyperglycemia, and hypercholesterolemia. The production of Monascus-fermented products could be modified by strain/substrate selection, and modification of fermentation conditions to enhance the contents of effective compounds such as monacolins, monascin, and ankaflavin. Therefore, further studies should be carried out to identify its bioactive components and their beneficial activity in other disease models.
The mechanism of Glycine max L. Merrill and its fermented products for improving obesity is not clear at present. However, several previous reports have suggested possible mechanisms: these include induction of thermogenesis in muscle, decreased fatty acid synthesis in adipose tissue, and lessened fatty acid uptake in intestinal tissue through activation of peroxisome proliferator-activated receptors (PPARs).50,51 In addition, a study has reported that soybean protein consumption stimulates insulin secretion and decreases PPAR-γ, GLUT-2, and SREBP-1 expression, and ameliorates hyperinsulinemia observed during obesity.52 Insulin contributes to increased synthesis and secretion of leptin in the adipose tissue.53 Leptin acts through the leptin receptor, and mutations of the leptin receptor cause obesity, hyperphagia, and reduce energy expenditure54; therefore, it can be used as an obesity marker. Administration of F-BS may act on the regulation of insulin secretion/sensitivity and leptin secretion, thus contributing to significant antiobesity effects in the HFD-induced obesity model. On the other hand, the glucose tolerance test, measurement of blood insulin level, and calculation of insulin homeostasis model assessment of insulin resistance may provide evidence for further studies to control the HFD-induced lipid metabolism disorder by similar methods.55
In conclusion, F-BS suppressed body weight and adipose tissue weight gains, and normalized blood glucose and T-CHO in C57BL/6 mice fed a HFD. Dietary F-BS attenuated body weight increase due to HFD feeding and suppressed the obesity-caused increase of liver weight. These effects of F-BS may contribute to improvements in energy consumption and glucose utilization. Our results indicate that F-BS has the potential to reduce the risk of obesity and hyperglycemia.
Acknowledgment
This work was supported by a grant from the Next-Generation bioGreen 21 Program (NO.PJ009643), Rural Development Administration, Republic of Korea.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Liu KS: Chemistry and nurtitional value of soybean components. In: Soybean: Chemistry, Technology, and Utilization, (Keshun Liu, ed.). Chapman & Hall, New York, 1997, pp. 25–113 [Google Scholar]
- 2.Kunyanga CN, Imungi JK, Okoth M, Momanyi C, Biesalski HK, Vadivel V: Antioxidant and antidiabetic properties of condensed tannins in acetonic extract of selected raw and processed indigenous food ingredients from Kenya. J Food Sci 2011;76:C560–C567 [DOI] [PubMed] [Google Scholar]
- 3.Itoh T, Hori Y, Atsumi T, Toriizuka K, Nakamura M, Maeyama T, Ando M, Tsukamasa Y, Ida Y, Furuichi Y: Hot water extract of adzuki (Vigna angularis) suppresses antigen-stimulated degranulation in rat basophilic leukemia RBL-2H3 cells and passive cutaneous anaphylaxis reaction in mice. Phytother Res 2012;26:1003–1011 [DOI] [PubMed] [Google Scholar]
- 4.Gundermann KJ, Kuenker A, Kuntz E, Droździk M: Activity of essential phospholipids (EPL) from soybean in liver diseases. Pharmacol Rep 2011;63:643–659 [DOI] [PubMed] [Google Scholar]
- 5.Dixit AK, Antony JIX, Sharma NK, Tiwari RK: Soybean constituents and their functional benefits. Oppor Challenge Scope Nat Prod Med Chem 2011;367–383 [Google Scholar]
