Abstract
Efforts to derive hematopoietic stem cells (HSCs) from human pluripotent stem cells (hPSCs) are complicated by the fact that embryonic hematopoiesis consists of two programs, primitive and definitive, that differ in developmental potential. As only definitive hematopoiesis generates HSCs, understanding how this program develops is essential for being able to produce this cell population in vitro. Here we show that both hematopoietic programs transition through hemogenic endothelial intermediates and develop from KDR+CD34−CD144− progenitors that are distinguished by CD235a expression. Generation of primitive progenitors (KDR+CD235a+) depends on stage-specific Activin-nodal signaling and inhibition of the Wnt-β-catenin pathway, whereas specification of definitive progenitors (KDR+CD235a−) requires Wnt-β-catenin signaling during this same time frame. Together, these findings establish simple selective differentiation strategies for the generation of primitive or definitive hematopoietic progenitors via Wnt-β-catenin manipulation, and in doing so provide access to enriched populations for future studies on hPSC-derived hematopoietic development.
Keywords: embryonic stem cell, pluripotent, hematopoiesis, KDR, CD235a, hemogenic endothelium, definitive hematopoiesis, Wnt
Most evidence suggests that primitive and definitive hematopoiesis in the mouse and human develop from distinct progenitors that are specified at different sites and times in the early embryo (reviewed in [1]). Primitive hematopoiesis is thought to derive from a multi-potent progenitor called the hemangioblast, which is characterized by co-expression of the receptor tyrosine kinase Flk-1/KDR (VEGFR2) and the primitive streak transcription factor Brachyury, and by its ability to generate both vascular and hematopoietic progeny2. This program rapidly transitions through a specialized population of endothelial cells with hemogenic potential, known as hemogenic endothelium3. In contrast, definitive hematopoiesis, is best defined by is development from hemogenic endothelium that is specified at different sites throughout the embryonic vasculature (reviewed in [1, 4]).
Studies with mouse and human embryonic stem cells (mESCs and hESCs) have provided compelling evidence that hematopoietic development in differentiation cultures recapitulates key stages of embryonic hematopoiesis5, 6. In both models, the onset of the primitive hematopoietic program is marked by upregulation of the Flk-1/KDR receptor and the development of hemangioblasts7, 8 followed by a transient wave of primitive hematopoiesis that gives rise to a limited spectrum of lineages, including primitive erythroid, macrophage and megakaryocyte (reviewed in [9]). Definitive hematopoiesis, as measured by T-lymphoid potential, emerges after the establishment of the primitive hematopoietic program and develops from a progenitor population that displays characteristics of hemogenic endothelium10–12. Analyses of the signaling pathways that regulate the development of the two hPSC-derived human hematopoietic programs revealed that they differ in their requirement for Activin-nodal signaling at the early stages of differentiation: whereas specification of primitive hematopoiesis is temporally dependent on the Activin-nodal pathway, definitive hematopoietic development is not12. These observations suggest that primitive and definitive hematopoiesis are specified early within hPSC differentiation cultures and raise the possibility that, with appropriate markers, it might be possible to physically separate the progenitors of these two programs during the stage of Activin-nodal dependence.
Results
CD235a is expressed on primitive hematopoietic progenitors
To identify surface antigens that define primitive and definitive progenitors, we screened hESC−derived mesoderm populations at different stages on an anti-CD antibody array containing ~370 known antibodies (http://www.ocigc.ca/antibody/; [13]). From this screen, Glycophorin A (CD235a) was the only antigen to show an interesting pattern as it was expressed on a subpopulation of cells within embryoid bodies during early mesoderm differentiation (not shown). This was an unexpected finding as Glycophorin A is considered to be an erythroid-specific antigen. It is, however, in line with the observation that Glycophorin A is expressed on hPSC-derived CD34+ cells14, suggesting that it is likely to be present on non-erythroid cells early in human development.
To determine whether CD235a marks cells with hematopoietic potential at this early time, we analyzed its expression and that of KDR on embryoid body populations of different stages generated from H1 hESCs using our previously described step-wise, serum-free approach12 (Figure 1A). Kinetic analyses revealed that CD235a was first expressed at day 3 of differentiation on a subset of KDR+ mesoderm, 24 hours after the emergence of the BRACHYURY/T+ primitive streak-like population (Figure 1B–D). At this stage the mesodermal cells do not express CD34 or CD144, markers found on hemogenic endothelium, hematopoietic or endothelial lineage cells (reviewed in [4, 15]). The co-expression with KDR at day 3 suggests that CD235a marks a subpopulation of hematopoietic mesoderm, possibly fated to the primitive lineage. To investigate this possibility, we analyzed the expression of CD235a after manipulation of the Activin-nodal signaling pathway, given that it is required for primitive hematopoietic development12, 16. Inhibition of Activin-nodal signaling by addition of the small-molecule antagonist SB-431542 (SB) between days 2 and 3 of differentiation prevented the development of the KDR+CD235a+ population (Figure 1E). In contrast, activation of the pathway by the addition of Activin A during the same period of time led to an increase in its size. These changes in response to manipulation of the Activin-nodal pathway support the hypothesis that expression of CD235a marks primitive hematopoietic progenitors.
Figure 1. Primitive hematopoiesis originates from a KDR+CD235a+ progenitor.
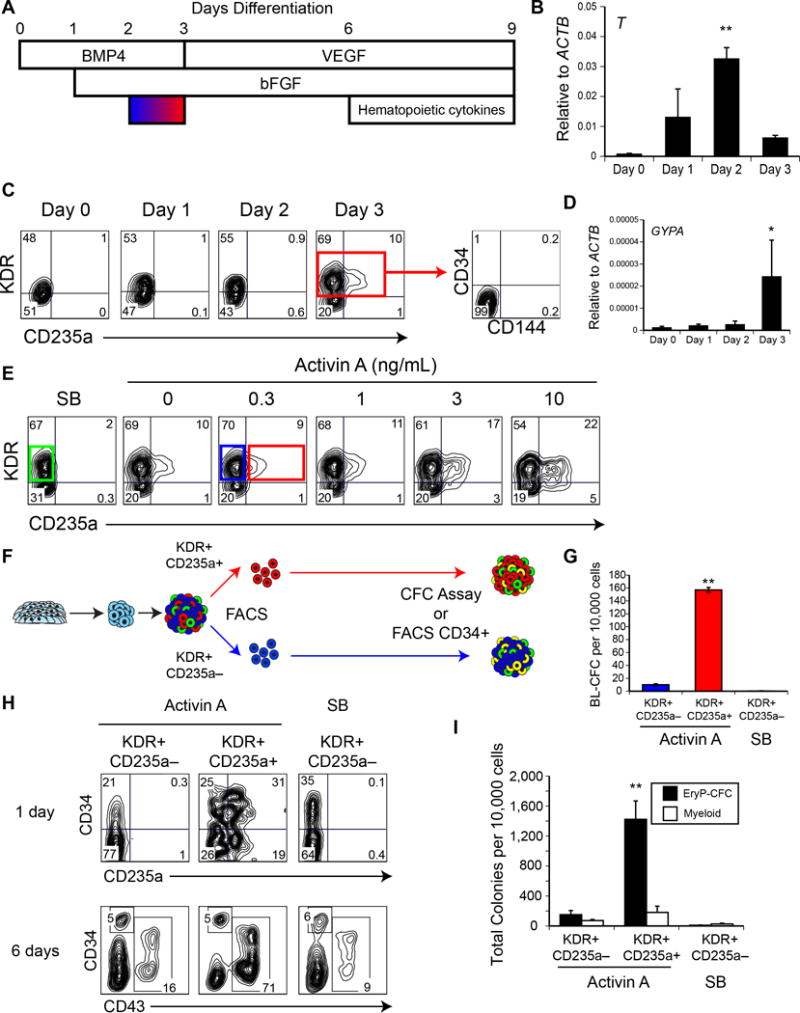
(A) Serum-free differentiation schematic. Embryoid bodies were differentiated in the presence of BMP4, followed by stage-specific addition of bFGF, VEGF and hematopoietic cytokines, as illustrated. Activin-nodal signaling was manipulated between days 2 and 3 (blue-red gradient). (B) qRT-PCR of BRACHYURY (T) expression during differentiation. (C) Representative flow cytometry analysis of KDR and CD235a expression during differentiation. Day 3 KDR+ cells did not express CD34 or CD144. (D) qRT-PCR of GLYCOPHORIN A (GYPA) expression during differentiation. (E) Representative flow cytometry analysis of KDR and CD235a expression on day 3 of differentiation, with Activin-nodal signal manipulation between days 2 and 3. (F) Schematic for primitive and definitive hematopoietic potential analysis. Day 3 KDR+ populations were isolated by FACS and either analyzed for hemangioblast potential or cultured an additional 6 days, with the resultant differentiation cultures either analyzed for CFC potential, or CD34+CD43− cells were isolated by FACS. (G) Hemangioblast (BL-CFC) potential of Activin-derived KDR+CD235a− (blue), KDR+CD235a+ (red), and SB-derived KDR+CD235a− (green) fractions, as in (F). n = 3. (Mean ± SEM). ** ANOVA p ≤ 0.0001. (H) KDR+ populations were isolated by FACS and reaggregated and cultured as in (F), and analyzed by flow cytometry for CD34 and either CD235a (top) or CD43 expression (bottom). (I) Colony-forming progenitor potential of day 9 reaggregates, as in (H). n = 3. (Mean ± SEM). ** ANOVA p = 0.001.
