Abstract
A novel, non-AT1, non-AT2 brain binding site for angiotensin peptides that is unmasked by p-chloromercuribenzoate (PCMB) has been identified as a membrane associated variant of neurolysin. The ability of different organic and inorganic oxidative and sulfhydryl reactive agents to unmask or inhibit 125I-Sar1Ile8 angiotensin II (SI-Ang II) binding to this site was presently examined. In tissue membranes from homogenates of rat brain and testis incubated in assay buffer containing losartan (10 μM) and PD123319 (10 μM) plus 100 μM PCMB, 5 of the 39 compounds tested inhibited 125I-SI Ang II binding in brain and testis. Mersalyl acid, mercuric chloride (HgCl2) and silver nitrate (AgNO3) most potently inhibited 125I-SI Ang II binding with IC50’s ~1–20 μM This HgCl2 inhibition was independent of any interaction of HgCl2 with angiotensin II (Ang II) based on the lack of effect of HgCl2 on the dipsogenic effects of intracerebroventricularly administered Ang II and 125I-SI Ang II binding to AT1 receptors in the liver. Among sulfhydryl reagents, cysteamine and reduced glutathione (GSH), but not oxidized glutathione (GSSG) up to 1 mM, inhibited PCMB-unmasked 125I-SI Ang II binding in brain and testis. Thimerosal and 4-hydroxymercuribenzoate moderately inhibited PCMB-unmasked 125I-SI Ang II binding in brain and testis at 100 μM; however, they also unmasked non-AT1, non-AT2 binding independent of PCMB. 4-hydroxybenzoic acid did not promote 125 I-SI Ang II binding to this binding site indicating that only specific organomercurial compounds can unmask the binding site. The common denominator for all of these interacting substances is the ability to bind to protein cysteine sulfur. Comparison of cysteines between neurolysin and the closely related enzyme thimet oligopeptidase revealed an unconserved cysteine (cys650, based on the full length variant) in the proposed ligand binding channel (Brown et al., 2001) [1] near the active site of neurolysin. It is proposed that the mercuric ion in PCMB and closely related organomercurial compounds binds to cys650, while the acidic anion forms an ionic bond with a nearby arginine or lysine along the channel to effect a conformational change in neurolysin that promotes Ang II binding.
Keywords: Neurolysin, Angiotensin II, Radioligand binding, p-chloromercuribenzoate, organomercurial, cysteine sulfhydryl, brain, testis
1. Introduction
The renin-angiotensin system (RAS) is critical for the regulation of blood pressure, fluid volume and electrolyte balance. It sustains cardiovascular homeostasis in both normal and pathophysiological conditions acting centrally as well as peripherally. The central actions of the RAS has been the subject of many reviews spanning more than 40 years [1–7]. When overactivated the brain RAS causes hypertension, through increased vasopressin release and increased sympathetic nerve activity. Despite decades of intensive research to address its pathophysiological actions in different tissues, questions remain regarding the functions and nature of the RAS [8–11].
Key components of the RAS include the angiotensin II type 1 (AT1) and angiotensin II type 2 (AT2) receptors, renin, angiotensinogen, angiotensin-converting enzyme (ACE), angiotensins I, II and III. Newly identified components (pro)renin receptor [12] [13], ACE2 [13,14], Mas protein (putative receptor for angiotensin (1–7) [15], and the AT4 receptor for angiotensin IV [16,17] have revolutionized our understanding of the functionality of the RAS. Components of the RAS have been found in a wide range of tissues and organs with classic cardiovascular functions as well as novel functions [18,19], In the brain the RAS also acts in regions associated with novel functions, such as cell differentiation, memory, and neurogenesis [7,10,20,21]. Newly discovered roles for the brain RAS in pathogenesis include the formation of intracranial aneurysms [22] mental retardation [23,24] and a role in epilepsy [24,25].
In the course of investigating RAS functionalities a novel non-AT1, non-AT2 binding site for angiotensin peptides was discovered [26,27]. This novel binding site is highly specific for Ang II and III and is present in rat, mouse, and human brain membranes [27–29], and mouse testis membranes, as well as other tissues [30] This novel binding site is not blocked by AT1 or AT2 receptor antagonists [26,27]. Interestingly, this novel binding site is unmasked in the presence of parachloromercuribenzoate (PCMB), an angiotensinase inhibitor [31,32] and to a lesser extent parachloromercuribenzenesulfonate (PCMBS), both of which react with sulfhydryls. Recently, we identified this binding site as neurolysin (E.C. 3.4.24.16) a member of the M3 zinc metallopeptidase family [33]
PCMB, is an organometallic agent that causes a conformational change in proteins most likely by reacting with free thiol groups of cysteine containing proteins and disulfide bonds resulting in the loss of biological activities by some of these proteins [34–36] and the activation of others [37]. PCMB and PCMBS are known protease/peptidase inhibitors [38–40]. While neurolysin is most well known for its ability to metabolize the neuropeptide neurotensin, it also metabolizes angiotensin I to form Ang (1–7) [41]. In addition, it metabolizes Ang II [42,43] reportedly at its Tyr-ile bond to form two tetrapeptides [44,45]. The enzymatic activity of neurolysin can be inhibited by the sulfhydryl reducing agent DTT, but not by the sulfhydryl alkylator iodoacetamide [43,46]. The rat liver soluble angiotensin binding protein, subsequently identified as neurolysin is inhibited by the organomercuric compound PCMBS [42]. Rat liver mitochondrial oligopeptidase, also identified as neurolysin, is inhibited by PCMB and N-ethyl maleimide [47].
This unmasking of the binding of Ang II is reversed by the disulfide-reducing agents DTT and 2- mercaptoethanol,[26] indicating possible involvement of cys-cys sulfhydryl bonds in maintaining the conformation of the protein to enable it to bind Ang II with high affinity. Alternatively, DTT is capable of dissociating PCMB from cysteines in proteins returning them to reduced sulfhydryls [48]. To further characterize the binding site, we presently report how this novel angiotensin binding protein interacts with different oxidative and sulfhydryl reactive agents in the presence and absence of PCMB in the brain and testis. These tissues were chosen because of their high abundance of this novel Ang II binding protein in the mouse [30]. Notably, neurolysin protein [49] and mRNA [50] have been reported to be abundantly expressed in the testis. Furthermore the two variants of neurolysin found in rat brain contain 12 and 13 cysteines [50].
While the novel binding site is unmasked by organomercurial agents, the primary question arising from this observation is whether such unmasking might occur under physiological or pathophysiological situations, in particular, altered redox states, or whether it is a pharmacological effect. There is considerable evidence suggesting that reversible oxidation of cysteine sulfhydryls is a fast-acting regulatory mechanism, enabling cells and tissues to respond to oxidative stress by activating or inhibiting enzymatic activity, see review [51]. This study’s main focus was to determine whether additional agents which may interact with cysteine sulfhydryls can also unmask or activate the binding site or reverse the unmasking effect of PCMB.
2. Materials and Methods
2.1 Animals
For behavioral experiments, male Sprague-Dawley rats (10–14 weeks of age) were individually housed in an AAALAC-approved vivarium and maintained on rat chow (Harland Tekland Rodent Diet, Madison WI) and water ad libitum except the night prior to surgery when food was removed. The vivarium was maintained at 22 ± 1° C on a 12:12 h light/dark cycle initiated at 07:00 h. For radioligand binding assays rat tissues were obtained from ongoing experiments at the University of Florida. Rats were sacrificed with an overdose of flurothane and the brain and testes were immediately harvested and frozen at −20° C until used for radioligand binding assays. All animal procedures were approved by the IACUC’s at Nova Southeastern University, University of Florida and Washington State University.
2.2 Radioligand Binding Assays
Binding of 125I-Sarcosine1, Isoleucine8 angiotensin II (125I-SI Ang II) to the novel, non-AT1, non-AT2 angiotensin binding site in the rat brain and testis as well as liver AT1 receptors was assessed by receptor binding assays based upon established procedures [26,52]. Briefly: frozen tissues were weighed and homogenized in ice-cold hypotonic buffer (20 mM NaPO4, pH 7.4) by mechanical homogenizer (Tissuemizer, Tekmar). All homogenates were centrifuged (40–48,000 × g for 10–20 min at 4–10° C) and the supernatants decanted. The membrane pellets were resuspended by homogenization in 25 ml assay buffer (150 mM NaCl, 5 mM EDTA, 0.1 mM bacitracin, 50 mM NaPO4, pH 7.1–7.2). The homogenates were recentrifuged as before and the pellets resuspended by homogenization in the assay buffer (50mg/ml initial wet tissue weight). Losartan and PD123319 (final concentration of 10 μM each) were added to the brain and testis membrane homogenates 10–15 minutes before incubation to eliminate binding of 125I-SI Ang II to AT1 or AT2 receptors in these tissues. When present in the brain and testis homogenates, parachloromercuribenzoic acid (PCMB, final concentration of 0.1 mM), derived from a 100 mM stock solution in 250 mM NaOH, was added to the membrane homogenate 10–15 minutes before incubation. Rat liver membrane homogenates were resuspended in assay buffer only at a concentration of (20 mg/ml initial wet tissue weight).
