Abstract
This study was conducted to investigate the preventive effects of different kanjangs (Korean soy sauces), including acid-hydrolyzed soy sauce (AHSS), fermented soy sauce (FSS), and fermented sesame sauce (FSeS), on 2% dextran sulfate sodium (DSS)-induced ulcerative colitis in C57BL/6J mice. The fermented sauces, particularly FSeS, significantly suppressed DSS-induced body weight loss, increased colon length, and decreased colon weight/length ratios. Histological observations suggested that the fermented sauces prevented edema, mucosal damage, and the loss of crypts induced by DSS compared to the control mice and animals fed AHSS. FSeS and FSS decreased the serum levels of tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), interleukin (IL)-6, and IL-17α. mRNA expression of these cytokines as well as that of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) in colon mucosa was also inhibited by the two sauces. Our results suggest that fermented sauces, especially FSeS, exert an anticolitic effect partially by reducing the serum levels of proinflammatory cytokines and inhibiting the mRNA expression of these factors in the colon tissue of mice treated with DSS. However, AHSS did not protect against DSS-induced colitis. In addition, low-dose treatment (4 mL/kg) with the fermented sauces resulted in greater anticolitic effects than consumption of a high quantity (8 mL/kg) of the sauces.
Key Words: : colitis, dextran sulfate sodium, fermented sesame sauce, fermented soy sauce, kanjang, pro-inflammatory cytokines
Introduction
Soy sauce is a traditional fermented soy-based liquid condiment with a salty taste that is widely consumed in Asia. This sauce is known as kanjang in South Korea, jiangyou in China, and shoyu in Japan. In South Korea, soy sauces can be classified as fermented soy sauce (FSS), acid-hydrolyzed soy sauce (AHSS; also called chemical soy sauce), and mixed soy sauce. FSS is prepared by fermenting protein-rich soybeans with Aspergillus oryzae (or Aspergillus sojae) in the presence of sodium chloride at 30°C for over 6 months. AHSS is manufactured from defatted soybeans or other protein-rich soybeans that are treated with 18% food-grade hydrochloric acid for 15–20 h with heating (107°C). The solution is then neutralized with sodium hydroxide or sodium carbonate, mixed with active carbon, and finally filtered to remove insoluble materials.1 Mixed soy sauce is produced by combining FSS and AHSS at an appropriate ratio. Fermented sesame sauce (FSeS), a new type of liquid condiment, is prepared in a manner similar to that of FSS2 and has also gained popularity in South Korea. FSS is known as a traditional functional food with antimutagenic3,4 and antioxidant5–7 properties. FSS was also found to exert antitumor effects against benzo[α]pyrene-induced forestomach neoplasia in female ICR mice,8,9 spontaneous liver tumors in female C3H/HeN mice,10 and liver tumors in neutron-irradiated male B6C3F1 mice.10 Despite these favorable properties, the anti-inflammatory activities of FSS and other fermented sauces have not been studied.
Inflammatory bowel diseases (IBDs) are defined as chronic inflammation of the gastrointestinal tract, and include ulcerative colitis (UC) and Crohn's disease (CD). These conditions represent a significant public health problem in Western societies and affect 1 in 1000 individuals.11 In South Korea, the incidence of UC is lower compared with Western nations but is increasing rapidly. According to an epidemiological study conducted from 1986 to 2005, the mean annual incidence rates of CD and UC in the Songpa-Kangdong district (Seoul, South Korea) significantly increased from 0.05 and 0.34 per 100,000 inhabitants, respectively, in 1986–1990 to 1.34 and 3.08 per 100,000 individuals, respectively, in 2001–2005.12
The etiology of UC remains unclear. Generally, UC pathogenesis is believed to involve complex interactions between the intestinal microbial environment, persistent pathogenic infections, defective mucosal barrier function, and dysregulation of the colonic mucosal immune system along with genetic and environment factors.13 Currently, it is widely accepted that both UC and CD are caused by inflammation-related cytokine-driven mixed infiltrates in the intestinal mucosa.14 In cases of animal and human UC, some proinflammatory cytokines such as tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), interleukin (IL)-1β, IL-6, and IL-17α also play crucial roles in disease pathogenesis.14–16
In the present study, a murine model of dextran sulfate sodium (DSS)-induced UC was used to evaluate the anti-inflammatory effects of AHSS, FSS, and FSeS. This mouse model exhibited many symptoms similar to those of human UC patients.17 In addition, the underlying mechanisms of action were explored.
Materials and Methods
Sauce samples and chemical reagents
AHSS, FSS, and FSeS were provided by Daesang Food Co., Ltd. (Echeon, South Korea). The FSS was prepared as follows: the cooked soybeans were mixed with roasted wheat and fermented for 72 h at 30°C with Aspergillus oryzae to prepare meju, which was mixed with a brine solution (15–20% NaCl) and fermented for 180 days at 30°C.1 The manufacturing process of FSeS was similar to the FSS. The roasted and crushed defatted sesame seeds were mixed with A. oryzae and fermented for 2–5 days at 15°C and 3–5 days at 45°C. The brine solution (15–20% NaCl) was added and fermented for 45 days at 30°C.2 The fermented soy or sesame sauce was finally processed by pressing, filtration, pasteurization, and packing. Different from the fermented sauces, the manufacturing process of acid dehydrolyzed soy sauce is produced by boiling defatted soybean or gluten in hydrochloric acid (18%, food grade) and then neutralizing the solution with sodium hydroxide. Following filtration and pasteurization, the acid dehydrolyzed soy sauce was prepared.1 The sauce samples were filtered through 0.45-μm syringe filters (Whatman International, Maidstone, Kent, United Kingdom) and stored at 4°C until further use. The Trizol reagent, oligodT18 primer, dNTPs, murine maloney leukemia virus (MMLV) reverse transcriptase, RNase inhibitor, ethidium bromide (EtBr), and agarose were purchased from Invitrogen Life Technologies (Carlsbad, CA, USA). DSS (molecular weight: 36,000–50,000) was obtained from MP Biomedical (Solon, OH, USA). Dinitrosalicylic acid (DNS) and Folin–Denis reagent were purchased from Sigma Chemical Co. (St. Louis, MO, USA). All reagents were of analytical grade.
