Abstract
IBNtxA (3′-iodobenzoyl-6β-naltrexamide) is a potent analgesic in mice lacking many traditional opioid side effects. In mice, it displays no respiratory depression, does not produce physical dependence with chronic administration, and shows no cross-tolerance to morphine. It has limited effects on gastrointestinal transit and shows no reward behavior. Biochemical studies indicate its actions are mediated through a set of μ-opioid receptor clone MOR-1 splice variants associated with exon 11 that lack exon 1 and contain only six transmembrane domains. Like the mouse and human, rats express exon 11–associated splice variants that also contain only six transmembrane domains, raising the question of whether IBNtxA would have a similar pharmacologic profile in rats. When given systemically, IBNtxA is a potent analgesic in rats, with an ED50 value of 0.89 mg/kg s.c., approximately 4-fold more potent than morphine. It shows no analgesic cross-tolerance in morphine-pelleted rats. IBNtxA displays no respiratory depression as measured by blood oxygen saturation. In contrast, oximetry shows that an equianalgesic dose of morphine lowers blood oxygen saturation values by 30%. IBNtxA binding is present in a number of brain regions, with the thalamus standing out with very high levels and the cerebellum with low levels. As in mice, IBNtxA is a potent analgesic in rats with a favorable pharmacologic profile and reduced side effects.
Introduction
Opiates have long been acknowledged as essential to treating pain clinically, but with a risk of diversion and addiction. Efforts to generate opioids that lack side effects and abuse potential go back over a century (Pasternak and Pan, 2013). Although many important analgesics and antagonists were developed, these agents did not dissociate pain relief from side effects. Current evidence suggests that this is possible, starting with the pharmacologic dissociation of morphine analgesia from respiratory depression and constipation by the antagonists naloxonazine and naloxazone (Pasternak et al., 1980; Wolozin and Pasternak, 1981; Hahn et al., 1982; Hahn and Pasternak, 1982; Ling et al., 1983, 1984, 1985, 1986).
After the cloning of the μ-opioid receptor (MOR-1) and its gene Oprm1, our group and others isolated and characterized a vast array of μ-receptor splice variants (for a review, see Pasternak and Pan, 2013). These variants fall into three major categories. The first includes the full-length variants containing the traditional μ-receptor variants with seven transmembrane (TM) domains that differ by 3′-splicing of the C terminus, leading to the replacement of exon 4 and its terminal 12 amino acids with a range of various combinations of alternative exons yielding from 1 to over 80 alternative unique amino acid sequences in their place (Bare et al., 1994; Zimprich et al., 1994, 1995; Pan et al., 1999, 2005; Pasternak et al., 2004). Another set of variants contains a single TM domain corresponding to TM1 in the traditional receptors (Du et al., 1997; Xu et al., 2013). Although this set does not directly bind opioids, it exerts significant pharmacologic actions through its chaperone-like function, which stabilizes the full-length variants and increases their expression levels in both tissue culture models and in vivo (Xu et al., 2013).
The final set of splice variants are associated with exon 11 and contain only six transmembrane (6TM) domains, lacking TM1 present in the traditional full-length receptors (Pan et al., 2001; Xu et al., 2011). Initially thought to be of little significance, they have proven very important in understanding μ-opioid pharmacology, as revealed by a series of knockout (KO) mice. A complete μ KO (E1/E11 KO) in which both exon 1 and exon 11 are disrupted is insensitive to all the μ drugs tested, confirming the importance of the Oprm1 gene in their actions (Y. X. Pan and G. W. Pasternak, unpublished data). An exon 1 MOR-1 KO mouse generated by Pintar eliminates the full-length 7TM variants while still expressing the truncated 6TM ones (Schuller et al., 1999). Conversely, an exon 11 MOR-1 KO mouse lacks the 6TM variants while still expressing the full-length ones (Pan et al., 2009; Majumdar et al., 2011b; Pasternak and Pan, 2013). As expected, loss of the traditional receptors in the exon 1 KO mouse eliminates morphine analgesia. In contrast, morphine and methadone retain full analgesic activity in the exon 11 KO animals. However, the analgesic activity of a number of other opioids with μ actions are significantly attenuated or completely lost in the exon 11 KO mice, including levorphanol, butorphanol, buprenorphine, and nalbuphine (Pan et al., 2009; Majumdar et al., 2011b, 2012; Pasternak and Pan, 2013). Thus, these KO models indicate the existence of two classes of μ opioids, the traditional morphinelike ones acting through full-length receptors and atypical ones working through the truncated 6TM splice variants.
Recently, we synthesized a highly potent analgesic, IBNtxA (3′-iodobenzoyl-6β-naltrexamide), with a very unusual pharmacologic profile (Majumdar et al., 2011b, 2012; Pasternak and Pan, 2013). Despite its high analgesic potency in mice, it exhibits no respiratory depression at doses up to 5-fold greater than its analgesic ED50, has little effect on gastrointestinal transit, and fails to show either reward or aversive behavior in a conditioned place preference paradigm. When administered chronically, it produces no physical dependence and shows no cross-tolerance to morphine. IBNtxA analgesia is fully retained in the Pintar exon 1 KO, clearly distinguishing its mechanisms from those of morphine. The analgesia was not the result of cross-labeling traditional δ or κ1 receptors because IBNtxA still retained full analgesic activity in a triple KO mouse lacking all traditional opioid receptors generated by crossing Pintar’s exon 1 MOR-1 KO with δ-type opioid receptor (DOR-1) KO and κ-type opioid receptor (KOR-1) KO animals.
Further studies using [125I]IBNtxA in the mouse identified a molecular target for the drug that depends on the truncated 6TM exon 11–associated variants. [125I]IBNtxA labels this site in the mouse brain with subnanomolar affinity and shows a similar affinity and Bmax value in the triple KO mice. Thus, IBNtxA acts through a novel opioid receptor target of action with a favorable side-effect profile in mice. Like the mouse, rats and humans also express truncated 6TM receptors associated with exon 11 (Pasternak et al., 2004; Xu et al., 2007; Pasternak and Pan, 2013). In this study, we examine the pharmacology of IBNtxA in the rat.
Materials and Methods
IBNtxA and [125I]BNtxA were synthesized and their structures confirmed as previously described elsewhere (Majumdar et al., 2011a,b, 2012). Opioids were obtained from the Research Technology Branch of the National Institutes of Health National Institute on Drug Abuse (Rockville, MD) or Tocris Bioscience (Bristol, UK). Miscellaneous chemicals and buffers were purchased from Sigma-Aldrich (St. Louis, MO).
Male Sprague Dawley rats were obtained from Charles River Laboratories (Hollister, CA). All rats were maintained on a 12-hour light/dark cycle with Purina rodent chow Diet 5053 and water available ad libitum, and they were housed in groups of two until testing. All animal studies were approved by the Institutional Animal Care and Use Committee of the Memorial Sloan-Kettering Cancer Center.
Binding Studies.
[125I]IBNtxA binding assays were performed in brain membrane homogenates prepared as previously described elsewhere at a concentration of 0.5–1.0 mg protein/ml (Clark et al., 1988, 1989; Majumdar et al., 2011b). To prevent binding to traditional opioid receptors, binding was performed in the presence of μ (CTAP [d-Phe-Cys-Tyr-d-Trp-Arg-Thr-Pen-Thr-NH2]), κ1 (U50,488H [(trans)-3,4-dichloro-N-methyl-N-[2-(1-pyrrolidinyl)-cyclohexyl]benzeneacetamide]), and δ (DPDPE [d-Pen2,d-Pen5-enkephalin]) blockers at a final concentration of 250 nM each. Nonspecific binding was determined in the presence of levallorphan (1 µM), and only specific binding is reported. Binding reached equilibrium at 25°C after 90 minutes (data not shown). Binding was performed for 90 minutes at 25°C using 0.5–1.0 ml of homogenate. Glass-fiber filters were soaked in 0.5% polyethyleneimine for at least 15 minutes before filtration to minimize the nonspecific binding to the filters.
KD, Bmax, and IC50 were fit by nonlinear regression using GraphPad Prism (GraphPad Software, La Jolla, CA). Each IC50 value was converted to give an apparent
as previously reported elsewhere (Cheng and Prusoff, 1973). Hill slopes were calculated using a linear regression of a probit transform from competition experiments using values from 15–85% inhibition (Litchfield and Wilcoxon, 1949). All experiments were replicated at least three times.
