Abstract
Several behavioral studies report that adolescent rats display a preference for nicotine compared with adults. However, age-related pharmacokinetic differences may confound the interpretation of these findings. Thus, differences in pharmacokinetic analyses of nicotine were investigated. Nicotine was administered via acute s.c. (1.0 mg base/kg) or i.v. (0.2 mg base/kg) injection to early adolescent (EA; postnatal day 25) and adult (AD; postnatal day 71) male Wistar rats. Nicotine and its primary metabolite, cotinine, and additional metabolites nornicotine, nicotine-1′-N-oxide, trans-3′-hydroxycotinine, and norcotinine were sampled from 10 minutes to 8 hours (plasma) and 2 to 8 hours (brain) post nicotine and analyzed by liquid chromatography–tandem mass spectrometry. Following s.c. nicotine, the EA cohort had lower levels of plasma nicotine, cotinine, and nicotine-1′-N-oxide at multiple time points, resulting in a lower area under the plasma concentration-time curve (AUC) for nicotine (P < 0.001), cotinine (P < 0.01), and nicotine-1′-N-oxide (P < 0.001). Brain levels were also lower for these compounds. In contrast, the EA cohort had higher plasma and brain AUCs (P < 0.001) for the minor metabolite nornicotine. Brain-to-plasma ratios varied for nicotine and its metabolites, and by age. Following i.v. nicotine administration, similar age-related differences were observed, and this route allowed detection of a 1.6-fold-larger volume of distribution and 2-fold higher plasma clearance in the EA cohort compared with the AD cohort. Thus, unlike in humans, there are substantial age differences in nicotine pharmacokinetics such that for a given nicotine dose, adolescent rats will have lower plasma and brain nicotine compared with adults, suggesting that this should be considered when interpreting animal model data.
Introduction
Tobacco dependence is primarily mediated through the pharmacological actions of nicotine (Benowitz et al., 2009; Caille et al., 2012). The study of the pharmacokinetics of nicotine provides insight into variation in nicotine levels and how this may subsequently influence nicotine-mediated behaviors. Among the factors that influence nicotine pharmacokinetics is age (Benowitz et al., 2009). For example, in humans over the age of 65, nicotine clearance (CL) is reduced (Molander et al., 2001), and in neonates, the nicotine half-life (t1/2) is longer compared with adults (Dempsey et al., 2000). No differences in nicotine pharmacokinetics have been observed between human adolescents and adults (Gourlay and Benowitz, 1996; Al Koudsi et al., 2010). However, differences in nicotine plasma levels between adolescent and adult rats given the same nicotine dose in a behavioral study were noted, suggesting potential age-related variation in nicotine pharmacokinetics (Shram et al., 2008).
Nicotine is inactivated and eliminated from the body by various metabolic pathways, primarily mediated by hepatic enzymes (Nakajima and Yokoi, 2005). Nicotine’s primary route of elimination in most mammalian species is via its conversion to cotinine by C-oxidation (Messina et al., 1997; Nakajima et al., 1998). Oxidative nicotine metabolism also produces minor nicotine metabolites including nornicotine (Green et al., 2001) and nicotine-1′-N-oxide (Ochiai et al., 2006). Measuring the pharmacokinetics of nicotine and its metabolites over time, preferably in both the plasma and brain, enhances the interpretation of nicotine’s effects (Hukkanen et al., 2005).
The majority of current adult smokers began smoking in adolescence, with 88% of first cigarette use occurring before age 18 (Liang et al., 2003; US Department of Health and Human Services, 2012). Human adolescents have a higher risk of developing nicotine dependence, relative to adults, even with limited tobacco exposure (Rose et al., 2012). Initiation of smoking during adolescence has been associated with a lower likelihood of quitting in adulthood, increased cigarette consumption, and increased difficulty quitting (Khuder et al., 1999). Animal models can be used to investigate aspects of the nicotine dependence cycle that cannot be easily investigated in humans, such as the initiation of use (Casey and Jones, 2010). For example, using the conditioned place preference paradigm, which assesses drug reward in rodents (Perna et al., 2011), acute low doses of s.c. nicotine elicited a preference for the nicotine-paired chamber in adolescent but not in adult rats (Vastola et al., 2002; Belluzzi et al., 2004). When first exposed to nicotine in adolescence in an i.v. self-administration paradigm, both male (Levin et al., 2007) and female (Levin et al., 2003) rats displayed enhanced rates of responding compared with animals first exposed in adulthood. These behavioral differences between adolescents and adults may be attributed to a reduced sensitivity to nicotine’s aversive effects (Vastola et al., 2002), differences in reward set points during development (Koob and Le Moal, 2001), and/or pharmacokinetic differences (Levin et al., 2003). Plasma nicotine and cotinine levels, measured at a single time point, were significantly lower in adolescent compared with adult rats (Shram et al., 2008). Adolescent rats also had enhanced upregulation of brain nicotinic acetylcholine receptors in response to nicotine and significantly lower brain nicotine levels than those reported in adults (Doura et al., 2008). These studies suggest that pharmacokinetic differences may contribute to the behavioral differences observed between adolescent and adult rats.
This study was conducted to directly assess these potential age-specific pharmacokinetic differences in nicotine and its metabolites and to assist in understanding the potential confound of extrapolating the results of rat behavioral studies to the interpretation of human nicotine-mediated behaviors, given the apparent species differences in nicotine pharmacokinetics as a function of age. Acute dosing of s.c. and i.v. nicotine was used to assess the impact of age on the plasma and brain levels of nicotine and its metabolites in early adolescent and adult rats.
Materials and Methods
Animals.
Male Wistar rats were purchased from Charles River Laboratories (St-Constant, QC, Canada). Rats were aged by postnatal days (PNDs); early adolescent (EA; PND 25) and adult (AD; PND 71) rats were used in each experiment. These age groups were selected according to the previously established developmental periods of EA (PND 21–34) and AD (PND >60) (Spear, 2000; McCormick and Mathews, 2010). Male rats were chosen, as they had been used in the behavioral studies and to avoid the sex-specific influence of sexually dimorphic growth hormone release on drug metabolism, which occurs in rats and is not relevant in humans (Shapiro et al., 1995); see Discussion. All EA (81 ± 8 g) and AD (349 ± 25 g) animals were housed in pairs or groups of three and maintained on a 12 hour light/dark and temperature-controlled cycle. Water and food were available ad libitum. All experimental procedures were conducted in accordance with the guidelines provided by the Canadian Council on Animal Care and approved by the Animal Care Committee of the University of Toronto.
Reagents.
(−)-Nicotine hydrogen tartrate salt was purchased from Sigma (St. Louis, MO). Nicotine-d4, rac-cotinine, rac-cotinine-d3, trans-3′-hydroxycotinine, trans-3′-hydroxycotinine-d3, (R,S)-norcotinine, (R,S)-nornicotine, (1′S,2′S)-nicotine-1′-oxide, and rac-trans-nicotine-1′-oxide-methyl-d3 were obtained from Toronto Research Chemicals (North York, ON, Canada). The purity of all compounds used was >98%.
In Vivo Nicotine Pharmacokinetics.
