Abstract
Background and objectives
Poor growth is a consequence of CKD, but can often be partially or fully prevented or corrected with the use of a number of medications. The extent of nonadherence with medications used to treat or mitigate growth failure in CKD has not been examined prospectively in children with CKD.
Design, setting, participants, & measurements
The prevalence of both prescription of and nonadherence to recombinant human growth hormone (rhGH), phosphate binders, alkali, active vitamin D, nutritional vitamin D, iron, and erythrocyte-stimulating agents was summarized over the first seven visits of the Chronic Kidney Disease in Children cohort study. The association between self-reported nonadherence to each medication group and the mean annual change in age- and sex-specific height z score was quantified using seven separate linear regression models with generalized estimating equations.
Results
Of 834 participants, 597 reported use of at least one of these medication groups and had adherence data available. Nonadherence ranged from 4% over all visits for erythrocyte-stimulating agents to 22% over all visits for nutritional vitamin D. Of the study participants, 451 contributed data to at least one of the analyses of adherence and changes in height z score. Children nonadherent to rhGH had no change in height z score, whereas those adherent to rhGH had a significant improvement of 0.16 SDs (95% confidence interval, 0.05 to 0.27); the effect size was slightly larger and remained significant after adjustment. Among participants with height≤3rd percentile and after adjustment, adherence to rhGH was associated with a 0.33 SD (95% confidence interval, 0.10 to 0.56) greater change in height z score. Nonadherence with other medication groups was not significantly associated with a change in height z score.
Conclusions
Self-reported nonadherence to rhGH was associated with poorer growth velocity in children with CKD, suggesting an opportunity for intervention and improved patient outcome.
Keywords: CKD, children, pediatric nephrology
Introduction
Impairment of linear growth velocity is a prominent clinical feature of CKD in children (1–3) and has been linked to decreased quality of life (4,5) and increased morbidity (6). In pediatric ESRD, poor growth has also been linked to mortality (7). In the Chronic Kidney Disease in Children (CKiD) study, the largest prospective study of pediatric patients with CKD to date, 15% of participants were below the third percentile for age- and sex-specific height at baseline (4,8) and the percentage of those with growth failure increased further as GFR declined (9). These findings exist despite the effectiveness of recombinant growth hormone (rhGH) therapy in children with CKD (10).
Medication nonadherence is highly prevalent and negatively affects outcomes in patients with chronic childhood medical conditions (11,12), including dialysis patients (13–15) and kidney transplant recipients (16,17). Treatment of patients with CKD includes multiple medications, high dosing frequency, numerous dose adjustments due to disease progression, and the need for coordination of medication administration timing with meals, all of which create a unique context for medication nonadherence in children (18–21).
It has been proposed that CKD mineral and bone disorder, acidosis, anemia, vitamin D deficiency, and rhGH resistance all have a deleterious effect on the growth velocity of children with CKD (22,23). Thus, one would expect that adherence to any class of medication aimed to control these relevant CKD comorbidities (i.e., phosphate [PO4] binders, active vitamin D, nutritional vitamin D, alkali, iron, erythrocyte-stimulating agents [ESAs], and rhGH) might improve growth; conversely, nonadherence to any of these medications could impair growth. To our knowledge, this premise has not been tested in a large-scale cohort of children with CKD. A recent Canadian study, based on the pharmacy refill rates in a pediatric CKD cohort, demonstrated that on average, patients missed 1 day of medications each week (20). A cross-sectional analysis of baseline CKiD data indicated that medications thought to support optimal growth were among those with the highest rates of nonadherence (21). However, the extent of medication nonadherence in children with CKD over time and its association with poor growth remains poorly understood.
This study describes the use of and nonadherence over time with the prescribed medications that may affect growth velocity and quantifies the strength of the association of nonadherence with the annualized change in the age- and sex-specific height z score.
Materials and Methods
Study Population and Design
From April 2005 through May 2013, 834 children with mild to moderate CKD were enrolled in the CKiD study, which is a multicenter, prospective cohort study conducted at 55 pediatric nephrology centers across North America. The study design and conduct were approved by an observational study monitoring board appointed by the National Institute of Diabetes and Digestive and Kidney Diseases and by the internal review boards of each participating center. Details of the CKiD study were previously published (9). Clinical and demographic information are collected at annual visits.
