Abstract
Background and objectives
Treatment with azathioprine within 3 months of remission induction with cyclophosphamide is a common treatment strategy for patients with ANCA-associated vasculitis. This study comprised patients undergoing long-term follow-up who were randomly allocated to azathioprine after 3–6 months or after 12 months of cyclophosphamide treatment.
Design, setting, participants, & measurements
Patients from 39 European centers between 1995 and 1997 with a new diagnosis of ANCA-associated vasculitis that involved the kidneys or another vital organ were eligible. At the time of diagnosis, participants were randomly allocated to convert to azathioprine after 3–6 months (the azathioprine group) or after 12 months of cyclophosphamide (the cyclophosphamide group). Patients who did not achieve a remission within 6 months were excluded. This study assessed relapses, ESRD, and death during long-term follow-up.
Results
Patients were allocated to the azathioprine group (n=71) and the cyclophosphamide group (n=73). Of these patients, 63 (43.8%) developed a relapse, 35 (24.3%) developed a renal relapse, 13 (9.0%) developed ESRD, and 21 (14.6%) died. Although there were worse outcomes in the azathioprine group, none were statistically significant. The subdistribution hazard ratio [sHR] for relapse was 1.63 (95% confidence interval [95% CI], 0.99 to 2.71), the composite of relapse or death hazard ratio [HR] was 1.59 (95% CI, 1.00 to 2.54), the ESRD sHR was 1.71 (95% CI, 0.56 to 5.19), and the death HR was 0.75 (95% CI, 0.32 to 1.79).
Conclusions
It remains uncertain whether converting to azathioprine after 3–6 months of induction cyclophosphamide therapy is as effective as converting after 12 months. Outcomes are still poor for this group of patients and further research is required to determine the optimal timing of maintenance therapy.
Keywords: vasculitis, GN, randomized controlled trials, epidemiology, outcomes
Introduction
Granulomatosis with polyangiitis (Wegener’s) and microscopic polyangiitis (MPA) are commonly associated with ANCA and are collectively referred to as ANCA-associated vasculitis (AAV). Initial immunosuppressive therapy with cyclophosphamide and glucocorticoids is the standard of care for patients with generalized disease (1,2). Although cyclophosphamide is effective at inducing remission of the disease, relapses are common irrespective of treatment. Furthermore, cyclophosphamide is associated with increasing toxicity as cumulative doses rise. The risks of infections in the short term and malignancy in the longer term are of particular concern (3–6).
Several strategies to reduce exposure to cyclophosphamide and mitigate its toxic effects while retaining disease control have been tested in randomized controlled trials (7–11). The Cyclophosphamide versus Azathioprine for Early Remission Phase of Vasculitis (CYCAZAREM) trial tested the effect of replacing daily oral cyclophosphamide with azathioprine after induction of remission rather than after 1 year of treatment in patients with generalized AAV and a serum creatinine≤5.6 mg/dl at presentation (7). At the time the trial closed (18 months after treatment started), 21 patients had a relapse of AAV with almost equal numbers observed between groups. Similarly, almost equal numbers experienced severe or life-threatening adverse events. Because no significant differences were observed between treatment groups, the trial concluded that cyclophosphamide can be withdrawn after induction of remission and a minimum of 3 months of cyclophosphamide therapy is given.
Whether the reduction in cyclophosphamide exposure in the first year of treatment affects the long-term course of AAV is uncertain. Recent experiences reducing cyclophosphamide exposure by use of pulses rather than daily dosing resulted in an increased risk for relapse, and replacing cyclophosphamide with methotrexate also suggested a higher risk and greater need for later immunosuppression (12,13). Similarly, introduction of azathioprine as early replacement for cyclophosphamide was associated with more relapses over 5 years in a retrospective cohort study (14). Given the uncertainty of the long-term consequences of early substitution of azathioprine for cyclophosphamide in generalized AAV, we conducted extended follow-up of patients enrolled in the CYCAZREM trial.
