Abstract
This study aims at evaluating the anticancer effects of berberine hydrochloride (berberine) and d-limonene, alone and in combination, on human gastric carcinoma cell line MGC803 to determine whether berberine and d-limonene work synergistically and elucidate their mechanisms. MGC803 cells were treated with berberine and d-limonene, alone and in combination, for 24–48 h. The inhibitory effects of these drugs on growth were determined by MTT assay. The combination index and drug reduction index were calculated with the Chou–Talalay method based on the median-effect principle. Flow cytometry and laser scanning confocal microscopy were employed to evaluate the effects of both drugs on cell-cycle perturbation and apoptosis, generation of reactive oxygen species (ROS), mitochondrial membrane potential, and expression of Bcl-2 and caspase-3 in MGC803 cells. Berberine or d-limonene alone can inhibit the growth of MGC803 cells in a dose- and time-dependent manner. Berberine and d-limonene at a combination ratio of 1:4 exhibited a synergistic effect on anti-MGC803 cells. The two drugs distinctly induced intracellular ROS generation, reduced the mitochondrial transmembrane potential (ΔΨm), enhanced the expression of caspase-3, and decreased the expression of Bcl-2. The combination of berberine and d-limonene showed more remarkable effects compared with drugs used singly in MGC803 cells. The combination of berberine and d-limonene exerted synergistic anticancer effects on MGC803 cells by cell-cycle arrest, ROS production, and apoptosis induction through the mitochondria-mediated intrinsic pathway.
Key Words: : anticancer, apoptosis, berberine, d-limonene, synergistic effect
Introduction
Cancer development involves multiple factors and genes. Combination therapy using multiple drugs is a common practice in the treatment of cancer to achieve higher efficacy and potency, with reduced toxicity. However, considering the explosive increase of drug combination quantities, selection of the appropriate drugs to be combined remains a great challenge.1,2 Fortunately, traditional Chinese medicine (TCM), which features the combinational use of herbs and has accumulated more than 100,000 formulae in the past 3000 years, provides important clues for identifying and developing synergistic drugs.3,4 A large number of TCM formulae contain Western drugs, and some components of Western drugs are structurally similar to TCM components. For instance, the Zuojinwan formula, containing Coptidis rhizoma and Evodia rutaecarpa and recorded in the book Danxixinfa, has been used for the treatment of gastric conditions for more than 700 years. The combined extracts of these herbs have been suggested to exhibit antitumor effects on rat subjects.5 Twelve and 45 components have been identified in C. rhizoma and E. rutaecarpa, respectively, through the TCM Database. Some Western counterparts (with structural similarity >85%) were obtained by structural comparison from the Comprehensive Medicinal Chemistry (CMC) database. Some of the obtained CMC drugs are annotated as antineoplastic (limonene), or related to antitumor treatments (berberine and others). Berberine, a component of C. rhizoma, has recently drawn a great deal of attention because of its antineoplastic and antitumor effects on numerous cancer cell lines. The underlying mechanisms of berberine involve cell-cycle arrest, apoptosis induction, and anti-inflammation.6 d-Limonene, a component of E. rutaecarpa, has been found to inhibit the growth of various cancer cells without toxicity by apoptosis induction and reactive oxygen species (ROS) generation,7 and has chemopreventive and chemotherapeutic activities against rodent mammary, liver, and pancreatic tumors.8–10 Thus, the evaluation of the anticancer effects of the combination of berberine and d-limonene would provide interesting results.
In the current study, we have evaluated the in vitro antitumor potential of berberine combined with d-limonene in MGC803 cells, with special emphasis on the mitochondrial pathway of apoptosis. We have also provided data to support our hypothesis that a combination treatment has greater antiproliferative and pro-apoptotic activities in vitro.
Materials and Methods
Cells and reagents
The human gastric carcinoma cell line MGC803 was purchased from China Center for Type Culture Collection. The cells were grown in RPMI-1640 medium (Gibco) supplemented with 10% newborn bovine serum in a humidified 5% CO2 incubator at 37°C. Berberine (purity ≥99%) was purchased from Shenzhen Medherb Biotechnology Co., Ltd., whereas d-limonene and cisplatin (purity ≥97%) were obtained from Sigma-Aldrich Co.
