Abstract
Advanced biomaterials and sophisticated processing technologies aim at fabricating tissue-engineering scaffolds that can predictably interact within a biological environment at the cellular level. Sterilization of such scaffolds is at the core of patient safety and is an important regulatory issue that needs to be addressed before clinical translation. In addition, it is crucial that meticulously engineered micro- and nano- structures are preserved after sterilization. Conventional sterilization methods involving heat, steam, and radiation are not compatible with engineered polymeric systems because of scaffold degradation and loss of architecture. Using electrospun scaffolds made from polycaprolactone, a low melting polymer, and employing spores of Bacillus atrophaeus as biological indicators, we compared ethylene oxide, autoclaving and 80% ethanol to a known chemical sterilant, peracetic acid (PAA), for their ability to sterilize as well as their effects on scaffold properties. PAA diluted in 20% ethanol to 1000 ppm or above sterilized electrospun scaffolds in 15 min at room temperature while maintaining nano-architecture and mechanical properties. Scaffolds treated with PAA at 5000 ppm were rendered hydrophilic, with contact angles reduced to 0°. Therefore, PAA can provide economical, rapid, and effective sterilization of heat-sensitive polymeric electrospun scaffolds that are used in tissue engineering.
Introduction
Tissue engineering is a rapidly evolving field that aims at developing functional tissue substitutes by integrating advanced engineering principles and improved understanding of cell behavior. The ultimate goal of tissue engineering and regenerative medicine is to improve the quality of life in patients by promoting true regeneration of structure and function of tissue compromised by disease or surgery.1 Scaffold-based tissue engineering is a popular strategy that involves the seeding and culture of specific cell types in an environment which mimics the native extracellular matrix (ECM). The ideal ECM analogs are engineered to be three-dimensional (3D) instructional matrices that possess the physical, chemical, and biological cues to promote tissue repair and regeneration2
Metals and alloys, ceramics and polymers, either alone or in combination, have been traditionally used to rehabilitate patients with failing or removed organs. While metals and ceramics are inherently strong and may possess favorable mechanical properties for orthopedic applications, they are designed to be nondegradable and possess limited processability. Polymers are being increasingly used in tissue engineering, because they are biocompatible, can be rendered biodegradable (by imparting appropriate chemistry), do not elicit host immune reactions (unlike natural polymers), and can be mass produced with little batch-to-batch variability. In addition, their composition, structure, mechanical properties, and degradation rates can be tailored to suit specific needs.3 Polycaprolactone (PCL) is a synthetic polymer, placed on the FDA's generally regarded as safe list, exhibits excellent biocompatibility, complete degradation in vivo, and has been approved for drug delivery and medical devices applications. PCL has been successfully used as micro- and nano-spheres in controlled drug delivery systems4–6; in sutures as a co-polymer with glycolide (Monacryl® by Ethicon); as a root canal filler7; and in tissue-engineering applications.8,9
Among different techniques available to generate 3D porous polymeric scaffolds, electrospinning is a versatile technique that consistently reproduces the sub-micron fibrous morphology of the native ECM. The process involves dissolving a biodegradable polymer in an organic solvent at high concentrations and subjecting this viscous solution to high voltage (tens of kilovolts). At a critical voltage, the electrostatic charge overcomes the surface tension of the polymer drop and polymer chains entangle to form a stable jet. As the charged jet travels toward a grounded target under the influence of electric field, the solvent evaporates and the fibers are collected as dry, fibrous, nonwoven mats. The scaffold composition, fiber diameters, and alignment can be readily controlled by the operator to tailor tissue-specific properties.10
Tissue-engineered products are devices intended to be in direct contact with living tissue and are regulated by the FDA for safety and efficacy. One of the fundamental requirements for such a device is the need to be completely sterile (and not merely disinfected) before implantation. The Center for Disease Control defines sterilization as a process that destroys or eliminates all forms of microbial life, while disinfection describes a process which eliminates many or all pathogenic microorganisms, except bacterial spores, on inanimate objects.11 Hence, it is imperative that an appropriate sterilization method is chosen to ensure safety as well as to maintain material properties and preserve engineered micro- and nano-scaled features of polymeric scaffolds. Current sterilization processes employed by the health care industry, including autoclave, gamma irradiation, and ethylene oxide (EtO), cannot be readily applied to tissue-engineered products because of the biomaterial involved, that is, synthetic polymers. Polyesters are the most widely used class of polymers because of their biocompatibility and biodegradability. However, being hydrolytically unstable, they cannot be subjected to moist heat (autoclaving); high-energy gamma radiation degrades polymeric backbone, reduces molecular weight, and alters degradation profiles.12 EtO alters scaffold properties by penetrating into polymeric networks and reacting with chemical groups.13
Given the translational nature of tissue-engineering research and constant innovation in polymer systems and their processing technologies, the issue of sterilization needs to be periodically revisited. In this study, we systematically explore the feasibility of using peracetic acid (PAA) as a chemical sterilant for polymeric tissue-engineered scaffolds and compared it with two accepted methods of sterilization (EtO and autoclaving) and a high-level disinfectant (80% ethanol). We included the latter because of its widespread use in tissue-engineering studies. We chose a widely used polymer, PCL, to represent polymers with low melting points and electrospun it to produce porous 3D scaffolds with defined nano-topography. Our aim was to identify the process conditions (concentration, contact time, and temperature) that are necessary to achieve sterility while maintaining scaffold integrity. Since spores are routinely used as biological indicators for sterilization,14 we inoculated electrospun scaffolds with spores of Bacillus atrophaeus and exposed them to different sterilization treatments. To our knowledge, this is the first study that evaluates the effectiveness of sterilization using spores as biological indicators in the context of electrospun polymeric scaffolds. In addition to assaying spore survival, the effects of sterilization processes on scaffold properties, including fiber morphology, permeability, hydrophilicity, and tensile modulus, were evaluated.
Materials and Methods
Electrospinning
PCL (Sigma; MW 80,000, melting point 59–64°C) was dissolved in 1,1,1,3,3,3-hexafluro-2-isopropanol (Oakwood Products) at a concentration of 100 mg/mL. Electrospinning apparatus (EC-DIG; IME Technologies) was used to generate nanofibers. Process conditions were optimized (rate: 7 mL/h, air-gap distance: 12.5 cm, applied needle voltage: +25 kV) to generate continuous nonwoven fibers that were collected onto a rotating cylindrical drum mandrel (100 mm diameter at 1000 rpm). After electrospinning, scaffolds were removed from the mandrel, dried in a fume hood for 30 min, and stored in an airtight desiccator until use. Electrospun PCL (e-PCL) scaffolds were cut using dermal biopsy punches (Acuderm) for use in experiments.