To formally test this hypothesis, we next isolated the KDR+CD235a+ and KDR+CD235a− fractions from day 3 Activin-induced embryoid bodies and the KDR+CD235a− fraction from day 3 SB-treated embryoid bodies (Figure 1E blue/red/green boxes, schematic in Figure 1F) and assayed them for hemangioblasts as a first measure of primitive hematopoietic potential. The Activin A-derived KDR+CD235a+ population contained ~10-fold more hemangioblasts than the corresponding KDR+CD235a− population (Figure 1G). No hemangioblasts were detected in the KDR− CD235a− fraction (not shown) or in the SB-treated KDR+CD235a− fraction (Figure 1G). These results demonstrate that CD235a is expressed on the hemangioblast and confirms that its expression marks an early stage of primitive hematopoietic development.
To further characterize the potential of the different KDR+ progenitors, we next aggregated the FACS-isolated KDR+CD235a− or KDR+CD235a+ populations and cultured the aggregates (as in Figure 1F) for up to 6 days (total of 9 days of culture) to replicate the timeframe used in our previous study to identify primitive and definitive hematopoiesis in hPSC-derived populations12. The derivative populations were analyzed for different cell surface markers including CD34 to monitor the emergence of hemogenic endothelium or hemogenic endothelial progenitors14, CD235a as a measure of primitive hematopoietic potential and CD43 as an indication of hematopoietic specification. CD43 is hematopoietic specific marker that is expressed on progenitors of both the primitive and definitive lineages12, 14, 17. All three KDR+ progenitors gave rise to CD34+ cells within 1 day of culture, likely reflecting specification of the hematopoietic and vascular lineages. The KDR+CD235+ mesoderm generated the largest CD34+ population and the only one that co-expressed CD235a (Figure 1H; top row). Following 6 days of aggregate culture, the proportion of CD34+ cells declined in all 3 populations. At this stage, the majority (>70%) of the cells in the KDR+CD235+ mesoderm–derived population expressed CD43, indicative of hematopoietic commitment. In contrast, fewer than 20% of the cells in the populations derived from the CD235a− progenitors were CD43+. This CD43+ population generated from the KDR+CD235a+ progenitors likely represents the expansion of the primitive hematopoietic program.
To test this, we next assayed the day 6 reaggregation cultures for the presence of primitive erythroid progenitors (EryP-CFCs) which, in methylcellulose, will give rise to small colonies of primitive erythroblasts that express the embryonic form of globin, HBE (reviewed in [9, 18]) Colony assays revealed that the KDR+CD235a+ mesoderm–derived aggregates contained a significantly higher frequency of EryP-CFCs than those generated from either the Activin A-induced or SB-treated KDR+CD235a− mesoderm (Figure 1I). Taken together with the hemangioblast and flow cytometric analyses, these findings demonstrate that the earliest identified progenitor of the human primitive hematopoietic program is marked by the co-expression of KDR and CD235a.
CD235a separates primitive and definitive hematopoietic mesoderm
In addition to the CD43+ cells, both the KDR+CD235a+ and KDR+CD235a− progenitors gave rise to a distinct CD34+CD43− population by day 9 of culture (Figure 1H). This was of interest, as until recently, this cell surface expression pattern was only associated with hPSC-derived progenitors the definitive hematopoietic program12. Given this, we assayed these populations for T-lymphoid and erythroid progenitors to assess their hematopoietic potential (as in Figure 1F). For the erythroid analyses, CD34+CD43− populations generated following the reaggregation of either day 3 CD235a+ or CD235a− cells were isolated by FACS and co-cultured with OP9-DL1 stromal cells for 7 days, as we have shown that this co-culture step is necessary to promote the development of erythroid progenitors from the embryoid body-derived CD34+CD43− population12. Following co-culture, the cells were harvested and assayed for colony- forming potential in methylcellulose. Notably, both CD34+CD43− populations generated similar numbers of medium to large size burst-like erythroid colonies (BFU-E; Figure 2A,B) that were morphologically distinct from the smaller EryP-CFC-derived colonies (Figure 1I, 2B). Analyses of the embryonic (HBE) and fetal (HBG) globin gene expression patterns (reviewed in [18]) in these erythroid colonies suggested that the KDR+CD235a− mesoderm–derived CD34+CD43− progenitors that generated the large erythroid colonies are different from EryP-CFC. The burst-like large colonies obtained from the CD34+CD43− population derived from the KDR+CD235a− mesoderm expressed significantly higher levels of globins associated with definitive hematopoiesis, including HBG (Figure 2Ci) and HBA (not shown) than the colonies generated from the KDR+CD235a+ mesoderm–derived CD34+CD43− cells (as in Figure 1I). Comparison of the ratios of the levels of HBG to HBE revealed that the cells in the KDR+CD235a+ mesoderm–derived colonies expressed more embryonic than fetal globin and in this regard were similar to the EryP-CFC-derived colonies. Roughly equal levels of HBG and HBE were detected in in the large colonies generated from the KDR+CD235a− mesoderm–derived CD34+CD43− progenitors (Figure 2Cii). These patterns indicate that the large colonies obtained from the CD34+CD43− population generated from the KDR+CD235a+ mesoderm contain primitive erythroblasts and develop from a progenitor that arises late in the culture after the emergence of the EryP-CFCs.
Figure 2. KDR+CD235a− mesoderm–derived CD34+CD43− cells possess definitive hematopoietic potential, but both CD34+ populations possess hemogenic endothelium-like potential.
