Abstract
Background and objectives
Calcitriol is used to treat secondary hyperparathyroidism in patients with CKD. Paricalcitol is less calcemic and phosphatemic in preclinical studies and in some trials in dialysis patients, but head-to-head comparisons in nondialysis patients are lacking. A large meta-analysis of trials concluded that these agents did not consistently reduce parathyroid hormone (PTH) and increased the risk of hypercalcemia and hyperphosphatemia. Therefore, the objective of this multicenter trial was to compare the rate of hypercalcemia between calcitriol and paricalcitol, while suppressing PTH 40%–60%.
Design, setting, participants, & measurements
Patients with stages 3–4 CKD (n=110) with a PTH level >120 pg/ml were recruited and randomized to 0.25 μg/d of calcitriol or 1 μg/d of paricalcitol between April 2009 and July 2011. Subsequent dose adjustments were by protocol to achieve 40%–60% PTH suppression below baseline. The primary endpoint was the rate of confirmed hypercalcemia of >10.5 mg/dl between groups.
Results
Forty-five patients in each group completed the 24 weeks of treatment. Both agents suppressed PTH effectively (−52% with paricalcitol and −46% with calcitriol; P=0.17), although the paricalcitol group reached a 40% reduction in PTH sooner at a median 8 weeks (interquartile range [IQR], 4, 12) versus 12 weeks (IQR, 8, 18; P=0.02) and had a lower pill burden of 240 (IQR, 180, 298) versus 292 (IQR, 231, 405; P=0.01). Confirmed hypercalcemia was very low in both groups (three with paricalcitol and one with calcitriol) and was not significantly different (P=0.36). Both groups had small increases in calcium and phosphorus levels (0.3–0.4 mg/dl in each electrolyte) and significant decreases in alkaline phosphatase, a marker of high bone turnover, with no significant differences between groups.
Conclusions
These results show that both calcitriol and paricalcitol achieved sustained PTH and alkaline phosphatase suppression in stages 3–4 CKD, with small effects on serum calcium and phosphorus and a low incidence of hypercalcemia.
Keywords: vitamin D, chronic renal disease, hyperparathyroidism, calcium
Introduction
Secondary hyperparathyroidism (SHPT) is common in patients with CKD, and is characterized by a progressive increase in parathyroid hormone (PTH) and growth of the parathyroid glands (1,2). A major factor in the development and progression of SHPT is the steady decline in serum calcitriol levels, the active form of vitamin D (1). The incidence and severity of SHPT increases as renal function worsens, with 35% of patients with stage 3 CKD and 70% of patients with stage 4 CKD carrying this diagnosis (1).
Exogenous administration of vitamin D receptor activators (VDRAs), such as calcitriol or the analogs paricalcitol, doxercalciferol, and alfacalcidol, suppresses PTH levels but can increase serum calcium and phosphorus (3–5). Major renal guidelines recommend use of active vitamin D for SHPT in stages 3–4 CKD, but comparison of different VDRAs in this population has been limited (6–8).
In addition to activating the vitamin D receptors (VDRs) in the parathyroid gland, calcitriol also activates the VDR in other tissues, including intestine and bone (9). Administration of calcitriol or its analogs requires regular monitoring of serum calcium and phosphorus levels (9). Induction of hypercalcemia or hyperphosphatemia can limit the dose of VDRA administered, thereby limiting its efficacy for PTH suppression.
In preclinical studies, at comparable PTH-suppressing doses, paricalcitol causes a significantly smaller increase in serum calcium and phosphorus levels than calcitriol (10,11). Crossover trials of these agents in hemodialysis patients have also demonstrated less intestinal calcium absorption and less stimulation of bone resorption with paricalcitol compared with calcitriol (12,13). These differences are thought to be the result of less activation of the intestinal and bone VDRs by paricalcitol. Two randomized trials comparing calcitriol with paricalcitol in hemodialysis patients have shown mixed results, with one trial reporting significantly less hypercalcemia and elevation of calcium–phosphate product with paricalcitol despite somewhat superior PTH suppression, whereas a smaller study showed no significant differences between agents (3,4).
In three placebo-controlled trials in patients with stages 3–4 CKD with SHPT, paricalcitol suppressed PTH by 42% at 6 months and caused hypercalcemia in 2% versus 0% of patients who received placebo (14). A small trial of calcitriol using doses of ≤0.5 μg/d reported that eight of 15 patients with CKD became hypercalcemic at least once during an 8-month treatment period (15).
Given the limited comparative data in patients with CKD with these agents, we performed a multicenter, open-label randomized trial to compare the incidence of hypercalcemia in patients treated with paricalcitol and calcitriol for SHPT. The clinical goal was to suppress and maintain PTH concentrations at 40%–60% below the mean pretreatment PTH value, and the primary endpoint was the difference in the incidence of hypercalcemia in each arm.