Medication Use and Adherence
As part of each annual visit, medications prescribed to the study participants were recorded. The percentage of participants who reported the use of any of the following medication groups was summarized at each of the first seven study visits: PO4 binders, active vitamin D, nutritional vitamin D, alkali, iron, ESAs, and rhGH. PO4 binders included both calcium-based and noncalcium-based preparations. For each medication reported, the participant was asked “Has (drug) been taken as prescribed in the past 7 days?,” with “yes” or “no” being the only two answer choices. A report of not taking the drug as prescribed in the past 7 days was classified as nonadherence. As such, patients who reported to miss even one dose of a particular medication were classified as nonadherent to this medication at a given visit.
Primary Outcome
At each visit, age- and sex-specific height z scores (hereafter referred to as height z scores) were calculated using the 2000 US Centers for Disease Control and Prevention standard growth charts for United States children (24). Pairs of consecutive visits served as the unit of analysis because our interest was in the annual change in height z score immediately after the exposure to medications and adherence reported at the first visit in the pair (index visit). The change in height z score within the pair of visits was standardized to a year.
Covariates
GFR was determined by the plasma iohexol disappearance (iGFR) (25) at the first two visits and every other visit thereafter. When this GFR value was not available (e.g., at odd-numbered visits after the initial baseline visit), the eGFR based on height/serum creatinine, cystatin C, and/or BUN, as well as sex and height, were calculated using CKiD-published estimating equations (26). All CKD diagnoses were categorized as either glomerular or nonglomerular. Data on the participants’ sex, annual household income (dichotomized into two categories: ≤$36,000 and >$36,000), and maternal education level (categorized as a high school graduate at most, some college, or college graduate) were collected at baseline. The percentage of a participant’s life with CKD was determined as the time between the CKD diagnosis and the index visit divided by age at the index visit.
Statistical Analyses
A linear trend test was used to assess the significance of changes over time in the following: (1) the use of each of the seven medication groups, and (2) the prevalence of overall nonadherence and nonadherence to each of the seven medication groups. The average annualized change in height z scores was compared between adherent and nonadherent participants using separate linear regression models for each of the seven medication groups. Generalized estimating equations were used to account for the statistical dependence incurred by repeated measures of the outcome (i.e., multiple visit pairs) on the same individual (27). In addition to the primary exposure of adherence, each regression model included index values of height z score, GFR, percentage of life with CKD, and age, as well as CKD cause (glomerular versus nonglomerular), sex, family income (baseline value), and maternal education (baseline value). We used 95% confidence intervals (95% CIs) as a measure of precision.
Results
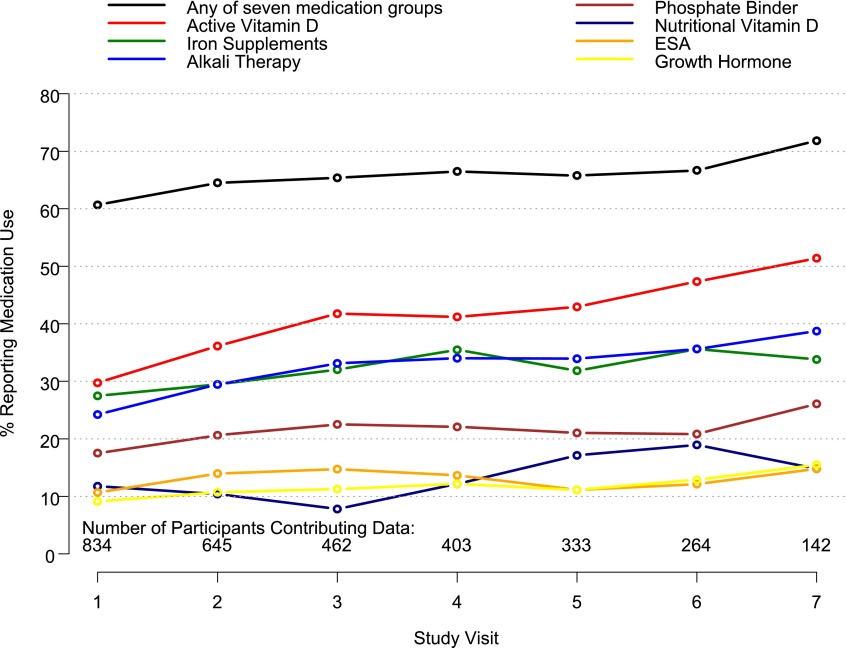
Figure 1 shows the prevalence of use of each of the seven medication groups at each visit. Of the study participants, 74% (618 of 834) reported use of at least one of the seven medication groups for at least one of the seven visits. The use of any of the seven medication groups ranged from 61% at baseline to 72% at the seventh visit. Active vitamin D (reported at 38% of all person-visits), iron (31%), and alkali (31%) were the three medications reported as most often administered. The prevalence of medication use increased significantly over time for all of the medication groups except rhGH and ESAs.
