Abstract
Introduction
Lack of enteral stimulation during PN impairs acquired (e.g. IgA) and innate mucosal immunity providing a cogent explanation for increased infections in PN fed patients compared to enteral feeding. Experimentally, BBS, a gastrin-releasing neuropeptide analogue, reverses PN-induced defects in gut and respiratory acquired immunity. Paneth cells produce and store the key bactericidal peptides of innate immunity for release into the lumen after cholinergic stimulation. We hypothesized that BBS during PN restores antimicrobial peptides (AMPs) and the bactericidal function of innate immunity.
Methods
IV cannulated male ICR mice were randomized to Chow, PN, or PN + 15 μg TID BBS (n=7 per group) for 5 days. Ileal tissue was analyzed for AMPs (Protein levels: sPLA2 by fluorescence, lysozyme and RegIII-γ by western, and cryptdin-4 by ELISA; mRNA: all by RT-PCR). Ileal tissue stimulated with a cholinergic agonist (100 μM bethanechol) assessed Pseudomonas bactericidal activity. Additional mice (Chow: n=7; PN: n=9; PN+BBS: n=8) were assessed for E. coli intestinal invasion in ex-vivo culture.
Results
Compared to chow, PN significantly decreased cellular levels of AMPs while BBS maintained them at Chow levels. Functionally, BBS prevented PN loss of bactericidal activity after cholinergic stimulation but failed to improve bacterial enteroinvasion in unstimulated tissue.
Conclusions
The ENS controls AMP levels in Paneth cells during PN but both ENS and parasympathetic stimulation are required for mucosal protection by innate immunity.
Keywords: Parenteral nutrition, mucosal immunity, Paneth cells, antimicrobial peptides, bombesin, neuropeptides, enteric nervous system
INTRODUCTION
Parenteral nutrition (PN) prevents progressive malnutrition due to starvation providing a lifesaving therapy for many of patients. However, PN renders patients susceptible to infectious complications and altered inflammatory responses.1, 2 Enteral feeding reduces this infectious risk.3–7 The reason for this PN-induced vulnerability appears at least in part related to aberrations in the adaptive and innate mucosal immune systems resulting from decreased enteral stimulation.8–11
For the past 15 years, our laboratory focused on the effects of PN on adaptive (acquired) mucosal immunity whereby antigen-specific IgA antibodies are produced in the lamina propria, transported into the gastrointestinal lumen and bind bacteria to prevent their mucosal attachment and subsequent invasion.12–15 PN with lack of enteral stimulation negatively affects numerous components of intestinal and extraintestinal (i.e. respiratory) adaptive immunity including decreases in: gastrointestinal-associated lymphoid tissue (GALT) size; Peyer’s patch, lamina propria, pulmonary, and salivary gland lymphocyte cellularity; GALT lymphocyte attractant and adhesion molecules,16–18 and small intestinal and Peyer’s patch chemokines19; lamina propria IgA-stimulating Th2 cytokines20; and the mucosal IgA transport protein, pIgR,21, 22 (Table 1).23–35 Together these PN-induced changes reduce secretory IgA (sIgA) at multiple mucosal surfaces, impairing IgA mediated mucosal immunity. The functional impact includes loss of both anti-viral and anti-bacterial immunity experimentally and provides a cogent explanation for the increase in pneumonia documented in PN fed patients.
Table 1. Effects of Chow, PN, and PN+BBS on the adaptive immune system.
Summary of current published data. All values are statistically significant (p<0.05).
| Adaptive Immune System | PN vs Chow | PN+BBS vs PN |
|---|---|---|
| GALT size | ↓23 | ↓25–27 |
| Peyer’s Patches lymphocytes | ↓23 | ↓26, 27 |
| Lamina Propria lymphocytes | ↓23 | ↓26, 27 |
| sIgA | ↓23, 24 | ↓26, 27 |
|
| ||
| Pulmonary lymphocytes | ↓28 | |
| sIgA | ↓24 | ↓27, 29 |
|
| ||
| Salivary Gland lymphocytes | ↓30 | ↓30 |
| sIgA | ↓30 | ↓30 |
|
| ||
| Functional | ||
| IgA mediated anti-viral immunity | ↓31–33 | ↓35 |
| Survival after bacterial challenge | ↓34 | ↓27 |
GALT, gastrointestinal-associated lymphoid tissue; PN, parenteral nutrition; BBS, bombesin; sIgA, secretory immunoglobulin A; pIgR, polymeric immunoglobulin receptor.
Recent work shows that PN with lack of enteral stimulation exerts deleterious effects upon innate immunity as well.36–45 Paneth cells located in the crypts of Lieberkühn in the small intestine and goblet cells scattered throughout the intestinal villi are the main effector cells of innate immunity. Paneth cells produce, store, and secrete antimicrobial peptides (AMPs) including: secretory phospholipase A2 (sPLA2), lysozymes, regenerating islet-derived protein 3 gamma (RegIII-γ), and alpha defensins (known as cryptdins in mice), among others.46 These AMPs are naturally occurring antibiotics which provide critical local bactericidal activity. Once released, they concentrate in the goblet cell mucus layer of the gastrointestinal tract, similar to sIgA, where they interact with intestinal bacteria preventing mucosal attachment and invasion. PN with lack of enteral stimulation reduces the intracellular and intraluminal levels of these antibacterial molecules as well as the size of the mucus layer. The functional effects of these innate immune aberrations are evident experimentally as PN-treated mice demonstrate decreased bactericidal activity of Paneth cell secretions, and increased vulnerability to enteric invasion by pathogenic bacteria.38 Enteral feeding prevents these morphologic and functional changes in both innate and adaptive mucosal immunity.
Unfortunately, not all patients can be safely fed enterally. However, exogenous administration of neuropeptides normally produced by the enteric nervous system (ENS) reverses most of the deleterious PN-induced changes in adaptive immunology. These results suggest the use of neuropeptides as a surrogate for enteral stimulation when enteral nutrition cannot be administered. The gastrointestinal tract is innervated by both the ENS and the autonomic nervous system, both of which play a role in gut function.47, 48 The ENS consist of an extensive network of 108 neurons with cell bodies located in the submucosal (Meissner’s) plexus and myenteric (Auerbach’s) plexus which control peristalsis, local changes in blood flow, and homeostasis of water and electrolytes.49 The ENS also influences mucosal immunity through neuropeptides which affect the proliferation, differentiation, and function of immune cells and prevent parenteral nutrition-induced defects in mucosal immunity in murine models.50, 51 Exogenous administration of bombesin (BBS), a gastrin-releasing peptide analogue, experimentally reverses PN-induced defects in gastrointestinal and respiratory adaptive mucosal immunity. It abrogates many of the PN-induced immune defects, and preserves established immunity to viral and bacterial pneumonia otherwise lost with PN in murine models (Table 1).
The current study examines the role and importance of the ENS and autonomic nervous system on the integrity of innate mucosal immunity. This work examines neuropeptide effects on Paneth cell products applying functional outcomes including bactericidal activity of intestinal secretions and mucosal resistance to bacterial enteroinvasion during PN both with and without BBS treatment. We hypothesized that administration of the ENS neuropeptide, BBS, during PN would restore tissue levels and luminal levels of innate immune AMPs. We also hypothesized that functional defects in bactericidal activity and mucosal resistance to bacterial invasion caused by PN with lack of enteral stimulation would be restored by this exogenous neuropeptide administration.
MATERIALS AND METHODS
Animals
All experimental protocols were approved by the Animal Care and Use Committee of the University of Wisconsin-Madison and Middleton Veterans Administration Hospital, Madison, WI. Male Institute of Cancer Research (Harlan, Indianapolis, IN) mice were used for experiments. Mice were housed in an American Association for Accreditation of Laboratory Animal Care- accredited facility with controlled temperature, humidity, and light cycle (12-hour light/12-hour dark). Mice were acclimatized for 1 week while housed 5 per covered/filtered box and fed a standard mouse chow (Rodent Diet 5001; LabDiet, PMI Nutrition International, St. Louis, MO) and water ad libitum before experiment protocol entry. After entry into the study protocol, mice were individually housed in metal metabolism cages with wire grid floors to eliminate coprophagia.
Surgical Procedure
Mice were anesthetized for surgical central line placement with an intra-peritoneal injection of a mixture of ketamine chloride (100 mg/kg) and acepromazine maleate (10 mg/kg) and weighed. A silicone rubber catheter (0.012″ I.D. by 0.025″ O.D.; Helix Medical Inc., Carpinteria, CA) was inserted into the vena cava through the right external jugular vein. The distal end of the catheter was tunneled subcutaneously and exited the midpoint of the tail. Mice were partially immobilized by tail restraint following this procedure to protect the catheter during infusion. This infusion technique in the mouse has been demonstrated to be an acceptable method of nutritional support, and this method does not produce physical or biochemical evidence of stress.52
Feeding Protocols
Four groups of six to eight week old mice were randomized to diets of Chow, parenteral nutrition (PN), or parenteral nutrition with bombesin (PN+BBS) with 6–10 mice per diet in each group. After catheterization mice were connected to infusion pumps and recovered for two days while receiving 4 mL 0.9% sodium chloride per day in addition to chow and water ad libitum. As previously described, this recovery period was chosen because prior work demonstrates that following 2 days, serum cytokines, corticosteroid levels, and IgA secretion induced by surgical stress return to baseline, and animals in all groups resume oral intake in normal amounts. Following this recovery period, experimental diets were initiated.
Chow mice continued to receive 0.9% sodium chloride at 4 mL/d with chow and water ad libitum throughout the study. PN and PN+BBS mice received PN solution at 4 mL/d (day 1), 7 mL/d (day 2), and 10 mL/d (day 3 to 5) because a graded infusion period is necessary for the mice to adapt to PN glucose and fluid loads. The PN solution contains 6.0% amino acids, 35.6% dextrose, electrolytes, and multivitamins for a total of 1440 kcal/L and a non-protein calorie/nitrogen ratio of 128:1. This formula meets the calculated nutrient requirements of mice weighing 25 to 30 g and was metabolically scaled to the average weight of mice in this experiment.53
In addition, each group received intravenous injections via the catheter three times a day (TID at 0700, 1500, and 2300). Chow and PN mice received 100 μL of vehicle 0.9% sodium chloride TID while the PN+BBS group received 15 μg of bombesin (Sigma, St. Louis, MO) dissolved in 100 μL of 0.9% sodium chloride TID.
Sample Collection
Following 5 days of experimental treatment (7 days post-catheterization), animals were anesthetized again by intra-peritoneal injection of a mixture of ketamine hydrochloride (100 mg/kg) and acepromazine maleate (10 mg/kg), weighed, and exsanguinated via left axillary artery transection. The small intestine was removed and mesenteric fat, lymph nodes, Peyer’s patches, and external vasculature were dissected away. 20 mL of cold calcium-magnesium-free Hanks’ balanced salt solution (CMF-HBSS; Bio Whittaker, Walkersville, MD) were flushed through the intestinal lumen (pylorus to terminal ileum) and collected. These intestinal lumen wash specimens were kept on ice until they were centrifuged at 2000 × g for 10 minutes at 4°C. 1 mL of supernate was frozen at −80°C for future protein analysis.
