Abstract
Background and objectives
Preoperative anemia adversely affects outcomes of cardiothoracic surgery. However, in patients with CKD, treating anemia to a target of normal hemoglobin has been associated with increased risk of adverse cardiac and cerebrovascular events. We investigated the association between preoperative hemoglobin and outcomes of cardiac surgery in patients with CKD and assessed whether there was a level of preoperative hemoglobin below which the incidence of adverse surgical outcomes increases.
Design, setting, participants, & measurements
This prospective observational study included adult patients with CKD stages 3–5 (eGFR<60 ml/min per 1.73 m2) undergoing cardiac surgery from February 2000 to January 2010. Patients were classified into four groups stratified by preoperative hemoglobin level: <10, 10–11.9, 12–13.9, and ≥14 g/dl. The outcomes were postoperative AKI requiring dialysis, sepsis, cerebrovascular accident, and mortality.
Results
In total, 788 patients with a mean eGFR of 43.5±13.7 ml/min per 1.73 m2 were evaluated, of whom 22.5% had preoperative hemoglobin within the normal range (men: 14–18 g/dl; women: 12–16 g/dl). Univariate analysis revealed an inverse relationship between the incidence of all adverse postoperative outcomes and hemoglobin level. Using hemoglobin as a continuous variable, multivariate logistic regression analysis showed a proportionally greater frequency of all adverse postoperative outcomes per 1-g/dl decrement of preoperative hemoglobin (mortality: odds ratio, 1.38; 95% confidence interval, 1.23 to 1.57; P<0.001; sepsis: odds ratio, 1.31; 95% confidence interval, 1.14 to 1.49; P<0.001; cerebrovascular accident: odds ratio, 1.31; 95% confidence interval, 1.00 to 1.67; P=0.03; postoperative hemodialysis: odds ratio, 1.38; 95% confidence interval, 1.11 to 1.75; P<0.01). Moreover, preoperative hemoglobin<12 g/dl was an independent risk factor for postoperative mortality (odds ratio, 2.6; 95% confidence interval, 1.1 to 7.3; P=0.04).
Conclusions
Similar to the general population, preoperative anemia is associated with adverse postoperative outcomes in patients with CKD. Whether outcomes could be improved by therapeutically targeting higher preoperative hemoglobin levels before cardiac surgery in patients with underlying CKD remains to be determined.
Keywords: anemia, chronic renal disease, cardiovascular disease
Introduction
Anemia is a common complication of CKD, and it is associated with adverse clinical outcomes and poor health–related quality of life (1–3). Surprisingly, normalization of hemoglobin in patients with CKD has not been associated with clear clinical benefits. Recently, several large randomized controlled trials testing the effect of using erythropoietin-stimulating agents (ESAs) to achieve various hemoglobin targets have shown that high hemoglobin target levels are associated with higher rates of adverse outcomes, including cardiovascular mortality, cerebrovascular events, and vascular access thrombosis (4–6). Therefore, the US Food and Drug Administration (FDA) recommends targeting the lowest hemoglobin that will prevent blood transfusion in patients with CKD, and the newest Kidney Disease Improving Global Outcomes and European guidelines state that ESAs should not be used to maintain hemoglobin concentrations>11.5 g/dl (7,8). At the present time, patients with CKD and ESRD die primarily because of cardiovascular events, and a substantial percentage of these patients will require cardiothoracic surgery during their lifespan (9,10). By following the current FDA and European recommendations, most of these patients with CKD will be anemic before surgery. Is the target hemoglobin between 10–12.0 g/dl optimal for these patients before such a stressful intervention? What is the preoperative hemoglobin that will be associated with the lowest incidence of adverse surgical outcomes in the CKD population? On the basis of current evidence we know that, in the general population, preoperative anemia adversely affects the outcomes of cardiothoracic surgery (11–16). However, in the growing population of patients with CKD, the optimal preoperative hemoglobin targets have not previously been explored. This subject has become especially relevant in light of new and relatively strict FDA recommendations, at least in ESA-treated patients with CKD, and the fact that preoperative hemoglobin is, potentially, a simply modifiable risk factor. The aims of this study were to (1) determine whether preoperative hemoglobin independently influences outcomes of cardiac surgery in patients with CKD and (2) define an optimum level of preoperative hemoglobin that is associated with the lowest incidence of adverse surgical outcomes in the CKD population undergoing cardiac surgery.
Materials and Methods
Study Population and Data Collection
This observational study included adult patients with CKD stages 3–5 (eGFR<60 ml/min per 1.73 m2) undergoing cardiac surgery from February of 2000 to January of 2010 at Shaare Zedek Medical Center, Jerusalem, Israel. All data were collected prospectively on a daily basis by research assistants who completed preoperative, intraoperative, and postoperative datasheets. The preoperative and intraoperative datasheets contain demographic data, variables related to the severity of disease, comorbid factors before surgery, and operative features. The postoperative datasheet contains variables related to in-hospital outcomes after surgery. The same research assistant, who was blinded to the patients’ preoperative hemoglobin levels, determined all postoperative outcomes according to standard definitions.
Eligible patients included individuals 18 years of age or older with CKD stages 3–5 (on the basis of the National Kidney Foundation CKD classification: stage 3, eGFR=30–59 ml/min per 1.73 m2; stage 4, eGFR=15–29 ml/min per 1.73 m2; stage 5, eGFR<15 ml/min per 1.73 m2) undergoing cardiac surgery from February of 2000 to January of 2010 in our center either electively or urgently. Patients on chronic dialysis were excluded.
Written consent was not obtained from the individual patients, because the study is on the basis of data collected for routine care. Local ethics committee approval was obtained.