Materials and Methods
Study Design and Oversight
The Paricalcitol and Calcitriol Endpoints study (ClinicalTrials.gov registry number NCT00823303) is an open-label, active comparator, multicenter, parallel group, phase 4 study of paricalcitol versus calcitriol for suppression of PTH in stages 3–4 CKD. The trial was conducted at four sites in the United States and was approved by each institutional review board. The trial design was investigator-initiated and was funded by Abbott, now AbbVie (Abbott Park, IL). Abbott was not involved in the design or conduct of the trial, but was able to review the manuscript before submission. The four trial sites were Washington University in St. Louis (St. Louis, MO), Henry Ford Hospital (Detroit, MI), Northwestern University (Chicago, IL), and the Northshore University Health System (Evanston, IL).
Eligible patients had stages 3–4 CKD and known or suspected SHPT. Inclusion criteria were aged>18 years, a stable dose of phosphate binder (if receiving a binder), eGFR of 15–59 ml/min per 1.73 m2 using the abbreviated Modification of Diet in Renal Disease equation, PTH>120 pg/ml, albumin-corrected calcium>8.5 mg/dl and <10.0 mg/dl, and phosphorus<4.6 mg/dl. Exclusion criteria were history of primary hyperparathyroidism, prednisone use for >30 days within the previous 6 months, administration of bisphosphonates or calcitonin within the previous 12 months, recent hospitalization, an expected need for dialysis or transplant in the next 6 months, history of renal or other organ transplant, parathyroidectomy, active alcohol or drug abuse, history of noncompliance, pregnancy or unwilling to use reliable birth control, use of a VDRA (calcitriol, doxercalciferol, paricalcitol, alfacalcidol) within 4 weeks of screening, ergocalciferol>50,000 IU/mo within the previous 30 days, cholecalciferol>1000 IU/d within the previous 30 days, or cinacalcet within 4 weeks before screening. Patients were permitted to undergo rescreening at a later time if they failed to meet all inclusion criteria upon initial screening.
Treatments
Patients were initially randomized to a daily capsule containing 1 μg/d paricalcitol or 0.25 μg/d calcitriol. The random sequence was computer generated and site specific without stratification. Sequentially numbered opaque sealed envelopes were provided to each site, with the next available envelope opened after the patient was confirmed to meet all inclusion and exclusion criteria. Patients each had their own PTH target goal of 40%–60% below their baseline PTH measurement. Dose adjustments were based on laboratory results at each visit (weeks 4, 8, 12, and 18). Albumin-corrected calcium (cCa) was calculated as follows: {serum calcium+[(4– serum albumin)×0.8]} if albumin was <4.0 g/dl. The dose was titrated up at any visit if PTH was suppressed <40% from baseline and cCa was <10.5 mg/dl. Dose increases were conducted in the following manner: one capsule alternating with two capsules daily, then two capsules daily, then three capsules daily, and then four capsules daily. Maximum possible doses were 4 μg/d paricalcitol or 1 μg/d calcitriol. Dosing was reduced one step if the PTH was suppressed by >60% from baseline; if the patient was already on the initial step of one capsule daily, the dose was reduced to one capsule every other day. If the cCa was ≥10.5 mg/dl, a confirmatory test was obtained as soon as possible but at least 1 day later; if hypercalcemia was verified, the dose was reduced by one step. Consistent with clinical practice, the use of dietary phosphorus restriction or phosphorus binders were encouraged if hyperphosphatemia (>4.6 mg/dl) developed. Study participation was over a 24-week period with active medication, with a 1-week follow-up.
Endpoints
The primary endpoint was the difference in the percentage of patients in each group developing a confirmed hypercalcemic event (≥10.5 mg/dl). The prespecified secondary objectives were to evaluate the differences between treatment groups in changes in serum calcium, incidence of hyperphosphatemia, change in serum phosphorus, change in alkaline phosphatase, percentage of patients achieving >40% PTH suppression, and mean PTH suppression. The primary endpoint was also the primary safety endpoint. Adverse events were also evaluated.
Statistical Analyses
The primary predefined analysis was based on an intention-to-treat analysis, which includes participants who received at least one study treatment and had at least one postbaseline calcium determination. Secondary endpoints were also assessed on an intention-to-treat basis. On the basis of prior published reports, we estimated a 5% rate of hypercalcemia with paricalcitol and a 30% rate with calcitriol (15,16). To have a 90% power to detect a difference at the P=0.05 confidence level, 42 patients per group were needed. Assuming a 30% dropout rate over the course of the study, we planned to randomize 110 patients. Data were analyzed using SigmaPlot software (version 11.0; Systat Software, San Jose, CA), and differences were considered significant at the P=0.05 level.
Normally distributed variables are expressed as the mean±SD and were compared using ANOVA. Non-normally distributed variables are expressed as the median with interquartile range (IQR) and were compared using the Mann–Whitney rank sum test. In comparing proportions of outcomes, the Fisher’s exact test was used to compare the primary outcome because of the low number of events, and the Pearson’s chi-squared test was used for secondary outcomes. The log-rank test was used to compare time to a 40% PTH reduction.
