Abstract
There is growing evidence that activation of metabotropic glutamate receptor 4 (mGlu4) leads to anxiolytic- and antipsychotic-like efficacy in rodent models, yet its relevance to depression-like reactivity remains unclear. Here, we present the pharmacological evaluation of ADX88178 [5-methyl-N-(4-methylpyrimidin-2-yl)-4-(1H-pyrazol-4-yl)thiazol-2-amine], a novel potent, selective, and brain-penetrant positive allosteric modulator of the mGlu4 receptor in rodent models of anxiety, obsessive compulsive disorder (OCD), fear, depression, and psychosis. ADX88178 dose-dependently reduced the number of buried marbles in the marble burying test and increased open-arm exploration in the elevated plus maze (EPM) test, indicative of anxiolytic-like efficacy. Target specificity of the effect in the EPM test was confirmed using male and female mGlu4 receptor knockout mice. In mice, ADX88178 reduced the likelihood of conditioned freezing in the acquisition phase of the fear conditioning test, yet had no carryover effect in the expression phase. Also, ADX88178 dose-dependently reduced duration of immobility in the forced swim test, indicative of antidepressant-like efficacy. ADX88178 reduced DOI (2,5-dimethoxy-4-iodoamphetamine)-mediated head twitches (albeit with no dose-dependency), and MK-801 [(5S,10R)-(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine]–induced locomotor hyperactivity in mice, but was inactive in the conditioned avoidance response test in rats. The compound showed good specificity as it had no effect on locomotor activity in mice and rats at efficacious doses. Thus, allosteric activation of mGlu4 receptors can be a promising new therapeutic approach for treatment of anxiety, OCD, fear-related disorders, and psychosis.
Introduction
Metabotropic glutamate (mGlu) receptors belong to the G protein–coupled receptor family and mediate slow, modulatory neurotransmission of glutamate, the primary excitatory neurotransmitter in the mammalian brain. The identified eight mGlu receptors (mGlu1–mGlu8) have been categorized into three groups (I, II, and III) based on sequence homology, second messenger coupling, and pharmacological characterization. The mGlu4 receptor, which, together with mGlu6, mGlu7, and mGlu8 receptors, belongs to group III mGlu receptors and is a predominantly presynaptic receptor, regulating release of glutamate (as an autoreceptor) or GABA (as a heteroreceptor). As abnormalities in the balance of glutamate and GABA neurotransmission have been linked to the etiology of several neuropsychiatric and movement disorders, activation of the mGlu4 receptor may help to restore the balance between these two key neurotransmission systems and lead to therapeutic outcomes. Activation of the mGlu4 receptor has been considered a promising therapeutic approach for the treatment of Parkinson’s disease, anxiety, schizophrenia, depression, chronic pain, epilepsy, and addiction (Celanire and Campo, 2012).
Although efforts to decipher the therapeutic potential of mGlu4 receptor modulators have focused on the role of this receptor in Parkinson’s disease (Duty, 2010; Celanire and Campo, 2012), there is growing evidence that its activators also show efficacy in models of anxiety and schizophrenia. For example, a nonselective group III receptor agonist, ACPT-I [(1S,3R,4S)-1-aminocyclopentane-1,3,4-tricarboxylic acid], showed an anticonflict effect in the rat Vogel test following intrahippocampal administration (Stachowicz et al., 2006) and reduced anxiety-like reactivity in the mouse elevated plus maze (EPM) and stress-induced hyperthermia tests following intraperitoneal administration (Stachowicz et al., 2009). In addition, ACPT-I has been shown to have an antipsychotic-like profile in rodents, reducing MK-801 [(5S,10R)-(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine]– and amphetamine-induced hyperactivity in rats, DOI (2,5-dimethoxy-4-iodoamphetamine)-induced head twitches in mice, and DOI-mediated excitatory postsynaptic potentials in the mouse cortex (Pałucha-Poniewiera et al., 2008).