After washing, small intestine tissue samples were taken as 2-cm segments of distal ileum that were either fixed in 4% paraformaldehyde for immunohistochemistry (IHC), snap frozen in RNAlater, or snap frozen with liquid nitrogen and stored at −80°C until processing for PCR and tissue protein analysis. Additional 2-cm ileal segments were opened longitudinally and either placed in 24-well multidishes (Nunc, Roskilde, Denmark), containing 1 mL CMF-HBSS on ice or placed in cold RPMI solution for imminent ex-vivo intestinal segment culture. Segments placed in the 24-well multidishes were randomized to incubate with either saline or 100 μM bethanechol (Cat# C5259, Sigma) for 1 hour at 37°C. Secretions were filtered through 0.2 μm filters (Cat# 09-719C, Fisherbrand) and frozen for analysis of AMP bethanechol stimulated secretion and bactericidal activity.38
Immunohistochemistry
To visually confirm the location and semi-quantitatively assess concentrations of sPLA2, lysozyme, and RegIII-γ in Paneth cells, we performed IHC on ileum segments from each treatment group (Chow, PN, PN+BBS). Following overnight fixation in 4% paraformaldehyde, samples were transferred to 70% ethanol, and stored at 4°C until processing. Samples were processed with a Tissue-Tek V.I.P. tissue processor with the following wash steps: 70% ETOH, 45 minutes; 80% ETOH, 45 minutes; 95% ETOH, 2x 1 hour; 100% ETOH, 2x 1 hour; Xylene, 2x 1 hour; Paraffin MP 60 °C (Surgipath, Richmond, IL), 2x 45 minutes; Paraffin, 2x 1 hour. Samples were then embedded in paraffin, sectioned at 5 μm with a microtome, and placed on Adhesive Coated Slides (White Aminosilane, Newcomer Supply, Madison, WI). Slides were deparaffinized in xylene and rehydrated in graded alcohol baths. Antigen retrieval was performed by boiling slides in a 10 mmol/L sodium citrate bath (pH 6.0). Slides were then blocked with 10% bovine serum albumin (BSA)-PBS for 1 hour. Samples were incubated with primary antibody for sPLA2 (1:200, sc-14468, goat polyclonal IgG, Santa Cruz Biotechnology), Lysozyme (1:400, ab108508, rat monoclonal IgG, Abcam), or RegIII-γ (1:500, PA5-25517, rabbit polyclonal IgG, Thermo Scientific) overnight in 1% BSA-PBS at 4°C in a humidified chamber. Remaining solutions were tapped off and samples were incubated with respective secondary antibodies in 1% BSA-PBS (1:1000, Alexa Fluor 594; Invitrogen, Grand Island, NY). Slides were imaged following DAPI (P36935, Invitrogen) and cover placement.
RT-PCR38
RNA Extraction
2-cm of distal ileum was collected and snap frozen in RNAlater (Cat # R0901, Sigma) and stored at −80°C until further use. The SV Total RNA Isolation System (Cat# Z3100, Promega) was used to isolate RNA from tissue samples following manufacturer instructions. A nanodrop spectrometer (Thermo Scientific) was used to confirm RNA samples were pure and of adequate concentration.
Reverse Transcription
Following RNA isolation, cDNA was made using ImPromII Reverse Transcription System (Cat# A3800, Promega) manufacturer guided instructions.
PCR
Specific primers were designed for sPLA2, lysozyme, RegIII-γ, and cryptdin-4, (Table 2). All reactions were run with 1 μL of cDNA and a β-actin control. PCR was performed using recombinant Taq DNA polymerase (Cat# 10342-020, Invitrogen). PCR reactions were assembled in a DNA-free environment and contained 10 μl of 10X PCR buffer, 3 μl of 50 mM MgCl2, 2 μl of 10 mM deoxynucleoside triphosphate (dNTP) mixture, 1 unit of recombinant Taq DNA polymerase, 1 μM of each primer, and 1 μL of cDNA each. The samples were amplified in a Hybaid PCR express system (Middlesex, UK) with the following program: initial denaturation at 94°C for 3 minutes, 25 thermal cycles of 94°C for 1 minute, 56°C for 30 seconds and 72°C for 45 seconds with a final extension at 72°C for 10 minutes. The size and quantity of PCR products was confirmed by analysis on a 3% agarose gel (weight/volume) electrophoresis stained with ethidium bromide.
Table 2.
Specific primers used for PCR assay.
| Antimicrobial Peptide | Forward primer (5′ to 3′) | Reverse Primer (5′ to 3′) |
|---|---|---|
| sPLA2 | ACAGGTCCAAGGGAACATTG | TCTGGTTTGCAGAACAGGTG |
| Lysozyme | ATGGCGAACACAATGTCAAA | GCGAGGAAGTGTGACCTCTC |
| RegIII-γ | AACAGAGGTGGATGGGAGTG | ATTTGGGATCTTGCTTGTGG |
| Cryptdin-4 | CCAGGCTGATCCTATCCAAA | ATTCCACAAGTCCCACGAAC |
Fluorescent Assay (sPLA2)36, 37, 54
Fluorescent assay for sPLA2 activity was performed as previously described by Tsao et al.54 with modification to substrate preparation as previously described. In brief, substrate was prepared by mixing 10 μL of Bis-BODipy FL C11-PN (Molecular Probes, Eugene, OR) fluorescently labeled probe in a 1 mL aliquot of phosphatidylglycerol (Sigma, St. Louis, MO) dissolved in chloroform (2 mg/mL) and evaporated under nitrogen. Substrate was dissolved in 100% ethanol, which is stable for storage for 1 month at −20°C.
Assay reaction mixture was subsequently prepared in glass culture tubes on ice with 10 μL of the stored substrate ethanol solution (20 μg of phospholipids) and 3 μL of sample, and 987 μL of 0.01 mol/L Tris HCL (pH 7.4) containing 10 mmol/L Ca2+ for a final volume of 1 mL. Assay blanks consisted of 10 μL of the substrate ethanol solution and 990 μL of Tris-HCL buffer. 300 μL of reaction mixture was transferred in triplicate to wells of a 96-well white polystyrene microplate (Porvair PS White, PerkinElmer, Norwalk, CT). Microplates were placed in a temperature-controlled (30°C) microplate reader (PerkinElmer) attached to a Luminescence Spectrometer LS50B (PerkinElmer). Fluorescence intensity (FL) of each well was recorded every 30 seconds for 60 cycles at 488 nm excitation (excitation slit 2.5 nm) and 530 nm emission (emission slit 5.0 nm). To confirm calcium-dependent sPLA2 activity, samples were also run with EGTA-Buffer (0.01 mol/L Tris HCL [pH 7.4] containing 10 mmol/L Ca2+ and 20 mmol/L EGTA), which contains ample EGTA for complete calcium sequestration. The reaction curve was fit to a second-order polynomial equation after all reactions reached equilibrium temperature. The first-degree coefficient was taken as the initial rate of reaction (expressed as change in FL/min/μL sample). Blank wells were used to identify background activity coefficient rates.
Western Blot (Lysozyme and RegIII-γ)
The amount of solubilized protein from each tissue homogenate was determined using the Bradford assay method. In sum, 40 μg of tissue homogenate protein, 15 μL of luminal wash fluid, or 15 μL of bethanechol stimulated secretions were used for western blotting. Samples were denatured at 95°C for 5 minutes with sodium dodecylsulfate and β-mercaptoethanol and separated in 10% agarose gels by electrophoresis at 150 V for 45 minutes at room temperature. Proteins were transferred to polyvinylidene fluoride membrane using Tris-glycine buffer plus 20% methanol at 80 V for 40 minutes at 4°C. Membranes were blocked with 5% nonfat dry milk prepared in TBS-Tween for 1 hour at room temperature with constant agitation. Membranes were then incubated with primary antibody, monoclonal lysozyme (ab108508, Abcam) diluted 1:5000 and Rabbit polyclonal RegIII-γ (PA5-25517, Thermo Scientific) diluted 1:250 overnight at 4°C with constant agitation. Membranes were then washed and incubated with secondary antibody, goat anti rabbit HRP diluted 1:20000 for lysozyme and 1:15000 for RegIII-γ for 1 hour at room temperature with constant agitation. After washing, membranes were incubated with HRP substrate (Super Signal West Femto maximum sensitivity substrate; Pierce, Rockford, IL) for 5 minutes, and bands were detected using ImageQuant4000 (GE Healthcare). Molecular weight bands of 14 kDa were used to verify lysozyme and molecular weight bands of 18 kDa were used to verify RegIII-γ size. NIH ImageJ software was used to determine relative concentrations with densitometry.
ELISA (enzyme-linked immunosorbent assay) (Cryptdin-4)
Mouse neutrophil defensin 4 (DEFA4) ELISA Kit (Cat# MBS904696, Cusabio) was used to determine cryptdin-4 protein levels in ileal tissue homogenates and ileal wash fluid following manufacturer provided instructions. Briefly pre-coated manufacturer provided plates were incubated for 2 hours at 37°C with 100 μL of standards, 100 μL of a 1:20 dilution of homogenized mouse ileal samples, and 100 μL of ileal wash fluid samples (neat). Following this incubation, liquid was removed from the wells and 100 μL of Biotin-antibody (1x) was added to each well and incubated at 37°C for 1 hour. Plates were then washed and incubated with 100 μL HRP-avidin at 37°C for 1 hour. Plates were again washed and then incubated with 90 μL of TMB Substrate for 15 minutes at 37°C before the reaction was stopped with 50 μL of Stop Solution. Plates were read at 450 nm using Molecular Devices Vmax kinetic microplate reader within 5 minutes of stopping the reaction.
Bacterial Preparation and Bactericidal Assay38
To determine bactericidal activity in small intestinal tissue samples obtained from the three diet treatment groups, Pseudomonas aeruginosa (P. aeruginosa) was cultured for 24 hours at 37°C in lysogeny broth (LB) (Fisher Scientific, Fair Lawn, NJ). Bacteria were then washed and suspended in CMF-HBSS as previously described and bacterial suspension concentration was adjusted to 2 × 103 colony forming units (CFU)/mL in CMF-HBSS. To evaluate bactericidal activity, 10 μL (~100 CFU) of bacterial suspension was added to both 200 μL of culture media containing bethanechol stimulated tissue and tissue stimulated with CMF-HBSS (controls). Samples were incubated at 37°C for 1 hour. Bacterial survival was assessed by plating bacteria incubated with bethanechol stimulated secretions in duplicate on LB agar plates (Fisher Scientific, Pittsburg, PA) under aerobic conditions at 37°C overnight. CFUs were counted and normalized to paired controls and results were expressed as bactericidal activity as measured by the reduction in percent surviving CFUs.
Bacterial Preparation and Ex-Vivo Intestinal Segment Culture38, 55
Escherichia coli 5011-Lux containing ampicillin resistance was grown in LB plus ampicillin (1:1000) for 24 hours at 37°C with 5 % CO2 and passed to new LB to grow for another 24 hours at 37°C with 5 % CO2 to increase virulence. Bacteria were washed and suspended in 1 mL DPBS 4°C as a bacterial stock solution as previously described. A spectrophotometer (DU640B, Beckman) at 450 nm wavelength was used to determine bacterial concentration and the stock solution was adjusted to 1×108 CFU/mL in RPMI plus ampicillin (100 μg/mL) based on previously established growth curves. Intestinal segments placed on a sterile surface were covered in a light layer of RPMI and opened longitudinally apical side up. Plastic rings (9 mm aperture) were lightly coated on one side with tissue glue (Dermabond, Ethicon, Cornelia, GA) and placed on the mucosal side of the intestinal segment. A second plastic ring was lightly coated with tissue glue and placed on the serosal side of the intestinal segment to sandwich the segment between the two discs. A light layer of tissue glue was then applied to the bottom of the serosal disc and the complex was lowered into a cell culture insert (Cat# 3292, 3.0 μM pore, 12 well format, BD Bioscience) mucosal side up. Gentle pressure was applied to the discs to ensure adherence of the bottom tissue disc to the cell culture insert. Cell culture inserts were placed into 12 well plates.
