Abstract
Background and objectives
Plasma copeptin, a marker of arginine vasopressin, is elevated in patients with autosomal dominant polycystic kidney disease and predicts disease progression. It is unknown whether elevated copeptin levels result from decreased kidney clearance or as compensation for impaired concentrating capacity. Data from patients with autosomal dominant polycystic kidney disease and healthy kidney donors before and after donation were used, because after donation, overall GFR decreases with a functionally normal kidney.
Design, setting, participants, & measurements
Data were obtained between October of 2008 and January of 2012 from healthy kidney donors who visited the institution for routine measurements predonation and postdonation and patients with autosomal dominant polycystic kidney disease who visited the institution for kidney function measurement. Plasma copeptin levels were measured using a sandwich immunoassay, GFR was measured as 125I-iothalamate clearance, and urine concentrating capacity was measured as urine-to-plasma ratio of urea. In patients with autosomal dominant polycystic kidney disease, total kidney volume was measured with magnetic resonance imaging.
Results
Patients with autosomal dominant polycystic kidney disease (n=122, age=40 years, men=56%) had significantly higher copeptin levels (median=6.8 pmol/L; interquartile range=3.4–15.7 pmol/L) compared with donors (n=134, age=52 years, men=49%) both predonation and postdonation (median=3.8 pmol/L; interquartile range=2.8–6.3 pmol/L; P<0.001; median=4.4 pmol/L; interquartile range=3.6–6.1 pmol/L; P<0.001). In donors, copeptin levels did not change after donation, despite a significant fall in GFR (from 105±17 to 66±10; P<0.001). Copeptin and GFR were significantly associated in patients with autosomal dominant polycystic kidney disease (β=−0.45, P<0.001) but not in donors. In patients with autosomal dominant polycystic kidney disease, GFR and total kidney volume were both associated significantly with urine-to-plasma ratio of urea (β=0.84, P<0.001; β=−0.51, P<0.001, respectively).
Conclusions
On the basis of the finding in donors that kidney clearance is not a main determinant of plasma copeptin levels, it was hypothesized that, in patients with autosomal dominant polycystic kidney disease, kidney damage and associated impaired urine concentration capacity determine copeptin levels.
Keywords: ADPKD, vasopressin, urea, cystic kidney
Introduction
In autosomal dominant polycystic kidney disease (ADPKD), arginine vasopressin (AVP) is assumed to be involved in cyst formation by promoting intracellular cAMP production, which leads to cyst growth and kidney function decline (1–3). Indeed, it has been found that blocking the AVP V2 receptor improves prognosis in both experimental ADPKD models (4,5) and patients with ADPKD (6).
AVP is difficult to measure (7,8). Copeptin consists of the C-terminal portion of Pro-AVP, the precursor of AVP, and is produced in equimolar amounts as AVP during precursor processing (9). Copeptin has been shown to be a relatively easy to measure, stable substitute for circulating AVP concentration (10,11).
Recently, we found cross-sectional associations between copeptin concentration and various markers of disease severity in ADPKD (12). In these patients, copeptin is elevated in proportion to the impairment of renal function. In addition, we showed that, in patients with ADPKD, a high copeptin concentration is associated with accelerated growth of total kidney volume (TKV) (13), more rapid loss of measured GFR during short-term follow-up, and loss of eGFR during long-term follow-up (14). These data suggest that copeptin is a risk marker or even risk factor for detrimental kidney outcome in ADPKD and that measurement of copeptin concentration may be of help to assess prognosis in this patient group.
The question arises of why copeptin is increased in some patients with ADPKD. We previously hypothesized that increased levels are the result of an impaired urine concentrating capacity caused by cyst-induced abnormality in medullary osmolar gradient. However, a role of diminished kidney clearance in elevated plasma copeptin levels cannot be excluded. Copeptin has a molecular mass of only 5 kD, which makes it subject to kidney clearance. Indeed, several studies showed inverse associations between kidney function and copeptin (15–18).
To gain more insight in the role of kidney function and urine concentrating capacity in determining copeptin levels in ADPKD, we assessed copeptin levels in living kidney donors before and after donation and patients with ADPKD. In these patients, kidney function was measured as clearance of 125I-iothalamate. In addition, we measured plasma osmolality, TKV, and urine-to-plasma concentration ratio of urea (U/P urea). U/P urea has been suggested to reflect the severity of the urine concentrating defect in ADPKD, with lower values reflecting more severe disease (19).
Materials and Methods
Study Population
For this study, data were used from 134 participants who donated a kidney for transplantation at our institution between October of 2008 and December of 2011 and had blood samples available. In these kidney donors, kidney function was routinely measured predonation and postdonation, which is described in more detail by Tent et al. (20). Furthermore, we used data from 122 patients with ADPKD who visited our institution for kidney function measurement between November of 2007 and January of 2012. Diagnosis of ADPKD was made using the criteria by Ravine et al. (21). Exclusion criteria for both groups were indications for kidney disease other than ADPKD, diabetes, or cardiovascular events. In addition, patients with ADPKD were excluded if they received kidney replacement therapy or were unable to undergo magnetic resonance imaging (MRI). This study was performed in adherence to the Declaration of Helsinki. All participants gave written informed consent.
Measurements and Definitions
The day before kidney function measurement, kidney donors and patients with ADPKD collected a 24-hour urine sample, in which concentrations of sodium, potassium, and urea were measured using standard methods. BP was assessed for 15 minutes with an automatic device (Dinamap) during the kidney function measurement. Blood samples were drawn in the morning without standardized fluid intake. Blood was used for immediate determination of hemoglobin, sodium, potassium, urea, and glucose using standard laboratory methods. Creatinine was measured with the Roche enzymatic creatinine assay. Furthermore, plasma samples were stored frozen at −80°C and thawed later to measure plasma copeptin levels using a sandwich immunoassay (Thermo Fisher Scientific BRAHMS, Hennigsdorf/Berlin, Germany) (10). All samples were analyzed for copeptin concentration at one single time point to eliminate interassay variation. The lower limit of detection was 0.4 pmol/L, and the functional assay sensitivity (interassay coefficient of variation<20%) was <1 pmol/L.
