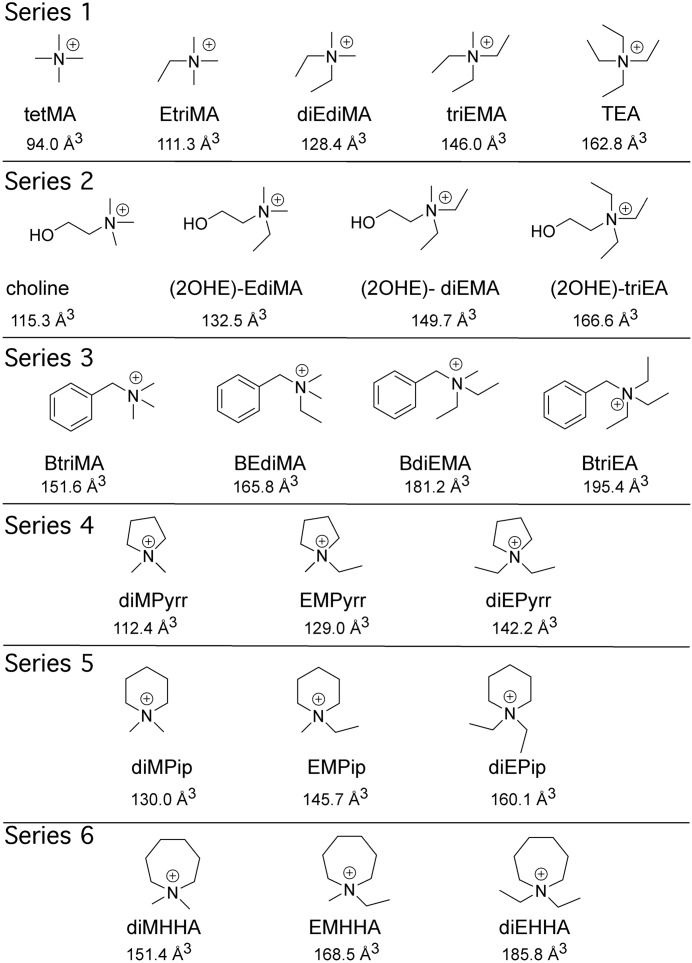
Abstract
The minimum pharmacophore for activation of the human α7 nicotinic acetylcholine receptor (nAChR) is the tetramethylammonium cation. Previous work demonstrated that larger quaternary ammonium compounds, such as diethyldimethylammonium or 1-methyl quinuclidine, were α7-selective partial agonists, but additional increase in the size of the ammonium cation or the quinuclidine N-alkyl group by a single carbon to an N-ethyl group led to a loss of efficacy for ion channel activation. We report that although such compounds are ineffective at inducing the normal channel open state, they nonetheless regulate the induction of specific conformational states normally considered downstream of channel activation. We synthesized several panels of quaternary ammonium nAChR ligands that systematically varied the size of the substituents bonded to the central positively charged nitrogen atom. In these molecular series, we found a correlation between the molecular volume of the ligand and/or charge density, and the receptor’s preferred distribution among conformational states including the closed state, the active state, a nonconducting state that could be converted to an activated state by a positive allosteric modulator (PAM), and a PAM-insensitive nonconducting state. We hypothesize that the changes of molecular volume of an agonist’s cationic core subtly impact interactions at the subunit interface constituting the orthosteric binding site in such a way as to regulate the probability of conversions among the conformational states. We define a new minimal pharmacophore for the class of compounds we have termed “silent agonists,” which are able to induce allosteric modulator-dependent activation but not the normal activated state.
Introduction
Binding of ligands to allosteric proteins, such as nicotinic acetylcholine receptors (nAChRs), regulates the probability with which the protein shifts among various conformational states (Papke, 2014). In the case of ligand-gated ion channels, it is customary to focus on the conformational change associated with channel activation. Agonists are drugs that promote conversion to the channel-activated state(s). In a specific experimental context, such as the evaluation of macroscopic currents from cells heterologously expressing a specific receptor subtype, drugs that are less effective at producing channel activation than a reference agonist, such as acetylcholine (ACh) in the case of nicotinic receptors, are classified as partial agonists.
The structural dissection of a series of compounds to define a receptor’s pharmacophore typically addresses efficacy first, defining the molecular elements required to activate the receptor. A second property to be considered is selectivity, defining what is required to activate one receptor subtype, but not other closely related subtypes. However, by considering the potential for differential induction of alternative conformational states, including nonconducting (desensitized) states, we can generate expanded and refined pharmacophores. The α7-type homomeric nAChR has an especially interesting array of conducting and nonconducting conformational states, regulated by both orthosteric and allosteric ligands (Williams et al., 2011a). It was recently proposed that the α7 receptor may mediate both channel activation–dependent and –independent forms of signaling (de Jonge and Ulloa, 2007; Skok, 2009; Kabbani et al., 2013). Therefore, understanding the pharmacophore for selectively inducing specific nonconducting states may be of equal interest and importance as understanding the pharmacophore for inducing open channel states.
The minimal pharmacophore for activating the ion channel of neuronal type nAChRs is tetramethylammonium (tetMA; see Fig. 1), and the minimal structure for selectively activating α7 nAChRs is diethyldimethylammonium (diEdiMA) (Horenstein et al., 2008), with the selectivity arising from replacement of a methyl group with an ethyl group, since ethyltrimethylammonium (EtriMA) is not α7-selective. In this article, we return to the minimal structures to define the requirements for producing what we have referred to as “silent agonists” (Chojnacka et al., 2013), molecules that have little or no efficacy as orthosteric agonists but can work in concert with positive allosteric modulators (PAMs) to produce channel activation through the induction of modulator-sensitive desensitized states. PAMs therefore serve to reveal the existence of these allosteric-potentiated open states, which otherwise might go undetected.
Fig. 1.
Structures of the quaternary ammonium compounds and their Connolly solvent-excluded volumes. The progressive replacement of methyl groups with ethyl groups, which characterized the first series of amines, was used to systematically generate five additional sets of compounds (series 2–6; see Materials and Methods for further details).
When activated by the binding of agonist alone, the openings of α7 receptors are extremely brief (<100 microseconds on average), and appear in isolation (Williams et al., 2012; Pesti et al., 2014). However, openings of receptors with both agonist and PNU-120596 [N-(5-chloro-2,4-dimethoxyphenyl)-N′-(5-methyl-3-isoxazolyl)-urea] bound, occur as protracted bursts with hundreds of intraburst closed events (Williams et al., 2011a), which suggested evidence for an agonist-bound closed state that is kinetically coupled with a PAM-dependent open state and is detectable only if the PAM is also bound. We hypothesized that such a PAM-sensitive closed state (Ds) could also contribute to the population of desensitized receptors under control conditions (Williams et al., 2011a). Comparison of the probability of the channel being open during these bursts to the steady-state probability of channels being open under the control condition (same population of channels in the very same patch) shows that the effect of the PAM is to produce large (100,000-fold) intermittent increases in the probability that a given channel will be open. However, the PAM effect on single channels is temporary, and there also exists a more stable closed state that we associate with a PAM-insensitive closed state (Di). Note that it is not likely that the long closed times between PAM-dependent bursts are strictly due to unbinding of ligands (although in some cases they must be), because at high concentrations of the agonist and PAM, the frequency of bursts decreases and the durations of the interburst closed times increase, associated with decreased macroscopic current (Williams et al., 2011a). In this study, we evaluate macroscopic current responses evoked by several series of structurally related compounds, either applied alone or in combination with PNU-120596 at a concentration that we determined in previous experiments to give us optimal potentiation of ACh-evoked currents (Williams et al., 2011a). The macroscopic currents we record reflect the integrated outcome of the dynamic induction of conducting and nonconducting states (Papke, 2010). The nonconducting states may encompass unliganded states as well as desensitized and possibly blocked states. We identify the minimal structural requirements for silent agonists, compounds that induce only nonconducting states in the absence of the PAM but produce significant currents in the presence of the PAM.
Materials and Methods
Commercial Reagents.
ACh, atropine, tetMA chloride, EtriMA iodide, diEdiMA hydroxide solution, TEA chloride, dimethylphenylpiperazinium (diMPP) iodide, and choline chloride were purchased from Sigma-Aldrich (St. Louis, MO). General reagents for chemical synthesis were purchased from Fisher Scientific (Pittsburgh, PA) or Sigma-Aldrich. Cell culture supplies were purchased from Life Technologies (Grand Island, NY). Triethylmethylammonium (triEMA) was purchased from Tokyo Chemical Industry (TCI America, Portland, OR). Fresh ACh stock solutions were made in Ringer’s solution each day of experimentation. Stock solutions of the test drugs were made in Ringer’s solution and kept at 4°C and used within 2 days. Working solutions were prepared freshly at the desired concentration from the stored stock.
Synthesis of Ammonium Compounds.
All compounds displayed satisfactory 1H- and 13C-NMR spectra and were found to be >95% purity by NMR methods (Supplemental NMR Spectra).
Quaternary ammonium salts were prepared by reacting commercially available methyl or ethyl amines with methyl iodide or ethyl iodide in tetrahydrofuran or ethanol and purified by recrystallization. (2-Hydroxyethyl)-ethyldimethylammonium iodide [(2OHE)-EdiMA] (Calas et al., 2000) was made from 2-dimethylaminoethanol and ethyl iodide, and is a white hygroscopic solid. (2-Hydroxyethyl)-diethylmethylammonium iodide [(2OHE)-diEMA] (Green et al., 2009) was made from 2-diethylaminoethanol and methyl iodide, and is a white solid. (2-Hydroxyethyl)-triethylammonium iodide [(2OHE)-triEA] (Kasuga et al., 1969) was made from 2-diethylaminoethanol and ethyl iodide, and is a white solid. Benzyltrimethylammonium iodide (BtriMA) (Ito et al., 2005) was made from dimethylbenzylamine and methyl iodide, and is a white solid. Benzylethyldimethylammonium iodide (BEdiMA) (Short and Biermacher, 1962) was made from dimethylbenzylamine and ethyl iodide, and is a white hygroscopic solid. Benzyldiethylmethylammonium iodide (BdiEMA) (Wempe, 2001) was made from ethylmethylbenzylamine and ethyl iodide, and is a white solid. Dimethylpyrrolidinium iodide (diMPyrr) (MacFarlane et al., 1999) was made from 1-methylpyrrolidine and methyl iodide, and is a white solid. Ethylmethylpyrrolidinium iodide (EMPyrr) (MacFarlane et al., 1999) was made from 1-methylpyrrolidine and ethyl iodide. Diethylpyrrolidinium iodide (diEPyrr) (Hünig and Baron, 1957) was made from 1-ethylpyrrolidine and ethyl iodide, and is a white solid. Dimethylpiperidinium iodide (diMPip) (Gamal-Eldin and Macartney, 2013) was made from 1-methylpiperidine and methyl iodide, and is a white solid. Ethylmethylpiperidinium iodide (EMPip) (von Braun and Buchman, 1931) was made from 1-methylpiperidine and ethyl iodide, and is a white solid. Diethylpiperidinium iodide (diEPip) (Lowe and Rendall, 1971) was made from 1-ethylpiperidine and ethyl iodide, and is a white solid. Dimethylhexahydroazepinium iodide (diMHHA) (Hünig and Baron, 1957) was made from 1-methylhexahydroazepine and methyl iodide, and is a white solid. Ethylmethylhexahydroazepinium iodide (EMHHA) (Hünig and Baron, 1957) was made from 1-methylhexahydroazepine and ethyl iodide, and is a white solid. Diethylhexahydroazepinium iodide (diEHHA) (Hünig and Baron, 1957) was made from 1-ethylhexahydroazepine and ethyl iodide, and is a white solid. 1-Methylhexahydroazepine was made from hexahydroazepine by the Eschweiler–Clarke reaction (formalin and formic acid) (Eschweiler, 1905; Clarke et al., 1933), and 1-ethylhexahydroazapine was prepared from hexahydroazepine using potassium carbonate and ethyl iodide. Ethylmethylphenylpiperazinium iodide (EMPP) was prepared from 1-ethyl-4-phenylpiperazine and methyl iodide, and is a white solid [1H-NMR (dimethylsulfoxide [DMSO]-d6, 500 MHz): δ 7.29–7.26 (m, 2H), 7.02 (d, 2H, 8.1 Hz), 6.87 (t, 1H, 7.3 Hz), 3.61–3.50 (m, 8H), 3.45–3.40 (m, 2H), 3.11 (s, 3H), and 1.29 (t, 3H, 7.1 Hz); and 13C-NMR (DMSO-d6, 125 MHz): δ 149.2, 129.0, 119.8, 115.6, 58.4, 58.3, 45.1, 41.8, and 7.1]. Diethylphenylpiperazinium iodide (diEPP) was made from 1-ethyl-4-phenylpiperazine and ethyl iodide, and is a white solid [1H-NMR (DMSO-d6, 500 MHz): δ 7.28 (t, 2H, 7.9 Hz), 7.01 (d, 2H, 8.3 Hz), 6.87 (t, 1H, 7.1 Hz, 7.5 Hz), 3.55–3.44 (m, 12H), 1.22 (t, 6H, 7.0 Hz, 7.2 Hz); and 13C-NMR (DMSO-d6, 125 MHz): δ 149.3, 129.0, 119.8, 115.5, 56.4, 51.8, 41.5, and 6.8].