Study Methods
Patients were classified into four groups stratified by preoperative hemoglobin level<10, 10–11.9, 12–13.9, and ≥14 g/dl (referred to as normal preoperative hemoglobin). The reference range for hemoglobin in our laboratory is 14–18 g/dl in men and 12–16 g/dl in women.
Preoperative eGFR was estimated in all patients by the four-component Modification of Diet in Renal Disease equation on the basis of preoperative creatinine levels (reference range=0.66–1.2 mg/dl; isotope dilution mass spectrometry traceable). In addition, to ensure the chronic stable nature of the kidney disease, in-hospital creatinine measurements during the preoperative period were compared with values obtained from either the family physician’s referral letter or previous in-hospital medical records. The following preoperative patient-related characteristics were recorded: age, sex, medical history of heart failure (according to New York Heart Association [NYHA] classification), left main coronary artery disease, diabetes mellitus (DM), hypertension, hyperlipidemia, peripheral vascular disease (PVD), cerebrovascular accident (CVA), and chronic obstructive pulmonary disease. Operative features included type of operation performed (coronary artery bypass graft [CABG], valvular or combined), incidence and indications for intra-aortic balloon pump use, surgery status (elective, emergent, emergent/salvage, and urgent), perfusion time, crossclamp time, and use of off-pump surgery. Postoperative complications recorded included AKI defined by RIFLE criteria (risk, injury, and failure stages), infections, prolonged mechanical ventilation (>24 hours), CVA, myocardial infarction, acute respiratory distress syndrome, in-hospital mortality incidence and causes, intensive care unit stay, and hospital stay.
Outcomes
The outcomes studied were postoperative AKI requiring dialysis, in-hospital mortality (regardless of length of stay), and major morbidities, including neurologic (CVA) and infectious (septic shock or other infections) morbidities requiring intravenous antibiotics.
Statistical Analyses
Analysis was performed using JMP Software, version 5.0 (SAS Institute). Data are expressed as mean±SD, except for parameters not normally distributed, which are shown as median (25th, 75th percentile). The association of potential risk factors with operative mortality or morbidity was assessed by univariate analysis; nominal and categorical variables were compared using the chi-squared likelihood ratio or Fisher exact test. Continuous variables were compared using the nonparametric Wilcoxon test. Factors with P value ≤0.05 in the univariate analysis were included in a stepwise multivariate logistic regression analysis. The criterion for entering factors into the stepwise regression model was a P value=0.25 to expose variables that were not related to the outcome but could be confounders. In the multivariate analysis, we used logistic regression analysis to determine the association between preoperative hemoglobin as a continuous variable and complications as dichotomous variables. We calculated the odds ratio (OR) for each 1-g/dl decrement of the preoperative hemoglobin in the logistic regression analysis. To find the cutoff values of preoperative hemoglobin potentially associated with adverse clinical outcomes, patients were divided into groups on the basis of their preoperative hemoglobin. Bar charts of ORs and P values were obtained through comparison of the selected group with the group with normal preoperative hemoglobin (≥14). Differences are reported as significant if P value is <0.05.
Results
During a 10-year period, 788 patients with preoperative CKD stages 3–5 underwent cardiac surgery. Tables 1 and 2 represent the baseline patient- and procedure-related characteristics of the whole study population and patients stratified by preoperative hemoglobin levels. The average age of the study population was 70±10 years; 60% of them were men. Mean plasma creatinine concentration was 1.9±1.5 mg/dl (range=0.96–9.7), and mean eGFR was 43.5±13.7 ml/min per 1.73 m2 (range=5.9–59.8). A high incidence of preoperative comorbidities was observed: severe heart failure (NYHA classification IV), 35%; DM, 42%; hypertension, 81%; hyperlipidemia, 70%; CVA, 12% (Table 1).
Table 1.
Patient-related characteristics stratified by preoperative hemoglobin levels
| Patient-related Characteristics | Patient, N | P Value | ||||
|---|---|---|---|---|---|---|
| All Patients (n=788) | Hb<10 (n=73) | Hb=10–11.9 (n=210) | Hb=12–13.9 (n=325) | Hb≥14 (n=180) | ||
| Sex (men) | 481 (60%) | 40 (55%) | 105 (50%) | 184 (57%) | 148 (82%) | <0.001 |
| Age, yr | 70±10 (26–91) | 68±9 | 70±11 | 71±10 | 69±9 | 0.19 |
| Preoperative creatinine, mg/dl | 1.9±1.5 (0.96–9.7) | 2.5±1.9 | 2.1±1.8 | 1.7±1.2 | 1.6±1 | <0.001 |
| eGFR, ml/min per 1.73 m2 | 43.5±13.7 (5.9–59.8) | 34±16 | 39±14 | 46±12 | 49±10 | <0.001 |
| CHF NYHA classification | ||||||
| I | 115 (16%) | 4 (5%) | 16 (8%) | 54 (17%) | 39 (22%) | <0.001 |
| II | 99 (13%) | 3 (4%) | 23 (11%) | 44 (13%) | 27 (15%) | 0.06 |
| III | 267 (36%) | 24 (33%) | 67 (32%) | 114 (35%) | 58 (32%) | 0.86 |
| IV | 259 (35%) | 38 (52%) | 91 (43%) | 83 (25%) | 43 (24%) | <0.001 |
| Left main art.>50% | 115 (14%) | 9 (12%) | 29 (14%) | 52 (16%) | 24 (13%) | 0.77 |
| PVD | 117(15%) | 16 (22%) | 34 (16%) | 46 (14%) | 20 (11%) | 0.15 |
| Hyperlipidemia | 554 (70%) | 48 (66%) | 151 (72%) | 219 (67%) | 127 (70%) | 0.62 |
| HTN | 645 (81%) | 63 (86%) | 179 (85%) | 256 (79%) | 139 (77%) | 0.08 |
| DM | 334 (42%) | 48 (66%) | 108 (51%) | 124 (38%) | 48 (27%) | <0.001 |
| CVA | 93 (12%) | 11 (15%) | 28 (13%) | 35 (11%) | 18 (10%) | 0.55 |
| COPD | 94 (12%) | 11 (15%) | 24 (11%) | 41 (13%) | 16 (9%) | 0.47 |
| Medications, n (%) | ||||||
| Diuretics | 389 (57%) | 34 (62%) | 126 (68%) | 159 (55%) | 70 (45%) | <0.001 |
| ACE inhibitors | 368 (54%) | 31 (56%) | 107 (58%) | 152 (53%) | 78 (50%) | 0.58 |
| ARBs | 81 (12%) | 8 (15%) | 24 (13%) | 36 (13%) | 13 (8%) | 0.45 |
| Statins | 381 (56%) | 32 (58%) | 106 (57%) | 150 (52%) | 93 (60%) | 0.46 |
| Aspirin | 496 (63%) | 41 (56%) | 123 (59%) | 208 (64%) | 124 (69%) | 0.11 |
| Ca channels blockers | 147 (19%) | 11 (15%) | 46 (22%) | 61 (19%) | 29 (16%) | 0.41 |
| β-Blockers | 455 (58%) | 36 (49%) | 123 (59%) | 200 (62%) | 96 (53%) | 0.14 |
CHF, congestive heart failure; NYHA, New York Heart Association; art, artery; PVD, peripheral vascular disease; HTN, hypertension; DM, diabetes mellitus; CVA, cerebrovascular accident; COPD, chronic obstructive pulmonary disease; ACE, angiotensin-converting enzyme; ARBs, angiotensin II receptor antagonists; Ca, calcium; Hb, hemoglobin
Table 2.
