Abstract
To support effective host defense, the T cell repertoire must balance breadth of recognition with sensitivity for antigen. The concept that T lymphocytes are positively selected in the thymus is established, but this achieves such a repertoire has not been resolved. Here we suggest that it is direct linkage between self and foreign antigen recognition that produces the necessary blend of T cell receptor (TCR) diversity and specificity in the mature peripheral repertoire, enabling responses to a broad universe of unpredictable antigens while maintaining an adequate number of highly sensitive T cells in a population of limited size. Our analysis also helps to explain how diversity and frequency of antigen-reactive cells in a T cell repertoire are adjusted in animals of vastly different size scale to enable effective anti-pathogen responses and suggest a possible binary architecture in the TCR repertoire that is divided between germline-related optimal binding and diverse recognition.
Introduction
For T lymphocytes to carry out their adaptive immune function, each must respond to antigen with exquisite specificity, yet as a population be able to recognize an enormous diversity of ligands. Both of these features are conferred on the population of αβ T cells in an individual by the repertoire of antigen specific receptors they express, with (for the most part) each lymphocyte bearing a unique T cell receptor (TCR) generated by a quasi-random process of somatic recombination of V(D)J gene segments and nucleotide insertion (Jung and Alt, 2004). This process can produce an excess of 1015 different TCR sequence combinations (Davis and Bjorkman, 1988; Murugan et al., 2012; Zarnitsyna et al., 2013). As the human naïve T cell pool contains ~1011 T cells and that of mice ~108 cells, it is clear that the entire combinatorial diversity of TCR sequences cannot be represented within the T cell repertoire (see Box 1) of one individual. Thus, a subset of all possible TCRs must be sufficient to provide an effective T cell contribution to pathogen resistance. It has been presumed that the primary feature of a T cell repertoire that supports this capacity is the enormous and random diversity of the TCRs represented (Davis and Bjorkman, 1988). However, the diversity of the mature T cell repertoire is far from random and additional factors are important to ensure that the population of T cells present in an individual constitutes an effective repertoire with which to prevent pathogen persistence and spread. Here we discuss what constitutes the structure of a useful T cell repertoire and use recently reported findings to suggest a new view of how thymic selection contributes to this goal.
Translating the Protecton Theory to T Cells
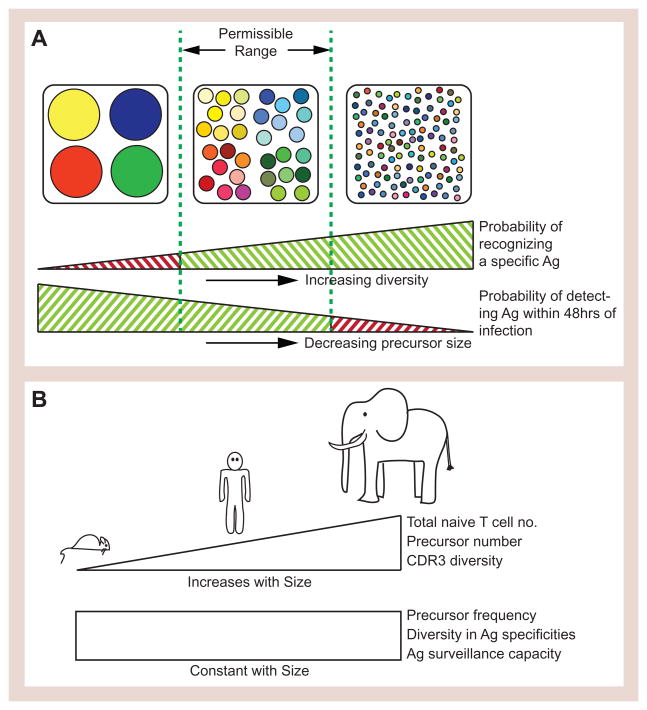
Conventional CD4+ and CD8+ T cells expressing αβ receptors recognize protein antigens in the form of a short peptide bound to host-encoded major histocompatibility complex molecules present on the cell surface (pMHC). T cells survey the body for MHC-presented antigen by continuously recirculating between blood and secondary lymphoid organs, the sites where T cell responses are initiated (von Andrian and Mempel, 2003). This mobility greatly improves the chances that a given T cell will encounter pMHC ligands matching its TCR. However, to rapidly detect rare antigen early in an infection, not only does an antigen-specific T cell have to be present in the T cell pool, a minimum number of T cells of a given specificity is also required. Given the size constraints of a T cell population, a tradeoff therefore arises. As the precursor frequency of a given antigen specificity increases, the time to detection of this antigen will decrease, but so will the overall diversity of the T cell repertoire, and the correct balance of frequency and diversity is needed for optimal immune function (Figure 1A). In contrast to what would be predicted if maximizing diversity was of prime importance, humans and mice have a similar precursor frequency of 1–100 cells per million naïve CD4+ or CD8+ T cells specific for a particular pMHC ligand despite the fact that humans have a higher absolute number of T cells than mice (Jenkins and Moon, 2012; Su et al., 2013). This highlights the scalability of the T cell repertoire with body size. The basic protective unit of a B cell repertoire, the “Protecton”, is defined as the number of B cells that allows timely production of sufficient amount of antibody/per unit of volume for efficient antigen neutralization and removal (Langman and Cohn, 1987). Larger animals with higher blood volumes need more cells of the same specificity to produce the same effective concentration of antibody in a similar time frame. Applying this to T cells, a Protecton is the number of T cells with a particular antigen specificity required to provide, in a timely manner, enough effectors per unit of body volume to effectively combat pathogens. The number of repetitions of the Protecton observed in each animal is therefore dependent on the absolute size of its T cell pool. The absolute number of precursors for each specificity will vary by orders of magnitude between an elephant and a mouse (Wiegel and Perelson, 2004). The Protecton concept predicts that the functional (antigen-specific) diversity and relative precursor frequency of T cells will be approximately the same in both species (Figure 1B).
Figure 1. Features of an effective T cell repertoire.
(A) Due to the fixed size of an individual’s naive T cell pool, a tradeoff between T cell diversity and the number of cells present of a given specificity (precursor number) arises. If the TCR repertoire is too diverse, the precursor frequency for a given specificity becomes so low that the response time to a replicating pathogen would be too slow. But if the repertoire is not diverse enough then foreign antigens may be missed entirely. The minimum unit repertoire that achieves an optimal balance of these two parameters is termed the Protecton. (B) To maintain an equal probability of successfully detecting a foreign antigen across species with vastly different body sizes, the absolute number of naïve T cells with a given TCR specificity has to be greater in larger animals to maintain the Protecton, but the precursor frequency for a given antigen stays constant as body size increases.
The T cell repertoire is not only structured to optimize both precursor frequency and diversity. Analysis of TCR sequences present within different individuals have demonstrated that the T cell repertoire is not a random sample of possible TCRs. TCR deep sequencing studies show a substantial degree of overlap among individuals bearing the same MHC alleles in the TCR sequences that contribute to a given antigen response, so-called “public” TCR clonotypes (Miles et al., 2011). Given the vast number of possible TCR sequences that can be generated during T cell development, strictly random inclusion of a subset of TCR into the mature T cell repertoire would make such overlap extremely unlikely, suggesting that there are active forces shaping the TCR sequence landscape of mature lymphocytes.
What mechanism(s) operate to establish the set of TCRs that fulfill the requirements of a Protecton and explain biased TCR patterns in the naïve repertoire? As the sequence of the TCR is fixed for the lifetime of the cell, the selection of useful TCRs has to take place prior to encounter with antigen and in the absence of experience of what these (foreign) antigens might be. In fact, it has long been appreciated that TCR interactions with self-peptide MHC (self-pMHC) in the thymus during T cell development provide the filtering criteria by which TCRs are selected. However, why this is an effective method to establish a useful T cell repertoire has been a matter of considerable debate.
Self-ligands and the selection of a T cell repertoire
Developing T cells undergo a filtering process in the thymus that establishes the particular T cell repertoire of an individual. Crucially, life or death decisions are dictated by the strength of TCR signaling resulting from interaction with the ensemble of pMHC present in the thymus, a ligand pool that is composed primarily of self-pMHC (Starr et al., 2003). The CD4+CD8+ double positive (DP) cells with a mature αβ TCR that pass the selection criteria develop into several subpopulations, the majority of which are MHC I-restricted CD8+ or MHC II-restricted CD4+ single positive (SP) cells. Only ~3–5% of (DP) thymocytes complete this maturation process (Scollay et al., 1980). DP or SP thymocytes with too great a reactivity for self-pMHC are induced to undergo apoptosis, a process termed negative selection or clonal deletion. Negative selection ensures that many (but not all) thymocytes whose TCRs respond too strongly to self-pMHC and thus present a risk of autoimmunity are purged from the repertoire (Palmer, 2003). It is estimated that between 3–30% of developing thymocytes are removed this way (Daley et al., 2013; Merkenschlager et al., 1997; Stritesky et al., 2013; van Meerwijk et al., 1997).