Heterologous Expression of nAChRs in Xenopus laevis Oocytes.
Human nAChR clones were obtained from Dr. J. Lindstrom (University of Pennsylvania, Philadelphia, PA). The human resistance-to-cholinesterase 3 clone, obtained from Dr. M. Treinin (Hebrew University, Jerusalem, Israel), was coinjected with α7 to improve the level and speed of α7-receptor expression without affecting the pharmacological properties of the receptors (Halevi et al., 2003). Subsequent to linearization and purification of the plasmid cDNAs, cRNAs, were prepared using the mMessage mMachine in vitro RNA transfection kit (Ambion, Austin, TX).
Oocytes were surgically removed from mature X. laevis frogs (Nasco, Ft. Atkinson, WI) and injected with appropriate nAChR subunit cRNAs as previously described (Papke and Stokes, 2010). Frogs were maintained in the Animal Care Service facility of the University of Florida (Gainesville, FL), and all procedures were approved by the University of Florida Institutional Animal Care and Use Committee. In brief, the frog was first anesthetized for 15–20 minutes in 1.5 liters of frog tank water containing 1 g of 3-aminobenzoate methanesulfonate buffered with sodium bicarbonate. The harvested oocytes were treated with 1.25 mg/ml collagenase (Worthington Biochemicals, Freehold, NJ) for 2 hours at room temperature in a calcium-free Barth’s solution (88 mM NaCl, 1 mM KCl, 2.38 mM NaHCO3, 0.82 mM MgSO4, 15 mM HEPES, and 12 mg/l tetracycline, pH 7.6) to remove the follicular layer. Stage V oocytes were subsequently isolated and injected with 50 nl of 5–20 ng nAChR subunit cRNA. Recordings were carried out 1–7 days after injection.
Two-Electrode Voltage-Clamp Electrophysiology.
Experiments were conducted using OpusXpress 6000A (Molecular Devices, Union City, CA) (Papke and Stokes, 2010). Both the voltage and current electrodes were filled with 3 M KCl. Oocytes were voltage-clamped at −60 mV. The oocytes were bath-perfused with Ringer’s solution (115 mM NaCl, 2.5 mM KCl, 1.8 mM CaCl2, 10 mM HEPES, and 1 μM atropine, pH 7.2) at 2 ml/min for α7 receptors and at 4 ml/min for other subtypes. To evaluate the effects of experimental compounds compared with ACh-evoked responses of various nAChR subtypes expressed in oocytes, baseline conditions were defined by two initial applications of ACh made before coapplications of experimental compounds with the control ACh. The agonist solutions were applied from a 96-well plate via disposable tips, and the test compounds were applied alone, coapplied with ACh, or coapplied with PNU-120596. For the concentration-response study, drug applications alternated between ACh controls and experimental compounds. Unless otherwise indicated, drug applications were 12 seconds in duration, followed by a 181-second washout period for α7 receptors and 6 seconds with a 241-second washout for other subtypes. A typical recording for each oocyte constituted two initial control applications of ACh, an experimental compound application, and then a follow-up control application of ACh to determine the desensitization or rundown of the receptors. The control ACh concentrations were 300 µM for α7 or 60 µM when followed up with PNU-120596 application, 100 μM for α3β4, and 30 μM for α4β2. The responses of α4β2- and α3β4-expressing cells were measured as peak current amplitudes, and the α7 data were calculated as net charge, as previously described (Papke and Porter Papke, 2002).
Data were collected at 50 Hz, filtered at 20 Hz, analyzed by Clampfit 9.2 (Molecular Devices) and Excel 2003 (Microsoft, Redmond, WA), and normalized to the averaged peak current or net-charge response of the two initial ACh controls (Papke and Porter Papke, 2002). Data were expressed as means ± S.E.M. from at least four oocytes for each experiment and were plotted by Kaleidagraph (Abelbeck Software, Reading, PA).
It should be noted that in our analyses of PNU-120596 potentiated currents, the test compounds were coapplied with 10 µM PNU-120596, a concentration we previously found to produce maximal potentiation with a single application (Williams et al., 2011a). Although the kinetics of the binding of the putative orthosteric ligands would be expected to be more rapid (especially at high concentrations) than the binding of the PAM, which may need to reach a binding site in the transmembrane domains (Young et al., 2008), contributions to the macroscopic response due to the binding of the orthosteric ligands alone would be small compared with amplified currents generated when the PAM sites are also bound. However, the faster binding of the orthosteric ligands at high concentrations may have contributed to the induction of the Di state, accounting for reduced PAM-potentiated currents at high concentrations and inverted “U” concentration-response functions.
Results
Responses of Neuronal nAChR to Series 1 Quaternary Amines.
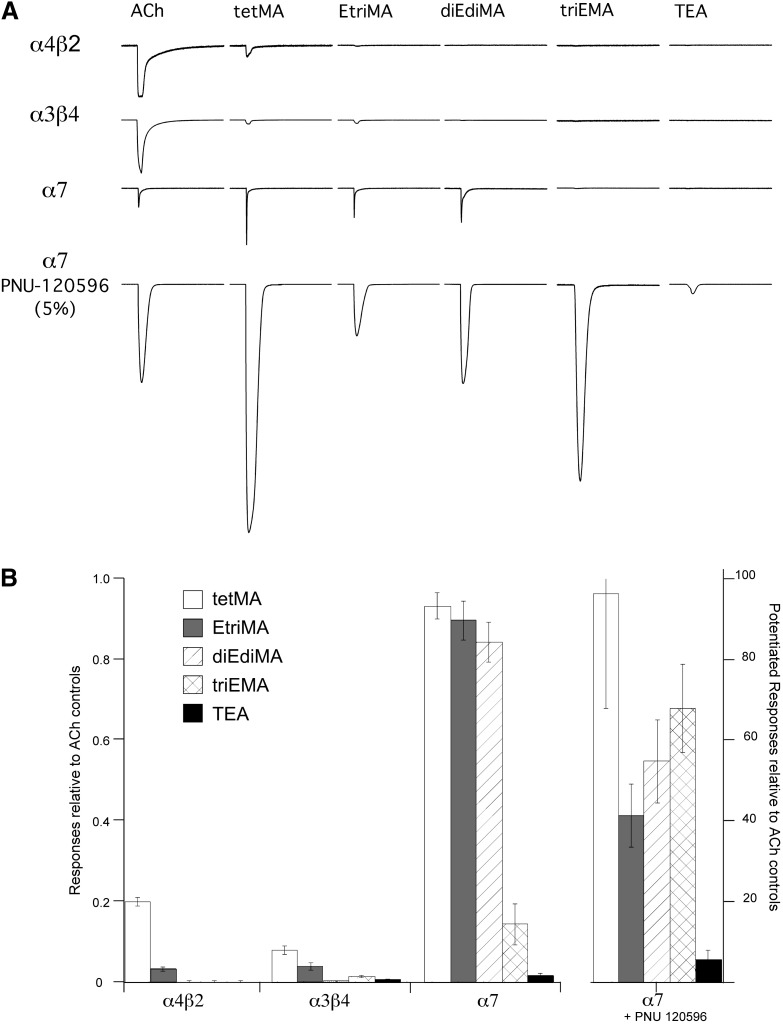
To expand our previous study of quaternary amines (Papke et al., 1996; Horenstein et al., 2008), we tested tetMA and the other series 1 amines on α4β2-, α3β4-, and α7 nAChR–expressing cells and compared those responses to control ACh-evoked responses. The α4β2-expressing cells only responded to tetMA with peak current responses ≤5% the ACh controls, whereas α3β4 receptors responded to both tetMA and EtriMA, and α7 receptors also responded well to diEdiMA (Fig. 2) and weakly to triEMA, at our limit of detection. None of the subtypes tested gave responses to the application of 100 µM TEA that were larger than stimulus artifacts associated with the application of Ringer’s solution alone. When coapplied with ACh, TEA functioned as a competitive antagonist for α7, decreasing currents evoked by 60 µM ACh with an IC50 of 80 ± 5 µM (not shown).
Fig. 2.
(A) Drug-evoked responses of human nAChR to ACh and series 1 quaternary amines. Cells expressing α4β2, α3β4, or α7 were first stimulated with ACh (30, 100, or 60 µM, respectively) to establish a basis for comparisons, and all representative evoked responses displayed have been scaled relative to the ACh responses from the individual cells. Each trace is 200 seconds in duration. The bottom row of traces shows the responses of α7 receptors to each of the compounds at 100 µM, coapplied with 10 µM of the α7 PAM PNU-120596. Responses to the various agents were first scaled relative to the ACh controls initially recorded from the same cells and then reduced by a factor of 20 for purposes of display. (B) The three groups of columns on the left show the average responses of α4β2-, α3β4-, and α7-expressing cells to 100 µM applications of the series 1 quaternary amines, expressed relative to ACh control responses recorded in the same cells. The α4β2 and α3β4 data are for peak current, and the α7 data are for net charge. The group of columns on the right gives the average net-charge responses to coapplications of the amines plus 10 µM PNU-120596 to α7-expressing cells, expressed relative to the net-charge responses to 60 µM ACh alone. Each bar shows the average normalized response of at least four cells (± S.E.M.).
The quaternary amines were then coapplied to α7-expressing cells with the efficacious type II PAM, PNU-120596, and all of them evoked significant responses. The revelation of TEA-induced expression of the Ds state identifies the structure of TEA as the minimal pharmacophore for an α7 silent agonist.