Discovery and characterization of more selective orthosteric and allosteric activators for the mGlu4 receptor offer much-needed pharmacological tools to further advance our understanding of the receptor in psychiatric disorders. For example, it has been shown that LSP1-2111 [(2S)-2-amino-4-[hydroxy[hydroxy(4-hydroxy-3-methoxy-5-nitro-phenyl)methyl]phosphoryl]butanoic acid], an orthosteric agonist with 30-fold higher potency at the mGlu4 receptor compared with other group III receptors, has an anxiolytic- and antipsychotic-like profile in several rodent models (Wierońska et al., 2012, 2013). Additionally, (−)-PHCCC [N-phenyl-7-(hydroxyimino)cyclopropa[b]chromen-1a-carboxamide], a mGlu4 receptor positive allosteric modulator (PAM), showed anticonflict efficacy in the rat Vogel test when administered to the basolateral amygdala (Stachowicz et al., 2004). Further, the selective mGlu4 receptor PAM Lu AF21934 [(+)-(1S,2R)-N1-(3,4-dichlorophenyl)cyclohexane-1,2-dicarboxamide] has been shown to have an anxiolytic-like profile in the stress-induced hyperthermia, four-plate, and marble burying (MB) tests, while having no antidepressant-like efficacy in the mouse tail suspension test (TST) (Sławińska et al., 2013a). Together with another mGlu4 receptor PAM, Lu AF32615 (4-[(E)-2-phenylvinyl]-2-pyrimidinamine), Lu AF21934 also reduced MK-801– and amphetamine-induced hyperactivity, DOI-induced head twitches, and restored MK-801–induced disruptions of social interactions and spatial delayed alternation tests in rats, indicative of broad efficacy in models relevant to positive, negative symptoms, and cognitive domains of schizophrenia (Sławińska et al., 2013b; Wierońska et al., 2013).
Here we tested a novel, potent, selective, and orally bioavailable mGlu4 receptor PAM, ADX88178 [5-methyl-N-(4-methylpyrimidin-2-yl)-4-(1H-pyrazol-4-yl)thiazol-2-amine] (Fig. 1), in models of anxiety, fear, depression, and psychosis. When assessed in vitro, ADX88178 enhances glutamate-mediated activation of the human and rat mGlu4 receptor with EC50 values of 4 and 9 nM, respectively (Le Poul et al., 2012). When evaluated in vivo, ADX88178 dose-dependently reduced haloperidol-induced catalepsy and potentiated effects of L-DOPA in the forelimb akinesia assay, when performed in rats with bilateral 6-hydroxydopamine lesions of the striatum (Le Poul et al., 2012). In the present report, the anxiolytic- and anti–obsessive compulsive disorder (OCD)–like efficacy of ADX88178 was assessed in the mouse MB test and the EPM test in mice and rats. The target specificity of the anxiolytic-like effect in the mouse EPM test was evaluated using male and female mGlu4 receptor knockout (KO) and wild-type (WT) controls. The potential efficacy of ADX88178 in fear- and stress-related disorders was evaluated in the cued fear conditioning test in mice. The antidepressant-like efficacy of ADX88178 was evaluated in the forced swim test (FST) in mice. The antipsychotic-like efficacy was assessed in the DOI-induced head twitch and MK-801–induced locomotor hyperactivity tests in mice, as well as the conditioned avoidance response (CAR) test in rats. Any potential nonspecific effects of ADX88178 on central nervous system activity were evaluated using the spontaneous locomotor activity (sLMA) test in mice and rats.
Fig. 1.
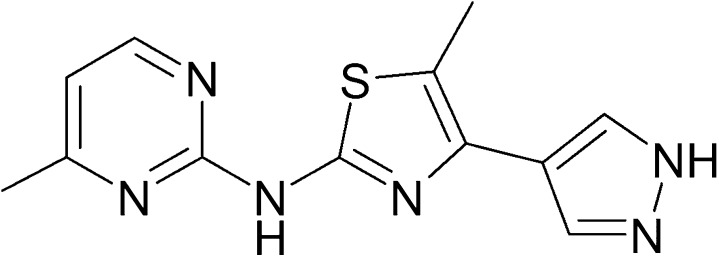
The chemical structure of ADX88178.