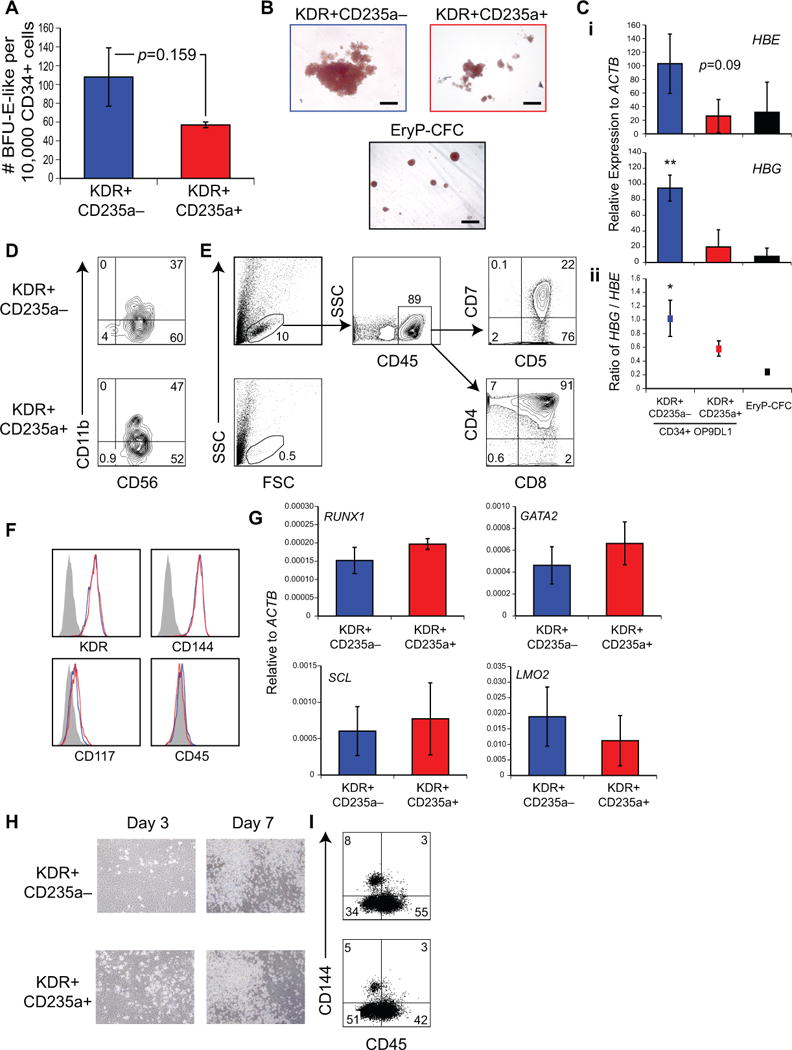
(A) Large burst-like erythroid colony-forming potential per 10,000 CD34+CD43− populations, derived as in (1F). CD34+CD43− cells derived from KDR+CD235a− (blue) or KDR+CD235a+ (red) were analyzed for erythroid colony-forming potential after 7 days of co-culture with OP9-DL1. n = 3 (Mean ± SEM). Student’s t-test p = 0.16. (B) Representative erythroid colony morphology obtained following CD34+CD43− isolation (upper panels), as in (A), or of EryP-CFC (lower panel), as in (1I). Scale bar 100 μm. (C) (i) qRT-PCR of erythroid colonies for globins HBE and HBG. n = 3. (Mean ± SD). ANOVA ** p = 0.002. (ii) Ratio of HBG/HBE expression n = 3. (Mean ± SEM). ANOVA * p = 0.04. (D-E) NK/T cell potential of CD34+CD43− populations, derived as in (1F). (D) CD34+CD43− cells were analyzed for NK cell potential after 21 days OP9-DL4 co-culture. (E) CD34+CD43− cells were analyzed for T cell potential after 30+ days OP9-DL4 co-culture. (F) Flow cytometry analysis of expression of KDR, CD144, CD117 and CD45 on CD34+CD43− populations, as in (1F). Blue; KDR+CD235a− derived, Red; KDR+CD235a+ derived. (G) qRT-PCR of RUNX1, GATA2, SCL and LMO2 on isolated CD34+CD43− populations, as in Figure (1F). n = 3. (Mean ± SD). Student’s t-test p > 0.05. (H,I) hemogenic endothelium potential of CD34+CD43− cells. KDR+CD235a− (top) and KDR+CD235a+ (bottom) mesoderm–derived CD34+CD43− cells were isolated and plated onto thin-layer-matrigel-coated plasticware for 7 days. Adherent endothelium and non-adherent hematopoietic cells were visible (H) and examined by flow cytometry analysis for CD144 and CD45 expression after 7 days (I).
As lymphoid potential is a distinguishing feature of definitive hematopoiesis1, we next analyzed each of the two aggregate-derived CD34+CD43− populations for T-lymphoid and natural killer (NK) cell potential using the OP9-DL4 co-culture assay12, 19. Both CD34+ populations efficiently gave rise to a CD56+CD11blow population, indicating that both possess NK cell potential (Figure 2D). In striking contrast, T cell potential was restricted to the KDR+CD235a− mesoderm-derived CD34+CD43− population (Figure 2E). Taken together with the above erythroid analyses, these results provide strong evidence that the KDR+CD235a− and KDR+CD235a+ mesoderm–derived CD34+ populations contain progenitors of definitive and primitive hematopoiesis, respectively.
Both programs transition through CD34+ hemogenic endothelium
Further characterization of the respective day 6 CD34+ CD43− populations revealed that both express the set of surface markers (CD144, KDR and CD117, but not CD45; Figure 2F) and transcription factors (RUNX1, GATA2, SCL and LMO2; Figure 2G) commonly used to identify hemogenic endothelium (reviewed in [4, 15]). When plated under appropriate conditions in vitro, hemogenic endothelium undergoes an endothelial-to-hematopoietic transition that can be measured by the emergence of round cells that express the pan-hematopoietic marker CD4514. Both CD34+CD43− populations gave rise to adherent monolayers within 3 days of hemogenic endothelium culture, and by day 7, non-adherent CD45+ populations were readily detected (Figure 2H,I). Collectively, these observations demonstrate that both the KDR+CD235a− and KDR+CD235a+ progenitors transition through a CD34+CD43− hemogenic endothelium stage that cannot be distinguished based on the markers currently used to identify this population15.
Wnt-β-catenin signaling regulates specification of either program
Next we investigated the role of specific signaling pathways in regulating primitive and definitive hematopoiesis with the goal of identifying strategies to promote one program or the other. We previously demonstrated that Activin-nodal signaling is required for specification of primitive hematopoiesis and that stage-specific inhibition of this pathway generates populations enriched in definitive progenitors12. However, modulation of this pathway did not negatively affect definitive hematopoietic development, indicating that other factors may regulate this program. In the initial studies, we chose to investigate Wnt-β-catenin given its role in the regulation of hematopoiesis in the mouse ESC differentiation model20–23. For these analyses, we focused on the mesoderm specification stage (day 2–3) immediately following BRACHYURY/T induction (Figure 1A). Inhibition of the pathway by the addition of the small molecule IWP224 led to 2-fold increase in the size of the CD235a+ population compared to the DMSO-treated control. In contrast, addition of the GSK-3 inhibitor CHIR99021 (CHIR), a Wnt agonist25, during the same timeframe inhibited development of the CD235a+ population (Figure 3A,B). The effect of Wnt-β-catenin signaling on the emergence of the KDR+CD235a+ population was observed with 2 hESC lines (H1 and HES2) and 1 hiPSC line (MSC-iPS1; Figure 3B), suggesting that it is a conserved mechanism for human hematopoietic specification. Analyses of hemangioblast potential showed that the IWP2-treated KDR+CD235a+ fraction was enriched for hemangioblasts, indicating that under these conditions, as in the unmanipulated cultures, CD235a expression marks the onset of primitive hematopoiesis (Figure 3C). When isolated and cultured as aggregates, the 3 different KDR+ progenitors gave rise to CD34+ cells within 24 hours of culture (Figure 3D). As expected, only the IWP2-treated KDR+CD235a+ progenitors generated a CD34+CD235a+ population. At day 6 of aggregate culture, more than 90% of the IWP2-treated KDR+CD235a+ mesoderm–derived population and almost 40% of the corresponding KDR+CD235a− mesoderm–derived population expressed CD43 (Figure 3E). Very few CD43+ cells were detected in the culture generated from the CHIR-treated KDR+CD235a− progenitors (Figure 3E), suggesting that CHIR treatment, similar to SB treatment (Figure 1I), inhibited primitive hematopoiesis. Primitive erythroid potential (EryP-CFC) correlated with the proportion of CD43+ cells and was found to be highly enriched in the population generated from the IWP2-derived KDR+CD235a+ progenitors (Figure 3F) indicating that inhibition of Wnt did not affect primitive hematopoiesis.