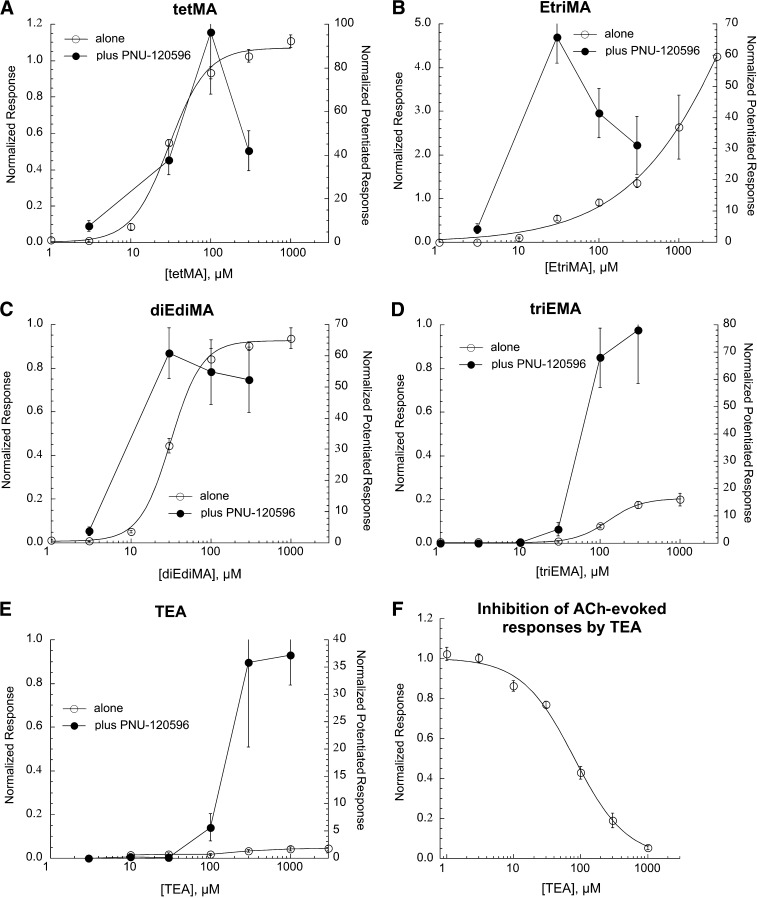
We tested the panel of series 1 quaternary amines on α7 across a range of concentrations, applied alone or in combination with PNU-120596 (Fig. 3). When applied alone, EtriMA was most efficacious (more so than ACh), but only at very high concentrations, so that the data were not well fit with the Hill equation. tetMA and diEdiMA were effectively full agonists relative to ACh (see Table 1 for EC50 values), whereas triEMA was a partial agonist, and TEA had negligible efficacy when applied alone. All of the series 1 amines synergized effectively with PNU-120596, although tetMA and EtriMA had pronounced inverted-U concentration-response functions, presumably related to the preferential induction of the Di state (Williams et al., 2011a) at higher concentrations. EtriMA and diEdiMA appeared notably more potent for generating PNU-120596–potentiated currents than when used alone. The efficacy of TEA as an orthosteric agonist was so low that it was effectively an antagonist of ACh-evoked α7 responses (Fig. 3F) with an IC50 of 80 ± 5 µM. These data are summarized in Table 1.
Fig. 3.
Concentration-response studies for the orthosteric (drug applied alone) and allosterically potentiated (drug coapplied with 10 µM PNU-120596) activation of α7 nAChR by series 1 quaternary amines tetMA (A), EtriMA (B), diEdiMA (C), triEMA (D), and TEA (E). In A–E, the left y-axes apply to the net-charge responses recorded when the drugs were applied alone (○), normalized to the responses to 300 µM ACh applied prior to the experimental application. The right y-axes apply to the net-charge responses when the drugs were coapplied with PNU-120596 (●), normalized to average net-charge responses to two prior applications of 60 µM ACh. The responses to the drugs applied alone in A–D were fit to the Hill equation, and the fit parameters are given in Table 1. (F) Since TEA did not evoke significant currents when applied alone, we tested its potency as an antagonist by coapplying it with 60 µM ACh. The TEA IC50 is reported in Table 1. Each point is the average normalized response of at least four cells (± S.E.M.).
TABLE 1.
Effects α7 nAChR
| Compound | Drug Alone |
Plus PNU-120596a |
||
|---|---|---|---|---|
| Imax | EC50 | IC50 | Normalized Response | |
| µM | ||||
| tetMA | 1.06 ± 0.03 | 30 ± 3 | 96 ± 28 | |
| EtriMA | >4 | >300 | 41 ± 8 | |
| diEdiMA | 0.9 ± 0.01 | 31 ± 1 | 55 ± 10 | |
| triEMA | 0.15 ± .01 | 107 ± 43 | 68 ± 11 | |
| TEA | 80 ± 5 | 5.6 ± 2.5 | ||
| Choline | 1.1 ± 0.1 | 270 ± 30 | 23 ± 7 | |
| (2OHE)-EdiMA | 1.5 ± 0.3 | 1500 ± 300 | 173 ± 50 | |
| (2OHE)-diEMA | >10,000 | 24 ± 9 | ||
| BtriMA | 0.47 ± 0.02 | 39 ± 5 | 38 ± 20 | |
| BEdiMA | 67 ± 2 | 27 ± 8 | ||
| BdiEMA | 42 ± 4 | 25 ± 12 | ||
| BtriEA | 26 ± 1 | 0.18 ± 0.07 | ||
| diMPyrr | 1.0 ± 0.1 | 18 ± 3 | 16 ± 3 | |
| EMPyrr | 0.9 ± 0.1 | 50 ± 1 | 46 ± 11 | |
| diEPyrr | 0.38 ± 0.03 | 180 ± 33 | 350 ± 100 | 24 ± 6 |
| diMPip | 0.8 ± 0.1 | 24 ± 2 | 49 ± 10 | |
| EMPip | 350 ± 100 | 31 ± 4 | ||
| diEPip | 200 ± 70 | 5.1 ± 1.9 | ||
| diMHHA | 0.29 ± 0.01 | 183 ± 7 | 15 ± 3.5 | |
| EMHHA | 159 ± 36 | 0.14 ± 0.06 | ||
| diEHHA | 40 ± 8 | 0.23 ± 0.15 | ||
Data for choline and related compounds were obtained at 1 mM plus 10 µM PNU-120596; data for all other compounds were obtained at 100 µM plus 10 µM PNU-120596. Values are for net charge relative to responses evoked by 60 µM ACh applied alone to the same cells.
The series 1 quaternary amines were also tested at a concentration of 100 µM for their antagonist activity on α3β4 and α4β2 receptors (Table 2). Although the large amines did not activate α4β2 receptors, they produced more than 40% inhibition of the α4β2 ACh–evoked response.
TABLE 2.
Effects on other neuronal nAChR
Single-concentration tests were conducted with the compounds applied alone to determine agonism or coapplied with ACh to determine antagonism. Compounds scored as positive for agonism produced responses that were above our limit of detection and >5% the amplitude of ACh controls. Compounds scored as positive (+ or ++) for antagonism reduced ACh responses so that the coapplication responses were <80% (+) or <40% (++) the amplitude of ACh controls. The compounds in the choline series were tested at 1 mM, and all other compounds were tested at 100 µM. Dashes indicate that no activity was detected.
| Compound |
α3β4 |
α4β2 |
||
|---|---|---|---|---|
| Agonism | Antagonism | IC50 | Antagonism | |
| µM | ||||
| tetMA | + | — | + | |
| EtriMA | + | — | + | |
| diEdiMA | — | — | ++ | |
| triEMA | — | + | ++ | |
| TEA | — | + | ++ | |
| Choline | — | — | — | |
| (2OHE)-EdiMA | — | — | + | |
| (2OHE)-diEMA | — | + | ++ | |
| BtriMA | — | + | — | |
| BEdiMA | — | ++ | 124 ± 13 | — |
| BdiEMA | — | ++ | 92 ± 33 | — |
| BtriEA | — | ++ | 39 ± 17 | + |
| diMPyrr | — | — | ++ | |
| EMPyrr | — | + | — | |
| diEPyrr | — | ++ | 59 ± 3 | + |
| diMPip | — | + | ++ | |
| EMPip | — | ++ | 90 ± 4 | — |
| diEPip | — | ++ | 51 ± 1 | + |
| diMHHA | — | ++ | 111 ± 14 | — |
| EMHHA | — | ++ | 55 ± 4 | + |
| diEHHA | — | +++ | 21 ± 1.5 | + |
Structural Analogs of the Series 1 Quaternary Amines.
Since we determined in the series of simple amines that there was a critical size between triEMA and TEA where orthosteric but not allosteric activity was lost, we tested the hypothesis that the same size criteria might apply to quaternary amines of more complex structure. Therefore, we synthesized five additional sets of compounds (Fig. 1), which progressively substituted ethyl for methyl groups onto the key charged nitrogen. The base of the series 2 compounds was choline. The root for the third series was benzyltrimethylamine. The remaining three sets had the charged nitrogen incorporated into 5, 6, or 7 member rings (Fig. 1). The Connolly solvent-excluded volumes for each of these quaternary ammonium compounds were calculated using Chem3D software (PerkinElmer, Waltham, MA) and are provided in Fig. 1.
Choline and Related Compounds.
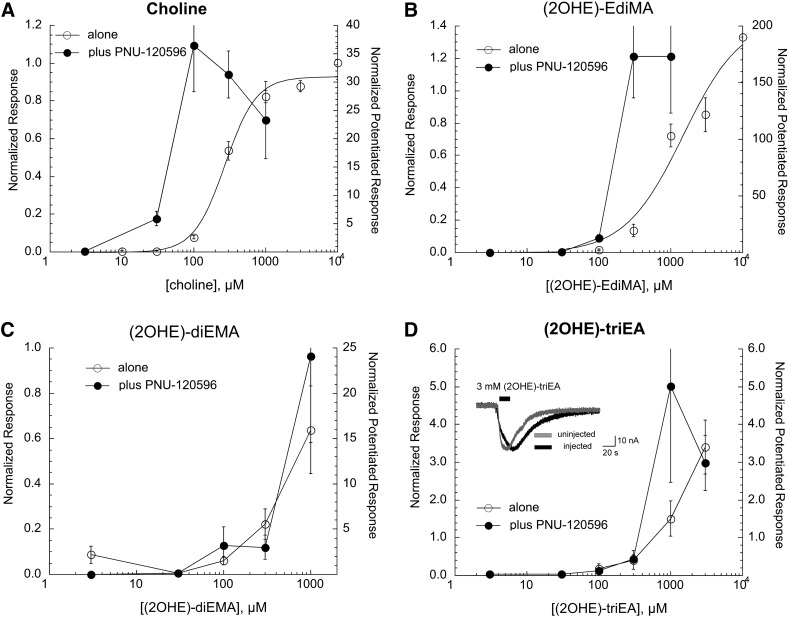
The concentration-response relationships for currents evoked in α7 nAChR–expressing oocytes by choline and related compounds (Fig. 1, series 2) applied alone, or in combination with 10 µM PNU-120596, are shown in Fig. 4. As previously reported (Papke and Porter Papke, 2002), choline is less potent than ACh but equally efficacious. Interestingly, choline appeared more potent when used in combination with PNU-120596. Similar results were obtained with (2OHE)-EdiMA, except that this compound was reduced in potency (Table 1) when applied alone and was more effective than choline at synergizing with the PAM. The diethylmethyl choline analog was further reduced in potency compared with the smaller compounds, with data that could not be fit well to the Hill equation. Responses to (2OHE)-diEMA were significantly increased by coapplication with PNU-120596 without a change in potency. Applications of (2OHE)-triEA at concentrations greater than 100 µM produced responses that were unlike normal α7-mediated responses, which rapidly decay to baseline (see Fig. 2A). The responses associated with the application of (2OHE)-triEA were not significantly increased when the compound was coapplied with PNU-120596 (note that the right and left scales in Fig. 4D are identical). To determine whether the (2OHE)-triEA responses were α7-mediated, (2OHE)-triEA was applied to oocytes that had not been injected with α7 RNA, and similar responses were observed (Fig. 4D, insert). Since (2OHE)-triEA responses were not receptor dependent, data from this compound were excluded from further pharmacophore analyses.
Fig. 4.
Concentration-response studies for the orthosteric (drug applied alone) and allosterically potentiated (drug coapplied with 10 µM PNU-120596) activation of α7 nAChR by choline (A) and related compounds (2OHE)-EdiMA (B), (2OHE)-diEMA (C), and (2OHE)-triEA (D). The left y-axes apply to the net-charge responses recorded when the drugs were applied alone (○), normalized to the responses to 300 µM ACh applied prior to the experimental application. The right y-axes apply to the net-charge responses when the drugs were coapplied with PNU-120596 (●), normalized to average net-charge responses to two prior applications of 60 µM ACh. The responses to the application of the drugs when applied alone in A–C were fit to the Hill equation, and the fit parameters are given in Table 1. Note that when (2OHE)-triEA was applied at concentrations of 300 µM or higher, there were relatively large responses that were slower than normal α7-mediated responses. These responses were not significantly increased when the drug was coapplied with PNU-120596. Similar responses were to (2OHE)-triEA were observed in oocytes that had not been injected with α7 RNA (insert), indicating that these were not α7 nAChR–mediated currents. Each point is the average normalized response of at least four cells (± S.E.M.).