Characterization of B. atrophaeus spores
B. atrophaeus spores (ATCC #9372) were purchased as suspensions in 20% ethanol (108/mL) from Moog Medical Devices Group and stored at 4°C. A total of 106 spores were diluted in 1 mL of de-ionized water (DI water), and two subsequent (1:50) serial dilutions were plated on tryptic soy-agar plates using the EddyJet2 Spiral Plating System (NeuTec Group). The plates were then transferred to the incubator at 35°C and checked for colonies after 18 h.
Sterilization efficacy of PAA
PAA was purchased as a 39% solution (Sigma) in acetic acid and hydrogen peroxide. Different concentrations of PAA (100, 500, 1000, 2500, and 5000 ppm) were obtained by diluting the stock (390,000 ppm) in an appropriate volume of DI water. Initial experiments to identify the minimal effective concentration were performed by directly exposing the spore suspensions to different concentrations of PAA for 5 min at room temperature, plating these solutions on solid agar and evaluating colonies, as described in the section “Characterization of B. atrophaeus spores”. The absence of colonies is a more important parameter while assessing terminal sterilization, as their evidence represents a failure to achieve sterility. Thus, the actual numbers of colonies are irrelevant and were not recorded. For experiments involving scaffolds, a second diluent was introduced; in addition to DI water, the PAA was also diluted in 20% ethanol. This is because we observed significantly better wetting of spore solution (in 20% ethanol) on scaffolds than DI water.
Scaffold inoculation with B. atrophaeus spores and culture
Our goal was to inoculate 106 spores onto each 10 mm disc of e-PCL fabric. We observed poor loading of spores onto scaffolds when used as dilute solution (as described in the section “Characterization of B. atrophaeus spores”) and, hence, chose to inoculate without dilution (10 μL spore suspension). Circular discs, placed into 24-well microplates, were inoculated with 106 spores and allowed to dry for 30 min. These spore-laden scaffolds were then subjected to different methods of sterilization (described in the section “Sterilization treatment and efficacy testing of electrospun scaffolds”). At the end of the defined sterilization cycle, scaffolds were transferred into 5 mL of tryptic soy broth (TSB) and cultured in a mechanical shaker for 3 days at 35°C. Turbidity of the broth indicated bacterial growth and was interpreted as an indicator of inadequate sterilization.
Scanning electron microscopy
Air-dried electrospun scaffolds (before and after various sterilization protocols) were mounted on aluminum stubs using standard double-sided tape, sputter coated with gold, and examined at an accelerating voltage of 20 kV using a JEOL JSM 5610LV scanning electron microscope (SEM). Average fiber diameters were calculated from a total of 50 randomly selected fibers from corresponding SEM images using Image J (NIH).
Sterilization treatment and efficacy testing of electrospun scaffolds
Dry, spore-laden PCL scaffolds were subjected to six different sterilization regimens: EtO; autoclaving; 80% ethanol; 1000, 2500, and 5000 ppm of PAA. EtO sterilization was carried out at 50°C for 16 h (including aeration time), while autoclaving was performed at 121°C at 15 psi for 15 min. Scaffolds for these treatments were placed in self-sealing pouches (Henry-Schein) containing appropriate chemical indicators to verify that conditions for sterilization were met. Scaffolds for ethanol treatment were immersed in an 80% solution (in DI water) for 30 min and rinsed thrice with phosphate-buffered saline for 10 min per wash. Stock solution of PAA was diluted in either DI water or 20% ethanol solution (in DI water) to prepare different concentrations, and PCL scaffolds were incubated for 15 min at room temperature on an orbital shaker. The contact times was increased from 5 min (section “Sterilization efficacy of PAA”) to account for 3D nature of the scaffold and was allowed adequate time for PAA to infiltrate the porous network and to come in contact with the spores. Scaffolds treated with each sterilization regimen, as well as untreated controls, were then transferred into 5 mL of TSB and cultured in a mechanical shaker for 3 days at 35°C. Again, an increase in culture turbidity, due to bacterial growth, was used as an indicator of inadequate sterilization.
Contact angle measurements
Changes in the surface properties of e-PCL scaffolds (treated and controls) were determined by measuring the contact angle using a Rame’-Hart 200 contact-angle goniometer. A sessile drop (2–4 μL volume) of DI water was placed on the surface of the scaffold using a micro-syringe and allowed to equilibrate for a period of 10 s. The image of the drop was captured and analyzed using DROPImage (Rame’-Hart Instrument) for contact angle measurements. A total of six readings were performed for each scaffold type.
Scaffold permeability
A modification of flow meter developed in our laboratory and previously described15 was used to calculate scaffold permeability. Instead of a steady hydrostatic pressure (provided by an elevated reservoir) and gravity-assisted flow, we adapted a micro-filtration assembly (EMD Millipore Corporation) and employed suction to provide the driving force for filtration. Electrospun scaffolds were cut into 25 mm circular discs, and their thickness was recorded using a micrometer (Mitutoyo America Corporation). Scaffolds were placed on top of a Type 316 stainless steel screen (100 mesh, filtration area of 2.1 cm2), with edges sealed using clear polytetrafluroethylene gaskets and the attachment secured to a 300 mL borosilicate glass funnel using an anodized aluminum clamp. The apparatus was attached to a vacuum pump that generated a suction of 25 inches mercury (corresponding to ∼4.9 inches mercury of positive pressure). The funnel was filled with 300 mL DI water, and the time required for 50 mL to flow through the membrane was recorded. Scaffold permeability was calculated from an average of four readings (for each scaffold type) and used in Darcy's equation (τ=Qηhs/Ftp), where τ represents scaffold permeability in darcy units (d), Q is the fluid volume passed through the scaffold in time t, η is the viscosity (0.89 cp for water at 25°C), hs is scaffold thickness, F is the filtration area, and p is the applied pressure.
Uniaxial tensile testing
Uniaxial tensile testing was performed according to our previous published studies.16 Briefly, scaffolds from each group (n=6) were punched into “dog-bones” (2.75 mm wide at their narrowest point with a gage length of 7.5 mm) and tested on an MTS Bionix 200 testing system with a 50 N load cell (MTS Systems Corp.) at an extension rate of 10.0 mm/min. Elastic modulus, strain at break, and energy to break were calculated and recorded by MTS TestWorks 4.0.
Stability of PAA
PAA was prepared in different concentrations from 100 to 2000 ppm in DI water as well as 20% ethanol solution and stored air tight at room temperature for approximately 3 weeks. The solutions were subsequently tested for PAA concentration every 3 days using colorimetric MQuant™ test strips that were specific for PAA and sensitive in 100–2000 mg/L (ppm) range (EMD Millipore). The manufacturer's instructions were followed to test PAA concentrations, and any changes over time were recorded.
