Abstract
In cultured renal cells and isolated perfused kidneys, extracellular guanosine augments extracellular adenosine and inosine (the major renal metabolite of adenosine) levels by altering the extracellular disposition of these purines. The present study addressed whether this “guanosine-adenosine mechanism” exists in vivo. In rats (n = 15), intravenous infusions of adenosine (1 µmol/kg per minute) decreased mean arterial blood pressure (MABP) from 114 ± 4 to 83 ± 5 mm Hg, heart rate (HR) from 368 ± 11 to 323 ± 9 beats/min), and renal blood flow (RBF) from 6.2 ± 0.5 to 5.3 ± 0.6 ml/min). In rats (n = 15) pretreated with intravenous guanosine (10 µmol/kg per minute), intravenous adenosine (1 µmol/kg per minute) decreased MABP (from 109 ± 4 to 58 ± 5 mm Hg), HR (from 401 ± 10 to 264 ± 20 beats/min), and RBF (from 6.2 ± 0.7 to 1.7 ± 0.3). Two-factor analysis of variance (2F-ANOVA) revealed a significant interaction (P < 0.0001) between guanosine and adenosine for MABP, HR, and RBF. In control rats, the urinary excretion rate of endogenous inosine was 211 ± 103 ng/30 minutes (n = 9); however, in rats treated with intravenous guanosine (10 µmol/kg per minute), the excretion rate of inosine was 1995 ± 300 ng/30 minutes (n = 12; P < 0.0001 versus controls). Because adenosine inhibits inflammatory cytokine production, we also examined the effects of intravenous guanosine on endotoxemia-induced increases in tumor necrosis factor-α (TNF-α). In control rats (n = 7), lipopolysaccharide (LPS; Escherichia coli 026:B6 endotoxin; 30 mg/kg) increased plasma TNF-α from 164 ± 56 to 4082 ± 730 pg/ml, whereas in rats pretreated with intravenous guanosine (10 µmol/kg per minute; n = 6), LPS increased plasma TNF-α from 121 ± 45 to 1821 ± 413 pg/ml (2F-ANOVA interaction effect, P = 0.0022). We conclude that the guanosine-adenosine mechanism exists in vivo and that guanosine may be a useful therapeutic for reducing inflammation.
Introduction
Because extracellular adenosine stimulates cell-surface adenosine receptors (A1, A2A, A2B, and A3) and thereby modulates cell function (Fredholm et al., 2011; Grenz et al., 2011), it is critical to elucidate the mechanisms regulating adenosine levels in the extracellular compartment of various organ systems. In this regard, adenosine influences many aspects of renal function (Vallon et al., 2006), and therefore it is particularly important to understand the determinants of extracellular adenosine levels in the kidney.
Our recent in vitro (cell culture) investigations (Jackson et al., 2013; Jackson and Gillespie, 2013) demonstrate that in many renal cell types, including preglomerular vascular smooth muscle cells, glomerular mesangial cells, and kidney epithelial cells, extracellular guanosine slows the removal of adenosine from the extracellular environment. We call this interaction the guanosine-adenosine mechanism and propose that this is an indirect mechanism by which extracellular guanosine can contribute to cell signaling without specific cell-surface G protein–coupled guanosine receptors, which may or may not exist (Traversa et al., 2003; Thauerer et al., 2012).
Our cell culture studies, however, left answered the question as to whether the guanosine-adenosine mechanism occurs in intact organs such as the kidney. To address this question, we recently performed experiments in isolated, perfused mouse kidneys (Jackson et al., 2014). These experiments show that the levels of guanosine and adenosine in the renal venous perfusate and in kidney tissue are highly correlated, that guanosine reduces the renal clearance of exogenous adenosine, and that inhibition of purine nucleoside phosphorylase (PNPase; an enzyme that metabolizes guanosine to guanine) increases the release of both guanosine and adenosine by the kidneys. These findings further support the conclusion that in the kidney guanosine modulates adenosine levels.
Although evidence for the guanosine-adenosine mechanism is accumulating, a critically important question is whether guanosine enhances the effects of adenosine on the cardiovascular and renal systems in vivo, as opposed to this mechanism being an artifact of in vitro experiments or merely a biochemical curiosity without functional significance. Therefore, one goal of the present study was to determine whether the guanosine-adenosine interaction can be observed in vivo.
Adenosine is an anti-inflammatory agent that inhibits both the innate and adaptive arms of the immune system (Hasko et al., 2007; Ohta and Sitkovsky, 2009; Colgan et al., 2013; Longhi et al., 2013; Poth et al., 2013). Because the present results indicate that guanosine can enhance adenosine signaling in vivo, a second objective of the present study was to test whether exogenous guanosine has potential as a pharmacologic agent for the management of inflammatory states.
Materials and Methods
Chemicals.
Adenosine, guanosine, and lipopolysaccharide (LPS; Escherichia coli 026:B6 endotoxin) were from Sigma-Aldrich (St. Louis, MO).
Animals.
This study used male Sprague-Dawley rats (Charles River, Wilmington, MA) that were between 15 and 20 weeks of age. The Institutional Animal Care and Use Committee approved all procedures. The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
Guanosine-Adenosine Interaction.
Thirty rats were anesthetized (Inactin, 90 mg/kg i.p.; Sigma-Aldrich), and body temperature was monitored with a rectal probe and stabilized at 37°C using an isothermal pad and a heat lamp. A cannula [polyethylene (PE)-240] was inserted into the trachea to maintain a patent airway. The left femoral artery was cannulated (PE-50), and mean arterial blood pressure (MABP) and heart rate (HR) were monitored using a digital blood pressure analyzer (Micro-Med, Inc., Louisville, KY). Next, two PE-50 cannulas (cannula A and cannula B) were placed in a jugular vein, and an infusion of 0.9% saline was initiated into each cannula at 25 μl/min. After inserting PE-10 tubing into the left ureter for urine collection, noncannulating, transit-time flow probes (Transonic Systems, Inc, Ithaca, NY) were positioned on both the left renal artery (1 mm) and mesenteric artery (2 mm). These were connected to a two-channel transit-time flowmeter (model T-206; Transonic Systems, Inc.) for recording of renal blood flow (RBF) and mesenteric blood flow (MBF).
After a 90-minute stabilization period, in the control group (n = 15), saline infusions were continued via both cannula A and cannula B. However, in the guanosine group (n = 15), guanosine was infused via cannula A at 10 µmol/kg per minute. After 15 minutes, a 30-minute urine collection was initiated while measuring MABP, HR, RBF, and MBF. Next, in both groups, cannula B was used to infuse adenosine at 0.3 µmol/kg per minute. After 15 minutes, another 30-minute urine collection was initiated while measuring MABP, HR, RBF, and MBF. This procedure was continued as the dose of adenosine was increased to 1 and then 3 µmol/kg per minute. Renal vascular resistance (RVR) and mesenteric vascular resistance (MVR) were calculated by dividing the MABP by the RBF or MBF, respectively. In nine control rats and 12 guanosine-treated rats, urine collected before the infusions of adenosine was analyzed for guanosine, adenosine, and inosine using high-performance liquid chromatography–tandem mass spectrometry as previously described (Jackson et al., 2009). It was not possible to compare the urine levels of these purines in the presence of adenosine because urine volumes were severely reduced in those rats receiving guanosine plus adenosine.
Guanosine on Endotoxin-Induced Cytokine Release.