400 μL of the adjusted bacterial stock solution in RMPI plus ampicillin was added to each insert and incubated for 1 hour at 37°C. Bacterial inoculum was removed and wells were gently rinsed 3 times with 700 μL of DPBS. 600 μL RPMI plus gentamicin (100 μg/mL) was added to each well and samples were incubated at 37°C for 1 hour. E. coli 5011-Lux is susceptible to gentamicin, and this incubation kills any extracellular bacteria in the wells. The RPMI and gentamicin solution was removed and wells were again washed as above. 500 μL of 0.1% Triton-X in PBS was added to each insert and plates were agitated on an orbital shaker (175 rpm; New Brunswick Scientific Classic Series C1 Shaker) for 30 minutes at 37°C to lyse cells. Serial dilutions (103–105) of cell lysate in DPBS were plated in duplicate on LB + ampicillin agar plates and grown for 18 hours at 37°C with 5 % CO2. Enteroinvasion was assessed by counting CFUs grown from cell lysate.
Statistical Analysis
All values are expressed as means ± standard error of the mean. Statistical analysis was performed by analysis of variance with ANOVA, followed by Fisher’s protected least significant difference post hoc test or Student’s t-test. Differences of p <0.05 were considered statistically significant. All statistical calculations were performed with StatView (Abacus Concepts, Berkley, CA). Bactericidal activity was measured by normalizing treatment group CFUs to the control group CFUs using pair analysis in each respective experiment. Treatment group CFUs were compared using analysis of variance.
RESULTS
The pre-experiment weights of mice in all groups were similar. There were no differences in body weight changes between treatment groups (p>0.05), (Table 3).
Table 3. Animal body weight and body weight change.
Weights are mean ± SEM. There are no statistically significant differences among groups (p>0.05).
| Group | n | Body Weight Before Feeding, g | Body Weight Change, g |
|---|---|---|---|
| Chow | 7 | 34.7 ± 1.0 | 3.2 ± 0.4 |
| PN | 9 | 34.3 ± 0.5 | 4.3 ± 0.2 |
| PN+BBS | 9 | 34.8 ± 0.8 | 3.9 ± 0.5 |
PN, parenteral nutrition; BBS, bombesin.
sPLA2
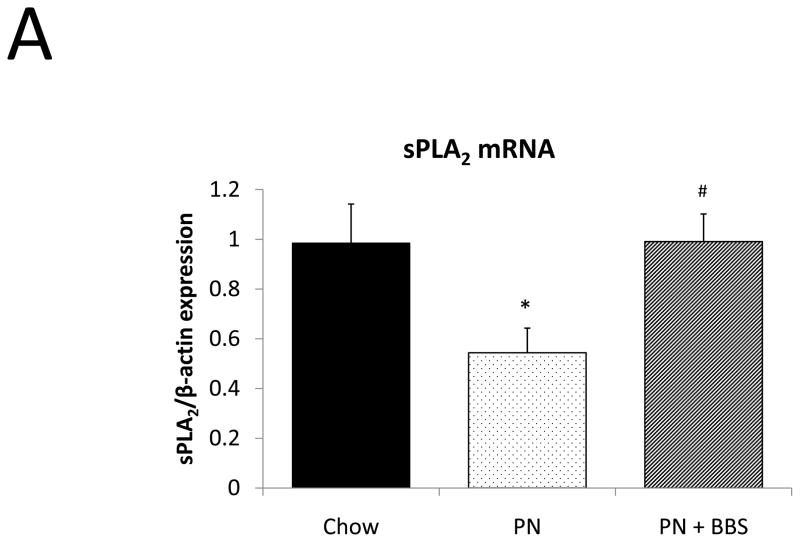
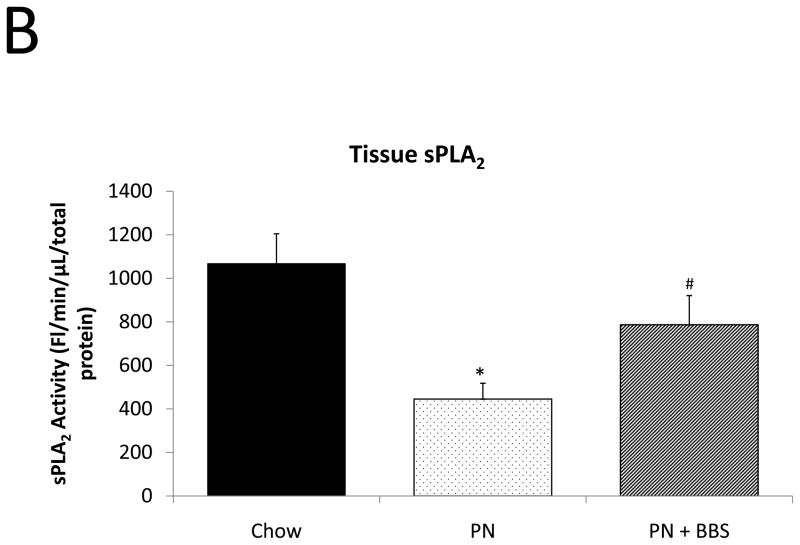
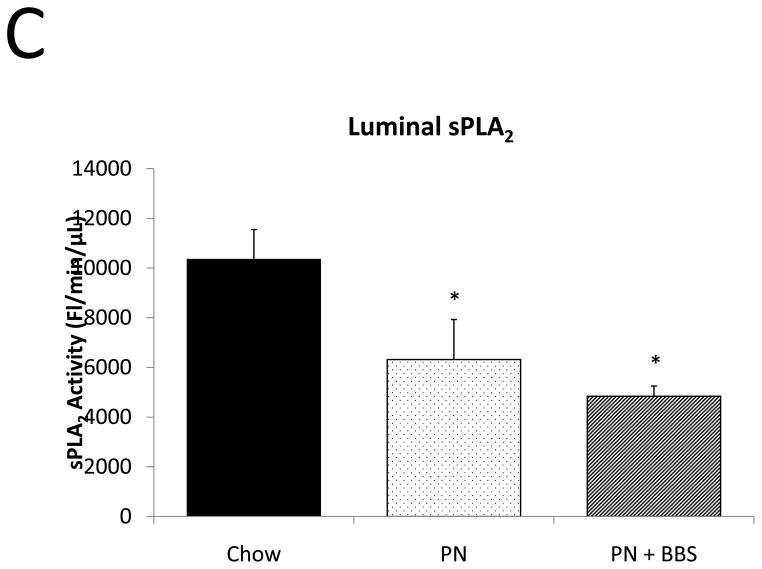
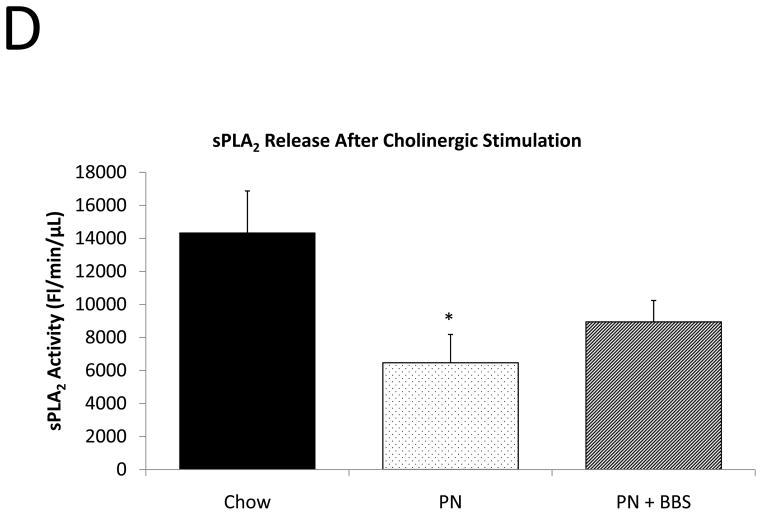
Representative IHC is shown in Figure 1 A–C. PN caused a significant decrease in sPLA2 mRNA transcription compared to Chow (p=0.02). PN+BBS significantly increased sPLA2 mRNA transcription compared to PN alone (p=0.02) to a level similar to Chow mice (p=0.97), (Fig. 2A). PN significantly decreased sPLA2 fluorescent activity in tissue, luminal fluid, and bethanechol stimulated secretions compared to Chow feeding (p=0.002; 0.02; 0.01 respectively), (Figs. 2B, C, D). The addition of BBS to PN restored tissue levels of sPLA2 activity toward Chow levels (p=0.049), (Fig. 2B), however luminal sPLA2 activity remained significantly decreased with PN+BBS compared to Chow (p=0.002) at levels similar to PN mice, (Fig. 2C). Bethanechol stimulated secretion of sPLA2 increased following PN+BBS compared to PN but this increase did not reach statistical significance (p=0.06), (Fig. 2D).
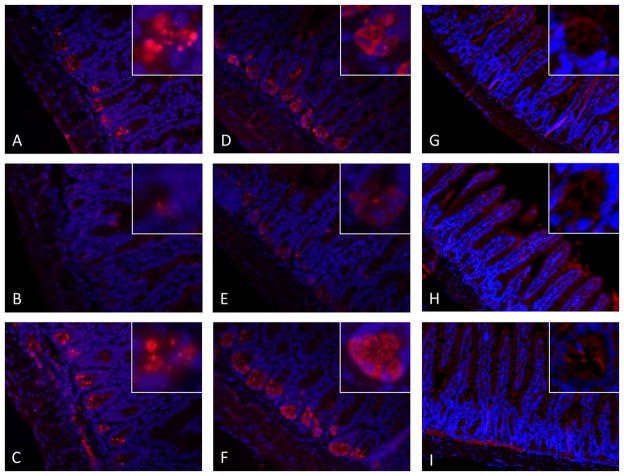
Figure 1. Representative immunohistochemistry (IHC) of antimicrobial peptides in ileum from Chow, PN, and PN+BBS-fed mice.
IHC of sPLA2 in A) Chow-fed mice; B) PN-fed mice; C) PN+BBS-fed mice. IHC of lysozyme in D) Chow-fed mice; E) PN-fed mice; F) PN+BBS-fed mice. IHC of RegIII-γ in G) Chow-fed mice; H) PN-fed mice; I) PN+BBS-fed mice. Images are 20X magnification of intestinal villi and crypts. Staining for sPLA2 and lysozyme is decreased in PN-fed mice and appears qualitatively similar in Chow and PN+BBS-fed mice. RegIII-γ staining appears cytosolic and localizes around intestinal villi. PN, parenteral nutrition; BBS, bombesin.
Figure 2. Chow, PN, and PN+BBS effects on sPLA2.
A) Effects on ileal tissue sPLA2 mRNA (n=7 per group); B) effects on ileal tissue sPLA2 fluorescent activity (Chow: n=6; PN: n=7; PN+BBS: n=7); C) effects on sPLA2 fluorescent activity in intestinal wash fluid (Chow: n=6; PN: n=7; PN+BBS: n=7); and D) effects on sPLA2 release after cholinergic stimulation with bethanechol (n=7 per group). Data are presented as mean ± SEM. *p<0.05 versus Chow; #p<0.05 versus PN. PN, parenteral nutrition; BBS, bombesin.