- 6.Sugano M, (ed.): Soy in Health and Disease Prevention. CRC Press, FL, 2006 [Google Scholar]
- 7.Choi YH, Lim H, Heo MY, Kwon DY, Kim HP: Anti-inflammatory activity of the ethanol extract of Chungkukjang, Korean fermented bean: 5-lipoxygenase inhibition. J Med Food 2008;11:539–543 [DOI] [PubMed] [Google Scholar]
- 8.Yang X, Dong C, Ren G: Effect of soyasaponins-rich extract from soybean on acute alcohol-induced hepatotoxicity in mice. J Agric Food Chem 2011;59:1138–1144 [DOI] [PubMed] [Google Scholar]
- 9.Van Horn L, McCoin M, Kris-Etherton PM, Burke F, Carson JA, Champagne CM, Karmally W, Sikand G: The evidence for dietary prevention and treatment of cardiovascular disease. J Am Diet Assoc 2008;108:287–331 [DOI] [PubMed] [Google Scholar]
- 10.Taku K, Lin N, Cai D, Hu J, Zhao X, Zhang Y, Wang P, Melby MK, Hooper L, Kurzer MS, Mizuno S, Ishimi Y, Watanabe S: Effects of soy isoflavone extract supplements on blood pressure in adult humans: systematic review and meta-analysis of randomized placebo-controlled trials. J Hypertens 2010;28:1971–1982 [DOI] [PubMed] [Google Scholar]
- 11.Morabito N, Crisafulli A, Vergara C, Gaudio A, Lasco A, Frisina N, D'Anna R, Corrado F, Pizzoleo MA, Cincotta M, Altavilla D, Ientile R, Squadrito FJ: Effects of genistein and hormone-replacement therapy on bone loss in early postmenopausal women: a randomised double-blind placebo-controlled study. J Bone Min Res 2002;17:1904–1912 [DOI] [PubMed] [Google Scholar]
- 12.Chen YM, Ho SC, Lam SSH, Ho SSS, Woo JLF: Soy isoflavones have a favorable effect on bone loss in Chinese postmenopausal women with lower bone mass: a double-blind, randomized, controlled trial. J Clin Endocrinol Metab 2003;88:4740–4747 [DOI] [PubMed] [Google Scholar]
- 13.Cameron M, Gagnier JJ, Little CV, Parsons TJ, Blümle A, Chrubasik S: Evidence of effectiveness of herbal medicinal products in the treatment of arthritis. Part I: Osteoarthritis. Phytother Res 2009;23:1497–1515 [DOI] [PubMed] [Google Scholar]
- 14.Holt S. Muntyan I, Likver L: Soya-based diets for diabetes mellitus. Altern Complement Ther 1996;2:79–82 [Google Scholar]
- 15.Ekor M, Emerole GO, Farombi EO: Phenolic extract of soybean (Glycine max) attenuates cisplatin-induced nephrotoxicity in rats. Food Chem Toxicol 2010;48:1005–1012 [DOI] [PubMed] [Google Scholar]
- 16.Azcoitia I, Moreno A, Carrero P, Palacios S, Garcia-Segura LM: Neuroprotective effects of soy phytoestrogens in the rat brain. Gynecol Endocrinol 2006;22:63–69 [DOI] [PubMed] [Google Scholar]
- 17.Kanamoto Y, Yamashita Y, Nanba F, Yoshida T, Tsuda T, Fukuda I, Nakamura-Tsuruta S, Ashida H: A black soybean seed coat extract prevents obesity and glucose intolerance by up-regulating uncoupling proteins and down-regulating inflammatory cytokines in high-fat diet-fed mice. J Agric Food Chem 2011;59:8985–8993 [DOI] [PubMed] [Google Scholar]
- 18.Adlercreutz H, Mazur W, Bartels P, Elomaa VV, Watanabe S, Wahala K, Landstrom M, Lundin E, Bergh A, Damber JE, Aman P, Widmark A, Johansson A, Zhang JX, Hallmans GJ: Phytoestrogens and prostate disease. J Nutr 2000;130:658–659 [DOI] [PubMed] [Google Scholar]
- 19.Davies MJ, Bowey EA, Adlercreutz H, Rowland IA, Rumsby PC: Effects of soy or rye supplementation of high-fat diets on colon tumour development in azoxymethane treated rats. Carcinogenesis 1999;20:927–931 [DOI] [PubMed] [Google Scholar]
- 20.Klein DW, Lansing M. Harley J: Microbiology (6thed.) McGraw-Hill, New York, 2006 [Google Scholar]
- 21.Shi YC, Pan TM: Beneficial effects of Monascus purpureus NTU 568-fermented products: a review. Appl Microbiol Biotechnol 2011;90:1207–1217 [DOI] [PubMed] [Google Scholar]