Statistical analysis
Values were presented as means and standard deviation, where appropriate. The scaffold types were compared using analysis of variance, and significant differences were described using Tukey's HSD. All analyses were performed using SAS software (JMP version 10; SAS Institute, Inc.).
Results
Electrospun scaffold and spore characterization
Porous, nanofibrous scaffolds were generated after the optimization of electrospinning conditions. SEM analyses revealed that the average fiber diameter was 0.92±0.52 μm. There was a broad distribution of fibers with fiber diameters ranging from 136 to 2100 nm. The SEM of spores showed a typical rod-shaped structure, with the smaller dimension <1 μm, and the size was small enough to penetrate into the depths of porous fibrous matrix. Spores loaded onto scaffolds could not be visualized even at high concentrations, possibly due to the porous nature of electrospun scaffolds as well as because of the lack of color contrast.
B. atrophaeus spore culture and sensitivity to PAA
Untreated spores promptly germinated on the surface of TSB agar to form discrete reddish-orange colonies within 18 h. Longer incubation times led to coalescence and difficulty distinguishing individual colonies. Exposure of spore suspensions to PAA (diluted in DI water) resulted in marked reductions in colony-forming units. The number of colonies decreased significantly at 100 ppm (visual), but isolated colonies could still be seen at 500 ppm. However, no colonies were found at 1000 ppm or above. Figure 1 is representative of the results obtained with three trials. Hence, we established that 1000 ppm was the minimal sporicidal concentration of PAA at room temperature.
FIG. 1.
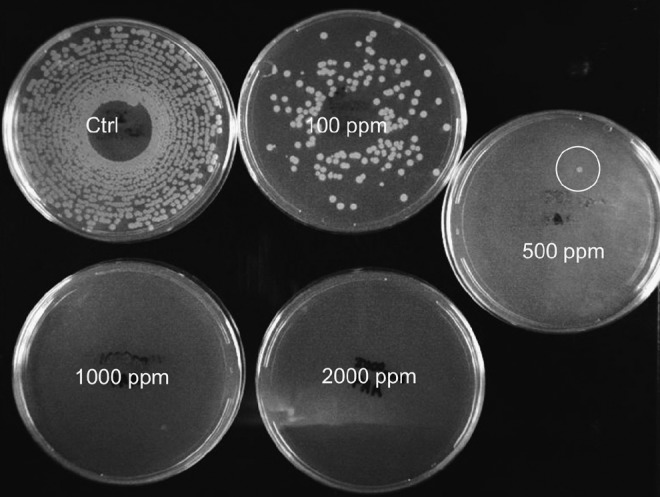
Effect of peracetic acid (PAA) (diluted in de-ionized water [DI water]) on spore viability. Spores were incubated with different concentrations of PAA for 5 min; suspensions were spiral-plated on solid agar and incubated for 18 h. Inadequate spore killing was observed at low concentrations (100 and 500 ppm) compared with controls, but complete sterility was seen at 1000 ppm and higher PAA concentrations.
Effects of sterilization
For each of the sterilization treatments on e-PCL scaffolds, we validated the sterilization process using the spores of B. atrophaeus as a biological indicator. In addition, we investigated the effects of the process on the physical and mechanical properties of the scaffolds. The results are discussed in the same order.
Sterilization efficacy
Both EtO and autoclaving are established methods of sterilization and expectedly destroyed all spores. Scaffolds treated with 80% ethanol demonstrated heavy bacterial loads similar to untreated controls. This is not surprising given that 80% ethanol is a high-level disinfectant that is incapable of killing spores and, hence, is not a viable option for terminal sterilization. Since 1000 ppm was identified to be the minimal sporicidal concentration of PAA, lower concentrations (100 and 500 ppm) were ignored and assays on e-PCL scaffolds were performed with 1000, 2500, and 5000 ppm only. Spore-inoculated scaffolds, challenged to different concentrations of PAA diluted in DI water, showed incomplete sterilization even at 1000 and 2500 ppm (data not shown). Lack of efficacy at these sporicidal concentrations was attributed to inadequate wetting of PCL scaffold and resulted in decreased access of PAA to spores within the scaffold. In order to improve the wetting characteristics of hydrophobic polymer scaffold, PAA was diluted in 20% ethanol, the same solution in which the spores were originally suspended. This modification dramatically improved the efficacy of PAA demonstrated by complete sterilization at 1000 ppm and above, which was consistent with our earlier observation with spore suspensions.
Effects of sterilization methods on physical and mechanical properties
Gross morphology and SEM:
EtO-treated electrospun scaffolds showed minimal gross dimensional change, but the scaffolds became translucent and brittle. Autoclaving induced massive melting and coalescence of polymer and completely destroyed the integrity of the scaffold. Further, scaffolds subjected to EtO and autoclaving showed complete loss of fibrous architecture and fusion of independent fibers under SEM (Fig. 2). Scaffolds treated with chemical sterilants (80% ethanol and different concentrations of PAA) did not show any appreciable change in either macroscopic (photographic imaging) or microscopic (SEM) scale compared with controls.
FIG. 2.
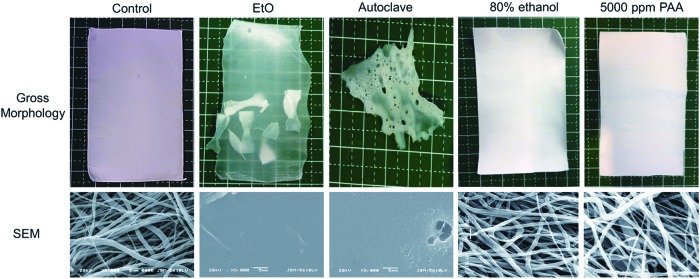
Optical (top) and scanning electron micrograph (bottom) images to illustrate morphology changes of electrospun polycaprolactone (e-PCL) scaffolds after sterilization treatments. Ethylene oxide (EtO) turned scaffolds into a solid, translucent film (dog-bone samples, placed in the same sterilization pouch, fused to the sheet), while autoclaving melted the scaffold. Complete loss of structure fibrous architecture can be observed with both standard sterilization methods. Chemical processing of scaffolds at room temperature (80% ethanol and PAA) did not induce any macro- or microscopic changes in scaffold morphology. Color images available online at www.liebertpub.com/tec
The scanning electron micrographs of scaffolds treated with PAA diluted in DI water and 20% ethanol are shown in Figure 3. The fibrous morphology of the scaffolds was significantly altered by treatment with PAA diluted in DI water in a concentration-dependent manner; individual fibers started fusing into bundles with evidence of fiber breakage at higher concentrations. PAA diluted in 20% ethanol showed a tendency toward thinning of fibers but preserved open porous architecture even at 5000 ppm. Statistical analyses confirmed significant effects of PAA concentration on fiber diameter depending on the diluent (p<0.001). Scaffolds treated with PAA diluted in DI water showed a significant difference in fiber diameter (p<0.001); fiber diameters at 2500 and 5000 ppm were larger than all other concentrations but were not different from one another (2500 ppm mean=2.03±1.02 μm vs. 5000 ppm mean=1.82±0.81 μm). Concentration-dependent effects on fiber diameter were not observed in scaffolds treated with PAA diluted in 20% ethanol (p>0.8).