Procedure-related characteristics stratified by preoperative hemoglobin levels
| Procedure Characteristics | All Patients (n=788) | Hb<10 (n=73) | Hb=10–11.9 (n=210) | Hb=12–13.9 (n=325) | Hb≥14 (n=180) | P Value |
|---|---|---|---|---|---|---|
| Type of procedure | ||||||
| CABG | 295 (37%) | 13 (18%) | 58 (28%) | 128 (39%) | 94 (52%) | <0.001 |
| CABG and valve | 180 (22%) | 17 (23%) | 54 (26%) | 71 (21%) | 23 (13%) | 0.23 |
| Valvular surgery | 185 (22%) | 22 (30%) | 52 (25%) | 69 (22%) | 38 (21%) | 0.33 |
| Other | 140 (17%) | 21 (29%) | 46 (21%) | 57 (18%) | 25 (16%) | 0.58 |
| Surgery status | ||||||
| Elective | 604 (75%) | 54 (74%) | 165 (79%) | 237 (73%) | 138 (77%) | 0.48 |
| Emergent | 45 (6%) | 5 (7%) | 11 (5%) | 20 (6%) | 7 (4%) | 0.68 |
| Emergent/salvage | 20 (3%) | 0 | 3 (1%) | 9 (3%) | 8 (4%) | 0.13 |
| Urgent | 131 (16%) | 14 (19%) | 31 (15%) | 59 (18%) | 27 (15%) | 0.62 |
| IABP incidence | 66 (8%) | 6 (8%) | 19 (9%) | 23 (7%) | 16 (9%) | 0.83 |
| Crossclamp time | ||||||
| 25th Percentile | 56 | 57 | 57 | 57 | 52 | 0.002 |
| Median | 75 | 76 | 82 | 77 | 69 | |
| 75th Percentile | 106 | 120 | 109 | 108 | 91 | |
| Perfusion time | ||||||
| 25th Percentile | 81 | 90 | 83 | 83 | 74 | <0.001 |
| Median | 109 | 120 | 116 | 111 | 98 | |
| 75th Percentile | 141 | 151 | 142 | 146 | 126 | |
| Inotrope use | 245 (31%) | 31 (42%) | 74 (35%) | 95 (29%) | 45 (25%) | 0.02 |
| Perioperative blood transfusion | 636 (81%) | 68 (93%) | 194 (92%) | 267 (82%) | 107 (59%) | <0.001 |
Crossclamp and perfusion times, which were not normally distributed, are given as medians, 25th percentiles, and 75th percentiles. CABG, coronary artery bypass graft; IABP, intra-aoritc balloon pump; Hb, hemoglobin.
In total, 37% of patients underwent CABG, 25% of patients had valvular surgery, and the remaining patients had a combination of both (Table 2).
After stratification by preoperative hemoglobin, four groups of patients were created. There were 73 patients in the first group (hemoglobin<10 g/dl), 210 patients in the second group (hemoglobin=10–11.9 g/dl), 325 patients in the third group (hemoglobin=12–13.9 g/dl), and 180 patients in the fourth group (hemoglobin≥14 g/dl). Lower preoperative hemoglobin was associated with higher prevalence of DM, severe heart failure (NYHA classification IV), advanced preoperative CKD, use of diuretics, higher incidence of perioperative (intraoperative and postoperative) blood transfusions, and prolonged crossclamp and perfusion times (Tables 1 and 2). In total, 636 patients received blood transfusions, and the incidence was significantly higher in patients with preoperative hemoglobin<14 g/dl (P<0.001). However, there were no statistically significant differences among groups in a number of important preoperative and intraoperative variables. Patients were similar in age; prevalence of chronic obstructive pulmonary disease, PVD, hypertension, hyperlipidemia, and CVA; preoperative medication use, including angiotensin-converting enzyme inhibitors, angiotensin II receptor antagonists, statins, aspirin, and β-blockers; incidence of valvular and CABG and valve operations; surgery status (elective, urgent, emergent, or emergent/salvage operations); and hemodynamic instability requiring intra-aortic balloon pump. Not unexpectedly, 82% of patients with hemoglobin>14 g/dl were men compared with about 50% of patients in other hemoglobin groups (P<0.001).