Lysozyme
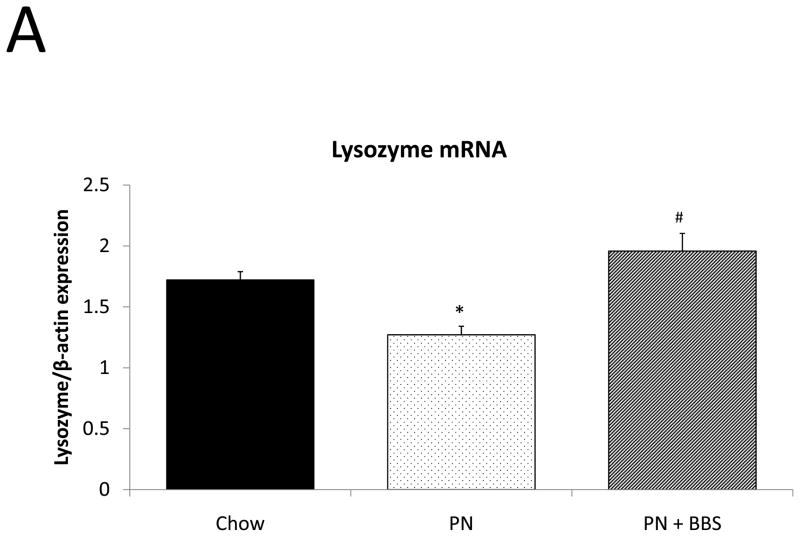
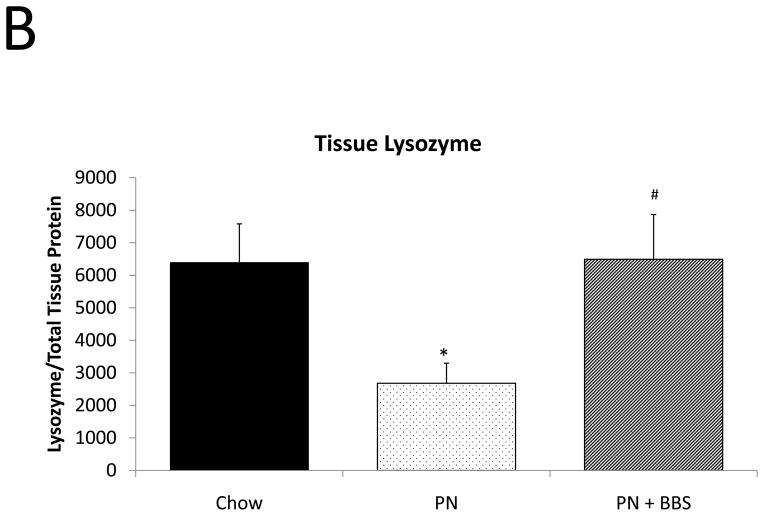
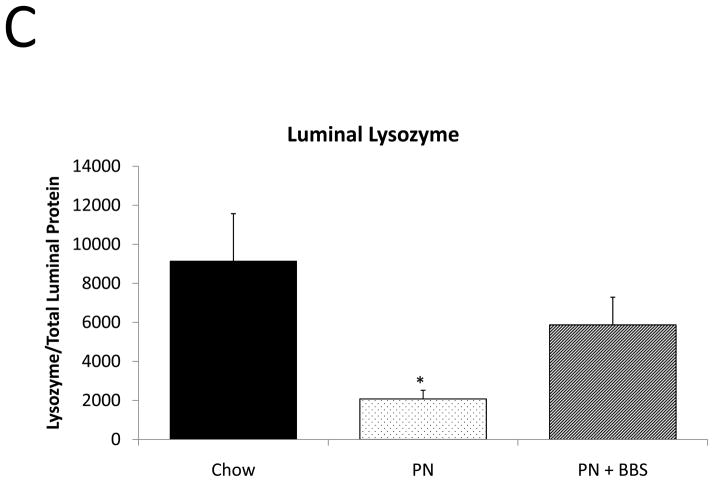
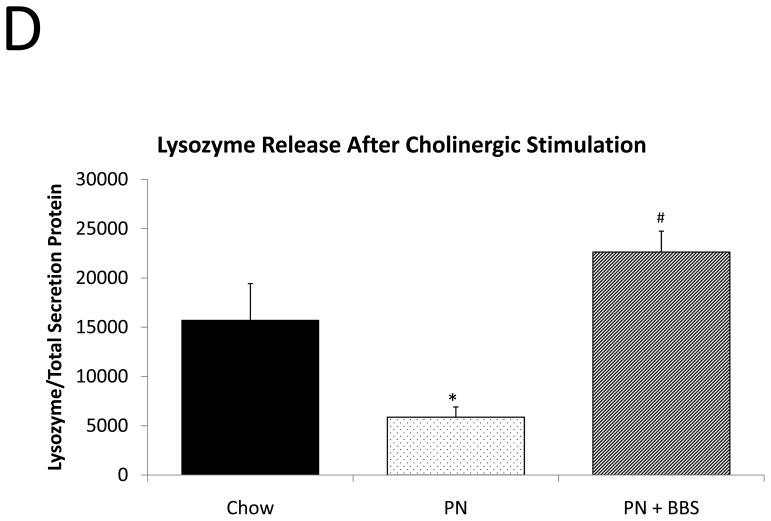
Representative IHC is shown in Figure 1 D–F. PN resulted in a significant decrease in lysozyme mRNA transcription compared to Chow (p=0.008). PN+BBS significantly increased lysozyme mRNA transcription compared to PN alone (p=0.0002) to values not significantly different from Chow (p=0.13), (Fig. 3A). PN resulted in a significant decrease in tissue lysozyme protein levels compared to Chow (p=0.02) while PN+BBS significantly improved tissue lysozyme protein levels from PN levels (p=0.01) to Chow levels, (Fig. 3B). Luminal protein levels of lysozyme significantly decreased with PN (p=0.008). Luminal protein levels of lysozyme increased with PN+BBS, but this increase failed to reach statistical significance (p=0.12), (Fig. 3C). Lysozyme protein levels in tissue secretions following bethanechol stimulation significantly decreased with PN compared to Chow feeding (p=0.015) but normalized to Chow levels with the addition of BBS to PN (p=0.0003), (Fig. 3D).
Figure 3. Chow, PN, and PN+BBS effects on Lysozyme.
A) Effects on ileal tissue lysozyme mRNA(Chow: n=6; PN: n=7; PN+BBS: n=7); B) effects on ileal tissue lysozyme relative protein concentration (Chow: n=6; PN: n=10; PN+BBS: n=7); C) effects on lysozyme protein in intestinal wash fluid (n=6 per group); and D) effects on lysozyme release after cholinergic stimulation with bethanechol (n=6 per group). Data are presented as mean ± SEM. *p<0.05 versus Chow; #p<0.05 versus PN. PN, parenteral nutrition; BBS, bombesin.
RegIII-γ
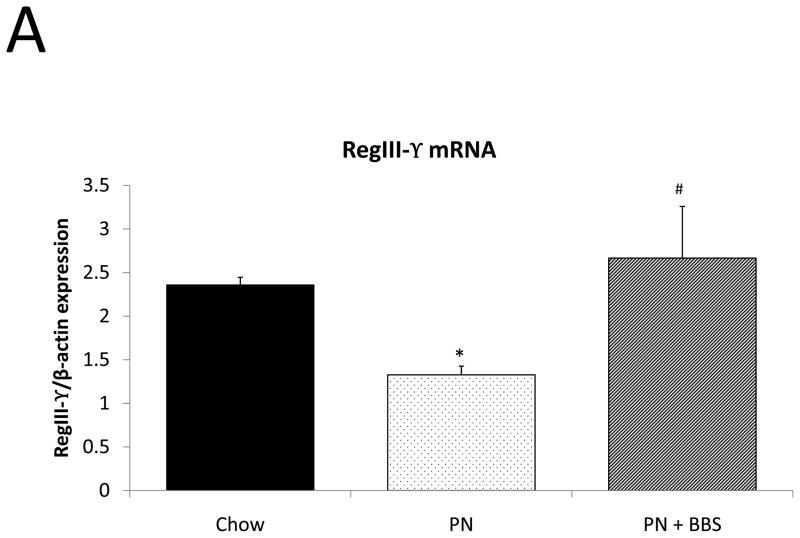
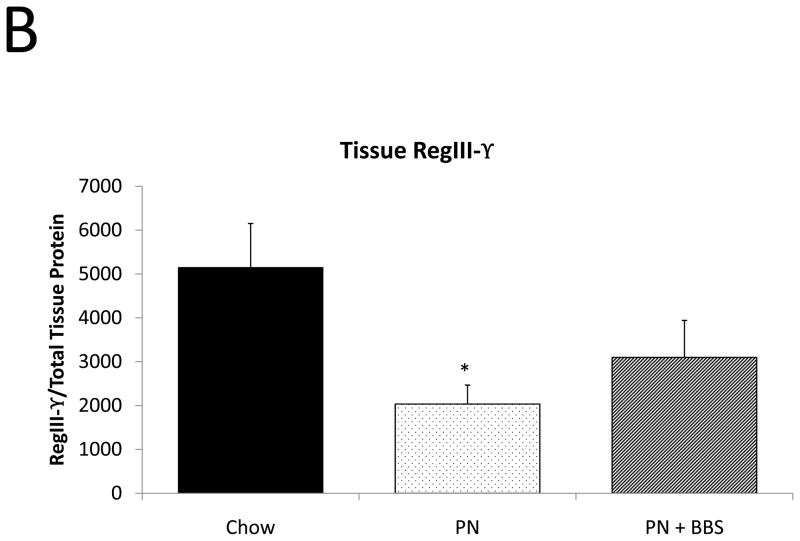
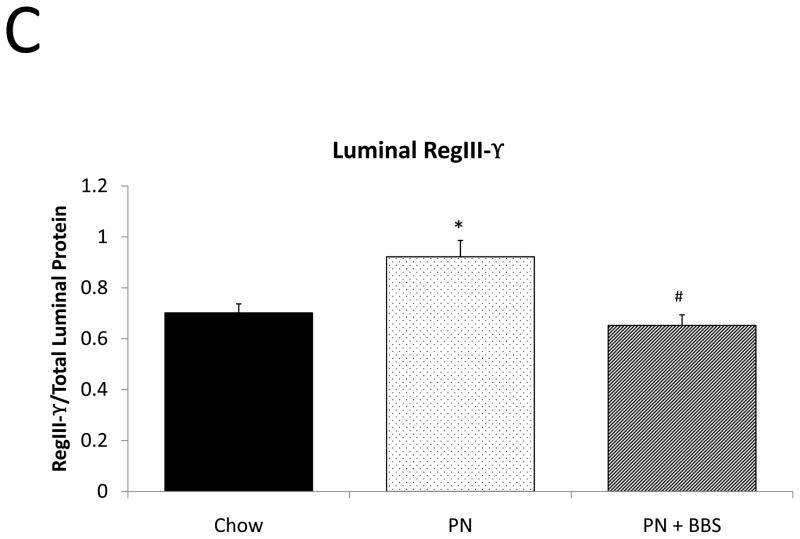
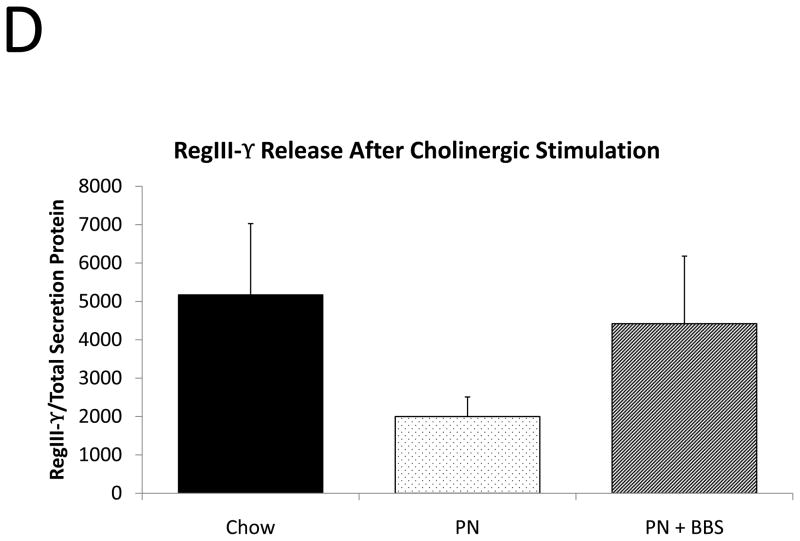
Representative IHC is shown in Figure 1 G–I. RegIII-γ mRNA transcription significantly decreases with PN compared to Chow (p=0.05). PN+BBS significantly increased RegIII-γ mRNA transcription compared to PN alone (p=0.015) and to values not significantly different from Chow mice (p=0.54), (Fig. 4A). PN significantly decreased RegIII-γ tissue protein levels compared to Chow (p=0.013). PN+BBS increased tissue protein levels of RegIII-γ, but not significantly, (p=0.38), (Fig. 4B). PN significantly increased RegIII-γ luminal protein levels (p=0.0037) which significantly decreased to Chow levels with the addition of BBS to PN (p=0.001), (Fig. 4C). RegIII-γ protein levels tended to decrease with PN after bethanechol stimulation of isolated intestinal segments and trend back toward Chow levels with the addition of BBS, however these changes were not statistically significant, (p=0.11; 0.23, respectively), (Fig. 4D).