Results
Study Patients
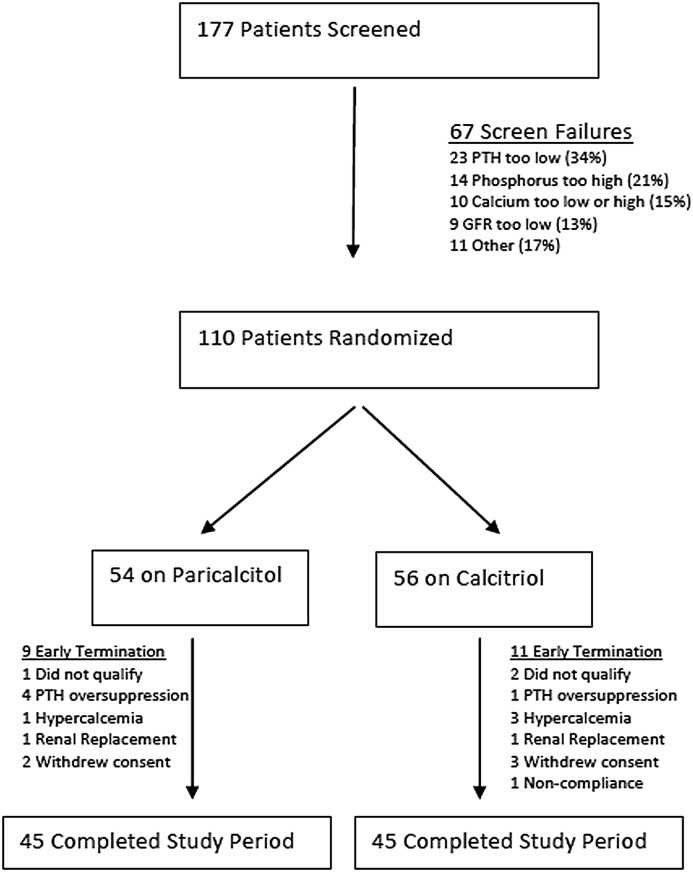
Patient disposition is shown in Figure 1. A total of 177 patients underwent screening between April 2009 and July 2011. There were 67 screen failures, and 18 patients successfully rescreened into the trial. The most common reasons for screen failures were as follows: PTH too low (34%), phosphorus too high (21%), calcium too low or too high (15%), and GFR too low (13%). A total of 110 patients were randomized: 54 to paricalcitol and 56 to calcitriol. Three patients identified as ineligible before the first follow-up visit were dropped from the trial. Forty-five patients in each group completed the 6-month trial. The reasons for early termination are shown in Figure 1 and did not differ significantly between arms. One death occurred during the screening period, but none occurred after randomization. The primary analyses were performed on 107 patients.
Figure 1.
Patient disposition. Enrollment, randomization, and follow-up of study participants. PTH, parathyroid hormone.
Baseline Characteristics
Table 1 shows the baseline demographic and clinical characteristics of the patients who underwent randomization. The two groups were well matched with the exception of the baseline PTH level, which was higher in the group receiving calcitriol.
Table 1.
Demographics and baseline data
| Characteristic | Paricalcitol Group (n=54) | Calcitriol Group (n=56) |
|---|---|---|
| Age (yr) | 66.6±13.2 | 64.7±12.6 |
| Weight (kg) | 92.4±21.7 | 97.6±28.4 |
| BP (mmHg) | ||
| Systolic | 132.5±16.5 | 136.5±18.2 |
| Diastolic | 71.1±11.9 | 71.7±11.7 |
| Race | ||
| African American | 33 (61) | 41 (73) |
| Caucasian | 18 (33) | 14 (25) |
| Other | 3 (6) | 1 (2) |
| Creatinine (mg/dl) | 2.49±0.72 | 2.63±0.83 |
| eGFR (ml/min per 1.73 m2) | 27.8±9.3 | 27.0±9.2 |
| cCa (mg/dl) | 9.32±0.35 | 9.36±0.40 |
| Phosphorus (mg/dl) | 3.66±0.56 | 3.74±0.52 |
| PTH (pg/ml) | 176 (142, 221) | 209 (158, 287) |
| Albumin (g/dl) | 3.9±0.36 | 3.75±0.38 |
| Alkaline phosphatase (U/L) | 80 (65, 104) | 77.5 (70.75, 94.5) |
| Urine phosphorus/creatinine ratio (mg/g) | 418 (341, 562) | 435 (322, 504) |
Data are expressed as the mean±SD, n (%), or median (interquartile range). cCa, albumin-corrected calcium; PTH, parathyroid hormone.
Primary Outcome
Table 2 shows the proportion of patients experiencing confirmed hypercalcemia, which was low in both groups (three receiving paricalcitol and one with calcitriol) and was not significantly different (P=0.36). When analyzed for all episodes of hypercalcemia, including unconfirmed hypercalcemia, the difference remained nonsignificant (seven with paricalcitol and four with calcitriol; P=0.36).
Table 2.