The choline compounds were tested at a concentration of 1 mM on α3β4- and α4β2-expressing cells. They did not activate receptors but, to varying degrees, produced some inhibition of ACh-evoked responses (Table 2).
Benzylic Quaternary Amines.
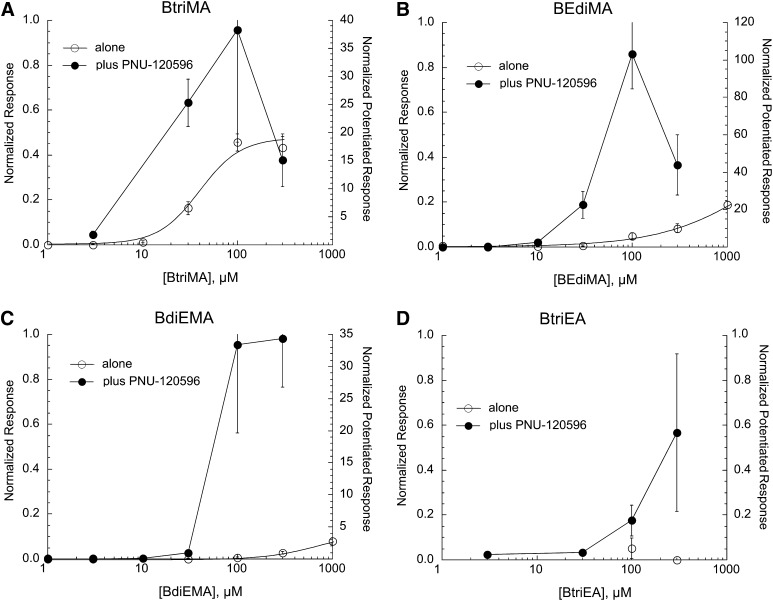
Shown in Fig. 5 are the concentration-response studies for the third series of compounds illustrated in Fig. 1. BtriMA was a reasonably potent and efficacious α7 partial agonist (Table 1) compared with the other compounds in the series. All of the compounds in this series except benzyltriethylammonium (BtriEA) synergized effectively with PNU-120596.
Fig. 5.
Concentration-response studies for the orthosteric (drug applied alone) and allosterically potentiated (drug coapplied with 10 µM PNU-120596) activation of α7 nAChR by the benzylic quaternary amines BtriMA (A), BEdiMA (B), BdiEMA (C), and BtriEA (D). The left y-axes apply to the net-charge responses recorded when the drugs were applied alone (○), normalized to the responses to 300 µM ACh applied prior to the experimental application. The right y-axes apply to the net-charge responses when the drugs were coapplied with PNU-120596 (●), normalized to average net-charge responses to two prior applications of 60 µM ACh. The responses to BtriMA when applied alone (A) were fit to the Hill equation, and the fit parameters are given in Table 1. Each point is the average normalized response of at least four cells (± S.E.M.).
The benzylic compounds were tested at a concentration of 100 µM on α3β4- and α4β2-expressing cells. They did not activate receptors but, to varying degrees, produced some inhibition of ACh-evoked responses (Table 2).
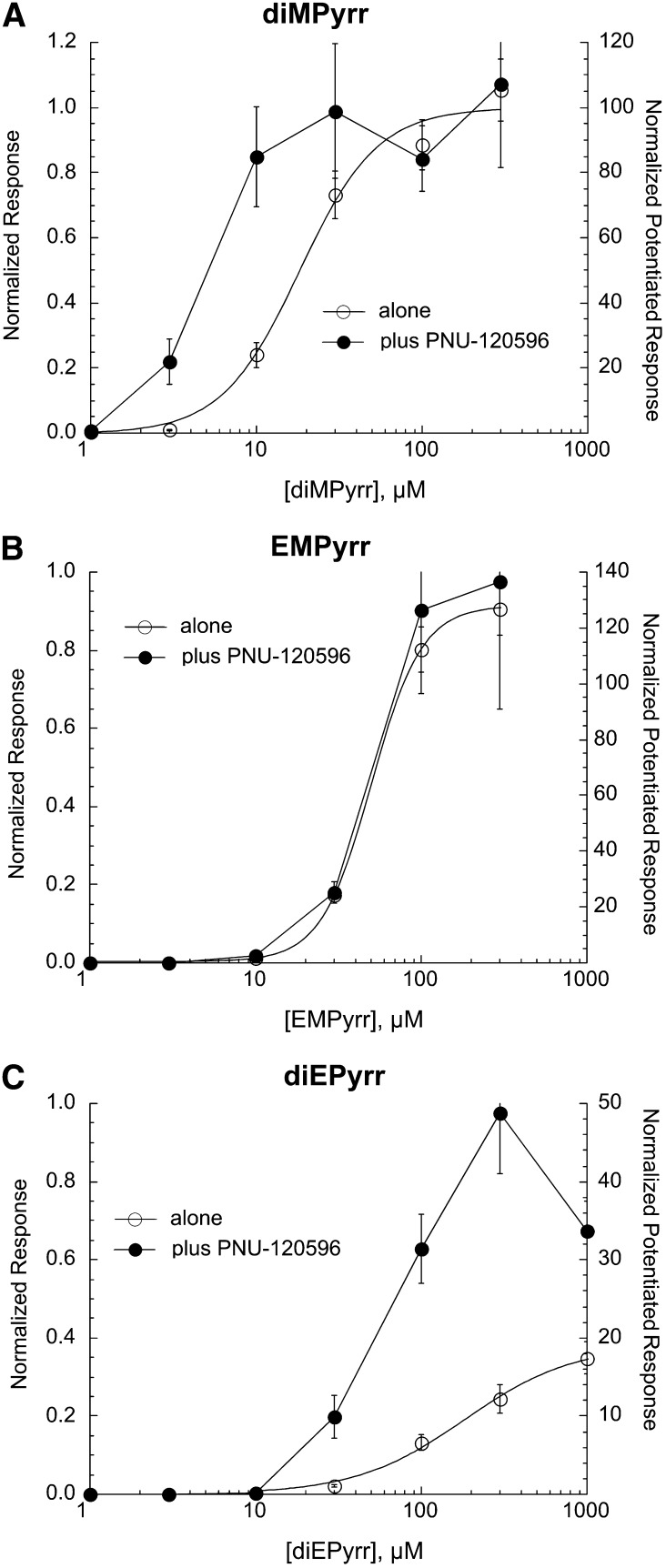
Pyrrolidinium Compounds.
Three compounds were tested with the core nitrogen contained within a pyrrolidinium ring (Fig. 1, series 4). As shown in Fig. 6, the dimethyl and ethylmethyl derivatives were full agonists, with diMPyrr being more potent than EMPyrr (Table 1). These compounds also synergized effectively with PNU-120596, and although the potentiated diMPyrr currents were shifted in potency compared with the orthosteric currents, the potency was similar for orthosteric and allosterically potentiated currents evoked by EMPyrr. The orthosteric and allosteric potency and the efficacy of diEPyrr were reduced compared with the other compounds in this series.
Fig. 6.
Concentration-response studies for the orthosteric (drug applied alone) and allosterically potentiated (drug coapplied with 10 µM PNU-120596) activation of α7 nAChR by the pyrrolidinium compounds diMPyrr (A), EMPyrr (B), and diEPyrr (C). The left y-axes apply to the net-charge responses recorded when the drugs were applied alone (○), normalized to the responses to 300 µM ACh applied prior to the experimental application. The right y-axes apply to the net-charge responses when the drugs were coapplied with PNU-120596 (●), normalized to average net-charge responses to two prior applications of 60 µM ACh. The responses to the application of the drugs when applied alone in A–C were fit to the Hill equation, and the fit parameters are given in Table 1. Each point is the average normalized response of at least four cells (± S.E.M.).
The pyrrolidinium compounds were tested at a concentration of 100 µM on α3β4- and α4β2-expressing cells. They did not activate receptors but, to varying degrees, produced some inhibition of ACh-evoked responses (Table 2).
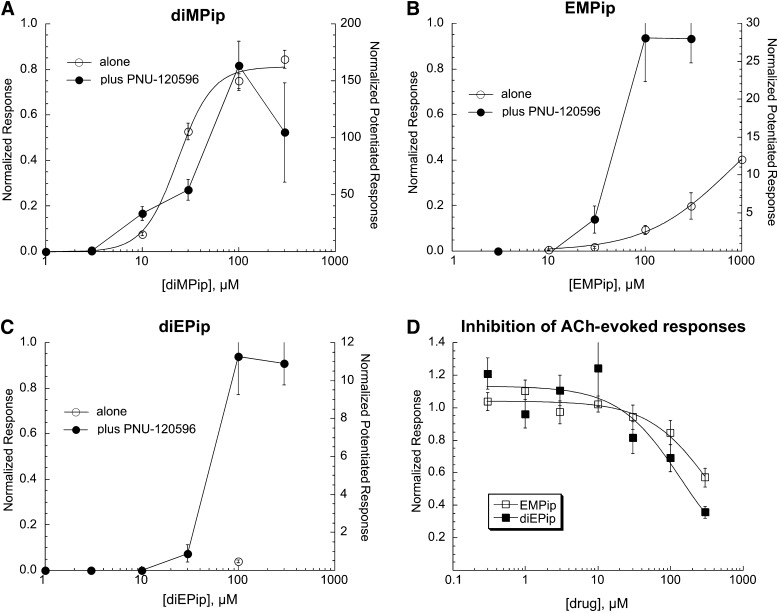
Piperidinium Compounds.
Three compounds were tested with the core nitrogen contained within a piperidinium ring (Fig. 1, series 5). As shown in Fig. 7, only the dimethyl derivative was an efficacious agonist (Table 1). To varying degrees, all three compounds synergized with PNU-120596. Coapplications of EMPip or diEPip with ACh significantly reduced the ACh-evoked responses of α7-expressing cells, as shown in Fig. 7D (IC50 values in Table 1).
Fig. 7.
Concentration-response studies for the orthosteric (drug applied alone) and allosterically potentiated (drug coapplied with 10 µM PNU-120596) activation of α7 nAChR by the piperidinium compounds diMPip (A), EMPip (B), and diEPip (C). The left y-axes apply to the net-charge responses recorded when the drugs were applied alone (○), normalized to the responses to 300 µM ACh applied prior to the experimental application. The right y-axes apply to the net-charge responses when the drugs were coapplied with PNU-120596 (●), normalized to average net-charge responses to two prior applications of 60 µM ACh. The responses to the application of the drugs when applied alone in A–C were fit to the Hill equation, and the fit parameters are given in Table 1. Each point is the average normalized response of at least four cells (± S.E.M.). (D) We tested the potency of EMPip and diEPip as antagonists by coapplying them with 60 µM ACh. The IC50 values are reported in Table 1.
The piperidinium compounds were tested at a concentration of 100 µM on α3β4- and α4β2-expressing cells. They did not activate receptors but, to varying degrees, produced some inhibition of ACh-evoked responses (Table 2).