Materials and Methods
Design
The CYCAZAREM trial was conducted in accordance with the Declaration of Helsinki and the protocol is previously described and available online (www.vasculitis.org) (7). Briefly, CYCAZAREM was an open-label, two-parallel-group randomized controlled trial that recruited from 39 centers in 11 European countries between 1995 and 1997. Patients were centrally allocated to one of two treatment groups by the method of minimization.
Patients
Eligible patients had granulomatosis with polyangiitis, MPA, or the renal limited form of MPA with renal involvement and/or threatened loss of other vital organ function. Patients had either a positive ANCA test by immunofluorescence or ELISA or vasculitis confirmed by a biopsy. Patients with severe renal involvement with a serum creatinine level of >5.6 mg/dl or that required dialysis were excluded. Registered patients that met the eligibility criteria and achieved disease remission, defined as the absence of disease activity documented with a Birmingham vasculitis activity score of zero, between 3 and 6 months went on to receive randomized treatment.
Treatment
All patients received the same remission-induction therapy composed of daily oral cyclophosphamide 2 mg/kg per day (reduced by 25 mg per day for age>60 years) and a tapering course of daily oral prednisolone (initially at 1 mg/kg per day) until remission. Those in remission between 3 and 6 months after trial registration were randomly assigned to either stop cyclophosphamide and commence 2 mg/kg azathioprine immediately (azathioprine group) or continue cyclophosphamide treatment until 12 months (cyclophosphamide group). All patients received 1.5 mg/kg azathioprine daily between months 12 and 18. After 18 months, patients were treated according to local standards of care both in terms of maintenance treatment and for the treatment of relapses.
The use of prophylaxis against Pneumocystis jiroveci, gastritis, fungal infection, and fractures was determined by local standards of practice.
Data Collection
Within-trial data were collected prospectively between 1995 and 1999 when the trial closed. Investigators completed standardized data abstraction forms between 2005 and 2008 to assess long-term outcomes. Data from the last in-trial follow-up were used when patients were lost to long-term follow-up.
Study Outcomes
The primary outcome was first relapse. Relapse was defined as new or worsened disease activity that occurred after an initial remission and required a change in therapy. Efficacy was further compared by assessing the outcomes of the composite of relapse and all-cause mortality, the incidence rate of relapses, renal relapse (i.e., relapse that included a renal manifestation), and medication exposure after the end of the trial. Safety was assessed by comparing the outcomes of all-cause mortality, ESRD (i.e., need for permanent dialysis or renal transplantation), and frequency of malignancies.
Statistical Analyses
All patients who entered remission between 3 and 6 months after trial registration were included in all analyses. Descriptive data are presented as the median (25th to 75th percentile) for continuous variables and as the frequency (percentage) for categorical data.
The outcomes were assessed using time-to-event analyses. The effect of treatment assignment on the primary outcome (relapse) was estimated using competing risk regression methods which jointly model the subdistributions of the primary and competing event (15). The subdistribution hazard ratio (sHR) was calculated for the time to first relapse for patients in the azathioprine group compared with those in the cyclophosphamide group while considering the competing risk of death. The competing risk paradigm was used because death may informatively censor patients (e.g., if one of the treatments increased the risk of early death, such as by infections, and thereby removed patients from the risk set for relapse). Similarly, we used competing risk models to assess renal relapses using both death and ESRD as competing risks and for the outcome of ESRD using death as a competing risk. We assessed all-cause mortality using a Cox proportional hazard model because there is no competing risk, and we calculated the hazard ratio (HR). We calculated the incidence rate ratio (IRR) for all relapses over the entire follow-up period.
The frequency of patients with at least one malignancy was compared using Fisher’s exact test. Medication exposure was assessed by comparing the frequency of any use of prednisone, cyclophosphamide, and other immunosuppressants during each predefined time period after the end of the original trial (i.e., 18–24, 25–36, 37–48, and 49–60 months of follow-up). Treatment groups were compared independently for each time period using Fisher’s exact test.