Paradoxically, while T cells that obtain too strong signals from self-pMHC are removed, those T cells that survive and complete their developmental program require a productive, weaker, signal from interaction with self-pMHC to do so. T cells incapable of such signaling die by ‘neglect’ and this requirement for a survival signal from TCR recognition of self-pMHC is known as positive selection. While the experimental evidence for positive selection is overwhelming, just what this process contributes to a useful T cell repertoire has been hard to establish. Experiments more than 40 years ago demonstrated that T cells respond maximally to foreign antigens displayed by cells that express the MHC haplotype of the host, now known to be specifically the MHC molecules present on the thymic stroma and involved in positive selection (Lo and Sprent, 1986; Rosenthal and Shevach, 1973; Zinkernagel and Doherty, 1974a, b). This feature of T cell antigen recognition was initially called MHC restriction because the allelic form of an MHC molecule ‘restricted’ the response to a nominal foreign antigen. From these classic experiments it was suggested that the process of positive selection provides a mechanism to ensure that only those thymocytes capable of recognizing antigen presented by the particular MHC alleles present in an individual would mature and occupy the T cell niche in the peripheral immune system (Bevan, 1977; Zinkernagel et al., 1978). This hypothesis was proposed prior to conclusive evidence that the specificity of the TCR for peptide and MHC is mediated by a single receptor (Kappler et al., 1981). Despite the fact that we now understand that the TCR has such a dual specificity for a conjoint, unimolecular pMHC ligand (Babbitt et al., 1985) the MHC restriction hypothesis has remained the predominant textbook explanation for positive selection.
After the molecular cloning of the antigen recognition (αβ) components of the TCR (Allison et al., 1982; Hedrick et al., 1984; Kappler et al., 1983) and characterizations of the structure of the TCR interacting with pMHC-I or pMHC-II (Garboczi et al., 1996; Reinherz et al., 1999), the MHC restriction hypothesis in which positive selection enforced MHC allelic specificity became difficult to understand as a fundamental model. Each TCR chain has 3 complementarity-determining regions (CDRs) that form the pMHC binding site. Of these, it is primarily, but not exclusively, CDR1 and 2 that engage the MHC molecule (Garcia et al., 2009). Given that CDR1 and 2 are germline encoded by the variable (V) gene segments, a prediction of the MHC restriction hypothesis in its simplest form is that there would be a substantial concordance between the MHC alleles of an individual and the Vα and Vβ gene segments predominantly expressed by mature T cells. However, such allele-specific, biased Vα and Vβ gene segment usage patterns is rarely been observed, while biased V segment usage for MHC-I versus MHC-II binding is well-documented (Garcia et al., 2009; Marrack et al., 2008; Sim et al., 1998). CDR1 and 2 do have conserved residues that play a role in MHC binding, but these interactions involve non-polymorphic, evolutionarily conserved residues on the MHC molecule (Scott-Browne et al., 2011; Yin et al., 2012). It is thought to be the two non-germline encoded CDR3s that contribute the most to the recognition of the peptide in the MHC binding groove, although the energy contributed to the interaction by these contacts varies among TCR-pMHC pairs studied (Borg et al., 2005; Burrows et al., 2010; Piepenbrink et al., 2013). Furthermore, as alloreactivity clearly demonstrates, TCRs are quite capable of specifically recognizing and responding to peptide presented by MHC alleles other than those on which they were selected.
A number of studies made clear that positive selection goes beyond simply selecting for T cells capable of making a functional interaction with pMHC and productively signaling. The self-peptides presented by MHC molecules that promote positive selection are not just bland space-fillers. In fact, not only is positive selection highly peptide-specific, as shown by studies that have identified only very few self-peptides that can select a particular TCR (Ebert et al., 2009; Lo et al., 2009), but the complexity of the peptide pool on which T cells are selected impacts the TCR repertoire and the foreign antigen recognition specificities that are represented. This was shown in vitro using fetal thymic organ cultures (Hogquist et al., 1993) and was also apparent from in vivo studies. In mice manipulated to present predominantly a single peptide by MHC-II molecules, CD4+ T cell numbers were still ~20% of a normal naïve CD4+ T cell pool and substantial diversity was nonetheless observed, initially seeming to contradict the idea that the self-pMHCs determined the diversity in foreign pMHC specificities (Ignatowicz et al., 1996; Surh et al., 1997; Tourne et al., 1997). However, the repertoire in such mice was unusually MHC-II reactive, was missing particular antigen specificities, and a residual amount of diversity in the pool of self peptides presented was key to thymic CD4+ T cell selection in these mice (Barton and Rudensky, 1999; Grubin et al., 1997; Lucas and Germain, 1996; Sant’Angelo et al., 1997; Surh et al., 1997). Furthermore, the description of unique proteolysis mechanisms that generate peptides in the thymus specialized for positive selection also emphasizes the importance of the composition of the self-peptide pool for the generation of a useful complement of TCRs (Viret et al., 2011; Xing et al., 2013).
A direct relationship between self- and agonist-pMHC binding by the TCR
So why positively select T cells if direct allele-specific MHC matching of the TCR is not at the core of the process, with the goal of filling the peripheral repertoire with ‘useful’ (self-MHC ‘restricted’) T cells? One important concept is that this filter criteria establishes a repertoire of specificities for sub-threshold self-pMHC ligands, enabling T cells to continue to interact in a biologically meaningful way with self-pMHC once they are released from the thymus, but without overt activation (Figure 2A). Several lines of evidence have shown that such continued interactions are important to both T cell homeostasis and in maintaining sensitivity of T cell responses to foreign antigen (Forsdyke, 1975; Krogsgaard et al., 2005; Mandl et al., 2012; Stefanova et al., 2002; Surh and Sprent, 2008). Interactions with peripheral self-pMHC provide extrinsic survival cues important to naïve T cell maintenance (Surh and Sprent, 2008). Self-pMHC recognition also leads to the partial phosphorylation of the TCR-associated ζ chain and promotes the polarization of TCRs on the cell surface, enhancing T cell responsiveness to foreign antigen (Stefanova et al., 2002). This is in contrast to chronic exposure of T cells to their agonist, which severely dampens TCR signaling and results in exhaustion or anergy (Banchereau and Pascual, 2006; Singh and Schwartz, 2003), emphasizing differences between self and agonist recognition (Morris and Allen, 2012). Furthermore, self-pMHC can enhance responses of monoclonal T cell blasts to foreign cognate antigen when the agonist pMHC is rare by acting as co-agonists and amplifying TCR signaling through increasing Lck recruitment (Krogsgaard et al., 2005). Self-peptides have been identified that can mediate both thymic positive selection and act as co-agonists during T cell activation of peripheral T cells (Ebert et al., 2009; Lo et al., 2009) (Figure 2A). Lastly, another non-mutually exclusive hypothesis for the role of positive selection is that the selection for T cells which are anti-”near-self” [AU: it might be immediately clear what is meant by ‘anti-“n”ear-self”’. Can this be explained in more detail or described differently] can act as an effective barrier to prevent evolution of pathogens toward the holes in the T cell repertoire caused by deletion of self-reactive T cells through negative selection (Forsdyke, 1975). Notably, none of these explanations addressed the relationship between self and foreign agonist peptide recognition. They posit a requirement for the recognition of self-pMHC in the periphery, but only distinguish between T cells that either see self-ligands or do not. The commonly held notion is that there is no predictable relationship between the strength of recognition of the self-pMHC and foreign pMHC ligands of a TCR. It is presumed that degenerate, heteroclitic cross-reactive recognition allows the receptors selected by weakly binding self to show the necessary stronger affinity for foreign pMHC.
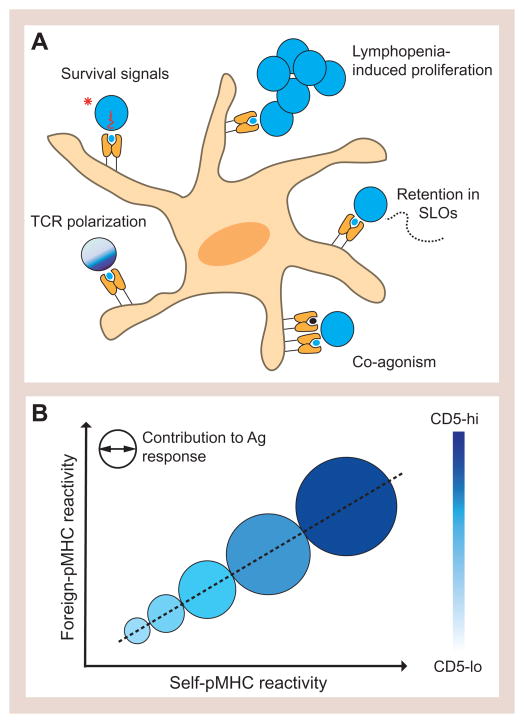
Figure 2. MHC restriction and the impact of self-recognition on peripheral T cell function.