For regional binding assays, rat brains were rapidly removed and placed in ice-cold phosphate-buffered saline (50 mM; pH 7.4) and dissected into the designated regions. Membrane preparations were made and binding performed with [125I]IBNtxA (0.1 nM) in the presence of CTAP, DPDPE, and U50,488 (250 nM each) to block traditional μ, δ, and κ binding. Binding was normalized to the whole brain. Results are the mean ± S.E.M. of three independent experiments. Regional binding was compared using a one-way analysis of variance followed by a Bonferroni multiple-comparison test.
Analgesia Assay.
ED50 values were determined using a cumulative dose-response approach in which rats were tested with escalating doses of IBNtxA 30 minutes after the prior subcutaneous injection using a radiant heat tail-flick paradigm as previously published elsewhere (Ling et al., 1985; Majumdar et al., 2011b) A maximal latency of 10 seconds was used to minimize tissue damage. ED50 values were determined by nonlinear regression analysis (GraphPad Prism). Results are expressed as the percentage of maximum possible effect (%MPE) for each individual rat according to the equation %MPE = [(Observed latency − Baseline latency)/(Maximal latency − Baseline latency)]. To assess for cross-tolerance between IBNtxA and morphine, rats were made tolerant to morphine by the subcutaneous implantation of three 75-mg pellets of morphine freebase each (total dose 225 mg) or placebo pellets under isoflurane anesthesia (Ling et al., 1984).
Respiratory Function Assay.
The effect of the drugs on respiration was assessed using oximetry. Blood oxygen saturations were assessed in awake, freely moving rats (175–225 g) with the MouseOx pulse oximeter system (Starr Life Sciences, Holliston, MA). A 5-second average oxygen saturation value was measured every 5 minutes. Each animal was habituated to the device for 30 minutes to establish a baseline before injection.
Results
In Vivo Actions of IBNtxA.
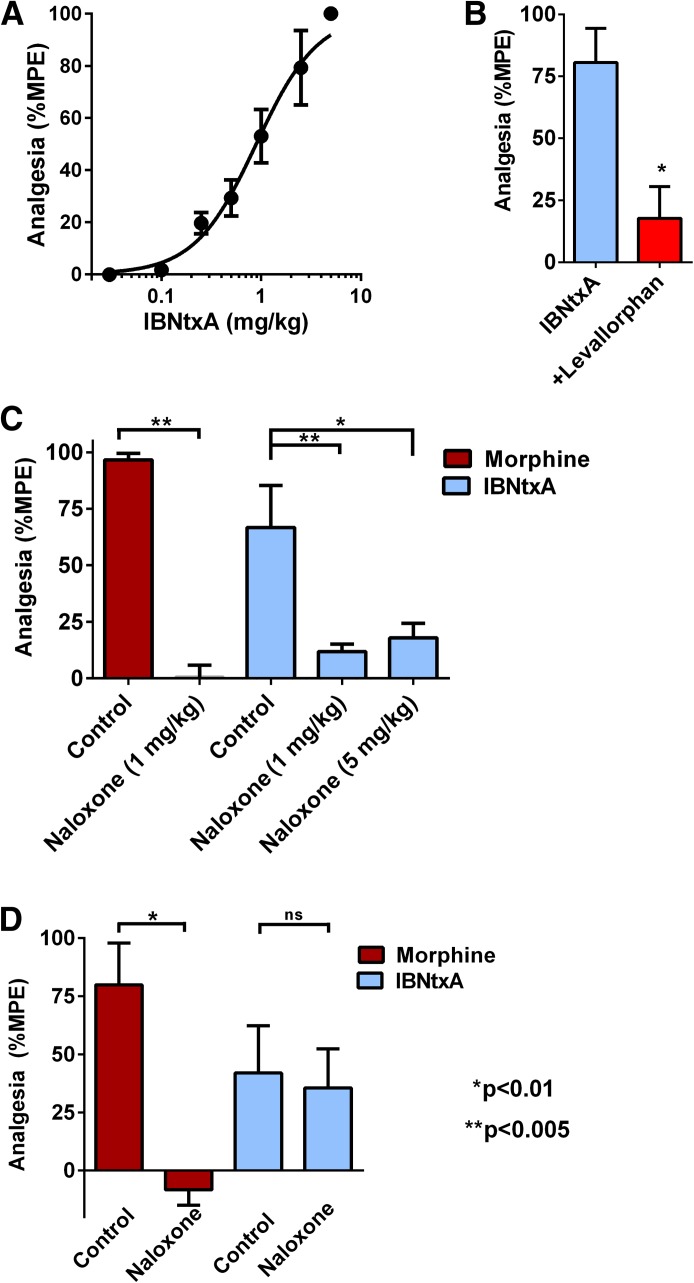
IBNtxA is a potent analgesic in mice in the radiant heat tail-flick assay. Similarly, it was an effective analgesic in rats (Fig. 1). Time action studies revealed a peak effect at 30 minutes after subcutaneous administration (not shown), similar to most opioids. At peak effect, IBNtxA had an analgesic ED50 of 0.89 mg/kg (95% confidence interval, 0.69–1.2) (Fig. 1A). This was approximately 2-fold less potent than in mice (ED50 = 0.48 ± 0.05 mg/kg) (Majumdar et al., 2011b) and approximately 4-fold more potent than morphine.
Fig. 1.
IBNtxA analgesia and sensitivity toward naloxone. (A) Groups of rats (n = 4) were assessed for IBNtxA analgesia at peak effect in three independent experiments (n = 12 total) in a cumulative dose-response paradigm where animals received escalating doses of IBNtxA to generate the analgesic dose-response curve. The ED50 was 0.89 mg/kg (95% confidence interval, 0.69–1.2). (B) Groups of rats (n = 8) received IBNtxA (2 mg/kg) and were tested for analgesia after 30 minutes, immediately given levallorphan (1 mg/kg s.c.), and tested 20 minutes later. (C) Groups of rats (n = 4) received either morphine (8 mg/kg s.c.) or IBNtxA (2 mg/kg s.c.) immediately after the injection of saline or the indicated dose of naloxone. Naloxone significantly reversed morphine analgesia (Bonferroni multiple comparison test, P < 0.0001) as well as IBNtxA (P < 0.005 at 1 mg/kg, P < 0.01 at 5 mg/kg). (D) To assess the effect of a low naloxone dose, groups of rats were injected with equivalent analgesic doses of morphine 8 mg/kg s.c. (n = 4) or IBNtxA 2 mg/kg s.c. (n = 5). Animals were tested in the tail-flick assay after 20 minutes for morphine and 30 minutes for IBNtxA. They were then immediately administered naloxone (0.1 mg/kg s.c.) and tested again in the tail-flick assay 20 minutes later. Results are reported as percentage of maximum possible effect (%MPE). The results for morphine revealed a significant reduction by naloxone (paired t test, P < 0.005). There was no significant (ns) naloxone effect against IBNtxA.
Morphine actions are readily reversed by the antagonist naloxone. The opioid antagonist levallorphan potently reverses IBNtxA in mice. Similarly, levallorphan effectively reversed IBNtxA in the rat (Fig. 1B). We next determined the sensitivity of IBNtxA analgesia to naloxone. First, we compared the ability of naloxone to reverse morphine and IBNtxA analgesia (Fig. 1C). At 1 mg/kg, naloxone fully reversed morphine’s response. Naloxone also effectively antagonized the actions of IBNtxA at both 1 and 5 mg/kg s.c., confirming the opioid nature of the response. However, IBNtxA analgesia was far less sensitive to naloxone than morphine (Fig. 1D). A lower naloxone dose (0.1 mg/kg s.c.) still fully reversed morphine analgesia but had no effect on IBNtxA activity. This relative insensitivity of IBNtxA analgesia to naloxone is similar to that previously observed in mice (Majumdar et al., 2011b).