Cell viability assay
Cell viability was determined by 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay (Sigma-Aldrich Co).11 The cells were seeded onto 96-well plates (1×105 cell/mL) at 100 μL/well, and then incubated overnight. The cells were treated with berberine and d-limonene, alone and in combination, for 24 and 48 h. Cisplatin was used as a positive control. Approximately 20 μL of the 5 mg/mL MTT solution was added to each well, and the plates were then incubated again for 4 h at 37°C. Subsequently, the media were removed, and 200 μL of dissolved solution (containing 10% sodium dodecyl sulfate, 5% isobutanol, and 0.012 M HCl) was added to each well. Absorbance at 570 nm was detected using a Thermo Multiskan MK3 (Thermo Fisher), with 630 nm as a reference wavelength. All samples were tested in six replicates, and the experiments were repeated at least thrice.
Synergy determination
The interaction between berberine and d-limonene was analyzed according the median-effect principle proposed by Chou and Talalay.12,13 The median effect equation is expressed as follows:
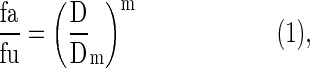 |
where D is the dose, fa and fu are the fractions affected and unaffected by the dose D, respectively, Dm is the dose that produces the median effect (inhibition by 50%), and m represents the shape of the dose-effect curve. Considering the logarithmic form of Equation (1), we derived the following equation:
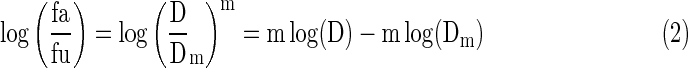 |
Equation (2) enables the calculation of m, Dm, and D (the dose required by a given effect produced by berberine and d-limonene alone or in combination). According to Chou and Talalay's method,12 the combination index (CI) between berberine and d-limonene can be valued by the following equation:
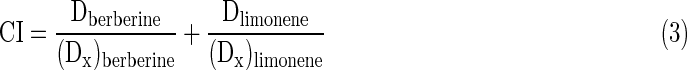 |
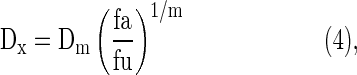 |
where CI smaller than 1, equal to 1, and greater than 1 indicates synergism, additive effect, and antagonism, respectively. (Dx)berberine and (Dx)limonene are the doses of berberine alone and d-limonene alone, providing x% growth inhibition, and Dberberine and Dlimonene are the concentrations of the combination of the two drugs with the same efficacy (x% inhibition). Dx can be calculated using Equation (4).
Chou used the dose-reduction index (DRI) to measure the number of reduction folds that may be produced in the dose by the synergistic combination of the two drugs at a given effect level compared with the doses of each drug alone.13 The DRI of berberine and d-limonene can be calculated using the following equations:
 |
 |
Nuclear morphology
MGC803 cells (1×105/mL) were seeded on cover slips and cultured with 20 μM of berberine or 80 μM of d-limonene, alone and in combination, for 48 h, using 20 μM of casplatin as a positive control. The cells were then washed with phosphate-buffered saline (PBS), fixed in 95% ethanol for 5 min, and stained with 0.01% acridine orange (AO) for 5 min. Nuclear morphology of apoptotic cells with condense/fragmented nuclei was observed under a fluorescent microscope.
Cell cycle and cell apoptosis by flow cytometry
Cell cycle and apoptosis analyses were performed by flow cytometry. The cells were seeded onto six-well plates and cultured with and without berberine and d-limonene for 24, 36, and 48 h. After the treatment, the floating and the attached cells in the medium were collected. The cells were then washed with cold PBS and fixed with 70% ethanol at 4°C overnight. Afterward, the cells were incubated with RNAase (100 μL, 5 mg/mL) for 30 min at 37°C, and stained with propidium iodide (100 μg/mL) for 30 min in the dark. Finally, the cells were analyzed by an FACSCalibur flow cytometer with the CellQuest and Modifit software (Becton Dickinson).
Determination of ROS generation
The oxidation-sensitive fluorescent probe 2′,7′-dichlorofluorescin diacetate (DCFH-DA) was used to assess the generation of intracellular ROS. Briefly, the cells (1×105/mL) were seeded on cover slips and cultured with 20 μM of berberine or 80 μM of d-limonene, alone and in combination, for 24 and 48 h. The cells were then washed with PBS and incubated with DCFH-DA (100 μL, 10 μM) for 50 min in the dark. DCFH-DA was deacetylated and became nonfluorescent DCFH after being collected by the cells. DCFH can be converted to green fluorescent DCF by intracellular ROS. The fluorescent images were recorded by a confocal microscope (Leica TCS SP2) with 100×objective and excitation and emission settings of 488 and 530 nm, respectively. The fluorescence intensity (FI) of 50 cells from each sample was quantified with the Leica software and Image J software.