Animals received either an acute s.c. (1.0 mg free base/kg body weight, a higher dose allowing better quantification of nicotine and metabolites) or i.v. (0.2 mg free base/kg body weight, a lower dose required to avoid toxicity) administration of (−)-nicotine hydrogen tartrate dissolved in sterile saline (0.9% NaCl). Fresh nicotine solutions were prepared and titrated to a pH of 7.4. Venous blood (saphenous vein, 100–200 μl) was collected at 10 minutes, 30 minutes, 1 hour, 2 hours, and 4 hours after nicotine injection, as well as trunk blood at sacrifice (2, 4, or 8 hours after nicotine injection). For each animal, two timed blood samples were obtained from the saphenous vein and the third sample from trunk blood at time of sacrifice. The sample timing and animal numbers were based on previous nicotine studies performed in our laboratory in adult rats, e.g., Micu et al. (2003), and pilot s.c. nicotine injection studies performed in adolescent rats. Thus, the total number of animals used was 30 for the EA and 14 for the AD groups for the s.c. study, and 25 for the EA and 23 for the AD groups for the i.v. dosing study; as each animal provided three of the six blood sampling times, this resulted in an average of 10, 9, 6, and 7 samples per time point, respectively. Blood samples were placed on ice, and plasma was collected after centrifugation at 3500g for 10 minutes and stored at −30°C until analyzed. Following sacrifice, brains were immediately dissected on ice, frozen on dry ice, and stored at −80°C.
Plasma Sample Preparation.
Sample preparation and extraction was adapted from a previously described method (Vieira-Brock et al., 2011). Briefly, 100 μl of 20 ng/ml deuterium-labeled internal standard in 0.01 M HCl, 900 μl of water, and 100 μl of 30% perchloric acid were added to 100 μl of plasma or 100 μl of standards, for a final volume of 1.2 ml. After vortex-mixing and centrifugation at 5400g for 5 minutes, the supernatant was added to Oasis MCX solid-phase extraction cartridges (3 cc, 60 mg) (Waters, Milford, MA) that had been preconditioned with 2 ml of high-performance liquid chromatography (HPLC)-grade methanol followed by 2 ml of 2% formic acid. The cartridges were washed with 1 ml of 2% formic acid and 1 ml of HPLC-grade methanol. Analytes were eluted with 1.5 ml of 5% (v/v) ammonium hydroxide in methanol and 1.5 ml of dichloromethane/isopropanol/ammonium hydroxide, 78:20:2 (v/v/v). The samples were fortified with 100 μl of 10% (v/v) hydrochloric acid in methanol to enhance nicotine recovery and then dried under a stream of nitrogen at 37°C. Dry samples were then reconstituted in 100 μl of 100 mM ammonium acetate in water/methanol/acetic acid, 79:20:1 (v/v/v), and 50 μl were injected onto a liquid chromatography–tandem mass spectrometer.
Brain Sample Preparation.
Brain samples (whole brain) were thawed on ice, weighed, homogenized in three volumes of ice-cold saline (0.9% NaCl), and centrifuged at 3000g for 10 minutes. The supernatant was collected and frozen at −80°C until time of analysis. The brain supernatant preparation was performed as described above using 1.0 ml of brain supernatant (without the addition of water), as previously described (Vieira-Brock et al., 2011). The mean recovery (n = 5) was >80% for all compounds assessed, using labeled standards. The recovery of nicotine was 85.4% in EA (range, 82.6–87.3%) and 85.3% in AD (range, 83.2–88.4%) brain samples; the recovery of cotinine was 85.5% in EA (range, 81.2–90.1%) and 85.8% in AD (range, 81.6–89.9%); the recovery of nornicotine was 89.0% in EA (range, 86.3–93.3%) and 90.3% in AD (range, 88.8–93.3%); the recovery of nicotine-1′-N-oxide was 86.7% in EA (range, 83.8–90.2%) and 84.6% in AD (range, 82.9–87.3%); the recovery of trans-3′-hydroxycotinine was 85.3% in EA (range, 84.5–86.6%) and 84.3% in AD (range, 82.8–86.5%); and the recovery of norcotinine was 81.1% in EA (range, 77.2–86.1%) and 80.7% in AD (range, 76.4–85.7%).
Liquid Chromatography–Mass Spectrometry Conditions.
The chromatographic conditions were adapted from an established method (Jacob et al., 2011) and were performed using an Agilent 1260 HPLC system (Agilent Technologies, Santa Clara, CA) equipped with a 4.6 mm × 150 mm Synergi Polar-RP column (4 μm) (Phenomenex, Torrance, CA). The mobile phase consisted of a 10 mM ammonium acetate/0.1% acetic acid in water (solvent A) and 10 mM ammonium acetate/0.1% acetic acid in methanol (solvent B). The flow rate was 0.7 ml/min, and the following gradient was used. The initial composition was 80% solvent A, changing to 100% solvent B over 6.5 minutes. From 6.5 to 8 minutes, 100% solvent B was maintained and was then changed to 80% solvent A at 8.1 minutes and maintained at this composition until the end of the run; total run time was 13 minutes. The spectrometric analysis was performed on an Agilent 6430 triple-quadruple mass spectrometer equipped with an atmospheric pressure chemical ionization ion source operated in the positive ion mode. The vaporizer temperature was optimized to 450°C, the gas temperature was 350°C, and the corona discharge current was set at 5 μA. The limit of quantification (LOQ) was 1 ng/ml for all analytes. Intraday coefficients of variation were assessed for all metabolites to determine intrarun assay precision. The coefficient of variation values were 2.3% for nicotine, 2.5% for cotinine, 1.9% for nornicotine, 1.6% for nicotine-1′-N-oxide, 1.1% for trans-3′-hydroxycotinine, and 3.0% for norcotinine.
Pharmacokinetic Analysis.
In vivo pharmacokinetic parameters were determined using noncompartmental analysis and were calculated using PK Functions for Microsoft Excel (J.I. Usansky, A. Desia, D. Tan-Liu, Department of Pharmacokinetics and Drug Metabolism, Allergan, Irvine, CA). The area under the concentration-time curves from 10 minutes to 4 hours (AUC(10min–4h)) or 2 to 8 hours (AUC(2–8h)) in plasma and the AUC(2–8h) in brain were determined using the log-linear trapezoidal rule. Half-life was estimated using the equation t1/2 = ln(2)/kel, where kel is the terminal elimination rate constant. The terminal elimination rate constant was estimated by linear regression of the terminal phase of the semilogarithmic concentration-time curve. The brain-to-plasma partition coefficient (KP) was calculated as a ratio of brain AUC(2–8h) to plasma AUC(2–8h). Following acute i.v. nicotine administration, the initial volume of distribution (VD) was calculated as Dose/C0, where C0 is the initial plasma nicotine concentration, extrapolated to time zero of the concentration-time curve. The plasma CL was calculated as ln(2) × VC/t1/2 where Vc is the initial volume of distribution (Toutain and Bousquet-Mélou, 2004c).
Statistical Analysis.
Statistical analyses were performed using GraphPad Prism 6.0 software (GraphPad Software Inc., La Jolla, CA). Variables were described by their mean ± S.D. and were compared between the EA and AD groups using a one-tailed, unpaired Student’s t-test with Welch’s correction. Due to the study design, whereby individual animals were sampled across varying time points, only group means are reported for t1/2, VD, and CL.