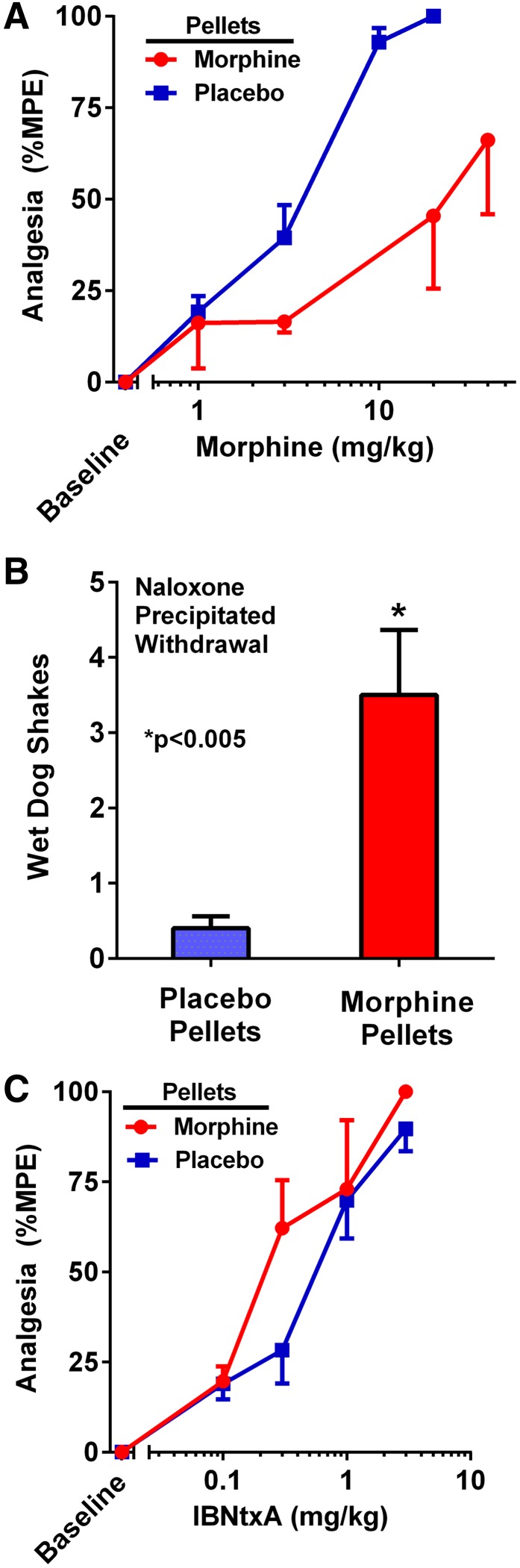
Cross-tolerance is often used to demonstrate a common mechanism of action. Traditionally, μ opioids show cross-tolerance to morphine, although this is often incomplete (for a review, see Pasternak and Pan, 2013). One standard paradigm is to implant rats with pellets of free-base morphine, resulting in its slow release over days (Cicero and Meyer, 1973; Yoburn et al., 1985). In this approach, rats are implanted with three morphine free-base pellets each (225 mg total), and the rats are assessed 3 days later. This paradigm leads to marked morphine tolerance and dependence. For the first few hours, all rats displayed full analgesia. When they were assessed after 3 days, no residual analgesia was detected despite the continued presence of the morphine pellets. The rats were both tolerant and dependent. When challenged with acute doses, the morphine-pelleted animals displayed a 6-fold decrease in morphine analgesia (P < 0.001), with the ED50 increasing from 3.3 to 20.5 mg/kg s.c. (Fig. 2A). The ability of naloxone to precipitate withdrawal (e.g., wet-dog shakes) indicated that the morphine-pelleted mice were physically dependent (Fig. 2B). However, the morphine-pelleted animals retained full analgesic sensitivity to IBNtxA (Fig. 2C), as shown by the absence of a shift of the dose-response curve. Furthermore, administration of IBNtxA to the morphine-pelleted animals failed to demonstrate any evidence of withdrawal (e.g., wet-dog shakes).
Fig. 2.
Assessment of cross-tolerance between morphine and IBNtxA. Analgesic cross-tolerance was assessed in groups of rats, each implanted with either three morphine (n = 4) or placebo (n = 10) pellets and tested with IBNtxA 72 hours later. (A) The next day cumulative morphine dose-response curves revealed a morphine ED50 of 20.5 mg/kg (95% confidence interval [CI], 7.7–54) in the morphine-pelleted rats compared with 3.3 mg/kg (95% CI, 2.7–4.0) in placebo-pelleted animals (P < 0.001). (B) After the morphine cumulative dose-response testing, animals received naloxone (1 mg/kg s.c.). Morphine-pelleted animals displayed a significantly greater number of wet-dog shakes relative to placebo-pelleted controls (P < 0.005). (C) In morphine-pelleted animals, the ED50 for IBNtxA was 0.26 mg/kg (95% CI, 0.15–0.45) compared with 0.52 mg/kg (95% CI, 0.37–0.75) in the placebo-pelleted rats. %MPE, percentage of maximum possible effect.
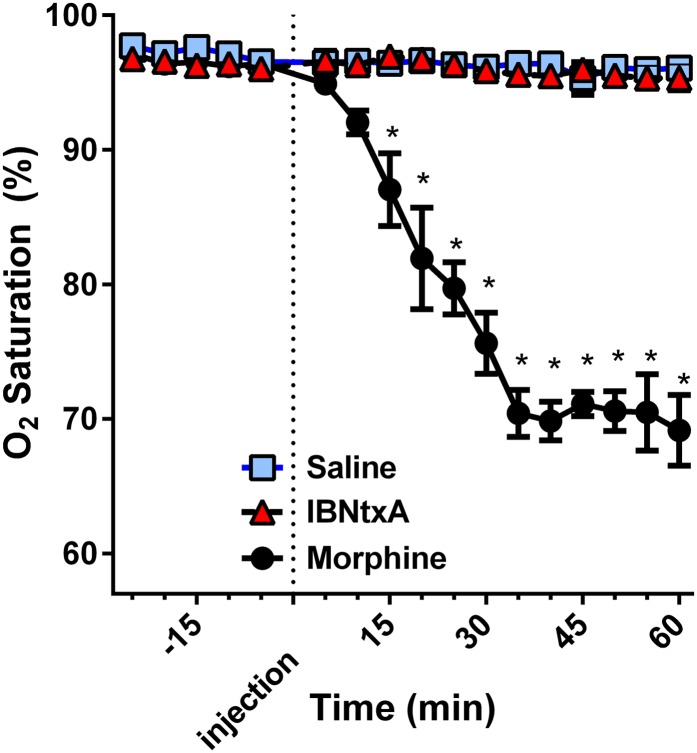
Respiratory depression remains one of the most problematic opioid side effects. Although low morphine doses are used to ameliorate dyspnea in a range of pulmonary problems, higher doses of opioids can be life threatening. In contrast to morphine, IBNtxA does not affect the respiratory rate in mice (Majumdar et al., 2011b). We now examined its effects in rats, using oximetry to determine oxygen saturation levels. Using doses of drug approximately 4- to 5-fold greater than their respective analgesic ED50, we compared IBNtxA to morphine (Fig. 3). As anticipated, the high morphine dose significantly depressed oxygen saturation, lowering it by 30%, a symptomatic clinical value. In marked contrast, an equianalgesic IBNtxA dose was indistinguishable from the saline group, displaying no evidence of any respiratory depression.
Fig. 3.
Effect of IBNtxA on respiratory function. Rats were randomly assigned to receive saline (n = 4), IBNtxA (4 mg/kg, n = 4), or morphine (20 mg/kg, n = 4). Using oximetry, each animal's O2 saturation was measured for 5 seconds every 5 minutes for 25 minutes before and 60 minutes after injection of the treatment. There were no differences between IBNtxA and saline at any time point, whereas morphine significantly depressed O2 saturation (*P < 0.0001 at each time point) from 15–60 minutes as determined by Bonferroni multiple-comparison test after a repeated-measures analysis of variance.
IBNtxA Binding Sites.