Chemical composition analyses
Salinity, pH, reducing sugar levels, amino type nitrogen concentrations, and total polyphenol contents of the three sauce samples were measured in triplicate. pH of the sauce samples was directly measured using a SevenEasy™ S20 pH meter (Mettler-Toledo GmbH, Schwerzenbach, Switzerland), and salinity was evaluated by volumetric titration with silver nitrate (AgNO3) using the Mohr method.18 Reducing sugar contents were analyzed with the Miller methods19 using the DNS reagent. Amino nitrogen levels were determined, as previously described, by Beddows et al.20 with some modifications. Diluted sauce samples (20 mL) were mixed with 20 mL of formalin solution (pH 7.4), and the pH was adjusted to 8.4 by titrating with 0.1 M NaOH. The volume of NaOH used was recorded to determine the total amino nitrogen content of the samples. Total polyphenolic contents of the sauces were measured, as previously described, by Taga et al.21 with modifications. A 0.5-mL aliquot of Folin–Denis reagent (previously diluted 50-fold with distilled water) was added to 200 μL of each sauce. Next, 0.75 mL of sodium carbonate (7.5%) was added before the solution was mixed and incubated at room temperature for 30 min. Absorbance was then measured at 750 nm using a UV-2401PC spectrophotometer (Shimadzu, Kyoto, Japan).
Animal studies
The animal protocol used in this study was reviewed by the Pusan National University Institutional Animal Care and Use Committee (PNU-IACUC; approval number PNU-2011-000408). Female C57BL/6J mice (6 weeks old, 16–18 g) were purchased from Korea Central Lab Animal, Inc. (Seoul, South Korea). The mice were housed in environmentally controlled conditions with a standard 12-h light/12-h dark cycle at room temperature and had free access to food and water. The animals were randomly divided into eight groups of seven mice each: group 1, the normal control treated with 0.9% normal saline; group 2, DSS-treated mice; and groups 3–8, DSS-treated animals given low (4 mL/kg) or high (8 mL/kg) doses of the different sauce samples. Sauce samples and vehicle were administered daily through an intragastric route starting 7 days before DSS treatment and continued until sacrifice. Colitis was induced in the mice by administration of 2% DSS in the drinking water for 7 days.
Histological observations
Four samples of the distal colon from each animal were subjected to histological examination. The colon tissues were fixed in 10% (v/v) neutral buffered formalin, dehydrated in ethanol, and embedded in paraffin. Sections (4 μm thick) were then prepared and stained with hematoxylin and eosin (H & E). Images were acquired using a Zeiss Axioskop 2 Plus microscope (Carl Zeiss MicroImaging, Thornwood, NY, USA) equipped with an AxioCam MRc5 CCD camera (Carl Zeiss).
Measurement of serum proinflammation cytokine levels
For the serum proinflammatory cytokine assay, blood collected from the inferior vena cava was transferred to a tube and centrifuged (3000 g for 10 min at 4°C). Serum levels of TNF-α, IFN-γ, IL-6, and IL-17α were measured with a commercial ELISA kit (ELISA MAX; Biolegend, San Diego, CA, USA) according to the manufacturer's protocol.
Semiquantitative reverse transcription-PCR assay
mRNA expression of TNF-α, IL-6, IL-17α, IFN-γ, inducible nitric oxide synthase (iNOS), and cyclooxygenase-2 (COX-2) in the colon mucosa was measured with a reverse transcription (RT)-PCR assay. Total RNA was isolated from the colonic tissue (100 mg) using the Trizol reagent according to the manufacturer's recommendations and centrifuged at 12,000 g for 15 min at 25°C after the addition of chloroform. Isopropanol was added to the supernatant at a 1: 1 ratio, and the RNA was pelleted by centrifugation (12,000 g for 15 min at 4°C). After washing the pellet with ethanol, the RNA was solubilized in diethyl pyrocarbonate-treated RNase-free water and quantified by measuring the absorbance at 260 nm using a UV-2401PC spectrophotometer (Shimadzu, Kyoto, Japan). Equal amounts of RNA (1 μg) were reverse transcribed in a master mix containing 1×reverse transcriptase buffer, 1 mM dNTPs, 500 ng of oligodT18 primers, 140 U of MMLV reverse transcriptase, and 40 U of RNase inhibitor for 45 min at 42°C. PCR was then carried out in an automatic thermocycler (Bioneer, Daejeon, South Korea) for 25 cycles (94°C for 30 s, 55°C for 30 s, and 72°C for 40 s) followed by an 8-min extension at 72°C. The PCR products were separated in 2% agarose gels and visualized by EtBr staining. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used for normalization. Gene expression was quantified using ImageJ software (http://rsbweb.nih.gov/ij/).
Statistical analyses
All data are presented as the mean±standard deviation (SD). Differences between the mean values for individual groups were assessed with a one-way analysis of variance (ANOVA) and Duncan's multiple range tests. Differences were considered significant when P<.05. SAS version 9.1 (SAS Institute, Inc., Cary, NC, USA) was used to perform these analyses.
Results
Chemical composition of the sauce samples
The chemical compositions of AHSS, FSS, and FSeS are shown in Table 1. The pH (4.9) and salinity (20.3%) of AHSS were similar to that of FSS (4.7% and 19.2%, respectively) and FSeS (5.1% and 18.6%, respectively). Reducing sugar contents of AHSS, FSS, and FSeS were 1.6, 3.7, and 4.4 g/L, respectively. Amino type nitrogen levels of the AHSS (1.4 g/100 mL) were higher than those in FSS (0.7 g/100 mL) and FSeS (0.8 g/100 mL). FSeS contained the highest polyphenol contents (122.6 mg/mL) compared to FSS (118.4 mg/mL) and AHSS (48.0 mg/mL).
Table 1.