Figure 3. Canonical Wnt signaling specifies definitive hematopoiesis.
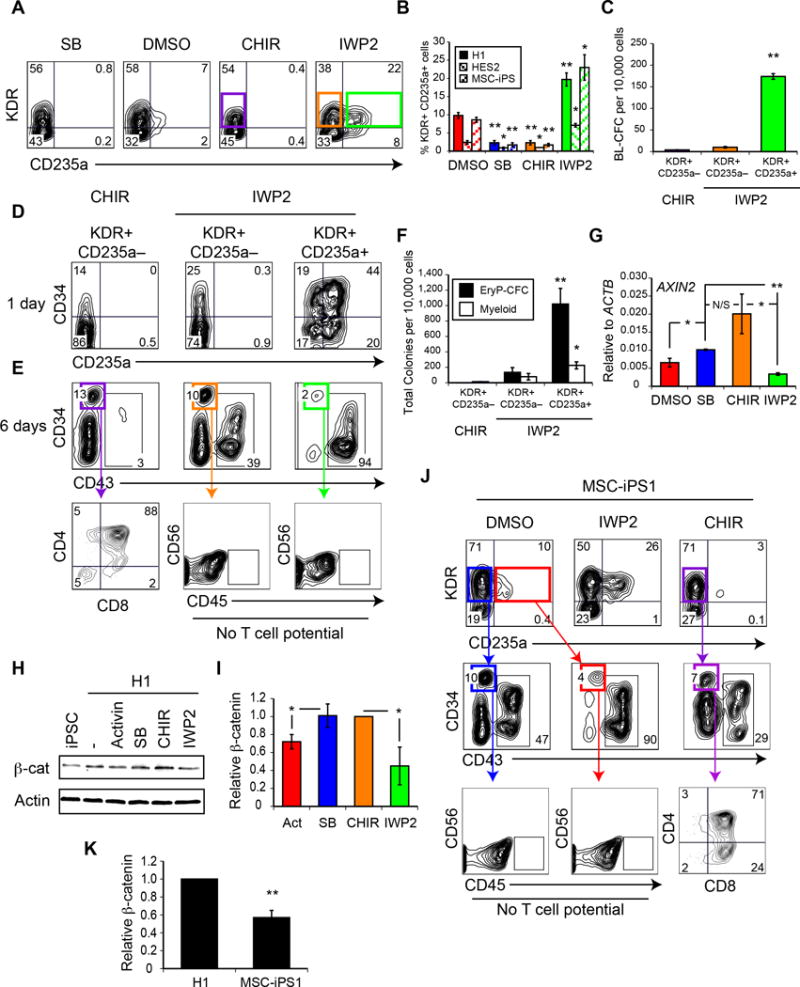
(A) Representative flow cytometric analysis of KDR and CD235a expression in day 3 of differentiation cultures, after manipulation of Wnt signaling between days 2 and 3, as in Figure 1A, by the GSK-3 inhibitor CHIR99021 (CHIR) or the Wnt-antagonist IWP2. (B) Quantification of percentage of KDR+CD235a+ cells obtained at day 3 of differentiation in 3 different hPSC lines. n = 3. (Mean ± SEM). Student’s t-test, relative to DMSO treatment * p ≤ 0.05 ** p ≤ 0.002. (C) Hemangioblast (BL-CFC) potential of the KDR+ populations, as in (A). n = 3. (Mean ± SEM). ANOVA ** p ≤ 0.0001. (D-E) Representative flow cytometry analysis of CD34 and either CD235a (D) or CD43 (E) expression of reaggregates derived from day 3 populations, as in (A). Lower panel; T-lymphoid potential of CD34+CD43− populations, as in (1F). (F) Colony-forming progenitor potential of day 9 reaggregates derived from day 3 populations, as in (A). n = 5. (Mean ± SEM). ANOVA * p ≤ 0.05 ** p ≤ 0.0001. (G) qRT-PCR of AXIN2 expression at day 3 of differentiation following different inhibitor treatment between days 2 and 3. n = 3. (Mean ± SEM). Student’s t-test * p ≤ 0.05 ** p ≤ 0.0001 N/S p = 0.144. (H–J) Western analysis (H) of nuclear β-catenin from day 3 differentiation cultures. (I) Quantification of nuclear β-catenin, as in (H) n = 3. (Mean ± SD). Student’s t-test * p ≤ 0.05. (J) T-lymphoid potential of MSC-iPS1 differentiation cultures. Upper panel; Representative KDR and CD235a expression on day 3 differentiation cultures of the hiPSC MSC-iPS1. Middle panel; Representative day 9 reaggregate flow cytometry analysis of CD34 and CD43 expression. Lower panel; T-lymphoid potential of CD34+CD43− populations, as in (1F). (K) Quantification of nuclear β-catenin in differentiation cultures of MSC-iPS1 or H1 hESC, as in (H) n = 3. (Mean ± SD). Student’s t-test ** p ≤ 0.0001.
Although Wnt-β-catenin inhibition did not alter the balance of primitive hematopoiesis between the KDR+CD235a+ and KDR+CD235a− mesoderm–derived populations, it did affect their definitive hematopoietic potential. As expected, the KDR+CD235a+ mesoderm–derived CD34+CD43− progenitors lacked T cell potential, as demonstrated by the absence of CD45+ cells in the co-culture (Figure 3E; bottom row). Surprisingly, when Wnt-β-catenin was inhibited from days 2–3, the KDR+CD235a− mesoderm–derived CD34+CD43− population was also devoid of T cell potential, suggesting that early inhibition of Wnt signaling blocks definitive hematopoietic development. Analyses of the other fractions (CD34+/−CD43+) derived following IWP2 treatment also failed to uncover any T cell potential (not shown), ruling out the possibility that inhibition of Wnt signaling induced a change in the surface marker phenotype of the definitive hematopoietic progenitors. T-lymphoid potential was detected in the CD34+CD43− population generated from KDR+CD235a− mesoderm treated with CHIR (Figure 3E). Taken together, these findings indicate that Wnt-β-catenin signaling at early stages of development plays a pivotal role in the generation of the two human hematopoietic programs, as it inhibits primitive hematopoiesis but appears to be required for specification of definitive hematopoiesis.
qRT-PCR analyses of the Wnt reporter target gene AXIN226 revealed a strong upregulation of expression after CHIR treatment, and repression after IWP2 treatment (Figure 3G), indicating that these small molecules are regulating canonical Wnt activity. Inhibition of Activin-nodal signaling with SB-431542 also led to an increase in AXIN2 expression compared to the DMSO control (Figure 3G), suggesting that endogenous Activin-nodal signaling may be affecting canonical Wnt activity. A relationship between the pathways is also supported by the observation that manipulation of both affects specification of the primitive and definitive hematopoietic programs. Western blot analyses revealed that nuclear β-catenin levels in the Activin A- or IWP2-treated day 3 embryoid bodies were significantly lower than those in the SB- or CHIR- treated cultures, further demonstrating that the Wnt-β-catenin pathway is downregulated during primitive hematopoietic specification with Activin-nodal signaling (Figure 3H,I). Manipulation of the Wnt pathway did not affect SMAD2 phosphorylation levels (not shown).