Figure 1.
Medication use at the first seven visits of the CKiD study. Medication use increased per visit as follows: active vitamin D, 7.5% (P<0.001); iron supplement, 3.9% (P=0.004); alkali therapy, 4.8% (P=0.002); phosphate binder, 7.9% (P<0.001); nutritional vitamin D, 17.6% (P<0.001); ESA, 2.6% (P=0.34); and rhGH, 3.1% (P=0.32). CKiD, Chronic Kidney Disease in Children; ESA, erythrocyte-stimulating agent; rhGH, recombinant human growth hormone.
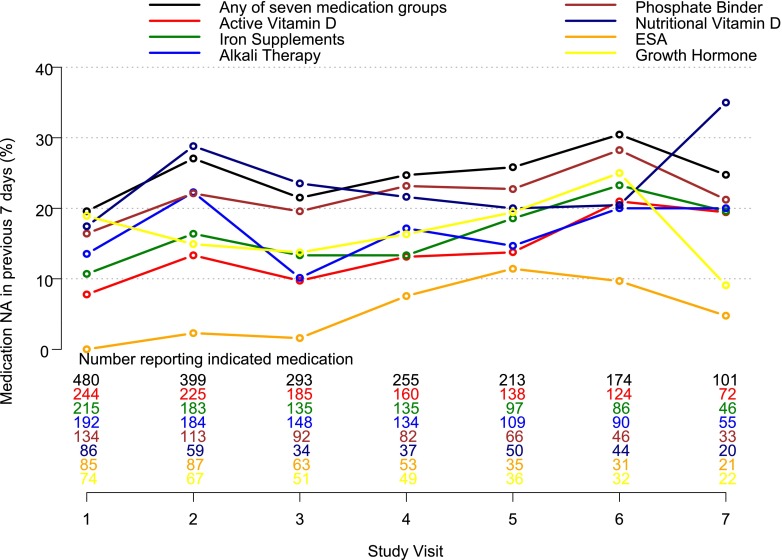
Figure 2 shows the percentage of children who reported nonadherence to each medication in the 7 days preceding the visit. Nonadherence to any of the seven medication groups prescribed ranged from 20% of the patients at baseline to 30% at the sixth visit. Nonadherence over all medication groups and all person-visits ranged from 0% for ESAs at visit one to 35% for nutritional vitamin D at visit seven. Nonadherence to rhGH was 17% over all person-visits. The prevalence of nonadherence significantly increased over time for all medication groups combined (P=0.01) as well as for the following medication groups: active vitamin D (P<0.001), iron supplements (P=0.01), and ESAs (P<0.001). None of the other medication groups had significant changes in the prevalence of nonadherence over time.
Figure 2.
Prevalence of nonadherence by medication group at each of the first seven visits of the CKiD study. The number of children reporting medication with adherence data available (i.e., the denominator of the percentage plotted) is given at the bottom of the figure for each of the medication groups. The percentages of medication nonadherence over all seven visits are as follows: nonadherence to any medication group, 24%; active vitamin D, 13%; iron supplements, 15%; alkali therapy, 16%; phosphate binders, 21%; nutritional vitamin D, 22%; ESA, 4%; and rhGH, 17%. NA, nonadherence.
Table 1 presents baseline descriptive statistics of the 451 children who reported taking at least one of the seven medication groups for at least one of the seven study visits, had adherence data available, and contributed data to at least one of the paired-visit analyses. The median height z score of −0.74 (interquartile range, −1.48, 0.04) indicates a substantial height deficit. The median GFR was 44.4 ml/min per 1.73 m2 (interquartile range, 33.5, 58.2) and 21%of participants had a glomerular cause of CKD.
Table 1.
Baseline characteristics of 451 CKiD study participants
| Characteristica | Value |
|---|---|
| Age-specific and sex-specific height z score | −0.74 (−1.48, 0.04) |
| GFR (ml/min per 1.73 m2) | 44.4 (33.5, 58.2) |
| Glomerular CKD cause | 93 (21) |
| Life with CKD (%) | 93 (41, 100) |
| Boys | 285 (63) |
| Age (yr) | 10.4 (6.6, 13.8) |
| White race | 302 (67) |
| Family income $36,000 | 263 (64) |
| Maternal education | |
| High school | 185 (42) |
| Some college | 124 (28) |
| College graduate | 129 (29) |
Data are presented as the median (interquartile range) or n (%).