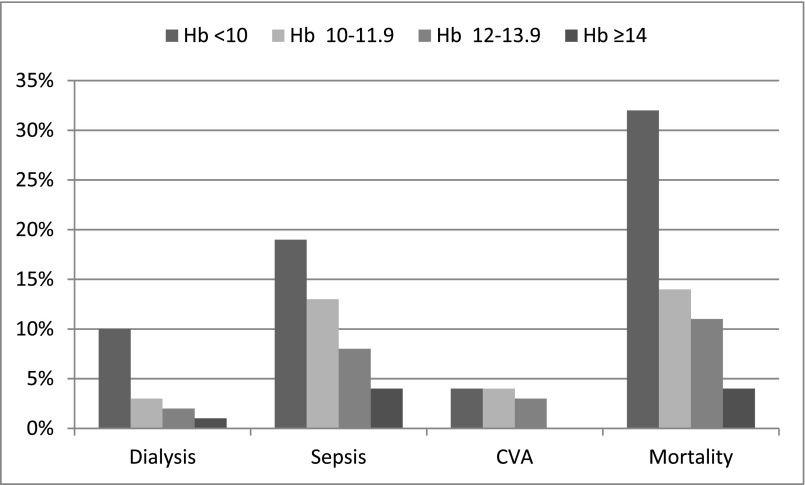
Overall, 26.6% of patients reached the study outcomes: 23 (2.8%) patients developed postoperative AKI requiring dialysis, 20 (2.5%) patients had a CVA, 74 (9.2%) patients had postoperative sepsis, and 96 (12%) patients died during the hospitalization. Figure 1 shows the unadjusted analysis of the incidence of adverse postoperative outcomes in groups of patients stratified by preoperative hemoglobin. A clear inverse association between the incidence of all four outcomes and preoperative hemoglobin values was detected.
Figure 1.
Inverse relationship between preoperative hemoglobin and a frequency of postoperative adverse outcomes was detected by univariate analysis. Incidence of postoperative AKI requiring dialysis, sepsis, cerebrovascular accident (CVA), and mortality according to preoperative hemoglobin (Hb) as detected by univariate analysis. For each outcome, bars are (from left to right) Hb<10, 10–11.9, 12–13.9, and ≥14 g/dl.
Only nine (1%) patients developed postoperative myocardial infarction: four (2%), one (0.3%), two (1%), and two (3%) patients with preoperative hemoglobin of ≥14, 12–13.9, 10–11.9, and <10 g/dl, respectively (P=0.10).
Postoperative AKI requiring dialysis was also inversely related to preoperative hemoglobin: 1%, 2%, 3%, and 10% (P=0.01 for trend) in patients with preoperative hemoglobin values of ≥14, 12–13.9, 10–11.9, and <10 g/dl, respectively. A similar relationship was seen for mortality: 4%, 11%, 14%, and 32% in the respective groups (P<0.001 for trend). Analogous trends were detected for other outcomes (P<0.001 for postoperative sepsis and P=0.01 for postoperative CVA) (Figure 1).
The association between preoperative hemoglobin and postoperative AKI defined by RIFLE criteria (risk, injury, and failure stages) is presented in Table 3.
Table 3.
Association between preoperative hemoglobin and postoperative AKI as defined by RIFLE criteria
| AKI Stages by RIFLE Criteria | Preoperative Hemoglobin, g/dl | P Value | |||
|---|---|---|---|---|---|
| <10 (n=73) | 10–11.9 (n=210) | 12–13.9 (n=325) | ≥14 (n=180) | ||
| Risk | 15 (21%) | 25 (12%) | 37 (11%) | 13 (7%) | 0.03 |
| Injury | 12 (16%) | 26 (12%) | 20 (6%) | 9 (5%) | 0.002 |
| Failure | 19 (26%) | 29 (14%) | 18 (6%) | 6 (3%) | <0.001 |
Of those patients who developed AKI, 90 patients were classified as risk, 67 patients were classified as injury, and 72 patients were classified as failure. The incidence of all stages of AKI was inversely related to preoperative hemoglobin, and these findings were statistically significant. The incidence of failure stage, for example, was 3%, 6%, 14%, and 26% (P<0.001) in patients with preoperative hemoglobin of ≥14, 12–13.9, 10–11.9, and <10 g/dl, respectively. Similar trends were detected for earlier stages (P=0.03 for risk and P=0.002 for injury) (Table 3).
