Highlights
-
•
Rapid adaptation of feeding behaviour to scheduled palatable diet access
-
•
No evidence of reduced feeding (hypophagia) prior to scheduled palatable meals
-
•
Mice exhibit food anticipatory activity (FAA) prior to scheduled palatable meals
-
•
Continuing presence of FAA when scheduled palatable mealsare withdrawn
-
•
Immediate hyperphagic response once the palatable meals are restored after 7 days
Keywords: Feeding pattern, Palatability, Food anticipation, Scheduled feeding, Binge-like eating, Mouse
Abstract
Male C57BL/6 mice fed ad libitum on control diet but allowed access to a palatable high fat diet (HFD) for 2 h a day during the mid-dark phase rapidly adapt their feeding behaviour and can consume nearly 80% of their daily caloric intake during this 2 h-scheduled feed. We assessed food intake microstructure and meal pattern, and locomotor activity and rearing as markers of food anticipatory activity (FAA). Schedule fed mice reduced their caloric intake from control diet during the first hours of the dark phase but not during the 3-h period immediately preceding the scheduled feed. Large meal/binge-like eating behaviour during the 2-h scheduled feed was characterised by increases in both meal number and meal size. Rearing was increased during the 2-h period running up to scheduled feeding while locomotor activity started to increase 1 h before, indicating that schedule-fed mice display FAA. Meal number and physical activity changes were sustained when HFD was withheld during the anticipated scheduled feeding period, and mice immediately binged when HFD was represented after a week of this “withdrawal” period. These findings provide important context to our previous studies suggesting that energy balance systems in the hypothalamus are not responsible for driving these large, binge-type meals. Evidence of FAA in HFD dark phase schedule-fed mice implicates anticipatory processes in binge eating that do not involve immediately preceding hypophagia or regulatory homeostatic signalling.
Introduction
Feeding is driven, in large part, by energy homeostasis – the balance between food intake and energy expenditure. Humans and many mammals consume their energy in the form of periodic bouts or meals. However, the initiation of a meal is not necessarily based on a general energy deficit or a specific need such as an inadequate glucose level. The impulse to initiate a meal may rather be based on factors such as time of the day, eating habits, social environment, or convenience (Woods, 2005). The ability to estimate time and anticipate critical events such as meal time is of relevance in nature, since it has clear implications for survival (Strubbe & Woods, 2004). In laboratory animals, restricted meal-feeding schedules may limit food availability to a single daily meal. Once habituated to these feeding conditions, animals have been shown to anticipate their next meal through adaptations such as increases in locomotor activity, body temperature and hormone release that precede the predicted meals (Verwey & Amir, 2009). The behavioural response is known as food anticipatory activity (FAA), and the 2 h to 3 h period preceding a daily scheduled meal is the relevant time frame (Challet, Mendoza, Dardente, & Pévet, 2009; Mistlberger, 1994; Shibata, Hirao, & Tahara, 2010). FAA is not just limited to restricted feeding schedules, i.e. where food is available for only a short time a day. The reward value of food and its motivational properties have also been implicated in food entrainment since FAA can also be induced in animals fed on palatable feeding schedules, where a stock diet is available for the remainder of the day (Mendoza, 2007; Mistlberger & Rusak, 1987).
A palatable scheduled feeding model, described by Berner et al. (Berner, Avena, & Hoebel, 2008), based on dietary manipulations by Corwin et al. (Corwin et al., 1998; Dimitriou, Rice, & Corwin, 2000) and Mistlberger et al. (Mistlberger & Rusak, 1987), induces substantial food intake over short periods of time in rats (Berner et al., 2008). Utilising this model, we provided scheduled access to a solid high fat palatable diet (HFD) for a 2-h period each day, without imposed caloric restriction during the remainder of the day, a manipulation that resulted in consumption of large, binge-type meals in both rats and mice (Bake, Duncan, Morgan, & Mercer, 2013). Interestingly, mice exhibited a more exaggerated response to the scheduled palatable diet manipulation, with about 80% of total daily calories consumed during the 2-h access (Bake et al., 2013). The present study further characterises the large meal/binge-like eating model at a behavioural level in mice, focussing on how palatable scheduled feeding influences food intake microstructure and meal patterns. We also measured activity patterns (locomotor activity and rearing) as markers of FAA in mice on scheduled palatable diet. In addition, we extended the model beyond the habituated response to palatable schedule feeding to assess food intake microstructure, meal patterns and activity patterns when the palatable scheduled feeding on HFD was withdrawn and then reintroduced.
Materials and methods
Animals
Six male C57BL/6 mice (Harlan, Bicester, UK), with initial body weights of approximately 22 g at 7 weeks of age, were placed under a reversed 12 h:12 h light/dark cycle (lights on at 16:00, ZT0; lights off at 04:00, ZT12; ZT, zeitgeber time) immediately upon arrival and were allowed to acclimatise as a group. After 2 weeks, mice were single housed in TSE PhenoMaster/LabMaster feeding/drinking monitoring cages (TSE Systems, Bad Homburg, Germany) and acclimatised for a week further before the start of 1 week of baseline food intake and locomotor activity measurements (phase 1). All mice were fed ad libitum standard pellet diet (Special Diet Services, Witham, UK; #871505 CRM (P); 22% protein, 69% carbohydrate, 9% fat by energy, 2.67 kcal/g) unless otherwise noted. Water was freely available at all times during the experiments. The ambient temperature and humidity in the animal room and in the wire-top experimental cages were c. 21°C and c. 50%, respectively. All procedures were licensed under the Animals (Scientific Procedures) Act of 1986 and received approval from the Ethical Review Committee at the Rowett Institute of Nutrition and Health.
Dietary manipulation
Following baseline measurements (phase 1), all mice underwent the same dietary manipulations, performed with pelleted HFD (Research Diets, New Brunswick, NJ, USA, #D12492; 20% protein, 20% carbohydrate, 60% fat by energy, 5.24 kcal/g). During phases 2 and 3, all mice had scheduled access to HFD for 2 h a day from ZT18 to ZT20 (6 h to 8 h into the dark phase, as employed by (Berner et al., 2008)) and standard pellet diet in the remaining time (phase 2, adaptation; phase 3, habituation). Due to the longitudinal development of binge-type feeding, phases 2 and 3 are termed “adaptation” and “habituation”, respectively. After 17 days of HFD scheduled feeding, for phase 4, the mice were switched back to standard pellet diet during scheduled feeding time (i.e. standard diet available 24 h a day; termed “replacement”). After a further 7 days, mice were returned to HFD during scheduled feeding time for 7 more days in phase 5, termed “refeeding”. Body weight was measured three times a week.
Food intake measurement and food intake microstructure analysis
During phases 1–5, food intake was measured using the TSE PhenoMaster/LabMaster system, which automatically records the weight of food eaten to a sensitivity of 0.01 g through a calibrated sensor. Food spillage was minimised by a catch tray underneath the food hopper. For assessing HFD intake during scheduled feeding, food hoppers containing the diet were exchanged using the “food refill” menu in the software at ZT18 and then again at ZT20. Food hoppers were also exchanged during baseline and replacement phases to standardise the amount of disturbance each day. Cumulative food intake was recorded at intervals of 5 min and summarised in 1 h bins and then averaged per mouse and study phase.
Meal pattern analysis
Data for meal analysis was collected as binary data every 10 s. Meal analysis was done as “so called” sequence analysis, whereby all meals occurring during the study period were recorded chronologically to allow the evaluation of single feeding episodes. The start of a meal was defined by food removal equal to or larger than 0.05 g and the meal was ended when no further food removal occurred before the end of the inter-meal interval of 15 min. The meal parameters (meal number and meal size) were then summarised over seven time periods – total day (ZT0–24), light phase (ZT0–12), dark phase (ZT13–24), early dark phase (ZT13–15), mid dark phase (ZT16–18), scheduled feeding time (ZT19–20), and late dark phase (ZT21–24), and then averaged per mouse and study phase. A 15 min inter-meal interval is commonly used in defining meals in mice (Atalayer & Rowland, 2011) and rats (Farley, Cook, Spar, Austin, & Kowalski, 2003).
Locomotor activity measurement and analysis
Activity was measured using a multicage activity monitoring system (Ugo Basile, Comerio, Italy). Each cage had a horizontal sensor frame for monitoring locomotor activity such as walking and running, and a vertical sensor frame for rearing and exploratory activity. Activity was measured as infrared beam breaks per 15 min interval, and was recorded via WinDas 2006 software (Ugo Basile). Horizontal and vertical activity data were separately summarised at 1 h intervals and then averaged per mouse and study phase.