To enable assessment and comparison of the effects of sulfhydryl reagents, reducing agents and oxidizing agents on PCMB unmasked and non-PCMB unmasked novel, non-AT1, non-AT2 angiotensin binding sites, these reagents were added to the tissue homogenates 10–15 minutes before the incubation period. After 1-hour incubation at 22–24° C the homogenates aspirated onto GF/B filters (prewetted with 1 mg/ml bovine albumin solution) using a cell harvester (Model M24R, Brandel, Gaithersburg, MD). The incubation tubes and filters were rinsed 3 times with (50 mM NaKPO4, pH 7.4), The filter disks upon which the tissue membranes were harvested were measured with a COBRA II gamma counter at a counting efficiency of ~70%.125I-SI Ang II was prepared at American Radiolabeled Chemicals (St. Louis, MO) or the University of Florida using the chloramine T procedure [53] and purified by HPLC [54].
Unless otherwise stated, all binding assays were performed by incubation of 40 μl 125I-SI Ang II, to achieve a final concentration of 250 pM, with 50 μl of membrane homogenate, in the absence or presence or 3 μM Ang II, in a total volume of 100 μl. Binding in the presence of 3 μM Ang II was defined as non-specific binding and subtracted from binding in the absence of Ang II, defined as total binding, to derive specific binding to the novel binding site.
Saturation binding assays were carried out with six concentrations of 125I-SI-Ang II (0.2–3 nM). Primary screening of test compounds were carried out in the presence of 250 pM 125I-SI Ang II and a single concentration (100 μM) of test compound. This concentration was similar to that of PCMB to unmask the binding of 125I-SI Ang II. It was anticipated that concentrations of the inactive test compounds lower than 100 μM would also be inactive. However, one cannot rule out the possibility that such compounds also demonstrated a U-shaped concentration effect relationship (albeit with higher potency than PCMB) which could have produced false negative results. If more than a 50% inhibition of 125I-SI-Ang II binding was observed, or if specific 125I-SI-Ang II binding was observed in the absence of PCMB, a secondary screen in the presence of 5 different concentrations of the test compound was carried out. Determination of Bmax (fmol of radioligand bound per mg initial wet weight), KD, IC50, and EC50 values were determined from specific binding using one-site saturation, competition binding or dose response stimulation models of Prism software (Graphpad Software, San Diego, CA). For competition binding analyses, a constraint of 100% was used for binding in the absence of competing ligand. If the data points did not accurately represent the one-site competition binding models, an additional constraint of no less than 0% inhibition of binding was used. The data was fitted to the variable Hill slope model (Prism) for comparison with the one-site model to determine if there was a statistically significant improvement with the more complex equation using the equation comparison algorithm of Prism. Comparisons of IC50 values were made using the curve comparison algorithm of Prism. For other statistical comparisons a two-way ANOVA was used with Bonferroni post hoc comparisons or a paired t test was used. Values reported are mean ± SEM.
2.3 Intracerebroventricular (ICV) Surgical Procedure
Six rats were each anesthetized with Ketamine/Xylazine (100 and 2 mg/kg, respectively, i.m.) and fitted with a chronic icv guide cannula (PE-60, Clay Adams, Sparks, MD) under aseptic stereotaxic surgery. The tip of the guide cannula was positioned just above the roof of the right lateral ventricle as previously described [55] Briefly, flat skull coordinates were 1.0 mm posterior to bregma and 1.5 mm lateral from midline. Behavioral testing for drinking responses began following one week of post-operative recovery. The drinking test was conducted between 07:00 and 10:00 under low light level in a quiet room painted black. ICV injections of Ang II (100 pmol in 2 μl artificial cerebrospinal fluid [aCSF]) or Ang II + HgCl2 (100 pmol and 2 μl of a100 μM solution prepared in aCSF, respectively) were given at least 3 days apart in a counterbalanced design such that 3 rats received AngII first followed by Ang II + HgCl2, and the other 3 rats received Ang II + HgCl2 first followed by Ang II. The icv injections were made using a 10 μl Hamilton syringe connected to a length of PE-20 tubing prepared with a 30 gauge stainless steel tubing injector that was passed within the PE-60 guide cannula. This injector extended 2 mm beyond the tip of the guide cannula, thus penetrating the lateral ventricle. The 2 μl infusant was delivered by hand over a period of 30 sec. The injector was left in place for 60 sec and then gently removed. The drinking test commenced immediately after the withdrawal of the injector. Latency to drink was measured as the time following removal of the injector until the first bubbles rose in the burette due to drinking. Water intake was measured to the nearest 0.1 ml using gas flow burettes prepared with stainless steel sipper tubes. The volume drunk was recorded every 5 min for 30 min.
At the conclusion of the drinking tests, correct cannula placement was checked by the infusion of 5 μl of fast green dye under Equithesin anesthesia (active ingredient pentobarbital 65 mg/ml, 2 ml/kg i.p.) followed by visual confirmation of dye in the lateral ventricles. All rats had correct icv guide cannula placements.
A paired t test was run to determine whether HgCl2 pretreatment reduced cumulative water consumption at 30 min. Values reported are mean ± SEM.
3. Results
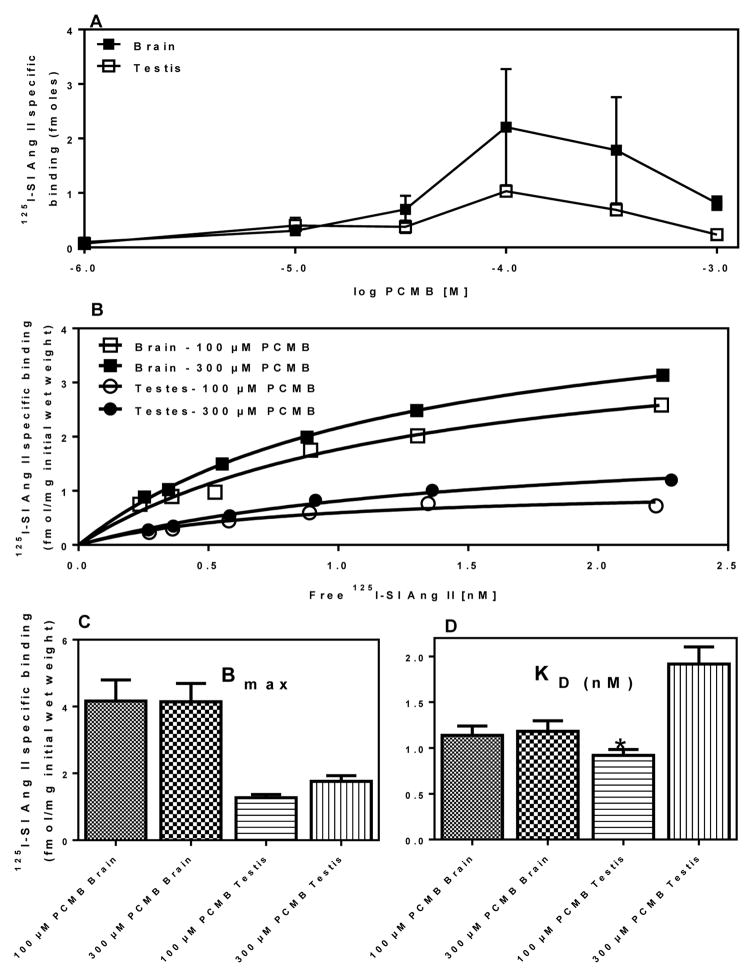
As shown previously [26], in rat brain and mouse tissues, PCMB unmasked a specific binding site for 125I-SI Ang II in the presence of concentrations of losartan (10 μM) and PD123319 (10 μM) sufficient to occupy > 99% of AT1 and AT2 receptors, respectively. As shown in Figure 1, optimal unmasking was observed at PCMB concentrations of 100–300 μM. The optimal PCMB concentration are slightly lower than our initial report [26], but is consistent with subsequent more recent studies [33,56]. Two-way ANOVA of the KD values indicated that the KD for testis was lower at 100 μM than at 300 μM (p<0.01) and that there was a main effect of higher affinity at 100 μM versus 300 μM (p=0.0033). Two-way ANOVA of Bmax values revealed a higher Bmax in brain than in testis (p=0.0003). Based upon these observations a concentration of 100 μM PCMB was used for subsequent screening assays.
Figure 1. Saturation isotherm for 125 I-SI Ang II binding to brain and testis with 100 μM and 300 μM PCMB.
Panel A: Optimization of PCMB concentration in testis (n=3) and brain (n=2). Panel B: representative plot of specific (3 μM Ang II displaceable) 125I-SI Ang II binding to brain and testis in the presence of 100 or 300 μM PCMB. Panel C: Bmax values for 125 I-SI Ang II binding to Brain and Testis with 100 μM and 300 μM PCMB n = 3. Panel D: KD values for 125 I-SI Ang II binding to Brain and Testis with 100 μM and 300 μM PCMB, n = 3. *Significantly higher affinity than 300 μM PCMB (p = 0.0164).
