Abstract
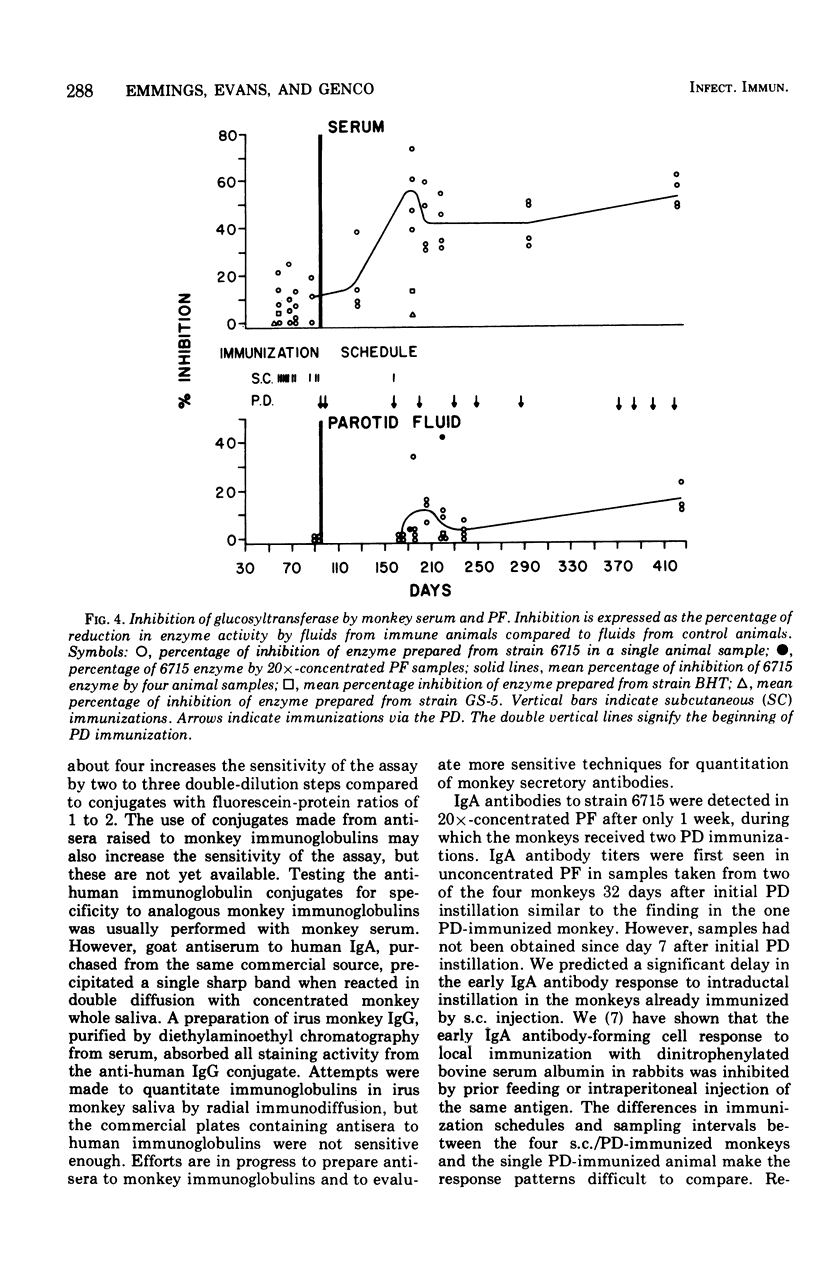
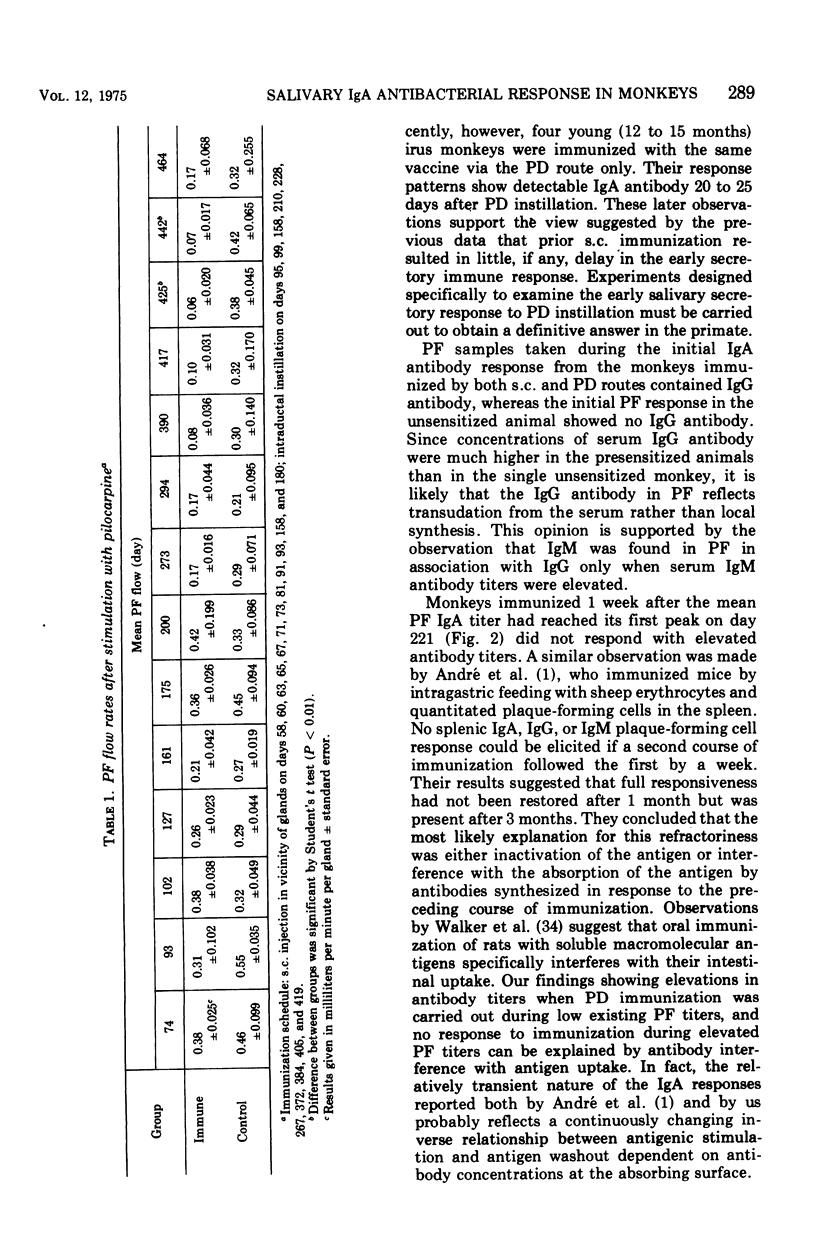
The antibody response of Macaca fascicularis in parotid saliva and serum to local immunization by two routes with Streptococcus mutans was studied and compared over 1 year. Antibodies were titrated and classified by indirect immunofluorescent staining using specific antiglobulin conjugates. Antiglucosyltransferase activity was assayed by an enzyme inhibition test. Animals were immunized first by injecting formalin-killed bacterial cells and cell products subcutaneously into the vicinity of the four major salivary glands. The monkeys were next immunized by retrograde instillation of antigen into the parotid duct. Extensive subcutaneous local immunization gave a serum response only. After parotid duct immunization, high titers of immunoglobulin A (IgA) antibody, along with traces of immunoglobulin G (IgG) immunoglobulin M (IgM) antibody, appeared in the parotid saliva, and in the serum high titers of IgG antibody were present along with lower titers of IgA and IgM. IgA antibodies in parotid fluid were shown by double immunofluorescent staining to be associated with antigenic determinants which cross-reacted with an antiserum directed to human secretory component. Titers in parotid fluids and sera fell sharply when immunization was stopped. This response pattern was reproducible. High concentrations of antibody capable of inhibiting glucosyltransferase prepared from S. mutans were found in the sera, but relatively little was detected in the parotid fluids. Extensive immunization via the parotid duct resulted in transient functional impairment of the gland, as evidenced by diminished salivary flow rates. We conclude that parotid ductal immunization can be an effective method for stimulating a salivary secretory IgA antibacterial antibody response.