Estimates of the effect of allocation to the azathioprine group, their 95% confidence intervals (95% CIs), and associated P values were calculated for each outcome. A two-tailed P value <0.05 was considered significant for all analyses with no adjustments made for multiple comparisons. Subgroup analyses were performed to determine whether treatment effects were different for patients who were positive for an anti-proteinase 3 (PR3) antibody compared with patients who were negative for an anti-PR3 antibody. Sensitivity analyses were performed by adjusting for anti-PR3 antibody status and for serum creatinine, important risk factors for relapse. All analyses were performed with Stata 11 MP software (College Station, TX).
Results
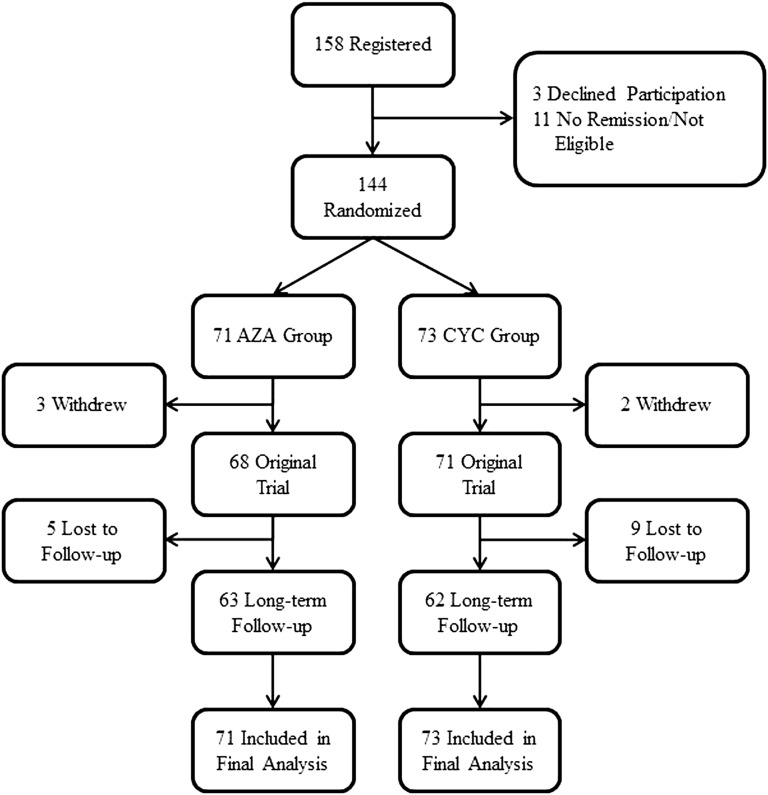
Figure 1 summarizes the flow of patients. Of the 158 patients registered, 144 (93%) were randomized to either the azathioprine group (71 allocated to azathioprine after 3–6 months of cyclophosphamide) or the cyclophosphamide group (73 allocated to azathioprine after 12 months of cyclophosphamide). Of these patients, 125 (87%) completed long-term follow-up (63 of 71 [89%] in the azathioprine group and 62 of 73 [85%] in the cyclophosphamide group). All analyses included all 144 patients. These 144 patients contributed a total of 1016 patient-years of follow-up with a median follow-up time of 8.5 years. Patient characteristics are summarized in Table 1.
Figure 1.
Patient flow. AZA, azathioprine group; CYC, cyclophosphamide group.
Table 1.
Patient characteristics
| Characteristic | AZA (n=71) | CYC (n=73) | All |
|---|---|---|---|
| Age (yr) | 56 (47–66) | 57 (45–67) | 57 (47–67) |
| Women | 41 (58) | 35 (48) | 76 (53) |
| GPA | 46 (65) | 46 (63) | 92 (64) |
| Anti-PR3+ | 44 (62) | 46 (63) | 90 (63) |
| Creatinine (mg/dl) | 1.6 (0.93–2.41) | 2.35 (1.21–3.51) | 1.79 (1.09–2.99) |
| Birmingham vasculitis activity score | 17.5 (7.5–24) | 16 (10–24) | 17 (10–24) |
Data are presented as the median (interquartile range; 25th to 75th percentile) or n (%). GPA, granulomatosis with polyangiitis/Wegener’s; anti-PR3+, anti-proteinase 3 antibody or positive for cytoplasmic ANCA if an ELISA was not performed; AZA, azathioprine group; CYC, cyclophosphamide group.