Rats were anesthetized and instrumented as described already herein, and an infusion of 0.9% saline (50 μl/min) was initiated via cannula A. During the first experimental period (period 1), urine was collected for 30 minutes and MABP, HR, RBF, and MBF were time-averaged during the 30-minute urine collection. Also 1 ml of blood (in heparin) was taken during the middle of the 30-minute urine collection. The midpoint blood sample was centrifuged, and the plasma was collected and stored for later analysis. Blood-volume loss was compensated by injecting 1 ml of 0.9% saline. In some animals, the saline infusion via cannula A was continued, whereas in other animals, guanosine (dissolved in 0.9% saline) was infused via cannula A at either 5 or 10 µmol/kg per minute. After 15 minutes, another 30-minute urine collection was performed with measurements of MABP, HR, RBF, and MBF and with another 1-ml midpoint blood sample (period 2). Next, in all three groups, an intravenous bolus of LPS (30 mg/kg) was administered via cannula B. After 60 minutes, yet another 30-minute urine collection was initiated (period 3), during which MABP, HR, RBF, and MBF were again measured, and another 1-ml midpoint blood sample was taken. Tumor necrosis factor-α (TNF-α) and IL-1β were measured in plasma using R&D Systems (Minneapolis, MN) kits [rat TNF-α Quantikine ELISA kit (catalog no. RTA00) and rat IL-1β/IL-1F2 Quantikine ELISA kit (catalog no. RLB00), respectively]. Creatinine was measured in urine and plasma using the QuantiChrom Creatinine Assay kit (catalog no. DICT-500; Bioassay Systems, Hayward, CA). As previously demonstrated, hemodyanmic parameters were stable over the time frame of the present study (Begany et al., 1996; Tofovic et al., 2001).
Statistics.
Data are presented as mean ± S.E.M. Data for MABP, HR, RBF, RVR, MBF, MVR, urine volume, creatinine clearance (glomerular filtration rate [GFR]), and plasma TNF-α and IL-1β were analyzed using a repeated-measures two-factor analysis of variance (2F-ANOVA), and Fisher’s least significant difference tests were applied for post hoc analyses. Comparisons of purine excretions between control and guanosine-treated groups were performed with unpaired, two-tailed Student’s t test. Survival analysis of control versus guanosine-treated groups was analyzed using the Gehan-Breslow-Wilcoxon test. A value of P < 0.05 was considered statistically significant.
Results
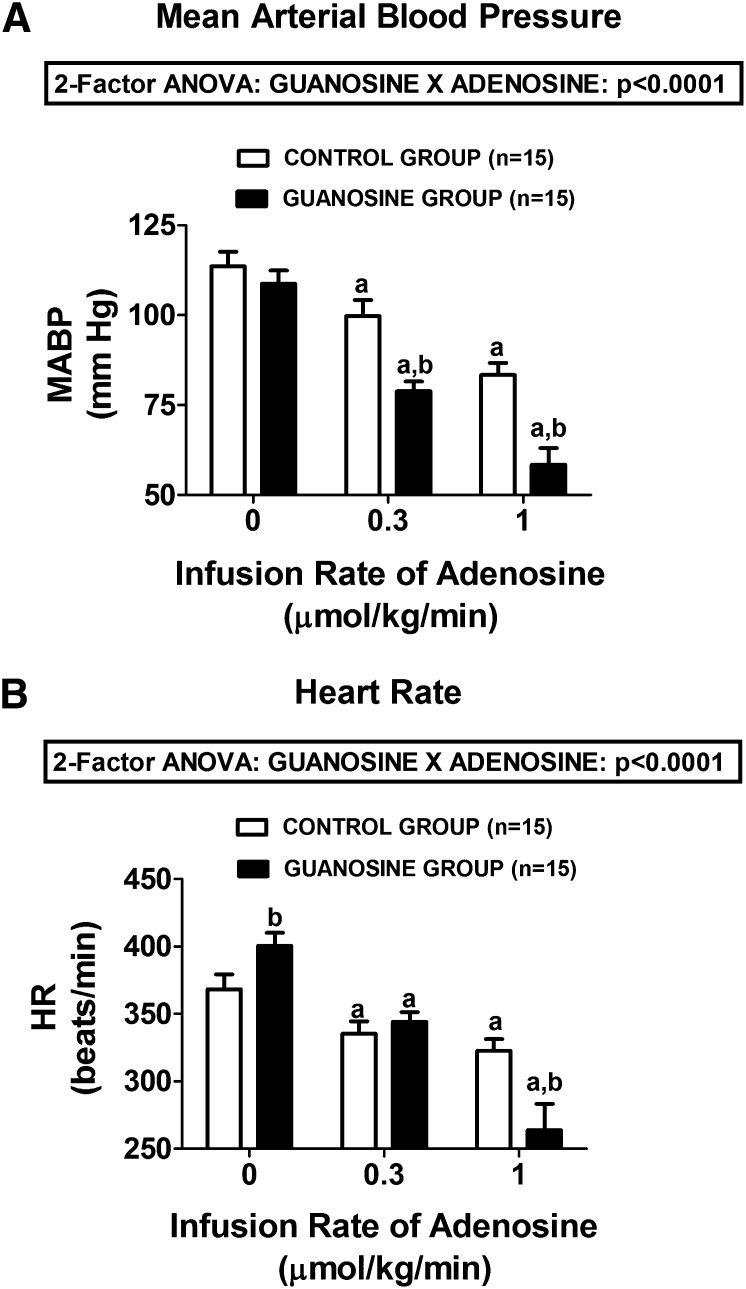
In control rats not pretreated with guanosine, intravenous infusions of adenosine at 0.3 and 1 µmol/kg per minute caused a dose-related decrease in both MABP (Fig. 1A) and HR (Fig. 1B). Pretreatment of rats with an intravenous infusion of guanosine (10 µmol/kg per minute) did not significantly alter basal MABP (114 ± 4 versus 109 ± 4 mm Hg in the control and guanosine-pretreated groups, respectively). However, basal HR was slightly but statistically significantly elevated in the guanosine-pretreated group (369 ± 11 versus 401 ± 10 beats/min in the control and guanosine-pretreated groups, respectively). As illustrated in Fig. 1, A and B, respectively, adenosine-induced decreases in MABP and HR were markedly augmented in guanosine-pretreated rats, and these effects were highly statistically significant (P < 0.0001; 2F-ANOVA).
Fig. 1.
Bar graphs summarize the dose-dependent effects of intravenous infusions of adenosine on (A) MABP and (B) HR in control rats and in rats treated with intravenous guanosine (10 µmol/kg per minute). Values represent means and S.E.M. aSignificant difference (P < 0.05) versus the respective basal (0) value. bSignificant difference (P < 0.05) between controls versus guanosine-treated rats at the same infusion rate of adenosine.