Determination of mitochondrial transmembrane potential
The changes in the mitochondrial membrane potential were measured with rhodamine 123 (Rh 123) by confocal scanning microscopy.14 The cells (1×105/mL) were seeded on cover slips and cultured with 20 μM of berberine or 80 μM of d-limonene, alone and in combination, for 24 and 48 h. The cells were then washed with PBS and incubated with Rh 123 (100 μL, 10 μM) for 30 min at 37°C. The fluorescent images were recorded by a confocal microscope (Leica TCS SP2) with 100×objective and excitation and emission settings of 505 and 520 nm, respectively. The FI of 50 cells from each sample was quantified with the Leica software and Image J software.
Immunofluorescence
The cells (1×105/mL) were seeded on cover slips and cultured with 20 μM of berberine or 80 μM of d-limonene, alone and in combination, for 24 and 48 h. After the treatment, the cells were washed with PBS and fixed with paraformaldehyde for 30 min. The fixed cells were permeabilized with 0.1% Triton X-100 in PBS and incubated with anti-Bcl-2 (1:200) and anti-Caspase-3 antibody (1:100) (Santa Cruz Biotechnology) at 4°C overnight. The proteins were then visualized on a confocal microscope after incubation, with fluorescein isothiocyanate (FITC)-conjugated secondary anti-mouse IgG antibody (Santa Cruz Biotechnology). The FI of 50 cells from each sample was quantified with the Leica software and Image J software.
Statistical analysis
All data are presented as means±standard deviation from more than three independent experiments. R 2.10.1 was used to analyze the data, and P<.05 was considered statistically significant.
Results
Cytotoxicity assay
The dose-effect relationship of berberine and d-limonene, alone and in combination, was determined on the growth of MGC803 cells after exposure for 24 and 48 h (Fig. 1). The median-effect analysis was carried out according to Chou's method.13
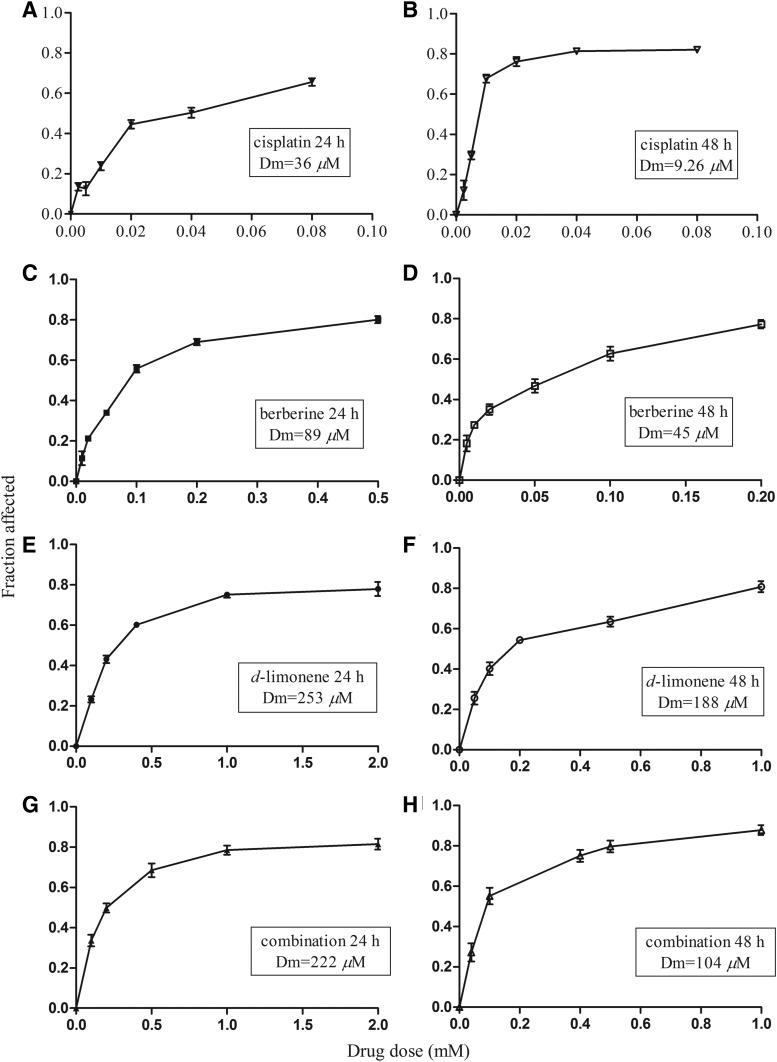
FIG. 1.