GFR was measured using a constant infusion method with 125I-iothalamate. The intrapatient day-to-day coefficient of variation of this method is 2.2% (22–25). GFR was adjusted for body surface area using the equation by Du Bois and Du Bois (26).
Patients with ADPKD underwent a standardized abdominal MRI protocol without the use of intravenous contrast. Scanning was performed on a 1.5-Tesla MRI Magnetom Avento (Siemens, Erlangen, Germany) with the use of body matrix and spine matrix coils. TKV was measured on T2-weighted coronal images using the commercially available software Analyze Direct 8.0 (Analyze Direct, Inc., Overland Park, KS), and adjusted for height. Intrareviewer and inter-reviewer coefficients of variation were 1.8% and 2.3%, respectively.
Urine osmolality was calculated using the equation 2×(urine sodium concentration+urine potassium concentration)+urine urea concentration (27). Plasma osmolality was calculated using the equation 1.9×(plasma sodium concentration+plasma potassium concentration)+plasma glucose concentration+plasma urea concentration×0.5+5 (28). U/P urea was also assessed.
Statistical Analyses
To display characteristics of participants, parametric values were expressed as mean±SD, whereas nonparametric variables were presented as median (interquartile range). Significance between donors predonation and postdonation was tested using a paired t test for parametric values or a Wilcoxon signed rank test for nonparametric values. Differences between donors and patients with ADPKD were analyzed using a t test, a chi-squared test, or a Mann–Whitney U test as appropriate.
Several regression analyses were performed to test associations between plasma copeptin, GFR, plasma osmolality, urine osmolality, U/P urea, and TKV. Copeptin, urine osmolality, U/P urea, urine volume, TKV, change in copeptin, change in plasma sodium, change in mean arterial pressure (MAP), and change in urine volume in donors from predonation to postdonation have a non-normal distribution and therefore, were In log transformed. Standardized β-values and P values are given for all regression analyses. Regression analyses were adjusted for age and sex when indicated, as well as copeptin analyses for variables that may influence AVP levels (i.e., plasma sodium concentration, urine volume [as indicator of fluid intake], MAP and use of diuretics). A sensitivity analysis was performed excluding all participants using diuretics. All analyses were performed using the statistical package IBM SPSS Statistics, version 20.0 (International Business Machines Corp., Chicago, IL). A two-sided P value <0.05 was considered to indicate statistical significance.
Results
Characteristics of participants are presented in Table 1. Overall, 134 donors and 122 patients with ADPKD were studied. The median time period between the predonation measurement and donation was 16 weeks (minimum=3 weeks, maximum=101 weeks) and between donation and postdonation measurement was 7 weeks (minimum=4 weeks, maximum=20 weeks). Plasma copeptin levels were significantly higher in patients with ADPKD compared with donors both predonation and postdonation. Patients with ADPKD had a significantly lower GFR compared with donors predonation but significantly higher GFR compared with donors postdonation. Men had higher copeptin levels than women in donors both predonation (men: 4.5 pmol/L; interquartile range=3.3–7.3 pmol/L; women: 3.3 pmol/L; interquartile range=2.2–5.7 pmol/L; P=0.003) and postdonation (men: 5.3 pmol/L; interquartile range=4.0–7.5 pmol/L; women: 3.5 pmol/L; interquartile range=2.5–5.2 pmol/L; P<0.001) as well as in patients with ADPKD (men: 10.7 pmol/L; interquartile range=4.8–21.8 pmol/L; women: 4.8 pmol/L; interquartile range=2.5–9.4 pmol/L; P<0.001.). Because sex is an independent predictor for copeptin, we adjusted all regression analyses for sex.
Table 1.
Characteristics of all participants
| Characteristics | Donors Predonation (n=134) | Donors Postdonation (n=134) | ADPKD (n=122) | P Value Predonation/Postdonation | P Value Predonation/ ADPKD | P Value Postdonation/ ADPKD |
|---|---|---|---|---|---|---|
| Age (yr) | 52±10 | 52±10 | 40±12 | <0.001 | <0.001 | <0.001 |
| Men (%) | 49 | 49 | 56 | — | 0.30 | 0.30 |
| Use of antihypertensives (%) | 15 | 15 | 85 | >0.99 | <0.001 | <0.001 |
| Use of diuretics (%) | 5 | 4 | 20 | 0.32 | <0.001 | <0.001 |
| Body surface area (m2) | 1.96±0.20 | 1.95±0.20 | 2.02±0.23 | <0.01 | 0.02 | <0.01 |
| Mean arterial pressure (mmHg) | 92±10 | 90±9 | 95±9 | 0.002 | <0.01 | <0.001 |
| Hemoglobin (mg/dl) | 14.3±1.1 | 13.5±1.1 | 13.4±1.5 | <0.001 | <0.001 | 0.51 |
| Plasma osmolality (mosM/kg) | 291±4 | 290±4 | 289±4 | 0.11 | <0.001 | 0.002 |
| Plasma sodium (mEq/L) | 142.3±1.9 | 141.6±1.8 | 140.4±1.8 | <0.001 | <0.001 | <0.001 |
| Plasma urea (mg/dl) | 16.0±3.4 | 19.3±4.2 | 24.1±12.6 | <0.001 | <0.001 | <0.001 |
| 24-h urine volume (L) | 2600 (2200–3050) | 2208 (1713–2700) | 2200 (1785–2673) | <0.001 | <0.001 | 0.51 |
| 24-h urine osmolality (mosM/kg) | 372 (320–459) | 412 (343–530) | 396 (314–474) | <0.001 | 0.54 | 0.06 |
| 24-h urine sodium (mmol/24 h) | 197 (156–249) | 169 (132–216) | 161 (128–215) | <0.001 | <0.001 | 0.34 |
| 24-h urine urea (mg/24 h) | 1140 (983–1350) | 1297 (1028–1669) | 1089 (905–1311) | <0.001 | <0.16 | <0.001 |
| GFR (ml/min per 1.73 m2) | 105±17 | 66±10 | 74±32 | <0.001 | <0.001 | 0.01 |
| U/P urea | 29.2 (23.3–35.1) | 27.1 (20.9–34.5) | 23.3 (15.2–31.1) | 0.10 | <0.001 | 0.001 |
| Total kidney volume (ml) | — | — | 1535 (931–2356) | — | — | — |
| Plasma copeptin (pmol/L) | 3.8 (2.8–6.3) | 4.4 (3.1–6.1) | 6.8 (3.4–15.7) | 0.17 | <0.001 | <0.001 |
Data are given as mean ± SD for parametric data or median (interquartile range) for nonparametric data. Significance between predonation and postdonation was tested using a paired t test or a Wilcoxon signed rank test when appropriate. Significance of difference between predonation and postdonation was tested using a paired t test or a Wilcoxon signed rank test when appropriate. Significance of differences between predonation/postdonation and ADPKD was tested using a t test, a chi-squared test, or a Mann–Whitney U test when appropriate. U/P urea, urine-to-plasma concentration ratio of urea; ADPKD, autosomal dominant polycystic kidney disease.