(A) Schematic of the role of self-pMHC interactions in the function of peripheral naïve CD4+ T cells that have been described. Clockwise from top left: T cells obtain trophic signals from interacting with self-pMHC that are required for their survival and, in lymphopenic conditions, lead to cell division; CD4+ T cells, but not CD8+ T cells, are retained in secondary lymphoid organs (SLOs) by contact with self-pMHCII-bearing dendritic cells; self-pMHC can act as co-agonists during activation with rare agonist pMHC; recognition of self-pMHC increases T cell sensitivity to foreign antigens by polarizing the TCR distribution in the cell membrane. (C) Graph depicting the direct relationship between self and foreign pMHC reactivity and the dominance of T cells with greater self-pMHC reactivity in a response to foreign antigen. Shades of blue are used to indicate surface CD5 expression levels prior to antigen recognition.
As TCR interactions with self ligands occur in an affinity range below that of TCR-agonist MHC interactions, biophysical methods have so far been unable to measure the strength of such interactions (Hedrick et al., 2005). However, the amount of CD5 expression provides a clone-specific read-out of the strength of self-pMHC reactivity, thus permitted probing the self-spectrum of naïve αβ T cells (Azzam et al., 1998; Mandl et al., 2012; Mandl et al., 2013; Smith et al., 2001). CD5 expression has a broad distribution in polyclonal naïve T cell populations, in sharp contrast to most surface molecules whose variation in expression is limited, such as CD4 or the TCR itself. Consistent with a T cell repertoire constructed of cells that are diverse in their self-reactivity as a function of their TCR specificity, CD5 on naïve monoclonal T cell populations has a much narrower distribution. Additionally, mean CD5 expression varies significantly among different populations of monoclonal T cells with distinct antigen specificities, strengthening the evidence for a link between TCR specificity and CD5 expression.
Utilizing CD5 as a marker for clone-specific self-reactivity and tetramer staining to examine foreign pMHC binding strength, it was found that CD4+ T cells with greater self-pMHC reactivity on average bound foreign agonist pMHC more strongly. This was true for several agonist pMHC ligands and indicated that a direct relationship exists between the strength of self and foreign pMHC binding (Figure 2B). It should be noted, however, that this relationship exists at the population level and among polyclonal T cells there can be specific TCRs with similar foreign pMHC affinity for the same epitope, yet distinct self-pMHC responsiveness, as recently described (Persaud et al., 2014). A general relationship between self- and foreign-pMHC binding makes it easy to see why self-peptides provide a critical “test set” on which to select useful TCRs and why the complement of self-peptides is essential for the predictive construction of a T cell repertoire. CD4+ T cells with higher self-reactivity predominate in acute responses to bacterial and viral pathogens, contributing disproportionately to the memory population after infection as a result (Mandl et al., 2013). CD5hi T cells do not expand preferentially to in vivo anti-CD3 stimulation, arguing that TCR-indifferent intrinsic differences in cell state are not the origin of their better performance in response to pathogens, although these findings do not exclude that there may be some differences in biologic state of the CD5lo and CD5hi T cells as a result of stronger signaling of the latter during thymic selection or in the periphery (Persaud et al., 2014).
The better performance under infection conditions of CD5hi cells with strong foreign antigen reactivity is consistent with previous studies that observed increased TCR binding strength for agonist pMHC among memory T cells (Busch and Pamer, 1999; Malherbe et al., 2004; Savage et al., 1999). This impacts the mean self-reactivity of the naïve CD4+ T cell population as an individual ages. In adults, CD5 expression is lower on naïve T cells than in neonates. This may be due to the selective recruitment of better ligand-binding CD5hi T cells into the memory population as a result of pathogen, vaccine, or commensal antigen exposure, and hence their removal from the naïve T cell pool (Mandl et al., 2013).
Whether there is also a direct relationship between self and foreign agonist pMHC binding among CD8+ T cells remains to be tested, but is complicated by the fact that co-receptor expression on CD8+ T cells, unlike in CD4+ T cells, is adjusted as a function of responsiveness to self and IL-7 (Park et al., 2007). This may disguise variation in the self-spectrum and explain why the CD5 distribution spread among naïve CD8+ T cells is narrower than for CD4+ T cells (Mandl et al., 2013). However, consistent with evidence from CD4+ T cells that there is a direct relationship between self and foreign pMHC binding strength, mutations improving TCR binding to an agonist of the CD8+ TCR clone 2C resulted in increased auto-reactivity even though the mutations were in the CDR3 regions of the TCR that contact peptide and not MHC (Holler et al., 2003).
Self and the architecture of a T cell repertoire
Polyspecificity and the Protecton
How does selection on self ensure the construction of a repertoire made of multiples of a Protecton? We hypothesize that given a similar size of the proteome in various vertebrate species, the number of self-peptides presented in the thymus is roughly the same in an elephant, a human, and a mouse. Assuming that a universe of self peptides of the same approximate magnitude and highly similar, although not identical, sequence composition would generate approximately the same range of specificities in the selected T cell pool, it would follow that more T cells of a given specificity would be selected in the larger host but the range of pMHC seen by the total T cell population would remain comparable. Consequently, positive selection would ensure that the frequency of a given specificity remains the same even if many more T cells populate an elephant than a mouse, fulfilling the requisites of the Protecton concept.
Theoretical and experimental studies estimate the number of self peptides on which a TCR repertoire is selected to lie between 103 and 105, substantially smaller than the predicted 106–1012 foreign peptides that a given MHC allele is able to present (Detours et al., 2000; Engelhard, 1994; Mason, 1998; Nikolich-Zugich et al., 2004). This makes apparent what has also been observed empirically (Hemmer et al., 1998; Su et al., 2013) a – TCR is able to bind not just a single epitope but a region of “shape space”, such that a Protecton contains many more specificities than available self ligands on which it was selected, and implying that a single self-pMHC is capable of selecting more than one agonist specificity. This property of TCR recognition, a form of degeneracy, plays a key role in ensuring that selection by a limited number of self pMHC results in the seeding of a T cell pool whose range of antigen recognition enables the requirements of the Protecton to be met.
How large the shape space seen by a single TCR needs to be and thus how many distinct peptides T cells are able to specifically recognize when presented by MHC, has been a matter of debate (Hemmer et al., 1998; Mason, 1998; Nikolich-Zugich et al., 2004; Wooldridge et al., 2012; Zarnitsyna et al., 2013). Recent data suggests that although a single TCR can recognize a large set of distinct peptides, this set is composed of closely related sequences (Birnbaum et al., 2014). Not all T cells are equally polyspecific (Kraj et al., 2001) and it is unclear whether there is any relationship between the strength of self-pMHC responsiveness and the promiscuity of a given TCR. Theoretical work suggests that extremely cross-reactive T cells are more likely to also bind strongly to self-pMHC and hence be removed by negative selection. Indeed, T cells produced in mice in the absence of negative selection are more promiscuous (Huseby et al., 2003). Conversely, T cell responses to foreign pMHC that are very similar to self-pMHC are demonstrably absent (Calis et al., 2012). Such repertoire holes increase in number with increases in the fraction of thymocytes that are negatively selected and therefore negative selection places an upper bound on the polyspecificity of T cells (Borghans and De Boer, 1998). This impact of negative selection on the T cell repertoire can have measurable consequences on T cell function. For instance, HLA alleles, such as HLA-B57, are associated with better control of highly mutable viruses such as HIV and may be more effective at generating a T cell response that is cross-reactive for point mutations in viral epitopes. This has been proposed to be a consequence of being able to bind and present a lower number of self peptides that contribute to negative selection (Kosmrlj et al., 2010). Thus these data lend credence to the idea that self-pMHC binding strength might be associated with polyspecificity.