Efficacy Outcomes
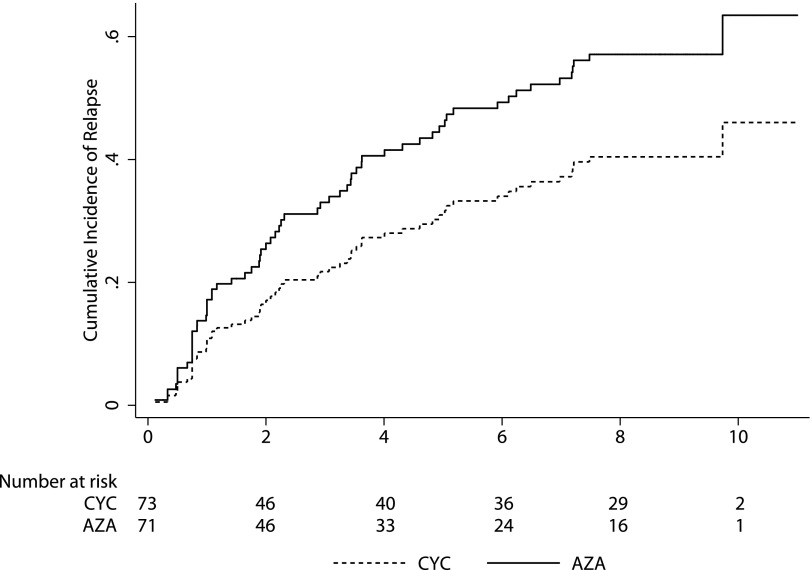
Sixty-three patients experienced a relapse: 37 (52%) in the azathioprine group and 26 (36%) in the cyclophosphamide group (sHR, 1.63; 95% CI, 0.99 to 2.71; P=0.06) (Figure 2). The composite outcome of relapse and death was also more common in the azathioprine group (HR, 1.59; 95% CI, 1.00 to 2.54; P=0.05). The incidence of relapse was not significantly higher in the azathioprine group (IRR, 1.25; 95% CI, 0.86 to 1.82; P=0.22). Of first relapses, 19 (27%) were renal in the azathioprine group compared with 16 (22%) in the cyclophosphamide group (sHR, 1.25; 95% CI, 0.65 to 2.43; P=0.50). ESRD occurred in eight patients (11%) in the azathioprine group and five patients (7%) in the cyclophosphamide group (sHR, 1.71; 95% CI, 0.56 to 5.19; P=0.35). No significant differences were observed between groups with regard to the proportion of patients receiving cyclophosphamide, corticosteroids, and/or other immunosuppressants during months 19–60 (Table 2).
Figure 2.
Cumulative incidence of relapse of ANCA-associated vasculitis by allocated maintenance treatment group. Cumulative incidence is adjusted for the competing risk of death. AZA, azathioprine group; CYC, cyclophosphamide group.
Table 2.
Therapy beyond the 18-month trial period for patients in the CYCAZAREM trial
| Time after Randomization (mo) | Cyclophosphamide | Other Immunosuppressantsa | Prednisolone | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CYC | AZA | P Value | CYC | AZA | P Value | CYC | AZA | P Value | |
| 19–24 | 9 (17) | 6 (11) | 0.58 | 37 (68) | 36 (69) | >0.99 | 47 (81) | 46 (82) | 0.99 |
| 25–36 | 4 (8) | 6 (12) | 0.74 | 28 (56) | 30 (64) | 0.53 | 35 (65) | 40 (77) | 0.20 |
| 37–48 | 2 (4) | 4 (8) | 0.68 | 23 (47) | 28 (59) | 0.23 | 28 (53) | 31 (61) | 0.43 |
| 49–60 | 1 (2) | 4 (8) | 0.36 | 20 (43) | 27 (60) | 0.14 | 21 (42) | 28 (56) | 0.23 |
Data are presented as n (%). During all time intervals, patients with missing data were excluded from analysis. P values were obtained by Fisher’s exact test. CYCAZAREM, Cyclophosphamide versus Azathioprine for Early Remission Phase of Vasculitis.