Results
Plasma Nicotine and Metabolite Levels Differ between Early Adolescent and Adult Rats
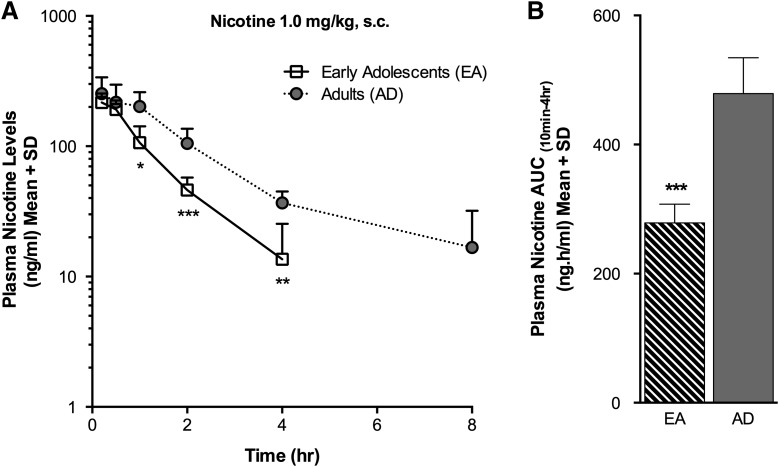
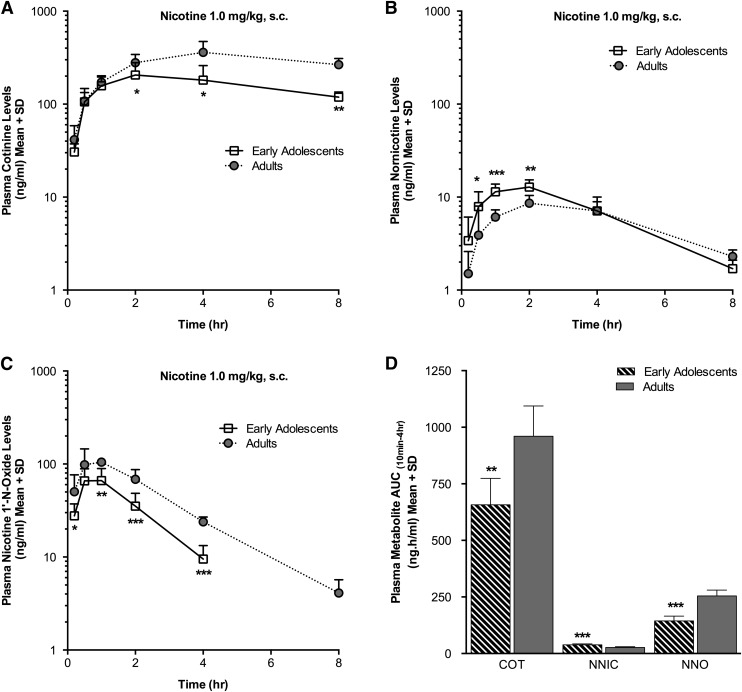
Plasma nicotine levels were lower in the EA(s.c.) group compared with the AD(s.c.) group at 1 hour (P < 0.05), 2 hours (P < 0.001), and 4 hours (P < 0.01) following acute s.c. nicotine administration (Fig. 1A); pharmacokinetic parameters are reported in Table 1. The mean plasma nicotine AUC(10min–4h) was also lower in the EA(s.c.) compared with the AD(s.c.) group (P < 0.001), indicative of lower total nicotine exposure in the EA(s.c.) animals (Fig. 1B). If the lower plasma nicotine levels in the EA(s.c.) group were due to an increased rate of conversion to cotinine, the major metabolic route for nicotine, higher plasma cotinine levels might be expected in the younger animals. However, plasma cotinine levels were lower in the EA(s.c.) group at 2 hours (P < 0.05), 4 hours (P < 0.05), and 8 hours (P < 0.01) compared with those in the AD(s.c.) group (Fig. 2A). The mean plasma cotinine AUC(10min–4h) was also lower in the EA(s.c.) compared with the AD(s.c.) group (P < 0.01) (Fig. 2D). We also investigated whether the formation of the minor metabolites nornicotine and nicotine-1′-N-oxide contributed to the observed age differences in nicotine levels. Plasma nornicotine levels were low, as expected, and higher at 30 minutes (P < 0.05), 1 hour (P < 0.001), and 2 hours (P < 0.01) in the EA(s.c.) compared with the AD(s.c.) group (Fig. 2B), resulting in a higher AUC(10min–4h) (P < 0.001) (Fig. 2D). The mean nornicotine Cmax was also higher in the EA(s.c.) versus the AD(s.c.) group (P < 0.001) (Table 1). The plasma levels of nicotine-1′-N-oxide were lower in the EA(s.c.) group at 10 minutes (P < 0.05), 1 hour (P < 0.01), 2 hours (P < 0.001), 4 hours (P < 0.001), and 8 hours (P < 0.015) following nicotine administration compared with the AD(s.c.) group (Fig. 2C), resulting in a lower mean nicotine-1′-N-oxide AUC(10min–4h) (P < 0.001) (Fig. 2D). The mean plasma Cmax for nicotine-1′-N-oxide was also lower in the EA(s.c.) compared with the AD(s.c.) group (P < 0.01) (Table 1). We also assessed the plasma levels of two additional minor nicotine metabolites. The mean plasma levels of trans-3′-hydroxycotinine and norcotinine were very low, near the LOQ (1 ng/ml), in both the EA(s.c.) and AD(s.c.) groups (Table 2).
Fig. 1.
Plasma nicotine levels and AUC(10min–4h) were lower in the EA(s.c.) group following s.c. nicotine administration. (A) Plasma nicotine levels measured over time. (B) Plasma nicotine AUC(10min–4h). *P < 0.05, **P < 0.01, ***P < 0.001 versus AD(s.c.) group.
TABLE 1.
Pharmacokinetic parameters of plasma nicotine and metabolites in EA and AD rats following s.c. nicotine
Data are presented as mean (S.D.).
| Parameter | NIC | COT | NNIC | NNO | ||||
|---|---|---|---|---|---|---|---|---|
| EA | AD | EA | AD | EA | AD | EA | AD | |
| Cmax (ng/ml) | 217 (37) | 253 (65) | 206 (73) | 361 (112) | 13** (2.5) | 9 (1.8) | 66** (23) | 105 (8.4) |
| t1/2 (min) | 62 | 74 | 446 | 543 | 123 | 181 | 66 | 89 |
| AUC(10min–4h) (ng⋅h/ml) | 278*** (29) | 479 (56) | 658** (52) | 960 (55) | 39*** (1.7) | 26 (1.7) | 144*** (9.4) | 255 (12) |
| AUC(2–8h) (ng⋅h/ml) | 87* (29) | 249 (47) | 988* (193) | 1893 (272) | 37 (4.8) | 34 (6.8) | 66** (16) | 148 (20) |
COT, cotinine; NIC, nicotine; NNIC, nornicotine; NNO, nicotine-1′-N-oxide.
P < 0.05, **P < 0.01, ***P < 0.001 versus AD(s.c.) group.
Fig. 2.
Plasma cotinine and nicotine-1′-N-oxide levels and AUC(10min–4h) were lower and plasma nornicotine levels and AUC(10min–4h) were higher in the EA(s.c.) group following s.c. nicotine administration. Plasma levels of cotinine (A), nornicotine (B), and nicotine-1′-N-oxide (C) measured over time. (D) Plasma cotinine (COT), nicotine-1′-N-oxide (NNO), and nornicotine (NNIC) AUC(10min–4h). *P < 0.05, **P < 0.01, ***P < 0.001 versus AD(s.c.) group.
TABLE 2.
Plasma trans-3′-hydroxycotinine (3HC) and norcotinine (NCOT) levels in EA and AD rats following s.c. nicotine
Data are presented as mean (S.D.).
| Time Point | Plasma 3HC Level | Plasma NCOT Level | ||
|---|---|---|---|---|
| EA | AD | EA | AD | |
| ng/ml plasma | ||||
| 10 min | < LOQ | < LOQ | < LOQ | < LOQ |
| 30 min | < LOQ | 1.4 (3.7) | 2.4 (0.8) | 2.7 (3.8) |
| 1 h | 1.4 (0.8) | 1.4 (0.1) | 3.5 (1.3) | 3.3 (0.3) |
| 2 h | 2.9 (1.6) | 2.8 (0.9) | 7.7 (3.1) | 5.2 (1.8) |
| 4 h | 2.9 (1.2) | 4.0 (1.9) | 7.6 (2.6) | 7.8 (3.1) |
| 8 h | 1.7 (0.4) | 4.0 (0.5) | 3.8 (0.7) | 6.3 (1.0) |
Brain Levels of Nicotine and its Metabolites Differ between Early Adolescent and Adult Rats.