Linking self-pMHC and foreign-pMHC reactivity
Reconciling the property of the TCR to bind different pMHCs with variable affinities (Hemmer et al., 1998), with the relationship between self-pMHC reactivity and foreign pMHC binding (Mandl et al., 2013) raises the question of what structural properties of the TCR-pMHC interaction could account for this relationship. Although CDR1 and CDR2 have been suggested to contribute to peptide binding directly or by stabilizing the TCR-pMHC interaction in some studies (Burrows et al., 2010), given that CDR1 and CDR2 are germline-encoded and thus invariant within particular α and β chains, it is difficult to see how differences in CDR1 and CDR2 could explain the observation that better self-pMHC binders also bind better to agonist pMHC when corresponding skews in α and β chain usage have not been observed (Garcia et al., 2009; Marrack et al., 2008). Thus, the binding of the CDR3 variable loops to the presented peptide and/or MHC residues must be important. This suggests that many if not most of the agonist ligands bound by a TCR, as well as being related to each other (Birnbaum et al., 2014), will involve peptides structurally related to the self-ligand, but different in crucial epitopic chemistry and/or positioning that affect binding strength. Alternative modes of TCR-pMHC binding with more substantive changes in the geometry of the CDRs on the pMHC, as has been seen particularly in the absence of explicit negative selection of a TCR, may in rarer instances also enable the recognition of peptides unrelated to the self-ligand or other agonists (Felix and Allen, 2007; Hemmer et al., 1998).
On the population level, the structure of a T cell repertoire with regard to both self-pMHC and foreign pMHC recognition also remains unclear and has been studied only for a few example TCRs (Singh et al., 2012). A simplistic model would be that T cells that recognize a given self-pMHC respond to the same foreign pMHC. However, as many more foreign pMHCs are recognized by a T cell population than self-pMHC, an exact correspondence between T cells of particular self-pMHC specificity and a foreign pMHC specificity is unlikely. Singh et al identified a specific TCR clonotype that is able to compete with CD4+ TCR Tg 5C.C7 T cells for self-pMHC, but does not recognize the 5C.C7 agonist peptide PCC (Singh et al., 2012). Thus, a model in which T cell recognition of a particular self-pMHC guarantees recognition of the same agonist pMHC would appear to be incorrect. Conversely, Singh et al also showed that AND and 5C.C7 TCR Tg CD4+ T cells, which have closely related specificities for agonist pMHC, do not recognize the same self-pMHC (Singh et al., 2012), consistent with the identification of distinct self-pMHC for AND and 5C.C7 cells that can act as co-agonists (Ebert et al., 2009; Lo et al., 2009). This implies that having the same agonist pMHC specificity does not guarantee a similar self-pMHC specificity either. Whether these results can be extrapolated to the TCR repertoire as a whole, what fraction of the repertoire can bind a given self-pMHC, and whether in some instances there is overlap between self and foreign specificity of distinct clonotypes will require further studies. However, it seems likely that TCRs that have somewhat different ways to weakly bind the same self-pMHC could have a high affinity for different foreign pMHC.
Evolutionary, thymic and peripheral selection biases
Biases have been described in the generation of a T cell repertoire. The first type of bias is inferred from the CDR3 sequences present in a T cell repertoire. Non-random events during DNA recombination and non-templated addition have the consequence that not all possible TCRs that can be made are equally represented in the unselected primary repertoire. TCRs encoded by a greater number of distinct recombination events are more frequent (Venturi et al., 2008). Additionally, the prevalence of junctional nucleotide insertions in CDR3 is negatively correlated with CDR3 sequence abundance (Robins et al., 2010). Thus, TCR sequences that are more near-germline are more likely to be shared among individuals and are present at greater frequencies within an individual (Yassai et al., 2002; Yassai and Gorski, 2000). Whether this is entirely explained by such generation biases and what other processes contribute to the greater abundance of particular clonotypes remains to be determined.
A second type of bias observed is in the self-pMHC reactivity of the TCRs that are included in the repertoire. Prior to selection, the population of DP thymocytes displays a log-normal distribution of CD5, the common distribution seen for expression of many different proteins measured in cell populations and that results from stochasticity in gene expression (Niepel et al., 2009). However, after selection the distribution of CD5 expression among single positive thymocytes and peripheral naïve CD4+ T cells has a negative skew and there is an overrepresentation of CD5hi cells in the mature naïve population (Figure 3) (Mandl et al., 2013). This suggests that thymic selection preferentially includes T cells with higher self-pMHC reactivity – those cells that also have a greater binding strength for agonist pMHC, as befits a useful repertoire. Peripheral processes may also act to impose biases in the T cell repertoire that is maintained over time. Heterogeneity among T cells in their self-reactivity may play an important role in the competitive hierarchy amongst T cells under homeostatic conditions as T cells seek self-pMHC trophic signals (Hataye et al., 2006). How this competition occurs in vivo within secondary lymphoid organs is not understood but it may impact the TCR repertoire in ways that have not yet been explored, particularly in adult humans where thymic output contributes little to the maintenance of the naïve T cell pool (den Braber et al., 2012).
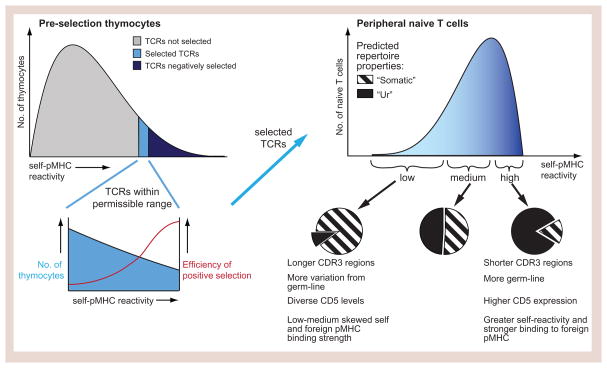
Figure 3. Trimming of the repertoire and TCR representation in the periphery.
Only a small fraction of TCRs are selected in the thymus from the TCRs that are generated (3–5%). The distribution shape of the self-spectrum prior to selection in the thymus is unknown, but it is likely positively skewed given that the majority of T cells die by neglect with most TCRs having at least some reactivity for pMHC. Data suggests that thymic selection favors greater self-pMHC reactivity (inset), with negative selection providing an upper threshold for self-pMHC binding strength of the TCRs that are selected. The self-pMHC reactivity distribution of selected TCRs that constitute the peripheral naïve T cell pool therefore has a negative skew. A prediction from current data is that TCRs from the high end of the self-spectrum in the periphery is enriched for germline TCRs which we term the “ur”-repertoire (these are the only type of TCRs produced in absence of TdT), while TCRs from the low end of the self-spectrum are enriched for CDR3s that have a variable number of nucleotide additions and constitute the “somatic” repertoire.
In addition to thymic and peripheral selection processes, most data suggest that there is evolutionary optimization of the TCR repertoire to enrich for TCRs that have a high likelihood of successfully binding to pMHC (Garcia, 2012). Even if the TCR repertoire was originally agnostic towards MHC, any germline encoded receptor gene segments that did not contribute to MHC recognition in a useful way would likely be lost over time due to neutral drift. In fact, evolutionarily conserved residues in the TCR binding region have been identified that confer such a germline TCR bias for recognizing MHC molecules (Yin et al., 2012). It needs to be noted that a very different conclusion has been reached by Singer and colleagues. They have suggested that the anti-MHC nature of mature T cell antigen recognition is imposed by the CD4 and CD8 co-receptors on an αβ TCR repertoire agnostic with respect to MHC binding (Van Laethem et al., 2012). However, there are alternative interpretations of these data entirely consistent with the results cited above at the structural and evolutionary level that suggest a germline anti-MHC bias in the TCR repertoire (Garcia, 2012).
Given the latter view of an evolved MHC bias, we propose that a Protecton may consist of two integral components. First, the “ur”-repertoire, which is made up of germline sequences without nucleotide additions and that optimally preserves this selected anti-MHC nature, and second, the TCRs that increase the diversity of the repertoire through the addition of random nucleotides in the V(D)J junctions by terminal deoxynucleotidyl transferase (TdT). These TCR constitute the “somatic” repertoire, which may overall show lower pMHC binding strength because of the introduced sequences (Figure 3). The ur-repertoire diversity is still substantial – with a possible 7.5×106 TCRs in humans and 1.2×106 in mice that can result from simple recombinations of the numerous V(D)J segments and the different α and β chains (Turner et al., 2006). In absence of TdT, the diversity of the T cell repertoire is reduced by 90–95% (Cabaniols et al., 2001), yet mice deficient in TdT nevertheless are not immunodeficient, but are able to clear a number of different infections normally (Gilfillan et al., 1995), consistent with the idea that germline TCRs have been evolutionarily optimized to be useful. If germline CDR3s already have a high probability of binding strongly to pMHC, this might predict that a greater fraction of CD5hi T cells are near-germline than are CD5lo T cells. Consistent with this, T cells during fetal development are initially produced in absence of TdT and CD4+ T cells in neonates express significantly greater levels of CD5 (Mandl et al., 2013). In addition, positive selection is more efficient in TdT-deficient mice (Gilfillan et al., 1994).