Chemical Composition of the Sauce Samples
| Group | pH | Salinity (%) | Reducing sugars (g/L) | Amino type nitrogen (g/100 mL) | Total polyphenols (mg/mL) |
|---|---|---|---|---|---|
| AHSS | 4.9±0.1b | 20.3±0.3a | 1.6±0.1c | 1.4±0.1a | 48.0±3.5b |
| FSS | 4.7±0.1c | 19.2±0.2b | 3.7±0.1b | 0.7±0.1c | 118.4±3.6a |
| FSeS | 5.1±0.1a | 18.6±0.3c | 4.4±0.2a | 0.8±0.1b | 122.6±4.5a |
Data are presented as the mean±SD.
Mean values in the same column with different superscript letters are significantly different (P<.05) according to Duncan's multiple range test.
AHSS, acid hydrolyzed soy sauce; FSS, fermented soy sauce; FSeS, fermented sesame sauce.
Anticolitic effect of the sauces in mice
As shown in Table 2, body weights of the DSS-treated mice were significantly decreased by day 15 (the end of the experiment). The fermented sauces more effectively prevented body weight loss compared to AHSS. Among the fermented sauces, FSeS had a greater ability to decrease DSS-induced body weight loss than the FSS. It is worth noting that the low dose (4 mL/kg) of the fermented sauces more effectively prevented DSS-induced body weight loss than the high dose (8 mL/kg).
Table 2.
Effects of FSeS, FSS, and AHSS on Body, Liver, Kidney, and Spleen Weight in C57bl/6j Mice with DSS-Induced Colitis
| Relative organ weight (g/100 g body weight) | |||||
|---|---|---|---|---|---|
| Group | Treatment | Final body weight (g) | Liver | Kidney | Spleen |
| 1 | Normal | 17.8±0.6a | 5.0±0.3ab | 1.5±0.2a | 0.6±01c |
| 2 | DSS+saline | 12.7±0.7f | 5.2±1.0ab | 1.1±0.4b | 0.9±0.2b |
| 3 | DSS+AHSS (4 mL/kg) | 13.5±0.3de | 5.3±0.3ab | 1.3±0.1ab | 0.8±0.1bc |
| 4 | DSS+FSS (4 mL/kg) | 13.9±0.5d | 5.4±0.5a | 1.4±0.2ab | 1.1±0.4a |
| 5 | DSS+FSeS (4 mL/kg) | 15.4±0.5b | 4.9±0.3ab | 1.3±0.2ab | 1.1±0.4a |
| 6 | DSS+AHSS (8 mL/kg) | 13.2±0.5ef | 4.7±0.2b | 1.3±0.1ab | 0.6±0.1c |
| 7 | DSS+FSS (8 mL/kg) | 13.7±0.5de | 4.9±0.7ab | 1.4±0.2ab | 0.7±0.1bc |
| 8 | DSS+FSeS (8 mL/kg) | 14.8±0.2c | 5.0±0.7ab | 1.3±0.4ab | 0.7±0.1bc |
Data are presented as the mean±SD.
abcdef: Mean values with the different superscript letters are significantly different (P<.05) according to Duncan's multiple range tests.
DSS, dextran sulfate sodium.
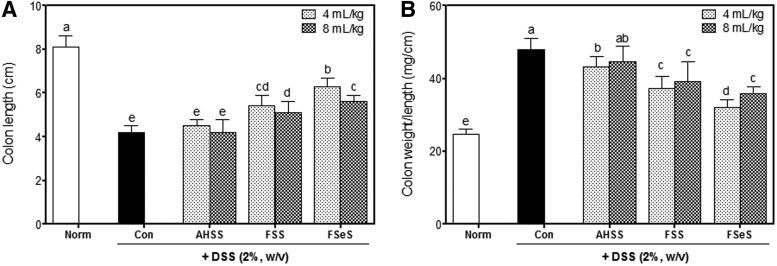
Shortening of the colon is a typical symptom of UC. As shown in Figure 1A, total colon length of the DSS-treated mice (4.2±0.3 cm) was significantly shorter compared with the normal control animals (8.1±0.5 cm). At doses of 4 and 8 mL/kg, the fermented sauces, especially FSeS (6.3±0.4 and 5.6±0.3 cm, respectively), more effectively mitigated colon length shortening induced by DSS than FSS (5.4±0.5 and 5.1±0.5 cm, respectively) and AHSS (4.5±0.3 and 4.2±0.6 cm, respectively).
FIG. 1.
Sauce samples attenuate clinical signs of dextran sulfate sodium (DSS)-induced colitis in mice. (A) Colon length and (B) colon weight/length ratio were evaluated. Data are expressed as the mean±SD. abcdeColumns with different letters are significantly different (P<.05) according to Duncan's multiple range test. AHSS, acid hydrolyzed soy sauce; FSS, fermented soy sauce; FSeS, fermented sesame sauce.
The ratio of colon weight to colon length was significantly elevated in the DSS-treated mice, indicating that DSS promoted edematous changes associated with colitis. Mice treated with DSS had a significantly increased colon weight/length ratio (48.0±3.1 mg/cm) compared with that of the normal mice (24.6±1.6 mg/cm). These DSS-associated changes were ameliorated by administering the fermented sauce samples at both low and high doses (Fig. 1B). Among the animals treated with 4 mL/kg of sauce, FSeS (31.9±2.2 mg/cm) significantly reduced edematous changes in the colon compared to FSS (37.2±3.4 mg/cm) and AHSS (43.1±2.9 mg/cm). Similar results were also observed for the mice treated 8 mL/kg of the sauces (35.9±1.9, 39.1±5.5, and 44.5±4.4 mg/cm for FSeS, FSS, and AHSS, respectively). In addition, all of the sauce samples were well tolerated by the mice and no obvious signs of systemic toxicity were observed during the entire treatment period. Toxicity was monitored by evaluating the general appearance of the animals along with differences in organ weight of the liver, kidney and spleen between normal mice and ones with DSS-induced colitis (Table 2).