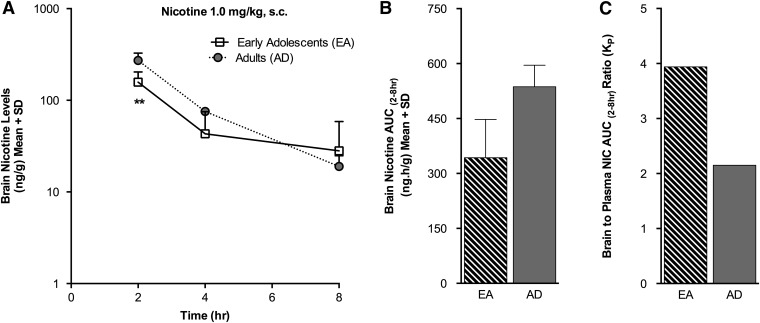
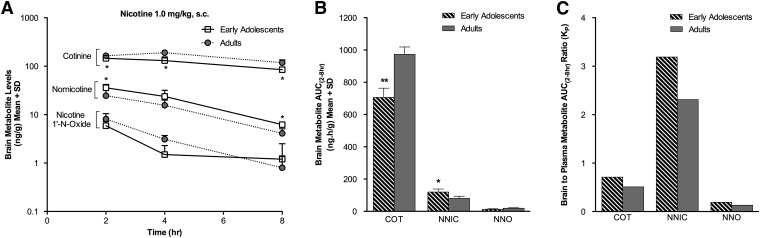
Brain nicotine levels were lower in the EA(s.c.) compared with the AD(s.c.) group at 2 hours (P < 0.01) following acute s.c. nicotine administration (Fig. 3A; Table 3), consistent with a modestly lower AUC(2–8h) (P = 0.06) (Fig. 3B). The brain nicotine KP ratio from 2 to 8 hours was 3.9 in the EA(s.c.) group and 2.2 in the AD(s.c.) group, indicating higher partitioning of nicotine in the brain versus the plasma (Fig. 3C). Brain cotinine levels were lower in the EA(s.c.) group at 2 hours (P < 0.05), 4 hours (P < 0.05), and 8 hours (P < 0.05) compared with the AD(s.c.) group (Fig. 4A), as was the brain cotinine AUC(2–8h) (P < 0.01) (Fig. 4B). The brain cotinine KP ratio from 2 to 8 hours was <1 for both the EA(s.c.) and AD(s.c.) groups, indicating that there was lower partitioning of cotinine into the brain (Fig. 4C). Brain nornicotine levels were higher in the EA(s.c.) group at 2 hours (P < 0.05) and 8 hours (P < 0.05) compared with the AD(s.c.) group (Fig. 4A), as was the brain nornicotine AUC(2–8h) (P < 0.05) (Fig. 4B). The brain nornicotine KP ratio from 2 to 8 hours was 3.2 in the EA(s.c.) group and 2.3 in the AD(s.c.) group, indicative of higher partitioning of nornicotine in the brain, as seen for nicotine (Fig. 4C). The brain nicotine-1′-N-oxide levels were relatively low and did not differ between the EA(s.c.) and AD(s.c.) animals (Fig. 4A); the KP ratio from 2 to 8 hours was also <1, as seen for cotinine (Fig. 4C). Mean brain levels of trans-3′-hydroxycotinine and norcotinine were low, near the LOQ (1 ng/g), in both the EA(s.c.) and AD(s.c.) groups (Table 4).
Fig. 3.
Brain nicotine levels were lower in the EA(s.c.) group following s.c. nicotine administration. (A) Brain nicotine levels measured over time. (B) There was no significant difference in mean brain nicotine AUC(2–8h), though a trend was noted (P = 0.06). (C) The brain-to-plasma partition coefficient (KP) was >1 in both the EA(s.c.) and AD(s.c.) groups. **P < 0.01 versus AD(s.c.) group.
TABLE 3.
Pharmacokinetic parameters of brain nicotine and metabolites in EA and AD rats following s.c. nicotine
Data are presented as mean (S.D.).
| Parameter | NIC | COT | NNIC | NNO | ||||
|---|---|---|---|---|---|---|---|---|
| EA | AD | EA | AD | EA | AD | EA | AD | |
| t1/2 (min) | 159 | 97 | 449 | 347 | 139 | 137 | 172 | 112 |
| AUC(2–8h) (ng⋅h/g) | 343 (105) | 534 (59) | 706** (58) | 972 (46) | 120* (20) | 78 (13) | 13 (3.4) | 19 (3.0) |
COT, cotinine; NIC, nicotine; NNIC, nornicotine; NNO, nicotine-1′-N-oxide.
P < 0.05, **P < 0.01 versus AD(s.c.) group.
Fig. 4.
Brain cotinine levels and AUC(2–8h) were lower while brain nornicotine levels and AUC(2–8h) were higher in the EA(s.c.) group following s.c. nicotine administration. (A) Brain cotinine, nornicotine, and nicotine-1′-N-oxide levels measured over time. (B) Brain cotinine (COT), nornicotine (NNIC), and nicotine-1′-N-oxide (NNO) AUC(2–8h). (C) The brain-to-plasma partition coefficient (KP) was <1 for COT and NNO in both age groups. In contrast, the KP for NNIC was >1 in both age groups. *P < 0.05, **P < 0.01 versus AD(s.c.) group.
TABLE 4.
Brain trans-3′-hydroxycotinine (3HC) and norcotinine (NCOT) levels in EA and AD rats following s.c. nicotine
Data are presented as mean (S.D.).
| Time Point | Brain 3HC Level | Brain NCOT Level | ||
|---|---|---|---|---|
| EA | AD | EA | AD | |
| ng/g brain tissue | ||||
| 2 h | < LOQ | < LOQ | 4.3 (1.1) | 3.1 (0.9) |
| 4 h | 1.0 (0.7) | 1.4 (0.2) | 4.9 (1.9) | 5.1 (0.4) |
| 8 h | < LOQ | 1.2 (0.3) | 2.7 (0.1) | 3.5 (0.6) |
Plasma and Brain Nicotine Levels Also Differed by Age Following i.v. Nicotine Administration.
Plasma and brain nicotine levels were compared between the EA and AD age groups following an acute i.v. nicotine administration. The use of the i.v. route of administration, with 100% bioavailability, permits investigation of whether the differences in plasma and brain nicotine levels between the EA and AD age groups following acute s.c. nicotine resulted from differences in absorption, due perhaps to differences in body composition between these ages. Intravenous injection can also be used to examine the VD and CL.