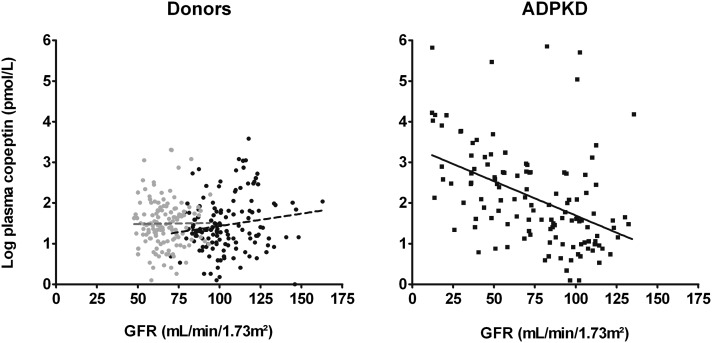
We found differences in the associations of copeptin with GFR between donors and patients with ADPKD. No significant association between copeptin and GFR was found in donors predonation (β=0.15, P=0.08) or postdonation (β=0.008, P=0.93), whereas a significant inverse association was found in patients with ADPKD (β=−0.45, P<0.001, which corresponds with a 19% increase in copeptin per 10-ml/min per 1.73 m2 decrease in GFR). In line, the interaction term between study group (ADPKD or donors predonation and postdonation) and GFR in the association between copeptin and GFR was significant (P<0.001 and P=0.03, respectively). Adjustment for age, sex, and variables that may influence AVP levels did not materially change our results (donors predonation and postdonation: P=0.85 and P=0.87, respectively; patients with ADPKD: β=−0.53, P<0.001) (Figure 1, Tables 2, 3, and 4). When only studying patients with ADPKD with a GFR in the range similar to the range in donors (GFR>48 ml/min per 1.73 m2, n=93), the association between copeptin and GFR remained significant (β=−0.26, P=0.01; i.e., a 13% increase in copeptin per 10-ml/min per 1.73 m2 decrease in GFR), even after additional adjustment for age, sex, and variables that may influence AVP levels (β=−0.28, P=0.01) (Tables 2–4).
Figure 1.
No association between plasma copeptin and GFR in donors whereas patients show a significant inverse association (β=−0.45, P<0.001).
Table 2.
Multivariable linear regression analyses showing the association of GFR and urine-to-plasma concentration ratio of urea with plasma copeptin (dependent variable) in 134 healthy kidney donors predonation and postdonation
| Donors | Model 1 | Model 2 | Model 3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Predonation | Postdonation | Predonation | Postdonation | Predonation | Postdonation | |||||||
| St. β | P Value | St. β | P Value | St. β | P Value | St. β | P Value | St. β | P Value | St. β | P Value | |
| GFR | 0.15 | 0.08 | 0.008 | 0.93 | 0.02 | 0.84 | −0.08 | 0.41 | 0.02 | 0.85 | −0.16 | 0.87 |
| Age (yr) | −0.25 | <0.01 | −0.13 | 0.18 | −0.27 | <0.01 | −0.11 | 0.26 | ||||
| Men | −0.18 | 0.04 | −0.35 | <0.001 | −0.14 | 0.10 | −0.32 | <0.001 | ||||
| Plasma sodium | 0.03 | 0.73 | 0.03 | 0.44 | ||||||||
| 24-h urine volume | −0.20 | 0.02 | −0.28 | 0.001 | ||||||||
| MAP | 0.02 | 0.79 | −0.09 | 0.29 | ||||||||
| Diuretics | 0.003 | 0.98 | 0.03 | 0.73 | ||||||||
| U/P urea | 0.19 | 0.03 | 0.39 | <0.001 | 0.08 | 0.40 | 0.29 | 0.001 | −0.09 | 0.41 | 0.12 | 0.35 |
| Age (yr) | −0.24 | <0.01 | −0.01 | 0.86 | −0.31 | 0.001 | −0.07 | 0.48 | ||||
| Men | −0.16 | 0.07 | −0.27 | 0.002 | −0.16 | 0.08 | −0.30 | 0.001 | ||||
| Plasma sodium | 0.02 | 0.78 | 0.06 | 0.45 | ||||||||
| 24-h urine volume | −0.26 | 0.02 | −0.20 | 0.09 | ||||||||
| MAP | 0.03 | 0.76 | −0.08 | 0.33 | ||||||||
| Diuretics | 0.006 | 0.94 | 0.02 | 0.79 | ||||||||
MAP, mean arterial pressure; U/P urea, urine-to-plasma concentration ratio of urea.