FIG. 3.
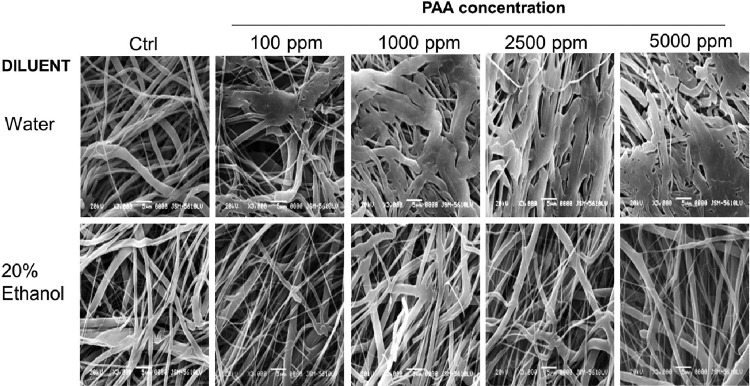
Scanning electron microscope images of e-PCL scaffolds treated with different concentrations of PAA diluted in DI water (top) or 20% ethanol (bottom) for 15 min. Significant changes in the fibrous structure were seen when DI water was used as a diluent. Such effects were not observed in 20% ethanol group, even at 5000 ppm.
Scaffold hydrophilicity:
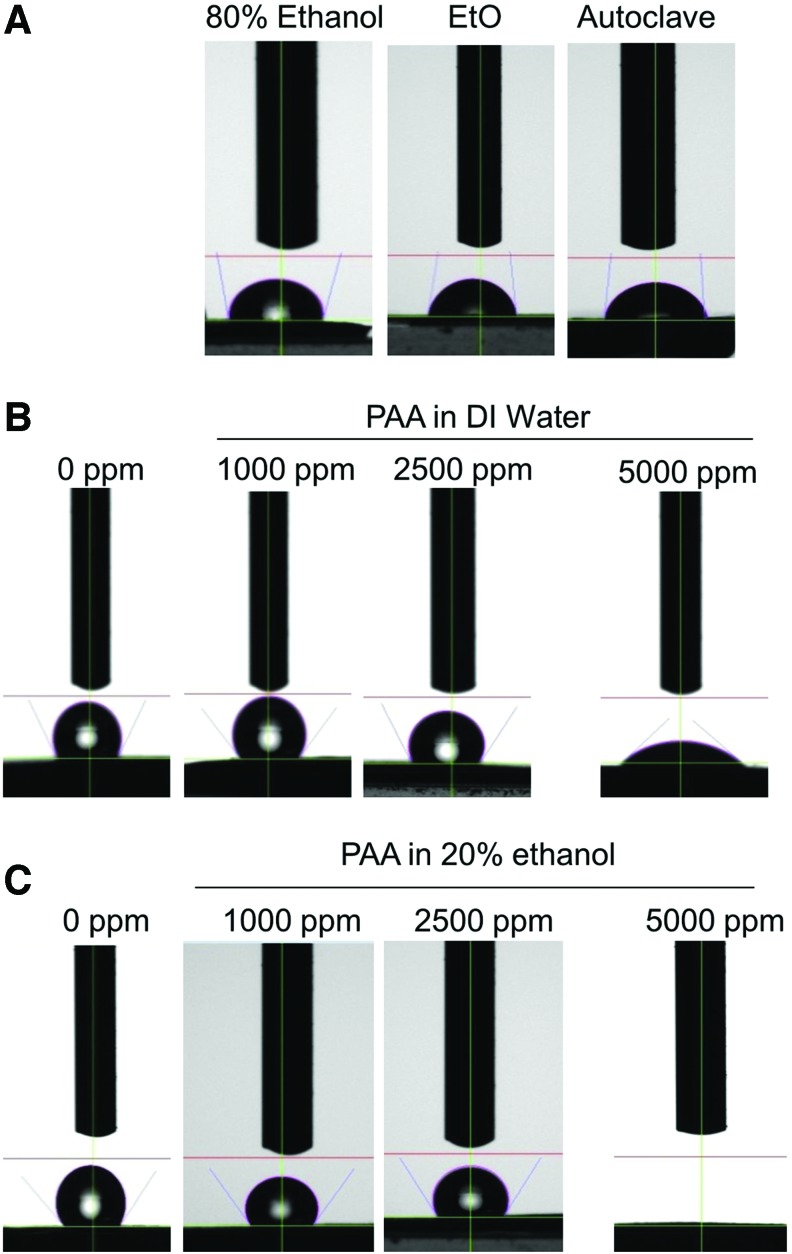
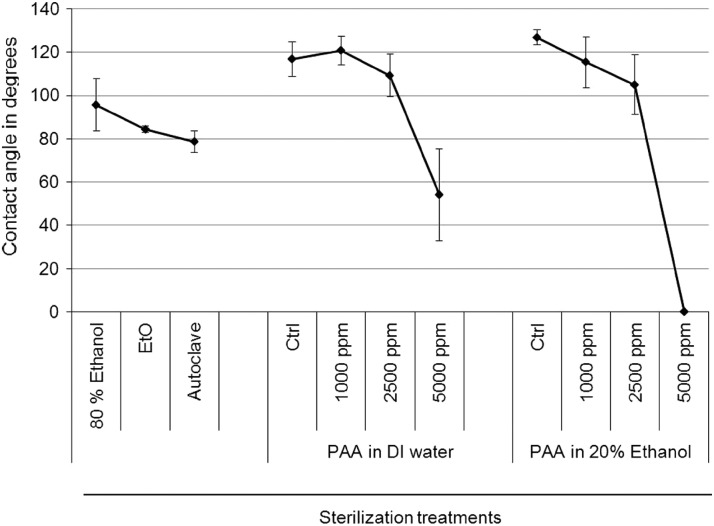
Contact angle measurements after different sterilization treatments were analyzed to indicate hydrophilicity or wettability of the scaffolds. Generally, surfaces are termed hydrophilic when the water contact angle is <90° and hydrophobic, if contact angle is more than 90°. Figure 4 shows representative image of an actual drop placed on differently treated surfaces. Untreated control PCL scaffolds are highly hydrophobic (contact angle around 120°); EtO, autoclaving, and 80% ethanol treatments make them hydrophilic as seen by reduced contact angles. Scaffolds treated with PAA at 1000 and 2500 ppm, in either diluent, did not significantly alter the wetting properties. However, at 5000 ppm, there was a dramatic decrease in the contact angles. Figure 5 is a quantitative representation of the average of contact angles measured from six replicates for each scaffold type. Scaffolds treated with PAA at 5000 ppm diluted in DI water decreased contact angles by more than half, whereas PAA in 20% ethanol completely soaked up the water and brought the contact angle to zero.