Plasma nicotine levels were lower in the EA(i.v.) compared with the AD(i.v.) group at 30 minutes (P < 0.05), 1 hour (P < 0.05), 2 hours (P < 0.001), and 4 hours (P < 0.05) (Fig. 5A), resulting in a lower plasma nicotine AUC(10min–4h) (P < 0.001) (Fig. 5B). Brain nicotine levels were not significantly different between the EA(i.v.) and AD(i.v.) groups at any time point (Fig. 6A); the mean brain nicotine AUC(2–8h) trended toward being lower in the EA(i.v.) group compared with the AD(i.v.) group (P = 0.09) (Fig. 6B). The brain nicotine KP ratio from 2 to 8 hours was 6.1 in the EA(i.v.) group compared with 3.2 the AD(i.v.) group (Fig. 6C), indicating increased nicotine partitioning into the brain of EA rats, as observed following s.c. nicotine administration. Pharmacokinetic parameters for nicotine and metabolites following acute i.v. administration were similar to those seen following s.c. administration, with comparable age-related differences, and are reported in Supplementary Table 1. Due to the relatively flat elimination curve for nicotine from the brain (Fig. 6A), we examined the later time points of 18 and 24 hours. The only metabolite detected in the plasma beyond 8 hours was cotinine, detected in both age groups above the LOQ at 18 and 24 hours after nicotine administration. Nicotine and cotinine were detectable in the brain at 18 and 24 hours while other metabolites were not, suggesting a long residence time for these two compounds.
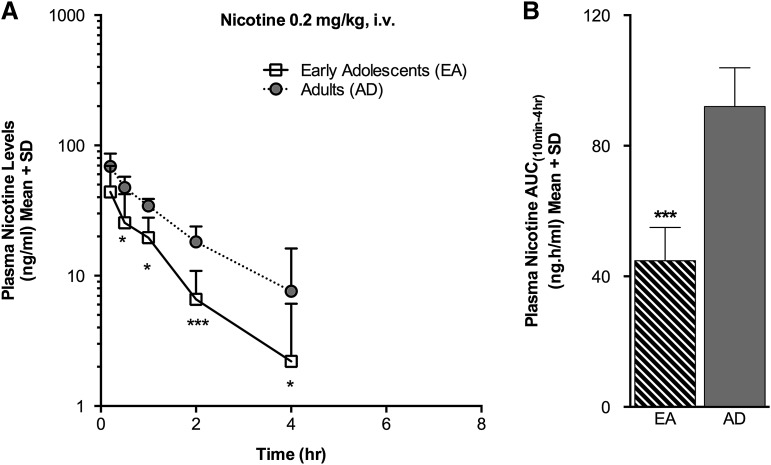
Fig. 5.
Plasma nicotine levels and AUC(10min–4h) were lower in the EA(i.v.) group following i.v. nicotine administration. (A) Plasma nicotine levels measured over time. (B) Plasma nicotine AUC(10min–4h). *P < 0.05, ***P < 0.001 versus AD(i.v.) group.
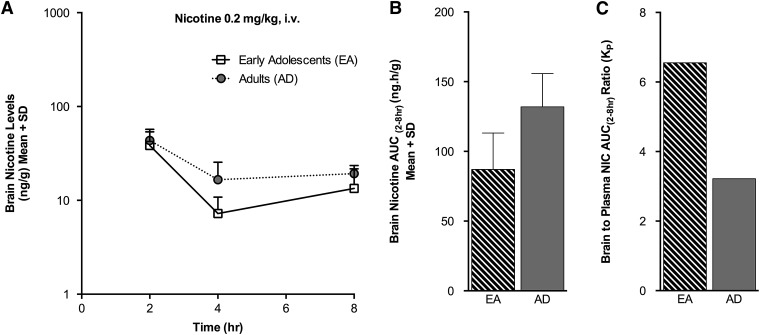
Fig. 6.
Brain nicotine levels were not significantly different in the EA(i.v.) compared with the AD(i.v.) group following i.v. nicotine administration. (A) Brain nicotine levels measured over time. (B) A trend toward a lower brain nicotine AUC(2–8h) was noted in the EA(i.v.) group (P = 0.09). (C) The KP for brain nicotine was >1 in both age groups.
Volume of Distribution and Clearance Differ between Early Adolescent and Adult Rats.
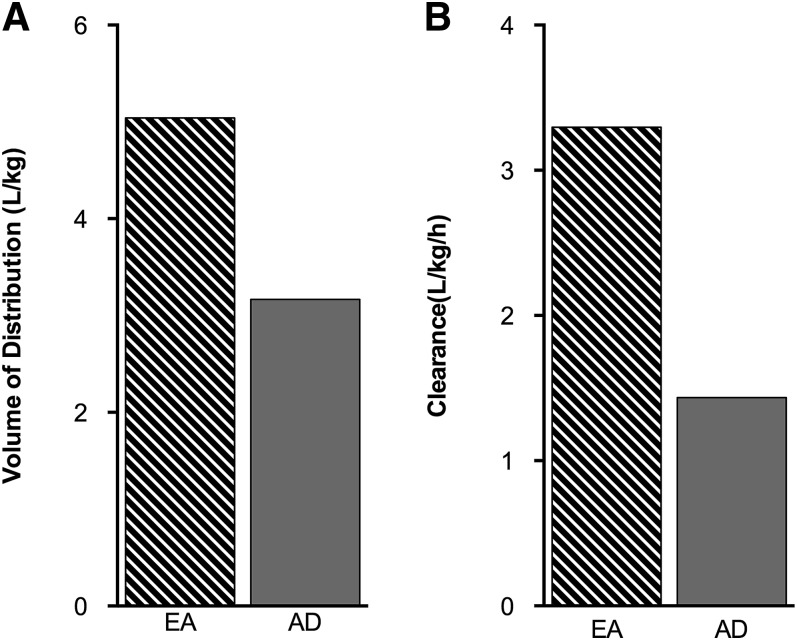
The EA(i.v.) group had a mean VD that was ∼1.6-fold larger than the AD(i.v.) group (Fig. 7A). Furthermore, the estimated mean CL rate was >2-fold faster in the EA(i.v.) group compared with that of the AD(i.v.) group (Fig. 7B).
Fig. 7.
Age-related differences in volume of distribution and nicotine clearance may alter pharmacokinetics between the two age groups. (A) The initial VD was ∼1.6-fold larger in the EA(i.v.) versus the AD(i.v.) group. (B) Nicotine CL was ∼2-fold faster in the EA(i.v.) group compared with the AD(i.v.) group.
Discussion
There are nicotine-mediated behavioral differences between adolescent and adult rats (Belluzzi et al., 2004; Levin et al., 2007). We have shown that rat age (adolescent versus adult) substantially impacts nicotine pharmacokinetics, and consequently the plasma and brain levels of nicotine and its metabolites, which may contribute to these observed behavioral differences.
Pharmacokinetic parameters including absorption (Mangoni and Jackson, 2004), drug distribution (Turnheim, 1998; Molander et al., 2001), excretion (McLean and Le Couteur, 2004), and metabolism (Klinger, 2005) can be altered by age. Characterization of nicotine pharmacokinetics using s.c. nicotine can be influenced by absorption (Hukkanen et al., 2005). However, the pharmacokinetic differences between early adolescent and adult rats are not due to differences in nicotine absorption, as we obtained similar differences following i.v. nicotine administration, where bioavailability is 100% (Toutain and Bousquet-Mélou, 2004a). Throughout development, body mass composition is altered in most mammalian species, with younger animals having a higher ratio of lean to fatty mass than older animals (Wolden-Hanson, 2010). As nicotine primarily distributes into lean mass, a change in this body mass composition could potentially impact nicotine’s volume of distribution (Molander et al., 2001). As adolescent animals tend to have primarily lean body mass, they would be expected to have a larger volume of distribution, relative to their body weight, for nicotine, resulting in lower plasma nicotine levels (Tutka et al., 2005). Of note, the substantial change in volume of distribution (∼1.6 times larger in EA) suggests that there are likely additional factors contributing to this difference.