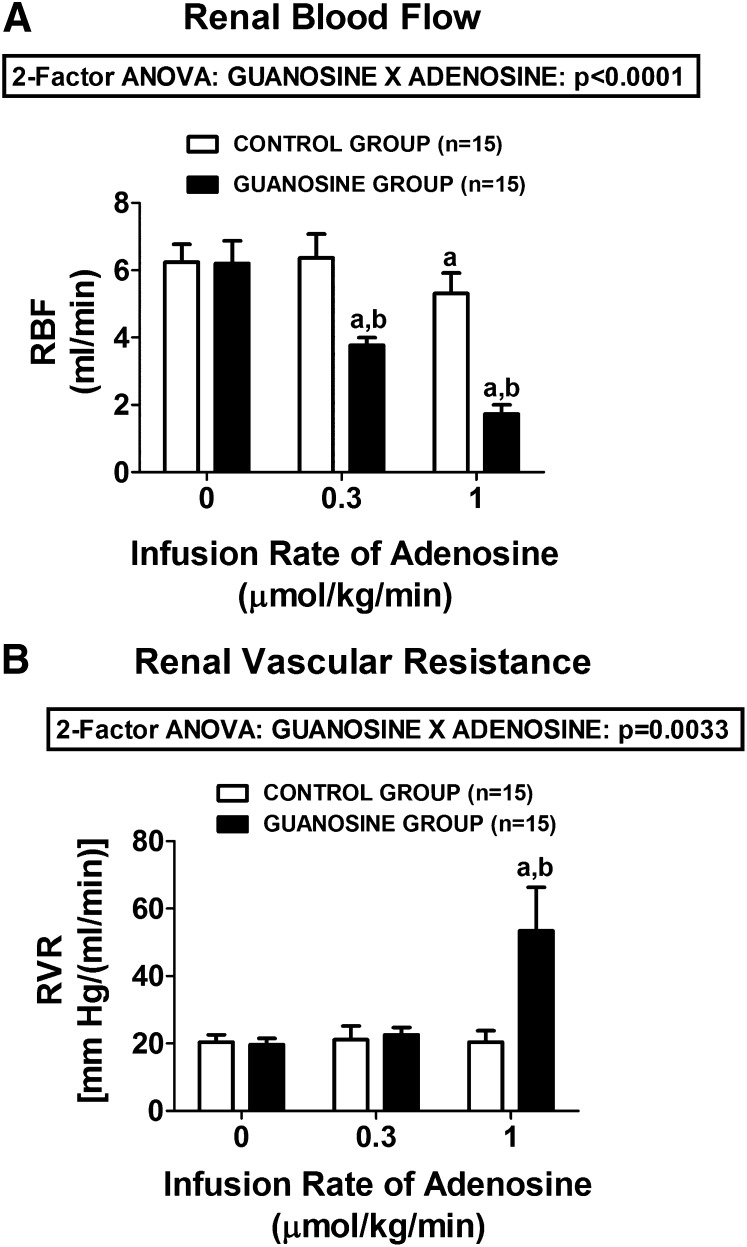
Basal RBF and basal RVR were similar in control versus guanosine-pretreated rats [for RBF, 6.2 ± 0.5 versus 6.2 ± 0.7 ml/min in the control and guanosine-pretreated groups, respectively; for RVR, 20.4 ± 2.2 versus 19.7 ± 0.7 mm Hg/(ml/min) in control versus guanosine-pretreated rats, respectively]. In control rats, intravenous adenosine had only a slight effect on RBF (from 6.2 ± 0.5 to 5.3 ± 0.6, basal versus 1 µmol/kg per minute of adenosine, respectively) (Fig. 2A) and no effect on RVR (from 20.4 ± 2.2 to 20.4 ± 3.3, basal versus 1 µmol/kg per minute of adenosine, respectively) (Fig. 2B). In contrast, in the guanosine-pretreated group, adenosine profoundly decreased RBF (from 6.2 ± 0.7 to 1.7 ± 0.3, basal versus 1 µmol/kg per minute of adenosine, respectively) (Fig. 2A) and profoundly increased RVR (from 19.7 ± 1.9 to 53.4 ± 12.9, basal versus 1 µmol/kg per minute of adenosine, respectively) (Fig. 2B). The interaction between guanosine and adenosine on RBF and RVR was highly statistically significant by 2F-ANOVA (P < 0.0001 and P = 0.0033, respectively).
Fig. 2.
Bar graphs illustrate the dose-dependent effects of intravenous infusions of adenosine on (A) RBF and (B) RVR in control rats and in rats treated with intravenous guanosine (10 µmol/kg per minute). Values represent means and S.E.M. aSignificant difference (P < 0.05) versus the respective basal (0) value. bSignificant difference (P < 0.05) between controls versus guanosine-treated at the same infusion rate of adenosine.
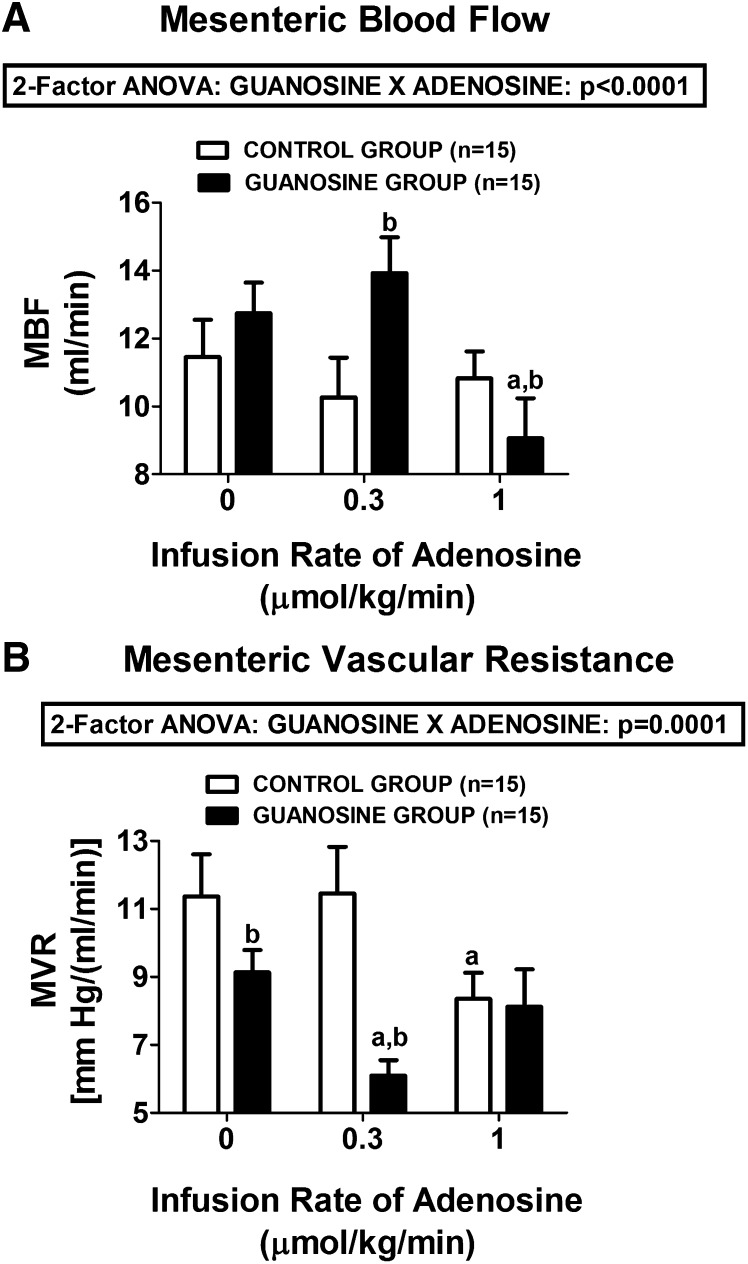
We also observed a statistically significant interaction between guanosine and adenosine on MBF (Fig. 3A) and MVR levels (Fig. 3B) (P < 0.0001 and P = 0.0001, respectively). In this regard, at 0.3 µmol/kg per minute, adenosine increased MBF and decreased MVR in guanosine-pretreated rats, but it had no effect on MBF or MVR in control rats. At 1 µmol/kg per minute, in guanosine-pretreated rats, adenosine decreased MBF and returned MVR back to baseline. This biphasic response to adenosine in guanosine-pretreated rats was likely due to the fact that at 1 µmol/kg per minute, adenosine profoundly reduced MABP (to 58 ± 5 mm Hg), which likely triggered a baroreceptor-mediated reflex increase in sympathetic tone to the mesentery. This biphasic response to adenosine was not observed in the control rats not receiving guanosine. Notably, even in the absence of adenosine, guanosine significantly reduced basal MVR.