Statistical analysis
Statistical analysis was performed with SigmaPlot 12.0 (Systat Software, Chicago, IL, USA). Diurnal differences in food intake microstructure and locomotor activity pattern during baseline were analysed with one-way repeated measures analysis of variance (one-way RM ANOVA). Longitudinal measurements of food intake and physical activity were analysed by two-way RM ANOVA for effect of “study phase” and “time point”, and interactions between these factors. Data for meal pattern were analysed by one-way RM ANOVA to reveal overall effects between study phases. When the data were not normally distributed and/or variances were not equal, a non-parametric ANOVA on ranks was performed. Post hoc and planned comparisons were assessed with Student–Newman–Keul Tests (SNK). Outcomes were considered statistically significant if P values were lower than 0.05. Data are presented as mean ± standard error of the mean (SEM).
Results
The study consisted of five phases: baseline measurements on standard pellet diet (phase 1), “adaptation” and “habituation” periods when pelleted HFD was fed by scheduled access for 2 h a day with standard pellet diet in the remaining time (phases 2 and 3, respectively), “replacement”, when mice were switched back to standard pellet diet during scheduled feeding time (i.e. standard diet available 24 h a day; phase 4), and “refeeding”, when mice were returned to HFD during scheduled feeding time (phase 5).
Food intake and body weight
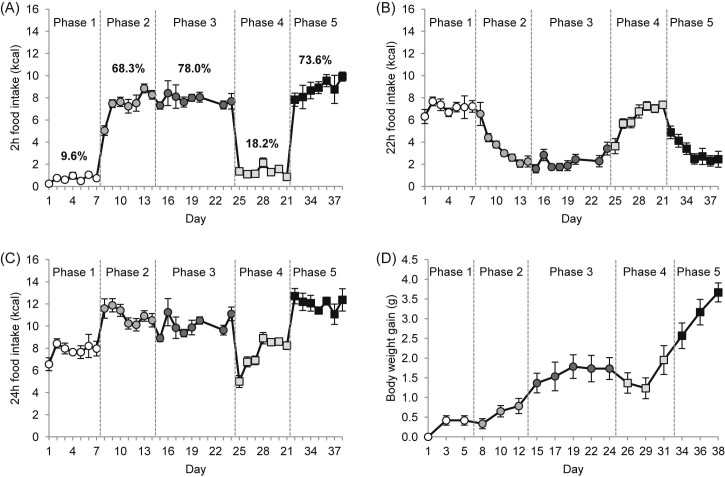
Study phase had a significant effect on mean caloric intake when analysed in 2 h, 22 h or 24 h bins (P < 0.001). When mice were schedule fed on HFD for 2 h a day in the middle of the dark phase to replicate the manipulation described by Berner et al. (Berner et al., 2008), they rapidly adapted their feeding behaviour to scheduled access conditions and binged on HFD, such that by the second day of HFD access, near maximal caloric intake was achieved (Fig. 1A). By contrast, the displacement of calories from standard diet in the remaining 22 h occurred more slowly, reaching a nadir after 7 days (Fig. 1B). For this reason the first 7 days are referred to as the adaptation phase. The following 10 days on scheduled HFD were termed the habituation phase since caloric intake during both 2 h and 22 h bins was relatively stable. The percentages of calories consumed from HFD during the adaptation or habituation phases were 68.3% and 78.0%, respectively, indicative of large meal/binge-like behaviour, compared with just 9.6% of total calories consumed during the same 2 h period in the baseline phase. Notably, compensation for calories from scheduled access was incomplete since total caloric intake was increased during adaptation and habituation phases (Fig. 1C) (SNK, P < 0.001 versus baseline). After 17 days of HFD scheduled feeding, mice were returned to baseline feeding conditions with standard diet during scheduled feeding. Two-hour caloric intake decreased immediately to a stable lower level whereas 22 h intake again adapted more slowly (Fig. 1A,B). Total caloric intake was minimal on the first day of the replacement phase and thereafter increased slowly to baseline levels (Fig. 1C). The overall percentage of calories consumed during the 2 h scheduled feeding time was 18.2%, significantly higher than that during baseline (SNK, P < 0.05). After a further 7 days, mice were again given scheduled access to HFD. Two-hour caloric intake increased immediately to a level comparable to the habituation phase, and continued to increase gradually across the 7-day phase and was higher on the last day of the refeeding phase compared with several days of the adaptation and habituation phases (day 38; P < 0.05 versus adaptation days 8, 9, 11 and 12, and habituation days 15 and 23; P < 0.1 versus adaptation day 10 and habituation days 18 and 24); overall percentage of calories from HFD was at 73.6%. The 22 h caloric intake from standard diet decreased slowly as observed previously in the adaptation phase. Total caloric intake during the refeeding phase was higher than in the adaptation and habituation phases (Fig. 1C) (SNK, P = 0.031 and P < 0.001).
Fig. 1.
Caloric intake (kcal) and body weight (g) of C57BL/6 mice during all study phases, i.e. mice have either 2-h scheduled access to a high fat diet (HFD) and standard diet in the remaining time (adaptation, habituation, refeeding) or 24-h access to standard diet (baseline, replacement). (A) Caloric intake from either HFD or standard diet during the 2 h scheduled feeding time. (B) Caloric intake from standard diet during the remaining 22 h. (C) Total daily caloric intake. (D) Body weight gain. Percentages above data line in A refer to calories consumed from HFD or control diet during schedule feeding time relative to total 24 h intake. Open circles, phase1, baseline; light grey circles, phase 2, adaptation; dark grey circles, phase 3, habituation; grey squares, phase 4, replacement; black squares, phase 5, refeeding. Data are presented as mean ± SEM.
Body weight reflected changes in caloric intake (one-way RM ANOVA, P < 0.001), slowly increasing during adaptation and habituation phases (habituation days 17 to 24, P < 0.05 versus baseline days 1 to 5, and adaptation days 8 to 12), stalling during the replacement phase before increasing again, more rapidly, in the final refeeding phase (Fig. 1D) (refeeding days 34 to 38, P < 0.05 versus all other days; P = 0.022 day 36 versus 34; P = 0.055 day 38 versus 36).
Food intake microstructure
Analysis of the baseline phase showed that mice displayed a clear diurnal rhythm of food intake (one-way RM ANOVA; P < 0.001; Fig. 2). Food intake started to increase during the last hour of the light phase at ZT12 (i.e. data from ZT11 to ZT12) (SNK; P < 0.05 versus all other ZT intervals) and was always higher during dark phase than during light phase (SNK; at ZT13 to ZT24, P < 0.05 versus ZT1 to ZT12). During mid-dark phase, food intake was at an intermediate level compared with other dark phase and light phase intervals (SNK; at ZT20, P < 0.05 versus all other ZT intervals).
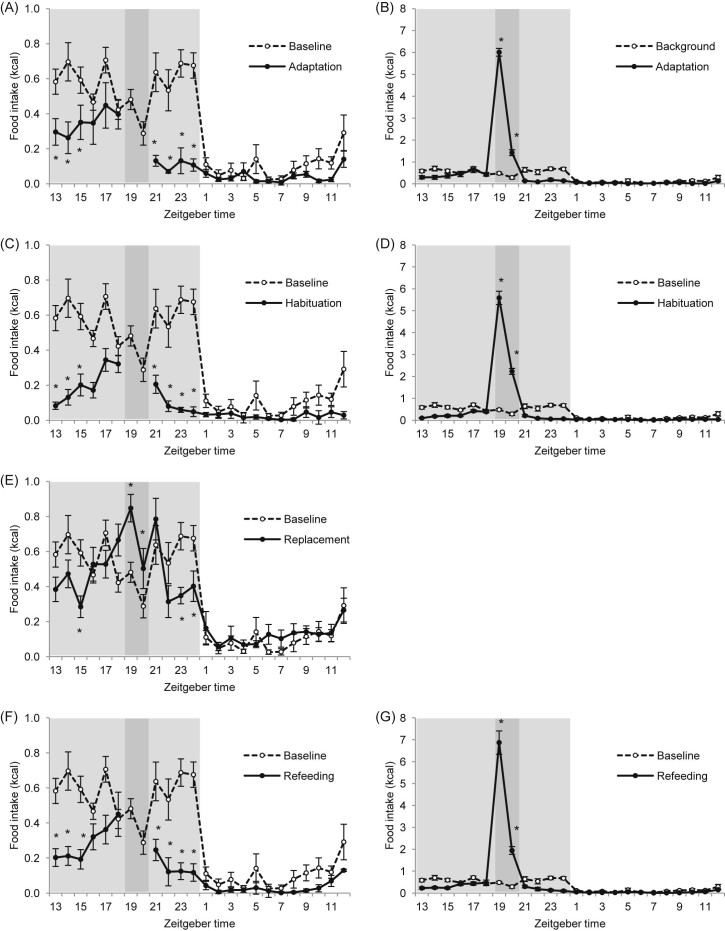
Fig. 2.