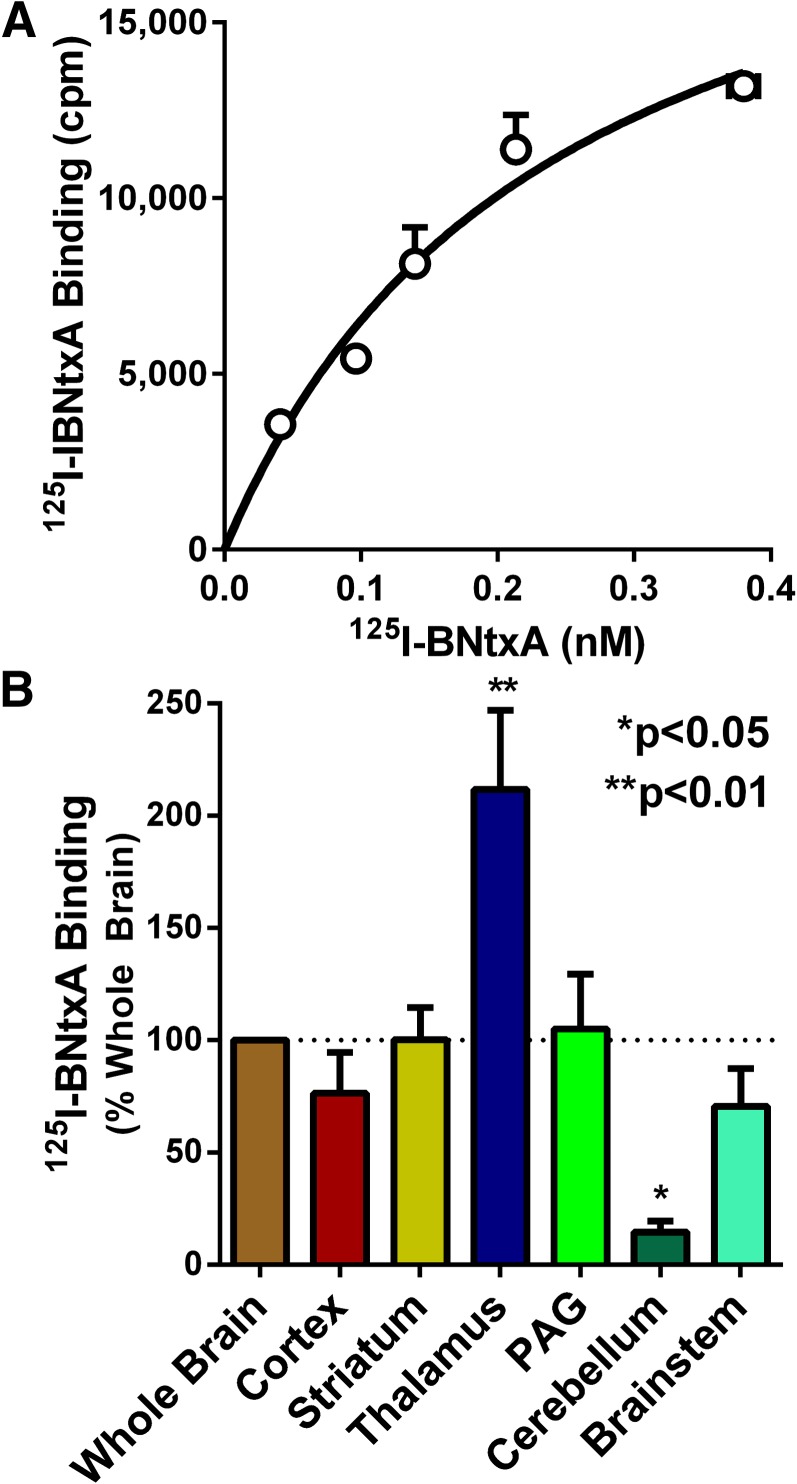
In the mouse, [125I]IBNtxA labels a target in the brain with high affinity that is pharmacologically distinct from any of the traditional opioid receptors (Majumdar et al., 2011b). Studies in a series of KO mice indicate that the binding site is dependent on a set of μ-opioid receptor splice variants associated with exon 11 that contain only six transmembrane domains. The binding site is insensitive to selective μ, δ, and κ1 opioids and persists in a triple KO mouse lacking δ (DOR-1), κ1 (KOR-1), and traditional full-length MOR-1 variants but which still expresses exon 11–associated truncated ones. [125I]IBNtxA binding in the rat brain displayed a unique binding site with similarities to the one in mice.
Because knockouts of the traditional μ, δ, and κ1 sites are not available with rats, we employed a blocking strategy to examine these sites. In the mouse, the selective agents CTAP (μ), DPDPE (δ), and U50,488H (κ1) showed minimal activity against [125I]IBNtxA binding in the triple KO animals (Majumdar et al., 2011b). We therefore examined [125I]IBNtxA binding in the presence of high concentrations of the three selective compounds (250 nM each). In the presence of these blocking concentrations of CTAP, DPDPE, and U50,488H, [125I]IBNtxA still labeled with high affinity a binding site in rat brain homogenates (KD = 0.22 ± 0.02 nM) (Fig. 4A). Although the observed affinity for the site is virtually identical to that seen in mouse triple KO brain (KD = 0.16 ± 0.04 nM), the Bmax in rats (15 ± 2 fmol/mg protein) was approximately 4-fold lower than in the triple KO mice (Bmax = 60.8 ± 1.6 fmol/mg protein) (Majumdar et al., 2011b), perhaps explaining the lower analgesic potency in the rat relative to the mouse.
Fig. 4.
[125I]IBNtxA binding in rat brain. (A) Saturation studies were performed using brain homogenates in the presence of μ (CTAP), δ (DPDPE), or κ1 (U50,488H) blockers at 250 nM. Nonspecific binding was defined by levallorphan (1 µM). Only specific binding is reported. The assay was replicated 3 times, and the results shown are from a representative experiment. Nonlinear regression analysis using GraphPad Prism generated an average KD from the three independent experiments of 0.22 ± 0.02 nM and a Bmax of 15 ± 2 fmol/mg protein. (B) Rat brains were rapidly removed and dissected into the indicated regions in ice-cold phosphate-buffered saline, and homogenates were prepared. Binding in each region was determined using [125I]IBNtxA (0.1 nM) in the presence of μ, δ, and κ1 blockers (250 nM each) and normalized to binding from the whole brain. Results are the mean of three independent experiments. Binding differed across regions (one-way analysis of variance, P = 0.0003), with the thalamus showing significantly more binding than the whole-brain control (Bonferroni multiple comparison test, P < 0.01) and the cerebellum showing significantly less binding than the whole-brain control (P < 0.05). PAG, periaqueductal gray.
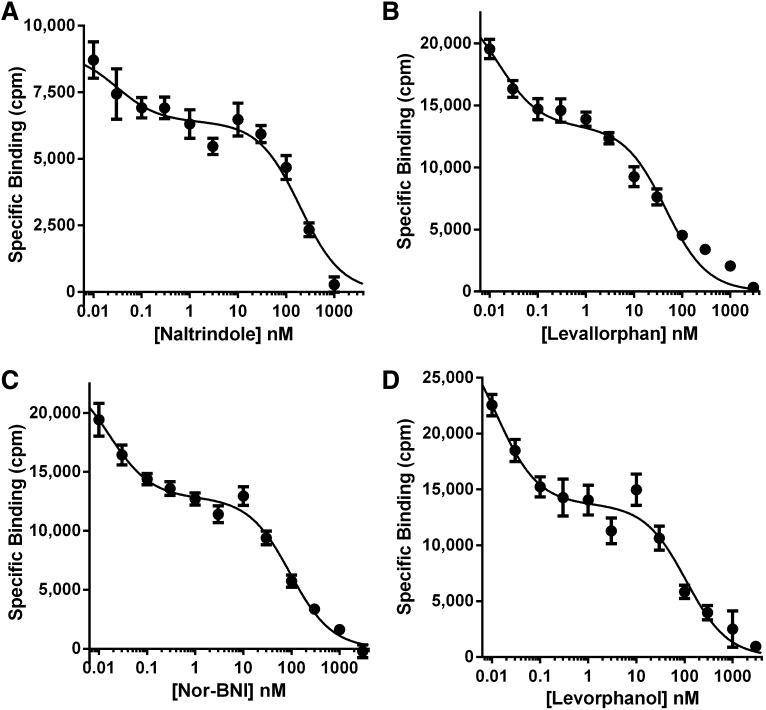
The binding selectivity profile of the [125I]IBNtxA site differed from traditional opioid receptors and showed similarities to those seen in the mouse (Table 1). Highly selective μ, δ, and κ1 drugs displayed very poor affinity for the site. Yet a variety of other opioids that had previously been associated with the κ3 site (Clark et al., 1989) displayed far greater affinity. Many of these drugs displayed shallow competition curves, as determined by logit plots (Hill slopes) suggesting that [125I]IBNtxA might be labeling more than one type of site. Selected compounds were chosen for detailed competition curves (Fig. 5; Table 2). The competition curves of the four compounds were clearly biphasic, with a high-affinity and a low-affinity component (Fig. 5). When submitted to nonlinear regression analysis, all four compounds were best fit to a two-site model, with the high-affinity component corresponding to approximately a third of the overall Bmax. The affinity of levallorphan for the mouse site corresponded closely to the high-affinity component in the rat. The high-affinity binding component of the other compounds in the rat displayed affinities at least as great as in the mouse. Of particular interest are naltrindole and norbinaltorphimine (norBNI). Although these drugs are considered highly selective δ and κ1 antagonists, respectively, they also competed a component of [125I]IBNtxA site with subnanomolar affinity.