Primary and secondary outcomes
| Outcome | Paricalcitol Group (n=53) | Calcitriol Group (n=54) | P Value |
|---|---|---|---|
| Primary outcome | |||
| Confirmed cCa>10.5 mg/dl | 3 (5.7) | 1 (1.9) | 0.36 |
| Secondary outcomes | |||
| Any cCa>10.5 mg/dl | 7 (13.2) | 4 (7.4) | 0.36 |
| >40% PTH reduction | 52 (98) | 47 (87) | 0.03 |
| >60% PTH reduction | 45 (83) | 28 (52) | <0.001 |
| Change in PTH, 24 wk (%)a | −52±23 | −46±21 | 0.17 |
| Total capsules | 240 (180, 298) | 292 (231, 405) | 0.01 |
| Change in cCa (mg/dl)a | +0.38 (0.10, 0.60) | +0.28 (0.14, 0.52) | 0.27 |
| Change in phosphorus (mg/dl)a | +0.2 (−0.1, 0.7) | +0.3 (0.0, 0.6) | 0.88 |
| Change in alkaline phosphatase (U/L)a | −9.0 (−22.2, 1.0) | −13.0 (−23.5, −8.0) | 0.32 |
| Any phosphorus>4.5 mg/dl | 21 (40) | 28 (52) | 0.21 |
| eGFR (ml/min per 1.73 m2), 24 wka | 24.0±8.6 | 22.6±9.6 | 0.45 |
| Urine phosphorus/creatinine ratio (mg/g), 24 wk | 377 (292, 528) | 414 (326, 514) | 0.62 |
Data are expressed as the mean±SD, n (%), or median (interquartile range).
These results are significantly different from baseline. See Results and Discussion sections for further details.
Efficacy
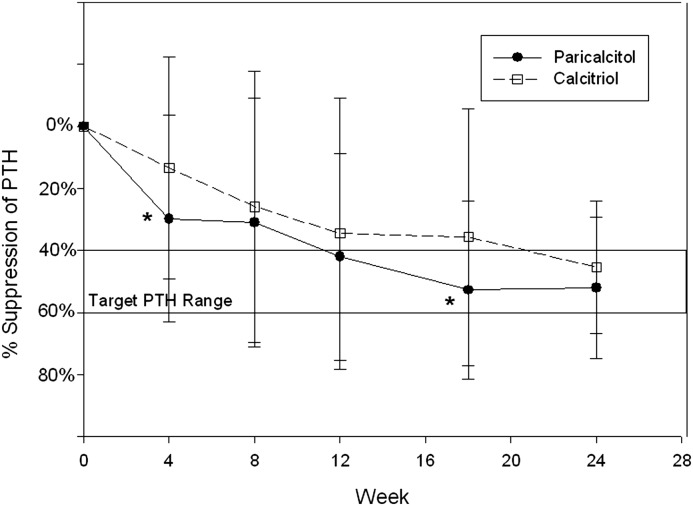
The changes in PTH over time are shown in Figure 2. PTH suppression in the paricalcitol arm was greater than in the calcitriol arm at each study time point and was significantly greater at 4 and 18 weeks. Overall PTH suppression at 24 weeks was similar in both groups (−52%±23% with paricalcitol versus −46%±21% with calcitriol; P=0.17). The mean final dosages were 1.3±0.8 μg/d for paricalcitol and 0.5±0.3 μg/d for calcitriol, for an approximate dose ratio of 2.6:1. The paricalcitol group required fewer capsules over the 24 weeks, with a median of 240 pills (IQR, 180, 298) versus 292 pills (IQR, 231, 405; P=0.01).
Figure 2.
Percentage change in mean PTH suppression over time. The target PTH suppression was 40%–60% below each patient’s baseline PTH measurement. Solid symbols are the paricalcitol group, and open symbols are the calcitriol group. All on-treatment results were significantly below baseline PTH in both groups (P<0.05). *P<0.01 (PTH suppression was significantly greater at these times in the paricalcitol group compared with the calcitriol group).
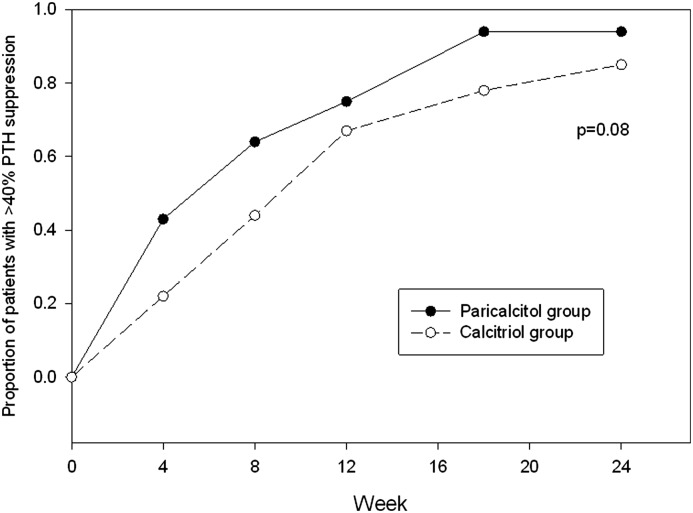
The target PTH goal during treatment was a 40%–60% reduction from each patient’s baseline PTH measurement. As shown in Table 2, the paricalcitol group was more likely to achieve at least 40% PTH suppression (52 of 53 [98%] versus 47 of 54 [87%]; P=0.03). Patients receiving paricalcitol were also more likely to have a >60% reduction in PTH at some point in the 24-week study period (45 of 53 [83%] versus 28 of 54 [52%]; P<0.001). The median time to at least a 40% reduction in PTH was earlier at 8 weeks (IQR, 4, 12) in the paricalcitol group compared with 12 weeks (IQR, 8, 18) in the calcitriol group (P=0.02; Figure 3).