Food intake microstructure of C57BL/6 mice during all study phases versus baseline food intake pattern: (A,B) adaptation, (C,D) habituation, (E) replacement and (F,G) refeeding phase. (A,C,E,F) Food intake microstructure showing caloric intake from standard diet. (B,D,G) Food intake microstructure showing total caloric intake including calories from HFD during scheduled feeding. Light shaded area indicates dark phase; dark shaded area indicates scheduled feeding time. *P < 0.05 versus baseline by two-way repeated measures ANOVA and Student–Newman–Keul post hoc test. For clarity, one asterisk also includes P < 0.01 and P < 0.001, and diagrams (B,D,G) display only differences during scheduled feeding time versus baseline (ZT19 and ZT20). Data are presented as mean ± SEM.
There were significant interactions between study phases and time intervals for dietary energy intake (two-way RM ANOVA; P < 0.001; Fig. 2), but no differences between any study phases during the light phase. For clarity, Fig. 2 shows post hoc comparisons of each 1 h ZT interval with baseline phase. These outcomes are summarised briefly before further analysis of relevant dark phase bins (Fig. 3). During adaptation and habituation phases, schedule-fed mice had increased caloric intake (from HFD) during scheduled feeding time (ZT19–20), a decreased caloric intake from standard diet during the first 3 hours of the dark phase (ZT13 to ZT15), and a very low caloric intake in the hours following the scheduled feed (ZT21 to ZT24). In contrast, during the 3-hour period running up to the scheduled feed, caloric intake did not differ from baseline (ZT16–18). During replacement, mice retained an increased caloric intake, but from standard diet, during the scheduled access period (ZT19–20), whereas during refeeding, food intake pattern resembled the adaptation and habituation phase.
Fig. 3.
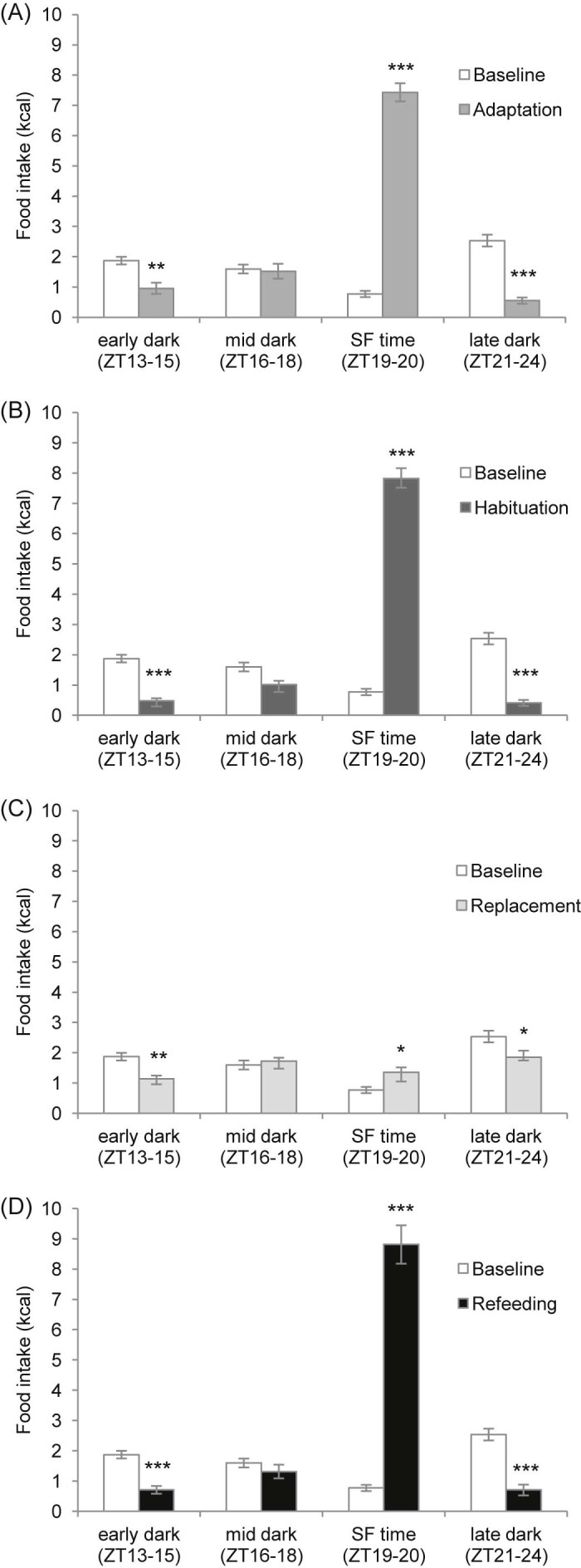
Food intake microstructure of C57BL/6 mice during all study phases versus baseline data over the dark phase: (A) adaptation, (B) habituation, (C) replacement and (D) refeeding phase. Relevant time bins from the analysis in Fig. 2 are depicted: early dark phase (ZT13–15, 3 h bin), mid dark phase (ZT16–18, 3 h bin), scheduled feeding (SF) time (ZT19–20, 2 h bin) and late dark phase (ZT21–24, 4 h bin). ***P < 0.001, **P < 0.01, *P < 0.05 versus baseline by two-way repeated measures ANOVA. Data are presented as mean ± SEM.
According to the main changes seen in the 1 h caloric intakes, data were then analysed in relevant time bins across the dark phase: early dark phase (ZT13–15), mid dark phase (ZT16–18), scheduled feeding time (ZT19–20) and late dark phase (ZT21–24) and the study phases were compared with baseline (Fig. 3). During all study phases – adaptation, habituation, replacement and refeeding – there were decreases in caloric intake from standard diet during early dark phase (P = 0.003, P < 0.001, P = 0.007 and P < 0.001, respectively) and late dark phase (P < 0.001, P < 0.001, P = 0.012 and P < 0.001, respectively) and an increase in caloric intake either from HFD or standard diet during scheduled feeding time (P < 0.001, P < 0.001, P = 0.031 and P < 0.001, respectively).
Mice consumed 7.85 ± 0.33 kcal HFD within the 2 h scheduled access during the habituation phase. Further analysis in 15-min bins revealed that approximately one-third of this intake occurred in the first 15 min (2.71 ± 0.24 kcal, 34.5% of the 2-h intake, 26.3% of total caloric intake; Fig. 4A). A similar pattern was observed in the refeeding phase, although more calories were consumed in the first 15 min (3.87 ± 0.17 kcal of a total of 8.81 ± 0.61 kcal, 43.7% of the 2-h intake, 34.2% of total caloric intake; one-way ANOVA, P = 0.003 versus habituation; Fig. 4B). On day 1 of the refeeding phase, a large proportion of calories was consumed in the first 15 min (4.62 ± 0.37 kcal of a total of 7.83 ± 0.60 kcal, 59.0% of the 2-h intake, 46.1% of total caloric intake; Fig. 4C).
Fig. 4.
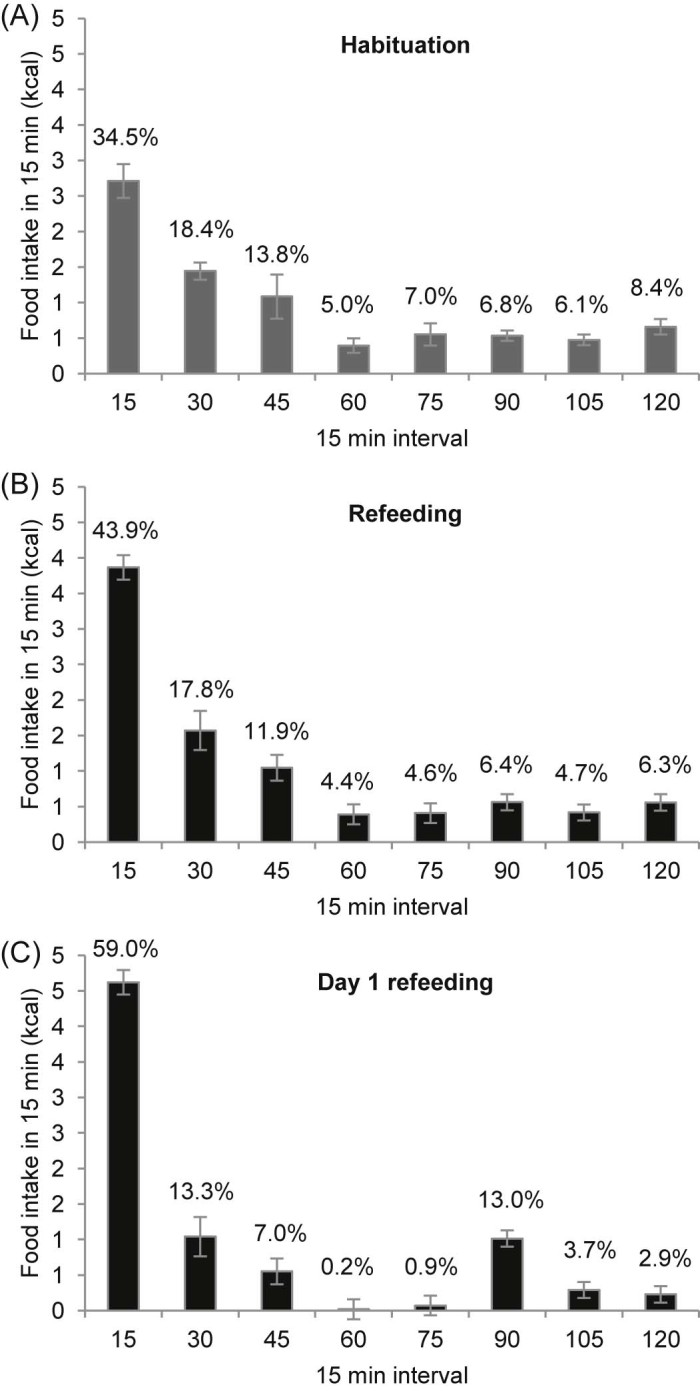
Microstructure of caloric intake from HFD during the 2 h scheduled access during (A) habituation phase, (B) refeeding phase and (C) day 1 of the refeeding phase. Data are shown as absolute values in 15-min bins. Percentages above bars refer to calories consumed in the 15 min bins relative to 2 h intake during scheduled feeding time. Data are presented as mean ± SEM.