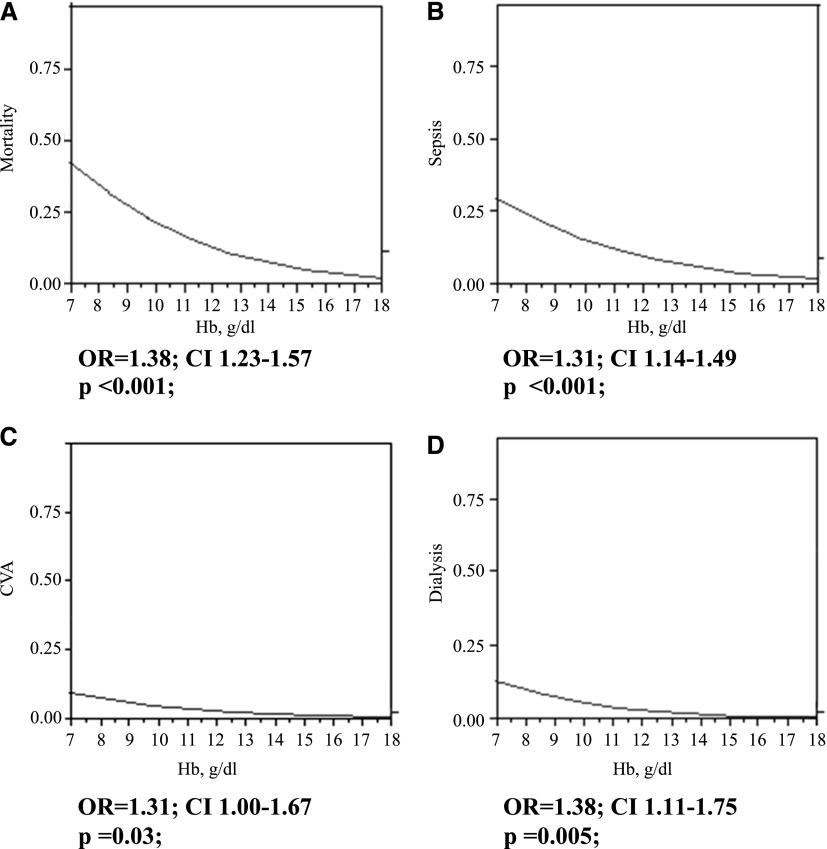
Figure 2 shows stepwise multivariate logistic regression analysis of the association between preoperative hemoglobin as a continuous variable and the four major postoperative adverse outcomes: sepsis, postoperative dialysis, CVA, and mortality. Adjustment was performed for all identified confounders with P value ≤0.05 in the univariate analysis and included sex, severity of preoperative CKD (on the basis of serum creatinine and eGFR), severity of preoperative heart failure (NYHA classifications I and IV), baseline DM, incidence of blood transfusion, type of surgery (CABG alone versus the combination of CABG and valve surgery), use of diuretics, intraoperative inotrope use, and crossclamp and perfusion times. It can be seen that the frequency of all adverse postoperative outcomes was inversely related to preoperative hemoglobin. For every 1-g/dl decrement of preoperative hemoglobin, there was a proportionally greater incidence of mortality (OR, 1.38; 95% confidence interval [95% CI], 1.23 to 1.57; P<0.001), sepsis (OR, 1.31; 95% CI, 1.14 to 1.49; P<0.001), CVA (OR, 1.31; 95% CI, 1 to 1.67; P=0.03), and postoperative hemodialysis (OR, 1.38; 95% CI, 1.11 to 1.75; P<0.01). No interaction by sex was detected in the above associations (P=0.90 for mortality, P=0.10 for sepsis, P=0.60 for CVA, and P=0.50 for dialysis).
Figure 2.
Lower preoperative hemoglobin is associated with proportionally greater incidence of postoperative adverse outcomes by multivariate analysis. Stepwise multivariate logistic regression analysis of association between preoperative hemoglobin (Hb) as the continuous variable and (A) mortality, (B) sepsis, (C) CVA, and (D) postoperative dialysis. Odds ratio (OR) is calculated per each 1-g/dl decrement of preoperative Hb as a continuous variable. Adjustment was performed for all identified confounders with P value ≤0.05 in the univariate analysis. CI, confidence interval.
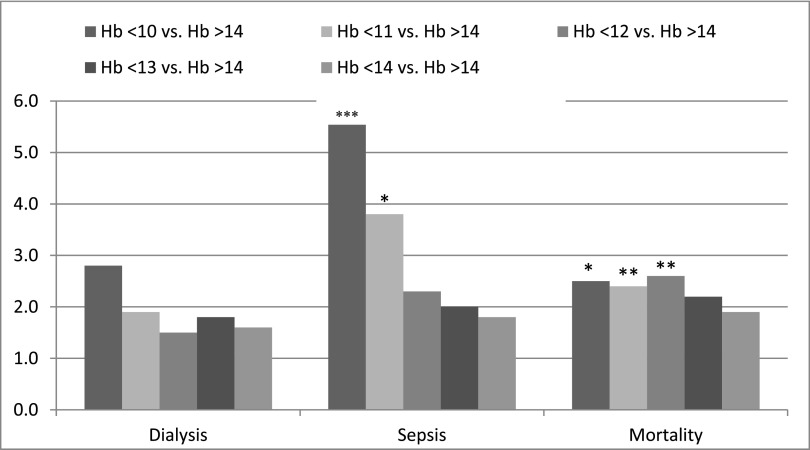
To determine threshold values of preoperative hemoglobin that independently predicted surgical outcomes, we calculated ORs (obtained after adjustment with the previously identified confounders) for postoperative dialysis, AKI according to RIFLE criteria, mortality, and sepsis in patients stratified by preoperative hemoglobin at cutoff points of 10, 11, 12, 13, and 14 g/dl compared with the reference group (Figure 3, Table 4). We showed that preoperative hemoglobin<12 g/dl was an independent risk factor for mortality (OR, 2.5; 95% CI, 1.1 to 7.3; P=0.04) and that preoperative hemoglobin<11 g/dl was independently associated with a higher incidence of both postoperative mortality (OR, 2.6; 95% CI, 1.1 to 5.3; P=0.04) and sepsis (OR, 3.8; 95% CI, 1.6 to 10; P=0.003). No significant association between hemoglobin and postoperative dialysis requirement was observed (OR, 1.6; 95% CI, 0.4 to 10.4; P=0.50). However, we found that preoperative hemoglobin<12 (OR, 2.7; 95% CI, 1.1 to 7.9; P=0.04) and <11 g/dl (OR, 2.6; 95% CI, 1.1 to 6.5; P=0.03) were independent risk factors for RIFLE stages failure and risk of postoperative AKI, respectively (Table 4). These associations were equally shown in both sexes. We could not calculate ORs for CVA, because there were no patients in the reference group who had a postoperative CVA.
Figure 3.