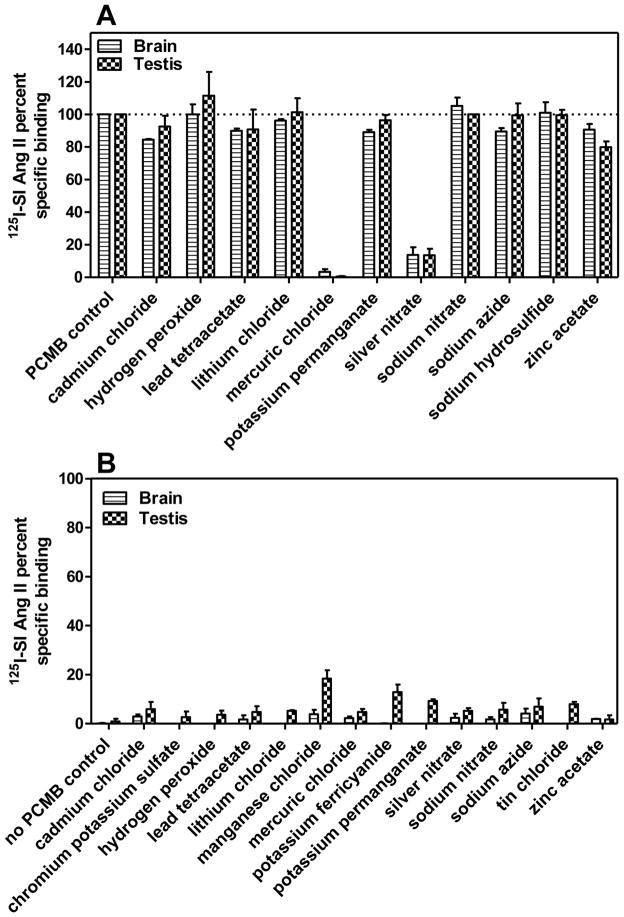
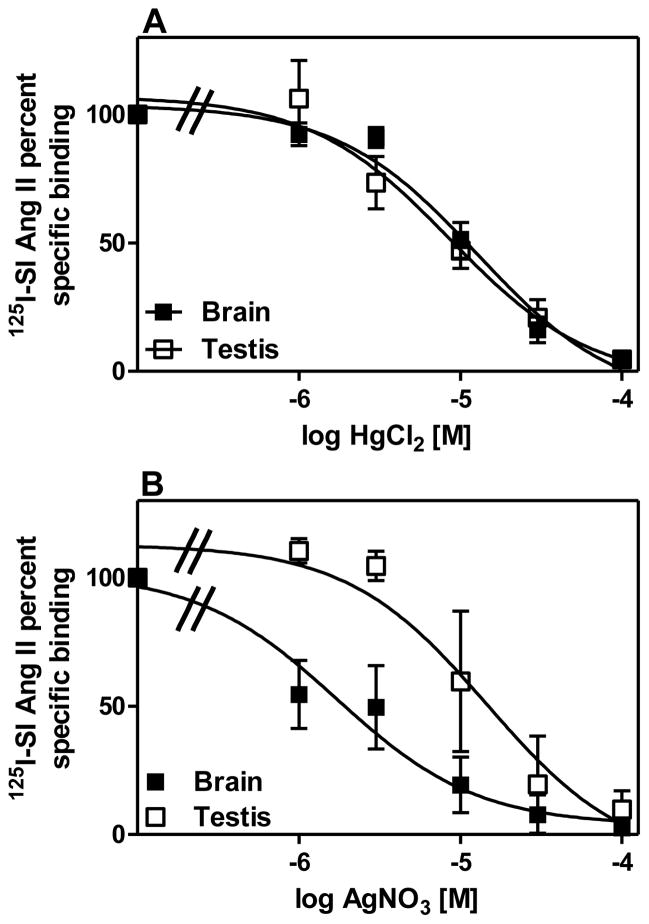
Figure 2A describes the primary screening of a series of inorganic compounds to compete for specific 125I-SI Ang II binding in the presence or absence of 100 μM PCMB. Of the 11 compounds tested, including the endogenous reactive oxygen species, hydrogen peroxide, only two: mercuric chloride (HgCl2) and silver nitrate (AgNO3) significantly reduced the unmasking of specific 125I-SI Ang II binding in the presence of PCMB at 100 μM. Secondary screening of these compounds to determine their IC50 values (Figure 3) indicated that they were capable of fully blocking the PCMB unmasking of specific 125I-SI Ang II binding at micromolar concentrations. The log molar IC50 values ± SEM for HgCl2 were −4.84 ± 0.10 and −4.96 ± 0.17 for brain and testis, respectively. The log molar IC50 values ± SEM for AgNO3 were −5.75 ± 0.18 and −4.67 ± 0.31 for brain and testis, respectively. Comparison of the log molar IC50 values for brain and testis by paired t test indicated that AgNO3 was a more potent inhibitor of PCMB unmasking (p=0.0094) in brain than in testis. Sodium nitrate did not show any inhibition of the unmasking suggesting that it was the silver ion that was the moiety of this salt that blocked the unmasking effect of PCMB.
Figure 2. Effect of different inorganic compounds at 100 μM concentrations on 125I-SI Ang II binding in the presence and absence of PCMB.
Panel A shows inorganic compounds in the presence of 100 μM PCMB, where only mercuric chloride and silver nitrate substantially inhibited the unmasking effects of PCMB. Panel B shows inorganic compounds in the absence of 100 μM PCMB. No compounds were able to unmask the binding site alone at ~100 μM concentrations. The small increase in specific 125I-SI Ang II binding in the presence of potassium permanganate was to a low affinity binding site that appears to be distinct from the binding site that is unmasked by PCMB.
Figure 3. Inhibition of 125I-SI Ang II binding to the novel Ang II binding site by HgCl2 and AgNO3.
Panel A shows the inhibition curves of HgCl2 for brain (IC50 = 14.3 μM) and testis (IC50 = 11.0 μM) (n = 3). Panel B shows the inhibition curves of AgNO3 for brain (IC50 =1.77 μM) and testis (IC50 = 21.3 μM) (n = 3).
In the absence of PCMB the 14 inorganic compounds tested showed limited ability to unmask specific 125I-SI Ang II binding (Figure 2B). There was less than 20% of the specific binding that was unmasked by PCMB. The small amount of unmasking observed appeared to be greater in the testis than in the brain. A two-way ANOVA (tissues and different compounds as factors) indicated a significantly greater unmasking in testis than in brain (p<0.0001). Post hoc Bonferroni comparisons indicated that manganese chloride and potassium ferricyanide showed a greater unmasking in testis compared to brain (p<0.05).
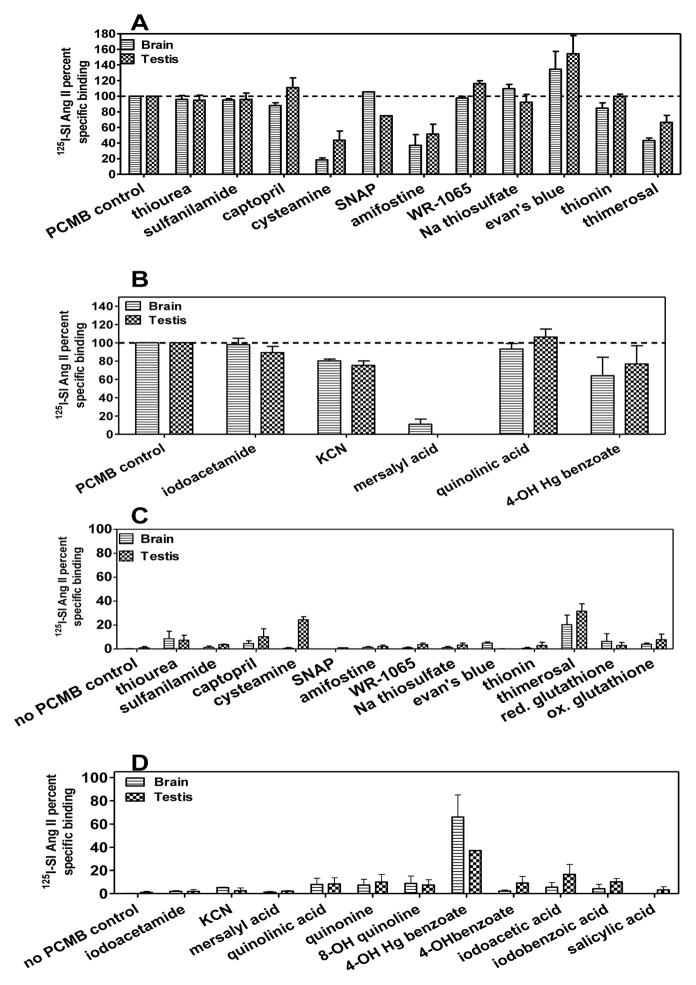
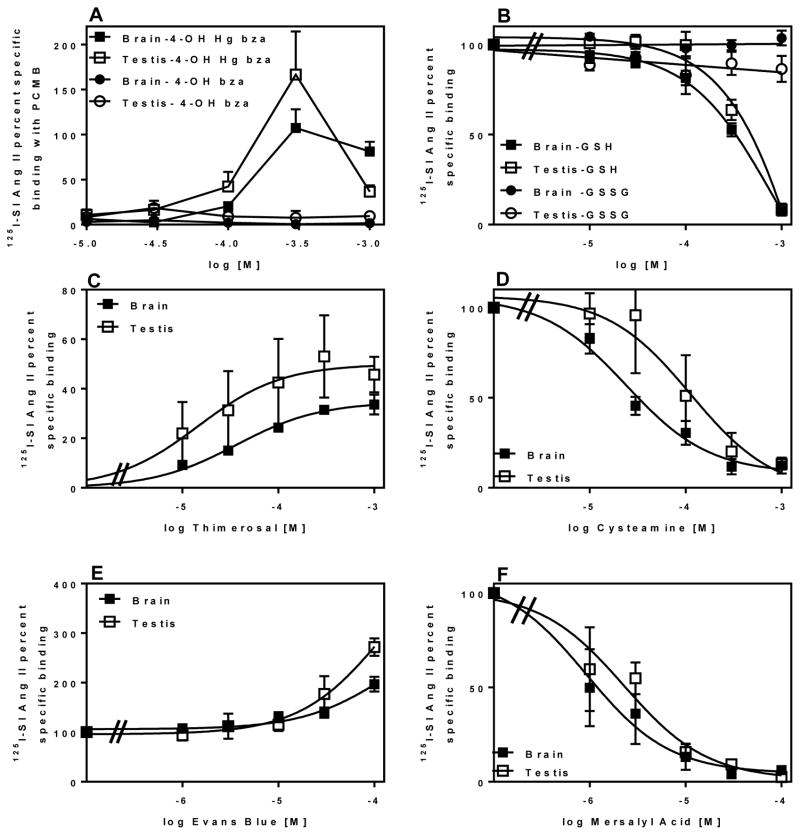
Figure 4 describes primary screening of a selection of organic compounds for their ability to inhibit unmasking of the novel non-AT1 non-AT2 binding site in brain and testis. Among the sulfur-containing organic compounds, only cysteamine and amifostine, inhibited the unmasking by more than 50% in both brain and testis. Glutathione was not included in the primary screening based on a previous observation of its inhibitory effect [56]. Among the non-sulfur containing organic compounds (KCN was included here because it contains a carbon), only mersalyl acid inhibited the unmasking by more than 50% in both brain and testis. In contrast, Evans blue enhanced 125I-SI Ang II binding in the presence of PCMB in both brain and testis. There was no difference in the extent of inhibition of binding in the brain and testis with the organic compounds.