Figure 4. Chow, PN, and PN+BBS effects on RegIII-γ.
A) Effects on ileal tissue RegIII-γ mRNA (n=7 per group); B) effects on ileal tissue RegIII-γ relative protein concentration (Chow: n=12; PN: n=10; PN+BBS: n=11); C) effects on RegIII-γ protein in intestinal wash fluid (Chow: n=11; PN: n=12; PN+BBS: n=9); and D) effects on RegIII-γ release after cholinergic stimulation with bethanechol (Chow: n=10; PN: n=12; PN+BBS: n=9). Data are presented as mean ± SEM. *p<0.05 versus Chow; #p<0.05 versus PN. PN, parenteral nutrition; BBS, bombesin.
Cryptdin-4
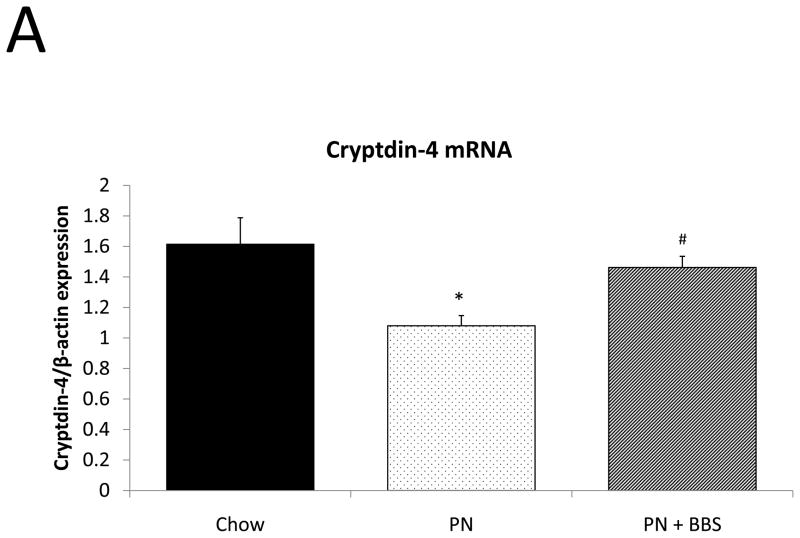
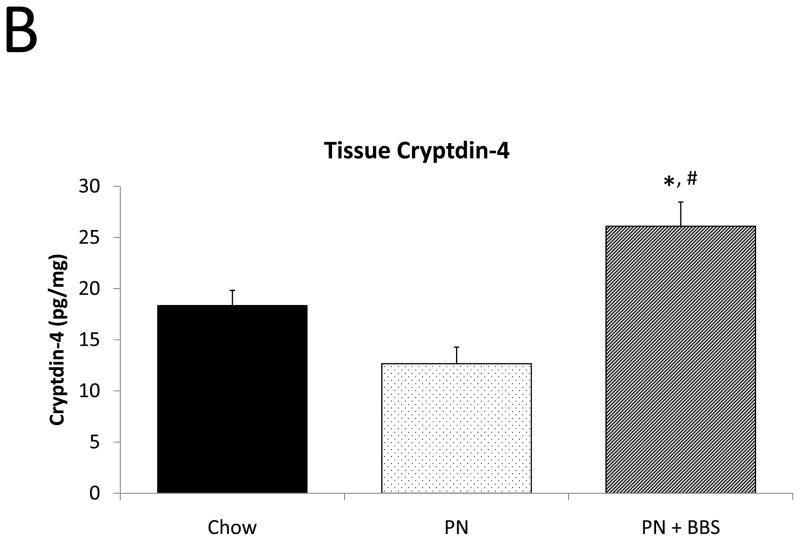
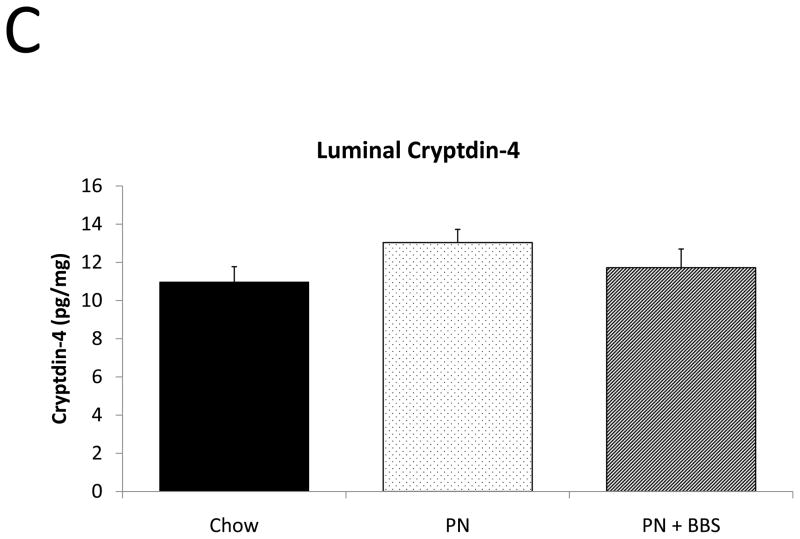
PN significantly decreased cryptdin-4 mRNA transcription compared to Chow (p=0.004). PN+BBS significantly increased cryptdin-4 mRNA transcription compared to PN alone (p=0.03) to values not significantly different from Chow levels (p=0.36), (Fig. 5A). Tissue levels of cryptdin-4 were not detectible in 5 of 11 PN samples. Comparing only the 6 samples detectable for cryptdin-4 to the other groups, PN decreased tissue levels of cryptdin-4 compared to Chow but not quite to statistical significance (p=0.07). PN+BBS significantly increased tissue cryptdin-4 protein in comparison to both PN and Chow (p=0.0002, and p=0.005 respectively), (Fig. 5B). There were no significant differences in luminal levels of cryptdin-4 between groups (p>0.05), (Fig. 5C). Bethanechol did not stimulate cryptdin-4 release to consistently detectable levels (data not shown).
Figure 5. Chow, PN, and PN+BBS effects on Cryptdin-4.
A) Effects on ileal tissue Cryptdin-4 mRNA (n=7 per group); B) effects on ileal tissue Cryptdin-4 relative protein concentration (Chow: n=13; PN: n=6; PN+BBS: n=11); C) effects on relative protein concentrations of Cryptdin-4 in intestinal wash fluid (Chow: n=15; PN: n=12; PN+BBS: n=9). Data are presented as mean ± SEM. *p<0.05 versus Chow; #p<0.05 versus PN. PN, parenteral nutrition; BBS, bombesin.
Bactericidal activity
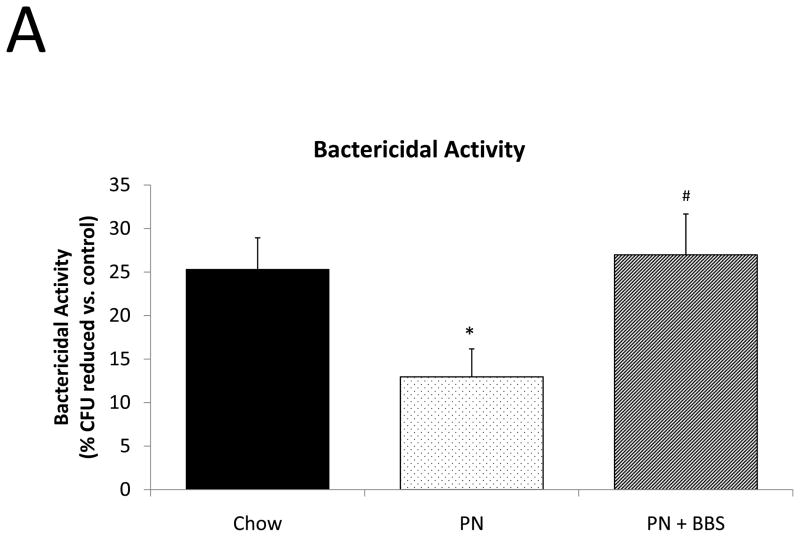
Functionally, PN feeding significantly decreased bactericidal activity in secretions obtained from small intestinal segments after bethanechol stimulation when compared to Chow feeding (p=0.039). Addition of BBS to PN restored bactericidal activity back to chow levels (BBS vs. Chow p=0.77; BBS vs. PN p=0.017), (Fig. 6A).
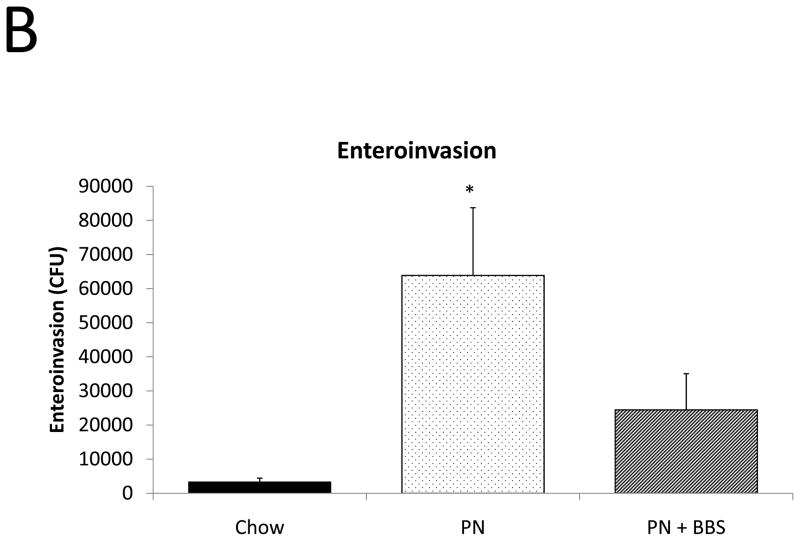
Figure 6. Functional effects of Chow, PN, and PN+BBS.
Functional effect of diet on A) bactericidal activity (Chow: n=6; PN: n=8; PN+BBS: n=7), and B) susceptibility to enteroinvasion (Chow: n=7; PN: n=9; PN+BBS: n=8). Data are presented as mean ± SEM. *p<0.05 versus Chow; #p<0.05 versus PN. PN, parenteral nutrition; BBS, bombesin.
Enteroinvasion
In a model of ex-vivo intestinal segment culture, PN demonstrated a significant increase in bacterial enteroinvasion when compared to Chow feeding (p=0.008). PN+BBS reduced bacterial enteroinvasion compared to PN levels, however this result barely failed to reach statistical significance (p=0.06), (Fig. 6B).