Meal pattern analysis
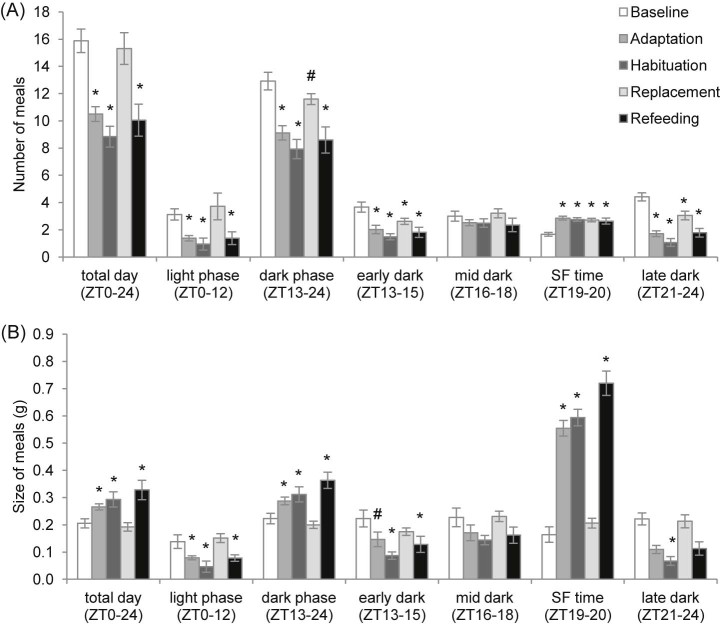
There were effects of study phase on both meal number (Fig. 5A) and meal size (Fig. 5B) when analysing over the complete day, the light phase, the whole dark phase, early dark phase, the scheduled feeding time or late dark phase (one-way RM ANOVA; P < 0.001, P < 0.001, P < 0.001, P < 0.001, P < 0.001 and P < 0.001 for meal number; P < 0.001, P = 0.001, P < 0.001, P = 0.004, P < 0.001 and P < 0.008 for meal size, respectively). There were no effects on meal number and meal size when analysing over the mid dark phase, the 3 h time period prior to scheduled feeding time. Meal number was decreased over the day, the light phase, the whole dark phase, early dark phase and late dark phase during adaptation, habituation and refeeding (SNK; day, P < 0.001, P < 0.001 and P < 0.001 versus baseline; light phase, P = 0.003, P = 0.003 and P = 0.009 versus baseline; whole dark phase, P < 0.001, P < 0.001 and P < 0.001 versus baseline; early dark phase, P < 0.001, P < 0.001 and P < 0.001 versus baseline; late dark phase, P < 0.001, P < 0.001 and P < 0.001 versus baseline) and also during replacement in the dark phase, early dark phase and late dark phase (SNK; P = 0.067, P = 0.015 and P < 0.001 versus baseline). However, during the scheduled feeding time, mice increased meal number during adaptation, habituation, replacement and refeeding (SNK; P < 0.001 versus baseline). Meal size increased over the day, the whole dark phase and scheduled feeding time when mice had scheduled access to HFD (SNK; P = 0.023, P < 0.05 and P < 0.001 versus baseline during adaptation; P = 0.005, P < 0.05 and P < 0.001 versus baseline during habituation; P < 0.001, P < 0.05 and P < 0.001 versus baseline during refeeding). Furthermore, meal size during scheduled feeding was largest during the refeeding phase (SNK; P = 0.002 and P = 0.006 versus adaptation and habituation). However when the dark phase was broken down further, there were decreases in meal size during the early dark phase (SNK; P = 0.056, P = 0.002 and P = 0.028 baseline versus adaptation, habituation and refeeding) and late dark phase (SNK; P < 0.05 baseline versus habituation). During the light phase, meal size decreased when mice had scheduled access to HFD (SNK; P = 0.007, P < 0.001 and P = 0.16 versus baseline during adaptation, habituation and refeeding).
Fig. 5.
Meal analysis of C57BL/6 mice during all study phases. (A) Daily meal number and (B) meal size, presented as daily total, and broken down into light phase (ZT0–12), dark phase (ZT13–24), early dark phase (ZT13–15), mid dark phase (ZT16–18), scheduled feeding (SF) time (ZT19–20) and late dark phase (ZT21–24). *P < 0.05, #P < 0.1 versus baseline by one-way repeated measures ANOVA and Student–Newman–Keul post hoc test. For clarity, one asterisk also includes P < 0.01 and P < 0.001. Data are presented as mean ± SEM.
Activity pattern
Analysis of the baseline phase demonstrated clear diurnal rhythms of both horizontal and vertical activity (one-way RM ANOVA; P < 0.001). Horizontal activity started to increase during the last hour of the light phase (SNK; at ZT12, P < 0.05 versus all other ZT intervals) and was elevated during the whole of the dark phase and the first hour of the light phase (SNK; at ZT13 to ZT1, P < 0.05 versus ZT2 to ZT12). There were three peaks in dark phase horizontal activity (SNK; at ZT13, ZT19 and ZT24, P < 0.05 versus remaining ZT intervals), whereas the lowest dark phase levels were observed around the mid dark phase (SNK; at ZT20 and ZT22, P < 0.05 versus all other ZT intervals). Vertical activity at baseline gave a similar picture, with an increase during the last hour of the light phase (SNK; at ZT12, P < 0.05 versus all other ZT intervals), elevated activity during most of the dark phase and in the first hour of the light phase (SNK; at ZT13 to ZT19, ZT21, ZT23 to ZT1, P < 0.005 versus ZT2 to ZT12), with the exception of ZT20 and ZT22.
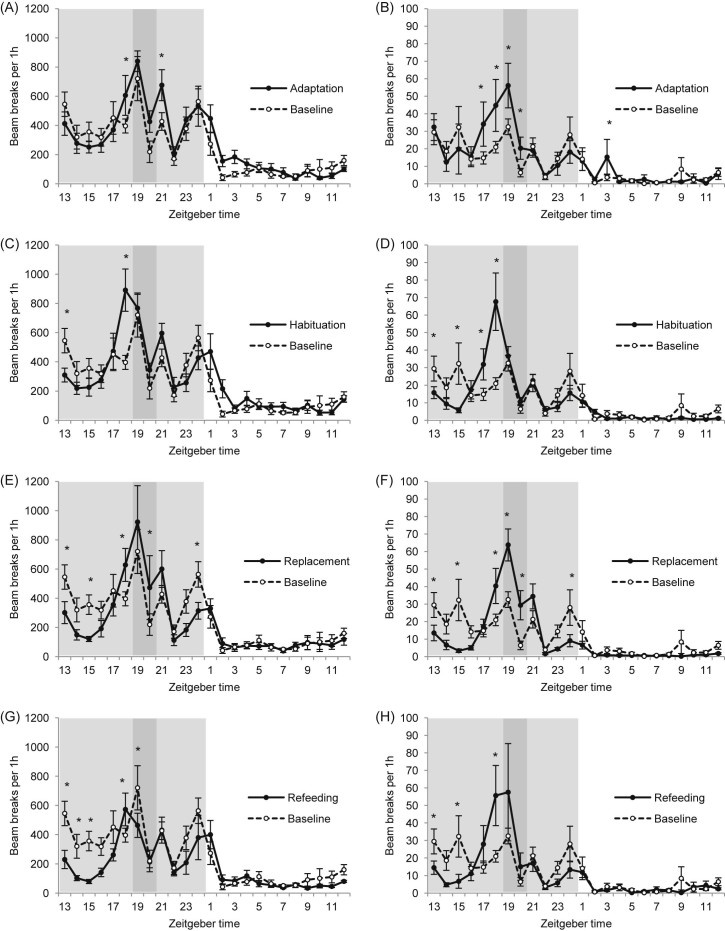
There were significant interactions between study phase and time interval for both horizontal activity (two-way RM ANOVA; P < 0.001; Fig. 6A,C,E,G) and vertical activity (P = 0.028; Fig. 6B,D,F,H). For clarity, Fig. 6 shows post hoc comparisons of each 1 h ZT interval with baseline phase. These outcomes are summarised briefly before further analysis of relevant dark phase bins (Fig. 7). For horizontal activity, there were no differences between study phases during the light phase. Increases in horizontal activity (versus baseline) were mainly observed during the mid-dark phase; the 1 h interval prior to scheduled feeding (ZT18) showed an increase in horizontal activity for all study phases. Decreases in horizontal activity (versus baseline) were mainly seen during the early dark phase, e.g. at ZT13 during habituation, replacement and refeeding. For vertical activity, there were similar patterns. Increases in vertical activity (versus baseline) were mainly observed during the mid-dark phase; the 1 h interval prior to scheduled feeding (ZT18) showed increases in vertical activity during all study phases and the interval 2 h prior to scheduled feeding (ZT17) also showed an increase during adaptation and habituation. Decreases in vertical activity (versus baseline) were mainly seen during the early dark phase; during habituation, replacement and refeeding, there were decreases at ZT13 and ZT15.