pMHC binding strength diversity, cell fate and function
Why risk the “dilution” of an effective germline repertoire with amino acids encoded by nucleotide additions? Several possible, non-mutually exclusive hypotheses might explain the evolutionary advantage of this TdT-mediated process. First, although non-germline CDR3s may have a higher probability of binding less well to many pMHC, in some instances TdT may result in the production of TCRs that are substantially better at binding to particular pMHC(s) and these TCR may contribute importantly to a T cell response (Messaoudi et al., 2002) (Figure 3). Second, the increased repertoire diversity resulting from the inclusion of CDR3s with nucleotide additions may be particularly critical for maintaining control of highly mutable pathogens prone to escape from oligoclonal T cell responses (Meyer-Olson et al., 2004; van Gisbergen et al., 2011). Third, incorporating not just the very highly responsive TCRs into the repertoire may have benefits during chronic infections, where such T cells are more likely to become exhausted and ineffective (Wherry, 2011). Indeed, a very highly self-pMHC reactive CD4+ T cell clone has been shown to expand poorly due to activation-induced cell death upon agonist ligand exposure (Persaud et al., 2014). Lastly, a growing body of work suggests that the strength of the TCR-pMHC interaction dictates not only the magnitude of the response, but also its quality by impacting cell fate and differentiation during an immune response (Corse et al., 2011). Therefore the inclusion of T cells with distinct propensities to make particular cell fate decisions as a result of properties of their TCR might critically influence the effectiveness of a T cell response.
On this theme, the differentiation of naïve CD4+ T cells into distinct helper lineages is influenced by pMHCII binding strength. Development of Th2 cells is favored by weaker TCR signals than Th1 development (Boyton and Altmann, 2002; Milner et al., 2010; van Panhuys et al., 2014). Indeed, Th2 cells are enriched for longer CDR3 Vα chains (Boyton et al., 2002). Conversely, regulatory T cells have been shown to have higher CD5 expression ((Moran et al., 2011) and our unpublished data) and are more readily induced from CD5hi cells (Martin et al., 2013). Follicular helper T cells have also been shown to preferentially develop from CD4+ T cell clonotypes with the highest pMHCII binding strength (Fazilleau et al., 2009). A recent study found that while extrinsic factors, such as cytokines, influence the fate of activated CD4+ T cells, there are clear clonotypic differences in effector cell patterning that are controlled by the TCR (Tubo et al., 2013). Such variability in effector responses among clonotypes will particularly come into play when the precursor frequency, and hence TCR diversity, is very low. Similarly, memory cell fate decisions of both CD4+ and CD8+ T cells are impacted by pMHC binding properties of the TCR (Kim et al., 2013; Knudson et al., 2013; Savage et al., 1999). Thus, the “somatic” repertoire may ensure a diversity of effector responses by broadening the range of TCR affinities. Whether any of these clonotypic properties are imparted by cell-intrinsic effects related to self-pMHC binding, set either during thymic development or as a result of peripheral interactions, remains to be examined in more detail. For CD8+ T cells there is evidence that self-pMHC interaction strength can impact properties not directly downstream of the TCR, such as their proliferation in response to IL-2 (Cho et al., 2010). For CD4+ T cells, recent data suggest that the level of IL-2 production upon activation by a mitogen or specific pMHC can be influenced by the strength of self-pMHC signaling during thymic selection (Persaud et al., 2014).
Concluding Remarks
The greatest challenge in the generation of a T cell repertoire is to construct an effective Protecton without knowledge of what foreign antigens will be encountered by peripheral T cells during the lifetime of an individual. Multiple factors play out the construction and architecture of a T cell repertoire. We hypothesize that important aspects of its structure arise from the use of self-ligands in the selection of the TCRs that go on to constitute the peripheral T cell population. Because there is a direct relationship between self-pMHC and foreign agonist binding, the ensemble of self-pMHC presented in the thymus acts as an essential “test set” predictive of the capacity of a T cell to recognize presented foreign antigen. A complement of TCRs is established that are heterogeneous in self-pMHC reactivity within a range that is set by thymic selection. This heterogeneity has an impact on the TCRs that respond to foreign antigen and contribute to the memory T cell repertoire. Viewing the architecture of a T cell repertoire from a self-centric perspective and defining the precise nature of the relationship between the repertoire of foreign specificities and of the self-pMHC ligands on which T cells are selected will provide additional insight into how biases in T cell responses to pathogen-derived antigens arise. Incorporating an understanding of how self-pMHC reactivity influences the structure of the TCR repertoire and the consequences of heterogeneity in this parameter among T cells into system models of T cell function and behavior will enable us to optimize the design of effective vaccines, manipulate the homeostasis of T cell populations, and develop interventions for autoimmune diseases.
Acknowledgments
We are grateful to Debbie van Baarle, Can Kesmir, Kristin Hogquist, and Nevil Singh for reading earlier versions of this manuscript and providing critical feedback. We would also like to thank Johannes Textor for immensely valuable discussions. This work was supported by the Intramural Research Program of NIAID at the National Institutes of Health.
Glossary
- Alloreactivity
ability of a given T cell to react to genetically mismatched MHC molecules
- MHC restriction
bias of T cell receptors to recognize and respond to peptide presented in the context of the major histocompatibility complex alleles present in an individual
- Precursor frequency
the frequency of T cells among the naïve T cell population of an individual with a given antigen specificity. It is important to note that this population is not clonal and will include a collection of CDR3 sequences
- Polyspecificity
ability of T cell receptor to bind specifically to different pMHC ligands
- Sub-threshold self-pMHC
specific self-pMHC ligands that do not lead to peripheral T cell activation and that induce a TCR signal distinct from that resulting from recognition of an agonist pMHC. Such self-pMHC ligands therefore do not include those that result in overt autoimmune responses. Throughout this review, we refer to such sub-threshold self-pMHC TCR ligands simply as self-pMHC unless otherwise specified
- T cell repertoire
the collection of all TCR clonotypes present in a T cell population of an individual at a given time
- T cell self-spectrum
the TCR affinities for self-pMHC and the frequency distribution of these affinities present in a T cell population of an individual at a given time
- TCR affinity
the strength of the binding between a given TCR and a given pMHC ligand, which is a function of the dissociation rate (Kd) as well as the association rate (Ka) for that interaction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allison JP, McIntyre BW, Bloch D. Tumor-specific antigen of murine T-lymphoma defined with monoclonal antibody. J Immunol. 1982;129:2293–2300. [PubMed] [Google Scholar]
- Azzam HS, Grinberg A, Lui K, Shen H, Shores EW, Love PE. CD5 expression is developmentally regulated by T cell receptor (TCR) signals and TCR avidity. J Exp Med. 1998;188:2301–2311. doi: 10.1084/jem.188.12.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babbitt BP, Allen PM, Matsueda G, Haber E, Unanue ER. Binding of immunogenic peptides to Ia histocompatibility molecules. Nature. 1985;317:359–361. doi: 10.1038/317359a0. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Pascual V. Type I interferon in systemic lupus erythematosus and other autoimmune diseases. Immunity. 2006;25:383–392. doi: 10.1016/j.immuni.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Barton GM, Rudensky AY. Requirement for diverse, low-abundance peptides in positive selection of T cells. Science. 1999;283:67–70. doi: 10.1126/science.283.5398.67. [DOI] [PubMed] [Google Scholar]