Our analyses of the day 3 populations (Figure 3B) showed that the effect of Wnt signaling on the development of the CD235a population was observed in different hPSC lines, including an iPSC line, MSC-iPS127. Given the application of iPSC technology to disease modeling and future cell therapy, we were interested in determining whether early-stage Wnt signaling would also affect definitive hematopoietic development from this iPSC line. In the absence of any manipulation of the Wnt-β-catenin pathway, we expected the KDR+CD235a− mesoderm–derived CD34+CD43− population would display T cell potential. This was not the case, however, as neither this population nor the primitive KDR+CD235a+ mesoderm–derived population contained T cell progenitors (Figure 3J; left panel). Given our observations that definitive hematopoietic specification is Wnt dependent, we speculated that these findings may be indicative of sub-optimal levels of endogenous Wnt signaling in the hiPSC-derived mesoderm. Indeed, western blot analyses revealed lower levels of nuclear β-catenin in day 3 hiPSC-derived embryoid bodies than in embryoid bodies generated from H1 (Figure 3H,K). Addition of CHIR to the hiPSC cultures between days 2 and 3 of differentiation reduced the size of the CD235a population (Figure 3B and 3J; right panel) and promoted the definitive hematopoietic fate as demonstrated by presence of T cell progenitors in the CD34+CD43− population (Figure 3J). These observations show that the lack T cell potential was due to suboptimal endogenous Wnt-β-catenin signaling in the day 2–3 population and that it is possible to ‘rescue’ definitive potential by activating the pathway during this time. Taken together, these findings further support the interpretation that specification of definitive hematopoiesis in hPSCs depends on stage-specific activation of the Wnt-β-catenin pathway.
To determine whether it is possible to direct primitive and definitive hematopoietic fates in the differentiation cultures without isolating the three KDR+ progenitors, we cultured the CHIR or IWP2-treated (days 2 – 3) embryoid bodies, as in Figure 1A, until day 9 and then assayed the entire population for EryP-CFC or the FACS isolated CD34+CD43− populations for T cell potential (Figure 4A). The cultures treated with either SB-431542 or CHIR99021 contained no CD43+ cells or EryP-CFC, indicating that these manipulations inhibited primitive hematopoiesis in the context of whole embryoid bodies (Figure 4B,C). In contrast, IWP2 treatment led to an increase in the size of the CD43+ population and the frequency of EryP-CFC in the differentiation cultures, demonstrating that inhibition of the Wnt-β-catenin pathway promotes primitive hematopoiesis in the embryoid bodies. The opposite patterns were observed for T cell potential as the CD34+CD43− population from the SB- and CHIR-treated embryoid bodies contained T-lymphoid progenitors whereas the IWP2 treated population did not (Figure 4D). These findings demonstrate that through the simple stage-specific manipulation of the Wnt-β-catenin pathway in embryoid bodies, it is possible to generate either primitive or definitive hematopoietic progenitors (Figure 4E).
Figure 4. Wnt manipulation allows for selective differentiation of primitive or definitive hematopoietic progenitors.
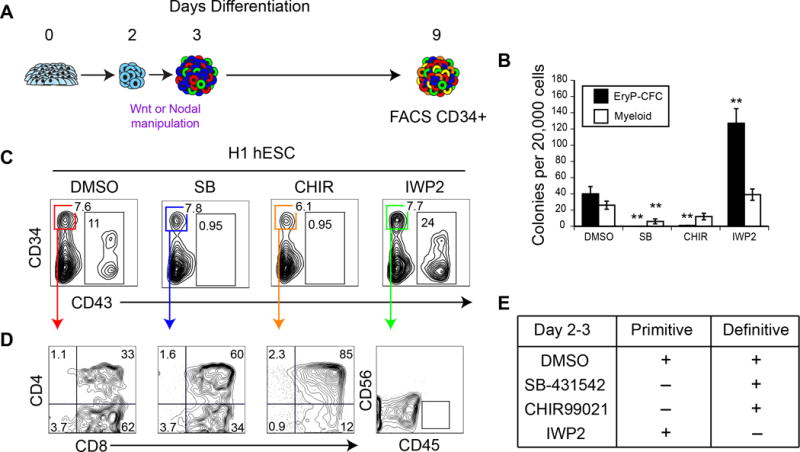
(A) Schematic for undisrupted embryoid body differentiation. hPSC are differentiated with Wnt activation or inhibition between days 2 and 3 of differentiation, followed by VEGF and cytokine supplementation until day 9, as in (1A). (B) Colony-forming progenitor potential of day 9 differentiation cultures, as in (A). n ≥ 5. (Mean ± SEM). Student’s t-test, relative to DMSO treatment * p ≤ 0.05 ** p ≤ 0.01. (C) Representative CD34 and CD43 expression of day 9 differentiation cultures, as in (A). (D) CD34+CD43− cells, as in (A) were analyzed for T cell potential after 28+ days OP9-DL4 co-culture. (E) Summary of hematopoietic potential following Wnt manipulation between days 2 and 3 of differentiation. CHIR99021 treatment prevents the specification of the primitive hematopoietic program while specifying the definitive program, while IWP2 prevents the specification of the definitive hematopoietic program while specifying the primitive hematopoietic program.
Discussion
The ability to produce HSCs from hPSCs will require methods for efficient specification of definitive hematopoietic progenitors. Here we investigated the earliest stage of hematopoietic commitment in vitro and gained the following insights that now enable us to derive populations consisting of either primitive or definitive progenitors. First, we found that expression of CD235a at day 3 of differentiation marks the KDR+ mesoderm fated to the primitive hematopoietic lineage and distinguishes it from the KDR+CD235a− mesoderm that gives rise to definitive hematopoiesis. Second, we showed that specification of the definitive program depends on Wnt-β-catenin signaling and that stage-specific manipulation of this pathway promotes definitive hematopoiesis while inhibiting primitive hematopoiesis. Third, we demonstrated that both programs transit through a hemogenic endothelial intermediate that undergoes an endothelial-to-hematopoietic transition and gives rise to CD45+ hematopoietic cells.
Together, these findings support a model (Figure 5) of human hematopoietic development in which the primitive hematopoietic program, defined by the emergence of KDR+CD235a+ mesoderm, is specified by the combination of Activin-nodal signaling and inhibition of the Wnt-β-catenin pathway, whereas specification of definitive KDR+CD235a− mesoderm is dependent on Wnt-β-catenin signaling. Within 24 hours of differentiation both mesoderm populations give rise to CD34+ cells that may represent the earliest stage of commitment to hemogenic endothelium. Following 6 days of culture (total of 9 days), the KDR+CD235a+ mesoderm generates a large CD43+ population that represents the emergence and expansion of primitive hematopoiesis, as demonstrated by the presence of EryP-CFC. While we identified CD34+CD43− hemogenic endothelium in in these reaggregation cultures after 6 days, it was clearly specified much earlier, likely within 24 hours with the emergence of the CD34+ population, as EryP-CFC are present by day 6. In this model, primitive hemogenic endothelium would generate hematopoietic progeny over a period of several days, possible generating different progenitors at different times. Hematopoietic commitment of the CHIR-treated KDR+CD235a− mesoderm did not occur in the 6-day aggregate cultures as indicated by the low number of CD43+ cells and lack of hematopoietic progenitors (Figure 3E, F). Hematopoietic potential was only revealed following culture of the day 6 CD34+ cells in hemogenic endothelial conditions (Figure 2H,I) or with Notch ligand expressing stromal cells (OP9-DL1 or OP9DL-4; Figure 2A,D,E), indicating that specification of these definitive progenitors may be Notch dependent, an interpretation consistent with previous studies showing that definitive hematopoietic development is dependent on Notch signaling (reviewed in [28]). Whether or not earlier stage CD34+ definitive populations would display similar potential remains to be determined.
Figure 5. Model of primitive and definitive hematopoietic specification from human pluripotent stem cells.