Cytotoxicity of berberine and d-limonene, alone and in combination, on the growth of MGC803 cells. MGC803 cells were treated with berberine (C, D) and d-limonene (E, F), alone and in combination (G, H), at 1: 4, for 24 (C, E, G) and 48 h (D, F, H), with cisplatin (A for 24 h, B for 48 h) as a positive control. The viability of cells was qualified by MTT assay, reported as fraction affected (fraction of cells affected by treatment), and data represent the mean±SD from three replicate wells. P<.01, compared with the control group.
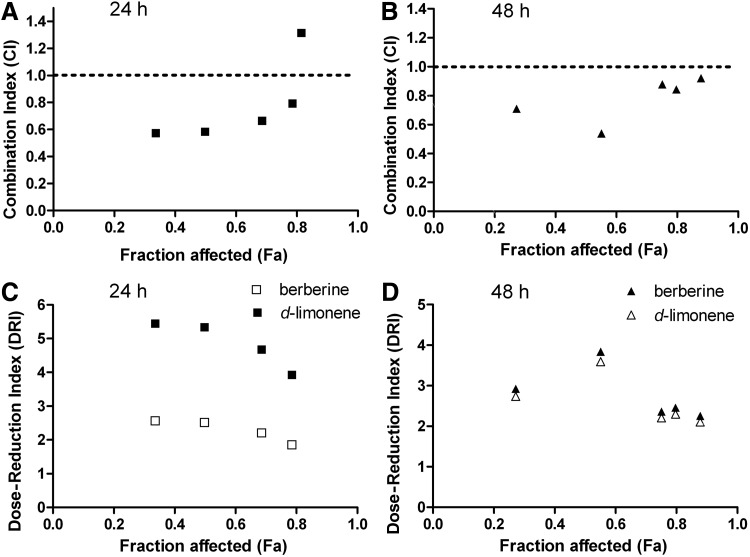
Berberine showed dose-dependent cytotoxic effects in MGC803 cells, with Dm (IC50) values of 89 μM at 24 h and 45 μM at 48 h, and good r values of 0.995 and 0.988, respectively (P<.01). Similarly, d-limonene also showed dose-dependent cytotoxic effects in MGC803 cells, with Dm (IC50) values of 253 μM at 24 h and 182 μM at 48 h, and good r values of 0.990 and 0.988, respectively (P<.01). The r value is the linear correlation coefficient of the median-effect plot and usually larger than 0.95 in vitro experiments.13 Since the Chou–Talalay model requires drugs to be used at a fixed dose ratio such as the ratio of (IC50)1/(IC50)2, we chose to use berberine and d-limonene at a fixed ratio (1:4) based on the ratio of their IC50 values at 48h. CIs were calculated to determine the antitumor effects of the combinations; the plots of CI versus fraction affected (Fa) are shown in Figure 2A and B. The combination of berberine and d-limonene exhibited a synergistic effect at 33–80 inhibition levels, where the combination doses were 0.1–1.0 mM at 24 h. The combination of the two drugs produced an antagonistic effect at a higher inhibition level, where the combination dose was 2.0 mM (CI=1.316). At 48 h, the combination treatment showed a synergistic effect when the combination dose was 40–100 μM (CI<1) and produced a nearly additive effect when the combination dose approached 200 μM (CI=0.92). The DRI values were also estimated to determine the drug dose reduction in the combination compared with the drugs alone at the given inhibition level. As shown in Figure 2C and D, the DRI values of berberine ranged from 1.8 to 3.8, whereas those of d-limonene ranged from 2.1 to 5.4. The results of the MTT assay suggested that both berberine and d-limonene can inhibit the growth of MGC803 cells. Berberine and d-limonene at the combination ratio of 1:4 exhibited a synergistic, cytotoxic effect in anti-MGC803 cells.
FIG. 2.
Synergistic anticancer effect of the combination of berberine and d-limonene in MGC803 cells. The combination index (CI) (A, B) and dose-reduction index (DRI) (C, D) of the combination of berberine and d-limonene were calculated as described. The data points below CI values of 1, denoted by horizontal dotted lines in (A) and (B), are indicative of synergistic effects.
Effect of berberine and d-limonene on the cell-cycle control of MGC803 cells
We analyzed the cell-cycle distribution using the flow cytometry technique to investigate whether the drugs have a regulatory role in cell cycle. As shown in Table 1, the percentage of G1 cells was higher in the MGC803 cells treated with berberine for 24, 36, and 48 h (P<.05) compared with the control. However, the combination of the drugs induced a remarkable accumulation of cells at the G1 and G2/M phases at 36 and 48 h; the number of cells at the S phase was distinctly inhibited (P<.05).