Table 3.
Multivariable linear regression analyses showing the association of GFR with plasma copeptin (dependent variable) in all 122 patients with ADPKD and 93 patients with ADPKD with a GFR>48 ml/min per 1.73 m2.
| ADPKD | Model 1 | Model 2 | Model 3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All Patients | GFR>48 ml/min per 1.73 m2 | All Patients | GFR>48 ml/min per 1.73 m2 | All Patients | GFR>48 ml/min per 1.73 m2 | |||||||
| St. β | P Value | St. β | P Value | St. β | P Value | St. β | P Value | St. β | P Value | St. β | P Value | |
| GFR | −0.45 | <0.001 | −0.26 | 0.01 | −0.50 | <0.001 | −0.31 | 0.01 | −0.53 | <0.001 | −0.28 | 0.01 |
| Age (yr) | −0.16 | 0.08 | −0.18 | 0.12 | −0.11 | 0.20 | −0.09 | 0.43 | ||||
| Men | −0.28 | <0.001 | −0.33 | 0.001 | −0.32 | <0.001 | −0.41 | <0.001 | ||||
| Plasma sodium | −0.13 | 0.08 | 0.17 | 0.08 | ||||||||
| 24-h urine volume | −0.31 | <0.001 | −0.36 | 0.001 | ||||||||
| MAP | 0.08 | 0.28 | 0.05 | 0.63 | ||||||||
| Diuretics | 0.004 | 0.96 | 0.03 | 0.77 | ||||||||
Table 4.
Multivariable linear regression analyses showing the association of urine-to-plasma concentration ratio of urea with plasma copeptin (dependent variable) in 122 patients with ADPKD.
| ADPKD | Model 1: All Patients | Model 2: All Patients | Model 3: All Patients | |||
|---|---|---|---|---|---|---|
| St. β | P Value | St. β | P Value | St. β | P Value | |
| U/P urea | −0.32 | <0.001 | −0.31 | 0.001 | −0.46 | <0.001 |
| Age (yr) | −0.06 | 0.53 | −0.04 | 0.63 | ||
| Men | −0.28 | 0.001 | −0.32 | <0.001 | ||
| Plasma sodium | 0.12 | 0.14 | ||||
| 24-h urine volume | −0.42 | <0.001 | ||||
| MAP | 0.10 | 0.23 | ||||
| Diuretics | 0.01 | 0.89 | ||||
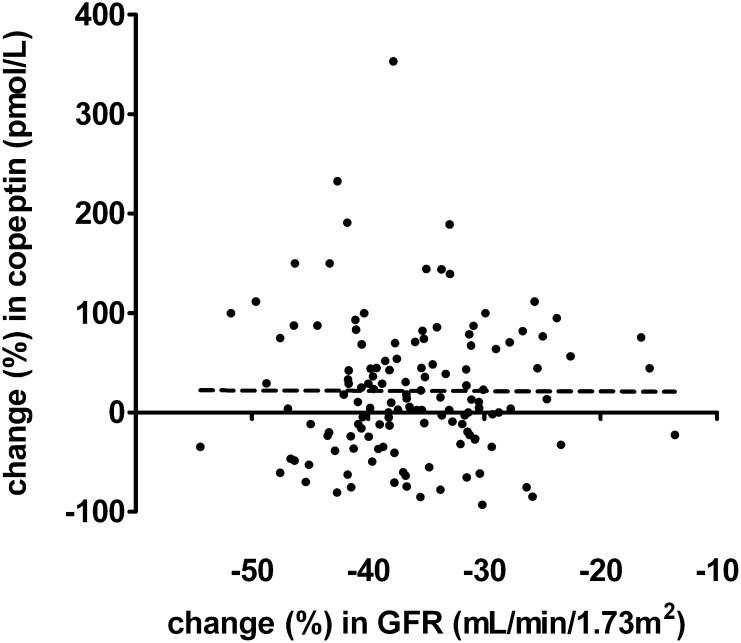
Because we wanted to test whether a decline in GFR influences copeptin values, we visualized change in GFR in relation to change in copeptin levels in percentages in donors from predonation to postdonation in Figure 2. Univariate regression analysis showed no association between change in GFR and change in copeptin (P=0.97). Also, when adjusting for change in plasma sodium, change in MAP, and change in 24-hour urine volume, no association was found between change in GFR and change in copeptin (P=0.92).
Figure 2.
No significant association between change in GFR and change in copeptin in donors comparing predonation with postdonation values (β=-0.02, p=0.84).
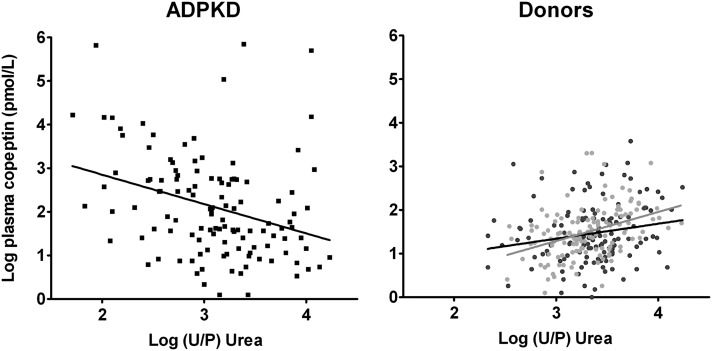
To assess the role of the urine concentrating capacity, we calculated the U/P urea and plotted it against copeptin levels and markers of disease severity in ADPKD (Figure 3). In patients with ADPKD, a negative association was found between U/P urea and copeptin (β=−0.32, P<0.001; i.e., a 52% decrease of copeptin per doubling in U/P urea): the lower the U/P urea and thereby the concentrating capacity, the higher the copeptin levels. This finding is in contrast to the positive association found in donors predonation (β=0.19, P=0.03; i.e., a 46% increase of copeptin per doubling in U/P urea) and postdonation (β=0.39, P<0.001; i.e., a doubling of copeptin per doubling in U/P urea) (Figure 3). In line, the interaction term between study group (ADPKD or donors predonation and postdonation) and U/P urea in the association between copeptin and U/P urea was significant (P<0.001 and P<0.001, respectively). After additional adjustment for age and sex, the associations between U/P urea and copeptin in patients with ADPKD (β=−0.31, P=0.001) and donors postdonation (β=0.29, P=0.001) remained similar, whereas in donors predonation, this association was no longer significant (P=0.40). Additional adjustment of the association between U/P urea and copeptin for factors that may influence AVP levels made no essential difference in patients with ADPKD (β=−0.46, P<0.001) and donors predonation (P=0.41), whereas in donors postdonation, the association lost significance (P=0.35) (Tables 2–4).