Figure 4. Effects of different sulfur and non-sulfur containing organic compounds at 100 μM concentrations on 125I-SI Ang II binding in the presence and absence of PCMB.
Panel A shows sulfur containing organic compounds in the presence of 100 μM PCMB. Greater than 50% inhibition of 125I-SI Ang II binding was observed with cysteamine, amifostine and thimerosal. Panel B shows non-sulfur containing organics in the presence of 100 μM PCMB. Mersalyl acid showed full inhibition of the unmasking of the binding site. Panel C shows sulfur containing organic compounds on 125I-SI Ang II binding in the absence of 100 μM PCMB. No compounds had a significant increase or unmasking effect on non-AT1, non-AT2 binding of 125I-SI Ang II although thimerosal (being a mercury containing compound) was later revisited for optimal unmasking effects. Panel D shows non-sulfur containing organic compounds in the absence of 100 μM PCMB. 4-hydroxymercuribenzoate (4-OH Hg bza) showed partial unmasking relative to 100 μM PCMB. Concentration of compounds tested was constant at ~ 100 μM.
Secondary screening of cysteamine, mersalyl acid, and reduced glutathione (GSH) to determine their IC50 values indicated that they were capable of fully blocking the PCMB unmasking of specific 125I-SI Ang II binding at micromolar concentrations. (Figure 5 panels A, D and E). Oxidized glutathione (GSSG) did not block the PCMB unmasking (Figure 5 panel A). Amifostine inhibition was < 50% at or above 100 μM in secondary screening (data not shown). Moreover, the active metabolite of amifostine WR-1065 did not inhibit the unmasking at 100 μM. For cysteamine the log molar IC50 ± SEM values were −4.55 ± 0.10 and −3.86 ± 0.34 for brain and testis, respectively. The log molar IC50 values ± SEM for mersalyl acid were −5.96 ± 0.19 and −5.64 ± 0.17 for brain and testis, respectively. Inhibition of the PCMB mediated unmasking of 125I-SI Ang II binding by GSH revealed a complex interaction. To evaluate the inhibition it was necessary to constrain both the top and bottom values of the fitted curves at 100% and 0%. Under such constraints, the inhibition curves for GSH were significantly different from −1 for both brain and testis. The Hill slope for testis (−2.62 ± 0.59) was steeper than the Hill slope for brain (−1.47 ± 0.17) (p<0.05). There was also a small difference in log IC50 with GSH being slightly more potent in the brain (−3.54±0.035) than in the testis (−3.42±0.037) (p<0.05).
Figure 5. Inhibition or enhancement curves of organic compounds for non-AT1, non-AT2 binding of 125I-SI Ang II.
Panel A shows comparison of the ability of 4-hydroxymercuribenzoate (4-OH Hg bza) and 4-hydroxybenzoate (4-OH bza) to unmask of the non-AT1, non-AT2 binding of 125I-SI Ang II relative to 100μM PCMB. Optimal binding with 4-OH Hg bza was observed at 300μM in brain (n=4) and testis (n=3). 4-OH bza had no significant effect on the binding site in brain (n=4) and testis (n=3).Panel B shows a comparison of reduced (GSH) and oxidized (GSSG) glutathione on the unmasking of non-AT1, non-AT2 binding of 125I-SI Ang II by PCMB (100 μM). GSH inhibited brain (IC50=285 μM) and testis (IC50= 376 μM) (n=5) non-AT1, non-AT2 binding of 125I-SI Ang II. GSSG had no effect on the binding (n=3). Panel C shows the ability of different concentrations of thimerosal to unmask the non-AT1, non-AT2 binding of 125I-SI Ang II relative to PCMB in brain(EC50=36.5 μM, Rmax= 34.7±1.6%) and testis (EC50=14.7 μM, Rmax= 49.9±9.2%) (n=3). Panel D shows inhibition of specific 125I-SI Ang II binding in the presence of PCMB by cysteamine in brain (IC50=28.1 μM) (n=5) and testis (IC50= 138 μM) (n=4). Panel E shows the enhancing effect on specific 125I-SI Ang II binding in the presence of PCMB by Evans blue in brain (EC50=71.2 μM, Rmax=163±56%) and testis (EC50= 156 μM, Rmax=442±340%)) (n=3). Panel F shows inhibition of specific 125I-SI Ang II binding in the presence of PCMB by mersalyl acid for brain (IC50=1.10 μM) and testis (IC50= 2.27 μM) (n=3).
The enhancement of 125I-SI Ang II binding in the presence of PCMB by Evans blue was assessed with a range of concentrations of Evans blue up to 100 μM. The enhancement was concentration dependent through the range of concentrations studied, however, the curves did not appear to attain an inflection point from which a reliable estimate of EC50 and Emax could be determined. As shown in Figure 4C, in the absence of PCMB Evans blue was unable to unmask 125I-SI Ang II binding.
In the absence of PCMB both the sulfur and non-sulfur containing organic compounds showed limited ability to unmask specific 125I-SI Ang II binding (Figure 4, panels C and D) with 2 exceptions. 4-hydroxymercuribenzoic acid (4-OH Hg bza) was capable of fully unmasking the 125I-SI Ang II binding in both brain and testis. As shown in Figure 5B it attained peak unmasking at ~300 μM which was not significantly different from the amount of unmasking generated by PCMB. Above 300 μM there was less unmasking of 125I-SI Ang II binding, reminiscent of the concentration-dependent pattern of unmasking of 125I SI Ang II binding by PCMB. Thimerosal which is cleaved to ethyl mercury upon binding to thiols [57] also unmasked 125I-SI Ang II binding in a concentration dependent manner. However, as shown in Figure 5C thimerosal unmasked only 34.7 ± 1.6 and 49.9 ± 9.2% of PCMB unmasked binding in brain and testis, respectively, with log molar EC50 values of −4.44 ± 0.09 in brain and −4.83 ± 0.42 in testis. In contrast to the other organic mercurials, mersalyl acid showed no ability to unmask 125I-SI Ang II in either brain and testis at concentrations up to 1 mM (data not shown).
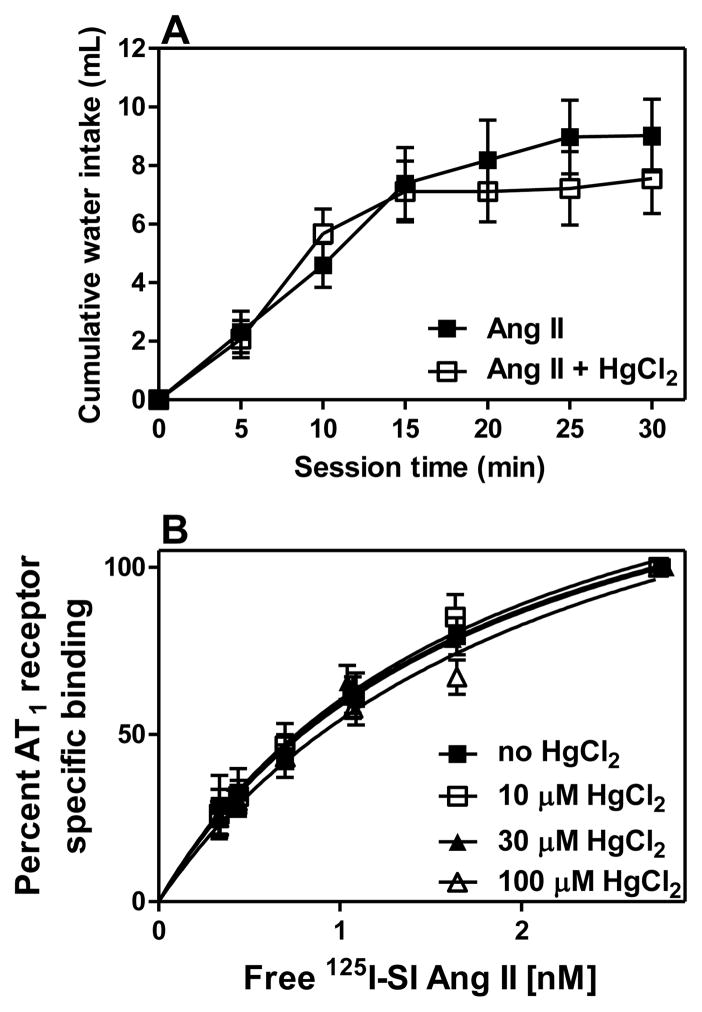
To determine if the inhibition of the PCMB unmasked 125I-SI Ang II binding by HgCl2 might be due to an interaction with Ang II rather than an interaction with cysteines in neurolysin, we examined the effect of HgCl2 on Ang II induced drinking and 125I-SI Ang II binding to liver. As shown in Figure 6A, ICV pretreatment of rats with 200 pmoles of HgCl2 prior to ICV administration of 100 pmoles of Ang II did not affect the dipsogenic response to Ang II. As shown in Figure 6B, HgCl2 in concentrations ranging from 10 to 100 μM did not impair 125I-SI Ang II binding to AT1 receptors in the rat liver. This suggests that HgCl2 does not inhibit the ability of Ang II to bind to and activate AT1 receptors.