Table 1.
Cell-Cycle Distribution Induced by Berberine and d-Limonene Alone and in Combination in MGC803 Cells
| Cell cycle (%) | ||||
|---|---|---|---|---|
| Treatment | Time (h) | G0/G1 | S | G2/M |
| Untreated control | 0 | 41.64±2.25 | 32.38±1.18 | 25.98±3.43 |
| Berberine | 24 | 48.61±0.91a | 28.3±1.48a | 23.09±2.40 |
| 36 | 47.69±0.92a | 27.77±1.38a | 24.54±2.31 | |
| 48 | 46.49±2.34a | 27.88±0.27a | 25.63±2.22 | |
| d-Limonene | 24 | 46.71±0.70a | 27.22±0.11a | 26.07±0.81 |
| 36 | 35.95±4.44a | 27.92±3.09a | 33.13±2.88a | |
| 48 | 58.81±4.15a | 12.84±0.78a | 28.36±3.36a | |
| Berberine+d-limonene | 24 | 66.58±0.92abc | 25.94±2.37a | 8.38±1.53abc |
| 36 | 49.96±1.36ac | 0abc | 50.04±1.36abc | |
| 48 | 62.19±1.49ab | 0.11±0.19abc | 37.7±1.31abc | |
Significantly different compared with the control group.
Significantly different compared with berberine group.
Significantly different compared with d-limonene group, P<.05.
Induction of apoptosis in MGC803 cells by berberine and d-limonene
Nuclear morphology of apoptotic cells with condense/fragmented nuclei was shown in Figure 3 by AO staining. The apoptosis induced by berberine and d-limonene was determined through flow cytometry to further investigate the decreased viability of the MGC803 cells. Figure 3F shows that berberine and d-limonene alone can increase the proportion of apoptosis cells after treatment for 36 and 48 h (P<.05). Compared with the effect of berberine or d-limonene alone, the apoptosis rate of the combination of the two compounds was much higher (29.03% vs. 12.2% or 19.6%, P<.05), indicating that the apoptotic effect was increased by the combination of the drugs.
FIG. 3.

Induction apoptosis of berberine and d-limonene, alone and in combination, in MGC803 cells. Morphological changes were shown by acridine orange after drug treatment for 48 h: (A) control, (B) 20 μM cisplatin, (C) 20 μM berberine, (D) 80 μM d-limonene, and (E) 20 μM berberine+80 μM d-limonene. The apoptosis induced by berberine and d-limonene at 24, 36, and 48 h was determined by flow cytometry (F). Data are mean±SD values. P<.05, asignificantly different compared with the control group; bsignificantly different compared with berberine group; csignificantly different compared with d-limonene group. The bar is 25 μm. Color images available online at www.liebertpub.com/jmf
Effect of berberine and d-limonene on intracellular ROS generation
We measured the intracellular ROS generation using the fluorescent probe DCFH-DA to investigate whether ROS is involved in mediating apoptosis induced by berberine and d-limonene. Treatment with each drug alone or in combination enhanced the ROS generation in MGC803 cells compared with the untreated cells (P<.05). The order of ROS generation at 24 h was combination (the mean Fl=241.70)>d-limonene (the mean Fl=168.44)>berberine (the mean Fl=101.62)>control (the mean Fl=32.34) (Fig. 4). Interestingly, the ROS generation at 48 h decreased compared with that at 24 h (P<.05), although both were remarkably higher than the control.
FIG. 4.