Figure 3.
Significant inverse association between U/P urea and copeptin in patients with ADPKD (β=−0.32, P<0.001) whereas donors show a significant positive association predonation and postdonation (β=0.19, P=0.03; β=0.39, P<0.001). Because the units of the numerator and the denominator are similar in the U/P urea, this ratio has no unit by itself.
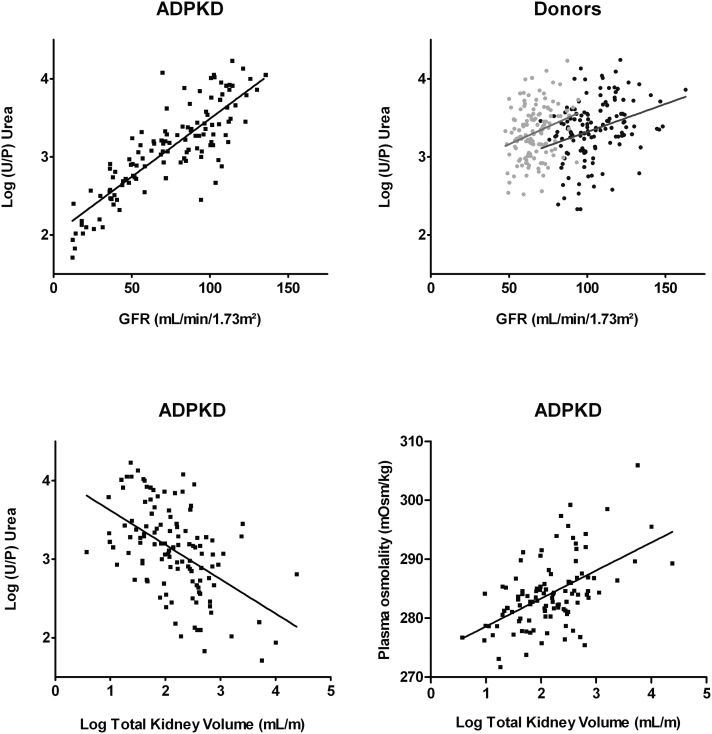
To link concentrating capacity to disease severity in patients with ADPKD, we investigated the associations of GFR and TKV with U/P urea. In patients with ADPKD, we found a strong positive association between GFR and U/P urea (β=0.84, P<0.001; i.e., a 14% decrease of U/P urea per 10-ml/min per 1.73 m2 decrease in GFR), that, in donors predonation and postdonation, was also present but considerably weaker (β=0.32, P<0.001; a 7% decrease of U/P urea per 10-ml/min per 1.73 m2 decrease in GFR; β=0.27, P=0.02; a 9% decrease of U/P urea per 10-ml/min per 1.73 m2 decrease in GFR, respectively) (Figure 4). In line, the interaction term between study group (ADPKD or donors predonation and postdonation) and GFR in the association between GFR and U/P urea was significant (P<0.001 and P<0.001, respectively). Adjustment for age and sex resulted in a similar significant association of GFR and U/P urea in patients with ADPKD (β=0.81, P<0.001) and donors predonation (β=0.23, P=0.02), whereas the association in donors postdonation was lost (β=0.13, P=0.16). In addition, in patients with ADPKD, an inverse association was found between TKV and U/P urea (β=−0.51, P<0.001; i.e., a 38% decrease in U/P urea when TKV doubles) (Figure 4), which remained significant after adjustment for age and sex (β=−0.43, P<0.001) and additional adjustment for GFR (β=−0.13, P=0.05) (Table 5).
Figure 4.
Significant positive associations between GFR and U/P urea in patients with ADPKD and donors and significant positive associations between total kidney volume, U/P urea and plasma osmolality in patients with ADPKD. Significant positive associations between GFR and U/P urea in patients with ADPKD (β=0.84, P<0.001) and donors predonation and postdonation (β=0.32, P<0.001; β=0.27, P=0.02). Also shown is the association between total kidney volume and U/P urea and total kidney volume and plasma osmolality in patients with ADPKD (β=−0.51, P<0.001; β=0.44, P<0.001).
Table 5.