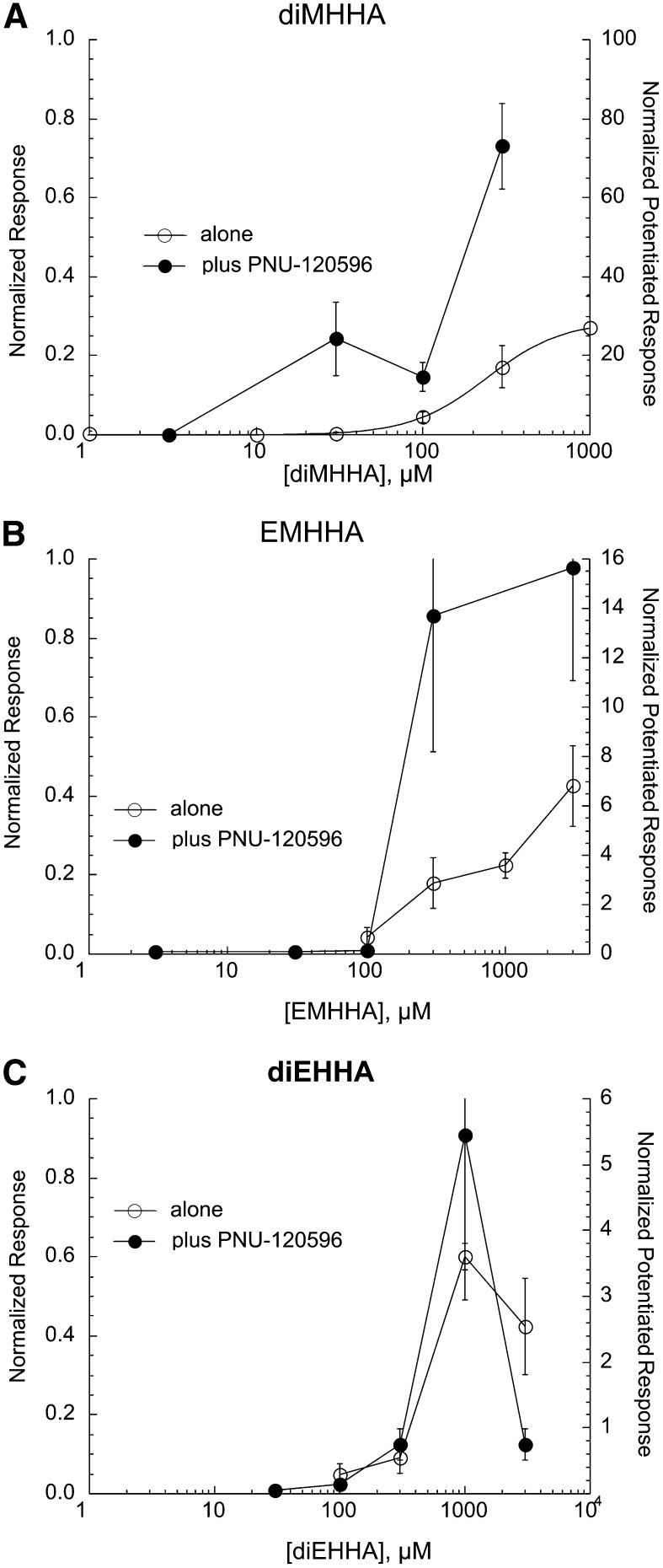
Hexahydroazepinium Compounds.
Three compounds with the core nitrogen contained within a hexahydroazepinium ring were tested (Fig. 1, series 6). As shown in Fig. 8, these compounds only evoked significant responses at concentrations greater than 100 µM when applied alone. The three compounds synergized with PNU-120596 although their efficacy was progressively reduced as the size of the compounds was increased, and only the dimethyl compound showed significant currents in combination with PNU-120596 at any concentration less than 300 µM. Note that although 1 mM diEHHA generated significant responses when coapplied with 10 µM PNU-120596, the responses to 3 mM diEHHA coapplied with 10 µM PNU-120596 were much smaller (Fig. 8C).
Fig. 8.
Concentration-response studies for the orthosteric (drug applied alone) and allosterically potentiated (drug coapplied with 10 µM PNU-120596) activation of α7 nAChR by the hexahydroazepinium compounds diMHHA (A), EMHHA (B), and diEHHA (C). The left y-axes apply to the net-charge responses recorded when the drugs were applied alone (○), normalized to the responses to 300 µM ACh applied prior to the experimental application. The right y-axes apply to the net-charge responses when the drugs were coapplied with PNU-120596 (●), normalized to average net-charge responses to two prior applications of 60 µM ACh. The responses to the application of the drugs when applied alone in A were fit to the Hill equation, and the fit parameters are given in Table 1. Each point is the average normalized response of at least four cells (± S.E.M.).
The hexahydroazepinium compounds were tested at a concentration of 100 µM on α3β4- and α4β2-expressing cells. They did not activate receptors but, to varying degrees, produced some inhibition of ACh-evoked responses (Table 2).
Constraints on Orthosteric and Allosteric Activation of α7 nAChR.
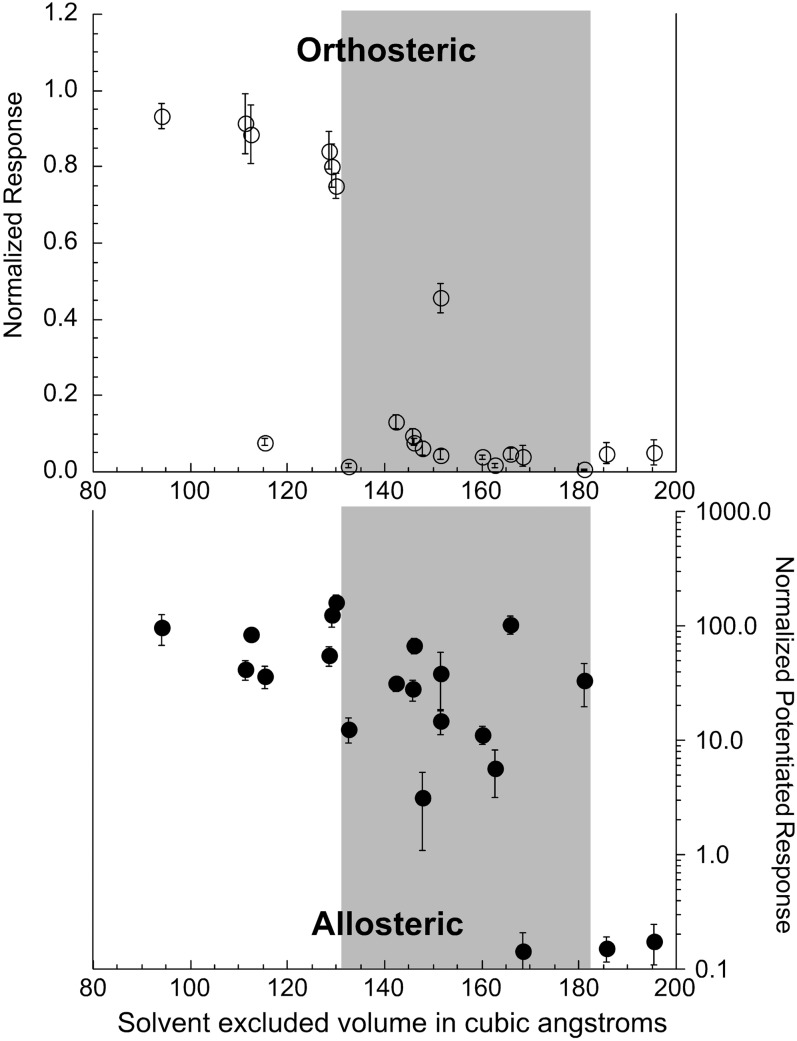
Our analysis of series 1 quaternary amines (Figs. 1–3) identified TEA as a minimal pharmacophore for silent agonism of α7. Some critical differences between triEMA and TEA led to the loss of orthosteric efficacy, yet with the retention of an ability to bind to the receptor and induce a nonconducting conformation that was sensitive to allosteric activation by PNU-120596. This was effectively an endpoint in a trend that was present through the first series of compounds tested, and similar trends were observed in our analyses of the additional compounds studied. Two features of the compounds were systematically altered in six compound sets: size and shielding of electrostatic interactions. With the substitution of ethyl for methyl groups, the compounds become bulkier, and the charge center of the nitrogen becomes shielded and presumably less effective for forming the cation-π bond interactions believed important for receptor activation (Zhong et al., 1998). Although the electrostatic effects will be relatively consistent from one series to the next, each series started out with a different bulk that was then increased. We evaluated the potential relationships between the estimated Connolly solvent-excluded volumes (Fig. 1) and the orthosteric and allosteric activity of all of the compounds by taking a cross section of the data at 100 µM, allowing both potency and efficacy to be reflected in the data (Fig. 9). We found that there was a marked trend for orthosteric activity to fall off for compounds with a volume greater than 130 Å3. The potential for allosteric activation was retained in compounds up to a size 180 Å3. The data therefore suggest that agonists may be tuned for α7 selectivity and preferential induction of PAM-sensitive desensitized states by designing their core pharmacophoric elements to be between 130 and 180 Å3.
Fig. 9.
Relationships between the size (estimated Connolly solvent-excluded volume) of the test compounds and the net-charge responses of α7-expressing cells stimulated by application of the agents at the concentration of 100 µM, normalized to the responses of the same cells to stimulation with 300 µM ACh (upper panel) and the net-charge responses application of 100 µM of the test compounds plus 10 µM PNU-120596 (lower panel) normalized to the responses of the same cells to control applications of 60 µM ACh alone.
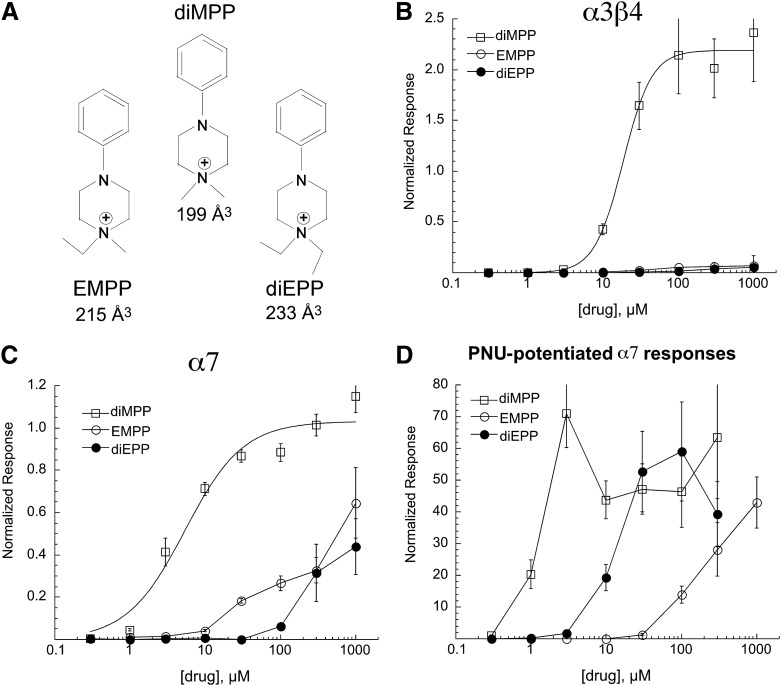
Tuning the Selectivity and Activity Profile of the Ganglionic Agonist diMPP.
diMPP, a ganglionic agonist first described in 1951 (Chen et al., 1951), is more efficacious than ACh for stimulating α3β4 receptors and is also a full agonist for α7 receptors. We created new analogs of diMPP by substituting ethyl groups for one or both of the diMPP methyl groups (Fig. 10A). As expected (Horenstein et al., 2008), the ethyl-substituted diMPP analogs showed no efficacy for α3β4 nAChR. Compared with diMPP, EMPP and, to a greater degree, diEPP had reduced efficacy and potency for activating α7. However, although diEPP was significantly compromised as an orthosteric agonist, it was relatively potent and efficacious in combination with PNU-120596. As shown in Fig. 10, 30 µM diEPP was ineffective when applied alone but activated net-charge responses in combination with PNU-120596 that were 50-fold larger than the 60 µM ACh controls. Note that all of these compounds have estimated solvent-excluded volumes larger than the 180 Å3 cut-off for allosteric activation suggested by the study of the more simple compounds (Fig. 9). However, approximately 70 Å3 of this space is accounted for by the phenyl group of the extended structure, suggesting that this part of the molecules is accommodated in space outside that which is critical for the core activity, such as in the cleft between the subunits, as suggested for the phenyl groups of benzylidene anabaseines (Hibbs et al., 2009). When the estimated volume of the phenyl groups is subtracted, sizes of these compounds fall well within the ranges illustrated in Fig. 9.
