Abstract
Background and objectives
Numerous uremic solutes are derived from the action of colon microbes. Two such solutes, indoxyl sulfate and p-cresol sulfate, have been associated with adverse outcomes in renal failure. This study tested whether increasing dietary fiber in the form of resistant starch would lower the plasma levels of these solutes in patients on hemodialysis.
Design, setting, participants, & measurements
Fifty-six patients on maintenance hemodialysis were randomly assigned to receive supplements containing resistant starch (n=28) or control starch (n=28) daily for 6 weeks in a study conducted between October 2010 and May 2013. Of these, 40 patients (20 in each group) completed the study and were included in the final analysis. Plasma indoxyl sulfate and p-cresol sulfate levels were measured at baseline and week 6.
Results
Increasing dietary fiber for 6 weeks significantly reduced the unbound, free plasma level of indoxyl sulfate (median −29% [25th percentile, 75th percentile, −56, −12] for fiber versus −0.4% [−20, 34] for control, P=0.02). The reduction in free plasma levels of indoxyl sulfate was accompanied by a reduction in free plasma levels of p-cresol sulfate (r=0.81, P<0.001). However, the reduction of p-cresol sulfate levels was of lesser magnitude and did not achieve significance (median −28% [−46, 5] for fiber versus 4% [−28, 36] for control, P=0.05).
Conclusions
Increasing dietary fiber in hemodialysis patients may reduce the plasma levels of the colon-derived solutes indoxyl sulfate and possibly p-cresol sulfate without the need to intensify dialysis treatments. Further studies are required to determine whether such reduction provides clinical benefits.
Keywords: uremia, intestine, chronic hemodialysis, nutrition
Introduction
Numerous solutes are derived from the action of colon microbes on peptides that escape digestion in the small intestine (1). Many of these solutes are excreted by the kidneys and accumulate in the plasma when the kidneys fail (2–5). Two such uremic solutes, indoxyl sulfate and p-cresol sulfate, are derived from the action of colon microbes on tryptophan and phenylalanine/tyrosine, respectively. High levels of these compounds have been associated with vascular injury and mortality in patients with renal failure, and experimental studies have provided further evidence that they are toxic (6–10).
Because indoxyl sulfate and p-cresol sulfate are bound to plasma proteins, they are poorly cleared by hemodialysis (11). An alternate means to lower their plasma levels is to reduce their production. The production of solutes by colon microbes may prove simpler to suppress than the production of other uremic solutes because they are made in an isolated compartment by processes foreign to mammalian metabolism. One potential means to suppress the production of such solutes is to increase dietary fiber intake (12,13). The term “fiber” comprises a variety of carbohydrates and related substances that are resistant to digestion in the small intestine (14). Colon microbes can break fiber down to short-chain fatty acids that provide energy to the host and for microbial growth (3). Increased fiber delivery to the colon may cause the microbes to utilize amino acids for growth, rather than convert them to uremic solutes. In addition, fiber may alter the colon’s microbial population in such a way as to decrease the production of undesirable solutes. Fiber may also reduce the colon transit time available for microbial metabolism (15–17). This study tested whether providing fiber in the form of resistant starch to hemodialysis patients would reduce their plasma levels of indoxyl sulfate and p-cresol sulfate.
Materials and Methods
A single-blinded prospective randomized trial compared the addition of resistant starch, a form of fiber, and digestible control starch to the diets of patients on hemodialysis. The study was conducted between October 2010 and May 2013 at two university-affiliated and four community-based hemodialysis facilities in northern California. The study (ClinicalTrials.gov NCT01186276) was approved by the institutional review board at each participating site and was conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from all participants.
Patients were recruited if they were aged >18 years and were stably maintained on outpatient hemodialysis. Patients were excluded if they had a measured residual urea clearance >2 ml/min or if they reported significant urine production, had active gastrointestinal disease, had used antibiotics within 4 weeks, had a record of skipping or shortening their hemodialysis treatments, or if changes in their hemodialysis prescription were planned.
Permuted-block randomization was utilized to assign patients to fiber or control starch. Fifty-six patients were randomized, with 28 assigned to the fiber group and 28 to the control group. As illustrated in Figure 1, 20 patients in each group were included in the final analysis. Two patients who were randomized to the control starch dropped out because of side effects (one due to bloating and the other due to loose stools). No patients in the fiber group dropped out due to side effects. Excluded patients either started antibiotics, missed a dialysis treatment within 1 week of the final sample collection, missed >3 days of supplements, or had undetectable solute levels.
Figure 1.