Multiple linear regression analyses showing the association of GFR, plasma osmolality and the urine-to-plasma ratio of urea with total kidney volume (dependent variable) in 122 Patients with ADPKD
| ADPKD | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| St. β | P Value | St. β | P Value | St. β | P Value | |
| GFR | −0.49 | <0.001 | −0.45 | <0.001 | ||
| Age (yr) | 0.02 | 0.82 | ||||
| Men | −0.29 | <0.001 | ||||
| Plasma osmolality | 0.44 | <0.001 | 0.34 | <0.001 | 0.22 | <0.01 |
| Age (yr) | 0.22 | 0.01 | 0.05 | 0.54 | ||
| Men | −0.28 | 0.001 | −0.27 | 0.001 | ||
| GFR | −0.41 | <0.001 | ||||
| U/P urea | −0.51 | <0.001 | −0.46 | <0.001 | −0.29 | 0.05 |
| Age (yr) | 0.03 | 0.76 | −0.001 | 0.99 | ||
| Men | −0.26 | 0.002 | −0.26 | 0.001 | ||
| GFR | −0.22 | 0.14 | ||||
TKV and GFR were also inversely associated (β=−0.49, P<0.001; i.e., an 11% increase of TKV corresponds with a 10-ml/min per 1.73 m2 decrease in GFR), and this association remained significant after adjustment for age and sex (β=−0.45, P<0.001). Lastly, a significant positive association was found between TKV and plasma osmolality (β=0.44, P<0.001; i.e., a doubling of TKV corresponds with a 3.3-mosM/kg increase in plasma osmolality) (Figure 4), which remained significant after adjustment for age and sex (β=0.34, P<0.001) and additional adjustment for GFR (β=0.22, P<0.01) (Table 5).
Interestingly, in the aforementioned multivariable model, it seemed that U/P urea and GFR were significantly associated with TKV. However, when both were entered in the model simultaneously, U/P urea was significantly associated with TKV (β=−0.29, P=0.05), whereas GFR was not (β=−0.22, P=0.14) (Table 5). This finding suggests that U/P urea may be an earlier and more sensitive sign of renal dysfunction in ADPKD than GFR. To further explore this finding, we substituted measured GFR with eGFR (Chronic Kidney Disease Epidemiology Collaboration), a measure for kidney function often used in clinical practice to evaluate disease severity. Again, we found that U/P urea was significantly associated with TKV (β=−0.34, P=0.02), whereas eGFR was not (β=−0.15, P=0.33).
Because use of diuretics can influence urine concentrating capacity and plasma osmolality and consequently, copeptin levels, we performed a sensitivity analysis excluding all participants using diuretics (25 patients with ADPKD, six donors predonation, and five donors postdonation). No differences were present between participants with or without the use of diuretics in copeptin values in donors predonation (P=0.68), donors postdonation (P=0.53), and patients with ADPKD (P=0.14). Using logistic regression adjusted for age and sex, we found, as may be expected, that patients with ADPKD with lower GFR and, thus, more advanced disease, were more likely to use diuretics (P<0.01). Importantly, excluding participants using diuretics did not materially change our results.
Discussion
In this study, we aimed to gain insight into the role of kidney function and urine concentrating capacity in determining plasma copeptin levels in patients with ADPKD. We found no association between GFR and copeptin levels in healthy kidney donors predonation or postdonation. Most importantly, in these participants, copeptin levels did not change after kidney donation, despite a significant decrease in GFR. In contrast, in patients with ADPKD, a significant association was found between GFR and copeptin levels. Moreover, in these patients, copeptin was associated with U/P urea, and in turn, U/P urea was associated with TKV and GFR. These data indicate that GFR, as such, is not a determinant of copeptin levels.
In the literature, the association between kidney function and plasma copeptin has been described in several studies, with higher copeptin concentrations when kidney function is lower (15–18). In our opinion, two mechanisms can underlie the negative association in these studies. First, it could be that copeptin is cleared by kidney excretion, leading to an increase in copeptin levels when kidney function deteriorates. Second, it could also be that, in patients with lower kidney function, more copeptin is released, because the AVP system is activated. When patients in these cross-sectional studies have a lower kidney function, it is caused by kidney damage, which is known to be associated with an impaired urine concentrating capacity (29). Patients with an impaired urine concentrating capacity show a compensatory increase in AVP to maintain water homeostasis (30). Our study results can distinguish between these two mechanisms. Kidney donors have a considerable decrease in GFR after the donation procedure but without a concomitant increase in kidney damage. Because in these participants no increase in copeptin levels was observed after the donation procedure, it is unlikely that kidney clearance plays a predominant role in determining plasma copeptin levels (at least at the range of GFR observed here). In line with this assumption are our findings of higher copeptin values in patients with ADPKD compared with kidney donors postdonation, despite better kidney function in these patients with ADPKD, as well as the significant inverse correlation between GFR and copeptin in patients with ADPKD.
Various studies in patients with ADPKD have shown that, before kidney function starts to decline, an impaired concentrating capacity can already be observed (31–33). Bankir and Bichet (19) suggested that this phenomenon is probably because of a urea-selective concentrating defect caused by a cystic distortion of the medullary countercurrent mechanism, and they proposed U/P urea as measure of impaired concentrating capacity (19). Using data from water deprivation tests in patients with ADPKD with normal and impaired kidney function, we found that, indeed, baseline U/P urea was strongly associated with maximal urine osmolality during water deprivation (β=0.82, P=0.001) (Supplemental Figure 1 and Supplemental Table 1 show patient characteristics) (30). These data support U/P urea as a marker for urine concentrating capacity in patients with ADPKD. In this study, we found a negative association between U/P urea and copeptin levels in patients with APDKD. Furthermore, TKV was strongly associated with U/P urea and plasma osmolality. We interpret these data as, when disease progresses in patients with ADPKD, the cystic kidney develops a concentrating defect with a compensatory rise in AVP and copeptin levels. Another possible explanation for the association between copeptin, U/P urea, and TKV is that patients with higher copeptin (i.e., AVP) show a more rapid disease progression and therefore, are more likely to have impaired kidney function and high TKV. In this explanation, it remains unresolved why many patients with ADPKD with relatively preserved GFR have such elevated copeptin levels.