Azathioprine, methotrexate, tacrolimus, gusperimus, dapsone, infliximab, and/or mycophenolate mofetil.
Subgroup analysis of patients who were positive for anti-PR3 antibody compared with those who were negative for anti-PR3 antibody did not reveal a significant difference between the azathioprine and cyclophosphamide groups (interaction P=0.71). Of patients who were positive for anti-PR3 antibody, 27 (61%) in the azathioprine group and 19 (41%) in the cyclophosphamide group had at least one relapse (sHR, 1.66; 95% CI, 0.93 to 2.98). Of patients who were negative for anti-PR3 antibody, 10 (37%) in the azathioprine group and seven (26%) in the cyclophosphamide group had at least one relapse (sHR, 1.57; 95% CI, 0.59 to 4.18). In the sensitivity analysis adjusted for serum creatinine and proteinase 3 status, the sHR for the azathioprine group was not substantially changed (sHR, 1.56; 95% CI, 0.93 to 2.63).
Safety Outcomes
The groups did not differ significantly with respect to any of the safety outcomes. Death occurred in nine patients (13%) in the azathioprine group and 12 patients (16%) in the cyclophosphamide group (HR, 0.75; 95% CI, 0.32 to 1.79; P=0.52). The distribution of causes of death did not differ significantly between groups (Table 3). Malignancies occurred in six patients (8%) in the azathioprine group and 11 patients (15%) in the cyclophosphamide group (P=0.30).
Table 3.
Contributing causes of death
| Cause of Death | CYC (n=12) | AZA (n=9) | P Valuea |
|---|---|---|---|
| Active vasculitis | 0 | 1 | 0.49 |
| Infection | 4 | 8 | 0.24 |
| Malignancy | 3 | 3 | 0.99 |
| Cardiovascular | 6 | 1 | 0.11 |
| Unknown | 4 | 2 | 0.68 |
Patients can have more than one contributing cause of death. CYC, cyclophosphamide group; AZA, azathioprine group.
P value from Fisher’s exact test.
Discussion
We assessed the long-term outcomes of patients with generalized AAV that started maintenance therapy with azathioprine immediately after remission induction or after 12 months of cyclophosphamide therapy. Earlier initiation of azathioprine did not result in a statistically significant loss of efficacy although relapses were numerically more frequent and immunosuppressive drugs and glucocorticoids were used more frequently. Although there is no clear disadvantage associated with early initiation of azathioprine, further research is required to determine whether this treatment regimen increases the long-term risk of relapse.
Cyclophosphamide-related side effects such as infections, hemorrhagic cystitis, infertility, and malignancy raised serious concerns over its safety and prompted several trials exploring treatment regimens to limit exposure in AAV. However, cyclophosphamide is recognized as one of the factors that transformed outcomes for patients with AAV, and reducing its use could inadvertently worsen disease activity–related outcomes. Two long-term follow-up studies based on randomized trials assessing strategies to reduce cyclophosphamide exposure in AAV demonstrated a higher relapse risk in the treatment groups with limited or no cyclophosphamide exposure (12,13). Furthermore, the relative increase in relapse risk observed after cyclophosphamide -sparing therapy in these studies was comparable with the increase in relapse risk observed in the azathioprine group in our study (12,13). The consistency of these findings suggests that low cumulative cyclophosphamide exposure during the initial 6–12 months of treatment may increase longer-term relapse risk despite the lack of statistical significance in individual trials.
If higher initial exposure to cyclophosphamide reduces the risk of relapse in the long-term, the choice of induction regimen may be tailored to patients’ risk of relapse and risk of cyclophosphamide-related comorbidity. For example, patients with a high risk of a relapse and a low risk of cyclophosphamide-induced complications may rationally opt for longer durations of cyclophosphamide therapy. In the past, the need for further cyclophosphamide exposure as a result of major relapses of AAV, and therefore higher lifetime cumulative doses, was part of the motivation for initial cyclophosphamide-sparing regimens. However, in the era of increasing choices of induction agents (e.g., rituximab) for major relapses, this may be less of a concern for the treatment of newly diagnosed disease. Furthermore, if higher initial exposure to cyclophosphamide results in fewer relapses, the lifetime exposure to cyclophosphamide may actually be reduced in selected patients.