Fig. 3.
Bar graphs demonstrate the dose-dependent effects of intravenous infusions of adenosine on (A) MBF and (B) MVR in control rats and rats treated with intravenous guanosine (10 µmol/kg per minute). Values represent means and S.E.M. aSignificant difference (P < 0.05) versus the respective basal (0) value. bSignificant difference (P < 0.05) between controls versus guanosine-treated at the same infusion rate of adenosine.
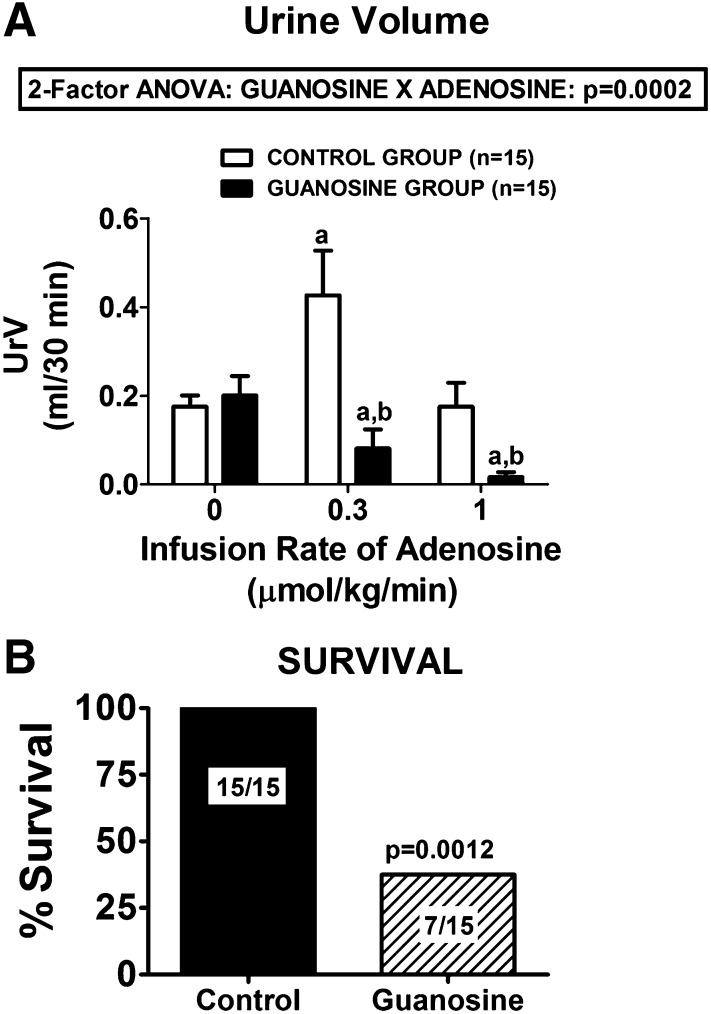
The guanosine-adenosine interaction was also observed with respect to urine excretion rate [urine volume (UrV)] (Fig. 4A), and this interaction was highly statistically significantly (P = 0.0002). Basal UrV was similar in control versus guanosine-pretreated rats (0.18 ± 0.03 versus 0.20 ± 0.04 ml/30 minutes in the control and guanosine-pretreated groups, respectively). In control rats, an infusion of adenosine at 0.3 µmol/kg per minute increased UrV to 0.43 ± 0.10 ml/30 minutes; in contrast, in guanosine-treated rats, the same dose of adenosine decreased UrV to 0.08 ± 0.04 ml/30 minutes. At a dose of 1 µmol/kg per minute, adenosine further decreased UrV in the guanosine-treated rats (to 0.02 ± 0.01 ml/30 minutes), whereas in the control rats, UrV returned to basal values (0.18 ± 0.05).
Fig. 4.
(A) Dose-dependent effects of intravenous infusions of adenosine on UrV in control rats and in rats treated with intravenous guanosine (10 µmol/kg per minute). Values represent means and S.E.M. aSignificant difference (P < 0.05) versus the respective basal (0) value. bSignificant difference (P < 0.05) between control versus guanosine-treated rats at the same infusion rate of adenosine. (B) Summary of the survival of rats receiving intravenous infusions of adenosine (3 µmol/kg per minute) only (Control) versus rats receiving intravenous infusions of both guanosine (10 µmol/kg per minute) and adenosine (3 µmol/kg per minute) simultaneously (Guanosine).
Our initial intention was to collect hemodynamic data in control versus guanosine-pretreated rats with an even higher dose of adenosine (i.e., 3 µmol/kg per minute). However, we discovered that although control rats could readily tolerate this dose of adenosine, within a few minutes after starting the infusion, many of the guanosine-pretreated rats rapidly died of shock levels of arterial blood pressure, and the ones that did not die were hemodynamically severely compromised. As shown in Fig. 4B, the mortality rate in guanosine-pretreated rats within 45 minutes of starting the higher infusion rate was 53% (P = 0.0012 compared with controls), with most expiring within a few minutes of initiating the higher dose of adenosine.
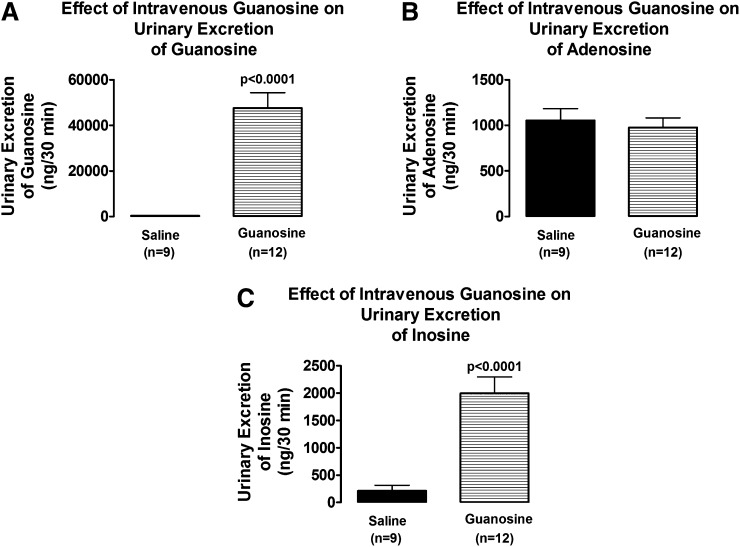
In 9 control rats and 12 guanosine-treated rats, a sufficient volume of urine was collected before the infusions of adenosine for analysis for guanosine, adenosine, and inosine using high-performance liquid chromatography–tandem mass spectrometry. It was not possible to compare the urine levels of these purines in the presence of adenosine because urine volumes were too low in the rats receiving guanosine plus adenosine. Intravenous infusions of guanosine vastly increased the renal excretion rate of guanosine (from 325 ± 22 to 47,597 ± 6748 ng/30 minutes; P < 0.0001; Fig. 5A). Although guanosine did not increase the urinary excretion rate of endogenous adenosine (Fig. 5B), guanosine profoundly increased the urinary excretion rate of the major endogenous metabolite of adenosine, namely, inosine (from 210 ± 103 to 1995 ± 300 ng/30 minutes; P < 0.0001; Fig. 5C).