DISCUSSION
PN prevents progressive protein-energy malnutrition in patients unable to be fed enterally, however it carries an increased risk of septic and inflammatory morbidity in critically ill patients compared to enteral feeding. While the cause of increased infectious complications and inflammation in PN-fed patients is likely multifactorial, significant changes in acquired and innate mucosal immunology remain cogent hypotheses to explain these clinical outcomes. Given that the ENS neuropeptide analogue, BBS, experimentally restores cellular and functional aspects of adaptive immunity, this work examined its effects on the innate immune system.
The surface area of adult gastrointestinal tract encompasses an area of over 200 m2, making it the largest surface of the body directly in contact with the outside world.56 The human gut contains 500–1000 species of bacteria and the total numbers of bacteria exceed the total number of human cells 150-fold.57–59 Normally, a symbiotic relationship exists between host and microbiome whereby the intestine provides nutrients to the bacteria, while the bacteria aid in food digestion, nutrient absorption, and vitamin production. In health, the gastrointestinal mucosal barrier effectively prevents bacterial invasion. The integrity of immunologic mechanisms which reduce bacterial attachment prevents mucosal invasion and subsequent infection despite the huge bacterial burden. This protection occurs through two basic immunologic systems. The adaptive immune system produces sIgA which concentrates within the mucus layer to provide protection against specific bacterial antigens by binding to these bacteria and preventing invasion.9 The innate immune system exists as a more teleologically ancient system that secretes antimicrobial peptides (AMPs), (Table 4).46, 60–68 PN with lack of enteral stimulation impairs both systems.
Table 4.
Mechanism of innate defense for studied antimicrobial peptides.
| Antimicrobial Peptide | Gram activity | Mechanism | Result | Production |
|---|---|---|---|---|
| sPLA260–64 | Both | phospholipid-sn-2 esterase | Hydrolyzes membrane phospholipids | Constitutive |
| Lysozyme65 | Both | β-1,4-glycosidase | Attacks peptidoglycan | Constitutive |
| RegIII-γ66, 67 | Positive only | Antimicrobial C-type lectin | Attacks peptidoglycan | Induced |
| Cryptdin-446, 68 | Both | Alpha defensin, antimicrobial peptide | Form membrane pores to promote osmotic lysis | Constitutive |
To our knowledge this is the first work examining interactions between the innate immune system and ENS. Prior work demonstrated that Paneth cell antimicrobial peptide (sPLA2, lysozyme, RegIII-γ, cryptdin-4) transcription decreased with PN compared to Chow feeding which coincided with alterations in microbiome composition,44, 69 decreased bactericidal activity of Paneth cell secretions, and increased intestinal susceptibility to enteroinvasion.38 This study expands on those studies to confirm those findings using IHC of in situ Paneth cell granules and measurements of tissue AMP production and levels. It also examines the ability of isolated tissues to release AMPs from the tissue and provides quantitative measurement of resistance of the tissues to bacterial invasion. While PN with lack of enteral stimulation reduced or impaired each of these variables, BSS supplementation of PN reversed most of these negative effects on the innate immune system defenses.
Paneth cells produce AMPs which constitute an integral component of the innate immune system.70 Mice lacking Paneth cells fail to clear infectious organisms and frequently develop a lethal colitis.71 By specifically ablating Paneth cells using a transgenic mouse model, Vaishnava et al concluded that Paneth cells products limit bacterial-mucosal contact and mucosal attachment by controlling the number of mucosa-associated bacteria.72 Paneth cells contain an extensive endoplasmic reticulum and Golgi networks ultrastructurally involved in protein production and secretion.46, 73 They produce, store, and secrete AMPs by means of large dense core granules which are apically released into the gastrointestinal lumen and concentrate in the mucin layer.74 This AMP production appears dependent on enteral stimulation. Starvation itself decreases the number of Paneth cell granules and distorts granule packaging and exocytosis, also reducing production of constitutively produced AMPs.40 These effects are likely related to the lack of enteral stimulation rather than malnutrition since PN prevents malnutrition while the results of starvation and lack of enteral feeding on Paneth cells appear similar. Our results show that PN with lack of enteral stimulation decreases AMP transcription and production which is evident in the alterations in mRNA, immunohistochemistry, and tissue levels.
After determining that BBS increased AMP production and storage within the Paneth cells, we examined AMP levels within the intestinal fluid washes obtained immediately after sacrifice of the animals. Surprisingly, intestinal fluid from BBS treated mice contained low levels of AMPs at levels similar to PN feeding. Since both IHC and tissue analysis demonstrated high levels of stored AMPs present in Chow and BBS treated mice, we examined the functional capacity of isolated intestinal segments to release AMPs from Paneth cells. Preliminary work demonstrated very limited AMP release from isolated segments incubated with saline or PBS. Previous investigators noted that bacterial products (lipopolysaccharide or muramyl dipeptide)75 or parasympathetic (cholinergic) stimulation76 triggered Paneth cell degranulation in vitro and in in vivo isolated intestinal perfusion models mediated through 2 independent mechanisms: 1) KCa3.1 calcium activated potassium channels77 and 2) activation of muscarinic receptors78 present on Paneth cells which result in G-protein-coupled receptor mediated calcium dependent intracellular signal transduction.79 Both mechanisms dynamically increase cytosolic calcium to result in granule secretion, a process common to many exocrine and endocrine cells.80 In the current work, cholinergic stimulation resulted in higher AMP levels in the incubation fluid and increased bactericidal activity in both Chow and BBS groups. PN treatment resulted in significantly lower AMP levels and lower bactericidal activity, presumably due to lower AMP storage within the smaller granules and possibly reduced cholinergic response of the muscarinic receptor. Taken in toto, BBS increases AMP production of sPLA2 and lysozyme but not their release. Secretion remains under cholinergic control.
Different mechanisms appear to control RegIII-γ release.81 Current evidence points to inducible expression of RegIII-γ through Toll-like receptor pathways activated by bacterial products. Its expression appears dependent on MyD88 signaling,66, 72 providing direct communication between enteric bacteria and the Paneth cells. RegIII-γ regulates bacterial association by maintaining a 50 μm zone of physical separation between the mucosal surface and bacteria.82 The higher luminal density of RegIII-γ with PN implicates RegIII-γ release as a compensatory response by the intestinal mucosa to maintain homeostasis due to significant changes in the luminal environment.66, 83 However, ENS activation presumably plays some role in this intestinal homeostasis since BBS treatment resulted in RegIII-γ levels returning to Chow levels.
Cryptdin-4 protein levels were undetectable in the incubation fluid after cholinergic stimulation in all dietary groups. mRNA data is consistent with results from Hodin et al. who found decreased production of constitutively expressed AMPs, including cryptdins, in a starvation model.40 A better method for cryptdin protein detection is necessary to further research its mechanism of production and secretion from Paneth cells.
In the final experiments of function, ex-vivo intestinal segment culture allowed analysis of tissue susceptibility and resistance to bacterial enteroinvasion. Significantly greater enteroinvasion occurred with PN than Chow consistent with decreased AMP production and secretion. Exogenous BBS reduced enteroinvasion, barely missing statistical significance compared to PN. While other factors than AMP production and secretion almost certainly affect this functional test, the observation that BBS failed to release AMPs from Paneth cells may play a role in the enteroinvasion experiment. Further research to test this hypothesis is currently underway.
It is not surprising that the nervous system is integrated into mucosal immunity- both acquired and innate. The gastrointestinal tract is richly innervated by both the ENS and the autonomic nervous system. The number of neurons in the ENS approximates the number of neurons within the spinal cord.84 Approximately 2 m of nervous tissue innervate every mm3 of intestinal tissue with most nerve tissue located within 13 μm of GALT mucosa.85, 86 The vagus nerve provides parasympathetic innervation to the upper gastrointestinal tract (through the ascending colon).87 Vagal neurons have long preganglionic fibers that project primarily to the myenteric and submucosal plexuses where they have extensive and highly divergent arbors that allow them to synapse in or near their targets.88 These fibers are also intricately interlaced with neurons of the ENS and can relay central input.
Primary limitations of this study reflect the inability to define intracellular mechanisms which, for AMP production in general, are not well defined in the literature. Our data show innate immune changes with administration of BBS to PN-fed mice, however these BBS effects may be indirect through signaling cascades. BBS is an analogue for gastrin-releasing peptide (GRP) which is normally secreted early after enteral stimulation. GRP triggers release of other peptides and hormones (i.e. gastrin, cholecystokinin, etc.).48 Our previous work demonstrate that individual neuropeptides such as gastrin, cholecystokinin and neurotensin induce direct, though more limited, effects on specific mucosal immunity.89 In regard to the functional studies, it is likely that other AMPs are involved. Quantitatively other AMPs exist which were not measured in this study although our prior work demonstrated that sPLA2 appeared responsible for approximately 70% of AMP derived antibacterial activity,37 although others have noted variation from this number.75 The principles defined in this work appear valid, nevertheless. Lastly, this series of experiments does not isolate innate immune function, so that some sIgA-based mucosal immunity as well as an altered mucin layer likely play a role in our functional results.
This work has significant clinical implications. Marshall considered the gut as the motor of organ dysfunction. In toto, our experimental work defines a weakening of gut barrier integrity during PN through deleterious effects on both acquired and innate immunity.90 Reductions in GALT cell mass, intestinal IgA levels, innate immune AMPs and mucin allow abnormal interaction between the epithelium and luminal bacteria. The RegIII-γ response with PN may depict just such an interaction: a likely explanation for the increased RegIII-γ release by Paneth cells during PN is though interaction between bacteria in the crypt and Toll-like receptors expressed on the epithelium. Our prior work demonstrated that PN primes neutrophils within the gut vasculature.91 These primed cells distribute themselves throughout the body- in particular the lung and liver- and generate an augmented inflammatory response to subsequent insults resulting in hepatic and pulmonary dysfunction. Thus, as the gut barrier loses integrity and generates a host response to bacteria, the stage is set for systemic inflammation and organ dysfunction with further insults to the host. BBS may prevent this.
In conclusion, this work provides evidence for the novel use of BBS to enhance innate immune function by increasing Paneth cell AMP levels and bolstering innate defenses against enteroinvasion. Our results indicate that BBS supplementation acting as a stimulus for the ENS, increases production of at least 4 AMPs: sPLA2, lysozyme, RegIII-γ, and cryptdin-4. Increases in tissue levels of AMPs do not translate into increases in luminal levels, their target destination; release requires cholinergic stimulation. These results suggest that PN+BBS treatment could provide the weapons to mount a defense against pathogens, but needs additional stimuli to release them. In vivo experiments are currently underway to test this hypothesis. Theoretically, PN feeding alone will not maintain complete innate defense even with cholinergic secretory stimuli due to limited AMP production. We conclude that ENS stimulation via specific neuropeptides enhances the innate immune system. However the autonomic nervous system, particularly the parasympathetic nervous system appears necessary to appropriately employ the bolstered immune system against pathogenic threats.
Acknowledgments
Source of Funding:
This research is supported by Award Number I01BX001672 from the Biomedical Laboratory Research & Development Service of the VA Office of Research and Development. The contents of this article do not represent the views of the Department of Veterans Affairs or the United States Government. This material is also based upon work supported in part by National Institute of Health (NIH) Grant T32 CA090217-13.
Footnotes
Conflicts of Interest:
The authors have no conflicts of interest to declare.
The work was originally presented April 10, 2014 at the American Surgical Association meeting in Boston, MA.