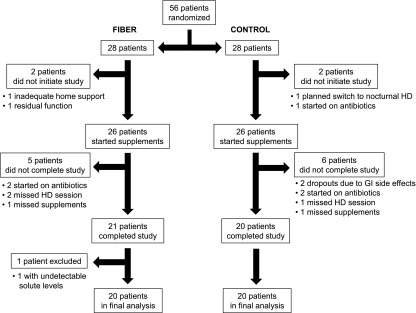
Patient disposition. There were two dropouts because of side effects in the control group and none in the fiber group. Each group had 20 patients included in the final analysis. GI, gastrointestinal; HD, hemodialysis.
Fiber and control supplements were provided as white powder in identical sachets and were supplied by Ingredion Incorporated (Bridgewater, NJ). Fiber sachets contained 15 g of high-amylose corn starch (Hi-maize 260), composed of approximately 40% digestible starch and 60% resistant starch, which being resistant to digestion is a form of fiber. Control sachets contained 15 g of waxy corn starch (AMIOCA), a high-amylopectin starch that is almost entirely digestible. The estimated energy provided by control sachets (60 calories per sachet) was only slightly higher than that provided by the fiber sachets (45 calories per sachet). Sachets were delivered to patients weekly and empty sachets were collected to assess compliance. Dialysis prescriptions, predialysis weights, and gastrointestinal symptoms including gas and loose stool were recorded weekly. Patients were instructed to mix the supplements in either liquid or food. They took the supplements for 6 weeks, consuming one sachet daily for the first week followed by two sachets daily for the remainder of the study. Five patients in the fiber group and five patients in the control group decreased consumption to one sachet per day for 3–5 weeks due to flatulence or loose stools.
Predialysis blood samples were obtained at baseline and week 6. Free solute levels were measured in plasma ultrafiltrate obtained using Nanosep 30K Omega separators (Pall, Ann Arbor, MI) and diluted 3-fold before analysis. Plasma samples were deproteinized 1:4 (vol/vol) with methanol and diluted 40-fold for measurement of total solute levels. Indoxyl sulfate and p-cresol sulfate were measured by stable isotope dilution liquid chromatography–tandem mass spectrometry as previously described (18). Plasma urea nitrogen, albumin, prealbumin, C-reactive protein (CRP), and phosphate were measured by clinical laboratories. The Kidney Disease Quality of Life (KDQOL)-36 questionnaire was administered at the time of the blood collections at the beginning and end of the study. Urea nitrogen generation was calculated as the predialysis urea nitrogen level multiplied by the monthly urea reduction ratio and the estimated volume of distribution.
The effect of fiber was assessed by comparing the fractional changes in free solute levels from baseline to week 6 in the two groups using analysis of covariance. According to a prespecified plan, the significance level was set at P value=0.025 to allow each of the two primary solutes tested, indoxyl sulfate and p-cresol sulfate, to be considered independently. The fractional changes in plasma levels of urea nitrogen, albumin, prealbumin, CRP, and phosphate and KDQOL scores from baseline to week 6 in the two groups were also compared using analysis of covariance. Characteristics of the two groups in Table 1 were compared using the unpaired t test and the chi-squared test (for sex and diabetic status). Linear regression was utilized to relate the fractional changes in plasma free levels of indoxyl sulfate and p-cresol sulfate. Statistical analyses were performed using SPSS software (version 21.0).
Table 1.
Patient characteristics
| Characteristic | Fiber Group (n=20) | Control Group (n=20) | P Value for Fiber versus Control Groups |
|---|---|---|---|
| Age (yr) | 54±14 | 58±13 | 0.29 |
| Men/women | 11/9 | 13/7 | 0.52 |
| Diabetes (n) | 9 | 9 | >0.99 |
| Dialysis vintage (yr) | 5 (2, 6) | 3 (2, 6) | 0.35 |
| Predialysis weight (kg) | 82±25 | 82±18 | >0.99 |
| Body mass index (kg/m2)a | 29±7 | 29±6 | 0.97 |
| Blood flow rate (ml/min) | 415±49 | 413±46 | 0.87 |
| Dialysate flow rate (ml/min) | 659±141 | 638±118 | 0.62 |
| Treatment time (min) | 223±92 | 212±68 | 0.68 |
| Albumin (g/dl)b | 4.0±0.3 | 3.9±0.3 | 0.17 |
| Urea reduction ratio (%) | 73±7 | 72±6 | 0.49 |
Data are presented as the mean±SD, n, or median (25th percentile, 75th percentile).
Body mass index (calculated on predialysis weight) was not obtained for one patient in each group.
Albumin levels were measured in predialysis samples.