Fig. 6.
Activity pattern of C57BL/6 mice during all study phases versus baseline activity pattern: (A,B) adaptation, (C,D) habituation, (E,F) replacement and (G,H) refeeding phase. (A,C,E,G) Locomotor activity pattern (horizontal activity). (B,D,F,H) Rearing pattern (vertical activity). Light shaded area indicates dark phase; dark shaded area indicates scheduled feeding time. *P < 0.05 versus baseline by two-way repeated measures ANOVA and Student–Newman–Keul post hoc test. For clarity, one asterisk also includes P < 0.01 and P < 0.001. Data are presented as mean ± SEM.
Fig. 7.
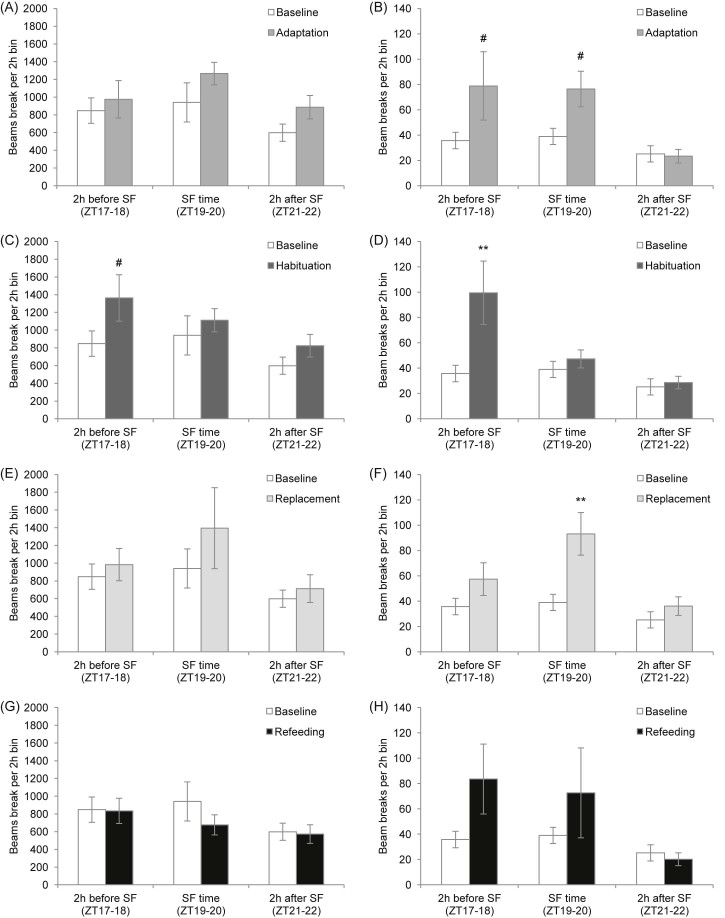
Activity pattern of C57BL/6 mice during all study phases versus baseline data over the dark phase: (A,B) adaptation, (C,D) habituation, (E,F) replacement and (G,H) refeeding phase. Relevant 2-h time bins from the analysis in Fig. 6 are depicted: 2 h before scheduled feeding (SF) (ZT17–18), SF time (ZT19–20) and 2 h after SF (ZT21–22). **P < 0.01, #P < 0.1 versus baseline by two-way repeated measures ANOVA. Data are presented as mean ± SEM.
According to the changes seen in the 1 h data for horizontal and vertical activity, data were then analysed in relevant 2 h bins over the dark phase: 2 h before scheduled feeding (ZT17–18), scheduled feeding time (ZT19–20) and 2 h after scheduled feeding (ZT21–22), and the study phases were compared with baseline (Fig. 7). Analysis revealed that during adaptation there were trends towards increases in vertical activity preceding and during scheduled feeding time (P = 0.066 and P = 0.063, respectively). During habituation, vertical activity was increased in the 2 h bin preceding scheduled feeding (P = 0.006) and there was a trend for increased horizontal activity preceding scheduled feeding (P = 0.094). During the replacement phase, vertical activity was increased during scheduled feeding time (P = 0.004).
Discussion
Providing mice with a palatable high fat diet for a 2-h period each day without caloric restriction is very effective in promoting hyperphagia during the access period (Fig. 1A). Consistent with previous reports, when control diet was replaced during scheduled feeding (Bake et al., 2013), mice rapidly adapted their feeding behaviours and binged on the palatable high fat diet (Fig. 1C), exhibiting a larger binge-like meal than rats under the same dietary regime (Bake et al., 2013; Bake, Morgan, & Mercer, 2014). Furthermore, mice showed an increase in body weight (Fig. 1D), as previously reported for feeding regimes offering an unrestricted amount of palatable diet (e.g. HFD, peanut butter, cheese) during a fixed time interval (Bake et al., 2013, 2014). This is in contrast to the effect on body weight of feeding paradigms that provide a fixed amount of a palatable food (corresponding to 30–35% of total caloric intake) at the same time every day, where there is a lack of body weight gain in mice (Gallardo, Gunapala, King, & Steele, 2012; Hsu, Patton, Mistlberger, & Steele, 2010a). Whereas mice in the fixed amount paradigm consumed similar or even higher amounts of calories each day, the relative proportion of palatable diet consumed each day (up to 78% in the current study) might be responsible for the differential effect.
To characterise this mouse model at a behavioural level, we focused on: (i) differences in the microstructure of feeding behaviour between schedule-feeding (adaptation and habituation phase) and control feeding (baseline phase), (ii) changes in meal size and number under these feeding regimes, (iii) assessment of activity patterns prior to scheduled feeding as a marker of food anticipatory activity (FAA), and (iv) consequences on feeding microstructure, meal patterns and activity patterns when palatable scheduled feeding is withdrawn in favour of control feeding (replacement phase) and then reintroduced again (refeeding phase).
Changes in feeding microstructure and meal pattern
As anticipated (Kohsaka et al., 2007), during baseline ad libitum feeding conditions, mice showed a clear diurnal rhythm of food intake, consuming most of their food (approximately 85%) during the dark phase (Fig. 2). Food intake was relatively consistent across the dark phase without clear peaks. This is in contrast to the three dark phase peaks observed in rats during early-, mid- and late-dark phase, the latter of which is the highest (Bake et al., 2014).
When mice were schedule-fed on palatable HFD, there was a shift in food intake towards the mid-dark phase. Schedule-fed mice showed a large reduction in control diet intake during the early hours of the dark phase and in the hours following the scheduled feed. However, notably, and in line with our previous observations in rats (Bake et al., 2014), schedule-fed mice did not significantly reduce their control diet intake when analysed in 1 h intervals (Fig. 2A,C,F) or change their meal pattern (Fig. 5A,B) during the 3 h period running up to scheduled feeding on HFD, the time frame for food anticipation and FAA. Analysing caloric intake as a corresponding 3 h bin during this time confirmed that there was no active anticipatory hypophagia during adaptation, habituation and refeeding (Fig. 3A,B,D). Overall, these observations suggest that schedule-fed mice were not in a hypocaloric, negative energy balance state immediately prior to schedule feeding.