References
- 1.Kudsk KA, Croce MA, Fabian TC, et al. Enteral versus parenteral feeding. Effects on septic morbidity after blunt and penetrating abdominal trauma. Ann Surg. 1992;215:503–511. doi: 10.1097/00000658-199205000-00013. discussion 511–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The Veterans Affairs Total Parenteral Nutrition Cooperative Study Group. Perioperative total parenteral nutrition in surgical patients. N Engl J Med. 1991;325:525–532. doi: 10.1056/NEJM199108223250801. [DOI] [PubMed] [Google Scholar]
- 3.Gramlich L, Kichian K, Pinilla J, et al. Does enteral nutrition compared to parenteral nutrition result in better outcomes in critically ill adult patients? A systematic review of the literature. Nutrition. 2004;20:843–848. doi: 10.1016/j.nut.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Barr J, Hecht M, Flavin KE, et al. Outcomes in critically ill patients before and after the implementation of an evidence-based nutritional management protocol. Chest. 2004;125:1446–1457. doi: 10.1378/chest.125.4.1446. [DOI] [PubMed] [Google Scholar]
- 5.Moore F, Moore E, Jones T, et al. TEN versus TPN following major abdominal trauma—reduced septic morbidity. J Trauma. 1989;29:916–922. doi: 10.1097/00005373-198907000-00003. discussion 922–923. [DOI] [PubMed] [Google Scholar]
- 6.Moore FA, Feliciano DV, Andrassy RJ, et al. Early enteral feeding, compared with parenteral, reduces postoperative septic complications. The results of a meta-analysis. Ann Surg. 1992;216:172–183. doi: 10.1097/00000658-199208000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore EE, Jones TN. Benefits of immediate jejunostomy feeding after major abdominal trauma--a prospective, randomized study. J Trauma. 1986;26:874–881. doi: 10.1097/00005373-198610000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Kudsk K, Minard G, Croce M, et al. A randomized trial of isonitrogenous enteral diets after severe trauma. An immune-enhancing diet reduces septic complications. Ann Surg. 1996;224:531–540. doi: 10.1097/00000658-199610000-00011. discussion 540–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kudsk KA. Jonathan E Rhoads lecture: Of mice and men... and a few hundred rats. JPEN J Parenter Enteral Nutr. 2008;32:460–473. doi: 10.1177/0148607108319795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wildhaber BE, Yang H, Spencer AU, et al. Lack of enteral nutrition--effects on the intestinal immune system. J Surg Res. 2005;123:8–16. doi: 10.1016/j.jss.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 11.Buchman AL, Moukarzel AA, Bhuta S, et al. Parenteral nutrition is associated with intestinal morphologic and functional changes in humans. JPEN J Parenter Enteral Nutr. 1995;19:453–460. doi: 10.1177/0148607195019006453. [DOI] [PubMed] [Google Scholar]
- 12.Kudsk KA. Current aspects of mucosal immunology and its influence by nutrition. Am J Surg. 2002;183:390–398. doi: 10.1016/s0002-9610(02)00821-8. [DOI] [PubMed] [Google Scholar]
- 13.Acheson DW, Luccioli S. Microbial-gut interactions in health and disease. Mucosal immune responses. Best Pract Res Clin Gastroenterol. 2004;18:387–404. doi: 10.1016/j.bpg.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Albanese CT, Smith SD, Watkins S, et al. Effect of secretory IgA on transepithelial passage of bacteria across the intact ileum in vitro. J Am Coll Surg. 1994;179:679–688. [PubMed] [Google Scholar]
- 15.Mestecky J, McGhee JR. Immunoglobulin A (IgA): molecular and cellular interactions involved in IgA biosynthesis and immune response. Adv Immunol. 1987;40:153–245. doi: 10.1016/s0065-2776(08)60240-0. [DOI] [PubMed] [Google Scholar]
- 16.Zarzaur B, Fukatsu K, Johnson C, et al. A temporal study of diet-induced changes in peyer patch MAdCAM-1 expression. Surgical Forum. 2001;52:194–196. [Google Scholar]
- 17.Gomez FE, Lan J, Kang W, et al. Parenteral nutrition and fasting reduces mucosal addressin cellular adhesion molecule-1 (MAdCAM-1) mRNA in Peyer’s patches of mice. JPEN J Parenter Enteral Nutr. 2007;31:47–52. doi: 10.1177/014860710703100147. [DOI] [PubMed] [Google Scholar]
- 18.Kang W, Gomez FE, Lan J, et al. Parenteral nutrition impairs gut-associated lymphoid tissue and mucosal immunity by reducing lymphotoxin Beta receptor expression. Ann Surg. 2006;244:392–399. doi: 10.1097/01.sla.0000234797.42935.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hermsen JL, Gomez FE, Maeshima Y, et al. Decreased enteral stimulation alters mucosal immune chemokines. JPEN J Parenter Enteral Nutr. 2008;32:36–44. doi: 10.1177/014860710803200136. [DOI] [PubMed] [Google Scholar]
- 20.Wu Y, Kudsk KA, DeWitt RC, et al. Route and type of nutrition influence IgA-mediating intestinal cytokines. Ann Surg. 1999;229:662–667. doi: 10.1097/00000658-199905000-00008. discussion 667–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sano Y, Gomez FE, Kang W, et al. Intestinal polymeric immunoglobulin receptor is affected by type and route of nutrition. JPEN J Parenter Enteral Nutr. 2007;31:351–356. doi: 10.1177/0148607107031005351. discussion 356–357. [DOI] [PubMed] [Google Scholar]
- 22.Sano Y, Gomez FE, Hermsen JL, et al. Parenteral nutrition induces organ specific alterations in polymeric immunoglobulin receptor levels. J Surg Res. 2008;149:236–242. doi: 10.1016/j.jss.2007.12.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J, Kudsk KA, Gocinski B, et al. Effects of parenteral and enteral nutrition on gut-associated lymphoid tissue. J Trauma. 1995;39:44–51. doi: 10.1097/00005373-199507000-00006. discussion 51–52. [DOI] [PubMed] [Google Scholar]
- 24.King BK, Li J, Kudsk KA. A temporal study of TPN-induced changes in gut-associated lymphoid tissue and mucosal immunity. Arch Surg. 1997;132:1303–1309. doi: 10.1001/archsurg.1997.01430360049009. [DOI] [PubMed] [Google Scholar]
- 25.Genton L, Reese SR, Ikeda S, et al. The C-terminal heptapeptide of bombesin reduces the deleterious effect of total parenteral nutrition (TPN) on gut-associated lymphoid tissue (GALT) mass but not intestinal immunoglobulin A in vivo. JPEN J Parenter Enteral Nutr. 2004;28:431–434. doi: 10.1177/0148607104028006431. [DOI] [PubMed] [Google Scholar]
- 26.Li J, Kudsk KA, Hamidian M, et al. Bombesin affects mucosal immunity and gut-associated lymphoid tissue in intravenously fed mice. Arch Surg. 1995;130:1164–1169. doi: 10.1001/archsurg.1995.01430110022005. discussion 1169–1170. [DOI] [PubMed] [Google Scholar]
- 27.DeWitt RC, Wu Y, Renegar KB, et al. Bombesin recovers gut-associated lymphoid tissue and preserves immunity to bacterial pneumonia in mice receiving total parenteral nutrition. Ann Surg. 2000;231:1–8. doi: 10.1097/00000658-200001000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hermsen JL, Gomez FE, Sano Y, et al. Parenteral feeding depletes pulmonary lymphocyte populations. JPEN J Parenter Enteral Nutr. 2009;33:535–540. doi: 10.1177/0148607109332909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zarzaur BL, Wu Y, Fukatsu K, et al. The neuropeptide bombesin improves IgA-mediated mucosal immunity with preservation of gut interleukin-4 in total parenteral nutrition-fed mice. Surgery. 2002;131:59–65. doi: 10.1067/msy.2002.118319. [DOI] [PubMed] [Google Scholar]
- 30.Pierre JF, Heneghan AF, Wang X, et al. Bombesin improves adaptive immunity of the salivary gland during parenteral nutrition. JPEN J Parenter Enteral Nutr. 2013 doi: 10.1177/0148607113507080. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kudsk KA, Li J, Renegar KB. Loss of upper respiratory tract immunity with parenteral feeding. Ann Surg. 1996;223:629–635. doi: 10.1097/00000658-199606000-00001. discussion 635–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Renegar KB, Kudsk KA, Dewitt RC, et al. Impairment of mucosal immunity by parenteral nutrition: depressed nasotracheal influenza-specific secretory IgA levels and transport in parenterally fed mice. Ann Surg. 2001;233:134–138. doi: 10.1097/00000658-200101000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Renegar KB, Johnson CD, Dewitt RC, et al. Impairment of mucosal immunity by total parenteral nutrition: requirement for IgA in murine nasotracheal anti-influenza immunity. J Immunol. 2001;166:819–825. doi: 10.4049/jimmunol.166.2.819. [DOI] [PubMed] [Google Scholar]
- 34.King BK, Kudsk KA, Li J, et al. Route and type of nutrition influence mucosal immunity to bacterial pneumonia. Ann Surg. 1999;229:272–278. doi: 10.1097/00000658-199902000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Janu PG, Kudsk KA, Li J, et al. Effect of bombesin on impairment of upper respiratory tract immunity induced by total parenteral nutrition. Arch Surg. 1997;132:89–93. doi: 10.1001/archsurg.1997.01430250091019. [DOI] [PubMed] [Google Scholar]
- 36.Pierre JF, Heneghan AF, Tsao FH, et al. Route and type of nutrition and surgical stress influence secretory phospholipase A2 secretion of the murine small intestine. JPEN J Parenter Enteral Nutr. 2011;35:748–756. doi: 10.1177/0148607111414025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Omata J, Pierre JF, Heneghan AF, et al. Parenteral nutrition suppresses the bactericidal response of the small intestine. Surgery. 2013;153:17–24. doi: 10.1016/j.surg.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heneghan AF, Pierre JF, Tandee K, et al. Parenteral nutrition decreases paneth cell function and intestinal bactericidal activity while increasing susceptibility to bacterial enteroinvasion. JPEN J Parenter Enteral Nutr. 2013 doi: 10.1177/0148607113497514. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heneghan AF, Pierre JF, Gosain A, et al. IL-25 improves luminal innate immunity and barrier function during parenteral nutrition. Ann Surg. 2014;259:394–400. doi: 10.1097/SLA.0b013e318284f510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hodin CM, Lenaerts K, Grootjans J, et al. Starvation compromises Paneth cells. Am J Pathol. 2011;179:2885–2893. doi: 10.1016/j.ajpath.2011.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Demehri FR, Barrett M, Ralls MW, et al. Intestinal epithelial cell apoptosis and loss of barrier function in the setting of altered microbiota with enteral nutrient deprivation. Front Cell Infect Microbiol. 2013;3:105. doi: 10.3389/fcimb.2013.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang H, Feng Y, Sun X, et al. Enteral versus parenteral nutrition: effect on intestinal barrier function. Ann N Y Acad Sci. 2009;1165:338–346. doi: 10.1111/j.1749-6632.2009.04026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feng Y, Ralls MW, Xiao W, et al. Loss of enteral nutrition in a mouse model results in intestinal epithelial barrier dysfunction. Ann N Y Acad Sci. 2012;1258:71–77. doi: 10.1111/j.1749-6632.2012.06572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miyasaka EA, Feng Y, Poroyko V, et al. Total parenteral nutrition-associated lamina propria inflammation in mice is mediated by a MyD88-dependent mechanism. J Immunol. 2013;190:6607–6615. doi: 10.4049/jimmunol.1201746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fishman JE, Sheth SU, Levy G, et al. Intraluminal nonbacterial intestinal components control gut and lung injury after trauma hemorrhagic shock. Ann Surg. 2014 doi: 10.1097/SLA.0000000000000631. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bevins CL, Salzman NH. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat Rev Microbiol. 2011;9:356–368. doi: 10.1038/nrmicro2546. [DOI] [PubMed] [Google Scholar]