Results
Characteristics of the 40 patients who completed the study are summarized in Table 1. Age, sex, diabetic status, dialysis vintage, weight, body mass index, dialysis prescriptions, and monthly albumin and urea reduction values were not significantly different in the two groups. Nine patients in each group were obese with a body mass index >30 kg/m2. The types of dialyzers utilized were similar in both groups and did not change throughout the study.
Plasma levels for the colon-derived solutes indoxyl sulfate and p-cresol sulfate are summarized in Table 2. In the patients randomized to the control starch, the levels of both solutes remained nearly constant. By contrast, in the patients randomized to increased fiber intake, the free level of indoxyl sulfate in the plasma was reduced by 27%±39% and the free level of p-cresol sulfate was reduced by 24%±44% at 6 weeks. Because the baseline levels for both solutes were slightly higher in the fiber group than the control group, significance was assessed using analysis of covariance to eliminate possible effects of regression to the mean. The effect of fiber on the free levels of indoxyl sulfate was significantly different from that of control starch, whereas the change in the free levels of p-cresol sulfate did not reach statistical significance. There was a tendency for the free fractions to fall in the fiber group so that the total solute levels did not decline as much as the free solute levels.
Table 2.
Solute measurements
| Solute | Fiber Group (n=20) | Control Group (n=20) | P Value |
|---|---|---|---|
| Indoxyl sulfate | |||
| Free concentration (mg/dl) | |||
| Baseline | 0.36±0.23 | 0.28±0.16 | 0.20 |
| Week 6 | 0.25±0.17 | 0.28±0.15 | |
| Percent change | −29 (−56, −12) | 0 (−20, 34) | 0.02 |
| Percent free | |||
| Baseline | 10.1±4.9 | 8.6±2.9 | 0.23 |
| Week 6 | 8.4±3.5 | 9.0±3.2 | |
| Percent change | −12 (−22, 1) | −1 (−17, 44) | 0.15 |
| Total concentration (mg/dl) | |||
| Baseline | 3.5±0.9 | 3.2±1.2 | 0.43 |
| Week 6 | 2.9±1.4 | 3.1±1.2 | |
| Percent change | −17 (−44, 1) | −5 (−16, 14) | 0.04 |
| p-Cresol sulfate | |||
| Free concentration (mg/dl) | |||
| Baseline | 0.27±0.15 | 0.22±0.13 | 0.26 |
| Week 6 | 0.21±0.14 | 0.23±0.14 | |
| Percent change | −28 (−46, 5) | 4 (−28, 36) | 0.05 |
| Percent free | |||
| Baseline | 8.5±4.8 | 6.9±2.0 | 0.19 |
| Week 6 | 6.6±2.4 | 7.1±2.2 | |
| Percent change | −14 (−28, −1) | 0 (−10, 37) | 0.04 |
| Total concentration (mg/dl) | |||
| Baseline | 3.3±1.5 | 3.2±1.3 | 0.82 |
| Week 6 | 2.9±1.6 | 3.1±1.4 | |
| Percent change | −8 (−34, 7) | −1 (−21, 8) | 0.63 |
Data are presented as the mean±SD or median (25th percentile, 75th percentile). The significance of differences at baseline was assessed using the unpaired t test. The significance of the effect of fiber was assessed using analysis of covariance.
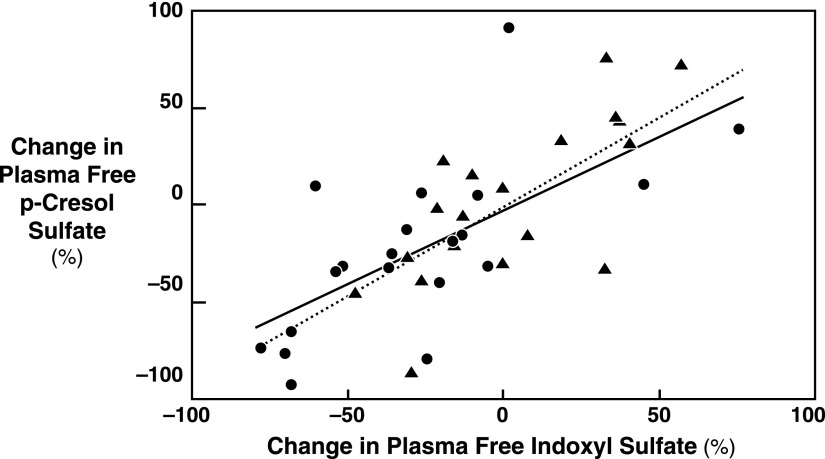
Figure 2 illustrates the relationship between the change in plasma free levels for indoxyl sulfate and p-cresol sulfate in individual patients. The change in the plasma free level of indoxyl sulfate was strongly correlated with the change in the plasma free level of p-cresol sulfate in individual patients in both the fiber (r=0.81, P<0.001) and control (r=0.94, P<0.001) groups and in the study population as a whole (r=0.76, P<0.001, not shown).