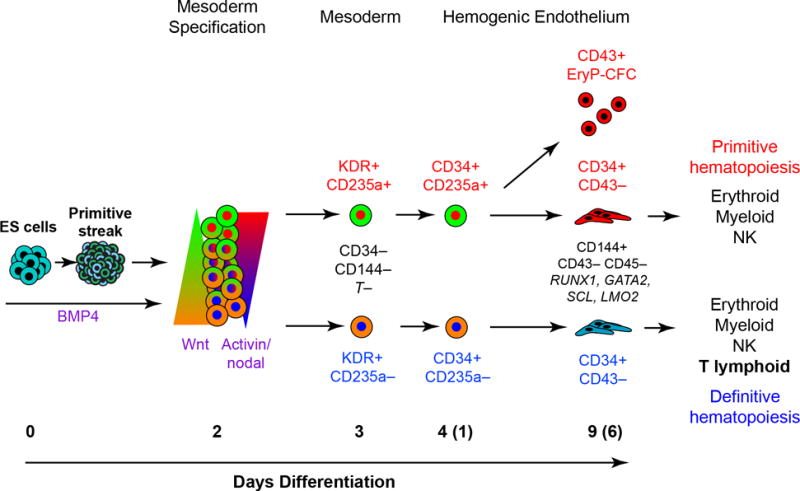
After formation of a primitive-streak-like population, KDR+CD235a+ mesoderm fated to the primitive hematopoietic lineage is specified by the combination of Activin-nodal signaling and Wnt inhibition, whereas activation of the Wnt/β-catenin specifies KDR+CD235a− definitive mesoderm. The KDR+CD235a+ mesoderm gives rise to a rapid burst of primitive hematopoiesis, marked by the emergence of a CD43+ population and the development of EryP-CFCs by day 6 of culture (9 days total). In contrast, the KDR+CD235a− mesoderm does not generate hematopoietic progeny during this timeframe. Following 6 days of culture, both mesodermal populations give rise to CD34+CD43− cells that expresses common markers of hemogenic endothelium, and display multi-lineage hematopoietic potential. However, only the KDR+CD235a− mesoderm–derived CD34+CD43− population shows T-lymphoid potential.
Hematopoietic development in various model organisms is typically thought to occur in distinct, successive ‘waves’ (reviewed in [1]). The observations that the primitive KDR+CD235a+ and definitive KDR+CD235a− mesoderm is specified at the same time within 72 hours of the onset of differentiation are not consistent with an exclusively temporal model of human hematopoietic development. Rather, they are more in line with findings from Runx1-Cre lineage tracing studies in the mouse that demonstrated HSC contribution from yolk sac progenitors specified as early as E7.5, a stage overlapping with the emergence of the primitive hematopoietic program29, 30. The ability to separate the primitive and definitive hematopoietic lineages at the KDR+ mesoderm stage of development has enabled us to monitor the progression of these two programs and demonstrate that they both transition through hemogenic endothelium intermediates that can be distinguished only on the basis of T-lymphoid potential, as both generate erythroid, myeloid (not shown) and NK lineage cells, and both display surface marker phenotypes and gene expression patterns currently used to define hemogenic endothelium. The observation that both KDR+ progenitors generated cells with a NK phenotype provides further evidence for the existence of distinct primitive and definitive NK cells, a concept supported by previous studies31, 32. The erythroid colonies generated from the two populations expressed different ratios of HBE and HBG globin, suggesting that the colony-forming cells they derived from represented different stages of human erythroid development. Although the expression pattern of the KDR+CD235a+ mesoderm–derived colonies is indicative of primitive erythroblasts, the pattern of the colonies generated from the KDR+CD235a− mesoderm is not consistent with the next stage of erythroid development, the fetal stage, as they retain high levels of HBE. While unexpected, this pattern may be indicative of a transition stage in human embryonic and fetal erythropoiesis as colonies generated from human 6-week fetal liver also co-express HBE and HBG globin33. The lack of markers to distinguish the primitive and definitive CD34+CD43− populations demonstrates that the identification of a hemogenic endothelium–like population without documentation of T-lymphoid potential can no longer be used as an indication of definitive hematopoiesis. It also raises the distinct possibility that hemogenic endothelium–like populations isolated from hPSC-differentiation cultures on the basis of existing markers such as CD34, CD144 and CD45 are mixtures of primitive and definitive hematopoietic cells.
The findings that Wnt-β-catenin signaling inhibits primitive hematopoiesis are consistent with the recent observations that activation of the pathway at early stages in hPSC differentiation cultures led to a reduction in hemangioblast potential and inhibition of primitive erythroid potential, respectively34, 35. This effect of Wnt-β-catenin signaling in the human system appear to be opposite to those described in the mouse ESC model, in which activation of the pathway during early mesoderm specification is required for establishment of the primitive program22. However, these differences may reflect subtle differences in the stage at which the populations were analyzed, as Lui et al. recently demonstrated that transient Wnt inhibition via Er71 expression is required for specification of murine primitive hemangiogenic mesoderm at a stage comparable to that evaluated in our study in the human cultures36. In addition to a role at the early mesoderm specification stage, several recent studies have demonstrated that Wnt signaling functions at the level of HSC development in the aorta-gonad-mesonephros region of the developing mouse embryo. We found that inhibition of the Wnt-β-catenin pathway by retinoic acid signaling was necessary for HSC development from hemogenic endothelium37, whereas the study of Ruiz-Herguido et al. provided evidence that Wnt signaling was required at a stage prior to HSC specification, possibly at the level of hemogenic endothelium development38. Collectively, these observations establish a role for Wnt signaling at multiple stages of definitive hematopoietic development, ranging from induction of the program to specification and/or maturation of the HSC.
Notably, endogenous levels of Wnt-β-catenin signaling in some hPSC lines such as H1 hESCs appear to be sufficient for specification of the definitive hematopoietic program, whereas in others they may be too low to establish this fate. Given the variability of endogenous Wnt signaling across different hPSCs lines39, these findings highlight the importance of establishing appropriate levels of Wnt-β-catenin signaling during the mesoderm specification step for generating the definitive hematopoietic program. With these observations, it is now possible, through the stage-specific manipulation of the Wnt-β-catenin pathway, to routinely generate hPSC-derived populations containing definitive but not primitive hematopoietic progenitors. Attempts to repopulate recipient animals with enriched hPSC-derived CD34+ definitive progenitors have not yet been successful, suggesting these cells may represent pre-HSCs that require additional maturation signals to engraft an animal. Progenitors with T cell potential have been identified in the mouse embryo at stages before the emergence of HSCs40, 41. Although the origin of these T-lymphoid cells has not yet been established, it is possible they derive from progenitors comparable to the hPSC-derived CD34+ hemogenic endothelium and similarly require additional signals to mature. hPSC-derived definitive hematopoietic progenitors, generated through the strategy described in this study, represent ideal targets for defining the pathways that regulate the generation of HSCs from the CD34+ hemogenic endothelium stage cells.
Methods
Methods and any associated references are available in the online version of this paper.
Maintenance and differentiation of human ES and iPS cells
The hESC lines H11 and HES-2, and human iPS cell line MSC-iPS12 were maintained on irradiated mouse embryonic fibroblasts in hESC media as described previously3. For differentiation, hPSC were cultured on matrigel-coated plasticware (BD Biosciences, Bedford, MA) for 24 hours, followed by embryoid body generation, as described previously4. Briefly, hPSCs dissociated with sequential collagenase B (1 mg/mL) and trypsin-EDTA (0.05%) treatment, followed by scraping. Aggregates were resuspended in SFD5 supplemented with L-glutamine (2 mM), ascorbic acid (1 mM), monothioglycerol (MTG, 4×10−4 M; Sigma), transferrin (150 μg/mL), and BMP-4 (10 ng/mL), bFGF (5 ng/mL), Activin A, SB-431542 (6 μM), CHIR99021 (3 μM), and/or IWP2 (3 μM). On the third day of differentiation, embryoid bodies were changed to StemPro-34 media supplemented as above, and VEGF (15 ng/mL), IL-6 (10 ng/mL), IGF-1 (25 ng/mL), IL-11 (5 ng/mL), SCF (50 ng/mL), EPO (2 U/mL final), TPO (30 ng/mL), IL-3 (30 ng/mL) and Flt-3L (10 ng/mL) were added as indicated. Cultures were maintained in a 5% CO2/5% O2/90% N2 environment for the first 8 days and then transferred to a 5% CO2/air environment. All recombinant factors are human and were purchased from R&D Systems (Minneapolis, MN).