Fig. 10.
(A) diMPP and ethyl-substituted analogs. (B) Responses of oocytes expressing human α3β4 receptors to the phenylpiperazinium compounds. Peak current responses were measured and compared with prior control applications of 100 µM ACh. Data were then normalized to ACh maximum by adjusting for the ratio of 100 µM ACh control responses to that of the ACh maximum responses determined in previous experiments (Stokes and Papke, 2012). (C) Responses of oocytes expressing human α7 receptors to the phenylpiperazinium compounds. Net-charge responses were measured and compared with prior control applications of 300 µM ACh, which correspond to the ACh maximum determined in previous experiments (Papke and Porter Papke, 2002). (D) Net-charge responses of oocytes expressing human α7 receptors to the phenylpiperazinium compounds coapplied with 10 µM PNU-120596. Responses were calculated relative to the average of two initial control responses to 60 µM ACh. Each cell was treated with a single concentration of a phenylpiperazinium compound in combination with PNU-120596. Each point is the average normalized response of at least four cells (± S.E.M.).
Channel Block as a Possible Limiting Factor for the Efficacy of Silent Agonists.
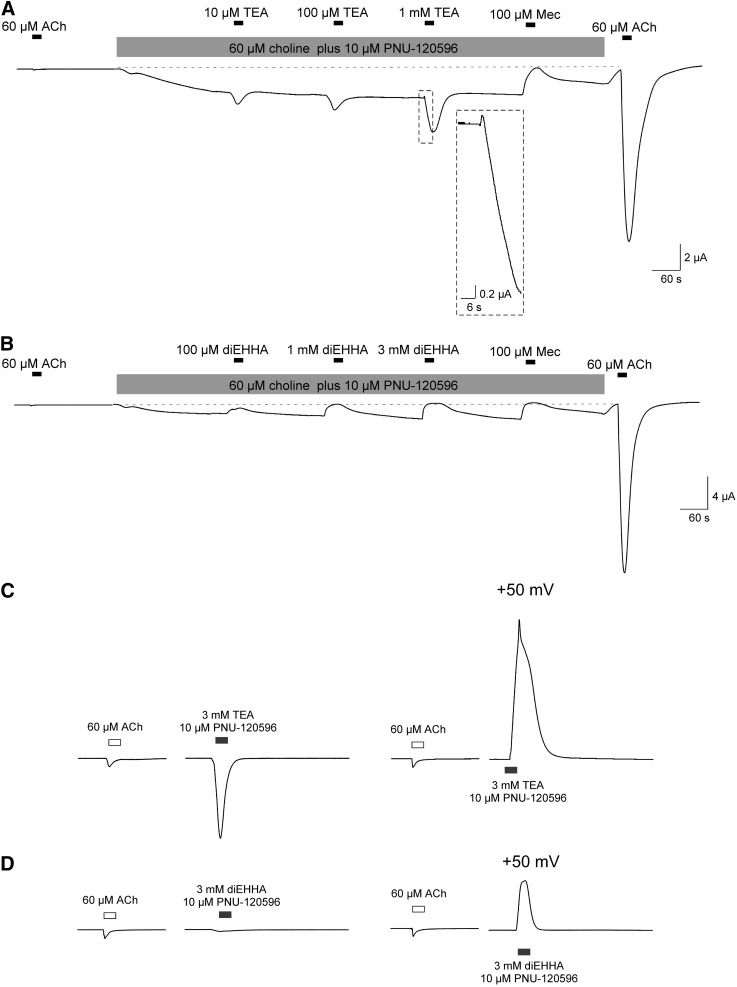
At high concentrations, ACh (Auerbach and Akk, 1998) and other agonists (Carter and Oswald, 1993) block muscle-type nAChR. In addition, TEA is known to be a blocker of voltage-gated potassium channels (Armstrong and Hille, 1972). Therefore, it is likely that, to varying degrees, the small charged molecules used in these experiments could act as channel blockers, and such activity might be a limiting factor, especially when the drugs are used at high concentration. To evaluate the degree to which channel block limited the α7 responses to TEA when coapplied with PNU-120596, we made applications of increasing concentrations of TEA during steady-state activation of receptors by the bath application of 60 µM choline plus 10 µM PNU-120596, a protocol previously used to evaluate the channel-blocking activity of benzylidene anabaseines (Papke et al., 2009). Within 4 minutes of starting the bath application, steady-state currents were established that were 22.1 ± 7.1 (n = 4) times larger than initial peak current responses to 60 µM ACh, and since choline has relatively low potency, the currents remained relatively stable throughout the course of the experiment. As shown in Fig. 11A, applications of TEA produced transient augmentations of the currents. Only when TEA was applied at 1 mM were the augmented currents preceded by a brief decrease in current likely to be associated with channel block. The inhibition produced by TEA was small compared with that produced by 100 µM mecamylamine. Note that once the bath application of 60 µM choline and PNU-120596 was terminated, the channel remained primed for potentiation, so that a follow-up application of 60 µM ACh alone produced a large response. These results with TEA were quite different from those obtained with 100 µM–3 mM diEHHA, which when applied under similar conditions produced only inhibition of the steady-state currents stimulated by 60 µM choline and 10 µM PNU-120596 (Fig. 11B).
Fig. 11.
Effects of silent agonists on steady-state activation in the presence of PNU-120596. To achieve a relatively steady level of α7 receptor activation, 10 µM PNU-120596 was bath applied with 60 µM of the low-potency agonist choline. This procedure produced currents that were increased from the original baseline about 20 times the peak current amplitudes of the initial control response to 60 µM ACh (22.1 ± 7.1 for the experiments represented by trace A and 19.3 ± 4.9 for the experiments represented by trace B, n = 4 in both experiments). (A) A representative trace illustrating the effects of increasing concentrations of TEA, which produced additional increases in the currents to peak amplitudes 12.8 ± 2.7, 9.3 ± 2.8, and 22.3 ± 5.0 times larger than initial ACh controls for 10 µM, 100 µM, and 1 mM TEA, respectively. The application of 100 µM mecamylamine (Mec) reduced the steady-state current to its original baseline. After the bath perfusion was stopped, the primed channels responded to 60 µM ACh with peak currents 82 ± 33 times larger than the original controls. The increased current in response to 1 mM TEA was preceded by small decreases in the steady-state current that was 1.5 ± 0.3 the amplitude of the initial ACh controls. This initial decrease is visible in the insert, which is amplified 5-fold relative to the main trace. (B) A representative trace illustrating the effects of increasing concentrations of diEHHA, which produced progressive decreases in the activation, measured as net charge, to 50 ± 8, 160 ± 16, and 230 ± 35 times larger than initial ACh controls for 100 µM, 1 mM, and 3 mM diEHHA, respectively. The applications of 1 mM diEHHA, 3 mM diEHHA, and 100 µM mecamylamine all reduced the steady-state current to the original baseline, with the mecamylamine effect comparable to that of 3 mM diEHHA (251 ± 32, relative to the ACh control net charge response). After the bath perfusion was stopped, the PNU-120596–primed receptors responded to 60 µM ACh with peak currents 167 ± 27 times larger than the original controls. (C) Representative traces illustrating the effects on holding potential on the potentiated currents evoked by 3 mM TEA. Responses measured were 4.8 ± 0.7 and 9.9 ± 1.4 (peak current and net charge, respectively, n = 7) relative to control responses to ACh alone at the standard holding potential of −60 mV, and the absolute value of the outward currents relative to the ACh controls were 13.1 ± 1.8 and 45 ± 10 (peak current and net charge, respectively, n = 6) at the depolarized potential of +50 mV. (D) Representative traces illustrating the effects of holding potential on the potentiated currents evoked by 3 mM diEHHA. Responses measured were 0.27 ± 0.11 and 0.72 ± 0.21 (peak current and net charge, respectively, n = 7) relative to control responses to ACh alone at the standard holding potential of −60 mV, and the absolute value of the outward currents relative to the ACh controls were 7.1 ± 2.4 and 13 ± 4 (peak current and net charge, respectively, n = 6) at the depolarized potential of +50 mV. The traces in C and D were scaled to have the same amplitude as the original ACh control responses.
We also evaluated the degree to which the responses to TEA and diEHHA coapplied with PNU-120596 could be limited by voltage-dependent channel block. The drugs were coapplied at 3 mM with 10 µM PNU-120596 at a holding potential of +50 mV and compared with data obtained at the normal holding potential of −60 mV. Consistent with the biphasic effects of 1 mM TEA on the steady-state currents, the results suggest that voltage-dependent channel block may be a factor limiting the potentiated responses to TEA. When measured relative to the ACh controls, the PNU-120596–potentiated outward currents at +50 mV had peak amplitudes 2.8 ± 0.5-fold larger and net charge values 4.6 ± 0.8-fold larger than the responses at −60 mV (Fig. 11C). When responses to 3 mM diEHHA plus 10 µM PNU-120596 were compared at the two voltages, the currents were relatively small compared with those evoked by 3 mM TEA, but that effect of voltage was greater. The peak amplitudes were 26 ± 9-fold larger and net charge values were 18 ± 5-fold larger than the responses at −60 mV (Fig. 11D). Although these data suggest that noncompetitive inhibition is an important factor limiting the diEHHA responses at high concentration, when 100 µM diEHHA was coapplied with ACh, its effects were most consistent with competitive activity since the inhibition of the net charge responses to 60 µM ACh (70% ± 2% inhibition, n = 7) was greater (P < 0.05) than the inhibition of 300 µM ACh responses (25% ± 13% inhibition, n = 5); in each case, 100 µM diEHHA had the effect of increasing peak current amplitudes (Papke, 2006). Therefore, although high concentrations of diEHHA clearly have mixed silent agonist/antagonist activity and a large portion of the antagonist activity is due to channel block, intrinsically weak silent agonism may also be a limiting factor.
Discussion
The discovery of the role of α7 nAChRs in vagal-mediated cholinergic anti-inflammatory response (Borovikova et al., 2000; van Westerloo et al., 2006; Pavlov et al., 2007; Rosas-Ballina and Tracey, 2009; Rosas-Ballina et al., 2009) has provided exciting impetus to discover drugs that might be used to treat sepsis, other inflammatory diseases, and various types of inflammation-related pain. This discovery provided a compelling motivation to reconsider our view of α7 and other nAChRs strictly as mediators of transmembrane signals relying on channel-mediated ion flux. The non-neuronal cells that mediate α7’s control of inflammation have not been shown to generate α7-mediated currents; moreover, some α7-targeting ligands that can effectively control inflammation have little or no efficacy as ion channel activators (van Maanen et al., 2009; Thomsen and Mikkelsen, 2012; Clark et al., 2014).
Even the α7 agonists that are most efficacious for producing channel activation produce only brief and infrequent ion channel currents and are far more effective at inducing and maintaining the receptors in nonconducting states, which have traditionally been dismissed as desensitized and functionally unimportant (Williams et al., 2011a). The time has come to discard the prejudice that the ligand-bound nonconducting states of nAChRs are all functionally unimportant. Proteomic analyses have indicated a large number of intracellular protein partners for α7 (Paulo et al., 2009), many of which are mediators of signal transduction. It has been proposed that desensitization is associated with an uncoupling of the amino terminal ligand binding domain from the pore-lining second transmembrane domain (Zhang et al., 2013). This effect may be specifically affected by PAM binding consistent with the proposed binding site for PNU-120596 being associated with a transmembrane site (Young et al., 2008). Just as conformational changes promoted by ligand binding spread through the transmembrane domains, they must also pass through to the intracellular domain and likely regulate signal transduction processes in both neuronal and non-neuronal cells.