Full text
PDF

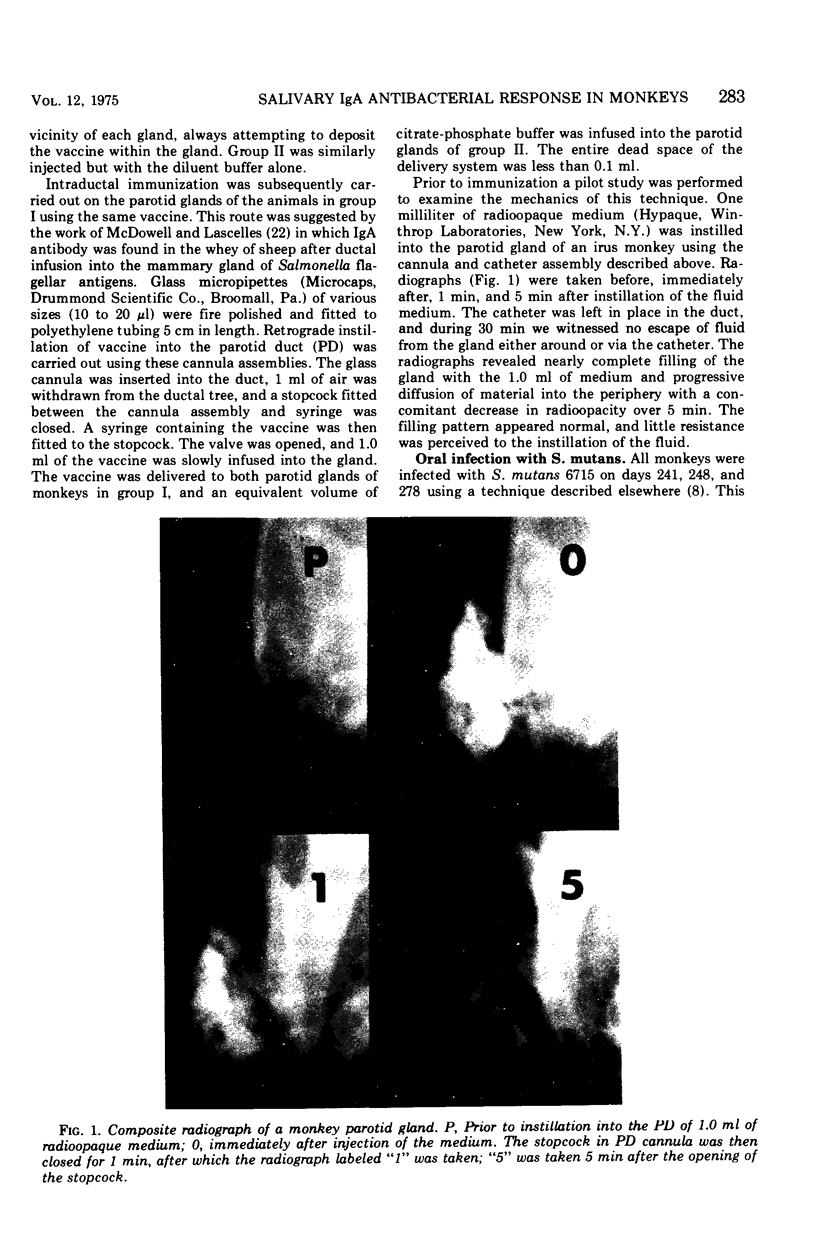

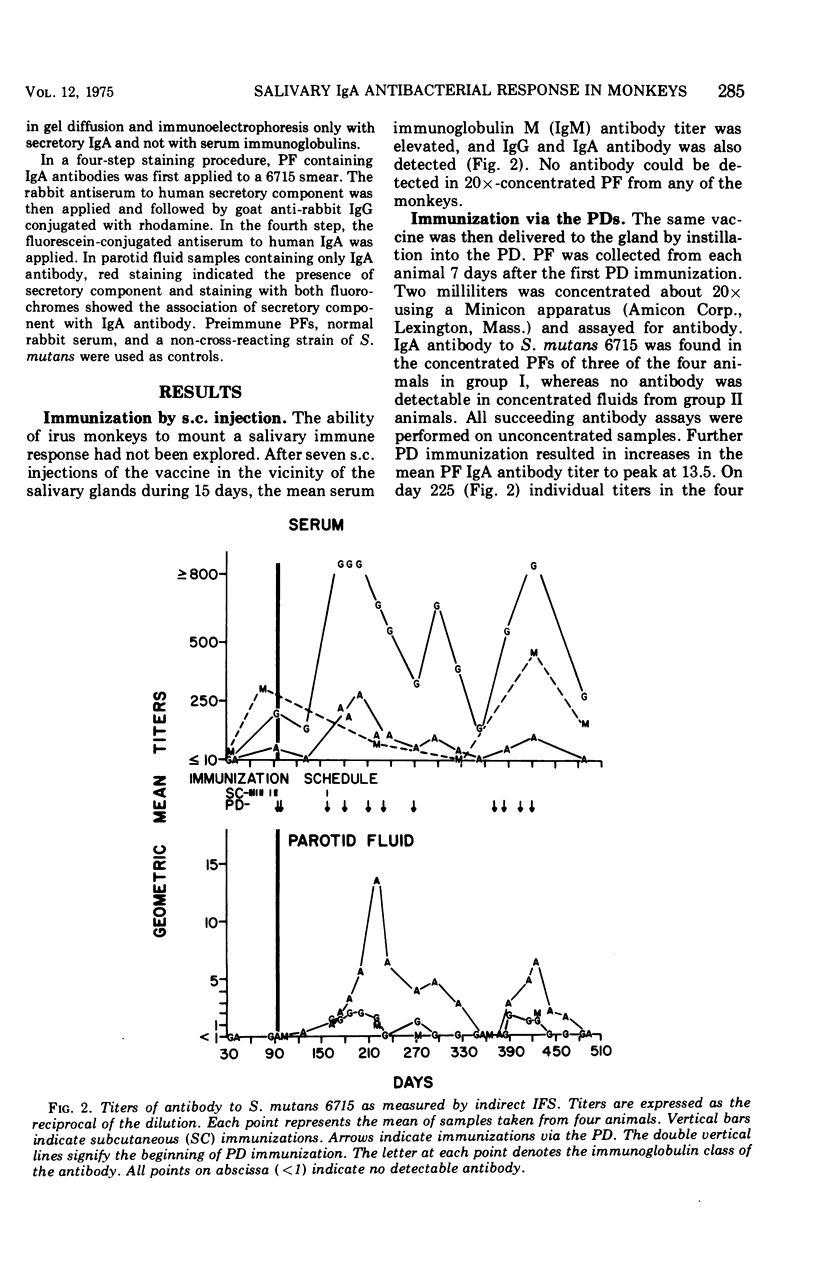
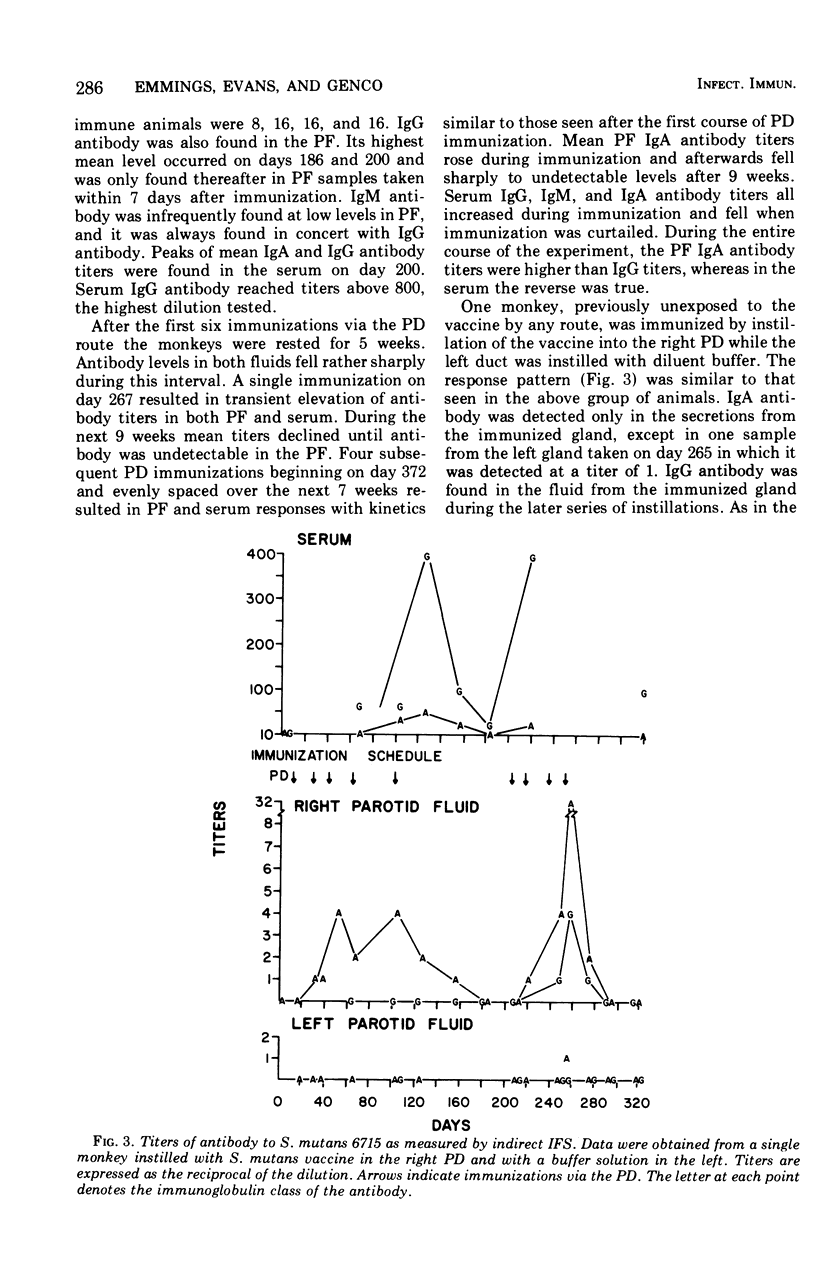




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- André C., Bazin H., Heremans J. F. Influence of repeated administration of antigen by the oral route on specific antibody-producing cells in the mouse spleen. Digestion. 1973;9(2):166–175. doi: 10.1159/000197442. [DOI] [PubMed] [Google Scholar]
- Bauer K. Cross-reactions between human and animal plasma proteins. I. The immunological evolution groups (IEG) I and II. Humangenetik. 1970;8(4):325–329. doi: 10.1007/BF00280332. [DOI] [PubMed] [Google Scholar]
- Bauer K. The antigenic determinants of several human plasma proteins. Their determination from the investigation of the cross-reactions between human and other mammalian plasmas. Int J Protein Res. 1970;2(3):137–145. [PubMed] [Google Scholar]
- Bidwell D., Voller A., Meuwissen J. H., Leeuwenberg A. D. Comparison of indirect haemagglutination and immunofluorescence tests for malaria antibody in Aotus monkeys infected with Plasmodium falciparum. Application of indirect haemagglutination tests for malaria. Bull World Health Organ. 1973;49(3):313–316. [PMC free article] [PubMed] [Google Scholar]
- Bowen W. H. A vaccine against dental caries. A pilot experiment in monkeys (Macaca irus). Br Dent J. 1969 Feb 18;126(4):159–160. [PubMed] [Google Scholar]
- Bowen W. H. The induction of rampant dental caries in monkeys (Macaca irus). Caries Res. 1969;3(3):227–237. doi: 10.1159/000259597. [DOI] [PubMed] [Google Scholar]