Nicotine is excreted by glomerular filtration and tubular secretion with variable absorption, depending on urinary pH. Renal clearance accounts for ∼5% of total nicotine clearance in humans (Benowitz et al., 2009). In vitro expression of rat organic cation transporters 1 and 2 indicated that they are basolaterally located transporters contributing to tubular secretion of cationic drugs, removing them from the plasma to be secreted in the urine (Urakami et al., 1998). In rats, renal transporter mRNA expression is increased in early adolescents (de Zwart et al., 2008). With developmental changes in transporter protein expression, it is predicted that the distribution and elimination of substrates would also change with age (de Zwart et al., 2008). The removal of a drug from the body is reflected by the plasma clearance, impacting plasma concentration (Toutain and Bousquet-Mélou, 2004b). The early adolescent animals have increased nicotine clearance, consistent with the lower plasma nicotine levels observed in this age group relative to the adult cohort.
In rats, nicotine is metabolized to cotinine by the hepatic enzyme CYP2B1, as the rat CYP2A family, chiefly responsible for nicotine metabolism in humans (Benowitz et al., 2006), is essentially inactive toward nicotine (Hammond et al., 1991; Nakayama et al., 1993). CYP2B expression changes through development; early adolescent rats had higher levels of hepatic CYP2B protein compared with adults (Yun et al., 2010). However, our data do not support a faster conversion of nicotine to cotinine in the early adolescent cohort, as both nicotine and cotinine levels are lower. Moreover, as nicotine is a high-extraction-ratio drug, its clearance is less affected by the expression and activity of hepatic enzymes (Benowitz et al., 2009). This, and the faster nicotine clearance in younger animals, may be attributed in part to increased hepatic blood flow (Woodhouse and Wynne, 1992). Furthermore, most of the other metabolites were present at relatively lower levels in the adolescent compared with the adult cohort, suggesting that an increased rate of metabolism to these metabolites is not responsible, at least in full, for the lower nicotine levels in the adolescent cohort. Together, this suggests that the lower levels of nicotine in the adolescent cohort are consistent with an effect of volume of distribution and clearance rather than with a change in metabolic pathways.
Sex-dependent differences in drug metabolism and pharmacokinetics have been demonstrated in several species, including rats (Czerniak, 2001). In general, female rats have rates of hepatic drug metabolism 3- to 5-fold lower than those of males (Kyerematen et al., 1988a); the longer nicotine half-life and lower plasma cotinine levels observed in female versus male rats are consistent with lower recovery of urinary nicotine metabolites (Kyerematen et al., 1988a). Nonmetabolic sex differences in nicotine pharmacokinetics include altered clearance and volume of distribution (Czerniak, 2001); female rats have a larger volume of distribution than males (Kyerematen et al., 1988a). However, despite the larger volume of distribution, and consistent with slower metabolism, female rats have higher plasma and brain nicotine levels compared with male rats, likely due to sex differences in nicotine distribution and metabolism (Rosecrans, 1972; Harrod et al., 2007). These sex differences in rat nicotine pharmacokinetics may also contribute to sex differences in nicotine-mediated behaviors, such as those seen for nicotine in the conditioned place preference paradigm (Torres et al., 2009), which may be related to the higher plasma and brain nicotine levels in females relative to males. Our findings of a potential role for differences in nicotine volume of distribution and clearance, and the known rodent sex differences in these parameters, suggest that differences between males and females of different ages may occur and should perhaps be considered when comparisons of nicotine-evoked behaviors are made.
Following acute nicotine administration, lower levels of nicotine were also observed in the brains of the adolescent animals. The lower brain nicotine levels may be important to the interpretation of differences in nicotine-mediated behaviors between adolescent and adult rats. Adolescent rats display increased rates of nicotine self-administration relative to adults (Levin et al., 2003, 2007), often attributed to an enhanced preference for nicotine. However, due to the lower brain nicotine levels observed in adolescent rats, it is possible that adolescent animals are self-administering more nicotine to attain rewarding brain levels comparable to those achieved by adults (Koob and Le Moal, 2001), effectively compensating for lower brain nicotine levels acquired per injection. We also observed a higher brain-to-plasma AUC(2–8h) ratio in the early adolescents compared with the adults. Understanding the differing brain levels of nicotine measured directly, or by age-specific extrapolations from plasma levels, may be useful when interpreting animal behavioral data.
In contrast to the lower plasma and brain levels of nicotine, plasma nornicotine levels were higher in early adolescent compared with adult rats. In both humans and rats, nicotine is metabolized to nornicotine by N-demethylation (Cundy and Crooks, 1984; Yamanaka et al., 2005), accounting for a urinary recovery of 2–3% of a given nicotine dose in humans and ∼8% in rats (Yamanaka et al., 2005). In this and former studies, nornicotine represents a more substantial proportion of the nicotine dose in the brain (Plowchalk et al., 1992; Crooks et al., 1997). Nornicotine has psychoactive properties and is self-administered by rats (Bardo et al., 1999); this metabolite also has a high affinity for nicotinic receptors and can evoke dopamine release in the striatum (Green et al., 2001). Nornicotine desensitizes nicotinic receptor subtypes with a lower potency than nicotine, although cross-sensitization was observed between nicotine and nornicotine, indicating common receptor subtypes (Dwoskin et al., 2001). We detected a significantly higher brain nornicotine AUC(2–8h) in the early adolescent cohort, indicating higher total brain nornicotine exposure. In this age group, in which, for a given dose of nicotine, brain nicotine levels are lower and brain nornicotine levels are higher than those in adults, the pharmacological actions of this metabolite may be contributing to observed age differences in nicotine-mediated behaviors.
Thus, given the same dose of nicotine, early adolescent rats have lower plasma and brain levels of nicotine, higher nicotine brain-to-plasma ratios, and differing levels of various nicotine metabolites in both plasma and brain compared with adult rats. Differences in volume of distribution and clearance are likely primarily responsible for these differences in nicotine levels. Additional contributing factors include differences in renal and brain transport, hepatic blood flow, and, possibly, minor contributions from differences in metabolic pathways. Adolescence is a key period of development in which, in humans, the majority of initiation of nicotine use occurs (Breslau and Peterson, 1996). Animal models are useful for examining the underlying neurobiological vulnerability to dependence in adolescence in ways that cannot be easily investigated in humans (Caille et al., 2012). However, the rat may not be a suitable animal model of human nicotine pharmacokinetics given species-specific age differences in nicotine pharmacokinetics (Gourlay and Benowitz, 1996; Benowitz et al., 2009), differences in the enzyme primarily responsible for nicotine metabolism (Nakayama et al., 1993; Benowitz et al., 2006), and differences in the relative importance of various metabolic pathways of nicotine (Kyerematen et al., 1988b; Hukkanen et al., 2005). Overall, the results presented here highlight both the importance of age as a factor contributing to differences in nicotine pharmacokinetics in rats and the importance of taking these differences, as well as potential pharmacodynamic differences, into account when interpreting the results of animal model data and extrapolating them to interpretations of human smoking behaviors.
Supplementary Material
Acknowledgments
The authors thank Andy Z.X. Zhu and Reo Tanoshima for helpful discussions of the pharmacokinetics and statistical analyses.
Abbreviations
- AD
adult
- AUC
area under the concentration-time curve
- CL
nicotine clearance
- EA
early adolescent
- HPLC
high-performance liquid chromatography
- KP
brain-to-plasma partition coefficient
- LOQ
limit of quantification
- PND
postnatal day
- t1/2
half-life
- VD
volume of distribution
Authorship Contributions
Participated in research design: Craig, Cui, Miksys, Tyndale.
Conducted experiments: Craig, Cui, Miksys, Zhao, Novalen.