Preoperative hemoglobin levels <12g/dl and <11 g/dl represent an independent risk factors for postoperative mortality and sepsis, respectively. Adjusted OR for postoperative outcomes in patients stratified by preoperative hemoglobin (Hb) levels. Values were obtained by multivariate analysis after adjustment for all identified confounders with P value ≤0.05 in the univariate analysis and included sex, severity of preoperative CKD (on the basis of serum creatinine and eGFR), severity of preoperative heart failure (NYHA classifications I and IV), baseline diabetes mellitus, incidence of blood transfusion, type of surgery (coronary artery bypass graft alone versus a combination of coronary artery bypass graft and valve surgery), use of diuretics, intraoperative inotrope use, and crossclamp and perfusion times. Selected groups were compared with the reference group with preoperative Hb>14 g/dl. Statistically significant values: *P=0.003; **P=0.04; ***P<0.005. NYHA, New York Heart Association.
Table 4.
Adjusted odd ratios for postoperative AKI defined by RIFLE criteria in patients stratified by preoperative hemoglobin levels (selected group compared with the reference group with preoperative hemoglobin>14 g/dl)
| AKI Stages by RIFLE Criteria | Preoperative Hemoglobin, g/dl | ||||
|---|---|---|---|---|---|
| <10 | <11 | <12 | <13 | <14 | |
| Risk | |||||
| Odds ratio; 95% confidence interval | 2.6; 1.1 to 6.0 | 2.6; 1.1 to 6.5 | 2.1; 1.0 to 5.0 | 2; 1.0 to 4.5 | 2; 1.0 to 4.7 |
| P value | 0.02 | 0.03 | 0.07 | 0.07 | 0.08 |
| Injury | |||||
| Odds ratio; 95% confidence interval | 1.6; 1.5 to 5.0 | 1.8; 1.0 to 4.6 | 2.2; 1.1 to 5.0 | 1.4; 1.0 to 3.3 | 1.3; 1.0 to 3.0 |
| P value | 0.40 | 0.20 | 0.06 | 0.30 | 0.50 |
| Failure | |||||
| Odds ratio; 95% confidence interval | 4.7; 1.6 to 15.5 | 4.5; 1.7 to 13.4 | 2.7; 1.1 to 7.9 | 2.4; 1.0 to 6.7 | 2.2; 1.0 to 6.1 |
| P value | 0.007 | 0.004 | 0.04 | 0.07 | 0.09 |
Values were obtained by multivariate analysis after adjustment for sex, severity of preoperative CKD, severity of preoperative heart failure (NYHA classifications I and IV), baseline diabetes mellitus, incidence of blood transfusion, type of surgery (CABG alone versus a combination of CABG and valve surgery), use of diuretics, intraoperative inotrope use, and crossclamp and perfusion times.
Analysis of mortality data revealed infection as the most common cause of death in patients with anemia, whereas cardiac death was the most common cause in patients with preoperative hemoglobin>14 g/dl.
Discussion
Our prospective cohort study showed several interesting findings related to preoperative hemoglobin in patients with significant CKD undergoing open heart surgery. First, low hemoglobin in patients with CKD is associated with increased prevalence of some but not all comorbidities. As expected, we found more severe anemia in patients with diabetes and advanced stages of heart and kidney diseases. However, anemia was not associated with age, hypertension, PVD, or preoperative CVA. Second, multivariate logistic regression analysis using hemoglobin as a continuous variable showed a remarkable and continuous inverse relationship between decreasing hemoglobin and the frequency of postoperative sepsis, CVA, AKI requiring hemodialysis, and in-hospital mortality. Third, we showed that, similar to patients without CKD, preoperative anemia in patients with CKD stages 3–5 is an independent risk factor for adverse postoperative outcomes, even after adjustment for CKD severity. Fourth, we defined threshold values of preoperative hemoglobin that were significantly and independently associated with adverse postoperative outcomes. Thus, preoperative hemoglobin<12 g/dl was an independent risk factor for postoperative failure stage of AKI and mortality, and hemoglobin<11 g/dl was independently associated with postoperative sepsis, risk, and failure stages of AKI, and higher mortality.
Recently, several studies addressed preoperative anemia as a predictor of poor outcomes after cardiac surgery (11–16). Most of them showed that low hemoglobin level, as both a continuous variable and a dichotomous variable, is a predictor of higher in-hospital and late mortality. In addition, both preoperative anemia and perioperative red blood cell transfusions were independently and strongly associated with postoperative AKI, which by itself, results in a >4-fold increase in the odds of death (13). However, none of these studies was focused on or specifically designed to detect a relationship between preoperative hemoglobin and outcomes of cardiac surgery in patients with significant CKD. Anemia is common in patients with CKD, and some studies suggested that treatment with ESAs and intravenous iron can improve left ventricular ejection fraction, renal function, and functional capacity in patients with heart failure and anemia (17–21). Moreover, the study by Cladellas et al. (21) showed that combined therapy with intravenous erythropoietin and iron administered before valvular cardiac surgery in patients with anemia was associated with a significant improvement in postoperative outcomes and a significant decrease in need for red blood cell transfusions. However, in the study, only 14% of patients in both intervention (75 patients) and observation (59 patients) cohorts had preoperative creatinine levels>1.5 mg/dl, and eGFR data were not provided (21).