Histological observations
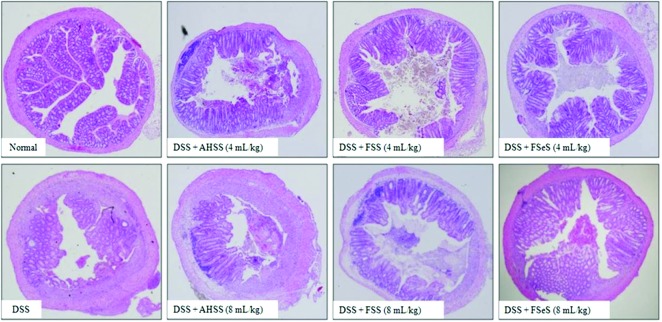
Colon tissues were histologically analyzed to evaluate DSS-induced intestinal injury. As shown in Figure 2, colon tissue from the DSS-treated mice showed typical acute inflammatory changes in the colonic architecture such as ulceration, crypt dilation, and goblet cell depletion. Infiltration of inflammatory cells was also observed. Conversely, colons from DSS-treated mice given the fermented sauces showed greatly reduced numbers of infiltrating cells and a lesser degree of mucosal injury and edema, particularly in the FSeS-treated groups. AHSS had a weaker ability to prevent DSS-induced inflammatory injury in the mice.
FIG. 2.
Histological evidence of DSS-induced colitis in mice treated with different sauce samples. Colon samples were obtained 7 days after DSS administration, sectioned, stained with H&E, and observed with a light microscope (original magnification, 40×). Color images available online at www.liebertpub.com/jmf
Effects of the fermented sauce samples on serum proinflammatory cytokine levels
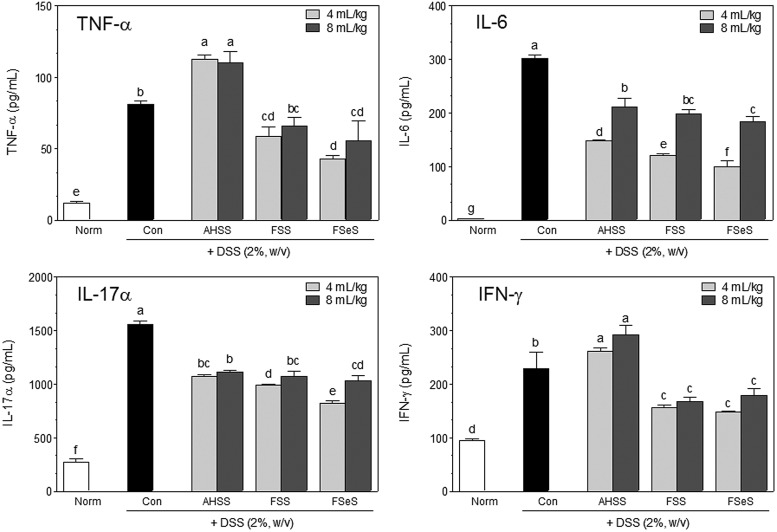
Increased serum proinflammatory cytokine levels are associated with UC pathogenesis.22,23 Therefore, effects of the fermented sauce samples on serum levels of TNF-α, IL-6, IL-17α, and IFN-γ were evaluated using an ELISA assay. As shown in Figure 3, DSS significantly increased the serum levels of TNF-α, IL-6, IL-17α, and IFN-γ. Fermented sauces, specifically FSeS, significantly decreased the levels of TNF-α (47%), IL-6 (67%), IL-17α (47%), and IFN-γ (35%) in serum compared to those found in mice treated with DSS alone (control group). At a concentration of 4 mL/kg, FSeS and FSS also decreased the serum levels of TNF-α (62% and 48%, respectively), IL-6 (32% and 18%, respectively), IL-17α (23% and 8%, respectively), and IFN-γ (40% and 35%, respectively) more effectively than AHSS.
FIG. 3.
Effects of the sauce samples on serum levels of proinflammatory cytokines (TNF-α, IL-6, IL-17α, and IFN-γ) in mice treated with 2% DSS. Data are expressed as the mean±SD. abcdefgColumns with different superscript letters are significantly different (P<.05) according to Duncan's multiple range test.
Effect of the sauces (4 mL/kg) on proinflammatory cytokine expression levels in the colonic mucosa
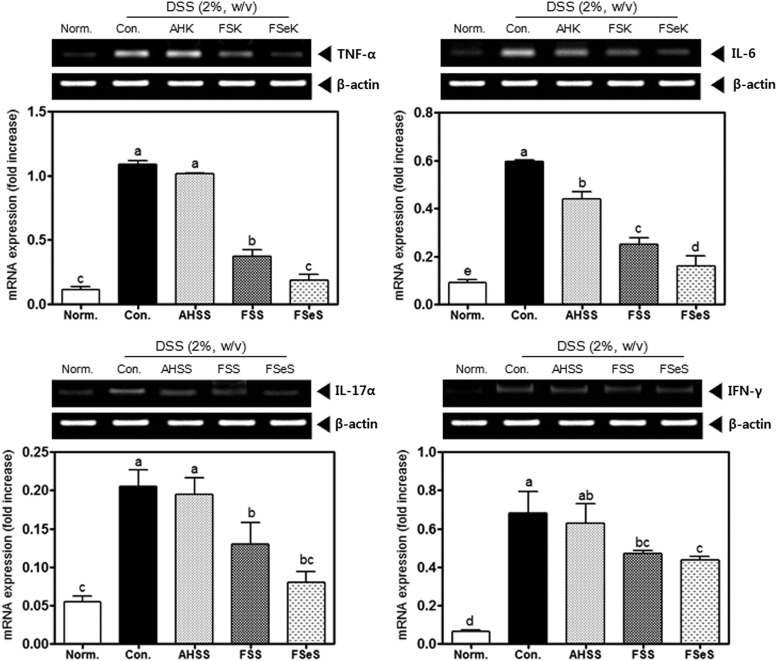
To further investigate the anti-inflammatory effects of fermented sauces on DSS-induced colitis in C57BL/6J mice, mRNA expression of TNF-α, IL-6, IL-17α, and IFN-γ in the colonic mucosa was analyzed by RT-PCR. As presented in Figure 4, colon inflammation induced by DSS resulted in elevated expression of all of the proinflammatory cytokines. FSeS and FSS significantly reduced the colonic mRNA levels of TNF-α (83% and 66%, respectively), IL-6 (73% and 58%, respectively), IL-17α (58% and 31%, respectively), and IFN-γ (28% and 21%, respectively) compared to the levels in mice treated with DSS alone. Our findings also indicated that the administration of FSeS and FSS more effectively reduced the mRNA expression of TNF-α (82% and 63%, respectively), IL-6 (61% and 43%, respectively), IL-17α (56% and 28%, respectively), and IFN-γ (30% and 24%, respectively) than AHSS in the mice with DSS-induced colitis.