Figure 6. HgCl2 does not affect responsivity to Ang II or AT1 receptor binding in liver.
Panel A indicates dipsogenic responses to icv Ang II with and without HgCl2 pretreatment (n=6). Panel B describes 125 I-SI Ang II binding to AT1 receptors in rat liver homogenate in the presence of varying concentrations of HgCl2 (10–100 μM) (n=2). Each tube had 1 mg of liver initial wet weight. Bmax values for liver containing no HgCl2 was 23.3±0.89, and Kd value of 3.57±0.20; for liver containing 10 μM HgCl2 Bmax was 20.8±1.8 and Kd of 2.93±0.40; liver containing 30 μM HgCl2 had a Bmax value of 20.6±2.4 and a Kd of 2.41±0.46; lastly, liver with 100 μM HgCl2 had a Bmax of 20.4±4.4 and a Kd of 3.15±1.1. Panel B shows a representative (n=1) values for the liver AT1 receptors saturation assays.
4. Discussion
These studies focused upon characterization of the PCMB-mediated unmasking of a hypothesized new member of the RAS, the non-AT1, non-AT2 angiotensin binding site discovered rat brain membranes [26], and found to be present in mouse, and human brain membranes as well [27,29]. Of primary interest for assessment of the PCMB activation of 125I-SI Ang II binding is whether PCMB mimics naturally occurring physiological or pathophysiological conditions. While these studies were in progress, we determined that this potential new member of the RAS was a membrane associated variant of neurolysin (E.C.3.4.24.16) based upon studies in neurolysin transfected cells and neurolysin knockout mouse brains [33]. Neurolysin has a broad tissue distribution [49] and is highly expressed in rat testis membranes as well as the brain [50]. It has been suggested that testis neurolysin may differ from brain neurolysin [58], which is consistent with the minor variations we observed between brain and testis membranes. However the difference seen by Rodd and Hersh might reflect a minor contamination of thimet oligopeptidase in the purified neurolysin so this matter remains unresolved.
Interestingly, we found a substantially lower concentration of the binding site in testis compared to brain. This contrasts with the observation of a greater amount of the binding site in mouse testis compared to mouse brain [30] and the greater amount of neurolysin found in rat testis compared to brain [59]. Comparison of Bmax values in rat and mouse brain suggests that the values are similar, assuming approximately 3 mg protein per 100 mg initial wet weight. However, the values in rat testis appear to be considerably lower than those reported for mouse testis [30]. The rat brain Bmax values are also similar to those previously reported for rat brain [26] albeit at lower concentrations of PCMB as noted above. With respect to the different distribution of neurolysin in brain and testis, [59] assayed both soluble and membrane associated neurolysin. This suggests that the ratio of membrane associated neurolysin to soluble neurolysin is much higher in brain than in testis.
Oxidation of sulfur containing amino acids is known to regulate the activities of a host of proteins [60,61] see review [62]. Oxidation of cys18 and cys 138 in angiotensinogen to form a disulfide bond has also been identified as a promoter of Ang I formation from angiotensinogen [63]. The functionalities of both the AT1 and AT2 receptors are affected by oxidation of extracellular cysteines to form disulfide bonds In the case of the AT1 receptor, it is essential that a disulfide bond between Cys 18 and cys 274 be intact because sulfhydryl reducing agents such as DTT and beta-mercaptoethanol impair its ability to bind Ang II [64–66]. Other G protein-coupled receptors also require an intact sulfhydryl linkage between cysteines in their extracellular domains to bind their agonists [67–69]. Conversely, sulfhydryl reducing agents increase the binding of Ang II to the AT2 receptor subtype presumably by disrupting a disulfide bond in the extracellular domain [65,66,70–72].
The inhibition of both PCMB unmasking [26] and inhibition of the enzymatic activity of neurolysin [43,50] by DTT suggests that disulfide bonded cysteines produce a conformation of neurolysin that favors substrate binding and cleavage. Neurolysin is reported to have a very narrow channel through which the substrate must traverse to reach the active site of the enzyme [45]. It is possible that one or more disulfide bonds are critical to maintaining the channel in the open position to admit substrate. If these bond(s) are broken then the channel may close and not allow the substrate to gain access to the active site. Under such conditions, any conformational alterations in the area of the active site would have no consequence for substrate binding.
The anomalous behavior of Evans blue to enhance 125I-SI Ang II binding unmasked by PCMB is surprising in view of its failure to unmask 125I-SI Ang II binding in the absence of PCMB. Evans blue is an azo dye, but it also has 4 sulfonate groups. Azo dyes are reported to bind to both methionines and cysteines on proteins [73], however, the negative charge of the sulfonate groups of Evans blue is not amenable to interaction with cysteine sulfhydryls. It is possible that a PCMB induced conformational change in neurolysin could facilitate an additional interaction with Evans blue that further enhances the ability of neurolysin to bind Ang II.
There can be little doubt that the mechanism whereby PCMB unmasks Ang II binding to neurolysin involves binding to a cysteine sulfhydryl. The ability of 4-hydroxymercuribenzoic acid, but not 4-hydroxybenzoic acid, to unmask 125I-SI Ang II binding strongly supports this conceptual mechanism. Assessment of the ability of 11 inorganic compounds to inhibit the unmasking effect of PCMB revealed only two that significantly inhibited the unmasking; mercuric chloride and silver nitrate. Both compounds displayed what appeared to be competitive inhibition of the unmasking effect. Protein cysteine sulfhydryls are a known target of HgCl2 [74,75]. Silver ion also binds avidly to the SH of protein cysteines, and the ability of AgNO3 to alter ion currents in frog oocytes is dependent on its ability to bind to free SH groups of cysteines [76]. Thus it is likely that silver ion also competes with PCMB for cysteine sulfhydryls, preventing PCMB from unmasking Ang II binding. Silver nitrate generates reactive oxygen species (ROS), which may also compete with PCMB for cysteines or promote disulfide formation. However, addition of hydrogen peroxide to the membranes did not inhibit the PCMB effect. An explanation for the lack of adverse reactive oxygen species effects is that the critical cysteine that mediates the unmasking by PCMB is not close enough to another cysteine residue to form a disulfide bond under conditions of oxidative stress.
The membrane binding of 125I-SI Ang II was further studied using reduced and oxidized glutathione (GSH and GSSG, respectively) to determine optimal environmental conditions and compare how the novel binding site interacts with these species (Figure 5, panel A). The addition of GSSG to the brain and testis did not inhibit the unmasking effects of PCMB. GSSG lacks the free thiol group that interacts with cysteine sulfhydryls, suggesting that the free thiol group of GSH glutathionylates the critical cysteine of neurolysin to compete with PCMB for binding to this cysteine. An alternative possibility, that GSH binds to PCMB seems unlikely in view of the ability of EDTA (present at 5 mM in these assays) to inhibit the binding of GSH to a model organomercurial compound that behaves similarly to PCMB [77].