Figure 3.
Proportion of patients achieving ≥40% suppression in PTH at any time during the trial by study week. The median time to at least a 40% reduction in PTH was earlier in the paricalcitol group (8 weeks; IQR, 4, 12) compared with the calcitriol group (12 weeks; IQR, 8, 18; P=0.02). IQR, interquartile range.
Secondary Outcomes
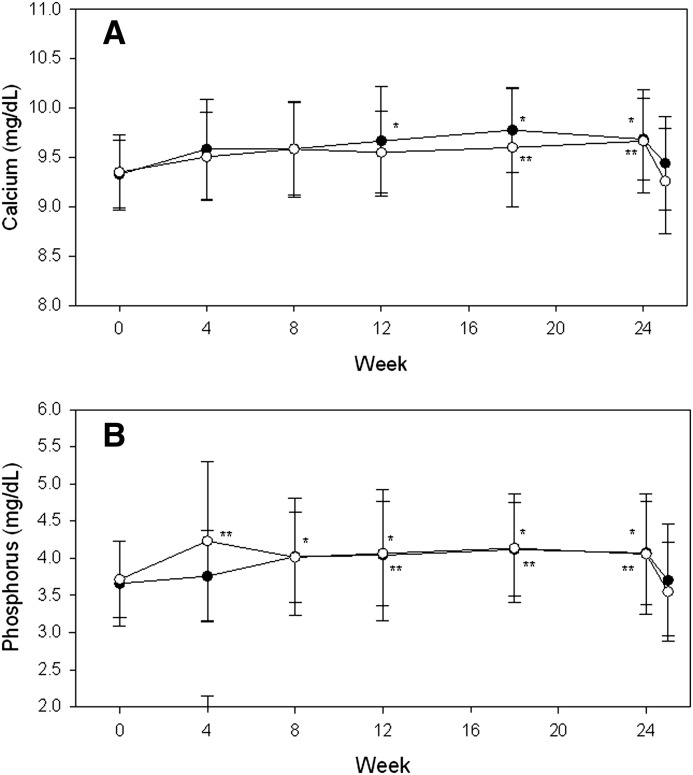
Secondary outcome measures at 24 weeks are shown in Table 2. Changes in calcium and phosphorus levels over time were similar between the two groups, with increases of <0.5 mg/dl in each electrolyte (Figure 4). Calcium and phosphorus increased significantly in both groups during treatment, but returned to baseline 1 week after drug withdrawal. Compared with the calcitriol arm, there were fewer patients in the paricalcitol arm with phosphorus>4.5 mg/dl at any time during the study (40% versus 52%) and at the end of the trial (17% versus 22%); however, these differences were not statistically significant. Alkaline phosphatase decreased by similar levels in the two groups, with –9.0 U/L (IQR, −22.2, 1.0) with paricalcitol versus −13.0 U/L (IQR, −23.5, −8.0) with calcitriol (P=0.32).
Figure 4.
Mean change in calcium and phosphorus during and after withdrawal of treatment. Data are shown as the mean values±SD from baseline in serum calcium (A) and phosphorus (B) during treatment and 1 week after treatment withdrawal at week 24. Solid symbols are the paricalcitol group, and open symbols are the calcitriol group. *P<0.05 versus baseline for paricalcitol; **P<0.05 versus baseline for calcitriol. Week 25 calcium and phosphorus values are also significantly lower than week 24 values (P<0.05), but are not different from baseline.
Safety
There were no significant differences in the rates, types, or severity of adverse events between groups (Table 3). Sixty-five percent of patients experienced at least one adverse event (70% on paricalcitol and 65% on calcitriol). Of these adverse events, only hypercalcemia and hyperphosphatemia were classified by the investigators as likely due to the study medication, and only electrolyte disturbances, upset stomach, constipation, viral syndrome, dry mouth, and rash were considered possibly related to study medications. Of the serious adverse events that occurred, only one in each group was considered possibly related to study drug (constipation in a patient receiving paricalcitol and hypercalcemia in a patient receiving calcitriol) and each associated with hospitalization.
Table 3.
Reported adverse events
| Adverse Event | Paricalcitol Group (n=53) | Calcitriol Group (n=54) |
|---|---|---|
| Any adverse event | 37 (70) | 35 (65) |
| Serious adverse event | 11 (21) | 14 (26) |
| Event type | ||
| Dermatologic | 7 (13) | 7 (13) |
| Neurologic | 11 (21) | 6 (11) |
| Gastrointestinal | 10 (19) | 4 (7) |
| Genitourinary | 7 (13) | 5 (9) |
| Endocrine | 4 (8) | 10 (19) |
| Respiratory | 7 (13) | 7 (13) |
| Musculoskeletal | 15 (28) | 12 (22) |
| Cardiac | 9 (17) | 7 (13) |
| Psychiatric | 2 (4) | 2 (4) |
| Other | 6 (12) | 6 (11) |
Data are expressed as n (%).