Missing data are as follows: height z score (n=9), percentage of life with CKD (n=7), family income (n=41), and maternal education (n=13).
Table 2 shows the unadjusted relationship between adherence status and the mean annualized change in height z score for each of seven medication groups. A total of 100 participants reported being prescribed rhGH with adherence data available during follow-up and contributed a total of 241 visit pairs in which rhGH use was reported at the index visit in the pair. Study participants who were nonadherent to rhGH had a mean annualized change of 0.00 in height z score (95% CI, −0.10 to 0.09), whereas those adherent to rhGH demonstrated a mean annualized change of 0.16 SDs in their height z score (95% CI, 0.10 to 0.22; P value for difference between groups, P=0.003). Although there was no difference in nonadherence among those with a height z score≤3rd percentile versus >3rd percentile (20% versus 17%; P=0.55), those who were adherent to rhGH had an average annualized change in height z score that was 0.30 SDs (95% CI, 0.10 to 0.49) greater than those who were nonadherent if the analysis was restricted to the 83 visit pairs with height z score<3rd percentile at the index visit. The mean changes for the two groups were 0.17 and −0.12, respectively. Participants who were adherent to alkali therapy, active vitamin D, or ESAs had significant increases in height z scores, although this was not statistically different from those who were nonadherent with those same medication groups.
Table 2.
Univariate analysis of annual growth velocity by adherence status
| Medication Group | Unique Study Participantsa; Visit Pairs Contributed to Analysis (n) | Visit Pairs for Which Study Participants Reported Nonadherence at First Visit in Visit Pair (%) | Mean Annualized Change (95% CI) in Age- and Sex-Specific Height z Score | P Value Comparing Nonadherent to Adherent Participants | |
|---|---|---|---|---|---|
| Nonadherent | Adherent | ||||
| Growth hormone | 100; 241 | 18.3 | 0.00 (−0.10 to 0.09) | 0.16 (0.10 to 0.22) | 0.003 |
| Phosphate binder | 156; 346 | 23.4 | 0.09 (0.00 to 0.18) | 0.02 (−0.02 to 0.06) | 0.13 |
| Alkali | 213; 615 | 16.7 | 0.03 (−0.03 to 0.09) | 0.04 (0.01 to 0.07) | 0.78 |
| Nutritional vitamin D | 105; 164 | 25.6 | 0.03 (−0.05 to 0.11) | 0.00 (−0.06 to 0.06) | 0.51 |
| Active vitamin D | 281; 796 | 12.9 | 0.01 (−0.05 to 0.06) | 0.04 (0.01 to 0.06) | 0.36 |
| Iron | 241; 609 | 15.6 | 0.00 (−0.05 to 0.04) | 0.03 (−0.01 to 0.06) | 0.22 |
| ESA | 102; 249 | 4.4 | 0.05 (−0.11 to 0.22) | 0.08 (0.02 to 0.13) | 0.78 |
ESA, erythrocyte-stimulating agent; 95% CI, 95% confidence interval.
Study participants with available adherence data at the first visit of the pair and age- and sex-specific height z scores at both visits of the pair.
Table 3 shows the effect of adherence on the annualized change in height z score for each of the seven medication groups after adjustment for covariates. Because of missing data on some of the covariates, both the number of participants contributing data and the number of visit pairs included in the multivariate analyses were less than the corresponding values for the unadjusted analyses (Table 2). Those adherent with rhGH had a mean annualized change in height z score that was 0.18 SDs greater (95% CI, 0.06 to 0.30) than those who were nonadherent. After adjustment, adherence to rhGH was associated with a 0.33 SD (95% CI, 0.10 to 0.56) greater annual change in height z score among participants with height z score<3rd percentile at the index visit. Conversely, no significant difference was found in height z scores between patients who were adherent versus those who were nonadherent for any of the other six medication groups.
Table 3.