FIG. 4.
Sessile drop images, after 10 s equilibration, on e-PCL scaffolds subjected to standard sterilization methods (A) and PAA diluted in DI water (B) or 20% ethanol (C). While conventional treatment reduced contact angles appreciably, PAA did not have any significant effect for approximately 2500 ppm. PAA at 5000 ppm induced a dramatic reduction in contact angle, irrespective of the diluent. The effect was more pronounced when 20% ethanol was used, as seen by complete absorption of the water drop. Color images available online at www.liebertpub.com/tec
FIG. 5.
Quantification of contact angle measurements of PCL scaffolds from Figure 7. Significant reduction in contact angles were seen after treatment with PAA at 5000 ppm. PAA dilution in DI water reduces the contact angle by half, while dilution in 20% ethanol brought it to zero.
Scaffold permeability:
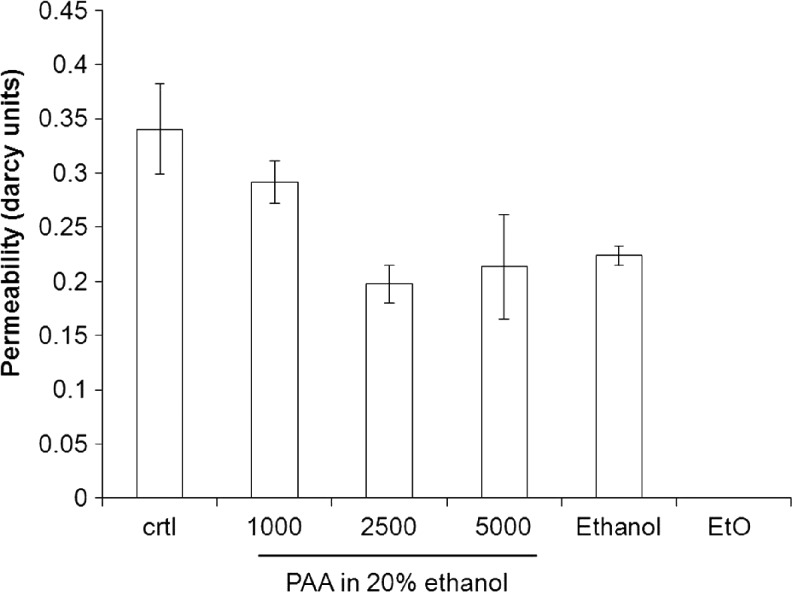
The permeability of the PCL scaffold to water was highest in the control-untreated PCL scaffold and decreased with increasing concentrations of PAA until the effect plateaued off at 2500 ppm (Fig. 6, p<0.001). The permeability of scaffolds treated with PAA at 2500 ppm was not significantly different than 5000 ppm, nor was it different than when using 80% ethanol. This correlates well with the observation on scaffold hydrophilicity; a hydrophilic scaffold is expected to interact with water and to decrease the flow rate. Scaffolds treated with PAA diluted in DI water demonstrated high variations in permeability, due to heterogeneity in wetting characteristics (data not shown).
FIG. 6.
Scaffold permeability (measured in darcy units) determined by flow rate of DI water through treated e-PCL scaffold. Decreased permeability was observed with increasing concentrations of PAA (p<0.001), while EtO-treated scaffolds formed a solid impermeable film.
Mechanical properties:
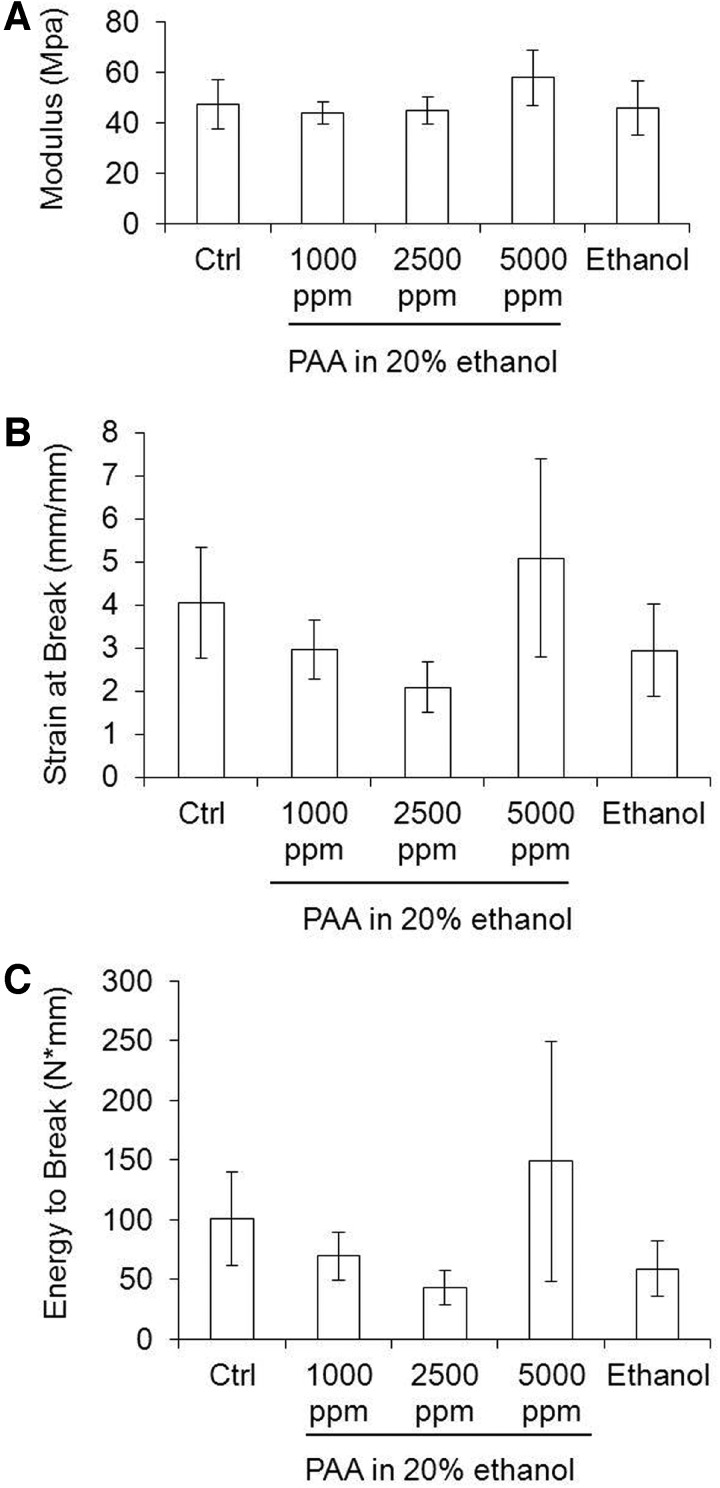
Since PAA diluted in DI water were not sporicidal at high concentrations, induced unfavorable changes in fiber morphology, and produced inconsistent data for scaffold permeability, we did not perform mechanical testing on these samples. The results of mechanical testing of scaffolds treated with 80% ethanol and different PAA concentrations are shown in Figure 7. EtO and autoclaved samples could not be mechanically tested because of loss of scaffold integrity. It is interesting to note that the modulus was not affected by the concentration of PAA used (p>0.06). Values for energy to break and strain at break indicate a tendency toward brittleness with increasing PAA concentrations for approximately 2500 ppm (statistically not significant). However, at 5000 ppm, the scaffolds were not statistically different from controls for the same properties (p=0.007 and p=0.010, respectively).