- Emmings F. G., Genco R. J. The IgA antibody-forming cell response in the rabbit submandibular gland following several different methods of immunization. Adv Exp Med Biol. 1974;45(0):467–471. doi: 10.1007/978-1-4613-4550-3_56. [DOI] [PubMed] [Google Scholar]
- Evans R. T., Emmings F. G., Genco R. J. Prevention of Streptococcus mutans infection of tooth surfaces by salivary antibody in Irus monkeys (Macaca fascicularis). Infect Immun. 1975 Aug;12(2):293–302. doi: 10.1128/iai.12.2.293-302.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. T., Genco R. J. Inhibition of glucosyltransferase activity by antisera to known serotypes of Streptococcus mutans. Infect Immun. 1973 Feb;7(2):237–241. doi: 10.1128/iai.7.2.237-241.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FITZGERALD R. J., KEYES P. H. Demonstration of the etiologic role of streptococci in experimental caries in the hamster. J Am Dent Assoc. 1960 Jul;61:9–19. doi: 10.14219/jada.archive.1960.0138. [DOI] [PubMed] [Google Scholar]
- Fukui K., Fukui Y., Moriyama T. Some Immunochemical Properties of Dextransucrase and Invertase from Streptococcus mutans. Infect Immun. 1974 Nov;10(5):985–990. doi: 10.1128/iai.10.5.985-990.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genco R. J., Evans R. T., Taubman M. A. Specificity of antibodies to Streptococcus mutans; significance in inhibition of adherence. Adv Exp Med Biol. 1974;45(0):327–336. doi: 10.1007/978-1-4613-4550-3_39. [DOI] [PubMed] [Google Scholar]