Fig. 5.
Bar graphs compare the effects of intravenous guanosine (10 µmol/kg per minute) on urinary excretion rates of (A) guanosine, (B) adenosine, and (C) inosine. Values represent means and S.E.M.
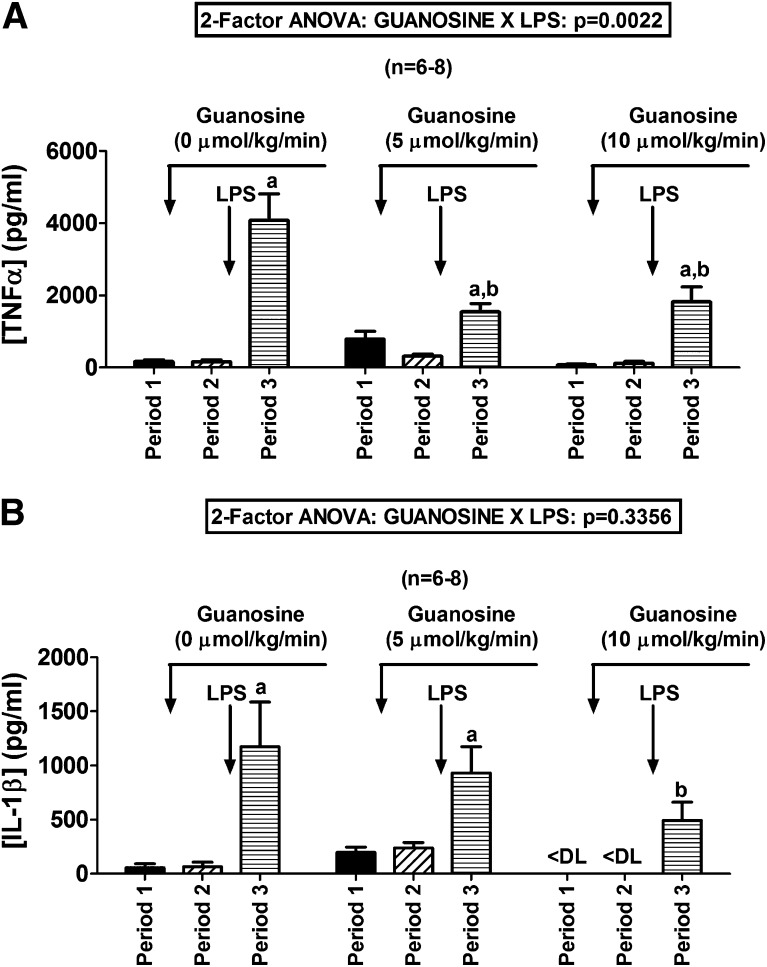
Because adenosine is anti-inflammatory and guanosine increases adenosine, we examined whether guanosine alters the effects of LPS (a proinflammatory endotoxin). In the absence of guanosine, LPS increased plasma levels of the proinflammatory cytokine TNF-α from 164 ± 56 to 4082 ±730 pg/ml (Fig. 6A). However, in animals pretreated with guanosine, this response was attenuated (guanosine at 5 µmol/kg per minute: from 318 ± 54 to 1550 ± 223 pg/ml; guanosine at 10 µmol/kg per minute: from 121 ± 45 to 1821 ± 413 pg/ml). The interaction between guanosine and LPS on plasma TNF-α was highly statistically significant by 2F-ANOVA (P = 0.0022; Fig. 6A). In the absence of guanosine, LPS increased plasma levels of the proinflammatory cytokine IL-1β from 65 ± 42 to 1173 ± 412 pg/ml (Fig. 6B). However, in animals pretreated with guanosine, this response tended to be attenuated (guanosine at 5 µmol/kg per minute: from 238 ± 52 to 931 ± 241 pg/ml; guanosine at 10 µmol/kg per minute: from less than detection limit to 493 ± 168 pg/ml). Although the interaction between guanosine and LPS on plasma IL-1β did not achieve statistical significance by 2F-ANOVA (Fig. 6B), the level of IL-1β in LPS-treated animals pretreated with guanosine at 10 µmol/kg per minute was significantly (P < 0.05) lower than the level in LPS-treated animals not pretreated with guanosine.
Fig. 6.
Bar graphs summarize the effects of LPS (30 mg/kg) on plasma levels of (A) TNF-α and (B) IL-1β in rats pretreated with intravenous infusions of guanosine at 0 (saline only), 5, or 10 µmol/kg per minute. Values represent means and S.E.M. aSignificant difference (P < 0.05) between period 2 and period 3 within the respective group. bSignificant difference (P < 0.05) versus period 3 of control (0 µmol/kg per minute of guanosine) group. <DL, less than detection limit.
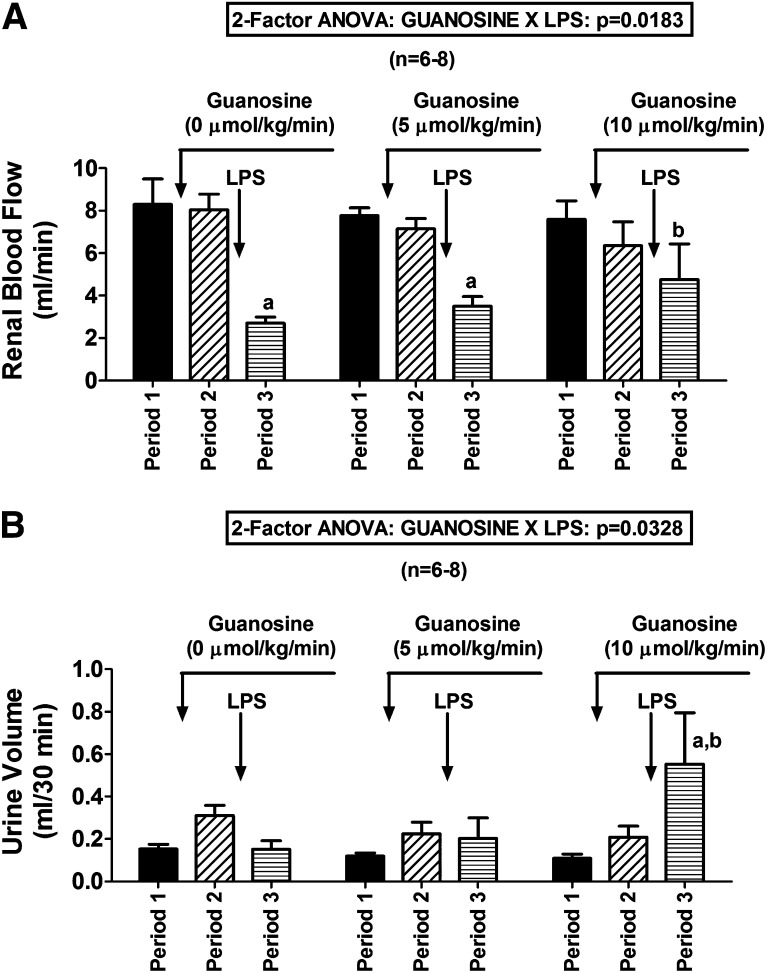
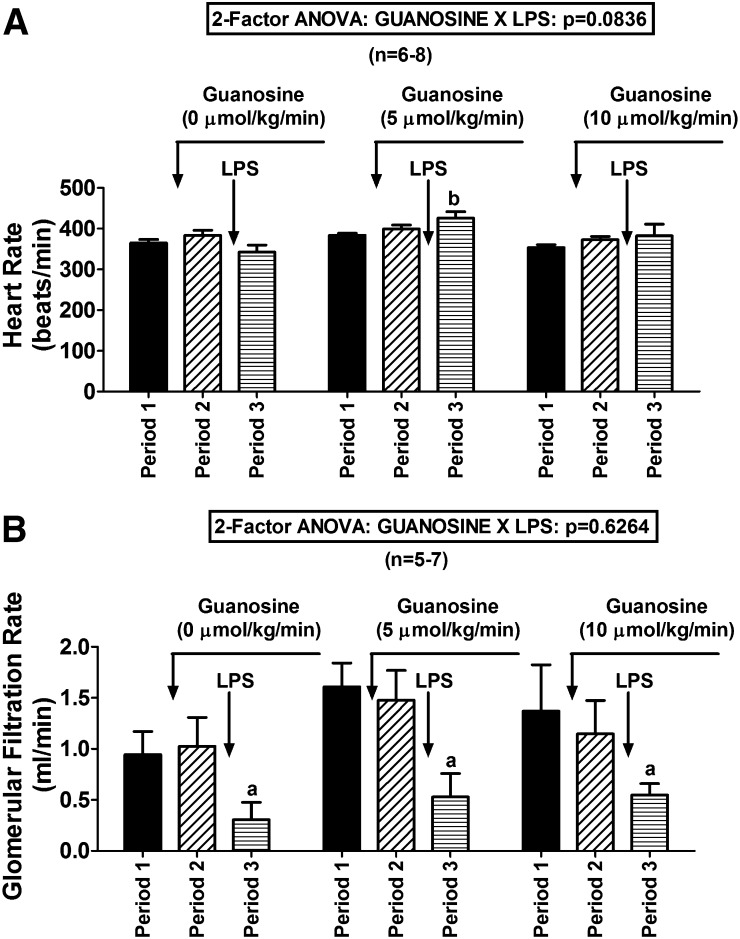
Guanosine not only reduced the cytokine response to LPS but also attenuated LPS-induced reductions in RBF (guanosine-LPS interaction on RBF by 2F-ANOVA, P = 0.0183; Fig. 7A). Also, LPS tended to decrease UrV in rats not treated with guanosine, yet in rats pretreated with guanosine, LPS either did not affect UrV (guanosine at 5 µmol/kg per minute) or increased UrV (guanosine at 10 µmol/kg per minute) (guanosine-LPS interaction on UrV by 2F-ANOVA, P = 0.0328; Fig. 7B). In the absence of guanosine, LPS tended to reduce HR, but in the presence of guanosine, LPS tended to increase HR. Although the guanosine-LPS interaction on HR was only near significant (2F-ANOVA, P = 0.0836; Fig. 8A), HR after LPS was significantly higher in guanosine (5 µmol/kg per minute)-pretreated rats compared with rats not pretreated with guanosine. Although guanosine did not significantly attenuate LPS-induced reductions in GFR, guanosine did not worsen LPS-induced decreases in GFR (Fig. 8B). LPS had little effect on MABP (Fig. 9A) or MBF (Fig. 9B); importantly, guanosine did not cause LPS to induce hypotension or mesenteric ischemia (Fig. 9, A and B).
Fig. 7.