Analysis of food intake microstructure in 15-min bins indicated that schedule-fed mice consumed HFD across the 2-h access period (Fig. 4A). The first 15-min bin saw the highest intake, yet only accounted for approximately one-third of HFD intake during the access period. The same analysis in rats suggested that a state approaching satiety was reached after 15 min of access since schedule-fed rats consumed approximately three-quarters of their intake of HFD in this time (Bake et al., 2014). Species differences in postprandial satiety with schedule-fed palatable diets may be an interesting avenue for further investigation.
Hyperphagia during scheduled feeding time was due to mice eating more frequent (Fig. 5A) and substantially larger meals (Fig. 5B). Larger meal sizes have been reported previously in rats fed ad libitum on high fat pellet diet (Melhorn et al., 2010) or high fat liquid diet (Warwick, McGuire, Bowen, & Synowski, 2000), as well as in rats prone to DIO compared with DIO-resistant rats fed on a high fat diet (Farley et al., 2003).
Entrainment of FAA
Despite the limited effect of scheduled feeding on food intake microstructure during the 3 h period prior to HFD access in both mice (current study) and rats (Bake et al., 2014), there were substantial changes in activity pattern in this time frame. The 2 h to 3 h period preceding a daily scheduled meal is regarded as the crucial time frame for FAA (Challet et al., 2009; Mistlberger, 1994; Shibata et al., 2010). Anticipation of “mealtime” can be observed in a range of behaviours, including wheel running, lever pressing, activity directed at feeders, general cage activity, and drinking, and represents a laboratory analogue of natural foraging behaviours (Mendoza, 2007; Mistlberger, 1994). In the current study, the diurnal rhythm seen in food intake during baseline was reflected in the pattern of both locomotor activity (Fig. 6A,C,E,G) and rearing activity (Fig. 6B,D,F,H), with peaks at the beginning, middle and end of the dark phase, a predictable nocturnal pattern (Kohsaka et al., 2007). However, it is important to note that whereas food consumption was recorded automatically, diet changes for scheduled feeding were done manually in the absence of automated access hardware. Consequently, activity peaks during the mid-dark phase (at ZT19 and ZT21) will have been influenced by the need to manually change the food hopper. To control for this disturbance effect, the physical manipulations were performed daily throughout all study phases even when no actual change of diet was required during baseline and replacement phases.
The diurnal pattern of activity seen during baseline persisted when mice were schedule-fed on HFD, albeit with a lower intensity during the early hours of the dark phase. There was no major shift in activity towards different time points. Crucially, rearing activity was increased in the 2 h period prior to scheduled feeding once the feeding behaviour was habituated to scheduled access conditions, and locomotor activity was increased during the 1-h period prior to scheduled feeding. This increase in activity is strongly indicative of FAA, with complementary analysis at each 1 h interval and in 2 h dark phase bins suggesting that FAA is strongest in the hour immediately before scheduled feeding. This may represent a novel finding in this model, i.e. FAA in mice prior to palatable meal feeding in the dark phase. Most studies investigating food anticipatory behaviour/FAA have employed restricted feeding schedules, which induce robust increases in activity in anticipation of the predicted meal, i.e. when food is not available. In rats, increases in locomotor activity are observed 2–3 h prior to meals of chow in the light phase (Escobar, Martínez-Merlos, Ángeles-Castellanos, Del Carmen Miñana, & Buijs, 2007; Mendoza, Ángeles-Castellanos, & Escobar, 2005; Verwey, Khoja, Stewart, & Amir, 2007). FAA has been shown in mice prior to daily meals of chow in the mid-light phase through an increase in the combined activity rate for walking, hanging, jumping and rearing during the 3 h period prior to a 2 h meal (Gunapala, Gallardo, Hsu, & Steele, 2011), as an increase in wheel running during the 3 h period prior to a 4 h meal (Blum et al., 2009), or as an increase in locomotor activity during the 2 h period prior to a 4 h meal (Davis, Choi, Clegg, & Benoit, 2011). Mice may be capable of anticipating 2 or 3 meals per day (Luby et al., 2012). However, fewer studies have investigated food anticipatory behaviour under palatable feeding schedules similar to the one used in the current study. In rats, FAA was observed on access to palatable food in the mid-light phase (Dailey, Stingl, & Moran, 2012; Merkestein et al., 2012). However, in some studies, FAA occurred with a lower intensity (Mendoza et al., 2005) or not in all animals of the study population (Verwey et al., 2007). In mice, it has been shown that FAA has some diet specificity; a palatable feeding schedule with high fat diet (Hsu et al., 2010a), peanut butter or cheese (Gallardo et al., 2012) induced a moderate increase in high intensity activity (walking, hanging, jumping and rearing) during the 2 h period prior to mealtime in the late light phase, whereas mice on a palatable feeding schedule with chocolate or fruit crunchies (nutritionally balanced fruit-flavoured pellets) did not exhibit FAA (Hsu et al., 2010a).
In most studies investigating FAA, the food is given in the light phase to make observation easier, although one study of mice investigated FAA prior to feeding for 2 h at the beginning of the dark phase (Liu et al., 2012). However, light-phase manipulations will disrupt the sleep–wake cycle (Eckel-Mahan & Sassone-Corsi, 2013). For example, feeding or forced activity for 8 h during the normal resting phase desynchronises the rhythm between the suprachiasmatic nucleus (SCN), the light-entrainable circadian oscillator, and the liver, disturbs molecular rhythms within the liver, and leads to a loss of blood glucose rhythm and to overweight in rats (Salgado-Delgado, Ángeles-Castellanos, Buijs, & Escobar, 2008; Salgado-Delgado et al., 2013). Similar consequences have been shown for mice sleep restricted for 6 h during the light phase or fed during the light phase only, with rhythms of metabolic genes or circadian genes in the liver being disturbed (Barclay et al., 2012; Damiola et al., 2000). Moreover, feeding mice with a high fat diet during the light phase was reported to contribute to weight gain in comparison to high fat diet feeding in the dark phase only (Arble, Bass, Laposky, Vitaterna, & Turek, 2009). In the current study, we show that it is possible to characterise FAA superimposed on normal activity during the active dark phase when mice are not food restricted.
The entrainment of FAA and the timing of meals have been linked to the food entrainable oscillator (FEO) that can work independently from the circadian clock in the SCN (Escobar, Cailotto, Ángeles-Castellanos, Delgado, & Buijs, 2009; Strubbe & Woods, 2004), although it requires a predictable gap between food presentations, an entrained circadian periodicity of 23–29 h, and will persist for several cycles despite continuous fasting, indicating the presence of an independent food clock (Challet et al., 2009; Stephan, 1981). Many studies have attempted to locate the FEO in the central nervous system, but its whereabouts still have to be determined (Challet et al., 2009; Mendoza, 2007). The FEO might be a distributed network of interacting nuclei each with a different function in the process of mediating FAA, rather than a single structure (Escobar et al., 2009; Mendoza, 2007).
Consequences of withdrawing and reintroducing palatable meals
Previous exposure to the palatable scheduled feeding regime had consequences for body weight, food intake microstructure and meal pattern, as well as activity pattern in the later study phases. After the initial increase during adaptation, body weight plateaued during habituation. However, body weight decreased after withdrawal of HFD during the replacement phase and then rapidly increased after the reintroduction of HFD. It is reasonable to assume that further cycles of replacement and refeeding would lead to a pattern of weight cycling (Barbosa-da-Silva, da Silva, Aguila, & Mandarim-de-Lacerda, 2013; Barbosa-da-Silva, Fraulob-Aquino, Lopes, Mandarim-de-Lacerda, & Aguila, 2012). It has also been shown that weight cycling under such conditions leads to substantial modification of blood lipids, glucose and insulin homeostasis, adipokine levels, and proinflammatory cytokines (Barbosa-da-Silva et al., 2012), and a structural remodelling of the liver (Barbosa-da-Silva et al., 2013), changes which were not reversed when mice lost body weight during the switch to chow feeding (Barbosa-da-Silva et al., 2012, 2013). In addition, the increase in adiposity resulting from high fat diet feeding cannot easily be reversed by reducing body weight when switching back to chow feeding; mice retained the increased number of adipocytes that were accumulated during high fat diet feeding, although adipocyte volumes were reduced (Shi et al., 2009). A decreased activity level had been suggested as a responsible mechanism for weight gain during weight cycling (Barbosa-da-Silva et al., 2012). However, since overall activity rate was not decreased during refeeding in the current study (data not shown), the increased body weight is likely due to the higher total daily caloric intake.
Both feeding microstructure and meal pattern showed that previous experience with scheduled access to HFD had consequences for behaviour during replacement and refeeding stages. When schedule-fed mice were switched back to control feeding conditions during the replacement phase, they retained a meal number appropriate to the consumption of larger amounts of food during that 2-h schedule-fed period (Fig. 5A), had increased caloric intake during that period (Fig. 2C), but returned to baseline meal size. When mice were then returned to HFD during scheduled feeding in the refeeding phase, they had an elevated caloric intake during scheduled feeding compared with habituation. Firstly, this was due to an increased meal size (Fig. 5B), and secondly, intake in the first 15 min following presentation of HFD was higher than during habituation (Fig. 4B). In particular, on day 1 of the refeeding phase, there was an elevated caloric intake during the first 15 min (Fig. 4C). It appears likely that the mice were still anticipating HFD since they continued to exhibit FAA prior to and during the scheduled feeding time throughout the replacement phase.