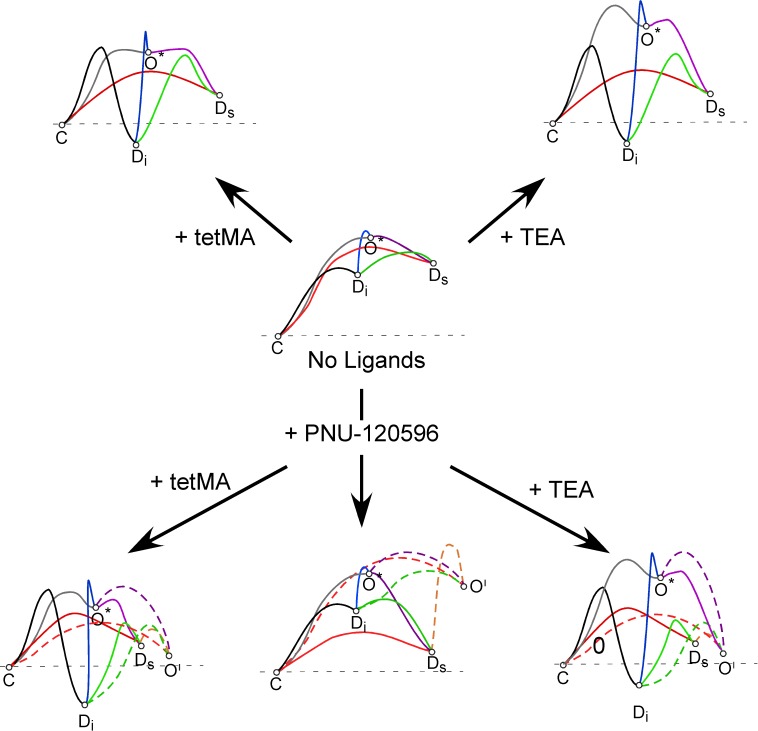
Our ability to probe the conformational landscape of α7 receptors has been greatly aided by the discovery of α7-selective PAMs. The selectivity of these PAMs for α7 appears to arise from their specific effects on the class of desensitized states that are unique to α7 and are induced at levels of agonist occupancy higher than those that most effectively lead to ion channel activation (Williams et al., 2011a). Based on the use of highly effective PAMs, such as PNU-120596, we have been able to use ion channel currents to detect and distinguish two classes of agonist-induced states that are nonconducting in the absence of the PAM. One functionally unique class of these desensitized states (Ds), is destabilized by the PAM and can convert to a novel conducting state, whereas the other class (Di) is PAM insensitive. Figure 12 provides a graphic representation of the thermodynamic relationship between the conformation states of α7 as regulated by the binding of agonists, silent agonists, and PAMs (Williams et al., 2011a; for further discussion of such models, see Williams et al., 2011b; Papke, 2014). Receptors that have bound neither orthosteric nor allosteric ligands are most stable in the resting closed state (C). Upon binding an efficacious agonist, such as tetMA, the energy barrier to the open state (O*) is sufficiently reduced so that some channels may open. However, for α7, this remains a low-probability event (Williams et al., 2011a) and receptors are more likely to enter Ds than to open. Upon binding a silent agonist such as TEA, the barrier to the open state remains high so that the receptors are more likely to enter one of the desensitized states than to open. However, the binding of PNU-120596 couples the Ds state to a PAM-dependent open state (O′) (Williams et al., 2011a), allowing both tetMA and TEA to stimulate bursts of channel activation.
Fig. 12.
Hypothetical energy landscapes for the conformational changes of α7 nAChR as affected by the binding the efficacious agonist tetMA or the silent agonist TEA, with or without the effects of PNU-120596. These models are based on our previous studies that identified the existence of two forms of α7 desensitization (Ds and Di), in addition to a resting closed state (C), a low-probability open state (O*), and the existence of a PAM-dependent open state (O′) that can be coupled to the Ds state. The models illustrated are those proposed for intermediate levels of ligand binding at both the orthosteric (agonist) and allosteric (modulator) sites, conditions that most effectively promote channel opening (Williams et al., 2011a). For each landscape, the absolute free energy of the various states is represented by the vertical displacement of the states, whereas the rate constants for transitions between the states would be inversely related to the height of the energy barriers, represented by the lines connecting the states (lower barriers predicting faster rate constants). In the absence of ligands, the C state is most stable and transitions from that state are thermodynamically unlikely. Once drugs are applied, the thermodynamic landscapes are changed and since all channels would be predicted to be in the C state prior to the drug applications, there is an initial phase of conformational change during which channels are to some degree synchronized as they move with highest probability from the C state to whatever state is connected to the C state by the lowest energy barrier. Note that in the absence of the PAM, the α7 channel opening probability is low, even for an efficacious agonist like ACh (Williams et al., 2012).
Our data indicate that although it is a common feature for all efficacious agonists to induce both Ds and Di to varying degrees depending on their applied concentration, ligands with such low efficacy that they are functional antagonists of normal channel activation can, in the case of compounds we classify as silent agonists, induce the Ds state and only appear efficacious when coapplied with a PAM or applied to receptors primed by previous exposure to PAMs. One of the first such compounds to be identified was NS-6740 (Thomsen and Mikkelsen, 2012), and we have also characterized the compounds KC-1 (5′-phenyl-3,4,5,6-tetrahydro-2,3′-bipyridine) (Chojnacka et al., 2013), R-47 [(R)-N-(4-methoxyphenyl)-2-((pyridin-3-yloxy)methyl)piperazine-1-carboxamide] (Clark et al., 2014), also known as CTI-15072 (van Maanen et al., 2009), as silent agonists. These compounds are large and structurally diverse, so here we start with the smallest α7 agonists to find the most rudimentary structure capable of silent agonism, which is that of TEA.
Through our systematic analysis of additional quaternary ammonium compounds, we identified unique size constraints on the effectiveness of ligands as orthosteric activators or as silent agonists, such that ligands with estimated solvent-excluded volumes around the cationic center between 130 and 180 Å3 show a tendency toward silent agonism. Although this correlation with molecular volume appears to be valid, the underlying molecular interactions remain to be elucidated. In the simplest way, the larger volume could induce and or preclude certain conformational configurations of the complex. Furthermore, when the central ammonium core is surrounded by increased volume associated with larger alkyl groups, the effective charge density at the alkyl ammonium group is lower, potentially lessening cation-π interactions, which could also have functional significance.
Previous studies of an agonist series binding to and activating the muscle-type nAChRs suggested that the steric bulk of an experimental agonist affected association and disassociation, whereas the ionic character of ligand with comparable size more specifically affected opening and closing rates (Papke et al., 1988). With the present set of compounds, both size and charge density are changed in parallel, so it would not be possible, even with single-channel data, to separate these factors. An alternative hypothesis is that binding of the PAM opens up the orthosteric binding site, allowing access to larger ligands. However, the silent agonist BdiEMA acts as an antagonist of orthosteric activation (Table 1) with approximately the same potency with which it generates currents in coapplication with PNU-120596 (Fig. 5), suggesting that access to the orthosteric binding site is similar in the absence and presence of the PAM.
We previously showed that the structural motifs that account for the α7 selectivity of specific agonists can be installed onto other nonselective agonists to make new α7-selective drugs (Horenstein et al., 2008). Our identification of triEA as a minimal element for being an α7 silent agonist encouraged us to generate diEPP, a modified form of the highly efficacious α3β4 agonist diMPP, that lost all activity for α3β4, showed reduced orthosteric activity for α7, and yet had good allosteric activity for α7. This indication that the minimal silent agonist pharmacophore might be moved on to other α7-selective agonists, such as quinuclidines or benzylidene anabaseines, large molecules with structural elements that are accommodated in the extended binding site, provides a promising new idea for potential drug development. Since it has been shown that silent agonists are promising agents for signal transduction, the development of such new compounds has the potential for more effective therapeutics selectively targeted to the roles of α7 in non-neuronal cells.
We have used PNU-120596 to distinguish silent agonists from simple antagonists of orthosteric activation, and we hypothesize that even in the absence of the PAM, receptors in the Ds and/or Di states are potential mediators of signal transduction. Some of the best support for this hypothesis comes from the documented anti-inflammatory activity of NS-6740 (Thomsen and Mikkelsen, 2012).
It is interesting to note the qualitative differences in the concentration-response functions for the PNU-120596–potentiated currents of the various agents. Although there are plateaus in the response data for some agents such as diEdiMA, TEA, diMPyrr, EMPyrr, EMPip, diEPip, and diMPP, other agents such as BEdiMA and tetMA show well potentiated responses over a very narrow range of concentration. These data suggest that the dynamic interconversion of receptors between the Ds state, associated with the potentiated currents, and the Di state, which limits such responses, varies depending on the specific ligand. If, in fact, these nonconducting states can be associated with signal transduction, it is unclear whether such signaling would preferentially arise from induction of Ds or Di or some other state that is independent of what can be detected by the use of the PAM.
The question also remains whether PAMs themselves induce novel conformational states that may also be effective modulators of signal transduction in non-neuronal cells. Some recent studies (Freitas et al., 2013) demonstrating analgetic effects of PNU-120596 suggest that this may be the case. However, the ubiquitous presence of choline in all in vivo and most in vitro experiments makes it impossible to conclude that such effects are due to the activity of the PAM alone. Therefore, the challenge remains to better define the relationship between α7 conformational states and the role of allosteric modulation in signal transduction by the receptor that does not rely on channel-mediated ionic currents.
Supplementary Material
Acknowledgments
The authors thank Clare Stokes, Shehd Abdullah Abbas Al Rubaiy, Matthew R. Kimbrell, Lu Wenchi Corrie, and Christopher W. Kinter for conducting the OpusXpress experiments.
Abbreviations
- (2OHE)-diEMA
(2-hydroxyethyl)-diethylmethylammonium iodide
- (2OHE)-EdiMA
(2-hydroxyethyl)-ethyldimethylammonium iodide
- (2OHE)-triEA
(2-hydroxyethyl)-triethylammonium iodide
- ACh
acetylcholine
- BdiEMA
benzyldiethylmethylammonium iodide
- BEdiMA
benzylethyldimethylammonium iodide
- BtriEA
benzyltriethylammonium
- BtriMA
benzyltrimethylammonium iodide
- Di
PAM-insensitive closed state
- diEdiMA
diethyldimethylammonium
- diEHHA
diethylhexahydroazepinium iodide
- diEPip
diethylpiperidinium iodide
- diEPP
diethylphenylpiperazinium iodide
- diEPyrr
diethylpyrrolidinium iodide
- diMHHA
dimethylhexahydroazepinium iodide
- diMPip
dimethylpiperidinium iodide
- diMPP
dimethylphenylpiperazinium
- diMPyrr
dimethylpyrrolidinium iodide
- DMSO
dimethylsulfoxide
- Ds
PAM-sensitive closed state
- EMHHA
ethylmethylhexahydroazepinium iodide
- EMPip
ethylmethylpiperidinium iodide
- EMPP
ethylmethylphenylpiperazinium iodide
- EMPyrr
ethylmethylpyrrolidinium iodide
- EtriMA
ethyltrimethylammonium
- KC-1
5′-phenyl-3,4,5,6-tetrahydro-2,3′-bipyridine
- nAChR
nicotinic acetylcholine receptor
- PAM
positive allosteric modulator
- PNU-120596
N-(5-chloro-2,4-dimethoxyphenyl)-N′-(5-methyl-3-isoxazolyl)-urea
- R-47
(R)-N-(4-methoxyphenyl)-2-((pyridin-3-yloxy)methyl)piperazine-1-carboxamide
- tetMA
tetramethylammonium
- triEMA
triethylmethylammonium
Authorship Contributions
Participated in research design: Papke, Chojnacka, Horenstein.
Conducted experiments: Chojnacka.
Contributed new reagents or analytic tools: Chojnacka, Horenstein.
Performed data analysis: Chojnacka, Papke.
Wrote or contributed to the writing of the manuscript: Papke, Chojnacka, Horenstein.
Footnotes
This research was supported by the National Institutes of Health National Institute of General Medical Sciences [Grant R01-GM57481].