The eGFR declined significantly in both groups over 24 weeks, but did not differ significantly between groups at any time point (see Tables 1 and 2). For all patients, the eGFR was 27.2±9.2 ml/min per 1.73 m2 at baseline, 23.9±9.6 ml/min per 1.73 m2 at 12 weeks (P<0.001 versus baseline), and 23.3±9.1 ml/min per 1.73 m2 at 24 weeks (P<0.001 versus baseline and P=0.01 versus 12 weeks).
At baseline, spot urine Calcium (UCa) measurements were below the limit of detection in most patients. Our results showed that 11% of calcitriol-treated patients had measureable UCa with a median value of 29.6 mg/g creatinine (IQR, 21.7, 40.9) and 21% of paricalcitol-treated patients with a median value of 33.0 mg/g creatinine value (IQR, 19.5, 52.7; between-group difference, P=0.58). At 24 weeks, the proportion of patients with measurable UCa had increased significantly in both groups: 33% of calcitriol-treated patients had a median value of 32.3 mg/g creatinine (IQR, 27.0, 58.9) and 51% of paricalcitol-treated patients had a median value of 54.5 mg/g creatinine (IQR, 37.9, 80.1). Median values were not significantly different between groups at 24 weeks (P=0.22). Urine phosphorus/creatinine values are shown in Tables 1 and 2 and did not change during treatment.
Discussion
This study is the first randomized trial to compare two VDRAs with the endpoint of development of hypercalcemia. The incidence of hypercalcemia was very low and there was no difference between groups. This finding may be partly explained by the lower starting doses used in this study (1 μg paricalcitol and 0.25 μg calcitriol) compared with other trials, and the avoidance of prolonged oversuppression of PTH (14–16). During the 24-week treatment period, the increase in serum calcium and phosphorus as well as the reduction in alkaline phosphatase were similar between the two groups. Thus, using a conservative dosing regimen with dose titration based on change in PTH and calcium, both VDRAs appear to be comparably safe in terms of development of hypercalcemia and hyperphosphatemia.
Treatment with vitamin D or VDRAs in stages 3–4 CKD has been controversial. A meta-analysis by Palmer et al. found no differences between calcitriol and newer VDRAs, but also highlighted the limited number of trials comparing these agents in CKD (8). They also reported that compared with placebo, calcitriol results in an increased risk of hypercalcemia and hyperphosphatemia and did not show a consistent reduction in PTH concentrations (8). However, both the Kidney Disease Outcomes Quality Initiative and Kidney Disease Improving Global Outcomes guidelines recommend treating patients with VDRA therapy if hyperparathyroidism persists after correction of calcidiol [25(OH)-vitamin D] deficiency by treatment with ergocalciferol or cholecalciferol (6,7). Several studies have demonstrated that correction of calcidiol deficiency does not resolve the SHPT (17–19). Thus, most patients with CKD require VDRA therapy to control hyperparathyroidism.
The available VDRAs (calcitriol and paricalcitol) and the prohormone VDRAs (doxercalciferol and alfacalcidol) suppress PTH in a dose-dependent fashion independent of the CKD stage (14,15,20,21). Studies of calcitriol in the late 1980s enrolled few patients and were designed to increase serum calcium and not specifically to suppress PTH. Nevertheless, calcitriol did significantly decrease PTH (20%–30%), and bone histology tended to improve (15,16). In a placebo-controlled trial in stages 3–4 CKD, doxercalciferol significantly suppressed PTH 46% with minimal hypercalcemia (defined as >10.7 mg/dl) and a slight increase in hypercalciuria (20). A double-blind placebo-controlled trial of paricalcitol suppressed PTH 42%, with hypercalcemia (defined as >10.5 mg/dl) occurring in 2% of patients without an increase in urinary calcium (14). Thus, current practice has been to use either paricalcitol or doxercalciferol in the belief that better PTH control could be obtained with a decreased risk of hypercalcemia.
In our trial, some differences between agents were noted with regard to their efficacy in lowering PTH. A greater proportion of paricalcitol-treated patients achieved a PTH reduction of at least 40% from baseline compared with calcitriol-treated patients (Figure 3), at a relatively lower dose of paricalcitol than anticipated. Whereas the ratio of 4:1 is the reported equivalency between paricalcitol and calcitriol (22), we observed a dose ratio equivalency of 3:1, and 2.6:1 by the trial end. Furthermore, suppression of PTH>60% of baseline occurred more frequently in the paricalcitol group.
In clinical practice, patients with high-normal serum calcium concentrations may not undergo dose titration of their VDRA for fear of developing hypercalcemia, thus limiting their ability to achieve optimal control of the SHPT. By contrast, this study called for a forced titration of the VDRA to achieve the target PTH as long as the albumin-corrected serum calcium level was <10.5 mg/dl. Despite this, few hypercalcemic events occurred.