Materials and Methods
Plasma Analysis.
The plasma analysis was performed using a tandem liquid chromatography–mass spectrometry method (ultra-performance liquid chromatography system) coupled with a mass spectrometer (API 3200; Applied Biosystems, Zug, Switzerland). To prepare the plasma sample, 150 μl of acetonitrile (protein precipitation) was added to 50 μl of plasma spiked with 10 μl of dimethylsulfoxide for unknown samples, or 10 μl of ADX88178 in dimethylsulfoxide for calibration and quality control (QC) samples. After vortexing and centrifugation (15 minutes, 4°C, 13,200g), 100 μl of supernatant was transferred into the 384-well analytical plate. The limit of quantification was 1–5 ng/ml. Five microliters of the supernatant was then injected into the ultra-performance liquid chromatography system (Waters, Milford, MA). Samples were injected onto an Acquity BEH C18 (Waters) reverse phase column (1.7 μm, 2.1 mm × 50 mm). Elution was performed with a high-pressure linear gradient from 25 to 100% acetonitrile in 10 mM ammonium formate solution at pH 3.5. Run time was 1.5 minutes (retention time = 0.43 minute). The electrospray positive ionization was used in multiple reaction monitoring mode (transition 273.04/132.2). Two calibration curves and 3 QC levels in duplicate were used to quantify and validate the run (Quadratic 1/x). The results generated with the analytical method were validated when 75% of back-calculated calibration points remained within ±20% of theoretical nominal concentrations and 66% of the QC samples were within ±20% of nominal theoretical concentrations.
Animals.
Unless otherwise specified, the studies involved adult male C57BL6/J mice (24–30 g) and Sprague-Dawley rats (240–300 g) purchased from Charles River (L’Arbresle, France). Upon arrival at the animal facility, mice were housed five per cage in type II cages (16 × 22 × 24 cm), whereas rats were housed two per cage in type III cages (22 × 37 × 18 cm). Animals were maintained on a 12-hour light/dark cycle (lights on from 07:00 to 19:00 hours) under constant temperature (22 ± 2°C) and humidity (>45%) conditions with food and water available ad libitum. They were acclimated for at least 5 days before experimentation. Experimental procedures were approved by the Ethical Committee of Addex Therapeutics and the Animal Care and Use Committee of Oregon Health & Science University, and were performed in full compliance with international European ethical standards (86/809-EEC), the French National Committee (Décret 87/848) for the care and use of laboratory animals, and the National Institutes of Health.
Elevated Plus Maze Test in Mice and Rats.
The procedure was performed as described previously (Kalinichev et al., 2013). Male mice (n = 8–10/group) were treated orally (p.o.) via gavage with vehicle [1% carboxymethyl cellulose (CMC)], ADX88178 (1, 3, 10 and 30 mg/kg) or diazepam (1.5 mg/kg). Male rats (n = 10/group) were treated p.o. with vehicle (1% CMC), ADX88178 (10, 30, and 100 mg/kg), or diazepam (3 mg/kg). After 60 minutes, animals were individually placed in the center of the maze (facing one of the closed arms) and left to explore for 5 minutes. A terminal blood sample was collected from all ADX88178-treated animals at the end of the experiment, and plasma was analyzed as described for the pharmacokinetic studies. The number of open-arm and closed-arm entries, as well as the time (seconds) spent in the open arms of the maze, was analyzed by one-way analysis of variance (ANOVA) followed by Dunnett’s test. As the variability across groups was not the same, we used square root transformation of the data prior to analysis to stabilize the variance (see Festing et al., 2002; Bate and Clark, 2014). The follow-up evaluation of the normal probability plot and predicted versus residual plot confirmed that the parametric assumptions, required for ANOVA, were satisfied once the transformation was made. Effects of diazepam were analyzed by t test.
EPM Test in mGlu4 Receptor KO and WT Mice.