TABLE 1.
Competition of [125I]IBNtxA binding in rat brain by a series of opioids
Rat brain homogenates were prepared and binding assays performed in the presence of μ, δ, and κ1 blockers, as described in Materials and Methods. Results are the mean ± S.E.M. of three independent determinations, each performed in triplicate. Hill slopes were determined on the binding between 15 and 85% inhibition. A number of compounds showed slopes far less than 1, consistent with binding heterogeneity, indicating that their Ki values may reflect the cumulative inhibition of different affinity sites. Several were examined in further detail (Table 2). The values for the triple KO mouse brain (Majumdar et al., 2011b), calf brain (Clark et al., 1989), and human neuroblastoma SK-N-BE(2)-C cell line (Standifer et al., 1994) are taken from the literature.
| Drug | [125I]IBNtxA |
[3H]NalBzoH (κ3) |
|||
|---|---|---|---|---|---|
| Rat |
Triple KO Mouse Brain |
Calf Striatum |
Human BE(2)-C Cells |
||
| Ki | Hill Slope | Ki | Ki | Ki | |
| nM | nM | nM | nM | ||
| μ | |||||
| Morphine | 530 ± 130 | −0.70 ± 0.18 | >1000 | 32.8 ± 2.2 | 11.1 ± 4 |
| Fentanyl | 201 ± 92 | −0.72 ± 0.23 | 226 ± 40 | ||
| DAMGO | >1000 | >1000 | 8.2 ± 1.9 | 18.3 ± 4.5 | |
| CTAP | >1000 | >1000 | |||
| Naloxone | 373 ± 156 | −0.83 ± 0.19 | 51.9 ± 1.4 | 8.4 ± 0.9 | 13.8 ± 6 |
| δ | |||||
| Naltrindole | 43.3 ± 11 | −0.50a | 26.3 ± 2.3 | 25.6 ± 8.7 | |
| DPDPE | >1000 | >1000 | >350 | >1000 | |
| κ1 | |||||
| U50,488 | >1000 | >1000 | >350 | >1000 | |
| norBNI | 651 ± 350 | −0.53 ± 0.25 | 3.3 ± 1.9 | 103 ± 85 | 71 ± 9.6 |
| Other opioid | |||||
| Levorphanol | 90 ± 42 | −0.55 ± 0.32 | 8.8 ± 2.5 | ||
| Ketocyclazocine | 150 ± 34 | −0.83 ± 0.18 | 0.04 ± 0.01 | 4.5 ± 0.8 | |
| Ethylketocyclazocine | 5.0 ± 1.5 | −0.74 ± 0.06 | 0.21 ± 0.11 | 1.4 ± 0.5 | |
| Nalorphine | 52 ± 19 | −0.63 ± 0.44 | 3.71 ± 1.45 | ||
| NalBzoH | 30 ± 7 | −0.75 ± 0.16 | 0.59 ± 0.15 | 0.9 ± 0.19 | |
| Levallorphan | 23 ± 13 | −0.49 ± 0.28 | 0.34 ± 0.02 | 2.2 ± 0.56 | |
| Buprenorphine | 5.7 ± 0.7 | −0.65 ± 0.24 | 1.8 ± 0.93 | ||
| σ1 | |||||
| (+)-Pentazocine | >1000 | >1000 | |||
DAMGO, Tyr-d-Ala-Gly-N-methyl-Phe-Gly-ol.
This value represents a single determination.
Fig. 5.
[125I]IBNtxA competition studies. Rat brain homogenate was incubated with [125I]IBNtxA (0.1 nM) with blockers at the indicated concentrations of the specified competitor. Nonspecific binding was determined using levallorphan (1 µM). Results are from three independent replications. Each data set was fit with both a one-site and two-site model using GraphPad Prism, and the models were compared using an extra-sum-of-squares F-test. The two-site model was preferred for each of the four drugs tested (P < 0.0001 for each).
TABLE 2.
Detailed competition studies of selected opioids on [125I]IBNtxA binding
Rat brain homogenates were prepared and binding assays performed as described in Materials and Methods. Results are the mean ± S.E.M. of three independent determinations, each performed in triplicate. Competitions were best fit by nonlinear regression analysis.
| Drug | Ki High Affinity | Ki Low Affinity | Fraction of High-Affinity Sites | Two-Site versus One-Site Model P Value | |
|---|---|---|---|---|---|
| nM | |||||
| Naltrindole | 0.06 ± 0.03 | 321 ± 160 | 27 ± 1% | <0.001 | |
| Levallorphan | 0.12 ± 0.03 | 119 ± 26 | 35 ± 5% | <0.001 | |
| norBNI | 0.08 ± 0.01 | 184 ± 23 | 34 ± 7% | <0.001 | |
| Levorphanol | 0.07 ± 0.01 | 219 ± 44 | 42 ± 8% | <0.001 | |
Finally, we examined the regional distribution of these binding sites. After the dissection of various regions, we performed homogenate binding studies (Fig. 4B). Binding was readily demonstrated in all the regions examined. The levels in cortex, striatum, periaqueductal gray, and brainstem were quite similar to those in whole brain. However, the binding in the thalamus was over 2-fold greater (P < 0.01) and in the cerebellum was 85% lower (P < 0.05).
Discussion
Much effort has been dedicated to the development of opioids that lack side effects and potential for abuse. The ability to dissociate morphine analgesia from many of its side effects, including respiratory depression, constipation, and physical dependence, were documented almost 30 years ago (Ling et al., 1983, 1984, 1985, 1989; Heyman et al., 1988; Paul and Pasternak, 1988). However, only recently have inroads been made in the synthesis of selective compounds. Two general approaches have been used. One involves the role of biased agonism, with biased drugs showing differences in pharmacologic actions based upon their relative activation of β-arrestin compared with G protein mechanisms (Bohn et al., 2002, 2003; Violin and Lefkowitz, 2007; Reiter et al., 2012; Dewire et al., 2013). The ability to differentiate opioid actions mediated through the same binding site with biased signaling offers major advantages in the design of ligands for G protein–coupled receptors.
The demonstration of heterodimerization of opioid receptors has yielded several interesting approaches toward the development of novel opioids. Opioid heterodimers can form targets with unique binding and pharmacologic profiles. MOR-1/DOR-1 dimers have been proposed (Gomes et al., 2013; Ferre et al., 2014), as has a DOR-1/KOR-1 dimer (Jordan and Devi, 1999) that corresponds to the pharmacologically defined κ2 receptor (Zukin et al., 1988). MOR-1 also dimerizes with opioid receptor–like 1 receptors to generate a target with a novel pharmacologic profile (Pan et al., 2002). More recently, an intriguing compound was reported that targets a MOR-1/KOR-1 heterodimer (Yekkirala et al., 2011). N-Naphthoyl-β-naltrexamine is a potent analgesic that lacks physical dependence or reward behavior. Structurally, it is similar to IBNtxA in that it, too, is based upon the 6β-naltrexamine scaffold. However, they differ in their mechanisms of action. N-Naphthoyl-β-naltrexamine acts through a heterodimer of MOR-1 and KOR-1 while IBNtxA retains full analgesic activity in triple KO mice that lack KOR-1 (Yekkirala et al., 2011).
The extensive alternative splicing of the μ-opioid receptor gene with its three classes of variants offers additional targets for drug development (for a review, see Pasternak and Pan, 2013). The full-length variants with their alternative 3′-splicing all bind traditional μ opioids. Their different C terminus sequences influence their distribution within the cell (Abbadie et al., 2001) and among regions (Abbadie et al., 2000a,b) and their intrinsic activity (Bolan et al., 2004) and even their ability to internalize (Caron et al., 2000; Abbadie and Pasternak, 2001; Tanowitz et al., 2008). Furthermore, each variant is also subject to biased signaling, further extending their possibilities.