- Bevan MJ. In a radiation chimaera, host H-2 antigens determine immune responsiveness of donor cytotoxic cells. Nature. 1977;269:417–418. doi: 10.1038/269417a0. [DOI] [PubMed] [Google Scholar]
- Birnbaum ME, Mendoza JL, Sethi DK, Dong S, Glanville J, Dobbins J, Ozkan E, Davis MM, Wucherpfennig KW, Garcia KC. Deconstructing the peptide-MHC specificity of T cell recognition. Cell. 2014 doi: 10.1016/j.cell.2014.03.047. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg NA, Ely LK, Beddoe T, Macdonald WA, Reid HH, Clements CS, Purcell AW, Kjer-Nielsen L, Miles JJ, Burrows SR, et al. The CDR3 regions of an immunodominant T cell receptor dictate the ‘energetic landscape’ of peptide-MHC recognition. Nat Immunol. 2005;6:171–180. doi: 10.1038/ni1155. [DOI] [PubMed] [Google Scholar]
- Borghans JA, De Boer RJ. Cross reactivity of the T -cell receptor. Immunology today. 1998;19:428–429. doi: 10.1016/s0167-5699(98)01317-6. [DOI] [PubMed] [Google Scholar]
- Boyton RJ, Altmann DM. Is selection for TCR affinity a factor in cytokine polarization? Trends in immunology. 2002;23:526–529. doi: 10.1016/s1471-4906(02)02319-0. [DOI] [PubMed] [Google Scholar]
- Boyton RJ, Zaccai N, Jones EY, Altmann DM. CD4 T cells selected by antigen under Th2 polarizing conditions favor an elongated TCR alpha chain complementarity-determining region 3. J Immunol. 2002;168:1018–1027. doi: 10.4049/jimmunol.168.3.1018. [DOI] [PubMed] [Google Scholar]
- Burrows SR, Chen Z, Archbold JK, Tynan FE, Beddoe T, Kjer-Nielsen L, Miles JJ, Khanna R, Moss DJ, Liu YC, et al. Hard wiring of T cell receptor specificity for the major histocompatibility complex is underpinned by TCR adaptability. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:10608–10613. doi: 10.1073/pnas.1004926107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch DH, Pamer EG. T cell affinity maturation by selective expansion during infection. J Exp Med. 1999;189:701–710. doi: 10.1084/jem.189.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabaniols JP, Fazilleau N, Casrouge A, Kourilsky P, Kanellopoulos JM. Most alpha/beta T cell receptor diversity is due to terminal deoxynucleotidyl transferase. J Exp Med. 2001;194:1385–1390. doi: 10.1084/jem.194.9.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calis JJ, de Boer RJ, Kesmir C. Degenerate T-cell recognition of peptides on MHC molecules creates large holes in the T-cell repertoire. PLoS Comput Biol. 2012;8:e1002412. doi: 10.1371/journal.pcbi.1002412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JH, Kim HO, Surh CD, Sprent J. T cell receptor-dependent regulation of lipid rafts controls naive CD8+ T cell homeostasis. Immunity. 2010;32:214–226. doi: 10.1016/j.immuni.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corse E, Gottschalk RA, Allison JP. Strength of TCR-Peptide/MHC Interactions and In Vivo T Cell Responses. J Immunol. 2011;186:5039–5045. doi: 10.4049/jimmunol.1003650. [DOI] [PubMed] [Google Scholar]
- Daley SR, Hu DY, Goodnow CC. Helios marks strongly autoreactive CD4+ T cells in two major waves of thymic deletion distinguished by induction of PD-1 or NF-kappaB. J Exp Med. 2013;210:269–285. doi: 10.1084/jem.20121458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MM, Bjorkman PJ. T-cell antigen receptor genes and T-cell recognition. Nature. 1988;334:395–402. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- den Braber I, Mugwagwa T, Vrisekoop N, Westera L, Mogling R, de Boer AB, Willems N, Schrijver EH, Spierenburg G, Gaiser K, et al. Maintenance of peripheral naive T cells is sustained by thymus output in mice but not humans. Immunity. 2012;36:288–297. doi: 10.1016/j.immuni.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Detours V, Mehr R, Perelson AS. Deriving quantitative constraints on T cell selection from data on the mature T cell repertoire. J Immunol. 2000;164:121–128. doi: 10.4049/jimmunol.164.1.121. [DOI] [PubMed] [Google Scholar]
- Ebert PJ, Jiang S, Xie J, Li QJ, Davis MM. An endogenous positively selecting peptide enhances mature T cell responses and becomes an autoantigen in the absence of microRNA miR-181a. Nat Immunol. 2009;10:1162–1169. doi: 10.1038/ni.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhard VH. Structure of peptides associated with class I and class II MHC molecules. Annu Rev Immunol. 1994;12:181–207. doi: 10.1146/annurev.iy.12.040194.001145. [DOI] [PubMed] [Google Scholar]
- Fazilleau N, McHeyzer-Williams LJ, Rosen H, McHeyzer-Williams MG. The function of follicular helper T cells is regulated by the strength of T cell antigen receptor binding. Nat Immunol. 2009;10:375–384. doi: 10.1038/ni.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix NJ, Allen PM. Specificity of T-cell alloreactivity. Nature reviews. 2007;7:942–953. doi: 10.1038/nri2200. [DOI] [PubMed] [Google Scholar]
- Forsdyke DR. Further implications of a theory of immunity. J Theor Biol. 1975;52:187–198. doi: 10.1016/0022-5193(75)90050-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garboczi DN, Ghosh P, Utz U, Fan QR, Biddison WE, Wiley DC. Structure of the complex between human T-cell receptor, viral peptide and HLA-A2. Nature. 1996;384:134–141. doi: 10.1038/384134a0. [DOI] [PubMed] [Google Scholar]
- Garcia KC. Reconciling views on T cell receptor germline bias for MHC. Trends in immunology. 2012;33:429–436. doi: 10.1016/j.it.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia KC, Adams JJ, Feng D, Ely LK. The molecular basis of TCR germline bias for MHC is surprisingly simple. Nat Immunol. 2009;10:143–147. doi: 10.1038/ni.f.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilfillan S, Bachmann M, Trembleau S, Adorini L, Kalinke U, Zinkernagel R, Benoist C, Mathis D. Efficient immune responses in mice lacking N-region diversity. Eur J Immunol. 1995;25:3115–3122. doi: 10.1002/eji.1830251119. [DOI] [PubMed] [Google Scholar]
- Gilfillan S, Waltzinger C, Benoist C, Mathis D. More efficient positive selection of thymocytes in mice lacking terminal deoxynucleotidyl transferase. International immunology. 1994;6:1681–1686. doi: 10.1093/intimm/6.11.1681. [DOI] [PubMed] [Google Scholar]
- Grubin CE, Kovats S, deRoos P, Rudensky AY. Deficient positive selection of CD4 T cells in mice displaying altered repertoires of MHC class II-bound self-peptides. Immunity. 1997;7:197–208. doi: 10.1016/s1074-7613(00)80523-3. [DOI] [PubMed] [Google Scholar]
- Hataye J, Moon JJ, Khoruts A, Reilly C, Jenkins MK. Naive and memory CD4+ T cell survival controlled by clonal abundance. Science. 2006;312:114–116. doi: 10.1126/science.1124228. [DOI] [PubMed] [Google Scholar]
- Hedrick SM, Cohen DI, Nielsen EA, Davis MM. Isolation of cDNA clones encoding T cell-specific membrane-associated proteins. Nature. 1984;308:149–153. doi: 10.1038/308149a0. [DOI] [PubMed] [Google Scholar]
- Hedrick SM, Cohen DI, Nielsen EA, Davis MM. Isolation of cDNA clones encoding T cell-specific membrane-associated proteins. 1984. J Immunol. 2005;175:2771–2775. [PubMed] [Google Scholar]
- Hemmer B, Vergelli M, Pinilla C, Houghten R, Martin R. Probing degeneracy in T-cell recognition using peptide combinatorial libraries. Immunology today. 1998;19:163–168. doi: 10.1016/s0167-5699(97)01217-6. [DOI] [PubMed] [Google Scholar]
- Hogquist KA, Gavin MA, Bevan MJ. Positive selection of CD8+ T cells induced by major histocompatibility complex binding peptides in fetal thymic organ culture. J Exp Med. 1993;177:1469–1473. doi: 10.1084/jem.177.5.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holler PD, Chlewicki LK, Kranz DM. TCRs with high affinity for foreign pMHC show self-reactivity. Nat Immunol. 2003;4:55–62. doi: 10.1038/ni863. [DOI] [PubMed] [Google Scholar]
- Huseby ES, Crawford F, White J, Kappler J, Marrack P. Negative selection imparts peptide specificity to the mature T cell repertoire. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:11565–11570. doi: 10.1073/pnas.1934636100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatowicz L, Kappler J, Marrack P. The repertoire of T cells shaped by a single MHC/peptide ligand. Cell. 1996;84:521–529. doi: 10.1016/s0092-8674(00)81028-4. [DOI] [PubMed] [Google Scholar]
- Jenkins MK, Moon JJ. The role of naive T cell precursor frequency and recruitment in dictating immune response magnitude. J Immunol. 2012;188:4135–4140. doi: 10.4049/jimmunol.1102661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung D, Alt FW. Unraveling V(D)J recombination; insights into gene regulation. Cell. 2004;116:299–311. doi: 10.1016/s0092-8674(04)00039-x. [DOI] [PubMed] [Google Scholar]