Recently, several randomized control trials have led to a major reassessment of anemia management in CKD: the Normal Hematocrit study in patients on hemodialysis with clinical signs of congestive heart failure or ischemic heart disease, studies from Europe (Cardiovascular Risk Reduction by Early Anemia Treatment with Epoetin-β) and the United States (Correction of Hemoglobin and Outcomes in Renal Insufficiency) in patients before dialysis, and finally, the Trial to Reduce Cardiovascular Events with Aranesp Therapy study on patients with diabetes, CKD, and anemia. These studies have provided evidence that the normalization of hemoglobin levels to 13.0–15.0 g/dl in patients with CKD stages 3 or 4 does not reduce cardiovascular events compared with the use of a lower target range (10.5–11.5 g/dl). Moreover, the use of darbepoetin alfa in patients with diabetes, CKD, and moderate anemia did not reduce the risk of either death or a cardiovascular event and was associated with an increased risk of stroke (4–6). The reasons for this finding are not entirely clear but may be caused by a combination of several factors: higher hemoglobin concentration itself, rapidity of the increase or oscillations in hemoglobin levels, overshoot of the target, or some off-target effect of ESAs, such as trophic effects on vascular endothelial or smooth-muscle cells (22). Interestingly, patients who achieved the higher hemoglobin had better outcomes, whereas a poor initial hematopoietic response to darbepoetin alfa was associated with an increased subsequent risk of death or cardiovascular events as doses were escalated to meet target hemoglobin levels (23). Regardless of pathogenetic mechanisms, clinical practice guidelines have been changed and recommend maintaining hemoglobin levels in the range of 10–11.9 g/dl in CKD. Our study showed that, before cardiac surgery, this range of preoperative hemoglobin was independently associated with a >2-fold greater risk of hospital mortality and postoperative AKI. It is not clear, however, whether the correction of preoperative hemoglobin by ESAs or blood transfusion will improve postoperative outcomes. Interestingly, the main cause of death in patients with anemia was infection, but patients with hemoglobin>14 g/dl were more likely to die from cardiovascular causes. Although anemia has been extensively shown as an independent risk factor for postoperative infection in both cardiac and noncardiac surgery (13,14,19,21,24), our study showed that patients with higher hemoglobin die mostly from cardiac complications. This fact, however, can be explained by a low rate of sepsis, sepsis-related, and overall mortality in this subgroup, leaving cardiovascular events as the leading cause of death.
It is not completely clear whether this finding is unique for the CKD population. In fact, our results are in line with the observation that patients with CKD with higher hemoglobin levels have increased cardiovascular mortality. Despite its complexity, a sufficiently powered randomized placebo controlled trial will be required to shed light on the phenomenon of optimal preoperative hemoglobin levels in patients with CKD undergoing cardiac surgery.
Our study has some limitations. First, it was on the basis of observational data, and we cannot entirely rule out known or unknown confounding factors as an explanation for our results. However, recall bias can be excluded in our study, because data collection was uniform in all patients; the same research assistants completed preoperative, intraoperative, and postoperative datasheets prospectively on a daily basis. In addition, no changes in definitions of comorbidities or studied outcomes were performed during the whole period of the study. Second, despite multivariate analysis, a powerful influence of lower preoperative eGFR by itself on postoperative morbidity and mortality in patients with greater anemia cannot be entirely ruled out. In addition, our data have been collected over a prolonged period of time, and therefore, the possibility of changes in both perioperative and surgical management strategies over these years should be considered. These strategies include myocardial preservation with crystalloid solutions containing tryptophan, histidine, and ketoglutarate instead of hyperkalemic solutions and a liberal postoperative fluid-management policy to reduce the need for inotropic and vasopressor support. Furthermore, our patient population has changed over the years, with an increase in age and other comorbidities. Despite these changes, no trend in the average Euroscore or intraoperative or postoperative characteristics could be detected between periods preceding and succeeding these major changes; also, there was no significant vintage effect on mortality or other outcomes.
One of the prominent limitations of our study is that data on preoperative ESA use and dose were not consistently recorded and therefore, could not be analyzed. Thus, the role of ESAs and their potential significant influence on patient outcomes in this specific surgical setting cannot be addressed. Finally, our definition of CKD was on the basis of eGFR and not measured GFR. Importantly, because of the observational design of our study, the possibility of residual confounding remains a serious limitation, and the only conclusion that can be drawn is an association between anemia and adverse postoperative outcomes. Although our study cannot show direct causality or help guide clinicians on treatment decisions, it can be considered as hypothesis-generating and serve as an important basis for future controlled trials exploring potential benefits of therapeutically targeting higher preoperative hemoglobin levels before cardiac surgery.
In summary, we evaluated a relatively large cohort of patients with CKD undergoing open heart surgery and focused specifically on the subject of preoperative hemoglobin and clinical outcomes. We showed a substantially greater risk of mortality and postoperative sepsis at threshold values of hemoglobin<12 and <11 g/dl, respectively. Although low hemoglobin levels were associated with a higher burden of comorbidities, multivariate analysis showed that anemia was an independent risk factor for adverse postoperative outcomes. Our findings raise several important questions that may have an effect on the care of patients with CKD undergoing cardiac surgery, especially in light of the easily modifiable nature of preoperative anemia. However, the role of therapeutic strategies aimed to increase preoperative hemoglobin and diminish postoperative complications in the CKD population undergoing cardiac surgery remains to be elucidated in a prospective randomized trial.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Barrett BJ, Fenton SS, Ferguson B, Halligan P, Langlois S, Mccready WG, Muirhead N, Weir RV, Canadian Society of Nephrology : Clinical practice guidelines for the management of anemia coexistent with chronic renal failure. J Am Soc Nephrol 10[Suppl 13]: S292–S296, 1999 [PubMed] [Google Scholar]