Figure 2.
The relation of indoxyl sulfate and p-cresol sulfate plasma free level changes in individual patients. The change in plasma free levels for indoxyl sulfate and p-cresol sulfate in individual patients was closely related for both the fiber and control groups. The patients receiving fiber are represented by circles (●) and the solid line (r=0.81, P<0.001) and the patients receiving control starch are represented by triangles (▲) and the dashed line (r=0.94, P<0.001). The changes in levels were also correlated in the study population as a whole (r=0.76, P<0.001, not shown).
Other measurements are summarized in Table 3. Body weight remained stable in both groups. Compared with the control group, the fiber group exhibited a tendency toward reduction of the plasma urea nitrogen (mean −7%±28% for fiber versus −2%±21% for control, P=0.62) and the plasma albumin as measured by the bromocresol purple assay (mean −4%±6% for fiber versus −1%±5% for control, P=0.12). The plasma albumin measured by nephelometry remained stable in both groups (mean −1.5%±14% for fiber versus −1.3±11% for control, P=0.79), whereas plasma prealbumin tended to decrease in the fiber group compared with the control group (mean −4%±18% for fiber versus 9%±19% for control, P=0.03). CRP levels were variable and no effect of fiber was apparent. Phosphate levels and total KDQOL scores remained stable.
Table 3.
Other measurements
| Parameter | Fiber Group (n=20) | Control Group (n=20) | P Value |
|---|---|---|---|
| Predialysis weight (kg) | |||
| Baseline | 82±25 | 82±18 | >0.99 |
| Week 6 | 83±25 | 82±19 | |
| Percent change | 0 (1, 2) | 1 (−1, 2) | 0.76 |
| Urea nitrogen (mg/dl) | |||
| Baseline | 65±22 | 61±19 | 0.61 |
| Week 6 | 56±14 | 60±19 | |
| Percent change | −12 (−29, 11) | 2 (−16, 7) | 0.62 |
| Albumin by bromocresol purple (g/dl) | |||
| Baseline | 3.7±0.3 | 3.6±0.3 | 0.37 |
| Week 6 | 3.5±0.3 | 3.5±0.4 | |
| Percent change | −3 (−6, 0) | −2 (−4, 3) | 0.12 |
| Albumin by nephelometry (g/dl) | |||
| Baseline | 4.2±0.4 | 4.1±0.5 | 0.69 |
| Week 6 | 4.1±0.4 | 4.0±0.4 | |
| Percent change | −3 (−7, 2) | −4 (−7, 1) | 0.79 |
| Prealbumin (mg/dl) | |||
| Baseline | 29±9 | 28±8 | 0.72 |
| Week 6 | 27±6 | 31±10 | |
| Percent change | −9 (−17, 11) | 7 (−3, 18) | 0.03 |
| CRP (mg/dl)a | |||
| Baseline | 1.0±1.1 | 0.6±0.5 | 0.22 |
| Week 6 | 1.1±1.6 | 0.8±1.1 | |
| Percent change | 47 (−28, 75) | 0 (−13, 46) | 0.11 |
| Phosphate (mg/dl) | |||
| Baseline | 6.3±2.5 | 5.5±1.9 | 0.31 |
| Week 6 | 6.3±1.8 | 5.6±2.2 | |
| Percent change | 2 (−11, 17) | 10 (−15, 23) | 0.79 |
| KDQOL-36 scoreb | |||
| Baseline | 72±15 | 73±16 | 0.91 |
| Week 6 | 73±12 | 75±16 | |
| Percent change | 0 (−4, 7) | 3 (−4, 6) | 0.68 |
Data are presented as the mean±SD, n, or median (25th percentile, 75th percentile). The significance of differences at baseline was assessed using the unpaired t test. The significance of the effect of fiber was assessed using analysis of covariance. CRP, C-reactive protein; KDQOL-36, Kidney Disease Quality of Life Short Form 36.
Six CRP values were <0.1 mg/dl (the limit of quantitation), but no participant had a value <0.1 mg/dl at both baseline and week 6.
One patient in the fiber group and four patients in the control group did not complete the KDQOL-36 at the end of the study.
The fiber supplement significantly reduced plasma free levels of indoxyl sulfate without causing gastrointestinal symptoms greater than those seen in control patients taking a similar amount of ordinary corn starch. Ten patients in the fiber group and 12 patients in the control group reported moderate gas. Six patients in the fiber group and eight patients in the control group reported transient loose stools. The two patients who dropped out of the study because of gastrointestinal symptoms were both taking the control supplement.