- Genco R. J., Flanagan T. D., Emmings F. G. Immunocyte response to experimental mumps virus infection in Rhesus monkeys. Infect Immun. 1973 Apr;7(4):520–525. doi: 10.1128/iai.7.4.520-525.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genco R. J., Taubman M. A. Secretory gamma-A antibodies induced by local immunization. Nature. 1969 Feb 15;221(5181):679–681. doi: 10.1038/221679a0. [DOI] [PubMed] [Google Scholar]
- Gerbrandy J. L., van Dura E. A. Anamnestic secretory antibody response in respiratory secretions of intranasally immunized mice. J Immunol. 1972 Nov;109(5):1146–1148. [PubMed] [Google Scholar]
- Gibbons R. J., Depaola P. F., Spinell D. M., Skobe Z. Interdental localization of Streptococcus mutans as related to dental caries experience. Infect Immun. 1974 Mar;9(3):481–488. doi: 10.1128/iai.9.3.481-488.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi J. A., Shklair I. L., Bahn A. N. Immunization with dextransucrases and glycosidic hydrolases. J Dent Res. 1972 Mar-Apr;51(2):436–442. doi: 10.1177/00220345720510023201. [DOI] [PubMed] [Google Scholar]
- KEYES P. H. The infectious and transmissible nature of experimental dental caries. Findings and implications. Arch Oral Biol. 1960 Mar;1:304–320. doi: 10.1016/0003-9969(60)90091-1. [DOI] [PubMed] [Google Scholar]