Adult male and female mGlu4 receptor KO mice (B6.129-Grm4tm1Hpn/J strain) and their WT controls (C57BL6/J strain) were purchased from The Jackson Laboratories (Bar Harbor, ME) and maintained in the facility of Merck Research Laboratories (West Point, PA) under standard laboratory conditions. This transgenic strain was selected because it had previously been used to investigate the effect of mGlu4 receptor in cerebellar synaptic plasticity and motor performance (Pekhletski et al., 1996). The EPM test in mice was performed as described earlier. Mice (n = 11–17 sex/genotype/treatment) were treated p.o. with vehicle (1% CMC) or ADX88178 (10 mg/kg) and tested in the EPM 60 minutes later. The number of open-arm and closed-arm entries, as well as the time (seconds) spent in open arms of the maze, were analyzed with a three-way ANOVA approach (with fixed factors treatment, sex, and genotype) followed by planned comparisons to compare ADX versus vehicle groups.
Marble Burying Test in Mice.
The experiment was performed as described previously (Kalinichev et al., 2013). Mice (n = 10/group) were treated (p.o.) with vehicle (1% CMC), ADX88178 (3, 10, 30, 100 mg/kg), or chlordiazepoxide (30 mg/kg). After a period of 60 minutes, animals were individually placed in experimental cages and left undisturbed for 30 minutes. A terminal blood sample was collected from all ADX88178-treated animals at the end of the experiment, and plasma was analyzed as described for the pharmacokinetic studies. The number of buried marbles was analyzed by the Kruskal-Wallis test followed by Dunn’s multiple comparisons.
Fear Conditioning in Mice.
Adult male C57BL6/J mice, purchased from Jackson Laboratories and maintained in the facility of Oregon Health & Science University under standard laboratory conditions, were used in this experiment. The Med Associates NIR Video and automated analysis system (Med Associates, St. Albans, VT), utilizing the Med Associates Video Freeze automated scoring system (freezing threshold: 18 motion, 15 frames) as described before (Davis et al., 2012), was used in the assessment. In this task, mice learn to associate a conditioned stimulus (CS; e.g., a tone) with an unconditioned stimulus (US; e.g., foot shock). CS-US pairings are preceded by a short habituation period, from which a baseline measure of motor activity is obtained. Freezing is defined as the absence of any movement, except for respiration. For the induction of fear conditioning (day 0), animals were placed inside a white LED-lit (100 lux) fear conditioning chamber, context A, which consisted of a metal grid floor with gray and white walls. A 60-second baseline was followed by 10 CS-US pairings. During acquisition, the 10-second tones (80 db, 2.8 Hz) coterminated with 2-second foot shocks (0.35 mA, US). The intertone interval was 20 seconds. Motor activity (arbitrary units) at baseline (before the first shock) and following each shock was measured to explore potential treatment-induced differences in response to the aversive stimulus. In the induction phase, mice were administered (p.o.) vehicle (1% CMC; n = 7), 10 mg/kg (n = 6), or 30 mg/kg (n = 10) ADX88178 60 minutes before the start of the fear conditioning session.
Subsequently, an evaluation was performed to see if ADX88178 had any lasting effects on the between-trial extinction. For this, 24 hours after the induction phase, mice were exposed to context B, a smooth white plastic floor with a “tented” black plastic ceiling and scented with a 10% isopropanol solution. After the initial 60-second baseline, animals were exposed to five 60-second tones with an intertone interval of 60 seconds. The session was repeated on 7 consecutive days. We analyzed the freezing during the first five tones on each day. Effects of ADX88178 on freezing were assessed using a two-way repeated-measures mixed-model approach, followed by planned comparisons.
Forced Swim Test in Mice.
The procedure was performed as described previously (Campo et al., 2011). On the test day, mice (n = 10/group) were treated p.o. with vehicle (1% CMC), ADX88178 (3, 10, 30, and 100 mg/kg), or imipramine (30 mg/kg). Sixty minutes later, animals were exposed to the test swim session for 6 minutes. A terminal blood sample was collected from all ADX88178-treated animals at the end of the experiment, and plasma was analyzed as described for the pharmacokinetic studies. The duration of immobility during the last 4 minutes of the session was analyzed using a one-way ANOVA, followed by Dunnett’s test. Effects of imipramine were analyzed with a t test.