Discussion
Conventional dialysis provides limited clearance of organic solutes that accumulate when the kidneys fail. High plasma levels of these solutes presumably contribute to poor health outcomes in dialysis patients. Major efforts have therefore been directed toward increasing the frequency and/or duration of treatment and to increasing solute clearances during treatment (19,20).
A possible complementary means to improve dialysis treatment is to suppress solute production. A large number of uremic solutes are derived from the action of colon microbes (2–5). Recent studies have delineated the size and complexity of the colon’s microbial population or “microbiome” (21,22). The microbiome exists in synergy with the human host, providing energy and nutrients and enhancing immune responsiveness (16,21,22). However, the microbiome also produces numerous waste solutes that are normally excreted by the kidney (2–4).
Indoxyl sulfate and p-cresol sulfate are the two most extensively studied solutes derived from colon microbes. Considerable evidence suggests that they are toxic (6–10). Because they are >90% bound to plasma proteins, their dialytic clearance is low and they accumulate to high levels in the plasma of hemodialysis patients (11,18).
One potential means to reduce the production of solutes derived from colon microbial breakdown of amino acids is to increase dietary fiber (12,23). Fiber was first utilized in patients with renal insufficiency as an adjunct to dietary protein restriction (24–26). It was known that fiber increases the bulk of the stool, which consists largely of microbes. It was hypothesized that incorporation of nitrogen into microbial proteins would reduce the production of “nitrogenous wastes” normally excreted in the urine and would thereby alleviate uremic symptoms and slow disease progression. Plasma levels of urea, the only solute measured in these studies, were shown to decline when fiber intake was increased. Studies in patients without renal disease, however, previously showed that increasing dietary fiber could also limit the production of p-cresol and phenol, which were under consideration as causes of colon cancer (27). Meijers et al. (12) subsequently showed that increasing fiber intake could reduce production of p-cresol in patients with renal failure. They supplemented the diet of hemodialysis patients with fiber in the form of oligofructose-enriched inulin. A dosage of 10 g daily for the first week followed by 10 g twice daily for the next 3 weeks reduced the production and plasma level of p-cresol sulfate by 20% but had no effect on indoxyl sulfate.
This study further tested the effect of increasing dietary fiber in hemodialysis patients. Fiber was given in the form of resistant starch, which has higher molecular weight than oligofructose-enriched inulin and other oligosaccharide forms of fiber. Fermentation of fiber in the colon produces gases including hydrogen and methane, which cause flatulence as observed by Meijers et al. (12). A potential advantage of resistant starch is that slower fermentation may limit flatulence and other gastrointestinal side effects (28). Using a randomized design, we found that resistant starch reduced free indoxyl sulfate levels at a dose which did not increase gastrointestinal symptoms above the level seen with a control starch.
The types and amounts of solutes produced in the colon depend upon the composition of the microbiome, which in turn is affected by diet (22,29,30). Indole and p-cresol, the precursors of indoxyl sulfate and p-cresol sulfate, are thought to be produced by different microbes, and prior studies have shown limited correlation between the generation rates of indoxyl sulfate and p-cresol sulfate both in healthy participants and hemodialysis patients (31,32). We found, however, that the changes in indoxyl sulfate and p-cresol sulfate in response to dietary fiber were closely related, as depicted in Figure 2. Because the patients’ prescriptions did not change, the dialytic solute clearances remained constant and the changes in plasma levels presumably reflected changes in generation. This suggests that although the generation of indoxyl sulfate and p-cresol sulfate is not linked chemically, their production was suppressed to the same degree. In assessing the effect of fiber, we believe that the changes in the free concentrations of indoxyl sulfate and p-cresol sulfate are more important than the changes in the total concentrations. First, we presume that it is the free concentration that is available for interaction with body tissues. This presumption is strongly supported by studies of pharmaceutical agents (33,34). Second, modeling indicates that for solutes that are largely bound to plasma proteins, the removal rate during dialysis is more closely proportional to the free solute concentration than the total solute concentration (35,36). In this study, the free fractions of both indoxyl sulfate and p-cresol sulfate tended to decline in the fiber group compared with the control group, so that the total solute levels fell less than the free solute levels. This increase in protein binding can be explained by a reduction of solute burden resulting in less competition for binding sites on albumin (37,38). Fiber may have reduced the overall bound solute burden by reducing the production of solutes other than indoxyl sulfate and p-cresol sulfate, which bind to albumin.