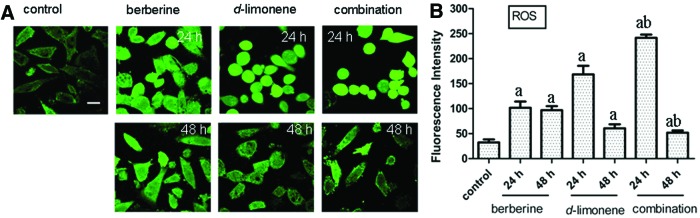
Induction of intracellular reactive oxygen species (ROS) generation in MGC803 cells after treatment with berberine and d-limonene, alone and in combination, for 24 and 48 h. Monitoring of the ROS production with DCF (A) in MGC803 cells; fluorescence intensity (FI) was measured by laser scanning confocal microscopy. Data presented as mean±SD values of three independent experiments (B). P<.05, asignificantly different compared with the control group; bsignificantly different compared with the single drug treatments. The bar is 15 μm. Color images available online at www.liebertpub.com/jmf
Effect of berberine and d-limonene on the mitochondrial transmembrane potential (ΔΨm)
The changes in ΔΨm have been linked to apoptosis; thus, we examined the influence of berberine and d-limonene, alone and in combination, on the ΔΨm of the MGC803 cells with Rh 123. Rh 123 is a mitochondria-binding specific cationic fluorescent dye that is proportional to the ΔΨm. The result showed a remarkable loss of ΔΨm when the MGC803 cells were exposed to berberine and d-limonene, alone or in combination, for 24 and 48 h, as revealed by the reduction of Rh 123 FI compared with the control (P<.05) (Fig. 5). The combination treatment showed a sharp decline of Rh 123 FI to 51.12 compared with the control (121.43), or berberine (78.22) and d-limonene (71.46) alone after treatment for 48 h.
FIG. 5.
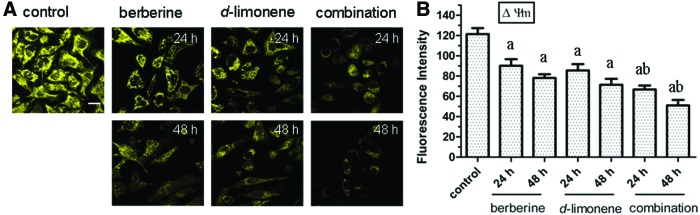
Loss of mitochondrial membrane potential (ΔΨm) in MGC803 cells after treatment with berberine and d-limonene, alone and in combination, for 24 and 48 h. Detection of ΔΨm changes with Rhodamine 123 (A) in MGC803 cells; FI was measured by laser scanning confocal microscopy. Data presented as mean±SD values of three independent experiments (B). P<.05, asignificantly different compared with the control group; bsignificantly different compared with the single drug treatments. The bar is 15 μm. Color images available online at www.liebertpub.com/jmf
Effect of berberine and d-limonene on the expression of Bcl-2
Bcl-2 is an inner mitochondrial membrane protein with an important role in preventing apoptosis. Thus, we examined the effect of berberine and d-limonene on Bcl-2 expression in the MGC803 cells. Compared with the control, the Bcl-2 protein expression decreased in a time-dependent manner in the MGC803 cells treated with berberine and d-limonene, alone and in combination, for 24 and 48 h (P<.05) (Fig. 6). The Bcl-2 expression in the cells treated with the combination of the drugs was lower than in those treated with the drugs alone (P<.05).
FIG. 6.
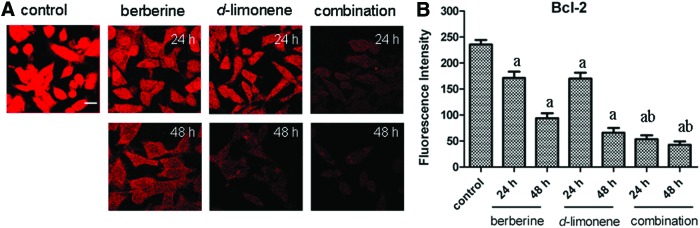
The changes of Bcl-2 protein expression in MGC803 cells after treatment with berberine and d-limonene, alone and in combination, for 24 and 48 h. The expression of Bcl-2 in MGC803 cells was detected by the FITC-conjugated secondary antibody (A), and FI was measured by laser scanning confocal microscopy. Data presented as mean±SD values of three independent experiments (B). P<.05, asignificantly different compared with the control group; bsignificantly different compared with the single drug treatments. The bar is 15 μm. Color images available online at www.liebertpub.com/jmf
Effect of berberine and d-limonene on the caspase-3 expression
Caspase-3 has been implicated as a key mediator of apoptosis, and it is required to trigger DNA fragmentation in apoptosis. The effect of berberine and d-limonene on the expression of caspase-3 was revealed through immunofluorescence (Fig. 7). After the incubation of the MGC803 cells with berberine and d-limonene, alone and in combination, for 24 and 48 h, the caspase-3 protein expression increased in a time-dependent manner compared with the control (P<.05). The caspase-3 expression in the cells treated with the combination of the drugs was higher than in those treated with the drugs alone (P<.05).
FIG. 7.