DOI-Induced Head Twitches in Mice.
Adult male C57BL6/J mice, purchased from Charles River (Margate, UK) and maintained at RenaSci Ltd. (Nottingham, UK) under standard laboratory conditions, were used in this experiment. Mice (n = 8/group) were treated with vehicle (1% CMC) or ADX88178 (3, 10, 30 mg/kg) administered p.o. or olanzapine (0.1 mg/kg) administered i.p. Sixty minutes following ADX88178 administration and 30 minutes following olanzapine administration, animals were challenged (i.p.) with either vehicle (saline) or DOI (3 mg/kg). Each animal was then placed in a novel cage, and the number of head twitches was counted for 6 minutes by a trained observer blind to the treatment. A terminal blood sample was collected from all ADX88178-treated animals at the end of the experiment, and plasma was analyzed as described for the pharmacokinetic studies. The number of head twitches was analyzed by one-way ANOVA followed by Dunnett’s test. The data were square root transformed prior to analysis to stabilize the variance. The effect of olanzapine was analyzed by t test.
MK-801–Induced Hyperactivity in Mice.
Adult male C57BL6/J mice, purchased from Charles River and maintained at RenaSci Ltd. under standard laboratory conditions, were used in the experiment. Mice (n = 10/group) were habituated in a set of clear Plexiglas activity arenas (48 × 26.5 × 30 cm) equipped with infrared photocell beams for 60 minutes. Subsequently, animals (n = 10/group) were treated (p.o.) with vehicle (1% CMC) or ADX88178 (3, 10, 30, mg/kg) and returned to the activity cage for 60 minutes. An additional group of mice (n = 10/group) were treated (i.p.) with olanzapine (1 mg/kg) and returned to the activity arena for 30 minutes. At the end of the respective pretreatment periods, animals were challenged (i.p.) with either MK-801 (0.6 mg/kg) or vehicle (saline), returned to the arenas, and monitored for activity for 60 minutes. A terminal blood sample was collected from all ADX88178-treated animals at the end of the experiment, and plasma was analyzed as described for the pharmacokinetic studies. The total distance traveled (centimeters) in the 0–30-minute and 30–60-minute periods post-treatment was analyzed using a one-way ANOVA followed by Dunnett’s test. The effects of olanzapine were analyzed by t test.
Conditioned Avoidance Response Test in Rats.
Adult male Wistar rats, purchased from Charles River and maintained at RenaSci Ltd. under standard laboratory conditions, were used in this experiment. The test was performed as described previously (Kalinichev et al., 2013). One week after the last validation experiment with an antipsychotic drug, animals (n = 8/group) were administered p.o. vehicle (1% CMC), ADX88178 (1, 3, 10, and 30 mg/kg), or olanzapine (3 mg/kg p.o.) 60 minutes before being evaluated in the test protocol as described earlier. A terminal blood sample was collected from all ADX88178-treated animals at the end of the experiment, and plasma was analyzed as described for the pharmacokinetic studies. The duration (seconds) of avoidance responses and the number of escapes were analyzed using a one-way ANOVA followed by Dunnett’s test. The effects of olanzapine were analyzed by t test.
Spontaneous Locomotor Activity in Mice and Rats.
Two sets of eight opaque Plexiglas arenas (35 × 35 × 50 cm for mice and 50 × 50 × 50 cm for rats) in conjunction with a video tracking and computerized analysis system (Viewpoint, Lyon, France) were used. Mice (n = 10/group) were treated p.o. with vehicle (1% CMC), ADX88178 (3, 10, 30, and 100 mg/kg), or baclofen (10 mg/kg). Rats (n = 10/group) were treated p.o. with vehicle (1% CMC), ADX88178 (1, 3, 10, 30, and 60 mg/kg), or baclofen (6 mg/kg). Sixty minutes after administration of compounds, animals were individually placed into activity arenas and monitored for sLMA for 30 minutes. Effects of ADX88178 on the total distance traveled were evaluated using one-way ANOVA followed by Dunnett’s test. The effect of baclofen was evaluated using t test.