- 22.Wild D, Toth G, Humpf HU: New Monascus metabolite isolated from red yeast rice (angkak, red koji). J Agric Food Chem 2002;50:3999–4002 [DOI] [PubMed] [Google Scholar]
- 23.Juslova P, Martinkova L, Kren V: Secondary metabolites of the fungus Monascus: a review. J Ind Microbiol 1996;16:163–170 [Google Scholar]
- 24.Ma J, Li Y, Ye Q, Li J, Hua Y, Ju D, Zhang D, Cooper R, Chang M: Constituents of red yeast rice, a traditional Chinese food and medicine. J Agric Food Chem 2000;48:5220–5225 [DOI] [PubMed] [Google Scholar]
- 25.Lee CL, Tsai TY, Wang JJ, Pan TM: In vivo hypolipidemic effects and safety of low dosage Monascus powder in a hamster model of hyperlipidemia. Appl Microbiol Biotechnol 2006;70:533–540 [DOI] [PubMed] [Google Scholar]
- 26.Chen CC, Liu IM: Release of acetylcholine by Hon-Chi to raise insulin secretion in Wistar rats. Neurosci Lett 2008;404:117–121 [DOI] [PubMed] [Google Scholar]
- 27.Kohama Y, Matsumoto S, Mimura T, Tanabe N, Inada A, Nakanishi T: Isolation and identification of hypotensive principles in red-mold rice. Chem Pharm Bull (Tokyo) 1987;35:2484–2489 [DOI] [PubMed] [Google Scholar]
- 28.Singewald N, Kouvelas D, Mostafa A, Sinner C, Philippu A: Release of glutamate and GABA in the amygdala of conscious rats by acute stress and baroreceptor activation: differences between SHR and WKY rats. Brain Res 2000;864:138–141 [DOI] [PubMed] [Google Scholar]
- 29.Ho BY, Pan TM: The Monascus metabolite monacolin K reduces tumor progression and metastasis of Lewis lung carcinoma cells. J Agric Food Chem 2009;57:8258–8265 [DOI] [PubMed] [Google Scholar]
- 30.Lee CL, Kuo TF, Wang JJ, Pan TM: Red mold rice ameliorates impairment of memory and learning ability in intracerebroventricular amyloid beta-infused rat by repressing amyloid beta accumulation. J Neurosci Res 2007;85:3171–3182 [DOI] [PubMed] [Google Scholar]
- 31.Lee CL, Wang JJ, Pan TM: Red mold rice extract represses amyloid beta peptide-induced neurotoxicity via potent synergism of anti-inflammatory and antioxidative effect. Appl Microbiol Biotechnol 2008;79:829–841 [DOI] [PubMed] [Google Scholar]
- 32.Jeon T, Hwang SG, Hirai S, Matsui T, Yano H, Kawada T, Lim BO, Park DK: Red yeast rice extracts suppress adipogenesis by down-regulating adipogenic transcription factors and gene expression in 3T3-L1 cells. Life Sci 2004;75:3195–3203 [DOI] [PubMed] [Google Scholar]
- 33.Chen WP, Ho BY, Lee CL, Lee CH, Pan TM: Red mold rice prevents the development of obesity, dyslipidemia and hyperinsulinemia induced by high-fat diet. Int J Obes (Lond) 2008;32:1694–1704 [DOI] [PubMed] [Google Scholar]
- 34.Wei Y, Chen K, Whaley-Connell AT, Stump CS, Ibdah JA, et al. : Skeletal muscle insulin resistance: role of inflammatory cytokines and reactive oxygen species. Am J Physiol Regul Integr Comp Physiol 2008;294:673–680 [DOI] [PubMed] [Google Scholar]
- 35.Hotamisligil GS: Inflammation and metabolic disorders. Nature 2006;444: 860–867 [DOI] [PubMed] [Google Scholar]
- 36.Cancello R, Clement K: Is obesity an inflammatory illness? Role of low-grade inflammation and macrophage infiltration in human white adipose tissue. BJOG 2006;113:1141–1147 [DOI] [PubMed] [Google Scholar]
- 37.Lumeng CN, Bodzin JL, Saltiel AR: Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 2007;117:175–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lo CW, Huang HP, Lin HM, Chien CT, Wang CJ: Effect of hibiscus anthocyanins-rich extract induces apoptosis of proliferating smooth muscle cell via activation of P38 MAPK and p53 pathway. Mol Nutr Food Res 2007;51:1452–1460 [DOI] [PubMed] [Google Scholar]
- 39.Nizamutdinova IT, Jin YC, Chung JI, Shin SC, Lee SJ, et al. : The anti-diabetic effect of anthocyanins in streptozotocininduced diabetic rats through glucose transporter 4 regulation and prevention of insulin resistance and pancreatic apoptosis. Mol Nutr Food Res 2009;53:1419–1429 [DOI] [PubMed] [Google Scholar]