Multivariate analyses of the effect of adherence to individual medication classes and annual changes in age- and sex-specific height z score
| Medication Group | Unique Study Participantsa; Visit Pairs Contributed to Analysis (n) | Visit Pairs for Which Study Participants Reported Nonadherence at First Visit in Visit Pair (%) | Mean Difference in Adherent versus Nonadherent Participants in Annualized Change in Age- and Sex-Specific Height z Score (95% CI) |
|---|---|---|---|
| Growth hormone | 86; 211 | 19.0 | 0.18 (0.06 to 0.30) |
| Phosphate binder | 134; 304 | 24.3 | −0.07 (−0.17 to 0.04) |
| Alkali | 192; 555 | 18.0 | 0.02 (−0.04 to 0.09) |
| Nutritional vitamin D | 92; 146 | 25.3 | −0.04 (−0.13 to 0.06) |
| Active vitamin D | 246; 703 | 14.1 | 0.02 (−0.04 to 0.08) |
| Iron | 212; 534 | 16.3 | 0.02 (−0.04 to 0.07) |
| ESA | 91; 221 | 4.5 | 0.08 (−0.10 to 0.27) |
Analyses are adjusted for age- and sex-specific height z scores at the index visit, GFR at the index visit, CKD diagnosis, percentage of life with CKD at index, sex, race, age at index, family income at baseline visit, and attained maternal education at baseline.
Study participants with available adherence data at the first visit of the pair and age- and sex-specific height z scores at both visits of the pair.
There were no statistically significant differences between participants who were adherent to rhGH compared with those who were nonadherent with respect to the following parameters: height z score, GFR, CKD diagnosis, percentage of life with CKD, sex, race, age, family income, maternal education, serum bicarbonate, and hemoglobin (data not shown).
Discussion
Nonadherence to prescribed treatment regimens is a major concern in the management of all chronic pediatric illnesses (11,12). However, there has been little investigation of the role that nonadherence plays in the outcomes of children with CKD (20). Growth impairment is a unique manifestation of pediatric CKD with major implications for quality of life, morbidity, and mortality during childhood and beyond (4,6,7). Despite the ability to modify many of the factors that contribute to growth delay, outcome studies demonstrate that optimal adult height is often not achieved in patients diagnosed with CKD during childhood (23,28). Thus, investigation of under-recognized risk factors for growth delay in pediatric CKD is critically important.
In this study utilizing the CKiD cohort, we reported the longitudinal use of prescribed medication groups that may affect growth velocity in pediatric patients with CKD. Active vitamin D was the most commonly prescribed medication across all visits, and the frequency of its use increased over time, which may be reflective of CKD progression. Of note, active vitamin D was used nearly twice as frequently as phosphate binders, which may reflect earlier changes in parathyroid hormone compared with serum phosphorus in the course of CKD. Iron supplements and alkali were the second and third most frequently prescribed medication groups, respectively. These frequency patterns are consistent with data from the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) (29). We observed the use of iron supplementation to be almost twice that of ESAs over time (Figure 1). This difference was not previously reported by NAPRTCS (29). Thus, further investigation of the prevalence of iron deficiency in the CKiD cohort in relation to the use of iron supplementation adjusted for nonadherence may be warranted.
The reported frequency of rhGH use was higher in the CKiD study (9.0% at study entry) compared with NAPRTCS (6.2% at entry, averaged for 1994–2007) despite the children in the NAPRTCS CKD cohort being markedly shorter than the children in the CKiD cohort (baseline height z scores of −1.44 versus −0.68, respectively). This may reflect a shift in the treatment philosophy of the pediatric nephrology community in the past decade toward earlier use of rhGH in children with CKD. In addition, the prevalence of rhGH use increased slightly over time in the CKiD cohort (Figure 1).
It is concerning that adherence worsened over time for some of the medication groups (Figure 2). In future analyses, it would be warranted to characterize the subgroup of CKiD study participants who were persistently nonadherent versus those who became nonadherent later in the study.
We observed substantial variability of nonadherence between individual classes of medications over time. Nonadherence to nutritional vitamin D was the highest, followed by phosphate binders. Nutritional vitamin D may be viewed as optional by some families because it is purchased over the counter. It may also require out-of-pocket payment and may be difficult for low-income families to afford. Phosphate binders need to be taken with meals three times per day, a requirement that presents unique challenges for adherence, as previously documented in other pediatric cohorts. Nonadherence to ESAs and rhGH was 4% and 17%, respectively, over all person-visits in which these medications were prescribed. This striking difference in the rates of nonadherence to two injectable medications may possibly be related to the differences in time required to see the effects of these therapies. Although missing ESA injections may result in a rapid drop in hemoglobin and potentially a need for transfusion, nonadherence to rhGH injections does not result in any easily detectable effect for months. In addition, the effect of therapy with rhGH may be seen as cosmetic to some, in contrast with ESAs, which may have a more obvious and crucial effect on health. Finally, rhGH requires daily injections, whereas ESAs may be prescribed from three times per week to once a week. Hence, rhGH is likely more burdensome and presents more frequent opportunities for nonadherence each week.