Drugs.
ADX88178 was synthesized at Addex Therapeutics [example 1.33, PCT Int. Appl. (2010) WO2010/0079239; Geneva, Switzerland]. Diazepam, olanzapine, DOI, and MK-801 were purchased from Tocris Bioscience (Bristol, UK). Chlordiazepoxide, imipramine, and baclofen [(R)-4-amino-3-(4-chloro-phenyl) butanoic acid] were purchased from Sigma-Aldrich (Buchs, Switzerland).
ADX88178 and baclofen were suspended in 1% CMC. Chlordiazepoxide was dissolved in water. Imipramine and olanzapine were dissolved in saline. Diazepam was dissolved in PEG400/water (30/70%). All drugs dosed p.o. in rats were administered at 5 ml/kg volume. All drugs dosed p.o. in mice were administered at 10 ml/kg volume. All drugs dosed i.p. in mice were administered at 3 ml/kg volume. All solutions and suspensions were prepared fresh daily. All doses were administered as free base.
Results
EPM in Mice and Rats.
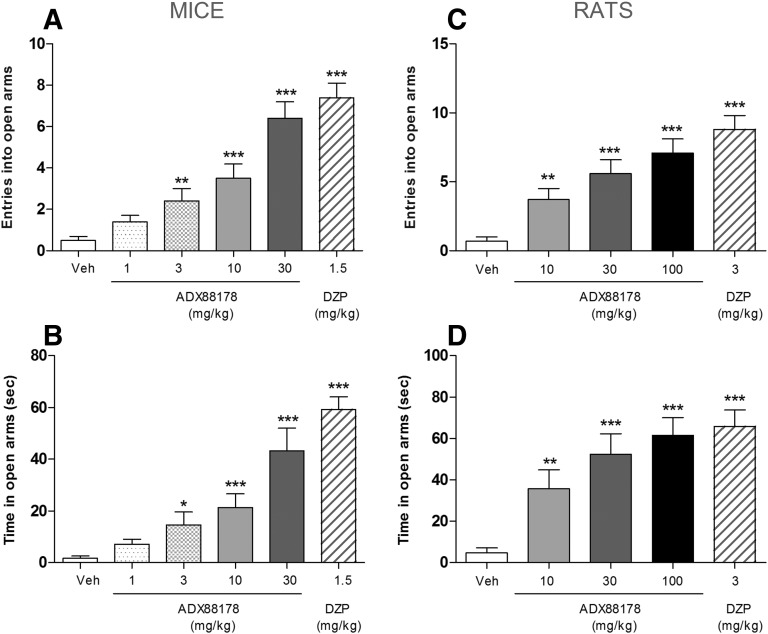
In mice, ADX88178 (1–30 mg/kg p.o.) dose-dependently increased the number of open-arm entries [F(4,45) = 16.59; P < 0.001] (Fig. 2A). Specifically, at 3, 10, and 30 mg/kg ADX88178, there were ∼5- (P < 0.01), 7-, and almost 13-fold increases (both P < 0.001) in the number of open-arm entries, respectively, when compared with the vehicle-treated controls (Fig. 2A). Also, ADX88178 dose-dependently increased the time spent in the open arms [F(4,45) = 13.43; P < 0.001]. Specifically, at 3, 10, and 30 mg/kg ADX88178, there were 8- (P < 0.01), 12-, and 24-fold increases (both P < 0.001) in the time spent in the open arms when compared with the vehicle-treated controls (Fig. 2B). Diazepam-treated animals showed almost 15- and 33-fold increases (both P < 0.001) in open-arm entries and the time spent in the open arms, respectively, when compared with vehicle-treated controls (Fig. 2, A and B). There was no effect of treatments on the number of closed-arm entries in the mouse EPM test (data not shown).
Fig. 2.