Flow Cytometry and Cell Sorting
The following antibodies were used for these studies: KDR (clone 89106), CD235a-APC (clone HIR2), CD4-PE-Cy7 (clone RPA-T4), CD5-PE-Cy7 (clone L17F12), CD7-FITC (clone M-T701), CD8-PE (clone RPA-T8), CD34-APC (clone 8G12), CD34-PE-CY7 (clone 4H11), CD43-PE or FITC (clone 1G10), CD45-APC-eFluor750 (clone 2D1), CD56-PE or APC (clone B159), CD117-APC (clone 104D2), CD144-PE (clone 123413). All antibodies were purchased from BD Biosciences (San Diego, CA) except CD34-PE-CY7 which was purchased from eBioscience (San Diego, CA), and KDR was purchased from R&D systems. Cells were sorted with FACSAria™II (BD) cell sorter at the Sick Kids/UHN Flow Cytometry Facility.
Hemogenic Endothelium analysis
2×104 CD34+CD43− cells were cultured for 7 days on thin-layer matrigel coated plasticware in StemPro-34 media, supplemented as described above, with an additional 10 ng/mL BMP4.
OP9-DL1 co-culture for BFU-E-like analysis
Isolated CD34+CD43− cells were cultured at a concentration of 2×104 cells per well on irradiated OP9-DL1 monolayers in OP9 media with VEGF (5 ng/mL), TPO (30 ng/mL), SCF (50 ng/mL), Flt3 (10 ng/mL), IL-11 (5 ng/mL), and BMP-4 (10 ng/mL) in 24-well plates for 7 days. Cells were harvested and plated in 1% methylcellulose as described below.
Hemangioblast Colony Assay
Analysis of hemangioblast colony forming potential was performed by plating 1×104 cells in 1% methylcellulose supplemented with 10% FCS (Atlas), L-glutamine (2 mM), ascorbic acid (1 mM), monothioglycerol (4×10−4 M), transferrin (150 μg/mL), 20% D4T endothelial cell-conditioned medium, bFGF (10 ng/mL), VEGF (10 ng/mL), SCF (100 ng/mL), IL-6 (20 ng/mL), EPO (2U/mL), IL-11 (5 ng/mL), IL-3 (40 ng/mL) and IGF-1 (25 ng/mL), as described in detail previously3. Colonies were quantified after 6 days of culture.
Hematopoietic Colony Assay
Analysis of hematopoietic colony potential was performed by plating 2×104 cells in 1% methylcellulose supplemented with 10% plasma-derived serum (Animal Technologies), 5% protein-free hybridoma media (Invitrogen), L-glutamine (2 mM), SCF (100 ng/mL), EPO (2U/mL), IL-6 (5 ng/mL), IL-3 (40 ng/mL), TPO (40 ng/mL), IL-11 (5 ng/mL), IGF-1 (25 ng/mL) and GM-CSF (1 ng/mL), as described in detail previously3. Colonies were quantified after 10–14 days.
OP9-DL4 co-culture for NK/T-lineage differentiation
OP9 cells expressing Delta-like 4 (OP9-DL4) were generated and described previously6, 7. 5–20 × 104 isolated CD34+CD43− cells were added to individual wells of a 6-well plate containing OP9-DL4 cells, and cultured with: T cells: rhFlt-3L (5 ng/mL) and rhIL-7 (5 ng/mL); NK cells: rhFlt-3L (5 ng/mL) and rhIL-15 (10 ng/mL) (Peprotech, Rocky Hill, NJ). rhSCF (100 ng/mL) was added to both cultures for the first 6 days. Every five days co-cultures were transferred onto fresh OP9-DL4 cells by vigorous pipetting and passaging through a 40μm cell strainer. Cells were analyzed by flow cytometry on the days indicated. Populations scored as positive yielded greater than 100 gated CD45+ events.
Acknowledgments
We would like to thank the SickKids–UHN Flow Cytometry Facility for their expert assistance with cell sorting, in particular A. Khandani. This work was supported by National Institutes of Health grants U01 HL100395 and CIHR MOP93569 to G.K and CIHR (HOP83070; MOP12927). C.M.S. and A.D. were supported by the McMurrich Post-Doctoral Fellowship and the Magna-Golftown Post-Doctoral Fellowship, respectively.
Footnotes
Author Contributions: C.M.S., A.D., G.A., M.K and G.K all participated in the design of the experiments. C.M.S, A.D., G.A. and M.K performed the experiments. C.M.S. and G.K. wrote the manuscript.
Competing Financial Interests: The authors declare no competing financial interests.
References
- 1.Medvinsky A, Rybtsov S, Taoudi S. Embryonic origin of the adult hematopoietic system: advances and questions. Development (Cambridge, England) 2011;138:1017–1031. doi: 10.1242/dev.040998. [DOI] [PubMed] [Google Scholar]
- 2.Huber TL, Kouskoff V, Fehling HJ, Palis J, Keller G. Haemangioblast commitment is initiated in the primitive streak of the mouse embryo. Nature. 2004;432:625–630. doi: 10.1038/nature03122. [DOI] [PubMed] [Google Scholar]
- 3.Lancrin C, et al. The haemangioblast generates haematopoietic cells through a haemogenic endothelium stage. Nature. 2009;457:892–895. doi: 10.1038/nature07679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antas VI, Al-Drees MA, Prudence AJ, Sugiyama D, Fraser ST. Hemogenic endothelium: a vessel for blood production. The international journal of biochemistry & cell biology. 2013;45:692–695. doi: 10.1016/j.biocel.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 5.Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132:661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 6.Smith AG. Embryo-derived stem cells: of mice and men. Annual review of cell and developmental biology. 2001;17:435–462. doi: 10.1146/annurev.cellbio.17.1.435. [DOI] [PubMed] [Google Scholar]
- 7.Choi K, Kennedy M, Kazarov A, Papadimitriou JC, Keller G. A common precursor for hematopoietic and endothelial cells. Development (Cambridge, England) 1998;125:725–732. doi: 10.1242/dev.125.4.725. [DOI] [PubMed] [Google Scholar]
- 8.Kennedy M, D’Souza SL, Lynch-Kattman M, Schwantz S, Keller G. Development of the hemangioblast defines the onset of hematopoiesis in human ES cell differentiation cultures. Blood. 2007;109:2679–2687. doi: 10.1182/blood-2006-09-047704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fraser ST. The Modern Primitives: Applying New Technological Approaches to Explore the Biology of the Earliest Red Blood Cells. ISRN hematology. 2013;2013:568928. doi: 10.1155/2013/568928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clarke RL, et al. The expression of Sox17 identifies and regulates haemogenic endothelium. Nature cell biology. 2013;15:502–510. doi: 10.1038/ncb2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Irion S, et al. Temporal specification of blood progenitors from mouse embryonic stem cells and induced pluripotent stem cells. Development (Cambridge, England) 2010;137:2829–2839. doi: 10.1242/dev.042119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kennedy M, et al. T lymphocyte potential marks the emergence of definitive hematopoietic progenitors in human pluripotent stem cell differentiation cultures. Cell reports. 2012;2:1722–1735. doi: 10.1016/j.celrep.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Dubois NC, et al. SIRPA is a specific cell-surface marker for isolating cardiomyocytes derived from human pluripotent stem cells. Nature biotechnology. 2011;29:1011–1018. doi: 10.1038/nbt.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi KD, et al. Identification of the hemogenic endothelial progenitor and its direct precursor in human pluripotent stem cell differentiation cultures. Cell reports. 2012;2:553–567. doi: 10.1016/j.celrep.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slukvin II. Deciphering the hierarchy of angiohematopoietic progenitors from human pluripotent stem cells. Cell cycle (Georgetown, Tex. 2013;12:720–727. doi: 10.4161/cc.23823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pearson S, Sroczynska P, Lacaud G, Kouskoff V. The stepwise specification of embryonic stem cells to hematopoietic fate is driven by sequential exposure to Bmp4, activin A, bFGF and VEGF. Development (Cambridge, England) 2008;135:1525–1535. doi: 10.1242/dev.011767. [DOI] [PubMed] [Google Scholar]