We found that patients reporting nonadherence to rhGH had on average no change in height z score, whereas adherent patients experienced improvement of growth velocity. To the best of our knowledge, this is the first formal evidence that confirms the critical role of adherence with rhGH in achieving improved growth outcomes. Furthermore, our results indicate that a simple method of adherence assessment with a single question based on self-report may be effective in identifying those children who would likely grow poorly. This sets the stage for a possible interventional quality assurance program to improve adherence and growth based on simple screening.
Assessment of potential causes of nonadherence to rhGH and other medications was beyond the scope of this study. Our multivariate analysis demonstrated, however, that index values of height and GFR, along with CKD diagnosis, percentage of life with CKD, sex, age, race, family income, and maternal education did not modify the observed association between nonadherence and poor growth. There is a perception that shorter children may be more motivated to take rhGH because of peer concerns; however, this was not supported by our data, because there was no difference in nonadherence among those with height z scores≤3rd percentile versus >3rd percentile. More detailed analysis of the possible association between socioeconomic status, medication nonadherence, and growth is warranted, especially in light of the recent data regarding linear growth in children of low socioeconomic status (30,31). Analysis of parental and patient self-perceived barriers to adherence may provide additional clues and help to develop an effective intervention.
Surprisingly, there was no significant association between growth velocity and nonadherence with other groups of medications, particularly alkali, active vitamin D, and PO4 binders. It is well accepted that CKD–mineral bone disorder contributes to growth delay in CKD. However, limited research has been conducted to formally assess the magnitude of the effect that interventions to correct these comorbidities have on growth. In the available studies, serum phosphorous, calcium, albumin, and parathyroid hormone levels were poor predictors of short stature (32–36). Thus, it is possible that some of the current treatment strategies are ineffective in improving growth or that self-reported nonadherence does not necessarily identify a substantial enough degree of medication nonadherence to affect growth. Therefore, further study to evaluate the effect of various degrees of nonadherence to the individual medication groups (e.g., alkali) on the corresponding clinical parameters or biomarkers (e.g., acidosis/serum bicarbonate) is warranted.
Our study has obvious limitations. Although the CKiD study is the largest pediatric prospective CKD cohort studied to date in North America, the number of patients receiving treatment with certain pharmacologic medication groups (especially ESAs and rhGH) was modest. The magnitude of nonadherence that we reported also likely underestimates the true frequency of nonadherence as demonstrated in other studies that utilized self-reporting (37). In addition, evaluation of medication adherence in the CKiD study was carried out only once a year, although the optimal frequency of evaluating medication adherence has not been established in the literature. Finally, our definition of nonadherence was dichotomous and we did not account for the severity of nonadherence (i.e., a patient that missed five doses was treated the same way as another patient who missed one dose).
In summary, we report longitudinal data on the frequency of prescribed medication use and self-reported nonadherence in the CKiD cohort. Patients adherent to treatment with rhGH therapy demonstrated greater growth velocity, especially for those with a height<3rd percentile, compared with patients who were nonadherent with this therapy. These data emphasize the fact that nonadherence must be considered and evaluated in the setting of poor and/or no response to rhGH therapy in patients with CKD. Efforts designed to determine risk factors for nonadherence and possible corrective measures may result in improved medication adherence and the opportunity for improved patient outcomes.
Disclosures
M.M.-M. is employed by the National Institute of Diabetes and Digestive and Kidney Diseases.
Supplementary Material
Acknowledgments
Data in this manuscript were collected by the CKiD prospective cohort study with clinical coordinating centers (principal investigators) at Children’s Mercy Hospital and the University of Missouri-Kansas City (Bradley Warady), Children’s Hospital of Philadelphia (Susan Furth), Central Biochemistry Laboratory (George Schwartz) at the University of Rochester Medical Center, and the data coordinating center (Alvaro Muñoz) at the Johns Hopkins Bloomberg School of Public Health.
The CKiD study is supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases, with additional funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Heart, Lung, and Blood Institute (U01-DK66143, U01-DK66174, U01-DK82194, U01-DK66116). This study is also supported by an award from the National Institutes of Health National Center for Advancing Translational Sciences (UL1-TR000454).