We did not analyze the composition of the colon microbiome in this study. Recent studies suggested, however, that the microbiome is altered in dialysis patients (39,40). It has been suggested that an altered microbiome in renal disease may not only be responsible for the production of uremic solutes, but may also cause increased intestinal permeability leading to translocation of bacteria and endotoxins, thereby promoting systemic inflammation (23,39). We did not observe an effect of fiber on CRP levels, which were quite variable.
Previous studies suggested that increasing fiber intake can reduce plasma urea levels in patients with renal failure by causing a larger portion of ingested nitrogen to be excreted as microbial proteins in the stool (12,24–27). A significant reduction in plasma urea levels between the fiber and control groups was not detected, but power to detect such a change was limited. We presume there was an increase in stool volume in the patients taking the fiber although the two groups reported no difference in symptoms.
In addition to fiber, other manipulations may reduce production of colon-derived uremic solutes (2,3,23). The charcoal sorbent AST-120 reduces absorption of indole into the body and thereby reduces production of indoxyl sulfate (41). The α-glucosidase inhibitor acarbose reduces p-cresol production by preventing digestion of carbohydrates in the small intestine and thus increasing carbohydrate delivery to the colon (42). Probiotics may alter the microbiome so as to reduce the production of undesirable compounds.
Malnutrition is a potential concern with any dietary intervention. We first measured plasma albumin using the bromocresol purple method routinely utilized by our clinical laboratory. This assay is based on the color change that occurs when the dye bromocresol purple binds to albumin. However, dye-binding assays may be affected by the accumulation of uremic solutes (43). We therefore further evaluated plasma albumin using nephelometry, an immune-based assay, and found no effect of diet on the levels. Prealbumin, however, was slightly reduced in the fiber group compared with the control group. This finding suggests the need for nutritional monitoring in further studies of fiber supplementation in dialysis patients. Of note, high dietary fiber intake was recently found to be associated with decreased mortality in patients with CKD (44).
In conclusion, we found that fiber reduced the plasma free level of the colon-derived solute indoxyl sulfate with no gastrointestinal symptoms greater than those seen in control patients taking a similar amount of corn starch. Because this control supplement is easily digestible and does not reach the colon, we presume the gastrointestinal symptoms reported by our patients were not caused by the supplements. We therefore suspect that we might have achieved a greater reduction in solute levels by administering a higher dose of resistant starch without excessive side effects. Our study has limitations. We could not perform an intention-to-treat analysis because solute measurements were not made at 6 weeks for most of the patients who did not complete the study. Our study was small, and more precise assessment of the effect of fiber on solute levels would require inclusion of larger numbers of participants. A larger and longer study would also be required to demonstrate that increasing fiber intake provides clinical benefit. However, given that average fiber intake is low in hemodialysis patients, fiber supplementation provides an appealing potential means to improve their health (45).
Disclosures
None.
Acknowledgments
The authors thank Dr. Christine Pelkman and Ingredion Incorporated for supplying the study supplements and Dr. Britt Burton Freeman for technical advice. The authors also thank the dietitians, nurses, technicians, and nephrologists of the participating dialysis units for their assistance. Statistical assistance was provided by Dr. Kevin F. Erickson of Stanford University and Dr. Alex McMillan of the Stanford Center for Clinical and Translation Research and Education (SPECTRUM).
The Stanford Nephrology fellowship program was supported by T32 DK007357. SPECTRUM is funded by the National Institutes of Health (NIH) National Center for Advancing Translational Sciences (UL1-TR000093). T.L.S. was supported by a Mitsubishi Tanabe Pharma Corporation/National Kidney Foundation Fellowship for the Study of Uremia. Other support was also provided by the NIH (R21-AT005123 to T.W.M.).
Part of this work was presented in abstract form at the American Society of Nephrology 2013 Annual Meeting, held November 7–10, 2013, in Atlanta, Georgia.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Can Oral Therapy Reduce Uremic Toxins?,” on pages 1513–1515.