In our long-term follow-up study, almost one half of all patients experienced a relapse and more than one half continued to receive immunosuppressive drugs up to 5 years after diagnosis. Furthermore, 21% of patients developed ESRD or died. These findings highlight that although outcomes have improved for patients with AAV, a need for more effective and safer treatment remains, which is a message that has not changed substantially in 20 years (15). Future clinical trials will need to evaluate longer-term outcomes.
Our study has several notable strengths. It builds on one of the few randomized controlled trials to assess early conversion from cyclophosphamide to azathioprine, a practice that has been widely adopted (1,2,16). Patient follow-up was largely complete and the consistency of results within and between trials strengthens the findings. Finally, our findings were consistent between subgroups and in sensitivity analyses, which suggests they are robust.
Our results must also be considered in the context of their limitations. The trial, although large for a rare disease, was not adequately powered to detect moderate differences in the risk of disease relapse. Thus, we are unable to exclude the possibility that a clinically important increase in the long-term risk of relapse with early conversion to azathioprine exists. Furthermore, there was a modest difference in the serum creatinine concentrations at the time of randomization. However, adjustment for potential confounders such as creatinine did not materially alter the results. We also lack information to determine the severity of the relapses that occurred after the 18-month trial period. A qualitative difference in the severity of relapses could make the observed difference in the frequency of relapses more or less clinically relevant. Data on cumulative drug doses during months 19–60 were not collected, and a more detailed comparison of drug exposures in the two treatment groups was not possible. Further follow-up and epidemiologic data, as well as data from other ongoing trials, will help further clarify the potential risks and benefits of early conversion to azathioprine and the risks of increased cyclophosphamide exposure (e.g., malignancy).
Uncertainty remains regarding whether initiating azathioprine 3–6 months after induction of remission with cyclophosphamide is less effective for the prevention of relapses than initiating azathioprine after 12 months of cyclophosphamide treatment. There may be a trade-off between the higher risk of relapse and the risk of other adverse outcomes that are likely associated with higher cyclophosphamide exposure. Further study is required to determine whether some patients should have the initiation of azathioprine therapy delayed after achieving a clinical remission.
Disclosures
None.
Acknowledgments
This trial was supported by grants from the European League against Rheumatism, the European Union European Community Systemic Vasculitis Trial project (BMH1-CT93-1078 and CIDP-CT94-0307), and the European Union Associated Vasculitis European Randomized Trial project (BMH4-CT97-2328 and IC20-CT97-0019). M.W. is supported by a Kidney Research Scientist Core Education National Training New Investigator Award from the Canadian Institutes of Health Research, Kidney Foundation of Canada, and Canadian Society of Nephrology. C.D.P. is supported by the National Institute for Health Research Biomedical Research Centre. D.R.W.J. is supported by the Cambridge Biomedical Research Centre.