Our data may have an additional consequence. It is generally acknowledged that, in patients with ADPKD, GFR remains near normal for a prolonged period of time, whereas disease actually progresses, with progressive development of cysts and an increase in TKV (34). In a multivariable regression analysis adjusted for age and sex, we found that both U/P urea and GFR were associated with TKV. However, when both were entered in the model simultaneously, only U/P urea, and not GFR, was associated with TKV. This finding implies that the urine concentrating capacity estimated by U/P urea or copeptin level may be an earlier and more sensitive marker of disease severity than GFR. This finding should be investigated in future longitudinal studies.
We acknowledge that this study has limitations. First, the study design comparing predonation and postdonation values has a fixed rather than a random order, and consequently, there may be potential time-related confounders. It is theoretically possible that adaptive changes postdonation modify the renal handling of copeptin beyond influencing GFR. However, we think that this design is the best available with the knowledge that we have on this topic. Second, the moment of data collection pre- and potsdonation was not standardized. Predonation, this variability is not likely to have influenced our results, because all participants involved were healthy and had stable kidney function. Postdonation, a period of at least 4 weeks was implemented between donation and patient visit to verify that patients reached steady state postdonation. Third, fluid and dietary intake was not standardized at the moment that blood was drawn for assessing copeptin levels. Urine output in kidney donors predonation was high compared with donors postdonation and patients with ADPKD. Because donors did not receive advice as to water load, we cannot explain this difference. However, it is not of major importance for our main conclusion. In case there was unanticipated water loading predonation, it may be expected to have resulted in a lower copeptin concentration predonation, which means that our finding that there is no rise in copeptin concentration postdonation is not threatened and may only be an underestimation. Fourth, living donors as a model for GFR reduction focuses on the upper GFR range. Therefore,we cannot draw conclusions about the role of kidney function on copeptin levels in the lower GFR spectrum. However, because a significant association was found between copeptin and GFR in patients with ADPKD, whereas GFR was significantly higher compared with donors postdonation who showed no association between copeptin and GFR, we can conclude that, in patients with ADPKD, other disease-related factors are of more importance in determining copeptin levels than GFR. Finally, this study did not include a control group with non-ADPKD. We, therefore, cannot conclude whether an increase in copeptin in more advanced disease is specific for ADPKD, or can also be found in other kidney diseases. However, answering this question is beyond the scope of this study and needs additional investigations. Strengths of this study are the use of a healthy population that donated a kidney, which offers a unique potential to disentangle the role of kidney function per se and urine concentrating impairment in determining plasma copeptin levels. The included study group was, furthermore, well phenotyped with a gold standard measurement of GFR (constant infusion of 125I- Iothalamate) and measurement of TKV by MRI.
In conclusion, our data indicate that kidney function per se is not the main determinant of plasma copeptin levels. We hypothesize that, in patients with ADPKD, disease severity is reflected by an impaired urine concentrating capacity, which causes disturbances in plasma osmolality stimulating AVP and copeptin release.
Disclosures
None.
Supplementary Material
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.08690813/-/DCSupplemental.
References
- 1.Grantham JJ: Lillian Jean Kaplan International Prize for advancement in the understanding of polycystic kidney disease. Understanding polycystic kidney disease: A systems biology approach. Kidney Int 64: 1157–1162, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Meijer E, Boertien WE, Zietse R, Gansevoort RT: Potential deleterious effects of vasopressin in chronic kidney disease and particularly autosomal dominant polycystic kidney disease. Kidney Blood Press Res 34: 235–244, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Hanaoka K, Guggino WB: cAMP regulates cell proliferation and cyst formation in autosomal polycystic kidney disease cells. J Am Soc Nephrol 11: 1179–1187, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Meijer E, Gansevoort RT, de Jong PE, van der Wal AM, Leonhard WN, de Krey SR, van den Born J, Mulder GM, van Goor H, Struck J, de Heer E, Peters DJ: Therapeutic potential of vasopressin V2 receptor antagonist in a mouse model for autosomal dominant polycystic kidney disease: Optimal timing and dosing of the drug. Nephrol Dial Transplant 26: 2445–2453, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Torres VE, Wang X, Qian Q, Somlo S, Harris PC, Gattone VH, 2nd: Effective treatment of an orthologous model of autosomal dominant polycystic kidney disease. Nat Med 10: 363–364, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, Perrone RD, Krasa HB, Ouyang J, Czerwiec FS, TEMPO 3:4 Trial Investigators : Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med 367: 2407–2418, 2012. 23121377 [Google Scholar]
- 7.Preibisz JJ, Sealey JE, Laragh JH, Cody RJ, Weksler BB: Plasma and platelet vasopressin in essential hypertension and congestive heart failure. Hypertension 5: I129–I138, 1983 [DOI] [PubMed] [Google Scholar]
- 8.Kluge M, Riedl S, Erhart-Hofmann B, Hartmann J, Waldhauser F: Improved extraction procedure and RIA for determination of arginine8-vasopressin in plasma: Role of premeasurement sample treatment and reference values in children. Clin Chem 45: 98–103, 1999 [PubMed] [Google Scholar]