- 47.Furness JB, Kunze WA, Clerc N. Nutrient tasting and signaling mechanisms in the gut. II. The intestine as a sensory organ: neural, endocrine, and immune responses. Am J Physiol. 1999;277:G922–892. doi: 10.1152/ajpgi.1999.277.5.G922. [DOI] [PubMed] [Google Scholar]
- 48.Genton L, Kudsk KA. Interactions between the enteric nervous system and the immune system: role of neuropeptides and nutrition. Am J Surg. 2003;186:253–258. doi: 10.1016/s0002-9610(03)00210-1. [DOI] [PubMed] [Google Scholar]
- 49.Furness JB, Costa M, Gibbins IL, et al. Neurochemically similar myenteric and submucous neurons directly traced to the mucosa of the small intestine. Cell Tissue Res. 1985;241:155–163. doi: 10.1007/BF00214637. [DOI] [PubMed] [Google Scholar]
- 50.Gabrilovac J, Marotti T. Gender-related differences in murine T- and B-lymphocyte proliferative ability in response to in vivo [Met(5)]enkephalin administration. Eur J Pharmacol. 2000;392:101–108. doi: 10.1016/s0014-2999(00)00118-7. [DOI] [PubMed] [Google Scholar]
- 51.Del Rio M, Hernanz A, de la Fuente M. Bombesin, gastrin-releasing peptide, and neuromedin C modulate murine lymphocyte proliferation through adherent accessory cells and activate protein kinase C. Peptides. 1994;15:15–22. doi: 10.1016/0196-9781(94)90164-3. [DOI] [PubMed] [Google Scholar]
- 52.Sitren HS, Heller PA, Bailey LB, et al. Total parenteral nutrition in the mouse: development of a technique. JPEN J Parenter Enteral Nutr. 1983;7:582–586. doi: 10.1177/0148607183007006582. [DOI] [PubMed] [Google Scholar]
- 53.National Academy of Science. Nutrient Requirements of Laboratory Animals. Washington, DC: National Academy of Science; 1978. National Research Publication No. 10. [Google Scholar]
- 54.Tsao FH, Shanmuganayagam D, Zachman DK, et al. A continuous fluorescence assay for the determination of calcium-dependent secretory phospholipase A2 activity in serum. Clin Chim Acta. 2007;379:119–126. doi: 10.1016/j.cca.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 55.Pierre J, Heneghan A, Meudt J, et al. Parenteral nutrition increases susceptibility of ileum to invasion by E coli. J Surg Res. 2013;183:583–591. doi: 10.1016/j.jss.2013.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dommett R, Zilbauer M, George JT, et al. Innate immune defence in the human gastrointestinal tract. Mol Immunol. 2005;42:903–912. doi: 10.1016/j.molimm.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 57.Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292:1115–1118. doi: 10.1126/science.1058709. [DOI] [PubMed] [Google Scholar]
- 58.Eckburg PB, Bik EM, Bernstein CN, et al. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Curtis MM, Sperandio V. A complex relationship: the interaction among symbiotic microbes, invading pathogens, and their mammalian host. Mucosal Immunol. 2011;4:133–138. doi: 10.1038/mi.2010.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weiss J, Inada M, Elsbach P, et al. Structural determinants of the action against Escherichia coli of a human inflammatory fluid phospholipase A2 in concert with polymorphonuclear leukocytes. J Biol Chem. 1994;269:26331–26337. [PubMed] [Google Scholar]
- 61.Harwig SS, Tan L, Qu XD, et al. Bactericidal properties of murine intestinal phospholipase A2. J Clin Invest. 1995;95:603–610. doi: 10.1172/JCI117704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weinrauch Y, Elsbach P, Madsen LM, et al. The potent anti-Staphylococcus aureus activity of a sterile rabbit inflammatory fluid is due to a 14-kD phospholipase A2. J Clin Invest. 1996;97:250–257. doi: 10.1172/JCI118399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Beers SA, Buckland AG, Koduri RS, et al. The antibacterial properties of secreted phospholipases A2: a major physiological role for the group IIA enzyme that depends on the very high pI of the enzyme to allow penetration of the bacterial cell wall. J Biol Chem. 2002;277:1788–1793. doi: 10.1074/jbc.M109777200. [DOI] [PubMed] [Google Scholar]
- 64.Koduri RS, Grönroos JO, Laine VJ, et al. Bactericidal properties of human and murine groups I, II, V, X, and XII secreted phospholipases A(2) J Biol Chem. 2002;277:5849–5857. doi: 10.1074/jbc.M109699200. [DOI] [PubMed] [Google Scholar]
- 65.Mukherjee S, Vaishnava S, Hooper LV. Multi-layered regulation of intestinal antimicrobial defense. Cell Mol Life Sci. 2008;65:3019–3027. doi: 10.1007/s00018-008-8182-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brandl K, Plitas G, Schnabl B, et al. MyD88-mediated signals induce the bactericidal lectin RegIII gamma and protect mice against intestinal Listeria monocytogenes infection. J Exp Med. 2007;204:1891–1900. doi: 10.1084/jem.20070563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Salzman NH, Underwood MA, Bevins CL. Paneth cells, defensins, and the commensal microbiota: a hypothesis on intimate interplay at the intestinal mucosa. Semin Immunol. 2007;19:70–83. doi: 10.1016/j.smim.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 68.Masuda K, Sakai N, Nakamura K, et al. Bactericidal activity of mouse α-defensin cryptdin-4 predominantly affects noncommensal bacteria. J Innate Immun. 2011;3:315–326. doi: 10.1159/000322037. [DOI] [PubMed] [Google Scholar]
- 69.Hodin CM, Visschers RG, Rensen SS, et al. Total parenteral nutrition induces a shift in the Firmicutes to Bacteroidetes ratio in association with Paneth cell activation in rats. J Nutr. 2012;142:2141–2147. doi: 10.3945/jn.112.162388. [DOI] [PubMed] [Google Scholar]
- 70.Ouellette AJ, Selsted ME. Paneth cell defensins: endogenous peptide components of intestinal host defense. FASEB J. 1996;10:1280–1289. doi: 10.1096/fasebj.10.11.8836041. [DOI] [PubMed] [Google Scholar]
- 71.Van der Sluis M, De Koning BA, De Bruijn AC, et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006;131:117–129. doi: 10.1053/j.gastro.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 72.Vaishnava S, Behrendt CL, Ismail AS, et al. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc Natl Acad Sci U S A. 2008;105:20858–20863. doi: 10.1073/pnas.0808723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ouellette AJ. Paneth cells and innate mucosal immunity. Curr Opin Gastroenterol. 2010;26:547–553. doi: 10.1097/MOG.0b013e32833dccde. [DOI] [PubMed] [Google Scholar]
- 74.Meyer-Hoffert U, Hornef MW, Henriques-Normark B, et al. Secreted enteric antimicrobial activity localises to the mucus surface layer. Gut. 2008;57:764–771. doi: 10.1136/gut.2007.141481. [DOI] [PubMed] [Google Scholar]
- 75.Ayabe T, Satchell DP, Wilson CL, et al. Secretion of microbicidal alpha-defensins by intestinal Paneth cells in response to bacteria. Nat Immunol. 2000;1:113–118. doi: 10.1038/77783. [DOI] [PubMed] [Google Scholar]
- 76.Qu XD, Lloyd KC, Walsh JH, et al. Secretion of type II phospholipase A2 and cryptdin by rat small intestinal Paneth cells. Infect Immun. 1996;64:5161–5165. doi: 10.1128/iai.64.12.5161-5165.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ayabe T, Wulff H, Darmoul D, et al. Modulation of mouse Paneth cell alpha-defensin secretion by mIKCa1, a Ca2+-activated, intermediate conductance potassium channel. J Biol Chem. 2002;277:3793–3800. doi: 10.1074/jbc.M107507200. [DOI] [PubMed] [Google Scholar]
- 78.Satoh Y, Ishikawa K, Oomori Y, et al. Bethanechol and a G-protein activator, NaF/AlCl3, induce secretory response in Paneth cells of mouse intestine. Cell Tissue Res. 1992;269:213–220. doi: 10.1007/BF00319611. [DOI] [PubMed] [Google Scholar]
- 79.Satoh Y, Habara Y, Ono K, et al. Carbamylcholine- and catecholamine-induced intracellular calcium dynamics of epithelial cells in mouse ileal crypts. Gastroenterology. 1995;108:1345–1356. doi: 10.1016/0016-5085(95)90681-9. [DOI] [PubMed] [Google Scholar]
- 80.Berridge MJ. Inositol trisphosphate and calcium signaling. Ann N Y Acad Sci. 1995;766:31–43. doi: 10.1111/j.1749-6632.1995.tb26646.x. [DOI] [PubMed] [Google Scholar]
- 81.Ogawa H, Fukushima K, Naito H, et al. Increased expression of HIP/PAP and regenerating gene III in human inflammatory bowel disease and a murine bacterial reconstitution model. Inflamm Bowel Dis. 2003;9:162–170. doi: 10.1097/00054725-200305000-00003. [DOI] [PubMed] [Google Scholar]
- 82.Vaishnava S, Yamamoto M, Severson KM, et al. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science. 2011;334:255–258. doi: 10.1126/science.1209791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cash HL, Whitham CV, Behrendt CL, et al. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science. 2006;313:1126–1130. doi: 10.1126/science.1127119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Anderson RB, Newgreen DF, Young HM. Neural crest and the development of the enteric nervous system. Adv Exp Med Biol. 2006;589:181–196. doi: 10.1007/978-0-387-46954-6_11. [DOI] [PubMed] [Google Scholar]
- 85.Ottaway C. Neuroimmunomodulation in the intestinal mucosa. Gastroenterol Clin North Am. 1991;20:511–529. [PubMed] [Google Scholar]
- 86.Debas HT, Mulvihill SJ. Neuroendocrine design of the gut. Am J Surg. 1991;161:243–249. doi: 10.1016/0002-9610(91)91139-a. [DOI] [PubMed] [Google Scholar]
- 87.Downing JE, Miyan JA. Neural immunoregulation: emerging roles for nerves in immune homeostasis and disease. Immunol Today. 2000;21:281–289. doi: 10.1016/s0167-5699(00)01635-2. [DOI] [PubMed] [Google Scholar]
- 88.Holst MC, Kelly JB, Powley TL. Vagal preganglionic projections to the enteric nervous system characterized with Phaseolus vulgaris-leucoagglutinin. J Comp Neurol. 1997;381:81–100. doi: 10.1002/(sici)1096-9861(19970428)381:1<81::aid-cne7>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 89.Keith Hanna M, Zarzaur BL, Fukatsu K, et al. Individual neuropeptides regulate gut-associated lymphoid tissue integrity, intestinal immunoglobulin A levels, and respiratory antibacterial immunity. JPEN J Parenter Enteral Nutr. 2000;24:261–268. doi: 10.1177/0148607100024005261. discussion 268–269. [DOI] [PubMed] [Google Scholar]
- 90.Marshall JC, Christou NV, Meakins JL. The gastrointestinal tract. The “undrained abscess” of multiple organ failure. Ann Surg. 1993;218:111–119. doi: 10.1097/00000658-199308000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kudsk KA. Effect of route and type of nutrition on intestine-derived inflammatory responses. Am J Surg. 2003;185:16–21. doi: 10.1016/s0002-9610(02)01146-7. [DOI] [PubMed] [Google Scholar]