- Linzer R., Slade H. D. Purification and characterization of Streptococcus mutans group d cell wall polysaccharide antigen. Infect Immun. 1974 Aug;10(2):361–368. doi: 10.1128/iai.10.2.361-368.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell G. H., Lascelles A. K. Local production of antibody by ovine mammary glands infused with Salmonella flagellar antigens. Aust J Exp Biol Med Sci. 1969 Dec;47(6):669–678. doi: 10.1038/icb.1969.164. [DOI] [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Extraction, purification, and chemical and immunological properties of the Streptococcus mutans group "a" polysaccharide cell wall antigen. Infect Immun. 1973 Aug;8(2):190–198. doi: 10.1128/iai.8.2.190-198.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Mechanism of adherence of Streptococcus mutans to smooth surfaces. I. Roles of insoluble dextran-levan synthetase enzymes and cell wall polysaccharide antigen in plaque formation. Infect Immun. 1973 Oct;8(4):555–562. doi: 10.1128/iai.8.4.555-562.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogra P. L., Karzon D. T. Distribution of poliovirus antibody in serum, nasopharynx and alimentary tract following segmental immunization of lower alimentary tract with poliovaccine. J Immunol. 1969 Jun;102(6):1423–1430. [PubMed] [Google Scholar]
- Sweeney E. A., Shaw J. H., Childs E. L. Effect of passive immunization on the dental caries incidence of caries-susceptible rats. J Dent Res. 1966 Jul-Aug;45(4):993–997. doi: 10.1177/00220345660450045401. [DOI] [PubMed] [Google Scholar]