- Kappler J, Kubo R, Haskins K, Hannum C, Marrack P, Pigeon M, McIntyre B, Allison J, Trowbridge I. The major histocompatibility complex-restricted antigen receptor on T cells in mouse and man: identification of constant and variable peptides. Cell. 1983;35:295–302. doi: 10.1016/0092-8674(83)90232-5. [DOI] [PubMed] [Google Scholar]
- Kappler JW, Skidmore B, White J, Marrack P. Antigen-inducible, H-2-restricted, interleukin-2-producing T cell hybridomas. Lack of independent antigen and H-2 recognition. J Exp Med. 1981;153:1198–1214. doi: 10.1084/jem.153.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Wilson T, Fischer KF, Williams MA. Sustained interactions between T cell receptors and antigens promote the differentiation of CD4(+) memory T cells. Immunity. 2013;39:508–520. doi: 10.1016/j.immuni.2013.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudson KM, Goplen NP, Cunningham CA, Daniels MA, Teixeiro E. Low-affinity T cells are programmed to maintain normal primary responses but are impaired in their recall to low-affinity ligands. Cell reports. 2013;4:554–565. doi: 10.1016/j.celrep.2013.07.008. [DOI] [PubMed] [Google Scholar]
- Kosmrlj A, Read EL, Qi Y, Allen TM, Altfeld M, Deeks SG, Pereyra F, Carrington M, Walker BD, Chakraborty AK. Effects of thymic selection of the T-cell repertoire on HLA class I-associated control of HIV infection. Nature. 2010;465:350–354. doi: 10.1038/nature08997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraj P, Pacholczyk R, Ignatowicz L. Alpha beta TCRs differ in the degree of their specificity for the positively selecting MHC/peptide ligand. J Immunol. 2001;166:2251–2259. doi: 10.4049/jimmunol.166.4.2251. [DOI] [PubMed] [Google Scholar]
- Krogsgaard M, Li QJ, Sumen C, Huppa JB, Huse M, Davis MM. Agonist/endogenous peptide-MHC heterodimers drive T cell activation and sensitivity. Nature. 2005;434:238–243. doi: 10.1038/nature03391. [DOI] [PubMed] [Google Scholar]
- Langman RE, Cohn M. The E-T (elephant-tadpole) paradox necessitates the concept of a unit of B-cell function: the protecton. Mol Immunol. 1987;24:675–697. doi: 10.1016/0161-5890(87)90050-2. [DOI] [PubMed] [Google Scholar]
- Lo D, Sprent J. Identity of cells that imprint H-2-restricted T-cell specificity in the thymus. Nature. 1986;319:672–675. doi: 10.1038/319672a0. [DOI] [PubMed] [Google Scholar]
- Lo WL, Felix NJ, Walters JJ, Rohrs H, Gross ML, Allen PM. An endogenous peptide positively selects and augments the activation and survival of peripheral CD4+ T cells. Nat Immunol. 2009;10:1155–1161. doi: 10.1038/ni.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas B, Germain RN. T-cell repertoire: political correctness in the immune system. Curr Biol. 1996;6:783–787. doi: 10.1016/s0960-9822(02)00594-8. [DOI] [PubMed] [Google Scholar]
- Malherbe L, Hausl C, Teyton L, McHeyzer-Williams MG. Clonal selection of helper T cells is determined by an affinity threshold with no further skewing of TCR binding properties. Immunity. 2004;21:669–679. doi: 10.1016/j.immuni.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Mandl JN, Liou R, Klauschen F, Vrisekoop N, Monteiro JP, Yates AJ, Huang AY, Germain RN. Quantification of lymph node transit times reveals differences in antigen surveillance strategies of naive CD4+ and CD8+ T cells. Proc Natl Acad Sci U S A. 2012;109:18036–18041. doi: 10.1073/pnas.1211717109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandl JN, Monteiro JP, Vrisekoop N, Germain RN. T cell-positive selection uses self-ligand binding strength to optimize repertoire recognition of foreign antigens. Immunity. 2013;38:263–274. doi: 10.1016/j.immuni.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrack P, Scott-Browne JP, Dai S, Gapin L, Kappler JW. Evolutionarily conserved amino acids that control TCR-MHC interaction. Annu Rev Immunol. 2008;26:171–203. doi: 10.1146/annurev.immunol.26.021607.090421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B, Auffray C, Delpoux A, Pommier A, Durand A, Charvet C, Yakonowsky P, de Boysson H, Bonilla N, Audemard A, et al. Highly self-reactive naive CD4 T cells are prone to differentiate into regulatory T cells. Nature communications. 2013;4:2209. doi: 10.1038/ncomms3209. [DOI] [PubMed] [Google Scholar]
- Mason D. A very high level of crossreactivity is an essential feature of the T-cell receptor. Immunol Today. 1998;19:395–404. doi: 10.1016/s0167-5699(98)01299-7. [DOI] [PubMed] [Google Scholar]
- Merkenschlager M, Graf D, Lovatt M, Bommhardt U, Zamoyska R, Fisher AG. How many thymocytes audition for selection? J Exp Med. 1997;186:1149–1158. doi: 10.1084/jem.186.7.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messaoudi I, Guevara Patino JA, Dyall R, LeMaoult J, Nikolich-Zugich J. Direct link between mhc polymorphism, T cell avidity, and diversity in immune defense. Science. 2002;298:1797–1800. doi: 10.1126/science.1076064. [DOI] [PubMed] [Google Scholar]
- Meyer-Olson D, Shoukry NH, Brady KW, Kim H, Olson DP, Hartman K, Shintani AK, Walker CM, Kalams SA. Limited T cell receptor diversity of HCV-specific T cell responses is associated with CTL escape. J Exp Med. 2004;200:307–319. doi: 10.1084/jem.20040638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles JJ, Douek DC, Price DA. Bias in the alphabeta T-cell repertoire: implications for disease pathogenesis and vaccination. Immunology and cell biology. 2011;89:375–387. doi: 10.1038/icb.2010.139. [DOI] [PubMed] [Google Scholar]
- Milner JD, Fazilleau N, McHeyzer-Williams M, Paul W. Cutting edge: lack of high affinity competition for peptide in polyclonal CD4+ responses unmasks IL-4 production. J Immunol. 2010;184:6569–6573. doi: 10.4049/jimmunol.1000674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran AE, Holzapfel KL, Xing Y, Cunningham NR, Maltzman JS, Punt J, Hogquist KA. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J Exp Med. 2011;208:1279–1289. doi: 10.1084/jem.20110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris GP, Allen PM. How the TCR balances sensitivity and specificity for the recognition of self and pathogens. Nat Immunol. 2012;13:121–128. doi: 10.1038/ni.2190. [DOI] [PubMed] [Google Scholar]
- Murugan A, Mora T, Walczak AM, Callan CG., Jr Statistical inference of the generation probability of T-cell receptors from sequence repertoires. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:16161–16166. doi: 10.1073/pnas.1212755109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niepel M, Spencer SL, Sorger PK. Non-genetic cell-to-cell variability and the consequences for pharmacology. Curr Opin Chem Biol. 2009;13:556–561. doi: 10.1016/j.cbpa.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolich-Zugich J, Slifka MK, Messaoudi I. The many important facets of T-cell repertoire diversity. Nature reviews. 2004;4:123–132. doi: 10.1038/nri1292. [DOI] [PubMed] [Google Scholar]
- Palmer E. Negative selection--clearing out the bad apples from the T-cell repertoire. Nature reviews. 2003;3:383–391. doi: 10.1038/nri1085. [DOI] [PubMed] [Google Scholar]
- Park JH, Adoro S, Lucas PJ, Sarafova SD, Alag AS, Doan LL, Erman B, Liu X, Ellmeier W, Bosselut R, et al. ‘Coreceptor tuning’: cytokine signals transcriptionally tailor CD8 coreceptor expression to the self-specificity of the TCR. Nat Immunol. 2007;8:1049–1059. doi: 10.1038/ni1512. [DOI] [PubMed] [Google Scholar]
- Persaud SP, Parker CR, Lo WL, Weber KS, Allen PM. Intrinsic CD4 T cell sensitivity and response to a pathogen are set and sustained by avidity for thymic and peripheral complexes of self peptide and MHC. Nat Immunol. 2014 doi: 10.1038/ni.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piepenbrink KH, Blevins SJ, Scott DR, Baker BM. The basis for limited specificity and MHC restriction in a T cell receptor interface. Nature communications. 2013;4:1948. doi: 10.1038/ncomms2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz EL, Tan K, Tang L, Kern P, Liu J, Xiong Y, Hussey RE, Smolyar A, Hare B, Zhang R, et al. The crystal structure of a T cell receptor in complex with peptide and MHC class II. Science. 1999;286:1913–1921. doi: 10.1126/science.286.5446.1913. [DOI] [PubMed] [Google Scholar]