Bar graphs summarize the effects of LPS (30 mg/kg) on (A) renal blood flow and (B) urine volume in rats pretreated with intravenous infusions of guanosine at 0 (saline only), 5, or 10 µmol/kg per minute. Values represent means and S.E.M. aSignificant difference (P < 0.05) between period 2 and period 3 within the respective group. bDenotes significant difference (P < 0.05) versus period 3 of control (0 µmol/kg per minute of guanosine) group.
Fig. 8.
Bar graphs summarize the effects of LPS (30 mg/kg) on (A) HR and (B) GFR in rats pretreated with intravenous infusions of guanosine at 0 (saline only), 5, or 10 µmol/kg per minute. Values represent means and S.E.M. aSignificant difference (P < 0.05) between period 2 and period 3 within the respective group. bSignificant difference (P < 0.05) versus period 3 of control (0 µmol/kg per minute of guanosine) group.
Fig. 9.
Bar graphs summarize the effects of LPS (30 mg/kg) on (A) MABP and (B) mesenteric blood flow in rats pretreated with intravenous infusions of guanosine at 0 (saline only), 5, or 10 µmol/kg per minute. Values represent means and S.E.M.
Discussion
The results of this study support the conclusion that the guanosine-adenosine mechanism is operative in vivo. This conclusion is based on the findings that infusions of guanosine remarkably increase the ability of adenosine to affect MABP, HR, RBF, RVR, MBF, MVR, and UrV. Also, guanosine infusions increase by approximately 10-fold the amount of endogenous inosine (the main metabolite of adenosine) excreted in the urine. The guanosine-adenosine interaction is so robust that a dose of adenosine that is nontoxic to normal rats kills within a few minutes over 50% of rats pretreated with a dose of guanosine that per se has little effect on the cardiovascular system or kidneys. These findings, particularly when coupled with our in vitro findings in cultured cells (Jackson et al., 2013; Jackson and Gillespie, 2013) and isolated, perfused kidneys (Jackson et al., 2014), leave little doubt that the guanosine-adenosine mechanism is operative and robust.
The guanosine-adenosine mechanism could explain previous protective effects reported for guanosine. There is now an overwhelming body of evidence in support of the conclusion that adenosine (Okusa et al., 1999, 2000, 2001; Lee and Emala, 2000, 2002; Okusa, 2002; Day et al., 2003, 2005; Lee et al., 2004a,b, 2007; Grenz et al., 2007a,b, 2008, 2011, 2012; Kim et al., 2009; Haskó et al., 2011) and inosine (Maggio et al., 1980; Marberger et al., 1980; Fitzpatrick et al., 1981; Rothwell et al., 1981; Mathur and Ramsey, 1983) protect the kidneys from acute injury. Less well known is the fact that guanosine is also renoprotective (Kelly et al., 2001). Likewise, adenosine is neuroprotective (for reviews, see Stone, 2002; Chen et al., 2007; Stone et al., 2007) and apparently inosine is as well (Shen et al., 2005). Importantly, there is a growing body of evidence that guanosine, like adenosine and inosine, is neuroprotective [for review of supporting evidence see Introduction in article by Dal-Cim and coworkers (Dal-Cim et al., 2013)]. The mechanism by which guanosine affords renoprotection and neuroprotection is unknown. The facts that adenosine and guanosine are both renoprotective and neuroprotective, that the existence of cell-surface guanosine receptors is questionable, and that guanosine increases both the extracellular levels of adenosine and inosine and augments the physiologic effects of adenosine strongly support the conclusion that the guanosine-adenosine mechanism mediates the protective effects of guanosine.