The truncated exon 11–associated 6TM variant class that we initially described over a decade ago (Pan et al., 2001) offers another approach toward the development of improved analgesics. Using a scaffold based upon either naltrexone (Majumdar et al., 2011a,b) or naloxone (Majumdar et al., 2012), we generated a series of compounds capable of acting through the 6TM variants of mice with very unique pharmacologic profiles, including IBNtxA. In mice, IBNtxA is a potent analgesic lacking many of the side effects of traditional opioids. To investigate whether this advantageous pharmacologic profile seen in mice extends to other species also expressing six transmembrane domain receptors, we examined the pharmacology of IBNtxA in rats.
Our results establish a similar pharmacology between rats and mice, although rats are somewhat less sensitive, which may be related to their lower density of [125I]IBNtxA-binding sites relative to mice. IBNtxA is a potent analgesic in rats. At doses as high as 5-fold its analgesic ED50, it fails to show any changes in oxygen saturation, a sensitive measure of respiratory depression, and it shows no cross-tolerance to morphine. Furthermore, when administered to highly morphine-dependent animals, it did not precipitate any withdrawal signs (Majumdar et al., 2011a).
The target responsible for the actions of IBNtxA remains unclear. In both mice and rats, [125I]IBNtxA labels a nontraditional site insensitive to traditional selective μ (morphine and Tyr-d-Ala-Gly-N-methyl-Phe-Gly-ol), δ (DPDPE), or κ1 (U50,488H) sites. The studies in mice, which have the advantage of a range of genetic models, strongly indicate a role for truncated 6TM splice variants of MOR-1 in the nontraditional [125I]IBNtxA binding sites, which persist in the triple KO mice and are lost in the exon 11 KO mouse. Like mice and humans, rats also express truncated 6TM variants. However, the relative abundance of these binding sites in the rat brain (15 fmol/mg protein) is only one-fourth that seen in the triple KO mice (60.8 fmol/mg protein) and far lower than levels seen in BE(2)-C human neuroblastoma cell lines (30–75 fmol/mg protein). The lower expression levels in the rat may help explain the lower analgesic potency of the drug.
Comparing the binding-selectivity profile in the mouse and rat reveals important similarities and some differences. In both species, the nontraditional [125I]IBNtxA binding site is not competed by many classic μ-, δ-, and κ1-selective agents. [125I]IBNtxA binding in the rat is heterogeneous, based upon the shallow competition curves and the Hill coefficients less than unity. The full curves appear biphasic, and curve fitting supports two sites. When dissected into two components by nonlinear regression analysis, approximately a third of [125I]IBNtxA binding is quite sensitive to naltrindole, levallorphan, norBNI, and levorphanol, with Ki values well below 1 nM, with the remainder of the binding relatively insensitive. The presence of more than one population of labeled sites in the rat illustrates how an overall IC50 that is used to generate an “apparent” Ki value can be misleading. The overall lower levels of [125I]IBNtxA binding in the rat may also contribute to the differences between the values in the mouse and rat, particularly if they change the relative abundance of the two sites. Thus, the estimations of the “Ki” values generated from total [125I]IBNtxA binding must be interpreted cautiously.
Naltrindole and norBNI are considered selective antagonists for δ and κ receptors, respectively. [125I]IBNtxA binding in both mice and rats is modestly sensitive to naltrindole. However, approximately a third of the binding in the rat is competed by naltrindole with a Ki of 60 pM. It seems unlikely that this reflects labeling of δ receptors, considering the inclusion of 250 nM DPDPE. A similar situation is revealed by norBNI competing a portion of [125I]IBNtxA binding very potently (Ki 80 pM). Again, the presence of 250 nM U50,488H argues against labeling traditional κ1 receptors, raising questions on the selectivity of both compounds.
The rat expresses two 6TM variants, rMOR-1G1 and rMOR-1G2. Both contain exon 4 but differ due to 5′-splicing. rMOR-1G2 yields a typical 6TM variant containing the 5′ coding sequence from exon 11. However, rMOR-1G1 splicing is more complicated because the AUG start codon within exon 11 yields a very short peptide of only seven amino acids due to a frame shift. It is still possible to generate a 6TM using the AUG start codon at the beginning of exon 2, in a manner similar to that previously reported for the μ3 receptor (Cadet et al., 2003; Pasternak and Pan, 2013). Although the diversity of binding might be due to these two variants, this seems unlikely because they only differ at the N terminus by the presence of seven amino acids encoded by exon 11 in rMOR-1G2 (MGSGPML) that would be absent in rMOR-1G1. An alternative explanation may involve heterodimerization of a 6TM variant with more than one nonopioid G protein–coupled receptor, leading to the possibility of a family of 6TM-related complexes.
In many respects, the [125I]IBNtxA target resembles the previously reported κ3 sites (Clark et al., 1989). Drugs earlier defined pharmacologically as κ3, including levorphanol (Moulin et al., 1988; Tive et al., 1992), nalorphine (Paul et al., 1991), and NalBzoH (naloxone benzoylhydrazone) (Luke et al., 1988; Gistrak et al., 1989; Paul et al., 1990), compete [125I]IBNtxA binding in the triple KO mouse quite potently (Majumdar et al., 2011b). Equally important, their analgesic actions are greatly diminished in the exon 11 KO mouse, as is IBNtxA (Majumdar et al., 2011b). Thus, NalBzoH analgesia is dependent upon 6TM variants. However, there are some differences between the κ3-binding sites defined with [3H]NalBzoH and the [125I]IBNtxA target, both of which are also seen in human BE(2)-C neuroblastoma cells (Clark et al., 1989; Standifer et al., 1994; Mathis et al., 2001).
The regional expression of [125I]IBNtxA binding varies. Homogenate binding studies show high levels in the thalamus and low levels in the cerebellum. The resolution of the anatomic distributions in the current autoradiography studies is relatively limited, but the pattern in the rat is similar to that in the triple KO mouse seen autoradiographically, which expresses none of the traditional opioid receptors (S. Grinnell, S. Majumdar, V. Le Rouzic, M. Ansonoff, J. E. Pintar, and G. W. Pasternak, submitted manuscript). Thus, the labeling in the rat is consistent with a target including 6TM variants.
In summary, [125I]IBNtxA has identified a unique target in the rat similar to the target in the triple KO mouse. Working through this target, IBNtxA produces a potent analgesia in the radiant heat tail-flick assay in both the rat and mouse. Additional studies in the mouse indicate that IBNtxA also is potent in inflammatory and neuropathic hypersensitivity and spontaneous pain (Wieskopf et al., in press). Unlike traditional opioids, IBNtxA produces its analgesia in the rat without producing respiratory depression or physical dependence. It shows no cross-tolerance to morphine and does not precipitate withdrawal in morphine-dependent animals. Together, these observations in both the rat and the mouse suggest that the truncated 6TM variants may provide a valuable target for the development of novel analgesics lacking the limitations of current drugs.
Abbreviations
- CTAP
d-Phe-Cys-Tyr-d-Trp-Arg-Thr-Pen-Thr-NH2
- DOR-1
δ-type opioid receptor
- DPDPE
d-Pen2,d-Pen5-enkephalin
- IBNtxA
3′-iodobenzoyl-6β-naltrexamdide
- KO
knockout
- KOR-1
κ-type opioid receptor
- MOR-1
μ-opioid receptor
- NalBzoH
naloxone benzoylhydrazone
- norBNI
norbinaltorphimine
- TM
transmembrane domain
- U50,488H
(trans)-3,4-dichloro-N-methyl-N-[2-(1-pyrrolidinyl)-cyclohexyl]benzeneacetamide
Authorship Contributions
Participated in research design: Grinnell, Majumdar, Pasternak.
Conducted experiments: Grinnell, Majumdar, Narayan, Le Rouzic, Ansonoff.
Contributed new reagents or analytic tools: Majumdar, Ansonoff, Pintar, Pasternak.
Performed data analysis: Grinnell, Narayan, Le Rouzic, Pasternak.
Wrote or contributed to the writing of the manuscript: Grinnell, Pasternak.
Footnotes
This work was supported, in part, by the National Institutes of Health National Institute on Drug Abuse [Grants R01-DA002615, R01-DA07242, and R01-DA06241]; the Harrington Research Institute; Mr. William H. Goodwin and Mrs. Alice Goodwin and the Commonwealth Foundation for Cancer Research; the Experimental Therapeutics Center of Memorial Sloan-Kettering Cancer Center (to G.W.P.); a fellowship from the PhRMA Foundation (to S.G.G.); and a core grant from the National Institutes of Health National Cancer Institute [Grant CA08748] (to the Memorial Sloan-Kettering Cancer Center).