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Armstrong CM, Hille B. (1972) The inner quaternary ammonium ion receptor in potassium channels of the node of Ranvier. J Gen Physiol 59:388–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach A, Akk G. (1998) Desensitization of mouse nicotinic acetylcholine receptor channels. A two-gate mechanism. J Gen Physiol 112:181–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. (2000) Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405:458–462 [DOI] [PubMed] [Google Scholar]
- Calas M, Ancelin ML, Cordina G, Portefaix P, Piquet G, Vidal-Sailhan V, Vial H. (2000) Antimalarial activity of compounds interfering with Plasmodium falciparum phospholipid metabolism: comparison between mono- and bisquaternary ammonium salts. J Med Chem 43:505–516 [DOI] [PubMed] [Google Scholar]
- Carter AA, Oswald RE. (1993) Channel blocking properties of a series of nicotinic cholinergic agonists. Biophys J 65:840–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Portman R, Wickel A. (1951) Pharmacology of 1,1-dimethyl-4-phenylpiperazinium iodide, a ganglion stimulating agent. J Pharmacol Exp Ther 103:330–336 [PubMed] [Google Scholar]
- Chojnacka K, Papke RL, Horenstein NA. (2013) Synthesis and evaluation of a conditionally-silent agonist for the α7 nicotinic acetylcholine receptor. Bioorg Med Chem Lett 23:4145–4149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RB, Lamppu D, Libertine L, McDonough A, Kumar A, LaRosa G, Rush R, Elbaum D. (2014) Discovery of novel 2-((pyridin-3-yloxy)methyl)piperazines as α7 nicotinic acetylcholine receptor modulators for the treatment of inflammatory disorders. J Med Chem 57:3966–3983 [DOI] [PubMed] [Google Scholar]
- Clarke HT, Gillespie HB, Weisshaus SZ. (1933) Action of formaldehyde on amines and amino acids. J Am Chem Soc 55:4571–4587 [Google Scholar]
- de Jonge WJ, Ulloa L. (2007) The alpha7 nicotinic acetylcholine receptor as a pharmacological target for inflammation. Br J Pharmacol 151:915–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschweiler W. (1905) Ersatz von an Stickstoff gebundenen Wasserstoffatomen durch die Methylgruppe mit Hulfe von formaldehyde. Ber Dtsch Chem Ges 38:880–882 [Google Scholar]
- Freitas K, Carroll FI, Damaj MI. (2013) The antinociceptive effects of nicotinic receptors α7-positive allosteric modulators in murine acute and tonic pain models. J Pharmacol Exp Ther 344:264–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamal-Eldin MA, Macartney DH. (2013) Cucurbit[7]uril host-guest complexes and [2]pseudorotaxanes with N-methylpiperidinium, N-methylpyrrolidinium, and N-methylmorpholinium cations in aqueous solution. Org Biomol Chem 11:1234–1241 [DOI] [PubMed] [Google Scholar]
- Green KD, Fridman M, Garneau-Tsodikova S. (2009) hChAT: a tool for the chemoenzymatic generation of potential acetyl/butyrylcholinesterase inhibitors. ChemBioChem 10:2191–2194 [DOI] [PubMed] [Google Scholar]
- Halevi S, Yassin L, Eshel M, Sala F, Sala S, Criado M, Treinin M. (2003) Conservation within the RIC-3 gene family. Effectors of mammalian nicotinic acetylcholine receptor expression. J Biol Chem 278:34411–34417 [DOI] [PubMed] [Google Scholar]
- Hibbs RE, Sulzenbacher G, Shi J, Talley TT, Conrod S, Kem WR, Taylor P, Marchot P, Bourne Y. (2009) Structural determinants for interaction of partial agonists with acetylcholine binding protein and neuronal alpha7 nicotinic acetylcholine receptor. EMBO J 28:3040–3051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horenstein NA, Leonik FM, Papke RL. (2008) Multiple pharmacophores for the selective activation of nicotinic alpha7-type acetylcholine receptors. Mol Pharmacol 74:1496–1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hünig S, Baron W. (1957) Abbau Quartarer Ammoniumsalze mit Athanolamin. Chem Ber 90:395–402 [Google Scholar]
- Ito K, Nagase K, Morohashi N, Ohba Y. (2005) Interaction between quaternary ammonium ions and dipeptides: positive anion allosteric effect. Chem Pharm Bull (Tokyo) 53:90–94 [DOI] [PubMed] [Google Scholar]
- Kabbani N, Nordman JC, Corgiat BA, Veltri DP, Shehu A, Seymour VA, Adams DJ. (2013) Are nicotinic acetylcholine receptors coupled to G proteins? BioEssays 35:1025–1034 [DOI] [PubMed] [Google Scholar]
- Kasuga K, Kato T, Kabata N, Handa M. (1969) Cycloaddition of carbon dioxide to 1,2-epoxypropane catalyzed by tetra-t-butylphthalocyaninatoaluminium(III) hydroxide. Bull Chem Soc Jpn 69:2885–2888 [Google Scholar]
- Lowe BM, Rendall HM. (1971) Dilute aqueous solutions of unsymmetrical quaternary ammonium iodides. Part 1.—Viscosity and density measurements. Trans Faraday Soc 67:2318–2327 [Google Scholar]
- MacFarlane DR, Meakin P, Sun J, Amini N, Forsyth M. (1999) Pyrrolidinium imides: a new family of molten salts and conductive plastic crystal phases. J Phys Chem B 103:4164–4170 [Google Scholar]
- Papke RL. (2006) Estimation of both the potency and efficacy of alpha7 nAChR agonists from single-concentration responses. Life Sci 78:2812–2819 [DOI] [PubMed] [Google Scholar]
- Papke RL. (2010) Tricks of perspective: insights and limitations to the study of macroscopic currents for the analysis of nAChR activation and desensitization. J Mol Neurosci 40:77–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL. (2014) Merging old and new perspectives on nicotinic acetylcholine receptors. Biochem Pharmacol 89:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Bencherif M, Lippiello P. (1996) An evaluation of neuronal nicotinic acetylcholine receptor activation by quaternary nitrogen compounds indicates that choline is selective for the α 7 subtype. Neurosci Lett 213:201–204 [DOI] [PubMed] [Google Scholar]
- Papke RL, Kem WR, Soti F, López-Hernández GY, Horenstein NA. (2009) Activation and desensitization of nicotinic alpha7-type acetylcholine receptors by benzylidene anabaseines and nicotine. J Pharmacol Exp Ther 329:791–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Millhauser G, Lieberman Z, Oswald RE. (1988) Relationships of agonist properties to the single channel kinetics of nicotinic acetylcholine receptors. Biophys J 53:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Porter Papke JK. (2002) Comparative pharmacology of rat and human alpha7 nAChR conducted with net charge analysis. Br J Pharmacol 137:49–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Stokes C. (2010) Working with OpusXpress: methods for high volume oocyte experiments. Methods 51:121–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulo JA, Brucker WJ, Hawrot E. (2009) Proteomic analysis of an alpha7 nicotinic acetylcholine receptor interactome. J Proteome Res 8:1849–1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov VA, Ochani M, Yang LH, Gallowitsch-Puerta M, Ochani K, Lin X, Levi J, Parrish WR, Rosas-Ballina M, Czura CJ, et al. (2007) Selective alpha7-nicotinic acetylcholine receptor agonist GTS-21 improves survival in murine endotoxemia and severe sepsis. Crit Care Med 35:1139–1144 [DOI] [PubMed] [Google Scholar]
- Pesti K, Szabo AK, Mike A, Vizi ES. (2014) Kinetic properties and open probability of α7 nicotinic acetylcholine receptors. Neuropharmacology 81:101–115 [DOI] [PubMed] [Google Scholar]
- Rosas-Ballina M, Goldstein RS, Gallowitsch-Puerta M, Yang L, Valdés-Ferrer SI, Patel NB, Chavan S, Al-Abed Y, Yang H, Tracey KJ. (2009) The selective alpha7 agonist GTS-21 attenuates cytokine production in human whole blood and human monocytes activated by ligands for TLR2, TLR3, TLR4, TLR9, and RAGE. Mol Med 15:195–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas-Ballina M, Tracey KJ. (2009) Cholinergic control of inflammation. J Intern Med 265:663–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short JH, Biermacher U. (1962) Sympathetic nervous system blocking agents. Investigation of ethyl-, hydroxyethyl-, vinyloxyethyl-, and propargyl-benzyldimethylammonium halides and related compounds. J Pharm Sci 51:881–884 [DOI] [PubMed] [Google Scholar]
- Skok MV. (2009) Editorial: To channel or not to channel? Functioning of nicotinic acetylcholine receptors in leukocytes. J Leukoc Biol 86:1–3 [DOI] [PubMed] [Google Scholar]
- Stokes C, Papke RL. (2012) Use of an α3β4 nicotinic acetylcholine receptor subunit concatamer to characterize ganglionic receptor subtypes with specific subunit composition reveals species-specific pharmacologic properties. Neuropharmacology 63:538–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen MS, Mikkelsen JD. (2012) The α7 nicotinic acetylcholine receptor ligands methyllycaconitine, NS6740 and GTS-21 reduce lipopolysaccharide-induced TNF-α release from microglia. J Neuroimmunol 251:65–72 [DOI] [PubMed] [Google Scholar]
- van Maanen MA, Papke RL, Koepke J, Bevaart L, Clark R, Lamppu D, Vervoordeldonk MJ, LaRosa GJ, and Tak PP (2009) Therapeutic effect of stimulating the nicotinic acetylcholine receptor in the collagen-induced model of rheumatoid arthritis: a role for ion channel activity and penetration of the central nervous system, in Cholinergic Nervous System as Therapeutic Approach for the Treatment of Arthritis. pp 77–97. Doctoral dissertation, University of Amsterdam, Amsterdam, The Netherlands. [Google Scholar]
- van Westerloo DJ, Giebelen IA, Florquin S, Bruno MJ, Larosa GJ, Ulloa L, Tracey KJ, van der Poll T. (2006) The vagus nerve and nicotinic receptors modulate experimental pancreatitis severity in mice. Gastroenterology 130:1822–1830 [DOI] [PubMed] [Google Scholar]
- von Braun J, Buchman ER. (1931) Über den Zerfall quartärer Ammoniumhydroxyde (V. Mitteil.). Chem Ber 64:2610–2617 [Google Scholar]
- Wempe MF. (2001) Quaternary ammonium ions can externally block voltage-gated K+ channels. Establishing a theoretical and experimental model that predicts KDs and the selectivity of K+ over Na+ ions. J Mol Struct 562:63–78 [Google Scholar]
- Williams DK, Peng C, Kimbrell MR, Papke RL. (2012) Intrinsically low open probability of α7 nicotinic acetylcholine receptors can be overcome by positive allosteric modulation and serum factors leading to the generation of excitotoxic currents at physiological temperatures. Mol Pharmacol 82:746–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DK, Wang J, Papke RL. (2011a) Investigation of the molecular mechanism of the α7 nicotinic acetylcholine receptor positive allosteric modulator PNU-120596 provides evidence for two distinct desensitized states. Mol Pharmacol 80:1013–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DK, Wang J, Papke RL. (2011b) Positive allosteric modulators as an approach to nicotinic acetylcholine receptor-targeted therapeutics: advantages and limitations. Biochem Pharmacol 82:915–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young GT, Zwart R, Walker AS, Sher E, Millar NS. (2008) Potentiation of alpha7 nicotinic acetylcholine receptors via an allosteric transmembrane site. Proc Natl Acad Sci USA 105:14686–14691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Xue F, Liu Y, Yang H, Wang X. (2013) The structural mechanism of the Cys-loop receptor desensitization. Mol Neurobiol 48:97–108 [DOI] [PubMed] [Google Scholar]
- Zhong W, Gallivan JP, Zhang Y, Li L, Lester HA, Dougherty DA. (1998) From ab initio quantum mechanics to molecular neurobiology: a cation-pi binding site in the nicotinic receptor. Proc Natl Acad Sci USA 95:12088–12093 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.