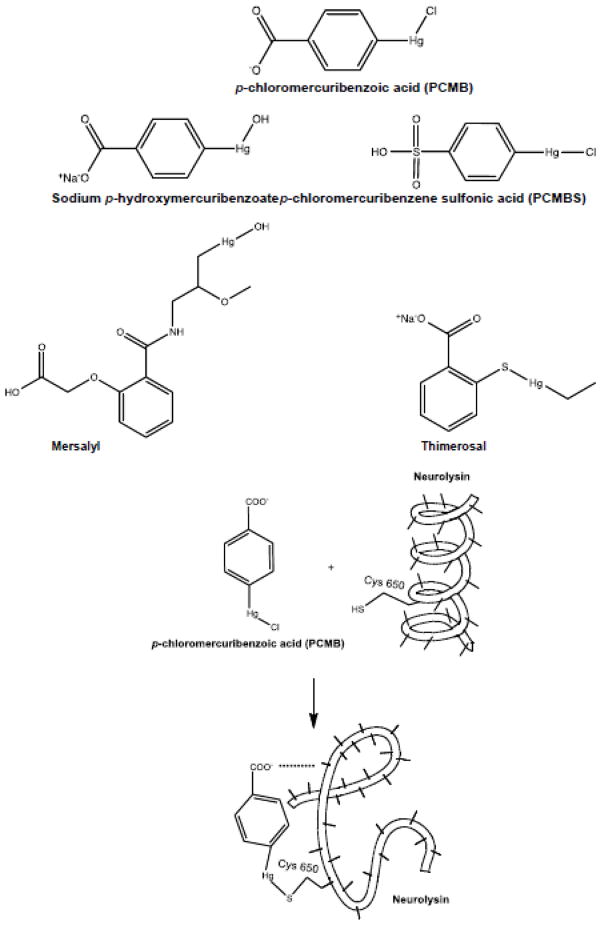
The relative or complete inability of most of these competing sulfhydryl compounds to unmask Ang II binding on their own can be explained based upon bivalency and size relative to those of the organomercurial compounds capable of unmasking the Ang II binding. Organomercurials can only react with a single cysteine sulfhydryl, however the sulfonate moiety of PCMBS can form an ionic bond with cationically charged amino acids adjacent to the cysteine targeted by the organomercurial [78]. In a similar manner, the carboxyl moiety of PCMB and 4-hydroxymercuribenzoate might also form an ionic bond with a basic amino acid. Of note, although mersalyl acid is also a carboxylic acid organomercurial it is considerably bulkier than PCMB, PCMBS and 4-hydroxymercuribenzoic acid (Figure 7) and may therefore be sterically unable to crosslink the critical basic amino acid moiety needed to effect the proposed conformational change in neurolysin that unmasks Ang II binding. Our observation that concentrations up to 1 mM mersalyl acid did not unmask binding suggests it does bind to the same cysteine as PCMB, but does not effect the same changes in protein function. In support of this hypothesis, it is noteworthy that mersalyl acid (Salyrgan®) was once used as a diuretic. PCMB and PCMBS are not only devoid of diuretic properties, they antagonize the diuretic actions of mersalyl acid and other mercurial diuretics [79,80].
Figure 7. Structures of organometallic compounds.
Structures of the compounds used in these studies and a schematic representation of the binding of PCMB to neurolysin at cysteine 650 inducing a conformational change in the protein structure by binding to a basic amino acid side chain with its carboxyl moiety.
Neurolysin has considerable amino acid homology with thimet oligopeptidase, to the point where substitution of only two amino acids at, or adjacent to their active zinc binding site domain of thimet oligopeptidase switches its cleavage site of neurotensin to that of neurolysin [81]. Since we have shown that PCMB does not unmask Ang II binding to thimet oligopeptidase [33], this suggests that the target cysteine in neurolysin is not conserved in thimet oligopeptidase. Of the 13 cysteines present in full-length (704 amino acids) variant of neurolysin [50] only five: Cys8, Cys88, Cys153, Cys256, and Cys650 are not conserved. According to the crystal structure of neurolysin reported by Brown et al., [45] only Cys 650 (cys627 using their numbering) is located near the zinc binding site in the substrate binding channel. This same cysteine is also unconserved in mouse and human thimet oligopeptidase. According to the crystal structure of Brown et al. [45], this cysteine is located on the α22 helix that crosses the binding channel, placing it in a position where its acidic domain could bind to a nearby arginine or lysine, producing a conformational change in the channel that facilitates the binding of Ang II. Another possibility is that the acidic moiety of PCMB could bind to the arginine2 of Ang II to increase the binding affinity of Ang II to neurolysin.
If this latter mechanism is correct, then the presence of arginine in the P3 position of the scissile site is necessary for unmasking of substrate binding. Neurotensin has an arginine in the P3 position. In our previous studies there was no evidence for an increased binding affinity for neurotensin in the presence of PCMB. In fact, the KI of neurotensin to compete for 125I-SI Ang II binding in rat brain [26]was 4 to 10-fold lower than its IC50, KI or KM as a neurolysin substrate or inhibitor [43,46]. However, the larger size of the decapeptide amino terminal cleavage product of neurotensin, which extends nearly to the bottom of the active site channel [45], may precludes its accommodation in the active site when the organomercurial compound is present. Ang II might still be easily accommodated because its amino terminal cleavage product is a much smaller tetrapeptide [44,45].
Based on these observations we propose that Cys650 of neurolysin is the target of PCMB and that the carboxy anion of PCMB reacts with a basic amino acid, either a lysine or arginine (or possibly a charged histidine) adjacent to Cys650 to effect a conformational change in enzyme to facilitate Ang II binding. Additional studies in which Cys650 is mutated to a different amino acid, e.g. alanine, will be needed to test this hypothesis.
Highlights.
Para-chloromercuribenzoate and other selected organomercurial agents unmask the ability of membrane associated neurolysin to bind angiotensin II with high affinity
The mechanism of this unmasking appears to involve the binding of para-chloromercuribenzoate to a cysteine sulfhydryl group at amino acid 650 that lines the active site channel of neurolysin
Acknowledgments
This work is supported by NHLBI HL-096357. The authors thank Dr. Eduardo Veliz for his contribution in knowledge of organic chemistry and biochemical reactions and Lancya Lansdowne for technical assistance. We thank Dr. Colin Sumners for providing the rat tissues used for the binding experiments. We also thank Dr. Syed Rizvi for preparing Figure 7 and the graphical abstract, and for critically reviewing this manuscript along with Drs. Michelle Clark and Vardan Karamyan.
Footnotes
Disclosures: The authors have no conflicts of interest to disclose regarding the work reported in this manuscript. All authors have reviewed and approved the final manuscript.
Author Contributions:
Kira L. Santos participated in the design of the radioligand binding assays, carried out all the radioligand binding assays, participated in the data analysis and graphical presentation of the data, and co-wrote the manuscript.
Megan A. Vento participated in the design of the behavioral experiments, carried out all the behavioral assays, participated in the behavioral data analysis and interpretation, and reviewed the manuscript.
John W. Wright participated in the design of the behavioral experiments, assisted in carrying out the behavioral assays, participated in the behavioral data analysis and interpretation, and co-wrote the manuscript.
Robert C. Speth participated in the design of the radioligand binding assays, data analysis and graphical presentation of the data, and co-wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Severs WB, Summy-Long J, Taylor JS, Connor JD. A central effect of angiotensin: release of pituitary pressor material. J Pharmacol Exp Ther. 1970;174:27–34. [PubMed] [Google Scholar]
- 2.Severs WB, Daniels-Severs AE. Effects of angiotensin on the central nervous system. Pharmacol Rev. 1973;25:415–49. [PubMed] [Google Scholar]
- 3.Phillips MI. Angiotensin in the brain. Neuroendocrinology. 1978;25:354–77. doi: 10.1159/000122756. [DOI] [PubMed] [Google Scholar]
- 4.Fitzsimons JT. Angiotensin, thirst, and sodium appetite. Physiological Reviews. 1998;78:583–686. doi: 10.1152/physrev.1998.78.3.583. [DOI] [PubMed] [Google Scholar]
- 5.Osborn JW, Fink GD, Sved AF, Toney GM, Raizada MK. Circulating angiotensin II and dietary salt: converging signals for neurogenic hypertension. Curr Hypertens Rep. 2007;9:228–35. doi: 10.1007/s11906-007-0041-3. [DOI] [PubMed] [Google Scholar]
- 6.Phillips MI, Sumners C. Angiotensin II in central nervous system physiology. Regul Pept. 1998;78:1–11. doi: 10.1016/s0167-0115(98)00122-0. [DOI] [PubMed] [Google Scholar]
- 7.Wright JW, Harding JW. The brain renin-angiotensin system: a diversity of functions and implications for CNS diseases. Pflugers Arch. 2012 doi: 10.1007/s00424-012-1102-2. [DOI] [PubMed] [Google Scholar]
- 8.Karamyan V, Speth R. Enzymatic pathways of the brain renin-angiotensin system: Unsolved problems and continuing challenges. Regul Pept. 2007;143:15–27. doi: 10.1016/j.regpep.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saavedra JM. Brain angiotensin II: new developments, unanswered questions and therapeutic opportunities. Cell Mol Neurobiol. 2005;25:485–512. doi: 10.1007/s10571-005-4011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phillips MI, de Oliveira EM. Brain renin angiotensin in disease. J Mol Med. 2008;86:715–22. doi: 10.1007/s00109-008-0331-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Speth RC, Karamyan VT. The significance of brain aminopeptidases in the regulation of the actions of angiotensin peptides in the brain. Heart Fail Rev. 2008;13:299–309. doi: 10.1007/s10741-007-9078-2. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen G, Delarue F, Burckle C, Bouzhir L, Giller T, Sraer JD. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest. 2002;109:1417–27. doi: 10.1172/JCI14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase 1. J Biol Chem. 2000;275:33238–43. doi: 10.1074/jbc.M002615200. [DOI] [PubMed] [Google Scholar]