FIG. 7.
Mechanical properties of e-PCL scaffolds treated with different concentrations of PAA diluted in 20% ethanol. (A) Tensile modulus, (B) strain at break, and (C) energy to break. Control scaffolds refer to scaffolds incubated with 20% ethanol with no PAA. Scaffolds treated with 80% ethanol are also shown. Scaffold properties were not significantly affected by PAA sterilization solution for an approximate concentration of 5000 ppm.
PAA stability
PAA is at equilibrium with acetic acid and hydrogen peroxide and is particularly unstable at low concentrations.17 PAA at 100 ppm started degrading around 7 days as determined by a visual comparison with manufacturer-provided shade guides. Higher concentrations of PAA (>200 ppm) did not show any degradation for a period of 3 weeks when stored air tight at room temperature. In addition, stability of PAA was not affected by the diluent used. Hence, PAA at concentrations necessary for sterilization (>1000 ppm) could be prepared in large volumes and stored for a minimum of 3 weeks.
Discussion
The aim of tissue engineering is to develop viable functional alternatives for failing tissues and organs. However, the strategies pursued have evolved from purely cell- or biomolecule-based approaches to the current paradigm of scaffold-based tissue engineering. This involves seeding and culturing specific cell types in engineered 3D matrices that are designed to simulate the ECM.2 Such matrices are expected to present appropriate physical, biological, and biochemical cues to predictably influence cell behavior.18,19 Synthetic polymers are widely used in tissue engineering, because they are biocompatible, biodegradable, and can be tailored to possess a wide range of properties. The growing list of polymers3,20 and emerging scaffold fabrication technologies,21 provide matrices with a variety of internal architecture and mechanical properties.
Intended to be in direct contact with living tissues, these scaffolds should be terminally sterilized before implantation. Product sterility cannot be assumed even if fabricated in a “clean room,” because the machinery and starting materials are not sterile. Moreover, usually, benign bacteria can become pathogenic when present on the surface of devices.22 These factors make scaffold sterilization a vital issue to be resolved before clinical translation. Synthetic polymers used in tissue-engineered scaffolds possess low melting points, are susceptible to hydrolysis, and possess intricate architecture at micro-or nano scale, all of which can be affected by the sterilization process. Mechanical and surface properties, toxicity, and biocompatibility of scaffolds can be potentially altered by sterilization. Hence, careful evaluation of scaffolds before and after sterilization is required to identify a sterilization method that is benign to the polymer, device, and the patient.22 In this context, it is important to realize that standard sterilization practices (including autoclaving, EtO, and use of high-energy irradiation) are not specifically suited for polymeric systems employed in tissue engineering.
Autoclaving with pressurized moist steam at 120°C for 15 min is not a viable option for sterilizing polymers with low melting points. Further, most biocompatible polymers are hydrolytically unstable, and exposure to moisture can accelerate degradation, reduce shelf life, and alter mechanical properties.23 EtO is a reactive gas that can penetrate into polymeric networks, react with their chemical groups, cause polymer degradation, and alter scaffold dimensions.24 In addition, EtO is carcinogenic and needs to be extensively degassed over many hours before packaging.13 High-energy irradiation is an efficient sterilization method that may preserve the morphology of 3D scaffolds, but it dramatically decreases the polymer molecular weight and, hence, accelerates degradation.11
Limitations of conventional modes of sterilization in tissue engineering have led researchers to explore alternatives, especially in the past few years. Shearer et al.25 found that PAA and antibiotic solutions were effective in sterilizing hollow fiber and flat sheets of poly (lactide:glycolide) but induced unfavorable changes in morphology but not mechanical properties. Rainer et al. compared the effects of different sterilization techniques (ethanol, dry heat, autoclave, UV, and plasma treatment) on morphology and crystallinity of electrospun poly-l-lactide scaffolds.26 Dry heat and autoclave treatments resulted in an increase in crystallinity, while low temperature UV and hydrogen peroxide plasma preserved the structural properties. Siritientong et al.27 evaluated the effects of sterilization on lyophilized sericin-polyvinyl alcohol scaffolds and concluded that gamma irradiation was the most appropriate method even though it degraded the scaffolds by almost 70% in 24 h. These early studies critically demonstrated that sterilization of tissue-engineering scaffolds is as much about maintenance of material properties and architecture as it is about killing microbes. Sterilization assumes greater significance in scaffolds containing proteins or biologics where the risk of denaturation is real and can result in decreased or loss of vital biological activity.
However, a common limitation in studies mentioned earlier included a lack of uniform model organisms tested; many did not specify the source or the identity of the contaminating bacteria. In cases where known bacteria were used, there is no consistency in the choice of the bacterial species. In addition, some groups used unsterilized material as a control, which can vary widely in their bacterial load or bio-burden. This makes a comparison of sterilization methods in the context of tissue-engineering scaffolds difficult.