M.W. and M.F. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
The CYCAZAREM investigators are: Belgium: Erasmus Hospital, Brussels—D. Abramowicz, M. Wissing; Universitaire Ziekenhuis, Leuven—D. Blockmans; Edith Cavell Medical Institute, Brussels—P. Madhoun; AZ VUB, Brussels—J. Sennesael; IMC de Tournai, Tournai—J. Stolear. Czech Republic: Charles University Hospital, Prague—V. Tesar, V. Chabova, I. Rychlik. Denmark: Rigshospitalet, Copenhagen—N. Rasmussen. Finland: University of Helsinki, Helsinki—A. Ekstrand, C. Grönhagen—Riska. France: Hôpital Necker, Paris—P. Lesavre; Centre Hospitalier, Valenciennes—P. Vanhille. Ireland: St. James Hospital, Dublin—C. Feighery. Germany: The Medical School, Hannover—K. de Groot; Heidelberg University Hospital, Heidelberg—K. Andrassy, O. Hergesell; Klinikum Mannheim, Mannheim—F. van der Woude, R. Nowack; Rheumaklinik, Bad Bramstedt—W. Gross, W. Schmitt; Heinrich Heine Universität, Düsseldorf—M. Schneider, C. Specker; Klinikum Nürnberg, Nürnberg—H. Rupprecht, P. Weber, S. Weidner. Italy: Spedali Civili, Brescia—G. Gregorini; Ospedale San Carlo Borromeo, Milan—A. Sinico. Lithuania: University fo Vilnius, Vilnius—J. Dadonieme. The Netherlands: University Hospital, Groningen—C. Kallenberg, C. Stegeman; Eemland Hospital, Amersfoort—C. Hagen, E. van Gurp; Leiden University Medical Center, Leiden—C. Siegert, C. Verburgh; University of Maastricht, Maastricht—J.W. Cohen Tervaert. Spain: Hospital Clinic I Provincal, Barcelona—E. Mirapeix; Hospital Germans Trias i Pujol, Badalona—A. Serra; Hospital Doctor Josep Trueta, Girona—M. Valles; Hospital Princeps d'España, Llobregat—R. Poveda; Fundació Puigvert, Barcelona—J. Ballarin. Sweden: Huddinge University Hospital, Huddinge—E. Pettersson, A. Bruchfeld; Danderyds Sjukhus, Danderyds—G. Germanis; University Hospital of Lund, Lund—D. Selga; Karolinska Sjukhuset, Stockholm—Z. Heigl, I. Lundberg, E. Svenungssen. United Kingdom: Imperial College, London—G. Gaskin; Southmead Hospital, Bristol—P. Mathieson; Ipswich Hospital, Ipswich—R. Watts; Leicester General Hospital, Leicester—J. Feehally; Royal Free Hospital, London—A. Burns; Western General Hospital, Edinburgh—R. Luqmani; Queen Elizabeth II Hospital, Birmingham—P. Bacon, D. Adu, C. Savage.
Footnotes
M.W. and M.F. contributed equally to this work.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Lapraik C, Watts R, Bacon P, Carruthers D, Chakravarty K, D’Cruz D, Guillevin L, Harper L, Jayne D, Luqmani R, Mooney J, Scott D, BSR and BHPR Standards, Guidelines and Audit Working Group : BSR and BHPR guidelines for the management of adults with ANCA associated vasculitis. Rheumatology (Oxford) 46: 1615–1616, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Mukhtyar C, Guillevin L, Cid MC, Dasgupta B, de Groot K, Gross W, Hauser T, Hellmich B, Jayne D, Kallenberg CG, Merkel PA, Raspe H, Salvarani C, Scott DG, Stegeman C, Watts R, Westman K, Witter J, Yazici H, Luqmani R, European Vasculitis Study Group : EULAR recommendations for the management of primary small and medium vessel vasculitis. Ann Rheum Dis 68: 310–317, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Talar-Williams C, Hijazi YM, Walther MM, Linehan WM, Hallahan CW, Lubensky I, Kerr GS, Hoffman GS, Fauci AS, Sneller MC: Cyclophosphamide-induced cystitis and bladder cancer in patients with Wegener granulomatosis. Ann Intern Med 124: 477–484, 1996 [DOI] [PubMed] [Google Scholar]