This trial has some limitations. One weakness is the use of albumin-corrected rather than ionized calcium levels, although this was done to mimic routine clinical practice. Another limitation is that the trial could be underpowered to detect differences between agents due to the low rate of hypercalcemia observed. We found a significant decrease in eGFR during treatment in our study. This could reflect progression of CKD, increased production of creatinine without a decrease in true GFR, or both. Agarwal et al. showed that short-term treatment with paricalcitol in patients with CKD increased serum creatinine and creatinine excretion, but did not alter iothalamate or creatinine clearance (23). A placebo-controlled trial of paricalcitol for SHPT found similar reductions in eGFR over 24 weeks, suggesting progression of CKD (14).
Two larger issues are the lack of patient-level outcomes in our trial and other studies, and the potential for harm from the increases in calcium and phosphorus from use of VDRAs. Our data show that the VDRAs increased serum calcium and may increase serum phosphorus and lower eGFR. On the basis of observational and preclinical data, hypotheses have been generated that hyperphosphatemia, hypercalcemia, and calcitriol deficiency contribute to cardiovascular events and deaths in patients with CKD (24–26). Use of calcitriol in patients with stages 3–4 CKD is associated with improved survival; paricalcitol was shown in blinded trials to reduce proteinuria, but failed to alter left ventricular remodeling (27–29). However, proteinuria and left ventricular remodeling are intermediate outcomes, and no randomized trials have assessed mortality during use of VDRAs. A large body of observational data links hyperphosphatemia to increased cardiovascular events and death, although a recent large study in patients with CKD failed to find this link (6,24,30). No trial to date has determined whether phosphate control in CKD lowers mortality. Because VDRAs increase calcium and phosphorus while treating SHPT, questions remain regarding whether VDRAs have a net positive, negative, or neutral effect on cardiovascular outcomes and death. Large outcomes trials are needed to test these competing hypotheses.
In summary, this study demonstrates that the use of either calcitriol or paricalcitol to suppress PTH by 40%–60% leads to a comparably low incidence of hypercalcemia in patients with stages 3–4 CKD. The important caveat is that dosing should be started with low doses, independent of the initial PTH concentration, and then titrated based on the PTH response.
Disclosures
D.W.C. and S.M.S. are consultants and speakers and have received honoraria from AbbVie.
Acknowledgments
This was an investigator-initiated trial, designed by D.W.C., S.M.S., and M.F., S.G., and C.G. served as additional site investigators. S.G. supervised and coordinated the data collection and analysis. All authors participated in trial subject management, data collection, and development of the manuscript.
Funding for this trial was provided by AbbVie. AbbVie was not involved in trial development, execution, data analysis or manuscript development.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Levin A, Bakris GL, Molitch M, Smulders M, Tian J, Williams LA, Andress DL: Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: Results of the study to evaluate early kidney disease. Kidney Int 71: 31–38, 2007 [DOI] [PubMed] [Google Scholar]
- 2.de Francisco AL: Secondary hyperparathyroidism: Review of the disease and its treatment. Clin Ther 26: 1976–1993, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Sprague SM, Lerma E, McCormmick D, Abraham M, Batlle D: Suppression of parathyroid hormone secretion in hemodialysis patients: Comparison of paricalcitol with calcitriol. Am J Kidney Dis 38[Suppl 5]: S51–S56, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Sprague SM, Llach F, Amdahl M, Taccetta C, Batlle D: Paricalcitol versus calcitriol in the treatment of secondary hyperparathyroidism. Kidney Int 63: 1483–1490, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Ross EA, Tian J, Abboud H, Hippensteel R, Melnick JZ, Pradhan RS, Williams LA, Hamm LL, Sprague SM: Oral paricalcitol for the treatment of secondary hyperparathyroidism in patients on hemodialysis or peritoneal dialysis. Am J Nephrol 28: 97–106, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group : KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl (113): S1–S130, 2009 [DOI] [PubMed] [Google Scholar]
- 7.National Kidney Foundation : K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 42[Suppl 3]: S1–S201, 2003 [PubMed] [Google Scholar]