- 14.Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ Res. 2000;87:E1–E9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 15.Santos RA, Simoes e Silva A, Maric C, Silva DM, Machado RP, de BI, et al. Angiotensin-(1–7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci U S A. 2003;100:8258–63. doi: 10.1073/pnas.1432869100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harding JW, Cook VI, Miller-Wing AV, Hanesworth JM, Sardinia MF, Hall KL, et al. Identification of an AII(3–8) [AIV] binding site in guinea pig hippocampus. Brain Res. 1992;583:340–3. doi: 10.1016/s0006-8993(10)80047-2. [DOI] [PubMed] [Google Scholar]
- 17.Albiston AL, McDowall SG, Matsacos D, Sim P, Clune E, Mustafa T, et al. Evidence that the angiotensin IV (AT4) receptor is the enzyme insulin regulated aminopeptidase. J Biol Chem. 2001;276:48623–6. doi: 10.1074/jbc.C100512200. [DOI] [PubMed] [Google Scholar]
- 18.Campbell DJ. Circulating and tissue angiotensin systems. J Clin Invest. 1987;79:1–6. doi: 10.1172/JCI112768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paul M, Poyan MA, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev. 2006;86:747–803. doi: 10.1152/physrev.00036.2005. [DOI] [PubMed] [Google Scholar]
- 20.McKinley MJ, Albiston AL, Allen AM, Mathai ML, May CN, McAllen RM, et al. The brain renin-angiotensin system: location and physiological roles. Int J Biochem Cell Biol. 2003;35:901–18. doi: 10.1016/s1357-2725(02)00306-0. [DOI] [PubMed] [Google Scholar]
- 21.Lazartigues E. A map and new directions for the (pro)renin receptor in the brain: focus on “A role of the (pro)renin receptor in neuronal cell differentiation”. Am J Physiol Regul Integr Comp Physiol. 2009;297:R248–R249. doi: 10.1152/ajpregu.00287.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shoja MM, Agutter PS, Tubbs RS, Payner TD, Ghabili K, Cohen-Gadol AA. The role of the renin--angiotensin system in the pathogenesis of intracranial aneurysms. J Renin Angiotensin Aldosterone Syst. 2011;12:262–73. doi: 10.1177/1470320310387845. [DOI] [PubMed] [Google Scholar]
- 23.Vervoort VS, Beachem MA, Edwards PS, Ladd S, Miller KE, de MX, et al. AGTR2 mutations in X-linked mental retardation. Sci. 2002;296:2401–3. doi: 10.1126/science.1072191. [DOI] [PubMed] [Google Scholar]
- 24.Cousin C, Bracquart D, Contrepas A, Nguyen G. Potential role of the (pro)renin receptor in cardiovascular and kidney diseases. J Nephrol. 2010;23:508–13. [PubMed] [Google Scholar]
- 25.Pereira MG, Becari C, Oliveira JA, Salgado MC, Garcia-Cairasco N, Costa-Neto CM. Inhibition of the renin-angiotensin system prevents seizures in a rat model of epilepsy. Clin Sci (Lond) 2010;119:477–82. doi: 10.1042/CS20100053. [DOI] [PubMed] [Google Scholar]
- 26.Karamyan VT, Speth RC. Identification of a novel non-AT1, non-AT2 angiotensin binding site in the rat brain. Brain Res. 2007;1143:83–91. doi: 10.1016/j.brainres.2007.01.051. [DOI] [PubMed] [Google Scholar]
- 27.Karamyan VT, Gembardt F, Rabey FM, Walther T, Speth RC. Characterization of the brain-specific non-AT(1), non-AT(2) angiotensin binding site in the mouse. Eur J Pharmacol. 2008;590:87–92. doi: 10.1016/j.ejphar.2008.05.035. [DOI] [PubMed] [Google Scholar]
- 28.Karamyan VT, Speth RC. Distribution of the Non-AT1, Non-AT2 Angiotensin-Binding Site in the Rat Brain: Preliminary Characterization. Neuroendocrinology. 2008;88:256–65. doi: 10.1159/000140635. [DOI] [PubMed] [Google Scholar]
- 29.Karamyan VT, Stockmeier CA, Speth RC. Human brain contains a novel non-AT1, non-AT2 binding site for active angiotensin peptides. Life Sci. 2008;83:421–5. doi: 10.1016/j.lfs.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rabey FM, Karamyan VT, Speth RC. Distribution of a novel binding site for angiotensins II and III in mouse tissues. Regul Pept. 2010;162:5–11. doi: 10.1016/j.regpep.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fujioka H, Okabe T, Yamaguchi H. Purification and characterization of angiotensin II degradation factor from porcine endothelial cells. Tohoku J Exp Med. 1995;177:183–92. doi: 10.1620/tjem.177.183. [DOI] [PubMed] [Google Scholar]
- 32.Kohara K, Tabuchi Y, Senanayake P, Brosnihan KB, Ferrario CM. Reassessment of plasma angiotensins measurement: effects of protease inhibitors and sample handling procedures. Peptides. 1991;12:1135–41. doi: 10.1016/0196-9781(91)90070-6. [DOI] [PubMed] [Google Scholar]
- 33.Wangler NJ, Santos KL, Schadock I, Hagen FK, Escher E, Bader M, et al. Identification of membrane-bound variant of metalloendopeptidase neurolysin (EC 3.4.24. 16) as the non-AT1, non-AT2 angiotensin binding site. J Biol Chem. 2012;287:1014–22. doi: 10.1074/jbc.M111.273052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Penefsky HS, Cross RL. Structure and mechanism of FoF1-type ATP synthases and ATPases. Adv Enzymol Relat Areas Mol Biol. 1991;64:173–214. doi: 10.1002/9780470123102.ch4. [DOI] [PubMed] [Google Scholar]
- 35.Pedersen PL. Adenosine triphosphatase from rat liver mitochondria - evidence for a mercurial-sensitive site for the activating anion bicarbonate. Biochem Biophys Res Commun. 1976;71:1182–8. doi: 10.1016/0006-291x(76)90778-6. [DOI] [PubMed] [Google Scholar]
- 36.Li Z, Neufeld GJ. Isolation and characterization of mitochondrial F(1)-ATPase from crayfish (Orconectes virilis) gills. Comp Biochem Physiol B Biochem Mol Biol. 2001;128:325–38. doi: 10.1016/s1096-4959(00)00330-4. [DOI] [PubMed] [Google Scholar]
- 37.Bublitz C, Lawler CA. The activation of glucose dehydrogenase by p-chloromercuribenzoate. Mol Cell Biochem. 1989;86:101–6. doi: 10.1007/BF00222609. [DOI] [PubMed] [Google Scholar]
- 38.Yoshimoto T, Simmons WH, Kita T, Tsuru D. Post-proline cleaving enzyme from lamb brain. J Biochem. 1981;90:325–34. doi: 10.1093/oxfordjournals.jbchem.a133477. [DOI] [PubMed] [Google Scholar]
- 39.Strom MS, Nunn DN, Lory S. Posttranslational processing of type IV prepilin and homologs by PilD of Pseudomonas aeruginosa. Methods Enzymol. 1994;235:527–40. doi: 10.1016/0076-6879(94)35168-6. [DOI] [PubMed] [Google Scholar]
- 40.Du PG, Kato S, Li YH, Maeda T, Yamane T, Yamamoto S, et al. Rat tripeptidyl peptidase I: molecular cloning, functional expression, tissue localization and enzymatic characterization. Biol Chem. 2001;382:1715–25. doi: 10.1515/BC.2001.207. [DOI] [PubMed] [Google Scholar]
- 41.Vincent B, Vincent JP, Checler F. Purification and characterization of human endopeptidase 3.4.24.16. Comparison with the porcine counterpart indicates a unique cleavage site on neurotensin. Brain Res. 1996;709:51–8. doi: 10.1016/0006-8993(95)01260-5. [DOI] [PubMed] [Google Scholar]
- 42.Kato A, Sugiura N, Hagiwara H, Hirose S. Cloning, amino acid sequence and tissue distribution of porcine thimet oligopeptidase. A comparison with soluble angiotensin-binding protein. Eur J Biochem. 1994;221:159–65. doi: 10.1111/j.1432-1033.1994.tb18725.x. [DOI] [PubMed] [Google Scholar]
- 43.Barelli H, Girard F, St PS, Kitabgi P, Vincent JP, Checler F. Further characterization of a neurotensin-degrading neutral metalloendopeptidase from rat brain. Neurochem Int. 1988;12:351–9. doi: 10.1016/0197-0186(88)90174-x. [DOI] [PubMed] [Google Scholar]
- 44.Dahms P, Mentlein R. Purification of the main somatostatin-degrading proteases from rat and pig brains, their action on other neuropeptides, and their identification as endopeptidases 24.15 and 24. 16. Eur J Biochem. 1992;208:145–54. doi: 10.1111/j.1432-1033.1992.tb17168.x. [DOI] [PubMed] [Google Scholar]
- 45.Brown CK, Madauss K, Lian W, Beck MR, Tolbert WD, Rodgers DW. Structure of neurolysin reveals a deep channel that limits substrate access. Proc Natl Acad Sci U S A. 2001;98:3127–32. doi: 10.1073/pnas.051633198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vincent B, Dauch P, Vincent JP, Checler F. Stably transfected human cells overexpressing rat brain endopeptidase 3.4.24. 16: biochemical characterization of the activity and expression of soluble and membrane-associated counterparts. J Neurochem. 1997;68:837–45. doi: 10.1046/j.1471-4159.1997.68020837.x. [DOI] [PubMed] [Google Scholar]