- 40.Nassiri-Asl M, Hosseinzadeh H: Review of the pharmacological effects of Vitis vinifera (grape) and its bioactive compounds. Phytother Res 2009;23:1197–1204 [DOI] [PubMed] [Google Scholar]
- 41.Todd JJ, Vodkin LO: Pigmented soybean (Glycine max) seed coats accumulate proanthocyanidins during development. Plant Physiol 1993;102:663–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Choung MG, Baek IY, Kang ST, Han WY, Shin DC, Moon HP, Kang KH: Isolation and determination of anthocyanins in seed coats of black soybean (Glycine max (L.) Merr.). J Agric Food Chem 2001;49:5848–5851 [DOI] [PubMed] [Google Scholar]
- 43.Spiegelman BM, Flier JS: Adipogenesis and obesity: rounding out the big picture. Cell 1996;87:377–389 [DOI] [PubMed] [Google Scholar]
- 44.Mandrup S, Lane MD: Regulating adipogenesis. J Biol Chem 1997;272:5367–5370 [DOI] [PubMed] [Google Scholar]
- 45.McNamara JR, Cohn JS, Wilson PW, Schaefer EJ: Calculated values for low-density lipoprotein cholesterol in the assessment of lipid abnormalities and coronary disease risk. Clin Chem 1990;36:36–42 [PubMed] [Google Scholar]
- 46.Remesar X, Fernández-López JA, Blay MT, Savall P, Salas A, Díaz-Silva M, Esteve M, Grasa MM, Alemany M: Effect of oral oleoyl-estrone on adipose tissue composition in male rats. Int J Obes Relat Metab Disord 2002;26:1092–1102 [DOI] [PubMed] [Google Scholar]
- 47.Laye MJ, Thyfault JP, Stump CS, Booth FW: Inactivity induces increases in abdominal fat. J Appl Physiol 2007;102:1341–1347 [DOI] [PubMed] [Google Scholar]
- 48.Kim J, Kyung J, Kim D, Choi EK, Bang P, Park D, Kim YB: Anti-obesity effects of Rapha diet® preparation in mice fed a high-fat diet. Lab Anim Res 2012;28:265–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bennett JW, Klich M: Mycotoxins. Clin Microbiol Rev 2003;16:497–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee JH, Jun HJ, Jia Y, Kim W, Choi SG, Lee SJ: Critical role of peroxisome proliferator activated receptor-δ on body fat reduction in C57BL/6J and human apolipoprotein E2 transgenic mice fed delipidated soybean. J Agric Food Chem 2011;59:11872–11881 [DOI] [PubMed] [Google Scholar]
- 51.Carrara VS, Amato AA, Neves FA, Bazotte RB, Mandarino JM, Nakamura CV, Filho BP, Cortez DA: Effects of a methanolic fraction of soybean seeds on the transcriptional activity of peroxisome proliferator-activated receptors (PPAR). Braz J Med Biol Res 2009;42:545–550 [DOI] [PubMed] [Google Scholar]
- 52.Noriega-López L, Tovar AR, Gonzalez-Granillo M, Hernández-Pando R, Escalante B, Santillán-Doherty P, Torres N: Pancreatic insulin secretion in rats fed a soy protein high fat diet depends on the interaction between the amino acid pattern and isoflavones. J Biol Chem 2007;282:20657–20666 [DOI] [PubMed] [Google Scholar]
- 53.Saladin R, De Vos P, Guerre-Millo M, Leturque A, Girard J, Staels B, Auwerx J: Transient increase in obese gene expression after food intake or insulin administration. Nature 1995;377:527–529 [DOI] [PubMed] [Google Scholar]
- 54.Clément K, Vaisse C, Lahlou N, Cabrol S, Pelloux V, Cassuto D, Gourmelen M, Dina C, Chambaz J, Lacorte JM, Basdevant A, Bougnères P, Lebouc Y, Froguel P, Guy-Grand B: A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature 1998;392:398–401 [DOI] [PubMed] [Google Scholar]
- 55.Davaatseren M, Hur HJ, Yang HJ, Hwang JT, Park JH, Kim HJ, Kim MJ, Kwon DY, Sung MJ: Taraxacum official (dandelion) leaf extract alleviates high-fat diet-induced nonalcoholic fatty liver. Food Chem Toxicol 2013;58:30–36 [DOI] [PubMed] [Google Scholar]