Consistent with the persistent increase in activity during the replacement phase, it has been shown previously that FAA can persist following withdrawal of palatable diet from a palatable scheduled feeding regime. In rats, for example, FAA persisted under ad libitum feeding conditions for at least 7 days at the expected time of a chocolate snack (Ángeles-Castellanos, Salgado-Delgado, Rodríguez, Buijs, & Escobar, 2008), and for mice, an increased food bin entry and high intensity activity continued after withdrawal of a palatable high fat treat (Hsu et al., 2010a). In contrast, FAA disappeared when a period of ad libitum feeding followed a restricted feeding schedule when chow was only available for 2 h or 3 h in the light phase, but FAA was reinstated when rats were fasted (Ángeles-Castellanos et al., 2008; Mistlberger, 1994). Mice, however, continued to exhibit limited FAA under ad libitum feeding conditions following the interruption of a restricted scheduled feeding regime of daily 4-h access to chow in the light phase (Blum et al., 2009). The FAA during habituation, as well as the persistent FAA during replacement, indicates that FAA might be driven by a FEO with a periodicity of 24 h but which does not depend on signals of either hunger or nutritional origin.
Conclusions and possible mechanistic underpinning of binge eating
Scheduled feeding on HFD stimulates a substantial binge eating episode in this mouse model. However, the period immediately before scheduled feeding is characterised by near normal levels of caloric intake from stock diet. We have previously observed the same phenomenon in schedule-fed rats (Bake et al., 2014). The absence of relative negative energy balance, in advance of the initiation of the binge, in either species, is in line with our previous findings (Bake et al., 2013) where there was no evidence of potentially causative perturbation in expression of hypothalamic homeostatic neuropeptide genes prior to consumption of large binge-type meals. Similarly, analysis of the gut hormones, ghrelin and glucagon-like peptide-1 indicated that these hormones were not involved in the anticipation of large palatable meals in rats (Bake et al., 2014), whereas they have been implicated in the anticipation of daily meals on restricted feeding schedules (Dailey et al., 2012; Drazen, Vahl, D'Alessio, Seeley, & Woods, 2006; Merkestein et al., 2012; Vahl, Drazen, Seeley, D'Alessio, & Woods, 2010). Two key findings of the current study were the continuing presence of FAA and sustained increase in meal frequency during the replacement phase, when only stock diet was available and the immediate hyperphagic response once HFD was restored after 7 days. The presence of FAA suggests that this could be part of the priming process for binge-like eating in the palatable schedule-fed model, which can be initiated very rapidly once HFD becomes available again. Although the mechanistic basis of FAA has not been definitively established, examination of mouse lines suggests that this behavioural profile is not critically dependent upon individual hormones or neuropeptides such as leptin (Gunapala et al., 2011; Ribeiro et al., 2011), ghrelin, NPY or orexin (Gunapala et al., 2011), or the histaminergic system (Liu et al., 2012), although ghrelin receptor signalling might be at least necessary to augment FAA (Blum et al., 2009; Davis et al., 2011), but may require functional dopaminergic (Liu et al., 2012), serotonergic (Hsu et al., 2010b) or melanocortin-3 receptor dependent signalling systems (Begriche et al., 2012; Sutton et al., 2008). This suggests an association between the mechanisms underlying binge-like eating on a palatable diet and those responsible for FAA, and highlights the value of palatable scheduled feeding models for further investigation as we seek to gain additional insight into the control of meal feeding and over-consumption of calories.
Footnotes
Acknowledgements: T.B. was funded by a CASE studentship from the BBSRC and AstraZeneca. The authors are also grateful for the funding from the Scottish Government and from the European Union Seventh Framework Programme (FP7/2007–2013) under grant agreements 266408 (Full4Health) and 245009 (NeuroFAST).
References
- Ángeles-Castellanos M., Salgado-Delgado R., Rodríguez K., Buijs R.M., Escobar C. Expectancy for food or expectancy for chocolate reveals timing systems for metabolism and reward. Neuroscience. 2008;155(1):297–307. doi: 10.1016/j.neuroscience.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Arble D.M., Bass J., Laposky A.D., Vitaterna M.H., Turek F.W. Circadian timing of food intake contributes to weight gain. Obesity (Silver Spring, Md.) 2009;17(11):2100–2102. doi: 10.1038/oby.2009.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atalayer D., Rowland N.E. Structure of motivation using food demand in mice. Physiology and Behavior. 2011;104(1):15–19. doi: 10.1016/j.physbeh.2011.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bake T., Duncan J.S., Morgan D.G.A., Mercer J.G. Arcuate nucleus homeostatic systems are not altered immediately prior to the scheduled consumption of large, binge-type meals of palatable solid or liquid diet in rats and mice. Journal of Neuroendocrinology. 2013;25(4):357–371. doi: 10.1111/jne.12008. [DOI] [PubMed] [Google Scholar]
- Bake T., Morgan D.G.A., Mercer J.G. Feeding and metabolic consequences of scheduled consumption of large, binge-type meals of high fat diet in the Sprague Dawley rat. Physiology and Behavior. 2014;128:70–79. doi: 10.1016/j.physbeh.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa-da-Silva S., da Silva N.C., Aguila M.B., Mandarim-de-Lacerda C.A. Liver damage is not reversed during the lean period in diet-induced weight cycling in mice. Hepatology Research. 2013 doi: 10.1111/hepr.12138. [DOI] [PubMed] [Google Scholar]
- Barbosa-da-Silva S., Fraulob-Aquino J.C., Lopes J.R., Mandarim-de-Lacerda C.A., Aguila M.B. Weight cycling enhances adipose tissue inflammatory responses in male mice. PLoS ONE. 2012;7(7) doi: 10.1371/journal.pone.0039837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay J.L., Husse J., Bode B., Naujokat N., Meyer-Kovac J., Schmid S.M. Circadian desynchrony promotes metabolic disruption in a mouse model of shiftwork. PLoS ONE. 2012;7(5) doi: 10.1371/journal.pone.0037150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begriche K., Marston O.J., Rossi J., Burke L.K., Mcdonald P., Heisler L.K. Melanocortin-3 receptors are involved in adaptation to restricted feeding. Genes, Brain, and Behavior. 2012;11(3):291–302. doi: 10.1111/j.1601-183X.2012.00766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berner L.A., Avena N.M., Hoebel B.G. Bingeing, self-restriction, and increased body weight in rats with limited access to a sweet-fat diet. Obesity (Silver Spring, Md.) 2008;16(9):1998–2002. doi: 10.1038/oby.2008.328. [DOI] [PubMed] [Google Scholar]
- Blum I.D., Patterson Z., Khazall R., Lamont E.W., Sleeman M.W., Horvath T.L. Reduced anticipatory locomotor responses to scheduled meals in ghrelin receptor deficient mice. Neuroscience. 2009;164(2):351–359. doi: 10.1016/j.neuroscience.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challet E., Mendoza J., Dardente H., Pévet P. Neurogenetics of food anticipation. The European Journal of Neuroscience. 2009;30(9):1676–1687. doi: 10.1111/j.1460-9568.2009.06962.x. [DOI] [PubMed] [Google Scholar]
- Corwin R.L., Wojnicki F.H.E., Fisher J.O., Dimitriou S.G., Rice H.B., Young M.A. Limited access to a dietary fat option affects ingestive behavior but not body composition in male rats. Physiology and Behavior. 1998;65(3):545–553. doi: 10.1016/s0031-9384(98)00201-7. [DOI] [PubMed] [Google Scholar]