The discussion herein underscores the possibility for therapeutic intervention with either guanosine per se or with drugs that modulate guanosine. In this regard, guanosine could be delivered either intravenously or intramuscularly (Shin et al., 2008) or via intraperitoneal administration (Giuliani et al., 2012). Importantly, the results of the present study demonstrate that guanosine per se has little effect on basal arterial blood pressure or renal blood flow and therefore likely would be safe. Yet by increasing endogenous levels of extracellular adenosine and inosine in an event- and site-specific manner, guanosine could prove useful for the treatment of such disorders as tissue ischemia-reperfusion, sepsis, and inflammation. Indeed, our present findings indicate that intravenous guanosine attenuates endotoxin-induced increases in plasma levels of TNF-α (and possibly IL-1β) in rats. Consistent with the reduction in LPS-induced cytokine levels, guanosine preserves RBF and UrV in endotoxemia. We hypothesize that the ability of guanosine to protect against RBF and UrV changes induced by LPS is mediated by the anti-inflammatory effects of guanosine via adenosine. Importantly, guanosine does not destabilize MABP, HR, or GFR in endotoxemia, suggesting that this compound would be safe to use in sepsis. Because guanosine is metabolized by PNPase to guanine, inhibition of PNPase increases extracellular guanosine levels associated with cellular injury (Jackson et al., 2013). Therefore, inhibition of PNPase is another possible therapeutic approach for treating diseases in which elevated levels of extracellular guanosine, adenosine, and inosine might be advantageous. In normal tissue, PNPase inhibitors likely would have few effects; in contrast, in damaged tissues (kidney injury or brain injury for example), PNPase inhibitors may be tissue protective.
Although the preceding discussion suggests that guanosine or PNPase inhibitors may be advantageous for the treatment of some disease states, it is conceivable that in other disorders one should focus on enhancing the metabolism of guanosine to prevent adverse effects of too much adenosine. The results of the present study show that guanosine can enhance the physiologic effects of adenosine so robustly that animals succumb to cardiovascular shock when higher doses of adenosine are infused. This finding suggests the possibility that guanosine could contribute to adverse outcomes following hemorrhagic or cardiogenic shock.
The mechanism of the guanosine-adenosine interaction remains unknown. Our initial studies (Jackson et al., 2013; Jackson and Gillespie, 2013) rule out the participation of many possible candidate systems, including adenosine deaminase, adenosine kinase, S-adenosylhomocysteine hydrolase, guanine deaminase, equilibrative nucleoside transporters, concentrative nucleoside transporters, SLC19A1, SLC19A2, SLC19A3, and SLC22A2. Given the striking results of the present study, our future studies will focus both on the therapeutic applications of the guanosine-adenosine interaction and the mechanism underlying this interaction.
In summary, the present study demonstrates that extracellular guanosine can regulate extracellular adenosine and inosine levels in vivo and that this interaction results in profound increases in the cardiorenal effects of adenosine. These results are of significance because they reveal an important but overlooked physiologic mechanism and suggest novel pharmacologic approaches to modify the endogenous adenosine system safely and effectively to treat diseases. These findings justify the dedication of resources to determine the mechanism of the guanosine-adenosine interaction and to determine whether manipulating this interaction could be of therapeutic advantage.
Abbreviations
- 2F-ANOVA
two-factor analysis of variance
- GFR
glomerular filtration rate
- HR
heart rate
- LPS
lipopolysaccharide
- MABP
mean arterial blood pressure
- MBF
mesenteric blood flow
- MVR
mesenteric vascular resistance
- PE
polyethylene
- PNPase
purine nucleoside phosphorylase
- RBF
renal blood flow
- RVR
renal vascular resistance
- TNF-α
tumor necrosis factor-α
- UrV
urine volume
Authorship Contributions
Participated in research design: Jackson.
Conducted experiments: Mi.
Performed data analysis: Jackson.
Wrote or contributed to the writing of the manuscript: Jackson, Mi.
Footnotes
This work was supported by the National Institutes of Health National Heart, Lung, and Blood Institute [Grants R01-HL109002 and R01-HL069846]; and the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grants R01-DK091190, R01-DK068575, and P30-DK079307].
References
- Begany DP, Carcillo JA, Herzer WA, Mi Z, Jackson EK. (1996) Inhibition of type IV phosphodiesterase by Ro 20-1724 attenuates endotoxin-induced acute renal failure. J Pharmacol Exp Ther 278:37–41 [PubMed] [Google Scholar]
- Chen J-F, Sonsalla PK, Pedata F, Melani A, Domenici MR, Popoli P, Geiger J, Lopes LV, de Mendonça A. (2007) Adenosine A2A receptors and brain injury: broad spectrum of neuroprotection, multifaceted actions and “fine tuning” modulation. Prog Neurobiol 83:310–331 [DOI] [PubMed] [Google Scholar]
- Colgan SP, Fennimore B, Ehrentraut SF. (2013) Adenosine and gastrointestinal inflammation. J Mol Med (Berl) 91:157–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal-Cim T, Ludka FK, Martins WC, Reginato C, Parada E, Egea J, López MG, Tasca CI. (2013) Guanosine controls inflammatory pathways to afford neuroprotection of hippocampal slices under oxygen and glucose deprivation conditions. J Neurochem 126:437–450 [DOI] [PubMed] [Google Scholar]
- Day Y-J, Huang L, McDuffie MJ, Rosin DL, Ye H, Chen J-F, Schwarzschild MA, Fink JS, Linden J, Okusa MD. (2003) Renal protection from ischemia mediated by A2A adenosine receptors on bone marrow-derived cells. J Clin Invest 112:883–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day Y-J, Huang L, Ye H, Linden J, Okusa MD. (2005) Renal ischemia-reperfusion injury and adenosine 2A receptor-mediated tissue protection: role of macrophages. Am J Physiol Renal Physiol 288:F722–F731 [DOI] [PubMed] [Google Scholar]
- Fitzpatrick JM, Wallace DM, Whitfield HN, Watkinson LE, Fernando AR, Wickham JE. (1981) Inosine in ischaemic renal surgery: long-term follow-up. Br J Urol 53:524–527 [DOI] [PubMed] [Google Scholar]
- Fredholm BB, IJzerman AP, Jacobson KA, Linden J, Müller CE. (2011) International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors: an update. Pharmacol Rev 63:1–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliani P, Ballerini P, Ciccarelli R, Buccella S, Romano S, D’Alimonte I, Poli A, Beraudi A, Peña E, Jiang S, et al. (2012) Tissue distribution and metabolism of guanosine in rats following intraperitoneal injection. J Biol Regul Homeost Agents 26:51–65 [PubMed] [Google Scholar]
- Grenz A, Bauerle JD, Dalton JH, Ridyard D, Badulak A, Tak E, McNamee EN, Clambey E, Moldovan R, Reyes G, et al. (2012) Equilibrative nucleoside transporter 1 (ENT1) regulates postischemic blood flow during acute kidney injury in mice. J Clin Invest 122:693–710 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Grenz A, Homann D, Eltzschig HK. (2011) Extracellular adenosine: a safety signal that dampens hypoxia-induced inflammation during ischemia. Antioxid Redox Signal 15:2221–2234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenz A, Osswald H, Eckle T, Yang D, Zhang H, Tran ZV, Klingel K, Ravid K and Eltzschig HK (2008) The reno-vascular A2B adenosine receptor protects the kidney from ischemia. PLoS Med 5:e137. [DOI] [PMC free article] [PubMed]