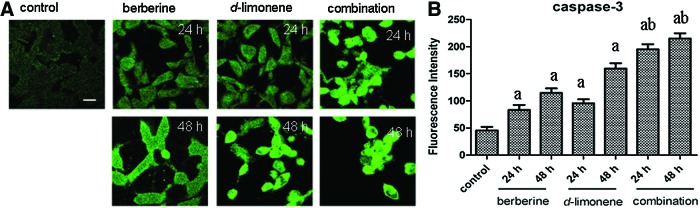
The changes in caspase-3 protein expression in MGC803 cells after treatment with berberine and d-limonene, alone and in combination, for 24 and 48 h. The expression of caspase-3 (A) in MGC803 cells was detected by the FITC-conjugated secondary antibody, and FI was measured by laser scanning confocal microscopy. Data presented as mean±SD values of three independent experiments (B). P<.05, asignificantly different compared with the control group; bsignificantly different compared with the single drug treatments. The bar is 15 μm. Color images available online at www.liebertpub.com/jmf
Discussion
In this study, we demonstrated that berberine and d-limonene exhibit cytotoxic effects on human gastric carcinoma (MGC803) cells in a dose- and time-dependent manner. Berberine was more potent than d-limonene in inhibiting the growth of MGC803 cells. Furthermore, the combination of berberine (8–200 μM) and d-limonene (1:4 ratio) exerted significant synergistic, cytotoxic effects on MGC803 cells in a dose- and time-dependent manner.
Berberine has been shown to produce anti-tumor activities against a wide spectrum of cancer cells6,15 with a relatively low IC50; for example, the IC50 for human gastric carcinoma SNU-5 cells is 48 μM.16 Our results showed that the IC50 of berberine in human gastric carcinoma cells after 48 h is 45 μM, consistent with previous reports. Berberine displays minimal toxicity in normal cells; it exhibits minimum toxicity in normal cells at 200 μM.17 d-Limonene has chemopreventive and chemotherapeutic activities without toxicity in several kinds of rodent tumor subjects.18 Moreover, d-limonene can promote the antitumor activity of other reagents, such as 4-hydroxyandrostenedione and docetaxel, in the combination therapy.7,19 The results of this study showed that d-limonene sensitizes berberine-induced cytotoxicity in human gastric cancer cells and reduces the dose of berberine by twofold.
The combined beneficial effect can be achieved partly through cell-cycle arrest, ROS production, and apoptosis induction in the mitochondria-mediated intrinsic pathway. Mitochondria have been considered targets of anticancer drugs and have a key function in the regulation of apoptosis.20
Berberine induces cell-cycle arrest at the G0/G1, G1, and/or G2/M phases in different cancer cells.6 Our results indicated that berberine caused G1 arrest in MGC803 cells, and that the combination of berberine and d-limonene led to an accumulation of cells in the G1 and G2/M phases at the expense of the S phase. These findings suggested that berberine and d-limonene may be useful for controlling cancer cell growth, because several cancer cells have defects in the cell-cycle checkpoints.
Mitochondria are the main production sites of ROS. The presence of excessive ROS in the mitochondria leads to the opening of the mitochondrial permeability transition pore, decline in ΔΨm, and release of cytochrome c, which, in turn, initiate caspase activation and trigger apoptosis.21 Several studies have provided evidence that berberine and d-limonene exhibit antitumor activity by ROS generation, caspase-3 activation, and apoptosis induction.7,22,23 Our results demonstrated that the incubation of MGC803 cells with berberine alone, d-limonene alone, and the combination of the two compounds increased the ROS generation, decreased ΔΨm, upregulated caspase-3 expression, downregulated the anti-apoptosis protein Bcl-2 expression, and induced the mitochondria-mediated apoptosis.
In conclusion, the findings presented in this study demonstrated, for the first time, that berberine and d-limonene synergistically inhibited the growth of MGC803 cells in vitro. This inhibition can be achieved partly by the generation of ROS and the induction of caspase-mediated apoptosis. Additional studies are needed to elucidate the mechanistic interactions of combinatorial drugs. Moreover, the idea of combining TCM active components should be given more focus, because it holds great potential in treating tumors with minimal side effects.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No. 31100987), Department of Education (No. 12JYC820084) Shandong University of Technology “4040-306018,” “4041-406022,” and projects for the development of young teachers.
Author Disclosure Statement
All the authors have no potential conflicts of interest to declare.