- 2.KDOQI. National Kidney Foundation : KDOQI clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease. Am J Kidney Dis 47[Suppl 3]: S11–S145, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, Reddan D, CHOIR Investigators : Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 355: 2085–2098, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Drüeke TB, Locatelli F, Clyne N, Eckardt KU, Macdougall IC, Tsakiris D, Burger HU, Scherhag A, CREATE Investigators : Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 355: 2071–2084, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Besarab A, Bolton WK, Browne JK, Egrie JC, Nissenson AR, Okamoto DM, Schwab SJ, Goodkin DA: The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med 339: 584–590, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, de Zeeuw D, Eckardt KU, Feyzi JM, Ivanovich P, Kewalramani R, Levey AS, Lewis EF, McGill JB, McMurray JJ, Parfrey P, Parving HH, Remuzzi G, Singh AK, Solomon SD, Toto R, TREAT Investigators : A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 361: 2019–2032, 2009 [DOI] [PubMed] [Google Scholar]
- 7.US Food and Drug Administration : Ongoing Safety Review Erythropoiesis-Stimulating Agents (ESAs) Epoetin Alfa (Marketed as Procrit, Epogen) and Darbepoetin Alfa (Marketed as Aranesp): Update, US Department of Health & Human Services, 2013, US Food and Drug Administration [Google Scholar]
- 8.KDIGO : KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl 2: 279–335, 2012 [Google Scholar]
- 9.Lassnigg A, Schmidlin D, Mouhieddine M, Bachmann LM, Druml W, Bauer P, Hiesmayr M: Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: A prospective cohort study. J Am Soc Nephrol 15: 1597–1605, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Cooper WA, O’Brien SM, Thourani VH, Guyton RA, Bridges CR, Szczech LA, Petersen R, Peterson ED: Impact of renal dysfunction on outcomes of coronary artery bypass surgery: Results from the Society of Thoracic Surgeons National Adult Cardiac Database. Circulation 113: 1063–1070, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Ranucci M, Di Dedda U, Castelvecchio S, Menicanti L, Frigiola A, Pelissero G, Surgical and Clinical Outcome Research (SCORE) Group : Impact of preoperative anemia on outcome in adult cardiac surgery: A propensity-matched analysis. Ann Thorac Surg 94: 1134–1141, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Karkouti K, Wijeysundera DN, Yau TM, Callum JL, Cheng DC, Crowther M, Dupuis JY, Fremes SE, Kent B, Laflamme C, Lamy A, Legare JF, Mazer CD, McCluskey SA, Rubens FD, Sawchuk C, Beattie WS: Acute kidney injury after cardiac surgery: Focus on modifiable risk factors. Circulation 119: 495–502, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Karkouti K, Wijeysundera DN, Beattie WS, Reducing Bleeding in Cardiac Surgery (RBC) Investigators : Risk associated with preoperative anemia in cardiac surgery: A multicenter cohort study. Circulation 117: 478–484, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Kulier A, Levin J, Moser R, Rumpold-Seitlinger G, Tudor IC, Snyder-Ramos SA, Moehnle P, Mangano DT, Investigators of the Multicenter Study of Perioperative Ischemia Research Group. Ischemia Research and Education Foundation : Impact of preoperative anemia on outcome in patients undergoing coronary artery bypass graft surgery. Circulation 116: 471–479, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Cladellas M, Bruguera J, Comín J, Vila J, de Jaime E, Martí J, Gomez M: Is pre-operative anaemia a risk marker for in-hospital mortality and morbidity after valve replacement? Eur Heart J 27: 1093–1099, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Zindrou D, Taylor KM, Bagger JP: Preoperative haemoglobin concentration and mortality rate after coronary artery bypass surgery. Lancet 359: 1747–1748, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Mancini DM, Katz SD, Lang CC, LaManca J, Hudaihed A, Androne AS: Effect of erythropoietin on exercise capacity in patients with moderate to severe chronic heart failure. Circulation 107: 294–299, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Bolger AP, Bartlett FR, Penston HS, O’Leary J, Pollock N, Kaprielian R, Chapman CM: Intravenous iron alone for the treatment of anemia in patients with chronic heart failure. J Am Coll Cardiol 48: 1225–1227, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Silverberg DS, Wexler D, Blum M, Tchebiner JZ, Sheps D, Keren G, Schwartz D, Baruch R, Yachnin T, Shaked M, Schwartz I, Steinbruch S, Iaina A: The effect of correction of anaemia in diabetics and non-diabetics with severe resistant congestive heart failure and chronic renal failure by subcutaneous erythropoietin and intravenous iron. Nephrol Dial Transplant 18: 141–146, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Silverberg DS, Wexler D, Blum M, Keren G, Sheps D, Leibovitch E, Brosh D, Laniado S, Schwartz D, Yachnin T, Shapira I, Gavish D, Baruch R, Koifman B, Kaplan C, Steinbruch S, Iaina A: The use of subcutaneous erythropoietin and intravenous iron for the treatment of the anemia of severe, resistant congestive heart failure improves cardiac and renal function and functional cardiac class, and markedly reduces hospitalizations. J Am Coll Cardiol 35: 1737–1744, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Cladellas M, Farré N, Comín-Colet J, Gómez M, Meroño O, Bosch MA, Vila J, Molera R, Segovia A, Bruguera J: Effects of preoperative intravenous erythropoietin plus iron on outcome in anemic patients after cardiac valve replacement. Am J Cardiol 110: 1021–1026, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Unger EF, Thompson AM, Blank MJ, Temple R: Erythropoiesis-stimulating agents—time for a reevaluation. N Engl J Med 362: 189–192, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Solomon SD, Uno H, Lewis EF, Eckardt KU, Lin J, Burdmann EA, de Zeeuw D, Ivanovich P, Levey AS, Parfrey P, Remuzzi G, Singh AK, Toto R, Huang F, Rossert J, McMurray JJ, Pfeffer MA, Trial to Reduce Cardiovascular Events with Aranesp Therapy (TREAT) Investigators : Erythropoietic response and outcomes in kidney disease and type 2 diabetes. N Engl J Med 363: 1146–1155, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Dunne JR, Malone D, Tracy JK, Gannon C, Napolitano LM: Perioperative anemia: An independent risk factor for infection, mortality, and resource utilization in surgery. J Surg Res 102: 237–244, 2002 [DOI] [PubMed] [Google Scholar]