- Robins HS, Srivastava SK, Campregher PV, Turtle CJ, Andriesen J, Riddell SR, Carlson CS, Warren EH. Overlap and effective size of the human CD8+ T cell receptor repertoire. Sci Transl Med. 2010;2:47ra64. doi: 10.1126/scitranslmed.3001442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal AS, Shevach EM. Function of macrophages in antigen recognition by guinea pig T lymphocytes. I. Requirement for histocompatible macrophages and lymphocytes. J Exp Med. 1973;138:1194–1212. doi: 10.1084/jem.138.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sant’Angelo DB, Waterbury PG, Cohen BE, Martin WD, Van Kaer L, Hayday AC, Janeway CA., Jr The imprint of intrathymic self-peptides on the mature T cell receptor repertoire. Immunity. 1997;7:517–524. doi: 10.1016/s1074-7613(00)80373-8. [DOI] [PubMed] [Google Scholar]
- Savage PA, Boniface JJ, Davis MM. A kinetic basis for T cell receptor repertoire selection during an immune response. Immunity. 1999;10:485–492. doi: 10.1016/s1074-7613(00)80048-5. [DOI] [PubMed] [Google Scholar]
- Scollay RG, Butcher EC, Weissman IL. Thymus cell migration. Quantitative aspects of cellular traffic from the thymus to the periphery in mice. Eur J Immunol. 1980;10:210–218. doi: 10.1002/eji.1830100310. [DOI] [PubMed] [Google Scholar]
- Scott-Browne JP, Crawford F, Young MH, Kappler JW, Marrack P, Gapin L. Evolutionarily conserved features contribute to alphabeta T cell receptor specificity. Immunity. 2011;35:526–535. doi: 10.1016/j.immuni.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim BC, Lo D, Gascoigne NR. Preferential expression of TCR V alpha regions in CD4/CD8 subsets: class discrimination or co-receptor recognition? Immunology today. 1998;19:276–282. doi: 10.1016/s0167-5699(98)01257-2. [DOI] [PubMed] [Google Scholar]
- Singh NJ, Bando JK, Schwartz RH. Subsets of Nonclonal Neighboring CD4(+) T Cells Specifically Regulate the Frequency of Individual Antigen-Reactive T Cells. Immunity. 2012;37:735–746. doi: 10.1016/j.immuni.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh NJ, Schwartz RH. The strength of persistent antigenic stimulation modulates adaptive tolerance in peripheral CD4+ T cells. J Exp Med. 2003;198:1107–1117. doi: 10.1084/jem.20030913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K, Seddon B, Purbhoo MA, Zamoyska R, Fisher AG, Merkenschlager M. Sensory adaptation in naive peripheral CD4 T cells. J Exp Med. 2001;194:1253–1261. doi: 10.1084/jem.194.9.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol. 2003;21:139–176. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- Stefanova I, Dorfman JR, Germain RN. Self-recognition promotes the foreign antigen sensitivity of naive T lymphocytes. Nature. 2002;420:429–434. doi: 10.1038/nature01146. [DOI] [PubMed] [Google Scholar]
- Stritesky GL, Xing Y, Erickson JR, Kalekar LA, Wang X, Mueller DL, Jameson SC, Hogquist KA. Murine thymic selection quantified using a unique method to capture deleted T cells. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:4679–4684. doi: 10.1073/pnas.1217532110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su LF, Kidd BA, Han A, Kotzin JJ, Davis MM. Virus-specific CD4(+) memory-phenotype T cells are abundant in unexposed adults. Immunity. 2013;38:373–383. doi: 10.1016/j.immuni.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surh CD, Lee DS, Fung-Leung WP, Karlsson L, Sprent J. Thymic selection by a single MHC/peptide ligand produces a semidiverse repertoire of CD4+ T cells. Immunity. 1997;7:209–219. doi: 10.1016/s1074-7613(00)80524-5. [DOI] [PubMed] [Google Scholar]
- Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Tourne S, Miyazaki T, Oxenius A, Klein L, Fehr T, Kyewski B, Benoist C, Mathis D. Selection of a broad repertoire of CD4+ T cells in H-2Ma0/0 mice. Immunity. 1997;7:187–195. doi: 10.1016/s1074-7613(00)80522-1. [DOI] [PubMed] [Google Scholar]
- Tubo NJ, Pagan AJ, Taylor JJ, Nelson RW, Linehan JL, Ertelt JM, Huseby ES, Way SS, Jenkins MK. Single naive CD4+ T cells from a diverse repertoire produce different effector cell types during infection. Cell. 2013;153:785–796. doi: 10.1016/j.cell.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner SJ, Doherty PC, McCluskey J, Rossjohn J. Structural determinants of T-cell receptor bias in immunity. Nat Rev Immunol. 2006;6:883–894. doi: 10.1038/nri1977. [DOI] [PubMed] [Google Scholar]
- van Gisbergen KP, Klarenbeek PL, Kragten NA, Unger PP, Nieuwenhuis MB, Wensveen FM, ten Brinke A, Tak PP, Eldering E, Nolte MA, et al. The costimulatory molecule CD27 maintains clonally diverse CD8(+) T cell responses of low antigen affinity to protect against viral variants. Immunity. 2011;35:97–108. doi: 10.1016/j.immuni.2011.04.020. [DOI] [PubMed] [Google Scholar]
- Van Laethem F, Tikhonova AN, Singer A. MHC restriction is imposed on a diverse T cell receptor repertoire by CD4 and CD8 co-receptors during thymic selection. Trends in immunology. 2012;33:437–441. doi: 10.1016/j.it.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meerwijk JP, Marguerat S, Lees RK, Germain RN, Fowlkes BJ, MacDonald HR. Quantitative impact of thymic clonal deletion on the T cell repertoire. J Exp Med. 1997;185:377–383. doi: 10.1084/jem.185.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Panhuys N, Klauschen F, Germain RN. TCR-dependent Signal Intensity Dominantly Controls CD4+ T Cell Polarization In Vivo. Immunity. 2014 doi: 10.1016/j.immuni.2014.06.003. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venturi V, Price DA, Douek DC, Davenport MP. The molecular basis for public T-cell responses? Nature reviews. 2008;8:231–238. doi: 10.1038/nri2260. [DOI] [PubMed] [Google Scholar]
- Viret C, Lamare C, Guiraud M, Fazilleau N, Bour A, Malissen B, Carrier A, Guerder S. Thymus-specific serine protease contributes to the diversification of the functional endogenous CD4 T cell receptor repertoire. J Exp Med. 2011;208:3–11. doi: 10.1084/jem.20100027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol. 2003;3:867–878. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- Wiegel FW, Perelson AS. Some scaling principles for the immune system. Immunology and cell biology. 2004;82:127–131. doi: 10.1046/j.0818-9641.2004.01229.x. [DOI] [PubMed] [Google Scholar]
- Wooldridge L, Ekeruche-Makinde J, van den Berg HA, Skowera A, Miles JJ, Tan MP, Dolton G, Clement M, Llewellyn-Lacey S, Price DA, et al. A single autoimmune T cell receptor recognizes more than a million different peptides. J Biol Chem. 2012;287:1168–1177. doi: 10.1074/jbc.M111.289488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y, Jameson SC, Hogquist KA. Thymoproteasome subunit-beta5T generates peptide-MHC complexes specialized for positive selection. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:6979–6984. doi: 10.1073/pnas.1222244110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassai M, Ammon K, Goverman J, Marrack P, Naumov Y, Gorski J. A molecular marker for thymocyte-positive selection: selection of CD4 single-positive thymocytes with shorter TCRB CDR3 during T cell development. J Immunol. 2002;168:3801–3807. doi: 10.4049/jimmunol.168.8.3801. [DOI] [PubMed] [Google Scholar]
- Yassai M, Gorski J. Thymocyte maturation: selection for in-frame TCR alpha-chain rearrangement is followed by selection for shorter TCR beta-chain complementarity-determining region 3. J Immunol. 2000;165:3706–3712. doi: 10.4049/jimmunol.165.7.3706. [DOI] [PubMed] [Google Scholar]
- Yin L, Scott-Browne J, Kappler JW, Gapin L, Marrack P. T cells and their eons-old obsession with MHC. Immunol Rev. 2012;250:49–60. doi: 10.1111/imr.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarnitsyna VI, Evavold BD, Schoettle LN, Blattman JN, Antia R. Estimating the Diversity, Completeness, and Cross-Reactivity of the T Cell Repertoire. Front Immunol. 2013;4:485. doi: 10.3389/fimmu.2013.00485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel RM, Callahan GN, Klein J, Dennert G. Cytotoxic T cells learn specificity for self H-2 during differentiation in the thymus. Nature. 1978;271:251–253. doi: 10.1038/271251a0. [DOI] [PubMed] [Google Scholar]
- Zinkernagel RM, Doherty PC. Immunological surveillance against altered self components by sensitised T lymphocytes in lymphocytic choriomeningitis. Nature. 1974a;251:547–548. doi: 10.1038/251547a0. [DOI] [PubMed] [Google Scholar]
- Zinkernagel RM, Doherty PC. Restriction of in vitro T cell-mediated cytotoxicity in lymphocytic choriomeningitis within a syngeneic or semiallogeneic system. Nature. 1974b;248:701–702. doi: 10.1038/248701a0. [DOI] [PubMed] [Google Scholar]