- 47.Serizawa A, Dando PM, Barrett AJ. Characterization of a mitochondrial metallopeptidase reveals neurolysin as a homologue of thimet oligopeptidase. J Biol Chem. 1995;270:2092–8. doi: 10.1074/jbc.270.5.2092. [DOI] [PubMed] [Google Scholar]
- 48.Shriver Z, Hu Y, Pojasek K, Sasisekharan R. Heparinase II from Flavobacterium heparinum. Role of cysteine in enzymatic activity as probed by chemical modification and site- directed mutagenesis. J Biol Chem. 1998;273:22904–12. doi: 10.1074/jbc.273.36.22904. [DOI] [PubMed] [Google Scholar]
- 49.Checler F, Barelli H, Vincent JP. Tissue distribution of a novel neurotensin-degrading metallopeptidase. An immunological approach using monospecific polyclonal antibodies. Biochem J. 1989;257:549–54. doi: 10.1042/bj2570549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dauch P, Vincent JP, Checler F. Molecular cloning and expression of rat brain endopeptidase 3.4.24. 16. J Biol Chem. 1995;270:27266–71. doi: 10.1074/jbc.270.45.27266. [DOI] [PubMed] [Google Scholar]
- 51.Leonard SE, Carroll KS. Chemical ‘omics’ approaches for understanding protein cysteine oxidation in biology. Curr Opin Chem Biol. 2011;15:88–102. doi: 10.1016/j.cbpa.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 52.Speth RC, Kim KH. Discrimination of two angiotensin II receptor subtypes with a selective analogue of angiotensin II, p-aminophenylalanine6 angiotensin II. Biochem Biophys Res Commun. 1990;169:997–1006. doi: 10.1016/0006-291x(90)91993-3. [DOI] [PubMed] [Google Scholar]
- 53.Hunter WM, Greenwood FC. Preparation of iodine-131 labeled human growth hormone of high specific activity. Nature. 1962;194:495–6. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- 54.Speth RC, Harding JW. Radiolabeling of angiotensin peptides. In: Wang DH, editor. Angiotensin Protocols. Humana Press; Totowa NJ: 2001. pp. 275–95. [DOI] [PubMed] [Google Scholar]
- 55.Wright JW, Stubley L, Pederson ES, Kramar EA, Hanesworth JM, Harding JW. Contributions of the brain angiotensin IV-AT4 receptor subtype system to spatial learning. J Neurosci. 1999;19:3952–61. doi: 10.1523/JNEUROSCI.19-10-03952.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rashid M, Arumugam TV, Karamyan VT. Association of the novel non-AT1, non-AT2 angiotensin binding site with neuronal cell death. J Pharmacol Exp Ther. 2010;335:754–61. doi: 10.1124/jpet.110.171439. [DOI] [PubMed] [Google Scholar]
- 57.Zareba G, Cernichiari E, Hojo R, Nitt SM, Weiss B, Mumtaz MM, et al. Thimerosal distribution and metabolism in neonatal mice: comparison with methyl mercury. J Appl Toxicol. 2007;27:511–8. doi: 10.1002/jat.1272. [DOI] [PubMed] [Google Scholar]
- 58.Rodd D, Hersh LB. Endopeptidase 24.16B. A new variant of endopeptidase 24. 16. J Biol Chem. 1995;270:10056–61. doi: 10.1074/jbc.270.17.10056. [DOI] [PubMed] [Google Scholar]
- 59.Dauch P, Masuo Y, Vincent JP, Checler F. Endopeptidase 24–16 in murines: tissue distribution, cerebral regionalization, and ontogeny. J Neurochem. 1992;59:1862–7. doi: 10.1111/j.1471-4159.1992.tb11021.x. [DOI] [PubMed] [Google Scholar]
- 60.Bonham CA, Vacratsis PO. Redox regulation of the human dual specificity phosphatase YVH1 through disulfide bond formation. J Biol Chem. 2009;284:22853–64. doi: 10.1074/jbc.M109.038612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tang XD, Daggett H, Hanner M, Garcia ML, McManus OB, Brot N, et al. Oxidative regulation of large conductance calcium-activated potassium channels. J Gen Physiol. 2001;117:253–74. doi: 10.1085/jgp.117.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guttmann RP. Redox regulation of cysteine-dependent enzymes. J Anim Sci. 2010;88:1297–306. doi: 10.2527/jas.2009-2381. [DOI] [PubMed] [Google Scholar]
- 63.Zhou A, Carrell RW, Murphy MP, Wei Z, Yan Y, Stanley PL, et al. A redox switch in angiotensinogen modulates angiotensin release. Nature. 2010;468:108–11. doi: 10.1038/nature09505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chiu AT, McCall DE, Nguyen TT, Carini DJ, Duncia JV, Herblin WF, et al. Discrimination of angiotensin II receptor subtypes by dithiothreitol. Eur J Pharmacol. 1989;170:117–8. doi: 10.1016/0014-2999(89)90145-3. [DOI] [PubMed] [Google Scholar]
- 65.Speth RC, Grove KL, Carter MR, Rowe BP. Differential effects of mercaptoethanol on angiotensin II receptor binding at multiple forebrain sites. FASEB J. 1990;4:A600. [Google Scholar]
- 66.Whitebread S, Mele M, Kamber B, de Gasparo M. Preliminary biochemical characterization of two angiotensin II receptor subtypes. Biochem Biophys Res Commun. 1989;163:284–91. doi: 10.1016/0006-291x(89)92133-5. [DOI] [PubMed] [Google Scholar]
- 67.Cook JV, McGregor A, Lee T, Milligan G, Eidne KA. A disulfide bonding interaction role for cysteines in the extracellular domain of the thyrotropin-releasing hormone receptor 1. Endocrinology. 1996;137:2851–8. doi: 10.1210/endo.137.7.8770906. [DOI] [PubMed] [Google Scholar]
- 68.Knudsen SM, Tams JW, Wulff BS, Fahrenkrug J. A disulfide bond between conserved cysteines in the extracellular loops of the human VIP receptor is required for binding and activation. Febs Letters. 1997;412:141–143. doi: 10.1016/s0014-5793(97)00714-x. Ref Type: Journal (Full) [DOI] [PubMed] [Google Scholar]
- 69.Gaibelet G, Capeyrou R, Dietrich G, Emorine LJ. Identification in the mu-opioid receptor of cysteine residues responsible for inactivation of ligand binding by thiol alkylating and reducing agents. FEBS Lett. 1997;408:135–40. doi: 10.1016/s0014-5793(97)00407-9. [DOI] [PubMed] [Google Scholar]
- 70.Aiyar N, Griffin E, Edwards R, Weinstock J, Samanen J, Nambi P. Characterization of bovine ovary angiotensin II receptors using subtype- selective antagonists 2. Pharmacology. 1993;46:1–8. doi: 10.1159/000139022. [DOI] [PubMed] [Google Scholar]
- 71.Gehlert DR, Gackenheimer SL, Schober DA. Angiotensin II receptor subtypes in rat brain: dithiothreitol inhibits ligand binding to AII-1 and enhances binding to AII-2. Brain Res. 1991;546:161–5. doi: 10.1016/0006-8993(91)91173-x. [DOI] [PubMed] [Google Scholar]
- 72.Heerding JN, Hines J, Fluharty SJ, Yee DK. Identification and function of disulfide bridges in the extracellular domains of the angiotensin II type 2 receptor. Biochem. 2001;40:8369–77. doi: 10.1021/bi002805p. [DOI] [PubMed] [Google Scholar]
- 73.Ketterer B, Christodoulides L. Identification of the amino acid residues that bind azo-dye in the soluble azo-dye carcinogen-binding proteins of rat liver. Biochem J. 1968;109:37P. doi: 10.1042/bj1090037pa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xu SZ, Zeng B, Daskoulidou N, Chen GL, Atkin SL, Lukhele B. Activation of TRPC cationic channels by mercurial compounds confers the cytotoxicity of mercury exposure. Toxicol Sci. 2012;125:56–68. doi: 10.1093/toxsci/kfr268. [DOI] [PubMed] [Google Scholar]
- 75.Carvalho CM, Lu J, Zhang X, Arner ES, Holmgren A. Effects of selenite and chelating agents on mammalian thioredoxin reductase inhibited by mercury: implications for treatment of mercury poisoning. FASEB J. 2011;25:370–81. doi: 10.1096/fj.10-157594. [DOI] [PubMed] [Google Scholar]
- 76.Schnizler MK, Bogdan R, Bennert A, Bury NR, Fronius M, Clauss W. Short-term exposure to waterborne free silver has acute effects on membrane current of Xenopus oocytes. Biochim Biophys Acta. 2007;1768:317–23. doi: 10.1016/j.bbamem.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 77.Sanyal G, Khalifah RG. Kinetics of organomercurial reactions with model thiols: sensitivity to exchange of the mercurial labile ligand. Arch Biochem Biophys. 1979;196:157–64. doi: 10.1016/0003-9861(79)90562-9. [DOI] [PubMed] [Google Scholar]
- 78.Schweri MM. Mercuric chloride and p-chloromercuriphenylsulfonate exert a biphasic effect on the binding of the stimulant [3H]methylphenidate to the dopamine transporter. Syn. 1994;16:188–94. doi: 10.1002/syn.890160304. [DOI] [PubMed] [Google Scholar]
- 79.Miller TB, Farah AE. Inhibition of mercurial diuresis by nondiuretic mercurials. J Pharmacol Exp Ther. 1962;135:102–11. [PubMed] [Google Scholar]
- 80.Miller TB, Farah AE. On the mechanism of the inhibition of mercurial diuresis by p-chloromercuribenzoic acid. J Pharmacol Exp Ther. 1962;136:10–9. [PubMed] [Google Scholar]
- 81.Lim EJ, Sampath S, Coll-Rodriguez J, Schmidt J, Ray K, Rodgers DW. Swapping the substrate specificities of the neuropeptidases neurolysin and thimet oligopeptidase. J Biol Chem. 2007;282:9722–32. doi: 10.1074/jbc.M609897200. [DOI] [PubMed] [Google Scholar]