Performed data analysis: Craig, Cui, Zhao.
Wrote or contributed to the writing of the manuscript: Craig, Tyndale.
Footnotes
This study was conducted with the support of an Endowed Chair in Addictions (to R.F.T.) and the Canadian Institute of Health Research [Grant MOP97751], National Institutes of Health [Grant U01-DA020830], Canada Foundation for Innovation [Grants 20289 and 16014], Campbell Family Mental Health Research Institute Centre for Addiction and Mental Health, and Ontario Ministry of Research and Innovation.
R.F.T. has participated in one-day consulting meetings for Novartis and McNeil.
Parts of this work were previously presented at the following meeting: Craig E, Khokhar JY, Zhao B, Novalen M, Miksys SM, and Tyndale RF (2013) Altered nicotine pharmacokinetics in adolescent versus adult rats impacts the interpretation of animal model data. Society for Research on Nicotine and Tobacco Annual Meeting; 2013 Mar 13–16; Boston, MA.
 This article has supplemental material available at dmd.aspetjournals.org.
This article has supplemental material available at dmd.aspetjournals.org.
References
- Al Koudsi N, Hoffmann EB, Assadzadeh A, Tyndale RF. (2010) Hepatic CYP2A6 levels and nicotine metabolism: impact of genetic, physiological, environmental, and epigenetic factors. Eur J Clin Pharmacol 66:239–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardo MT, Green TA, Crooks PA, Dwoskin LP. (1999) Nornicotine is self-administered intravenously by rats. Psychopharmacology (Berl) 146:290–296 [DOI] [PubMed] [Google Scholar]
- Belluzzi JD, Lee AG, Oliff HS, Leslie FM. (2004) Age-dependent effects of nicotine on locomotor activity and conditioned place preference in rats. Psychopharmacology (Berl) 174:389–395 [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Hukkanen J, Jacob P., 3rd (2009) Nicotine chemistry, metabolism, kinetics and biomarkers, in Nicotine Psychopharmacology (Henningfield JE, London ED, Pogun S. eds) pp 29–60, Springer, Berlin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Swan GE, Jacob P, 3rd, Lessov-Schlaggar CN, Tyndale RF. (2006) CYP2A6 genotype and the metabolism and disposition kinetics of nicotine. Clin Pharmacol Ther 80:457–467 [DOI] [PubMed] [Google Scholar]
- Breslau N, Peterson EL. (1996) Smoking cessation in young adults: age at initiation of cigarette smoking and other suspected influences. Am J Public Health 86:214–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caille S, Clemens K, Stinus L, Cador M. (2012) Modeling nicotine addiction in rats. Methods Mol Biol 829:243–256 [DOI] [PubMed] [Google Scholar]
- Casey BJ, Jones RM. (2010) Neurobiology of the adolescent brain and behavior: implications for substance use disorders. J Am Acad Child Adolesc Psychiatry 49:1189–1201, quiz 1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooks PA, Li M, Dwoskin LP. (1997) Metabolites of nicotine in rat brain after peripheral nicotine administration. Cotinine, nornicotine, and norcotinine. Drug Metab Dispos 25:47–54 [PubMed] [Google Scholar]
- Cundy KC, Crooks PA. (1984) High-performance liquid chromatographic method for the determination of N-methylated metabolites of nicotine. J Chromatogr A 306:291–301 [DOI] [PubMed] [Google Scholar]
- Czerniak R. (2001) Gender-based differences in pharmacokinetics in laboratory animal models. Int J Toxicol 20:161–163 [DOI] [PubMed] [Google Scholar]
- Dempsey D, Jacob P, 3rd, Benowits NL. (2000) Nicotine metabolism and elimination kinetics in newborns. Clin Pharmacol Ther 67:458–465 [DOI] [PubMed] [Google Scholar]
- de Zwart L, Scholten M, Monbaliu JG, Annaert PP, Van Houdt JM, Van den Wyngaert I, De Schaepdrijver LM, Bailey GP, Coogan TP, Coussement WC, et al. (2008) The ontogeny of drug metabolizing enzymes and transporters in the rat. Reprod Toxicol 26:220–230 [DOI] [PubMed] [Google Scholar]
- Doura MB, Gold AB, Keller AB, Perry DC. (2008) Adult and periadolescent rats differ in expression of nicotinic cholinergic receptor subtypes and in the response of these subtypes to chronic nicotine exposure. Brain Res 1215:40–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwoskin LP, Teng LH, Crooks PA. (2001) Nornicotine, a nicotine metabolite and tobacco alkaloid: desensitization of nicotinic receptor-stimulated dopamine release from rat striatum. Eur J Pharmacol 428:69–79 [DOI] [PubMed] [Google Scholar]
- Gourlay SG, Benowitz NL. (1996) The benefits of stopping smoking and the role of nicotine replacement therapy in older patients. Drugs Aging 9:8–23 [DOI] [PubMed] [Google Scholar]
- Green TA, Crooks PA, Bardo MT, Dwoskin LP. (2001) Contributory role for nornicotine in nicotine neuropharmacology: nornicotine-evoked [3H]dopamine overflow from rat nucleus accumbens slices. Biochem Pharmacol 62:1597–1603 [DOI] [PubMed] [Google Scholar]
- Hammond DK, Bjercke RJ, Langone JJ, Strobel HW. (1991) Metabolism of nicotine by rat liver cytochromes P-450. Assessment utilizing monoclonal antibodies to nicotine and cotinine. Drug Metab Dispos 19:804–808 [PubMed] [Google Scholar]
- Harrod SB, Booze RM, Mactutus CF. (2007) Sex differences in nicotine levels following repeated intravenous injection in rats are attenuated by gonadectomy. Pharmacol Biochem Behav 86:32–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hukkanen J, Jacob P, 3rd, Benowitz NL. (2005) Metabolism and disposition kinetics of nicotine. Pharmacol Rev 57:79–115 [DOI] [PubMed] [Google Scholar]
- Jacob P, 3rd, Yu L, Duan M, Ramos L, Yturralde O, Benowitz NL. (2011) Determination of the nicotine metabolites cotinine and trans-3′-hydroxycotinine in biologic fluids of smokers and non-smokers using liquid chromatography-tandem mass spectrometry: biomarkers for tobacco smoke exposure and for phenotyping cytochrome P450 2A6 activity. J Chromatogr B Analyt Technol Biomed Life Sci 879:267–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khuder SA, Dayal HH, Mutgi AB. (1999) Age at smoking onset and its effect on smoking cessation. Addict Behav 24:673–677 [DOI] [PubMed] [Google Scholar]
- Klinger W. (2005) Developmental pharmacology and toxicology: biotransformation of drugs and other xenobiotics during postnatal development. Eur J Drug Metab Pharmacokinet 30:3–17 [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. (2001) Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology 24:97–129 [DOI] [PubMed] [Google Scholar]
- Kyerematen GA, Owens GF, Chattopadhyay B, deBethizy JD, Vesell ES. (1988a) Sexual dimorphism of nicotine metabolism and distribution in the rat. Studies in vivo and in vitro. Drug Metab Dispos 16:823–828 [PubMed] [Google Scholar]
- Kyerematen GA, Taylor LH, deBethizy JD, Vesell ES. (1988b) Pharmacokinetics of nicotine and 12 metabolites in the rat. Application of a new radiometric high performance liquid chromatography assay. Drug Metab Dispos 16:125–129 [PubMed] [Google Scholar]