- 4.Westman KW, Bygren PG, Olsson H, Ranstam J, Wieslander J: Relapse rate, renal survival, and cancer morbidity in patients with Wegener’s granulomatosis or microscopic polyangiitis with renal involvement. J Am Soc Nephrol 9: 842–852, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Little MA, Nightingale P, Verburgh CA, Hauser T, De Groot K, Savage C, Jayne D, Harper L, European Vasculitis Study (EUVAS) Group : Early mortality in systemic vasculitis: Relative contribution of adverse events and active vasculitis. Ann Rheum Dis 69: 1036–1043, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Heijl C, Harper L, Flossmann O, Stücker I, Scott DG, Watts RA, Höglund P, Westman K, Mahr A, European Vasculitis Study Group (EUVAS) : Incidence of malignancy in patients treated for antineutrophil cytoplasm antibody-associated vasculitis: Follow-up data from European Vasculitis Study Group clinical trials. Ann Rheum Dis 70: 1415–1421, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Jayne D, Rasmussen N, Andrassy K, Bacon P, Tervaert JW, Dadoniené J, Ekstrand A, Gaskin G, Gregorini G, de Groot K, Gross W, Hagen EC, Mirapeix E, Pettersson E, Siegert C, Sinico A, Tesar V, Westman K, Pusey C, European Vasculitis Study Group : A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. N Engl J Med 349: 36–44, 2003 [DOI] [PubMed] [Google Scholar]
- 8.De Groot K, Rasmussen N, Bacon PA, Tervaert JW, Feighery C, Gregorini G, Gross WL, Luqmani R, Jayne DR: Randomized trial of cyclophosphamide versus methotrexate for induction of remission in early systemic antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum 52: 2461–2469, 2005 [DOI] [PubMed] [Google Scholar]
- 9.de Groot K, Harper L, Jayne DR, Flores Suarez LF, Gregorini G, Gross WL, Luqmani R, Pusey CD, Rasmussen N, Sinico RA, Tesar V, Vanhille P, Westman K, Savage CO, EUVAS (European Vasculitis Study Group) : Pulse versus daily oral cyclophosphamide for induction of remission in antineutrophil cytoplasmic antibody-associated vasculitis: A randomized trial. Ann Intern Med 150: 670–680, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Jones RB, Tervaert JW, Hauser T, Luqmani R, Morgan MD, Peh CA, Savage CO, Segelmark M, Tesar V, van Paassen P, Walsh D, Walsh M, Westman K, Jayne DR, European Vasculitis Study Group : Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med 363: 211–220, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Stone JH, Merkel PA, Spiera R, Seo P, Langford CA, Hoffman GS, Kallenberg CG, St Clair EW, Turkiewicz A, Tchao NK, Webber L, Ding L, Sejismundo LP, Mieras K, Weitzenkamp D, Ikle D, Seyfert-Margolis V, Mueller M, Brunetta P, Allen NB, Fervenza FC, Geetha D, Keogh KA, Kissin EY, Monach PA, Peikert T, Stegeman C, Ytterberg SR, Specks U, Group R-IR, RAVE-ITN Research Group : Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med 363: 221–232, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faurschou M, Westman K, Rasmussen N, de Groot K, Flossmann O, Höglund P, Jayne DR, European Vasculitis Study Group : Brief report: Long-term outcome of a randomized clinical trial comparing methotrexate to cyclophosphamide for remission induction in early systemic antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum 64: 3472–3477, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Harper L, Morgan MD, Walsh M, Hoglund P, Westman K, Flossmann O, Tesar V, Vanhille P, de Groot K, Luqmani R, Flores-Suarez LF, Watts R, Pusey C, Bruchfeld A, Rasmussen N, Blockmans D, Savage CO, Jayne D, EUVAS investigators : Pulse versus daily oral cyclophosphamide for induction of remission in ANCA-associated vasculitis: Long-term follow-up. Ann Rheum Dis 71: 955–960, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Sanders JS, Slot MC, Stegeman CA: Maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. N Engl J Med 349: 2072–2073, author reply 2072–2073, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Hoffman GS, Kerr GS, Leavitt RY, Hallahan CW, Lebovics RS, Travis WD, Rottem M, Fauci AS: Wegener granulomatosis: An analysis of 158 patients. Ann Intern Med 116: 488–498, 1992 [DOI] [PubMed] [Google Scholar]
- 16.Menahem S, Hiremagalur B, Mudge D, Toussaint N, Walters G, Caring for Australians with Renal Impairment (CARI) : The CARI guidelines. Induction and maintenance therapy in ANCA-associated systemic vasculitis. Nephrology (Carlton) 13[Suppl 2]: S24–S36, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Fine JP: Regression modeling of competing crude failure probabilities. Biostatistics 2: 85–97, 2001 [DOI] [PubMed] [Google Scholar]