Preliminary results of this study were presented at the 16th Congress of the International Pediatric Nephrology Association, held August 30–September 3, 2013, in Shanghai, China, and were previously reported in abstract form (O.Akchurin et al., Pediatr Nephrol 28: 1652, 2013).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.01150114/-/DCSupplemental.
References
- 1.Rees L, Mak RH: Nutrition and growth in children with chronic kidney disease. Nat Rev Nephrol 7: 615–623, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Fine RN: Etiology and treatment of growth retardation in children with chronic kidney disease and end-stage renal disease: A historical perspective. Pediatr Nephrol 25: 725–732, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Mahesh S, Kaskel F: Growth hormone axis in chronic kidney disease. Pediatr Nephrol 23: 41–48, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Uzri A, Matheson M, Gipson DS, Mendley SR, Hooper SR, Yadin O, Rozansky DJ, Moxey-Mims M, Furth SL, Warady BA, Gerson AC, Chronic Kidney Disease in Children Study Group : The impact of short stature on health-related quality of life in children with chronic kidney disease. J Pediatr 163: 736–, e1., 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerson AC, Wentz A, Abraham AG, Mendley SR, Hooper SR, Butler RW, Gipson DS, Lande MB, Shinnar S, Moxey-Mims MM, Warady BA, Furth SL: Health-related quality of life of children with mild to moderate chronic kidney disease. Pediatrics 125: e349–e357, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Furth SL, Stablein D, Fine RN, Powe NR, Fivush BA: Adverse clinical outcomes associated with short stature at dialysis initiation: A report of the North American Pediatric Renal Transplant Cooperative Study. Pediatrics 109: 909–913, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Wong CS, Gipson DS, Gillen DL, Emerson S, Koepsell T, Sherrard DJ, Watkins SL, Stehman-Breen C: Anthropometric measures and risk of death in children with end-stage renal disease. Am J Kidney Dis 36: 811–819, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Wong CJ, Moxey-Mims M, Jerry-Fluker J, Warady BA, Furth SL: CKiD (CKD in children) prospective cohort study: a review of current findings. Am J Kidney Dis 60: 1002–1011, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furth SL, Abraham AG, Jerry-Fluker J, Schwartz GJ, Benfield M, Kaskel F, Wong C, Mak RH, Moxey-Mims M, Warady BA: Metabolic abnormalities, cardiovascular disease risk factors, and GFR decline in children with chronic kidney disease. Clin J Am Soc Nephrol 6: 2132–2140, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hodson EM, Willis NS, Craig JC: Growth hormone for children with chronic kidney disease. Cochrane Database Syst Rev 2: CD003264, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dean AJ, Walters J, Hall A: A systematic review of interventions to enhance medication adherence in children and adolescents with chronic illness. Arch Dis Child 95: 717–723, 2010 [DOI] [PubMed] [Google Scholar]
- 12.McGrady ME, Hommel KA: Medication adherence and health care utilization in pediatric chronic illness: A systematic review. Pediatrics 132: 730–740, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simoni JM, Asarnow JR, Munford PR, Koprowski CM, Belin TR, Salusky IB: Psychological distress and treatment adherence among children on dialysis. Pediatr Nephrol 11: 604–606, 1997 [DOI] [PubMed] [Google Scholar]
- 14.Lam LW, Twinn SF, Chan SW: Self-reported adherence to a therapeutic regimen among patients undergoing continuous ambulatory peritoneal dialysis. J Adv Nurs 66: 763–773, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Chua AN, Warady BA: Adherence of pediatric patients to automated peritoneal dialysis. Pediatr Nephrol 26: 789–793, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Dew MA, Dabbs AD, Myaskovsky L, Shyu S, Shellmer DA, DiMartini AF, Steel J, Unruh M, Switzer GE, Shapiro R, Greenhouse JB: Meta-analysis of medical regimen adherence outcomes in pediatric solid organ transplantation. Transplantation 88: 736–746, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dobbels F, Ruppar T, De Geest S, Decorte A, Van Damme-Lombaerts R, Fine RN: Adherence to the immunosuppressive regimen in pediatric kidney transplant recipients: A systematic review. Pediatr Transplant 14: 603–613, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Ibrahim N, Wong IC, Patey S, Tomlin S, Sinha MD, Jani Y: Drug-related problem in children with chronic kidney disease. Pediatr Nephrol 28: 25–31, 2013 [DOI] [PubMed] [Google Scholar]