- Grenz A, Zhang H, Eckle T, Mittelbronn M, Wehrmann M, Köhle C, Kloor D, Thompson LF, Osswald H, Eltzschig HK. (2007a) Protective role of ecto-5′-nucleotidase (CD73) in renal ischemia. J Am Soc Nephrol 18:833–845 [DOI] [PubMed] [Google Scholar]
- Grenz A, Zhang H, Hermes M, Eckle T, Klingel K, Huang DY, Müller CE, Robson SC, Osswald H, Eltzschig HK. (2007b) Contribution of E-NTPDase1 (CD39) to renal protection from ischemia-reperfusion injury. FASEB J 21:2863–2873 [DOI] [PubMed] [Google Scholar]
- Haskó G, Csóka B, Koscsó B, Chandra R, Pacher P, Thompson LF, Deitch EA, Spolarics Z, Virág L, Gergely P, et al. (2011) Ecto-5′-nucleotidase (CD73) decreases mortality and organ injury in sepsis. J Immunol 187:4256–4267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskó G, Pacher P, Deitch EA, Vizi ES. (2007) Shaping of monocyte and macrophage function by adenosine receptors. Pharmacol Ther 113:264–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson EK, Cheng D, Jackson TC, Verrier JD, Gillespie DG. (2013) Extracellular guanosine regulates extracellular adenosine levels. Am J Physiol Cell Physiol 304:C406–C421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson EK, Cheng D, Mi Z, Gillespie DG. (2014) Guanosine regulates adenosine levels in the kidney. Physiol Rep 2:e12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson EK, Gillespie DG. (2013) Regulation of cell proliferation by the guanosine–adenosine mechanism: role of adenosine receptors. Physiol Rep 1:e00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson EK, Ren J, Mi Z. (2009) Extracellular 2′,3′-cAMP is a source of adenosine. J Biol Chem 284:33097–33106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly KJ, Plotkin Z, Dagher PC. (2001) Guanosine supplementation reduces apoptosis and protects renal function in the setting of ischemic injury. J Clin Invest 108:1291–1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Chen SWC, Park SW, Kim M, D’Agati VD, Yang J, Lee HT. (2009) Kidney-specific reconstitution of the A1 adenosine receptor in A1 adenosine receptor knockout mice reduces renal ischemia-reperfusion injury. Kidney Int 75:809–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HT, Emala CW. (2000) Protective effects of renal ischemic preconditioning and adenosine pretreatment: role of A1 and A3 receptors. Am J Physiol Renal Physiol 278:F380–F387 [DOI] [PubMed] [Google Scholar]
- Lee HT, Emala CW. (2002) Adenosine attenuates oxidant injury in human proximal tubular cells via A1 and A2A adenosine receptors. Am J Physiol Renal Physiol 282:F844–F852 [DOI] [PubMed] [Google Scholar]
- Lee HT, Gallos G, Nasr SH, Emala CW. (2004a) A1 adenosine receptor activation inhibits inflammation, necrosis, and apoptosis after renal ischemia-reperfusion injury in mice. J Am Soc Nephrol 15:102–111 [DOI] [PubMed] [Google Scholar]
- Lee HT, Kim M, Jan M, Penn RB, Emala CW. (2007) Renal tubule necrosis and apoptosis modulation by A1 adenosine receptor expression. Kidney Int 71:1249–1261 [DOI] [PubMed] [Google Scholar]
- Lee HT, Xu H, Nasr SH, Schnermann J, Emala CW. (2004b) A1 adenosine receptor knockout mice exhibit increased renal injury following ischemia and reperfusion. Am J Physiol Renal Physiol 286:F298–F306 [DOI] [PubMed] [Google Scholar]
- Longhi MS, Robson SC, Bernstein SH, Serra S, Deaglio S. (2013) Biological functions of ecto-enzymes in regulating extracellular adenosine levels in neoplastic and inflammatory disease states. J Mol Med (Berl) 91:165–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio AJ, Jr, Das S, Smith RB, Kaufman JJ. (1980) Renal preservation with inosine. Urology 16:343–345 [DOI] [PubMed] [Google Scholar]
- Marberger M, Günther R, Alken P, Rumpf W, Ranc M. (1980) Inosine: alternative or adjunct to regional hypothermia in the prevention of post-ischemic renal failure? Eur Urol 6:95–102 [DOI] [PubMed] [Google Scholar]
- Mathur VK, Ramsey EW. (1983) Comparison of methods for preservation of renal function during ischemic renal surgery. J Urol 129:163–165 [DOI] [PubMed] [Google Scholar]
- Ohta A, Sitkovsky M. (2009) The adenosinergic immunomodulatory drugs. Curr Opin Pharmacol 9:501–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okusa MD. (2002) A2A adenosine receptor: a novel therapeutic target in renal disease. Am J Physiol Renal Physiol 282:F10–F18 [DOI] [PubMed] [Google Scholar]
- Okusa MD, Linden J, Huang L, Rieger JM, Macdonald TL, Huynh LP. (2000) A2A adenosine receptor-mediated inhibition of renal injury and neutrophil adhesion. Am J Physiol Renal Physiol 279:F809–F818 [DOI] [PubMed] [Google Scholar]
- Okusa MD, Linden J, Huang L, Rosin DL, Smith DF, Sullivan G. (2001) Enhanced protection from renal ischemia-reperfusion [correction of ischemia-reperfusion] injury with A2A-adenosine receptor activation and PDE 4 inhibition. Kidney Int 59:2114–2125 [DOI] [PubMed] [Google Scholar]
- Okusa MD, Linden J, Macdonald T, Huang L. (1999) Selective A2A adenosine receptor activation reduces ischemia-reperfusion injury in rat kidney. Am J Physiol 277:F404–F412 [DOI] [PubMed] [Google Scholar]
- Poth JM, Brodsky K, Ehrentraut H, Grenz A, Eltzschig HK. (2013) Transcriptional control of adenosine signaling by hypoxia-inducible transcription factors during ischemic or inflammatory disease. J Mol Med (Berl) 91:183–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell D, Bartley J, James M. (1981) Preservation of the ischaemic canine kidney with inosine. Urol Res 9:75–78 [DOI] [PubMed] [Google Scholar]
- Shen H, Chen G-J, Harvey BK, Bickford PC, Wang Y. (2005) Inosine reduces ischemic brain injury in rats. Erratum appears in Stroke. 2005 May;36 5 :1106 Note: Dosage error in article text Stroke 36:654–659 [DOI] [PubMed] [Google Scholar]
- Shin DH, Choi KS, Cho BS, Song S, Moon D-C, Hong JT, Lee C-K, Chung YB. (2008) Pharmacokinetics of guanosine in rats following intravenous or intramuscular administration of a 1:1 mixture of guanosine and acriflavine, a potential antitumor agent. Arch Pharm Res 31:1347–1353 [DOI] [PubMed] [Google Scholar]
- Stone TW. (2002) Purines and neuroprotection. Adv Exp Med Biol 513:249–280 [DOI] [PubMed] [Google Scholar]
- Stone TW, Forrest CM, Mackay GM, Stoy N, Darlington LG. (2007) Tryptophan, adenosine, neurodegeneration and neuroprotection. Metab Brain Dis 22:337–352 [DOI] [PubMed] [Google Scholar]
- Thauerer B, Zur Nedden S, Baier-Bitterlich G. (2012) Purine nucleosides: endogenous neuroprotectants in hypoxic brain. J Neurochem 121:329–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tofovic SP, Zacharia L, Carcillo JA, Jackson EK. (2001) Inhibition of adenosine deaminase attenuates endotoxin-induced release of cytokines in vivo in rats. Shock 16:196–202 [DOI] [PubMed] [Google Scholar]
- Traversa U, Bombi G, Camaioni E, Macchiarulo A, Costantino G, Palmieri C, Caciagli F, Pellicciari R. (2003) Rat brain guanosine binding site. Biological studies and pseudo-receptor construction. Bioorg Med Chem 11:5417–5425 [DOI] [PubMed] [Google Scholar]
- Vallon V, Mühlbauer B, Osswald H. (2006) Adenosine and kidney function. Physiol Rev 86:901–940 [DOI] [PubMed] [Google Scholar]