- Levin ED, Lawrence SS, Petro A, Horton K, Rezvani AH, Seidler FJ, Slotkin TA. (2007) Adolescent vs. adult-onset nicotine self-administration in male rats: duration of effect and differential nicotinic receptor correlates. Neurotoxicol Teratol 29:458–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Rezvani AH, Montoya D, Rose JE, Swartzwelder HS. (2003) Adolescent-onset nicotine self-administration modeled in female rats. Psychopharmacology (Berl) 169:141–149 [DOI] [PubMed] [Google Scholar]
- Liang L, Chaloupka F, Nichter M, Clayton R. (2003) Prices, policies and youth smoking, May 2001. Addiction 98 (Suppl 1):105–122 [DOI] [PubMed] [Google Scholar]
- Mangoni AA, Jackson SH. (2004) Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol 57:6–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick CM, Mathews IZ. (2010) Adolescent development, hypothalamic-pituitary-adrenal function, and programming of adult learning and memory. Prog Neuropsychopharmacol Biol Psychiatry 34:756–765 [DOI] [PubMed] [Google Scholar]
- McLean AJ, Le Couteur DG. (2004) Aging biology and geriatric clinical pharmacology. Pharmacol Rev 56:163–184 [DOI] [PubMed] [Google Scholar]
- Messina ES, Tyndale RF, Sellers EM. (1997) A major role for CYP2A6 in nicotine C-oxidation by human liver microsomes. J Pharmacol Exp Ther 282:1608–1614 [PubMed] [Google Scholar]
- Micu AL, Miksys S, Sellers EM, Koop DR, Tyndale RF. (2003) Rat hepatic CYP2E1 is induced by very low nicotine doses: an investigation of induction, time course, dose response, and mechanism. J Pharmacol Exp Ther 306:941–947 [DOI] [PubMed] [Google Scholar]
- Molander L, Hansson A, Lunell E. (2001) Pharmacokinetics of nicotine in healthy elderly people. Clin Pharmacol Ther 69:57–65 [DOI] [PubMed] [Google Scholar]
- Nakajima M, Iwata K, Yamamoto T, Funae Y, Yoshida T, Kuroiwa Y. (1998) Nicotine metabolism in liver microsomes from rats with acute hepatitis or cirrhosis. Drug Metab Dispos 26:36–41 [PubMed] [Google Scholar]
- Nakajima M, Yokoi T. (2005) Interindividual variability in nicotine metabolism: C-oxidation and glucuronidation. Drug Metab Pharmacokinet 20:227–235 [DOI] [PubMed] [Google Scholar]
- Nakayama H, Okuda H, Nakashima T, Imaoka S, Funae Y. (1993) Nicotine metabolism by rat hepatic cytochrome P450s. Biochem Pharmacol 45:2554–2556 [DOI] [PubMed] [Google Scholar]
- Ochiai Y, Sakurai E, Nomura A, Itoh K, Tanaka Y. (2006) Metabolism of nicotine in rat lung microvascular endothelial cells. J Pharm Pharmacol 58:403–407 [DOI] [PubMed] [Google Scholar]
- Perna MK, Henderson YO, Bruner CL, Brown RW. (2011) An analysis of nicotine conditioned place conditioning in early postweanling and adolescent rats neonatally treated with quinpirole. Behav Brain Res 220:254–261 [DOI] [PubMed] [Google Scholar]
- Plowchalk DR, Andersen ME, deBethizy JD. (1992) A physiologically based pharmacokinetic model for nicotine disposition in the Sprague-Dawley rat. Toxicol Appl Pharmacol 116:177–188 [DOI] [PubMed] [Google Scholar]
- Rose JS, Lee CT, Dierker LC, Selya AS, Mermelstein RJ. (2012) Adolescent nicotine dependence symptom profiles and risk for future daily smoking. Addict Behav 37:1093–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosecrans JA. (1972) Brain area nicotine levels in male and female rats with different levels of spontaneous activity. Neuropharmacology 11:863–870 [DOI] [PubMed] [Google Scholar]
- Shapiro BH, Agrawal AK, Pampori NA. (1995) Gender differences in drug metabolism regulated by growth hormone. Int J Biochem Cell Biol 27:9–20 [DOI] [PubMed] [Google Scholar]
- Shram MJ, Siu EC, Li Z, Tyndale RF, Lê AD. (2008) Interactions between age and the aversive effects of nicotine withdrawal under mecamylamine-precipitated and spontaneous conditions in male Wistar rats. Psychopharmacology (Berl) 198:181–190 [DOI] [PubMed] [Google Scholar]
- Spear LP. (2000) The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev 24:417–463 [DOI] [PubMed] [Google Scholar]
- Torres OV, Natividad LA, Tejeda HA, Van Weelden SA, O’Dell LE. (2009) Female rats display dose-dependent differences to the rewarding and aversive effects of nicotine in an age-, hormone-, and sex-dependent manner. Psychopharmacology (Berl) 206:303–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toutain PL, Bousquet-Mélou A. (2004a) Bioavailability and its assessment. J Vet Pharmacol Ther 27:455–466 [DOI] [PubMed] [Google Scholar]
- Toutain PL, Bousquet-Mélou A. (2004b) Plasma clearance. J Vet Pharmacol Ther 27:415–425 [DOI] [PubMed] [Google Scholar]
- Toutain PL, Bousquet-Mélou A. (2004c) Volumes of distribution. J Vet Pharmacol Ther 27:441–453 [DOI] [PubMed] [Google Scholar]
- Turnheim K. (1998) Drug dosage in the elderly. Is it rational? Drugs Aging 13:357–379 [DOI] [PubMed] [Google Scholar]
- Tutka P, Mosiewicz J, Wielosz M. (2005) Pharmacokinetics and metabolism of nicotine. Pharmacol Rep 57:143–153 [PubMed] [Google Scholar]
- Urakami Y, Okuda M, Masuda S, Saito H, Inui KI. (1998) Functional characteristics and membrane localization of rat multispecific organic cation transporters, OCT1 and OCT2, mediating tubular secretion of cationic drugs. J Pharmacol Exp Ther 287:800–805 [PubMed] [Google Scholar]
- US Department of Health and Human Services (2012) Preventing Tobacco Use Among Youth and Young Adults: A Report of the Surgeon General, US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, Atlanta, GA: [PubMed] [Google Scholar]
- Vastola BJ, Douglas LA, Varlinskaya EI, Spear LP. (2002) Nicotine-induced conditioned place preference in adolescent and adult rats. Physiol Behav 77:107–114 [DOI] [PubMed] [Google Scholar]
- Vieira-Brock PL, Miller EI, Nielsen SM, Fleckenstein AE, Wilkins DG. (2011) Simultaneous quantification of nicotine and metabolites in rat brain by liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 879:3465–3474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolden-Hanson T. (2010) Changes in body composition in response to challenges during aging in rats. Interdiscip Top Gerontol 37:64–83 [DOI] [PubMed] [Google Scholar]
- Woodhouse K, Wynne HA. (1992) Age-related changes in hepatic function. Implications for drug therapy. Drugs Aging 2:243–255 [DOI] [PubMed] [Google Scholar]
- Yamanaka H, Nakajima M, Fukami T, Sakai H, Nakamura A, Katoh M, Takamiya M, Aoki Y, Yokoi T. (2005) CYP2A6 AND CYP2B6 are involved in nornicotine formation from nicotine in humans: interindividual differences in these contributions. Drug Metab Dispos 33:1811–1818 [DOI] [PubMed] [Google Scholar]
- Yun KU, Oh SJ, Oh JM, Kang KW, Myung C-S, Song GY, Kim B-H, Kim SK. (2010) Age-related changes in hepatic expression and activity of cytochrome P450 in male rats. Arch Toxicol 84:939–946 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.