References
- 1.Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, Siuzdak G: Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci U S A 106: 3698–3703, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schepers E, Glorieux G, Vanholder R: The gut: The forgotten organ in uremia? Blood Purif 29: 130–136, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Evenepoel P, Meijers BK, Bammens BR, Verbeke K: Uremic toxins originating from colonic microbial metabolism. Kidney Int Suppl 114: S12–S19, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Meyer TW, Hostetter TH: Uremic solutes from colon microbes. Kidney Int 81: 949–954, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Aronov PA, Luo FJ, Plummer NS, Quan Z, Holmes S, Hostetter TH, Meyer TW: Colonic contribution to uremic solutes. J Am Soc Nephrol 22: 1769–1776, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niwa T: Indoxyl sulfate is a nephro-vascular toxin. J Ren Nutr 20[Suppl]: S2–S6, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Meijers BK, Bammens B, De Moor B, Verbeke K, Vanrenterghem Y, Evenepoel P: Free p-cresol is associated with cardiovascular disease in hemodialysis patients. Kidney Int 73: 1174–1180, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Liabeuf S, Barreto DV, Barreto FC, Meert N, Glorieux G, Schepers E, Temmar M, Choukroun G, Vanholder R, Massy ZA, European Uraemic Toxin Work Group (EUTox) : Free p-cresylsulphate is a predictor of mortality in patients at different stages of chronic kidney disease. Nephrol Dial Transplant 25: 1183–1191, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Meijers BK, Van Kerckhoven S, Verbeke K, Dehaen W, Vanrenterghem Y, Hoylaerts MF, Evenepoel P: The uremic retention solute p-cresyl sulfate and markers of endothelial damage. Am J Kidney Dis 54: 891–901, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Schepers E, Meert N, Glorieux G, Goeman J, Van der Eycken J, Vanholder R: P-cresylsulphate, the main in vivo metabolite of p-cresol, activates leucocyte free radical production. Nephrol Dial Transplant 22: 592–596, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Lesaffer G, De Smet R, Lameire N, Dhondt A, Duym P, Vanholder R: Intradialytic removal of protein-bound uraemic toxins: Role of solute characteristics and of dialyser membrane. Nephrol Dial Transplant 15: 50–57, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Meijers BK, De Preter V, Verbeke K, Vanrenterghem Y, Evenepoel P: p-Cresyl sulfate serum concentrations in haemodialysis patients are reduced by the prebiotic oligofructose-enriched inulin. Nephrol Dial Transplant 25: 219–224, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Evenepoel P, Meijers BK: Dietary fiber and protein: Nutritional therapy in chronic kidney disease and beyond. Kidney Int 81: 227–229, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Cummings JH, Stephen AM: Carbohydrate terminology and classification. Eur J Clin Nutr 61[Suppl 1]: S5–S18, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Burkitt DP, Walker AR, Painter NS: Effect of dietary fibre on stools and the transit-times, and its role in the causation of disease. Lancet 2: 1408–1412, 1972 [DOI] [PubMed] [Google Scholar]
- 16.Flint HJ: The impact of nutrition on the human microbiome. Nutr Rev 70[Suppl 1]: S10–S13, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Kalmokoff M, Zwicker B, O’Hara M, Matias F, Green J, Shastri P, Green-Johnson J, Brooks SP: Temporal change in the gut community of rats fed high amylose cornstarch is driven by endogenous urea rather than strictly on carbohydrate availability. J Appl Microbiol 114: 1516–1528, 2013 [DOI] [PubMed] [Google Scholar]
- 18.Sirich TL, Aronov PA, Plummer NS, Hostetter TH, Meyer TW: Numerous protein-bound solutes are cleared by the kidney with high efficiency. Kidney Int 84: 585–590, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raimann JG, Thijssen S, Ramos R, Levin NW: More frequent hemodialysis: What do we know? Where do we stand? Contrib Nephrol 171: 10–16, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Canaud B, Bowry SK: Emerging clinical evidence on online hemodiafiltration: Does volume of ultrafiltration matter? Blood Purif 35: 55–62, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Dethlefsen L, McFall-Ngai M, Relman DA: An ecological and evolutionary perspective on human-microbe mutualism and disease. Nature 449: 811–818, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tremaroli V, Bäckhed F: Functional interactions between the gut microbiota and host metabolism. Nature 489: 242–249, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Ramezani A, Raj DS: The gut microbiome, kidney disease, and targeted interventions. J Am Soc Nephrol 25: 657–670, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rampton DS, Cohen SL, Crammond VD, Gibbons J, Lilburn MF, Rabet JY, Vince AJ, Wager JD, Wrong OM: Treatment of chronic renal failure with dietary fiber. Clin Nephrol 21: 159–163, 1984 [PubMed] [Google Scholar]