FIG. 4.
Effects of the sauce samples (4 mL/kg) on mRNA expression of proinflammatory cytokines (TNF-α, IL-6, IL-17α, and IFN-γ) in the colonic mucosa of mice treated with 2% DSS. The mRNA levels of inflammatory cytokines were measured using a reverse transcription (RT)-PCR assay, as described in the Materials and Methods section. The PCR products were quantified and normalized relative to GAPDH (internal control). Data are expressed as the mean±SD. a–dBars with different letters are significantly different (P<.05) according to Duncan's multiple range test.
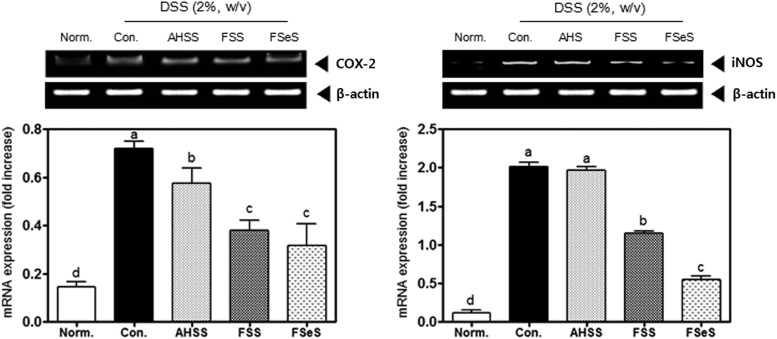
Effect of the sauces (4 mL/kg) on iNOS and COX-2 expression in the colonic mucosa
DSS significantly increased the mRNA expressions of iNOS and COX-2 in the colonic mucosa of the mice (Fig. 5). Subsequent treatment with 4 mL/kg of the fermented sauces caused a marked decrease in iNOS and COX-2 mRNA levels in the colonic mucosa. FSeS and FSS effectively decreased the mRNA levels of iNOS (72% and 42%, respectively) and COX-2 (57% and 48%, respectively) compared to the levels found in mice treated with DSS alone. AHSS only weakly inhibited mRNA expression of iNOS and COX-2 in colonic mucosa compared to the fermented sauces.
FIG. 5.
Effects of the sauce samples (4 mL/kg) on mRNA expression of iNOS and COX-2 in the colonic mucosa of mice treated with 2% DSS. The mRNA levels of iNOS and COX-2 were measured with an RT-PCR assay, as described in the Materials and Methods section. The PCR products were quantified and normalized relative to GAPDH (internal control). Data are expressed as the mean±SD. a–dBars with different letters are significantly different (P<.05) according to Duncan's multiple range test.
Discussion
Kanjang (fermented soy sauce) has been traditionally used as both a condiment and a health food with antioxidant, antimutagenic, and antitumor activities.3–10 Both FSS and FSeS are prepared by fermenting soybeans or sesame seeds as main ingredients, respectively, with A. oryzae or Aspergillus sojae at 30°C for over 6 months. Soybeans, a raw material of soy sauce, contain high concentrations of soy isoflavones, including daidzin, genistin, daidzein, genistein, and glycitein.24,25 During the fermentation process, daidzin and genistin are transformed into daidzein and genistein, respectively, by A. oryzae and other fungi of this genus.26,27 It has been reported that FSS is rich in phytochemicals, including free daidzein (0.9–23.5 μg/g) and free genistein (2.8–17.9 μg/g).28,29 Sesame, an oil-rich crop, has been traditionally considered a health food in Asia. Some studies reported that sesame seeds contain high concentrations of sesamin and sesaminol along with their glycosides.30,31 During the fermentation of sesame sauce, sesamin is transformed by starters of the genus Asperillus into sesaminol monoglucoside (13.7 μg/g), sesaminol triglucoside (0.2 μg/g), sesaminol 6-catechol (1.1 μg/g),2 and epsisesamin 2,6-dicatechol.32 We also previously found that FSeS is enriched with sesamin (13 μg/g).2
Unlike fermented sauces, AHSS is a product prepared from defatted soybeans (or other protein-rich materials) and 18% food-grade hydrochloric acid by heating (107°C) for 15–20 h. This sauce mainly contains reducing sugars and amino acids.1 Acid hydrolysis of defatted soybeans is an important process in AHSS manufacturing.1 During this procedure, residual fatty acid esters (glycerol) exist in the raw soy sauce materials in the form of chloropropanols.33 Among these compounds, 1,3-dichloropropan-2-ol (1,3-DCP) and 3-chloropropane-1,2-diol (3-MCPD) are two of the most toxic.34,35 1,3-DCP has been deemed a genotoxic carcinogen and has mutagenic effects on bacterial and mammalian cells in vitro.36 3-MCPD is absorbed by the gastrointestinal tract and metabolized into glycidol, a genotoxic carcinogen that induces mutagenesis in humans and rodents.37 As shown in Table 1, total phenol contents of FSS and FSeS were higher than those of AHSS. It has been suggested that more free-form and aglycone phenolic compounds can be formed during fermentation. However, the phenolic compounds can exist as complex forms without fermentation in AHSS. Shao38 reported that the level of phenolic compounds is increased during soy sauce fermentation and showed that in vitro antioxidant, anticancer, and antimutagenic activities are increased. Jung et al.39 also reported that doenjang (Korean fermented soy paste) exerts stronger antitumor and antimetastatic effects when fermented for longer periods of time. Our data indicated that fermentation is able to increase the production of bioactive phenolic compounds and could play an important role in preventing colitis.