- 8.Palmer SC, McGregor DO, Macaskill P, Craig JC, Elder GJ, Strippoli GF: Meta-analysis: Vitamin D compounds in chronic kidney disease. Ann Intern Med 147: 840–853, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Sprague SM, Coyne D: Control of secondary hyperparathyroidism by vitamin D receptor agonists in chronic kidney disease. Clin J Am Soc Nephrol 5: 512–518, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Brown AJ, Finch J, Slatopolsky E: Differential effects of 19-nor-1,25-dihydroxyvitamin D(2) and 1,25-dihydroxyvitamin D(3) on intestinal calcium and phosphate transport. J Lab Clin Med 139: 279–284, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Finch JL, Dusso AS, Pavlopoulos T, Slatopolsky EA: Relative potencies of 1,25-(OH)(2)D(3) and 19-Nor-1,25-(OH)(2)D(2) on inducing differentiation and markers of bone formation in MG-63 cells. J Am Soc Nephrol 12: 1468–1474, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Coyne DW, Grieff M, Ahya SN, Giles K, Norwood K, Slatopolsky E: Differential effects of acute administration of 19-Nor-1,25-dihydroxy-vitamin D2 and 1,25-dihydroxy-vitamin D3 on serum calcium and phosphorus in hemodialysis patients. Am J Kidney Dis 40: 1283–1288, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Lund RJ, Andress DL, Amdahl M, Williams LA, Heaney RP: Differential effects of paricalcitol and calcitriol on intestinal calcium absorption in hemodialysis patients. Am J Nephrol 31: 165–170, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Coyne D, Acharya M, Qiu P, Abboud H, Batlle D, Rosansky S, Fadem S, Levine B, Williams L, Andress DL, Sprague SM: Paricalcitol capsule for the treatment of secondary hyperparathyroidism in stages 3 and 4 CKD. Am J Kidney Dis 47: 263–276, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Nordal KP, Dahl E: Low dose calcitriol versus placebo in patients with predialysis chronic renal failure. J Clin Endocrinol Metab 67: 929–936, 1988 [DOI] [PubMed] [Google Scholar]
- 16.Baker LR, Abrams L, Roe CJ, Faugere MC, Fanti P, Subayti Y, Malluche HH: 1,25(OH)2D3 administration in moderate renal failure: A prospective double-blind trial. Kidney Int 35: 661–669, 1989 [DOI] [PubMed] [Google Scholar]
- 17.Zisman AL, Hristova M, Ho LT, Sprague SM: Impact of ergocalciferol treatment of vitamin D deficiency on serum parathyroid hormone concentrations in chronic kidney disease. Am J Nephrol 27: 36–43, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Chandra P, Binongo JN, Ziegler TR, Schlanger LE, Wang W, Someren JT, Tangpricha V: Cholecalciferol (vitamin D3) therapy and vitamin D insufficiency in patients with chronic kidney disease: A randomized controlled pilot study. Endocr Pract 14: 10–17, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al-Aly Z, Qazi RA, Gonzalez EA, Zeringue A, Martin KJ: Changes in serum 25-hydroxyvitamin D and plasma intact PTH levels following treatment with ergocalciferol in patients with CKD. Am J Kidney Dis 50: 59–68, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Coburn JW, Maung HM, Elangovan L, Germain MJ, Lindberg JS, Sprague SM, Williams ME, Bishop CW: Doxercalciferol safely suppresses PTH levels in patients with secondary hyperparathyroidism associated with chronic kidney disease stages 3 and 4. Am J Kidney Dis 43: 877–890, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Hamdy NA, Kanis JA, Beneton MN, Brown CB, Juttmann JR, Jordans JG, Josse S, Meyrier A, Lins RL, Fairey IT: Effect of alfacalcidol on natural course of renal bone disease in mild to moderate renal failure. BMJ 310: 358–363, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin KJ, González EA, Gellens ME, Hamm LL, Abboud H, Lindberg J: Therapy of secondary hyperparathyroidism with 19-nor-1alpha,25-dihydroxyvitamin D2. Am J Kidney Dis 32[Suppl 2]: S61–S66, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Agarwal R, Hynson JE, Hecht TJ, Light RP, Sinha AD: Short-term vitamin D receptor activation increases serum creatinine due to increased production with no effect on the glomerular filtration rate. Kidney Int 80: 1073–1079, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM: Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 15: 2208–2218, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Wolf M, Shah A, Gutierrez O, Ankers E, Monroy M, Tamez H, Steele D, Chang Y, Camargo CA, Jr, Tonelli M, Thadhani R: Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int 72: 1004–1013, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Moe SM, Thadhani R: What have we learned about chronic kidney disease-mineral bone disorder from the EVOLVE and PRIMO trials? Curr Opin Nephrol Hypertens 22: 651–655, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shoben AB, Rudser KD, de Boer IH, Young B, Kestenbaum B: Association of oral calcitriol with improved survival in nondialyzed CKD. J Am Soc Nephrol 19: 1613–1619, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Zeeuw D, Agarwal R, Amdahl M, Audhya P, Coyne D, Garimella T, Parving HH, Pritchett Y, Remuzzi G, Ritz E, Andress D: Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): A randomised controlled trial. Lancet 376: 1543–1551, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Thadhani R, Appelbaum E, Pritchett Y, Chang Y, Wenger J, Tamez H, Bhan I, Agarwal R, Zoccali C, Wanner C, Lloyd-Jones D, Cannata J, Thompson BT, Andress D, Zhang W, Packham D, Singh B, Zehnder D, Shah A, Pachika A, Manning WJ, Solomon SD: Vitamin D therapy and cardiac structure and function in patients with chronic kidney disease: The PRIMO randomized controlled trial. JAMA 307: 674–684, 2012 [DOI] [PubMed] [Google Scholar]
- 30.Mehrotra R, Peralta CA, Chen SC, Li S, Sachs M, Shah A, Norris K, Saab G, Whaley-Connell A, Kestenbaum B, McCullough PA, Kidney Early Evaluation Program (KEEP) Investigators : No independent association of serum phosphorus with risk for death or progression to end-stage renal disease in a large screen for chronic kidney disease. Kidney Int 84: 989–997, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]