References
- Abbadie C, Pan Y, Drake CT, Pasternak GW. (2000a) Comparative immunohistochemical distributions of carboxy terminus epitopes from the mu-opioid receptor splice variants MOR-1D, MOR-1 and MOR-1C in the mouse and rat CNS. Neuroscience 100:141–153 [DOI] [PubMed] [Google Scholar]
- Abbadie C, Pan Y, Pasternak GW. (2000b) Differential distribution in rat brain of mu opioid receptor carboxy terminal splice variants MOR-1C-like and MOR-1-like immunoreactivity: evidence for region-specific processing. J Comp Neurol 419:244–256 [DOI] [PubMed] [Google Scholar]
- Abbadie C, Pasternak GW. (2001) Differential in vivo internalization of MOR-1 and MOR-1C by morphine. Neuroreport 12:3069–3072 [DOI] [PubMed] [Google Scholar]
- Abbadie C, Pasternak GW, Aicher SA. (2001) Presynaptic localization of the carboxy-terminus epitopes of the mu opioid receptor splice variants MOR-1C and MOR-1D in the superficial laminae of the rat spinal cord. Neuroscience 106:833–842 [DOI] [PubMed] [Google Scholar]
- Bare LA, Mansson E, Yang D. (1994) Expression of two variants of the human μ opioid receptor mRNA in SK-N-SH cells and human brain. FEBS Lett 354:213–216 [DOI] [PubMed] [Google Scholar]
- Bohn LM, Gainetdinov RR, Sotnikova TD, Medvedev IO, Lefkowitz RJ, Dykstra LA, Caron MG. (2003) Enhanced rewarding properties of morphine, but not cocaine, in beta(arrestin)-2 knock-out mice. J Neurosci 23:10265–10273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn LM, Lefkowitz RJ, Caron MG. (2002) Differential mechanisms of morphine antinociceptive tolerance revealed in (beta)arrestin-2 knock-out mice. J Neurosci 22:10494–10500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolan EA, Pan YX, Pasternak GW. (2004) Functional analysis of MOR-1 splice variants of the mouse mu opioid receptor gene Oprm. Synapse 51:11–18 [DOI] [PubMed] [Google Scholar]
- Cadet P, Mantione KJ, Stefano GB. (2003) Molecular identification and functional expression of mu 3, a novel alternatively spliced variant of the human mu opiate receptor gene. J Immunol 170:5118–5123 [DOI] [PubMed] [Google Scholar]
- Caron MG, Bohn LM, Barak LS, Abbadie C, Pan Y-X and Pasternak GW (2000) Mu opioid receptor spliced variant, MOR1D, internalizes after morphine treatment in HEK-293 cells in Abstracts: Society for Neuroscience 30th Annual Meeting; 2000 Nov 4–9; New Orleans, LA. no. 783.3, Society for Neuroscience, Washington, DC.
- Cheng Y, Prusoff WH. (1973) Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol 22:3099–3108 [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Meyer ER. (1973) Morphine pellet implantation in rats: quantitative assessment of tolerance and dependence. J Pharmacol Exp Ther 184:404–408 [PubMed] [Google Scholar]
- Clark JA, Houghten R, Pasternak GW. (1988) Opiate binding in calf thalamic membranes: a selective mu 1 binding assay. Mol Pharmacol 34:308–317 [PubMed] [Google Scholar]
- Clark JA, Liu L, Price M, Hersh B, Edelson M, Pasternak GW. (1989) Kappa opiate receptor multiplicity: evidence for two U50,488-sensitive kappa1 subtypes and a novel kappa3 subtype. J Pharmacol Exp Ther 251:461–468 [PubMed] [Google Scholar]
- DeWire SM, Yamashita DS, Rominger DH, Liu G, Cowan CL, Graczyk TM, Chen XT, Pitis PM, Gotchev D, Yuan C, et al. (2013) A G protein-biased ligand at the μ-opioid receptor is potently analgesic with reduced gastrointestinal and respiratory dysfunction compared with morphine. J Pharmacol Exp Ther 344:708–717 [DOI] [PubMed] [Google Scholar]
- Du Y-L, Elliot K, Pan Y-X, Pasternak GW, Inturrisi CE. (1997) A splice variant of the mu opioid receptor is present in human SHSY-5Y cells in Abstracts: Society for Neuroscience 27th Annual Meeting; 1997 Oct 25–29; New Orleans, LA. p 1206, Society for Neuroscience, Washington, DC [Google Scholar]
- Ferré S, Casadó V, Devi LA, Filizola M, Jockers R, Lohse MJ, Milligan G, Pin JP, Guitart X. (2014) G protein-coupled receptor oligomerization revisited: functional and pharmacological perspectives. Pharmacol Rev 66:413–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gistrak MA, Paul D, Hahn EF, Pasternak GW. (1989) Pharmacological actions of a novel mixed opiate agonist/antagonist: naloxone benzoylhydrazone. J Pharmacol Exp Ther 251:469–476 [PubMed] [Google Scholar]
- Gomes I, Fujita W, Gupta A, Saldanha SA, Negri A, Pinello CE, Eberhart C, Roberts E, Filizola M, Hodder P, et al. (2013) Identification of a μ-δ opioid receptor heteromer-biased agonist with antinociceptive activity. Proc Natl Acad Sci USA 110:12072–12077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn EF, Carroll-Buatti M, Pasternak GW. (1982) Irreversible opiate agonists and antagonists: the 14-hydroxydihydromorphinone azines. J Neurosci 2:572–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn EF, Pasternak GW. (1982) Naloxonazine, a potent, long-lasting inhibitor of opiate binding sites. Life Sci 31:1385–1388 [DOI] [PubMed] [Google Scholar]
- Heyman JS, Williams CL, Burks TF, Mosberg HI, Porreca F. (1988) Dissociation of opioid antinociception and central gastrointestinal propulsion in the mouse: studies with naloxonazine. J Pharmacol Exp Ther 245:238–243 [PubMed] [Google Scholar]
- Jordan BA, Devi LA. (1999) G-protein-coupled receptor heterodimerization modulates receptor function. Nature 399:697–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling GSF, MacLeod JM, Lee S, Lockhart SH, Pasternak GW. (1984) Separation of morphine analgesia from physical dependence. Science 226:462–464 [DOI] [PubMed] [Google Scholar]
- Ling GSF, Paul D, Simantov R, Pasternak GW. (1989) Differential development of acute tolerance to analgesia, respiratory depression, gastrointestinal transit and hormone release in a morphine infusion model. Life Sci 45:1627–1636 [DOI] [PubMed] [Google Scholar]
- Ling GSF, Simantov R, Clark JA, Pasternak GW. (1986) Naloxonazine actions in vivo. Eur J Pharmacol 129:33–38 [DOI] [PubMed] [Google Scholar]
- Ling GSF, Spiegel K, Lockhart SH, Pasternak GW. (1985) Separation of opioid analgesia from respiratory depression: evidence for different receptor mechanisms. J Pharmacol Exp Ther 232:149–155 [PubMed] [Google Scholar]
- Ling GSF, Spiegel K, Nishimura SL, Pasternak GW. (1983) Dissociation of morphine’s analgesic and respiratory depressant actions. Eur J Pharmacol 86:487–488 [DOI] [PubMed] [Google Scholar]
- Litchfield JT, Jr, Wilcoxon F. (1949) A simplified method of evaluating dose-effect experiments. J Pharmacol Exp Ther 96:99–113 [PubMed] [Google Scholar]
- Luke MC, Hahn EF, Price M, Pasternak GW. (1988) Irreversible opiate agonists and antagonists: V. Hydrazone and acylhydrazone derivatives of naltrexone. Life Sci 43:1249–1256 [DOI] [PubMed] [Google Scholar]