In contrast, we adopted a standardized format to test the sterilization of electrospun scaffolds in terms of microbe identity and number. Spores of B. atrophaeus were appropriately chosen as the model organism, considering their routine use (as biological indicators) to validate sterilization processes. We ensured a consistency in bio-burden by inoculating 10 mm discs of e-PCL scaffolds with 106 B. atrophaeus spores. We employed EtO and autoclaving as positive controls of sterilization and 80% ethanol because of their widespread use in tissue-engineering studies. We then systematically identified the process conditions for effective sterilization using PAA, a known chemical sterilant at room temperature. Varying the concentration, contact times, we were able to demonstrate that e-PCL scaffolds can be effectively sterilized using PAA without subjecting them to harsh processes. This is especially important in the context of tissue-engineering scaffolds, because sterilization provides a higher standard of care as well as the highest margin of safety for patients, compared with high-level disinfection. We reasoned that spores being the most resistant form of life14 and present in large numbers on scaffolds, a negative spore test would indicate complete elimination of bio-burden11 and a sterile scaffold.
The use of chemical agents to reduce bacterial load in polymeric scaffolds is attractive, because it allows processing at low temperatures and short duration. PAA has long been in use as a chemical sterilizing agent because of its strong oxidizing properties. It is available as an equilibrium mixture of acetic acid and hydrogen peroxide and has been extensively used in the food industry because of its high potency and low residual toxicity. PAA denatures proteins, disrupts cell wall permeability, and is effective against all known microbes (including spores) even in the presence of organic matter.17,28 PAA is effective at low concentrations, low temperatures, and reduced contact times compared with traditional methods. PAA is also economical, degrades into nontoxic end products (water, oxygen, and carbon dioxide), and can be safely disposed of without affecting the environment.11
The efficacy of PAA is affected by concentration, contact time, pH, and temperature. A commercially available system (Steris Corporation) employs 35% PAA diluted to 200 ppm in water (pH 6.4), at 50–56°C for 23 min. This automated system has been approved for sterilizing medical, surgical, and dental instruments, including those made from heat-sensitive materials.29 The STERIS system is optimized for sterilization at near neutral pH to reduce the tendency of PAA to corrode metals; hence, the use of PAA at low concentrations (200 ppm).
We needed to significantly deviate from the FDA-approved STERIS protocol to account for differences in materials being processed. First, since polymeric scaffolds are sensitive to heat (PCL degraded with EtO exposure at 50°C), our priority was to perform sterilization at room temperature. Second, in contrast to traditional solid surfaces (tubes and instruments), engineered scaffolds are 3D, nanofibrous, and porous structures. The enormous surface-to-volume ratio offered by electrospun scaffolds is not only a huge advantage in tissue engineering but also presents an opportunity for colonization and survival of spores/bacteria in the depths of the scaffold. Third, the hydrophobicity of the polymer coupled with highly porous structure makes the scaffold difficult to be wet by PAA. It is known that the device should be completely immersed and all surfaces should be in direct contact with PAA for effective sterilization.
Having established that 1000 ppm PAA killed spores in suspension within 5 min at room temperature, we extended the contact time to 15 min in experiments with scaffolds, taking into account their 3D porous structure. We also found that wettability of the scaffold affected the ability of PAA to kill spores. Since our model polymer (PCL) was hydrophobic, diluting PAA in water (as has been done in STERIS) yielded incomplete sterilization. However, the use of 20% ethanol (in DI water) as a diluent for PAA significantly improved the wetting characteristic of the scaffold and restored the efficacy of PAA at previously established concentrations. This finding reinforces the fact that PAA needs to be in physical contact with the surface to be effective.
PAA at high concentrations (5000 ppm) also induced favorable surface properties on electrospun scaffolds. Control PCL scaffolds were significantly hydrophobic as reflected by very high contact angles. However, when scaffolds were treated with PAA at 5000 ppm, the scaffold surface became markedly hydrophilic. The extent of this effect was dependent on the diluent, with contact angles dropping to zero when 20% ethanol was used. This was not unexpected, because polyester polymers are known to undergo acid- or alkali-mediated hydrolysis, resulting in increased hydrophilicity. Since hydrophilicity directly affects scaffold biocompatibility and favorable host response,30,31 the use of PAA offers the dual advantage of sterilization and inducing favorable surface properties.
Since acid-mediated hydrolysis can potentially affect morphological characteristics and mechanical properties of electrospun scaffolds, we sought to evaluate these effects by SEM and tensile testing. We found that individual fibers tended to fuse and scaffolds demonstrated decreased porosity when PAA was diluted in DI water, yet no appreciable change occurred when PAA was diluted in 20% ethanol, even at 5000 ppm. In addition, mechanical properties were not significantly affected by the PAA treatment.
The limitations of the current study include investigating the effects on one polymer type (PCL) processed by one fabrication technique (electrospinning) and, hence, cannot be generalized. PAA is a potent chemical agent that can sterilize any surface, yet its practicality needs to be ascertained for various polymers processed differently. Conditions for sterilization and effects on scaffolds will vary and need to be optimized for specific systems. Future work will investigate the biological response of PAA-sterilized scaffolds in vitro and in vivo.
Conclusions
Our primary goal was to identify and systematically evaluate the feasibility of using PAA as an alternative to conventional methods to effectively sterilize e-PCL scaffolds. We deliberately chose PCL because of its low melting point and electrospun it to confer defined nanoscale features, whose integrity can be followed during processing. We report that PAA at 1000 ppm (diluted in 20% ethanol) for 15 min at room temperature renders the scaffold sterile and at 5000 ppm, it dramatically alters the hydrophilicity of the scaffold as well. More importantly, these effects are observed while preserving the morphological and mechanical properties of the scaffold.
Novel biomaterials (smart polymers, carbon nanotubes) and fabrication technologies (including electrospinning, solid freeform fabrication, stereolithography, and 3D printing) are being introduced at a rapid pace to develop tissue-specific scaffolds. Scaffold sterilization, through the last physical step in product processing, should be at the forefront of scaffold development to ensure that neither the novel properties of biomaterials nor the nanoscale architecture that are painstakingly built are altered or lost.
The authors confirm that there are no known conflicts of interest associated with this publication, and there has been no significant financial support for this work that could have influenced its outcome.
Acknowledgments
The project was supported by CTSA (UL1TR000058) from the National Center for Advancing Translational Sciences and the CCTR Endowment Fund of Virginia Commonwealth University. The contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Disclosure Statement
No competing financial interests exist.