We also observed that 4 mL/kg of fermented sauce had a greater preventive effect against DSS-induced colon shortening compared to 8 mL/kg. In addition, the lower dose of fermented sauces increased the ratios of colon weight to colon length and effectively ameliorated DSS-induced edema, crypt dilation, goblet cell depletion, and infiltration of inflammatory cells in the colonic mucosa.
Both fermented sauces as well as AHSS contained high levels of salt (approximately 18–20% of NaCl). High salt intake can result in gastrointestinal damage, increased DNA replication, and cell proliferation.40 High levels of NaCl also exert a comutagenic effect in the Ames test.41 In our previous studies, FSS showed an in vitro antioxidant activity (approximately 28%); however, the salt used to make soy sauce was not found to have the antioxidant activity (<1%) at the same concentrations (data not shown). Thus, kanjang is a more healthy condiment compared to salt alone when used for cooking and as a food flavoring.
In active cases of UC, the inflamed colonic mucosa has increased electrical conductivity and enhanced permeability to monovalent ions.42 In the descending/sigmoid colon and rectum, inflammation causes a notable decrease or loss of the lumen-negative transmucosal potential difference, a consequence of both increased epithelial permeability and the virtual absence of electrogenic Na+ transport.43,44 We found that 8 mL/kg of the sauces did not possess effective anticolitic activity (compared to 4 mL/kg of the sauces) in the mice treated with DSS. This difference is thought to be associated with the greater NaCl levels in the higher dose of fermented sauces. This finding emphasizes the importance of identifying appropriate concentrations of kangjang or NaCl to prevent UC.
Imbalances between pro- and anti-inflammatory cytokines are associated with the development of UC and are dramatically shifted toward the proinflammatory factors.22,23 Downregulating the production of proinflammatory cytokines such as TNF-α, IL-1β, IL-6, IL-17α, and IFN-γ in the intestine can inhibit inflammatory responses and successfully ameliorate UC, as indicated by a previous clinical study, in humans and animal models of DSS-induced UC.45 In the present investigation, we found that FSeS and FSS both reduced the serum levels of proinflammatory cytokines (TNF-α, IL-6, IL-17α, and IFN-γ) and also inhibited colonic mRNA expression of these cytokines compared to AHSS. A number of studies have reported that the main bioactive compounds in soy sauce, including genistein and daidzein, effectively reduce the levels of TNF-α, an important inflammatory mediator, elevated by IBD in experimental rodent models.46–48 Genistein also fails to decrease T-cell proliferation and reduces the production of IL-1β and IL-6 in lipopolysaccharide (LPS)-treated mice.49 Sesamin and sesamolin have been shown to inhibit TNF-α, IL-1β, and IL-6 activity in vitro and in vivo.50–52
In addition to proinflammatory cytokines, overexpression of iNOS and COX-2 in the colonic mucosa is associated with UC pathogenesis.53,54 Following treatment with 4 mL/kg of fermented sauces (FSeS or FSS), the colonic mRNA expressions of iNOS and COX-2 were reduced compared to the levels found in AHSS-treated mice with DSS-induced colitis. The main bioactive compounds in FSeS and FSS, such as genistein, daidzein, sesamin, and sesamolin, can decrease LPS-induced production of nitric oxide (NO), an important proinflammatory mediator, during IBD pathogenesis in vitro49,55,56 and in vivo.50 Genistein and daidzein can also inhibit LPS-induced iNOS expression by decreasing the activities of nuclear factor-κB (NF-κB) as well as signal transducer and activator of transcription-1 (STAT-1), two important transcription factors, in murine J774 macrophages.55 In addition, genistein, daidzein, and sesamin reduce COX-2 activity in vitro.57–59 Based on these results, we suggest that fermented sauces have anti-inflammatory effects on DSS-induced UC.
In conclusion, the results from the present study indicated that fermented sauces, FSeS and FSS, administered orally at 4 mL/kg significantly prevent DSS-induced body weight loss, colonic shortening, and intestinal wall thickening in mice. FSeS and FSS administration also decreased the serum and colonic mRNA levels of proinflammatory cytokines (TNF-α, IFN-γ, IL-6, and IL-17α), iNOS, and COX-2. Furthermore, 4 mL/kg of the fermented sauce samples more effectively attenuated DSS-induced colitis than 8 mL/kg of the sauces. FSeS exhibited more potent anticolitic effects than FSS, indicating that raw materials for the fermentation process are important for increasing the functionality of the sauces. AHSS could not confer protection against colitis. Taken together, the results from the current investigation suggest that fermented sauces, particularly FSeS, have a potent anticolitic efficacy. Our findings also indicated that the fermentation process is very important for enhancing the levels of functional compounds and improving the beneficial properties of kangjang. Finally, appropriate intake of fermented sauce is important to maximize the anticolitic activity.