- 17.Vodyanik MA, Thomson JA, Slukvin II. Leukosialin (CD43) defines hematopoietic progenitors in human embryonic stem cell differentiation cultures. Blood. 2006;108:2095–2105. doi: 10.1182/blood-2006-02-003327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sankaran VG, Xu J, Orkin SH. Advances in the understanding of haemoglobin switching. British journal of haematology. 2010;149:181–194. doi: 10.1111/j.1365-2141.2010.08105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.La Motte-Mohs RN, Herer E, Zuniga-Pflucker JC. Induction of T-cell development from human cord blood hematopoietic stem cells by Delta-like 1 in vitro. Blood. 2005;105:1431–1439. doi: 10.1182/blood-2004-04-1293. [DOI] [PubMed] [Google Scholar]
- 20.Davis RP, et al. Targeting a GFP reporter gene to the MIXL1 locus of human embryonic stem cells identifies human primitive streak-like cells and enables isolation of primitive hematopoietic precursors. Blood. 2008;111:1876–1884. doi: 10.1182/blood-2007-06-093609. [DOI] [PubMed] [Google Scholar]
- 21.Jackson SA, Schiesser J, Stanley EG, Elefanty AG. Differentiating embryonic stem cells pass through ’temporal windows’ that mark responsiveness to exogenous and paracrine mesendoderm inducing signals. PloS one. 2010;5:e10706. doi: 10.1371/journal.pone.0010706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nostro MC, Cheng X, Keller GM, Gadue P. Wnt, activin, and BMP signaling regulate distinct stages in the developmental pathway from embryonic stem cells to blood. Cell stem cell. 2008;2:60–71. doi: 10.1016/j.stem.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sumi T, Tsuneyoshi N, Nakatsuji N, Suemori H. Defining early lineage specification of human embryonic stem cells by the orchestrated balance of canonical Wnt/beta-catenin, Activin/Nodal and BMP signaling. Development (Cambridge, England) 2008;135:2969–2979. doi: 10.1242/dev.021121. [DOI] [PubMed] [Google Scholar]
- 24.Chen B, et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nature chemical biology. 2009;5:100–107. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Polychronopoulos P, et al. Structural basis for the synthesis of indirubins as potent and selective inhibitors of glycogen synthase kinase-3 and cyclin-dependent kinases. Journal of medicinal chemistry. 2004;47:935–946. doi: 10.1021/jm031016d. [DOI] [PubMed] [Google Scholar]
- 26.Jho EH, et al. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Molecular and cellular biology. 2002;22:1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park IH, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 28.Bigas A, Robert-Moreno A, Espinosa L. The Notch pathway in the developing hematopoietic system. The International journal of developmental biology. 2010;54:1175–1188. doi: 10.1387/ijdb.093049ab. [DOI] [PubMed] [Google Scholar]
- 29.Samokhvalov IM, Samokhvalova NI, Nishikawa S. Cell tracing shows the contribution of the yolk sac to adult haematopoiesis. Nature. 2007;446:1056–1061. doi: 10.1038/nature05725. [DOI] [PubMed] [Google Scholar]
- 30.Tanaka Y, et al. Early ontogenic origin of the hematopoietic stem cell lineage. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:4515–4520. doi: 10.1073/pnas.1115828109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Phillips JH, et al. Ontogeny of human natural killer (NK) cells: fetal NK cells mediate cytolytic function and express cytoplasmic CD3 epsilon, delta proteins. The Journal of experimental medicine. 1992;175:1055–1066. doi: 10.1084/jem.175.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tavian M, Robin C, Coulombel L, Peault B. The human embryo, but not its yolk sac, generates lympho-myeloid stem cells: mapping multipotent hematopoietic cell fate in intraembryonic mesoderm. Immunity. 2001;15:487–495. doi: 10.1016/s1074-7613(01)00193-5. [DOI] [PubMed] [Google Scholar]
- 33.Peschle C, et al. Embryonic—Fetal Hb switch in humans: studies on erythroid bursts generated by embryonic progenitors from yolk sac and liver. Proceedings of the National Academy of Sciences of the United States of America. 1984;81:2416–2420. doi: 10.1073/pnas.81.8.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gertow K, et al. WNT3A Promotes Hematopoietic or Mesenchymal Differentiation from hESCs Depending on the Time of Exposure. Stem cell reports. 2013;1:53–65. doi: 10.1016/j.stemcr.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paluru P, et al. The negative impact of Wnt signaling on megakaryocyte and primitive erythroid progenitors derived from human embryonic stem cells. Stem cell research. 2013;12:441–451. doi: 10.1016/j.scr.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu F, et al. ER71 specifies Flk-1+ hemangiogenic mesoderm by inhibiting cardiac mesoderm and Wnt signaling. Blood. 2012;119:3295–3305. doi: 10.1182/blood-2012-01-403766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chanda B, Ditadi A, Iscove NN, Keller G. Retinoic acid signaling is essential for embryonic hematopoietic stem cell development. Cell. 2013;155:215–227. doi: 10.1016/j.cell.2013.08.055. [DOI] [PubMed] [Google Scholar]
- 38.Ruiz-Herguido C, et al. Hematopoietic stem cell development requires transient Wnt/beta-catenin activity. The Journal of experimental medicine. 2012;209:1457–1468. doi: 10.1084/jem.20120225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang W, Zhang D, Bursac N, Zhang Y. WNT3 Is a Biomarker Capable of Predicting the Definitive Endoderm Differentiation Potential of hESCs. Stem Cell Reports. 2013;1:46–52. doi: 10.1016/j.stemcr.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoshimoto M, et al. Autonomous murine T-cell progenitor production in the extra-embryonic yolk sac before HSC emergence. Blood. 2012;119:5706–5714. doi: 10.1182/blood-2011-12-397489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boiers C, et al. Lymphomyeloid contribution of an immune-restricted progenitor emerging prior to definitive hematopoietic stem cells. Cell stem cell. 2013;13:535–548. doi: 10.1016/j.stem.2013.08.012. [DOI] [PubMed] [Google Scholar]
References
- 1.Thomson JA, et al. Embryonic stem cell lines derived from human blastocysts. Science (New York, NY. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 2.Park IH, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 3.Kennedy M, D’Souza SL, Lynch-Kattman M, Schwantz S, Keller G. Development of the hemangioblast defines the onset of hematopoiesis in human ES cell differentiation cultures. Blood. 2007;109:2679–2687. doi: 10.1182/blood-2006-09-047704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kennedy M, et al. T lymphocyte potential marks the emergence of definitive hematopoietic progenitors in human pluripotent stem cell differentiation cultures. Cell reports. 2012;2:1722–1735. doi: 10.1016/j.celrep.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Sturgeon CM, et al. Primitive erythropoiesis is regulated by miR-126 via nonhematopoietic Vcam-1+ cells. Developmental cell. 2012;23:45–57. doi: 10.1016/j.devcel.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 6.La Motte-Mohs RN, Herer E, Zuniga-Pflucker JC. Induction of T-cell development from human cord blood hematopoietic stem cells by Delta-like 1 in vitro. Blood. 2005;105:1431–1439. doi: 10.1182/blood-2004-04-1293. [DOI] [PubMed] [Google Scholar]
- 7.Schmitt TM, et al. Induction of T cell development and establishment of T cell competence from embryonic stem cells differentiated in vitro. Nature immunology. 2004;5:410–417. doi: 10.1038/ni1055. [DOI] [PubMed] [Google Scholar]