References
- 1.Lynch S.E., Marx R.E., Nevins M., and Wisner-Lynch L.A. (eds.). Tissue Engineering: Applications in Oral and Maxillofacial Surgery and Periodontics. 2nd edn. Hanover Park, IL: Quintessence Publishing Co, Inc., 2008 [Google Scholar]
- 2.Hutmacher D.W., and Cool S.Concepts of scaffold-based tissue engineering—the rationale to use solid free-form fabrication techniques. J Cell Mol Med 11,654, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu X.H., and Ma P.X.Polymeric scaffolds for bone tissue engineering. Ann Biomed Eng 32,477, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Lemmouchi Y., Schacht E., Kageruka P., De Deken R., Diarra B., Diall O., et al. Biodegradable polyesters for controlled release of trypanocidal drugs: in vitro and in vivo studies. Biomaterials 19,1827, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Vasir J.K., Tambwekar K., and Garg S.Bioadhesive microspheres as a controlled drug delivery system. Int J Pharm 255,13, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Zhang S.F., and Uludag H.Nanoparticulate systems for growth factor delivery. Pharm Res 26,1561, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Alani A., Knowles J.C., Chrzanowski W., Ng Y.L., and Gulabivala K.Ion release characteristics, precipitate formation and sealing ability of a phosphate glass-polycaprolactone-based composite for use as a root canal obturation material. Dent Mater 25,400, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Dalton P.D., Woodfield T., and Hutmacher D.W.SnapShot: polymer scaffolds for tissue engineering (vol 30, pg 701, 2009). Biomaterials 30,2420, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Dash T.K., and Konkimalla V.B.Poly-ɛ-caprolactone based formulations for drug delivery and tissue engineering: a review. J Control Release 158,15, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Sell S., Barnes C., Smith M., McClure M., Madurantakam P., Grant J., et al. Extracellular matrix regenerated: tissue engineering via electrospun biomimetic nanofibers. Polym Int 56,1349, 2007 [Google Scholar]
- 11.Rutala W.A., and Weber D.J.Guideline for Disinfection and Sterilization in Healthcare Facilities. Center for Disease Control and Prevention, ed., Atlanta, GA: CDC, 2008, p. 64 [Google Scholar]
- 12.Bosworth L.A., Gibb A., and Downes S.Gamma irradiation of electrospun poly(e-caprolactone) fibers affects material properties but not cell response. J Polym Sci Pol Phys 50,870, 2012 [Google Scholar]
- 13.Phillip E., Murthy N.S., Bolikal D., Narayanan P., Kohn J., Lavelle L., et al. Ethylene oxide's role as a reactive agent during sterilization: Effects of polymer composition and device architecture. J Biomed Mater Res B 101B,532, 2013 [DOI] [PubMed] [Google Scholar]
- 14.McDonnell G.E. (ed.). Antisepsis, Disinfection, and Sterilization: Types, Action, and Resistance. Washington, DC: ASM Press, 2007 [Google Scholar]
- 15.Sell S., Barnes C., Simpson D., and Bowlin G.Scaffold permeability as a means to determine fiber diameter and pore size of electrospun fibrinogen. J Biomed Mater Res A 85A,115, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Madurantakam P.A., Rodriguez I.A., Garg K., McCool J.M., Moon P.C., and Bowlin G.L.Compression of multilayered composite electrospun scaffolds: a novel strategy to rapidly enhance mechanical properties and three dimensionality of bone scaffolds. Adv Mater Sci Eng 2013. http://dx.doi.org/10.1155/2013/561273
- 17.Block S.S.Peroxygen compounds. In: Block S.S., ed. Disinfection, Sterilization, and Preservation, 5th edn. Philadelphia, PA: Lippincott, Williams and Wilkins, 2001, pp 185–204 [Google Scholar]
- 18.Lutolf M.P., Gilbert P.M., and Blau H.M.Designing materials to direct stem-cell fate. Nature 462,433, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guilak F., Cohen D.M., Estes B.T., Gimble J.M., Liedtke W., and Chen C.S.Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell 5,17, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martina M., and Hutmacher D.W.Biodegradable polymers applied in tissue engineering research: a review. Polym Int 56,145, 2007 [Google Scholar]
- 21.Liu C., Xia Z., and Czernuszka J.T.Design and development of three-dimensional scaffolds for tissue engineering. Chem Eng Res Design 85,1051, 2007 [Google Scholar]
- 22.Labarre D., Ponchels G., and Vauthire C. (ed.). Biomedical and Pharmaceutical Polymers. London, Chicago: Pharmaceutical Press, 2011 [Google Scholar]
- 23.Athanasiou K.A., Niederauer G.G., and Agrawal C.M.Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers. Biomaterials 17,93, 1996 [DOI] [PubMed] [Google Scholar]
- 24.Holy C.E., Cheng C., Davies J.E., and Shoichet M.S.Optimizing the sterilization of PLGA scaffolds for use in tissue engineering. Biomaterials 22,25, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Shearer H., Ellis M.J., Perera S.P., and Chaudhuri J.B.Effects of common sterilization methods on the structure and properties of poly(D,L lactic-co-glycolic acid) scaffolds. Tissue Eng 12,2717, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Rainer A., Centola M., Spadaccio C., Gherardi G., Genovese J.A., Licoccia S., et al. Comparative study of different techniques for the sterilization of poly-L-lactide electrospun microfibers: effectiveness vs. material degradation. Int J Artif Organs 33,76, 2010 [PubMed] [Google Scholar]
- 27.Siritientong T., Srichana T., and Aramwit P.The effect of sterilization methods on the physical properties of silk sericin scaffolds. AAPS PharmSciTech 12,771, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sagripanti J.-L., and Bonifacino A.Comparative sporicidal effect of liquid chemical germicides on three medical devices contaminated with spores of Bacillus subtilis. Am J Infect Control 24,364, 1996 [DOI] [PubMed] [Google Scholar]
- 29.Crow S.Peracetic acid sterilization: a timely development for a busy healthcare industry. Infect Control Hosp Epidemiol 13,111, 1992 [DOI] [PubMed] [Google Scholar]
- 30.Boland E.D., Telemeco T.A., Simpson D.G., Wnek G.E., and Bowlin G.L.Utilizing acid pretreatment and electrospinning to improve biocompatibility of poly(glycolic acid) for tissue engineering. J Biomed Mater Res Part B Appl Biomater 71,144, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Valence S.D., Tille J.-C., Chaabane C., Gurny R., Bochaton-Piallat M.-L., Walpoth B.H., et al. Plasma treatment for improving cell biocompatibility of a biodegradable polymer scaffold for vascular graft applications. Eur J Pharm Biopharm 85,78, 2013 [DOI] [PubMed] [Google Scholar]