Acknowledgments
This work was supported by a research grant from the Daesang Corporation and a grant (BCUM-B11025) from the National Center of Efficacy Evaluation for the Development of Health Products Targeting Digestive Disorders (NCEED) funded by the Ministry of Health and Welfare, South Korea.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Liu K: Soybean as Functional Foods and Ingredients. AOAC Press, Champaign, IL, 2004, p. 269 [Google Scholar]
- 2.Song JL: Anticancer effects of fermneted sesame sauce. Dcotoral Thesis, Pusan National University, Busan, Korea, 2012 [Google Scholar]
- 3.Ham SS, Kim SH, Yoo SJ, et al. : Biological activities of soybean sauce (Kanjang) supplemented with deep sea water and sea tangle. Korean J Food Preserv 2008;15:274–279 [Google Scholar]
- 4.Yoon KD, Kwon DJ, Hong SS, Kim SI, Chung KS: Inhibitory effect of soybean and fermented soybean products on the chemically induced mutagenesis. Korean J Appl Microbiol Biotechnol 1996;24:525–528 [Google Scholar]
- 5.Aoshima H, Ooshima S: Anti-hydrogen peroxide activity of fish and soy sauce. Food Chem 2009;112:339–343 [Google Scholar]
- 6.Wang H, Jenner AM, Lee CY, et al. : The identification of antioxidants in dark soy sauce. Free Radic Res 2007;41:479–488 [DOI] [PubMed] [Google Scholar]
- 7.Song JL, Choi JH, Seo JH, Kil JH, Park KY: Antioxidative effects of fermented sesame sauce against hydrogen peroxide-induced oxidative damage in LLC-PK1 porcine renal tubule cells. Nutr Res Pract 2014;8:138–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benjamin H, Storkson J, Nagahara A, Pariza MW: Inhibition of benzo (a) pyrene-induced mouse forestomach neoplasia by dietary soy sauce. Cancer Res 1991;51:2940–2942 [PubMed] [Google Scholar]
- 9.Nagahara A, Benjamin H, Storkson J, et al. : Inhibition of benzo [a] pyrene-induced mouse forestomach neoplasia by a principal flavor component of Japanese-style fermented soy sauce. Cancer Res 1992;52:1754–1756 [PubMed] [Google Scholar]
- 10.Ito A, Watanabe H, Basaran N: Effects of soy products in reducing risk of spontaneous and neutron-induced liver-tumors in mice. Int J Oncol 1993;2:773–776 [DOI] [PubMed] [Google Scholar]
- 11.Steidler L, Hans W, Schotte L, et al. : Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science 2000;289:1352–1355 [DOI] [PubMed] [Google Scholar]
- 12.Yang SK, Yun S, Kim JH, et al. : Epidemiology of inflammatory bowel disease in the Songpa-Kangdong district, Seoul, Korea, 1986–2005: A KASID study. Inflamm Bowel Dis 2008;14:542–549 [DOI] [PubMed] [Google Scholar]
- 13.Abraham C, Cho JH: Inflammatory Bowel Disease. N Engl J Med 2009;361:2066–2078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baumgart DC, Carding SR: Inflammatory bowel disease: cause and immunobiology. Lancet 2007;369:1627–1640 [DOI] [PubMed] [Google Scholar]
- 15.Mudter J, Neurath MF: IL-6 signaling in inflammatory bowel disease: Pathophysiological role and clinical relevance. Inflamm Bowel Dis 2007;13:1016–1023 [DOI] [PubMed] [Google Scholar]
- 16.Rovedatti L, Kudo T, Biancheri P, et al. : Differential regulation of interleukin 17 and interferon γ production in inflammatory bowel disease. Gut 2009;58:1629–1636 [DOI] [PubMed] [Google Scholar]
- 17.Okayasu I, Hatakeyama S, Yamada M, et al. : A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology 1990;98:694–702 [DOI] [PubMed] [Google Scholar]
- 18.Fischer RB, Peters DG: Basic Theory and Practice of Quantitative Chemical Analysis, 3rd edition. WB Saunders, Philadelphia, PA, USA: 1968, pp. 375–396 [Google Scholar]
- 19.Miller GL: Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 1959;31:426–428 [Google Scholar]
- 20.Beddows C, Ismail M, Steinkraus K: The use of bromelain in the hydrolysis of mackerel and the investigation of fermented fish aroma. Int J Food Sci Technol 1976;11:379–388 [Google Scholar]
- 21.Taga MS, Miller E, Pratt DE: Chia seeds as a source of natural lipid antioxidants. J Am Oil Chem Soc 1984;61:928–931 [Google Scholar]
- 22.Fiocchi C: Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology 1998;115:182–205 [DOI] [PubMed] [Google Scholar]
- 23.Hendrickson BA, Gokhale R, Cho JH: Clinical aspects and pathophysiology of inflammatory bowel disease. Clin Microbiol Rev 2002;15:79–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kudou S, Fleury Y, Welti D, et al. : Malonyl isoflavone glycosides in soybean seeds (Glycine max Merrill). Agric Biol Chem 1991;55:2227–2233 [Google Scholar]
- 25.Wang H, Murphy PA: Isoflavone composition of American and Japanese soybeans in Iowa: effects of variety, crop year, and location. J Agric Food Chem 1994;42:1674–1677 [Google Scholar]
- 26.Chang TS, Ding HY, Tai SS, Wu CY: Metabolism of the soy isoflavones daidzein and genistein by fungi used in the preparation of various fermented soybean foods. Biosci Biotechnol Biochem 2007;71:1330–1333 [DOI] [PubMed] [Google Scholar]
- 27.Lee IH, Chou CC: Distribution profiles of isoflavone isomers in black bean kojis prepared with various filamentous fungi. J Agric Food Chem 2006;54:1309–1314 [DOI] [PubMed] [Google Scholar]
- 28.Choi YB, Sohn HS: Isoflavone content in Korean fermented and unfermented soybean foods. Korean J Food Sci Technol 1998;30:745–750 [Google Scholar]
- 29.Kang CS, Lee YS, Kim JS, Hahn YH: High performance liquid chromatographic analysis of isoflavones in soybean foods. Korean J Food Sci Technol 2000;32:25–30 [Google Scholar]
- 30.Katsuzaki H, Kawakishi S, Osawa T: Sesaminol glucosides in sesame seeds. Phytochemistry 1994;35:773–776 [DOI] [PubMed] [Google Scholar]
- 31.Moazzami AA, Andersson RE, Kamal-Eldin A: HPLC analysis of sesaminol glucosides in sesame seeds. J Agric Food Chem 2006;54:633–638 [DOI] [PubMed] [Google Scholar]