- 25.Younes H, Alphonse JC, Behr SR, Demigné C, Rémésy C: Role of fermentable carbohydrate supplements with a low-protein diet in the course of chronic renal failure: Experimental bases. Am J Kidney Dis 33: 633–646, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Bliss DZ, Stein TP, Schleifer CR, Settle RG: Supplementation with gum arabic fiber increases fecal nitrogen excretion and lowers serum urea nitrogen concentration in chronic renal failure patients consuming a low-protein diet. Am J Clin Nutr 63: 392–398, 1996 [DOI] [PubMed] [Google Scholar]
- 27.Birkett A, Muir J, Phillips J, Jones G, O’Dea K: Resistant starch lowers fecal concentrations of ammonia and phenols in humans. Am J Clin Nutr 63: 766–772, 1996 [DOI] [PubMed] [Google Scholar]
- 28.Grabitske HA, Slavin JL: Gastrointestinal effects of low-digestible carbohydrates. Crit Rev Food Sci Nutr 49: 327–360, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Smith EA, Macfarlane GT: Formation of phenolic and indolic compounds by anaerobic bacteria in the human large intestine. Microb Ecol 33: 180–188, 1997 [DOI] [PubMed] [Google Scholar]
- 30.Sonnenburg ED, Zheng H, Joglekar P, Higginbottom SK, Firbank SJ, Bolam DN, Sonnenburg JL: Specificity of polysaccharide use in intestinal bacteroides species determines diet-induced microbiota alterations. Cell 141: 1241–1252, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poesen R, Viaene L, Verbeke K, Claes K, Bammens B, Sprangers B, Naesens M, Vanrenterghem Y, Kuypers D, Evenepoel P, Meijers B: Renal clearance and intestinal generation of p-cresyl sulfate and indoxyl sulfate in CKD. Clin J Am Soc Nephrol 8: 1508–1514, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patel KP, Luo FJ, Plummer NS, Hostetter TH, Meyer TW: The production of p-cresol sulfate and indoxyl sulfate in vegetarians versus omnivores. Clin J Am Soc Nephrol 7: 982–988, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmidt S, Gonzalez D, Derendorf H: Significance of protein binding in pharmacokinetics and pharmacodynamics. J Pharm Sci 99: 1107–1122, 2010 [DOI] [PubMed] [Google Scholar]
- 34.von Winckelmann SL, Spriet I, Willems L: Therapeutic drug monitoring of phenytoin in critically ill patients. Pharmacotherapy 28: 1391–1400, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Walther JL, Bartlett DW, Chew W, Robertson CR, Hostetter TH, Meyer TW: Downloadable computer models for renal replacement therapy. Kidney Int 69: 1056–1063, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Meyer TW, Leeper EC, Bartlett DW, Depner TA, Lit YZ, Robertson CR, Hostetter TH: Increasing dialysate flow and dialyzer mass transfer area coefficient to increase the clearance of protein-bound solutes. J Am Soc Nephrol 15: 1927–1935, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Gulyassy PF, Depner TA: Impaired binding of drugs and endogenous ligands in renal diseases. Am J Kidney Dis 2: 578–601, 1983 [DOI] [PubMed] [Google Scholar]
- 38.Klammt S, Wojak HJ, Mitzner A, Koball S, Rychly J, Reisinger EC, Mitzner S: Albumin-binding capacity (ABiC) is reduced in patients with chronic kidney disease along with an accumulation of protein-bound uraemic toxins. Nephrol Dial Transplant 27: 2377–2383, 2012 [DOI] [PubMed] [Google Scholar]
- 39.Vaziri ND: CKD impairs barrier function and alters microbial flora of the intestine: A major link to inflammation and uremic toxicity. Curr Opin Nephrol Hypertens 21: 587–592, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vaziri ND, Wong J, Pahl M, Piceno YM, Yuan J, DeSantis TZ, Ni Z, Nguyen TH, Andersen GL: Chronic kidney disease alters intestinal microbial flora. Kidney Int 83: 308–315, 2013 [DOI] [PubMed] [Google Scholar]
- 41.Schulman G, Vanholder R, Niwa T: AST-120 for the management of progression of chronic kidney disease. Int J Nephrol Renovasc Dis 7: 49–56, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Evenepoel P, Bammens B, Verbeke K, Vanrenterghem Y: Acarbose treatment lowers generation and serum concentrations of the protein-bound solute p-cresol: A pilot study. Kidney Int 70: 192–198, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Mabuchi H, Nakahashi H: Underestimation of serum albumin by the bromcresol purple method and a major endogenous ligand in uremia. Clin Chim Acta 167: 89–96, 1987 [DOI] [PubMed] [Google Scholar]
- 44.Krishnamurthy VM, Wei G, Baird BC, Murtaugh M, Chonchol MB, Raphael KL, Greene T, Beddhu S: High dietary fiber intake is associated with decreased inflammation and all-cause mortality in patients with chronic kidney disease. Kidney Int 81: 300–306, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kalantar-Zadeh K, Kopple JD, Deepak S, Block D, Block G: Food intake characteristics of hemodialysis patients as obtained by food frequency questionnaire. J Ren Nutr 12: 17–31, 2002 [DOI] [PubMed] [Google Scholar]