- 19.So TY, Layton JB, Bozik K, Farrington E, Gipson PE, Gibson K, Primack W, Conley W, 3rd, Gipson DS, Ferris M: Cognitive pharmacy services at a pediatric nephrology and hypertension clinic. Ren Fail 33: 19–25, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Dionne JM, Lou K, Er L, Collin K, White CT: Pharmaceutical cost distribution in childhood chronic kidney disease. Pediatr Nephrol 27: 1531–1539, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Blydt-Hansen TD, Pierce CB, Cai Y, Samsonov D, Massengill S, Moxey-Mims M, Warady BA, Furth SL: Medication treatment complexity and adherence in children with CKD. Clin J Am Soc Nephrol 9: 247–254, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janjua HS, Mahan JD: Growth in chronic kidney disease. Adv Chronic Kidney Dis 18: 324–331, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Bacchetta J, Harambat J, Cochat P, Salusky IB, Wesseling-Perry K: The consequences of chronic kidney disease on bone metabolism and growth in children. Nephrol Dial Transplant 27: 3063–3071, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, Wei R, Curtin LR, Roche AF, Johnson CL: 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat 11 246: 1–190, 2002 [PubMed] [Google Scholar]
- 25.Schwartz GJ, Furth S, Cole SR, Warady B, Muñoz A: Glomerular filtration rate via plasma iohexol disappearance: Pilot study for chronic kidney disease in children. Kidney Int 69: 2070–2077, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Schwartz GJ, Schneider MF, Maier PS, Moxey-Mims M, Dharnidharka VR, Warady BA, Furth SL, Muñoz A: Improved equations estimating GFR in children with chronic kidney disease using an immunonephelometric determination of cystatin C. Kidney Int 82: 445–453, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diggle P, Heagerty P, Liang KY, Zeger S: Analysis of Longitudinal Data, New York, Oxford University Press, 2002 [Google Scholar]
- 28.Franke D, Winkel S, Gellermann J, Querfeld U, Pape L, Ehrich JH, Haffner D, Pavičić L, Zivičnjak M: Growth and maturation improvement in children on renal replacement therapy over the past 20 years. Pediatr Nephrol 28: 2043–2051, 2013 [DOI] [PubMed] [Google Scholar]
- 29.North American Pediatric Renal Trials and Collaborative Studies : NAPRTCS 2008 Annual Report, Rockville, MD, NAPRTCS, 2008 [Google Scholar]
- 30.Silva LM, van Rossem L, Jansen PW, Hokken-Koelega AC, Moll HA, Hofman A, Mackenbach JP, Jaddoe VW, Raat H: Children of low socioeconomic status show accelerated linear growth in early childhood; results from the Generation R Study. PLoS ONE 7: e37356, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hidalgo G, Ng DK, Moxey-Mims M, Minnick ML, Blydt-Hansen T, Warady BA, Furth SL. Association of income level with kidney disease severity and progression among children and adolescents with CKD: a report from the Chronic Kidney Disease in Children (CKiD) Study. Am J Kidney Dis. 2013 Dec;62(6):1087–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seikaly MG, Salhab N, Gipson D, Yiu V, Stablein D: Stature in children with chronic kidney disease: Analysis of NAPRTCS database. Pediatr Nephrol 21: 793–799, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Waller SC, Ridout D, Cantor T, Rees L: Parathyroid hormone and growth in children with chronic renal failure. Kidney Int 67: 2338–2345, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Waller S: Parathyroid hormone and growth in chronic kidney disease. Pediatr Nephrol 26: 195–204, 2011 [DOI] [PubMed] [Google Scholar]
- 35.Seeherunvong W, Abitbol CL, Chandar J, Zilleruelo G, Freundlich M: Vitamin D insufficiency and deficiency in children with early chronic kidney disease. J Pediatr 154: 906–, e1., 2009 [DOI] [PubMed] [Google Scholar]
- 36.Rodig NM, McDermott KC, Schneider MF, Hotchkiss HM, Yadin O, Seikaly MG, Furth SL, Warady BA: Growth in children with chronic kidney disease: a report from the Chronic Kidney Disease in Children Study. Pediatr Nephrol, 2014. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shi L, Liu J, Fonseca V, Walker P, Kalsekar A, Pawaskar M: Correlation between adherence rates measured by MEMS and self-reported questionnaires: A meta-analysis. Health Qual Life Outcomes 8: 99, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.