- 32.Miyake Y, Fukumoto S, Okada M, et al. : Antioxidative catechol lignans converted from sesamin and sesaminol triglucoside by culturing with Aspergillus. J Agric Food Chem 2005;53:22–27 [DOI] [PubMed] [Google Scholar]
- 33.Collier P, Cromie DDO, Davies A: Mechanism of formation of chloropropanols present in protein hydrolysates. J Am Oil Chem Soc 1991;68:785–790 [Google Scholar]
- 34.Crews C, Le Brun G, Hough P, Harvey D, Brereton P: Chlorinated propanols and levulinic acid in soy sauces. Czech J Food Sci 2000;18:276–277 [Google Scholar]
- 35.Lynch BS, Bryant DW, Hook GJ, Nestmann ER, Munro IC: Carcinogenicity of monochloro-1, 2-propanediol (α-chlorohydrin, 3-MCPD). Int J Toxicol 1998;17:47–76 [Google Scholar]
- 36.BIBRA Working Group: 1,3-dichloro-2-propanol. In Toxicity profile. TNO-BIBRA International Ltd., Carshalton, Surrey, UK: 1999 [Google Scholar]
- 37.Habermeyer M, Guth S, Eisenbrand G: Identification of gaps in knowledge concerning toxicology of 3‐MCPD and glycidol esters. Eur J Lipid Sci Technol 2011;113:314–318 [Google Scholar]
- 38.Shao LN: Studies on physicochemical changes and cancer preventive effects of fermented soy sauce during ripening period. Master Thesis, Pusan National University, Busan, Korea, 2009 [Google Scholar]
- 39.Jung KO, Park SY, Park KY: Longer aging time increases the anticancer and antimetastatic properties of doenjang. Nutrition 2006;22:539–545 [DOI] [PubMed] [Google Scholar]
- 40.Tuyns AJ: Salt and gastrointestinal cancer. Nutr Cancer 1988;11:229–232 [DOI] [PubMed] [Google Scholar]
- 41.Kim SH, Park KY, Suh MJ: Comutagenic effect of sodium chloride in the Salmonella/mammalian microsome assay. Food Biotechnol 1995;4:264–267 [Google Scholar]
- 42.Sandle G, Higgs N, Crowe P, et al. : Cellular basis for defective electrolyte transport in inflamed human colon. Gastroenterology 1990;99:97–105 [DOI] [PubMed] [Google Scholar]
- 43.Sandle G, Hayslett J, Binder H: Effect of glucocorticoids on rectal transport in normal subjects and patients with ulcerative colitis. Gut 1986;27:309–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Irving PM, Shanahan F, Rampton DS: Drug interactions in inflammatory bowel disease. Am J Gastroenterol 2008;103:207–219 [DOI] [PubMed] [Google Scholar]
- 45.Morimoto M, Watanabe T, Yamori M, Takebe M, Wakatsuki Y: Isoflavones regulate innate immunity and inhibit experimental colitis. J Gastroenterol Hepatol 2009;24:1123–1129 [DOI] [PubMed] [Google Scholar]
- 46.Sakai T, Furoku S, Nakamoto M, et al. : Soy isoflavone equol perpetuates dextran sulfate sodium-induced acute colitis in mice. Biosci Biotechnol Biochem 2011;75:593–595 [DOI] [PubMed] [Google Scholar]
- 47.Seibel J, Molzberger AF, Hertrampf T, Laudenbach-Leschowski U, Diel P: Oral treatment with genistein reduces the expression of molecular and biochemical markers of inflammation in a rat model of chronic TNBS-induced colitis. Eur J Nutr 2009;48:213–220 [DOI] [PubMed] [Google Scholar]
- 48.Jeng KCG, Hou RCW: Sesamin and sesamolin: natures therapeutic lignans. Curr Enzyme Inhib 2005;1:11–20 [Google Scholar]
- 49.Kao TH, Wu WM, Hung CF, Wu WB, Chen BH: Anti-inflammatory effects of isoflavone powder produced from soybean cake. J Agric Food Chem 2007;55:11068–11079 [DOI] [PubMed] [Google Scholar]
- 50.Jeng KCG, Hou RCW, Wang JC, Ping LI: Sesamin inhibits lipopolysaccharide-induced cytokine production by suppression of p38 mitogen-activated protein kinase and nuclear factor-κB. Immunol Lett 2005;97:101–106 [DOI] [PubMed] [Google Scholar]
- 51.Utsunomiya T, Chavali SR, Zhong WW, Forse RA: Effects of sesamin-supplemented dietary fat emulsions on the ex vivo production of lipopolysaccharide-induced prostanoids and tumor necrosis factor α in rats. Am J Clin Nutr 2000;72:804–808 [DOI] [PubMed] [Google Scholar]
- 52.Kankuri E, Asmawi MZ, Korpela R, Vapaatalo H, Moilanen E: Induction of iNOS in a rat model of acute colitis. Inflammation 1999;23:141–152 [DOI] [PubMed] [Google Scholar]
- 53.Sakamoto C: Roles of COX-1 and COX-2 in gastrointestinal pathophysiology. J Gastroenterol 1998;33:618–624 [DOI] [PubMed] [Google Scholar]
- 54.Hämäläinen M, Nieminen R, Vuorela P, Heinonen M, Moilanen E: Anti-inflammatory effects of flavonoids: genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-κB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-κB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediat Inflamm 2007;2007:45673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim AR, Cho JY, Zou Y, Choi JS, Chung HY: Flavonoids differentially modulate nitric oxide production pathways in lipopolysaccharide-activated RAW264.7 cells. Arch Pharm Res 2005;28:297–304 [DOI] [PubMed] [Google Scholar]
- 56.Liang YC, Huang YT, Tsai SH, et al. : Suppression of inducible cyclooxygenase and inducible nitric oxide synthase by apigenin and related flavonoids in mouse macrophages. Carcinogenesis 1999;20:1945–1952 [DOI] [PubMed] [Google Scholar]
- 57.Mutoh M, Takahashi M, Fukuda K, et al. : Suppression of cyclooxygenase-2 promoter-dependent transcriptional activity in colon cancer cells by chemopreventive agents with a resorcin-type structure. Carcinogenesis 2000;21:959–963 [DOI] [PubMed] [Google Scholar]
- 58.Harikumar KB, Sung B, Tharakan ST, et al. : Sesamin manifests chemopreventive effects through the suppression of NF-κB–regulated cell survival, proliferation, invasion, and angiogenic gene products. Mol Cancer Res 2010;8:751–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hsieh PF, Hou CW, Yao PW, et al. : Sesamin ameliorates oxidative stress and mortality in kainic acid-induced status epilepticus by inhibition of MAPK and COX-2 activation. J Neuroinflammation 2011;8:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]