References
- 1.Morphy R, Kay C, Rankovic Z: From magic bullets to designed multiple ligands. Drug Discov Today 2004;9:641–651 [DOI] [PubMed] [Google Scholar]
- 2.Morphy R, Rankovic Z: Designed multiple ligands. An emerging drug discovery paradigm. J Med Chem 2005;48:6523–6543 [DOI] [PubMed] [Google Scholar]
- 3.Li XJ, Zhang HY: Synergy in natural medicines: implications for drug discovery. Trends Pharmacol Sci 2008;29:331–332 [DOI] [PubMed] [Google Scholar]
- 4.Wang L, Zhou GB, Liu P, et al. : Dissection of mechanisms of Chinese medicinal formula Realgar-Indigo naturalis as an effective treatment for promyelocytic leukemia. Proc Natl Acad Sci USA 2008;105:4826–4831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qiu J: Traditional medicine: a culture in the balance. Nature 2007;448:126–128 [DOI] [PubMed] [Google Scholar]
- 6.Tang J, Feng Y, Tsao S, et al. : Berberine and Coptidis rhizoma as novel antineoplastic agents: a review of traditional use and biomedical investigations. J Ethnopharmacol 2009;126:5–17 [DOI] [PubMed] [Google Scholar]
- 7.Rabi T, Bishayee A: d-Limonene sensitizes docetaxel-induced cytotoxicity in human prostate cancer cells: generation of reactive oxygen species and induction of apoptosis. J Carcinog 2009;8:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elegbede JA, Elson CE, Qureshi A, et al. : Inhibition of DMBA-induced mammary cancer by the monoterpene d-limonene. Carcinogenesis 1984;5:661–664 [DOI] [PubMed] [Google Scholar]
- 9.Nakaizumi A, Baba M, Uehara H: d-Limonene inhibits N-nitrosobis(2-oxopropyl)amine induced hamster pancreatic carcinogenesis. Cancer Lett 1997;117:99–103 [DOI] [PubMed] [Google Scholar]
- 10.Kaji I, Tatsuta M, Iishi H, et al. : Inhibition by d-limonene of experimental hepatocarcinogenesis in Sprague-Dawley rats does not involve p21(ras) plasma membrane association. Int J Cancer 2001;93:441–444 [DOI] [PubMed] [Google Scholar]
- 11.Mosmann T: Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983;65:55–63 [DOI] [PubMed] [Google Scholar]
- 12.Chou TC, Talalay P: Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul 1984;22:27–55 [DOI] [PubMed] [Google Scholar]
- 13.Chou TC: Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev 2006;58:621–681 [DOI] [PubMed] [Google Scholar]
- 14.Scaduto RC, Jr, Grotyohann LW: Measurement of mitochondrial membrane potential using fluorescent rhodamine derivatives. Biophys J 1999;76:469–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun Y, Xun K, Wang Y, et al. : A systematic review of the anticancer properties of berberine, a natural product from Chinese herbs. Anticancer Drugs 2009;20:757–769 [DOI] [PubMed] [Google Scholar]
- 16.Lin JP, Yang JS, Lee JH, et al. : Berberine induces cell cycle arrest and apoptosis in human gastric carcinoma SNU-5 cell line. World J Gastroenterol 2006;12:21–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chidambara Murthy KN, Jayaprakasha GK, Patil BS: The natural alkaloid berberine targets multiple pathways to induce cell death in cultured human colon cancer cells. Eur J Pharmacol 2012;688:14–21 [DOI] [PubMed] [Google Scholar]
- 18.Crowell PL: Prevention and therapy of cancer by dietary monoterpenes. J Nutr 1999;129:775S–778S [DOI] [PubMed] [Google Scholar]
- 19.Chander SK, Lansdown AG, Luqmani YA, et al. : Effectiveness of combined limonene and 4-hydroxyandrostenedione in the treatment of NMU-induced rat mammary tumours. Br J Cancer 1994;69:879–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hockenbery DM: Targeting mitochondria for cancer therapy. Environ Mol Mutagen 2010;51:476–489 [DOI] [PubMed] [Google Scholar]
- 21.Wang X: The expanding role of mitochondria in apoptosis. Genes Dev 2001;15:2922–2933 [PubMed] [Google Scholar]
- 22.Meeran SM, Katiyar S, Katiyar SK: Berberine-induced apoptosis in human prostate cancer cells is initiated by reactive oxygen species generation. Toxicol Appl Pharmacol 2008;229:33–43 [DOI] [PubMed] [Google Scholar]
- 23.Inoue K, Kulsum U, Chowdhury SA, et al. : Tumor-specific cytotoxicity and apoptosis-inducing activity of berberines. Anticancer Res 2005;25:4053–4059 [PubMed] [Google Scholar]