- TOMASI T. B., Jr, TAN E. M., SOLOMON A., PRENDERGAST R. A. CHARACTERISTICS OF AN IMMUNE SYSTEM COMMON TO CERTAIN EXTERNAL SECRETIONS. J Exp Med. 1965 Jan 1;121:101–124. doi: 10.1084/jem.121.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbman M. A., Smith D. J. Effects of local immunization with Streptococcus mutans on induction of salivary immunoglobulin A antibody and experimental dental caries in rats. Infect Immun. 1974 Jun;9(6):1079–1091. doi: 10.1128/iai.9.6.1079-1091.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzer J. M., Hageage G. J., Jr, Larson R. H. Variable experiences in immunization of rats against Streptococcus mutans-associated dental caries. Arch Oral Biol. 1973 Nov;18(11):1425–1439. doi: 10.1016/0003-9969(73)90117-9. [DOI] [PubMed] [Google Scholar]
- Taubman M. A., Genco R. J. Induction and properties of rabbit secretory A antibody directed to group A streptococcal carbohydrate. Immunochemistry. 1971 Dec;8(12):1137–1155. doi: 10.1016/0019-2791(71)90392-2. [DOI] [PubMed] [Google Scholar]
- Tomasi T. B., Grey H. M. Structure and function of immunoglobulin A. Prog Allergy. 1972;16:81–213. [PubMed] [Google Scholar]
- Uhr J. W., Möller G. Regulatory effect of antibody on the immune response. Adv Immunol. 1968;8:81–127. doi: 10.1016/s0065-2776(08)60465-4. [DOI] [PubMed] [Google Scholar]
- Walker W. A., Isselbacher K. J., Bloch K. J. Intestinal uptake of macromolecules: effect of oral immunization. Science. 1972 Aug 18;177(4049):608–610. doi: 10.1126/science.177.4049.608. [DOI] [PubMed] [Google Scholar]
- Wood J. M. A dextransucrase activity from Streptococcus FA-1. Arch Oral Biol. 1967 Dec;12(12):1659–1660. doi: 10.1016/0003-9969(67)90202-6. [DOI] [PubMed] [Google Scholar]