- Majumdar S, Burgman M, Haselton N, Grinnell S, Ocampo J, Pasternak AR, Pasternak GW. (2011a) Generation of novel radiolabeled opiates through site-selective iodination. Bioorg Med Chem Lett 21:4001–4004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar S, Grinnell S, Le Rouzic V, Burgman M, Polikar L, Ansonoff M, Pintar J, Pan YX, Pasternak GW. (2011b) Truncated G protein-coupled mu opioid receptor MOR-1 splice variants are targets for highly potent opioid analgesics lacking side effects. Proc Natl Acad Sci USA 108:19778–19783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar S, Subrath J, Le Rouzic V, Polikar L, Burgman M, Nagakura K, Ocampo J, Haselton N, Pasternak AR, Grinnell S, et al. (2012) Synthesis and evaluation of aryl-naloxamide opiate analgesics targeting truncated exon 11-associated μ opioid receptor (MOR-1) splice variants. J Med Chem 55:6352–6362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis JP, Mandyam CD, Altememi GF, Pasternak GW, Standifer KM. (2001) Orphanin FQ/nociceptin and naloxone benzoylhydrazone activate distinct receptors in BE(2)-C human neuroblastoma cells. Neurosci Lett 299:173–176 [DOI] [PubMed] [Google Scholar]
- Moulin DE, Ling GSF, Pasternak GW. (1988) Unidirectional analgesic cross-tolerance between morphine and levorphanol in the rat. Pain 33:233–239 [DOI] [PubMed] [Google Scholar]
- Pan YX, Bolan E, Pasternak GW. (2002) Dimerization of morphine and orphanin FQ/nociceptin receptors: generation of a novel opioid receptor subtype. Biochem Biophys Res Commun 297:659–663 [DOI] [PubMed] [Google Scholar]
- Pan YX, Xu J, Bolan E, Abbadie C, Chang A, Zuckerman A, Rossi G, Pasternak GW. (1999) Identification and characterization of three new alternatively spliced mu-opioid receptor isoforms. Mol Pharmacol 56:396–403 [DOI] [PubMed] [Google Scholar]
- Pan YX, Xu J, Bolan E, Moskowitz HS, Xu M, Pasternak GW. (2005) Identification of four novel exon 5 splice variants of the mouse mu-opioid receptor gene: functional consequences of C-terminal splicing. Mol Pharmacol 68:866–875 [DOI] [PubMed] [Google Scholar]
- Pan YX, Xu J, Mahurter L, Bolan E, Xu M, Pasternak GW. (2001) Generation of the mu opioid receptor (MOR-1) protein by three new splice variants of the Oprm gene. Proc Natl Acad Sci USA 98:14084–14089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan YX, Xu J, Xu M, Rossi GC, Matulonis JE, Pasternak GW. (2009) Involvement of exon 11-associated variants of the mu opioid receptor MOR-1 in heroin, but not morphine, actions. Proc Natl Acad Sci USA 106:4917–4922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak DA, Pan L, Xu J, Yu R, Xu MM, Pasternak GW, Pan YX. (2004) Identification of three new alternatively spliced variants of the rat mu opioid receptor gene: dissociation of affinity and efficacy. J Neurochem 91:881–890 [DOI] [PubMed] [Google Scholar]
- Pasternak GW, Childers SR, Snyder SH. (1980) Opiate analgesia: evidence for mediation by a subpopulation of opiate receptors. Science 208:514–516 [DOI] [PubMed] [Google Scholar]
- Pasternak GW, Pan YX. (2013) Mu opioids and their receptors: evolution of a concept. Pharmacol Rev 65:1257–1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul D, Levison JA, Howard DH, Pick CG, Hahn EF, Pasternak GW. (1990) Naloxone benzoylhydrazone (NalBzoH) analgesia. J Pharmacol Exp Ther 255:769–774 [PubMed] [Google Scholar]
- Paul D, Pasternak GW. (1988) Differential blockade by naloxonazine of two μ opiate actions: analgesia and inhibition of gastrointestinal transit. Eur J Pharmacol 149:403–404 [DOI] [PubMed] [Google Scholar]
- Paul D, Pick CG, Tive LA, Pasternak GW. (1991) Pharmacological characterization of nalorphine, a kappa3 analgesic. J Pharmacol Exp Ther 257:1–7 [PubMed] [Google Scholar]
- Reiter E, Ahn S, Shukla AK, Lefkowitz RJ. (2012) Molecular mechanism of β-arrestin-biased agonism at seven-transmembrane receptors. Annu Rev Pharmacol Toxicol 52:179–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuller AG, King MA, Zhang J, Bolan E, Pan YX, Morgan DJ, Chang A, Czick ME, Unterwald EM, Pasternak GW, et al. (1999) Retention of heroin and morphine-6 beta-glucuronide analgesia in a new line of mice lacking exon 1 of MOR-1. Nat Neurosci 2:151–156 [DOI] [PubMed] [Google Scholar]
- Standifer KM, Cheng J, Brooks AI, Honrado CP, Su W, Visconti LM, Biedler JL, Pasternak GW. (1994) Biochemical and pharmacological characterization of mu, delta and kappa3 opioid receptors expressed in BE(2)-C neuroblastoma cells. J Pharmacol Exp Ther 270:1246–1255 [PubMed] [Google Scholar]
- Tanowitz M, Hislop JN, von Zastrow M. (2008) Alternative splicing determines the post-endocytic sorting fate of G-protein-coupled receptors. J Biol Chem 283:35614–35621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tive L, Ginsberg K, Pick CG, Pasternak GW. (1992) Kappa 3 receptors and levorphanol-induced analgesia. Neuropharmacology 31:851–856 [DOI] [PubMed] [Google Scholar]
- Violin JD, Lefkowitz RJ. (2007) Beta-arrestin-biased ligands at seven-transmembrane receptors. Trends Pharmacol Sci 28:416–422 [DOI] [PubMed] [Google Scholar]
- Wieskopf JS, Pan Y-X, Marcovitz J, Tuttle AH, Majumdar S, Pidakala J, Pasternak GW, Mogil JS. (2014) Broad spectrum analgesic efficacy of IBNtxA is mediated by exon 11-associated splice variants of the mu-opioid receptor gene. Pain, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolozin BL, Pasternak GW. (1981) Classification of multiple morphine and enkephalin binding sites in the central nervous system. Proc Natl Acad Sci USA 78:6181–6185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Xu MM, Matulonis J, Rossi G, Pasternak G, Pan Y-X. (2007) Identification and characterization of seven new alternatively spliced variants from the rat mu opioid receptor gene, OPRM in Abstracts: Society for Neuroscience 37th Annual Meeting; 2007 Nov 3–7; San Diego, CA. no. 353.10/G35, Society for Neuroscience, Washington, DC [Google Scholar]
- Xu J, Xu M, Brown T, Rossi GC, Hurd YL, Inturrisi CE, Pasternak GW, Pan YX. (2013) Stabilization of the μ-opioid receptor by truncated single transmembrane splice variants through a chaperone-like action. J Biol Chem 288:21211–21227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Xu M, Rossi GC, Pasternak GW, Pan YX. (2011) Identification and characterization of seven new exon 11-associated splice variants of the rat μ opioid receptor gene, OPRM1. Mol Pain 7:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yekkirala AS, Lunzer MM, McCurdy CR, Powers MD, Kalyuzhny AE, Roerig SC, Portoghese PS. (2011) N-naphthoyl-beta-naltrexamine (NNTA), a highly selective and potent activator of μ/kappa-opioid heteromers. Proc Natl Acad Sci USA 108:5098–5103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoburn BC, Chen J, Huang T, Inturrisi CE. (1985) Pharmacokinetics and pharmacodynamics of subcutaneous morphine pellets in the rat. J Pharmacol Exp Ther 235:282–286 [PubMed] [Google Scholar]
- Zimprich A, Bacher B, Höllt V. (1994) Cloning and expression of an isoform of the rmu-opioid receptor (rmuOR1B). Regul Pept 54:347–348 DOI:10.1016/0167-0115(94)90531-2. [Google Scholar]
- Zimprich A, Simon T, Höllt V. (1995) Cloning and expression of an isoform of the rat μ opioid receptor (rMOR1B) which differs in agonist induced desensitization from rMOR1. FEBS Lett 359:142–146 [DOI] [PubMed] [Google Scholar]
- Zukin RS, Eghbali M, Olive D, Unterwald EM, Tempel A. (1988) Characterization and visualization of rat and guinea pig brain kappa opioid receptors: evidence for kappa 1 and kappa 2 opioid receptors. Proc Natl Acad Sci USA 85:4061–4065 [DOI] [PMC free article] [PubMed] [Google Scholar]