- Dailey M.J., Stingl K.C., Moran T.H. Disassociation between preprandial gut peptide release and food-anticipatory activity. Endocrinology. 2012;153(1):132–142. doi: 10.1210/en.2011-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiola F., Le Minli N., Preitner N., Kornmann B., Fleury-Olela F., Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes and Development. 2000;14(23):2950–2961. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J.F., Choi D.L., Clegg D.J., Benoit S.C. Signaling through the ghrelin receptor modulates hippocampal function and meal anticipation in mice. Physiology and Behavior. 2011;103(1):39–43. doi: 10.1016/j.physbeh.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitriou S.G., Rice H.B., Corwin R.L. Effects of limited access to a fat option on food intake and body composition in female rats. The International Journal of Eating Disorders. 2000;28(4):436–445. doi: 10.1002/1098-108x(200012)28:4<436::aid-eat12>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Drazen D.L., Vahl T.P., D'Alessio D.A., Seeley R.J., Woods S.C. Effects of a fixed meal pattern on ghrelin secretion. Evidence for a learned response independent of nutrient status. Endocrinology. 2006;147(1):23–30. doi: 10.1210/en.2005-0973. [DOI] [PubMed] [Google Scholar]
- Eckel-Mahan K., Sassone-Corsi P. Metabolism and the circadian clock converge. Physiological Reviews. 2013;93(1):107–135. doi: 10.1152/physrev.00016.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar C., Cailotto C., Ángeles-Castellanos M., Delgado R.S., Buijs R.M. Peripheral oscillators. The driving force for food-anticipatory activity. The European Journal of Neuroscience. 2009;30(9):1665–1675. doi: 10.1111/j.1460-9568.2009.06972.x. [DOI] [PubMed] [Google Scholar]
- Escobar C., Martínez-Merlos M.T., Ángeles-Castellanos M., Del Carmen Miñana M., Buijs R.M. Unpredictable feeding schedules unmask a system for daily resetting of behavioural and metabolic food entrainment. The European Journal of Neuroscience. 2007;26(10):2804–2814. doi: 10.1111/j.1460-9568.2007.05893.x. [DOI] [PubMed] [Google Scholar]
- Farley C., Cook J.A., Spar B.D., Austin T.M., Kowalski T.J. Meal pattern analysis of diet-induced obesity in susceptible and resistant rats. Obesity Research. 2003;11(7):845–851. doi: 10.1038/oby.2003.116. [DOI] [PubMed] [Google Scholar]
- Gallardo C.M., Gunapala K.M., King O.D., Steele A.D. Daily scheduled high fat meals moderately entrain behavioral anticipatory activity, body temperature, and hypothalamic c-Fos activation. PLoS ONE. 2012;7(7) doi: 10.1371/journal.pone.0041161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunapala K.M., Gallardo C.M., Hsu C.T., Steele A.D. Single gene deletions of orexin, leptin, neuropeptide Y, and ghrelin do not appreciably alter food anticipatory activity in mice. PLoS ONE. 2011;6(3) doi: 10.1371/journal.pone.0018377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C.T., Patton D.F., Mistlberger R.E., Steele A.D. Palatable meal anticipation in mice. PLoS ONE. 2010;5(9) doi: 10.1371/journal.pone.0012903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu J.L., Yu L., Sullivan E., Bowman M., Mistlberger R.E., Tecott L.H. Enhanced food anticipatory activity associated with enhanced activation of extrahypothalamic neural pathways in serotonin2C receptor null mutant mice. PLoS ONE. 2010;5(7) doi: 10.1371/journal.pone.0011802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohsaka A., Laposky A.D., Ramsey K.M., Estrada C., Joshu C., Kobayashi Y. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metabolism. 2007;6(5):414–421. doi: 10.1016/j.cmet.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Liu Y., Liu T., Qu W., Hong Z., Urade Y., Huang Z. Dopamine is involved in food-anticipatory activity in mice. Journal of Biological Rhythms. 2012;27(5):398–409. doi: 10.1177/0748730412455913. [DOI] [PubMed] [Google Scholar]
- Luby M.D., Hsu C.T., Shuster S.A., Gallardo C.M., Mistlberger R.E., King O.D. Food anticipatory activity behavior of mice across a wide range of circadian and non-circadian intervals. PLoS ONE. 2012;7(5) doi: 10.1371/journal.pone.0037992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melhorn S.J., Krause E.G., Scott K.A., Mooney M.R., Johnson J.D., Woods S.C. Acute exposure to a high-fat diet alters meal patterns and body composition. Physiology and Behavior. 2010;99(1):33–39. doi: 10.1016/j.physbeh.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza J. Circadian clocks. Setting time by food. Journal of Neuroendocrinology. 2007;19(2):127–137. doi: 10.1111/j.1365-2826.2006.01510.x. [DOI] [PubMed] [Google Scholar]
- Mendoza J., Ángeles-Castellanos M., Escobar C. Entrainment by a palatable meal induces food-anticipatory activity and c-Fos expression in reward-related areas of the brain. Neuroscience. 2005;133(1):293–303. doi: 10.1016/j.neuroscience.2005.01.064. [DOI] [PubMed] [Google Scholar]
- Merkestein M., Brans M.A.D., Luijendijk M.C.M., de Jong J.W., Egecioglu E., Dickson S.L. Ghrelin mediates anticipation to a palatable meal in rats. Obesity (Silver Spring, Md.) 2012;20(5):963–971. doi: 10.1038/oby.2011.389. [DOI] [PubMed] [Google Scholar]
- Mistlberger R.E. Circadian food-anticipatory activity. Formal models and physiological mechanisms. Neuroscience and Biobehavioral Reviews. 1994;18(2):171–195. doi: 10.1016/0149-7634(94)90023-x. [DOI] [PubMed] [Google Scholar]
- Mistlberger R.E., Rusak B. Palatable daily meals entrain anticipatory activity rhythms in free-feeding rats. Dependence on meal size and nutrient content. Physiology and Behavior. 1987;41(3):219–226. doi: 10.1016/0031-9384(87)90356-8. [DOI] [PubMed] [Google Scholar]
- Ribeiro A.C., Ceccarini G., Dupré C., Friedman J.M., Pfaff D.W., Mark A.L. Contrasting effects of leptin on food anticipatory and total locomotor activity. PLoS ONE. 2011;6(8) doi: 10.1371/journal.pone.0023364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgado-Delgado R., Ángeles-Castellanos M., Buijs M.R., Escobar C. Internal desynchronization in a model of night-work by forced activity in rats. Neuroscience. 2008;154(3):922–931. doi: 10.1016/j.neuroscience.2008.03.066. [DOI] [PubMed] [Google Scholar]
- Salgado-Delgado R.C., Saderi N., Basualdo M.D.C., Guerrero-Vargas N.N., Escobar C., Buijs R.M. Shift work or food intake during the rest phase promotes metabolic disruption and desynchrony of liver genes in male rats. PLoS ONE. 2013;8(4) doi: 10.1371/journal.pone.0060052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H., Akunuru S., Bierman J.C., Hodge K.M., Mitchell M.C., Foster M.T. Diet-induced obese mice are leptin insufficient after weight reduction. Obesity (Silver Spring, Md.) 2009;17(9):1702–1709. doi: 10.1038/oby.2009.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S., Hirao A., Tahara Y. Restricted feeding-induced entrainment of activity rhythm and peripheral clock rhythm. Sleep and Biological Rhythms. 2010;8(1):18–27. doi: 10.1177/0748730409352782. [DOI] [PubMed] [Google Scholar]
- Stephan F.K. Limits of entrainment to periodic feeding in rats with suprachiasmatic lesions. Journal of Comparative Physiology – A. 1981;143(4):401–410. [Google Scholar]
- Strubbe J.H., Woods S.C. The timing of meals. Psychological Review. 2004;111(1):128–141. doi: 10.1037/0033-295X.111.1.128. [DOI] [PubMed] [Google Scholar]
- Sutton G.M., Perez-Tilve D., Nogueiras R., Fang J., Kim J.K., Cone R.D. The melanocortin-3 receptor is required for entrainment to meal intake. Journal of Neuroscience. 2008;28(48):12946–12955. doi: 10.1523/JNEUROSCI.3615-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahl T.P., Drazen D.L., Seeley R.J., D'Alessio D.A., Woods S.C. Meal-anticipatory glucagon-like peptide-1 secretion in rats. Endocrinology. 2010;151(2):569–575. doi: 10.1210/en.2009-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verwey M., Amir S. Food-entrainable circadian oscillators in the brain. The European Journal of Neuroscience. 2009;30(9):1650–1657. doi: 10.1111/j.1460-9568.2009.06960.x. [DOI] [PubMed] [Google Scholar]
- Verwey M., Khoja Z., Stewart J., Amir S. Differential regulation of the expression of Period2 protein in the limbic forebrain and dorsomedial hypothalamus by daily limited access to highly palatable food in food-deprived and free-fed rats. Neuroscience. 2007;147(2):277–285. doi: 10.1016/j.neuroscience.2007.04.044. [DOI] [PubMed] [Google Scholar]
- Warwick Z.S., McGuire C.M., Bowen K.J., Synowski S.J. Behavioral components of high-fat diet hyperphagia. Meal size and postprandial satiety. American Journal of Physiology – Regulatory Integrative and Comparative Physiology. 2000;278(1):R196–R200. doi: 10.1152/ajpregu.2000.278.1.R196. [DOI] [PubMed] [Google Scholar]
- Woods S.C. Signals that influence food intake and body weight. Physiology and Behavior. 2005;86(5):709–716. doi: 10.1016/j.physbeh.2005.08.060. [DOI] [PubMed] [Google Scholar]