- 9.de Bree FM, Burbach JP: Structure-function relationships of the vasopressin prohormone domains. Cell Mol Neurobiol 18: 173–191, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morgenthaler NG, Struck J, Alonso C, Bergmann A: Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin Chem 52: 112–119, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Szinnai G, Morgenthaler NG, Berneis K, Struck J, Müller B, Keller U, Christ-Crain M: Changes in plasma copeptin, the c-terminal portion of arginine vasopressin during water deprivation and excess in healthy subjects. J Clin Endocrinol Metab 92: 3973–3978, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Meijer E, Bakker SJ, van der Jagt EJ, Navis G, de Jong PE, Struck J, Gansevoort RT: Copeptin, a surrogate marker of vasopressin, is associated with disease severity in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 6: 361–368, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boertien WE, Meijer E, Li J, Bost JE, Struck J, Flessner MF, Gansevoort RT, Torres VE, Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease CRISP : Relationship of copeptin, a surrogate marker for arginine vasopressin, with change in total kidney volume and GFR decline in autosomal dominant polycystic kidney disease: Results from the CRISP cohort. Am J Kidney Dis 61: 420–429, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boertien WE, Meijer E, Zittema D, van Dijk MA, Rabelink TJ, Breuning MH, Struck J, Bakker SJ, Peters DJ, de Jong PE, Gansevoort RT: Copeptin, a surrogate marker for vasopressin, is associated with kidney function decline in subjects with autosomal dominant polycystic kidney disease. Nephrol Dial Transplant 27: 4131–4137, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Bhandari SS, Loke I, Davies JE, Squire IB, Struck J, Ng LL: Gender and renal function influence plasma levels of copeptin in healthy individuals. Clin Sci (Lond) 116: 257–263, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Meijer E, Bakker SJ, Halbesma N, de Jong PE, Struck J, Gansevoort RT: Copeptin, a surrogate marker of vasopressin, is associated with microalbuminuria in a large population cohort. Kidney Int 77: 29–36, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Przybylowski P, Malyszko J, Malyszko JS: Copeptin in heart transplant recipients depends on kidney function and intraventricular septal thickness. Transplant Proc 42: 1808–1811, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Nigro N, Müller B, Morgenthaler N, Fluri F, Schütz P, Neidert S, Stolz D, Bingisser R, Tamm M, Christ-Crain M, Katan M: The use of copeptin, the stable peptide of the vasopressin precursor, in the differential diagnosis of sodium imbalance in patients with acute diseases. Swiss Med Wkly 141: w13270, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Bankir L, Bichet DG: Polycystic kidney disease: An early urea-selective urine-concentrating defect in ADPKD. Nat Rev Nephrol 8: 437–439, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Tent H, Rook M, Stevens LA, van Son WJ, van Pelt LJ, Hofker HS, Ploeg RJ, van der Heide JJ, Navis G: Renal function equations before and after living kidney donation: A within-individual comparison of performance at different levels of renal function. Clin J Am Soc Nephrol 5: 1960–1968, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ravine D, Gibson RN, Walker RG, Sheffield LJ, Kincaid-Smith P, Danks DM: Evaluation of ultrasonographic diagnostic criteria for autosomal dominant polycystic kidney disease 1. Lancet 343: 824–827, 1994 [DOI] [PubMed] [Google Scholar]
- 22.Donker AJ, van der Hem GK, Sluiter WJ, Beekhuis H: A radioisotope method for simultaneous determination of the glomerular filtration rate and the effective renal plasma flow. Neth J Med 20: 97–103, 1977 [PubMed] [Google Scholar]
- 23.Apperloo AJ, de Zeeuw D, Donker AJ, de Jong PE: Precision of glomerular filtration rate determinations for long-term slope calculations is improved by simultaneous infusion of 125I-iothalamate and 131I-hippuran. J Am Soc Nephrol 7: 567–572, 1996 [DOI] [PubMed] [Google Scholar]
- 24.Zietse R, Blankestijn PJ, Pos B, Balk AH, Derkx FH, Weimar W, Schalekamp MA: Optimising glomerular filtration rate and effective renal plasma flow measurements using a simple pharmacokinetic model. Clin Nephrol 43: 29–34, 1995 [PubMed] [Google Scholar]
- 25.Michels WM, Grootendorst DC, Rozemeijer K, Dekker FW, Krediet RT: Glomerular filtration rate measurements by 125I-iothalamate should be corrected for inaccurate urine collections with 131I-hippuran. Clin Nephrol 72: 337–343, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Du Bois D, Du Bois EF: A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition 5: 303–311, discussion 312–313, 1989 [PubMed] [Google Scholar]
- 27.Rose BD, Post TW: Hyperosmolal States, Hypernatremia. Clinical Physiology of Acid-Base and Electrolyte Disorders, 5th Ed., McGraw-Hill Companies, New York, NY: 2001 [Google Scholar]
- 28.Fazekas AS, Funk GC, Klobassa DS, Rüther H, Ziegler I, Zander R, Semmelrock HJ: Evaluation of 36 formulas for calculating plasma osmolality. Intensive Care Med 39: 302–308, 2013 [DOI] [PubMed] [Google Scholar]
- 29.Vize PD, Smith HW: A Homeric view of kidney evolution: A reprint of H.W. Smith’s classic essay with a new introduction. Evolution of the kidney. 1943. Anat Rec A Discov Mol Cell Evol Biol 277: 344–354, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Zittema D, Boertien WE, van Beek AP, Dullaart RP, Franssen CF, de Jong PE, Meijer E, Gansevoort RT: Vasopressin, copeptin, and renal concentrating capacity in patients with autosomal dominant polycystic kidney disease without renal impairment. Clin J Am Soc Nephrol 7: 906–913, 2012 [DOI] [PubMed] [Google Scholar]
- 31.Fick GM, Duley IT, Johnson AM, Strain JD, Manco-Johnson ML, Gabow PA: The spectrum of autosomal dominant polycystic kidney disease in children. J Am Soc Nephrol 4: 1654–1660, 1994 [DOI] [PubMed] [Google Scholar]
- 32.Seeman T, Dusek J, Vondrák K, Bláhová K, Simková E, Kreisinger J, Dvorák P, Kyncl M, Hríbal Z, Janda J: Renal concentrating capacity is linked to blood pressure in children with autosomal dominant polycystic kidney disease. Physiol Res 53: 629–634, 2004 [PubMed] [Google Scholar]
- 33.Gabow PA, Kaehny WD, Johnson AM, Duley IT, Manco-Johnson M, Lezotte DC, Schrier RW: The clinical utility of renal concentrating capacity in polycystic kidney disease. Kidney Int 35: 675–680, 1989 [DOI] [PubMed] [Google Scholar]
- 34.Franz KA, Reubi FC: Rate of functional deterioration in polycystic kidney disease. Kidney Int 23: 526–529, 1983 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.