Open-arm entries (A and C) and time spent in open arms (seconds) (B and D) in male C57BL6/J mice (A and B) and Sprague-Dawley rats (C and D) in the EPM test. Before exposure to the maze, mice (n = 10/group) were treated p.o. with vehicle (Veh; 1% CMC), ADX88178 (1, 3, 10, 30 mg/kg), or diazepam (DZP; 1.5 mg/kg), whereas rats (n = 10/group) were treated p.o. with vehicle (1% CMC), ADX88178 (10, 30, 100 mg/kg), or DZP (3 mg/kg). Each point represents the observed mean (±S.E.M.). *P < 0.05; **P < 0.01; ***P < 0.001 compared with the corresponding Veh group.
In rats, ADX88178 (10–100 mg/kg p.o.) dose-dependently increased the number of open-arm entries [F(3,36) = 15.18; P < 0.001] in the rat EPM test (Fig. 2C). Specifically, at 10, 30, and 100 mg/kg ADX88178, there were ∼5- (P < 0.01), 8-, and more than 10-fold increases (both P < 0.001) in the number of open-arm entries, respectively, when compared with the vehicle-treated controls (Fig. 2C). Also, ADX88178 dose-dependently increased the time spent in the open arms [F(3,36) = 8.81; P < 0.001]. Specifically, at 10, 30, and 100 mg/kg ADX88178, there were 7.5- (P < 0.01), 11-, and 13-fold increases (both P < 0.001) in time spent in the open arms when compared with the vehicle-treated controls (Fig. 2D). Diazepam-treated rats showed almost 12- and 14-fold increases (both P < 0.001) in open-arm entries and the time spent in open arms, respectively, when compared with vehicle-treated controls (Fig. 2, C and D). There was no effect of treatments on the number of closed-arm entries in the rat EPM test (data not shown). The terminal concentrations of ADX88178 in plasma in mice and rats are presented in Table 1.
TABLE 1.
Measured concentrations of ADX88178 in mice and rats at the end of experiments
| Treatment | Species | Route | Dose | n | Mean Plasma Exposure |
|---|---|---|---|---|---|
| mg/kg | ng/ml | ||||
| EPMa | |||||
| ADX88178 | Mice | p.o. | 1 | 10 | 10 |
| ADX88178 | Mice | p.o. | 3 | 10 | 229 |
| ADX88178 | Mice | p.o. | 10 | 10 | 1287 |
| ADX88178 | Mice | p.o. | 30 | 10 | 2865 |
| ADX88178 | Rats | p.o. | 10 | 10 | 1129 |
| ADX88178 | Rats | p.o. | 30 | 10 | 2295 |
| ADX88178 | Rats | p.o. | 100 | 10 | 3513 |
| MBb | |||||
| ADX88178 | Mice | p.o. | 3 | 10 | 22 |
| ADX88178 | Mice | p.o. | 10 | 10 | 541 |
| ADX88178 | Mice | p.o. | 30 | 10 | 1619 |
| ADX88178 | Mice | p.o. | 100 | 10 | 4395 |
| FSTc | |||||
| ADX88178 | Mice | p.o. | 3 | 10 | 167 |
| ADX88178 | Mice | p.o. | 10 | 10 | 1776 |
| ADX88178 | Mice | p.o. | 30 | 10 | 2994 |
| ADX88178 | Mice | p.o. | 100 | 10 | 6028 |
| DOId | |||||
| ADX88178 | Mice | p.o. | 3 | 8 | 33 |
| ADX88178 | Mice | p.o. | 10 | 8 | 338 |
| ADX88178 | Mice | p.o. | 30 | 8 | 1146 |
| MK-801e | |||||
| ADX88178 | Mice | p.o. | 3 | 10 | 23 |
| ADX88178 | Mice | p.o. | 10 | 10 | 133 |
| ADX88178 | Mice | p.o. | 30 | 10 | 624 |
| CARf | |||||
| ADX88178 | Rats | p.o. | 1 | 8 | 106 |
| ADX88178 | Rats | p.o. | 3 | 8 | 408 |
| ADX88178 | Rats | p.o. | 10 | 8 | 1273 |
| ADX88178 | Rats | p.o. | 30 | 8 | 3030 |
Plasma sample taken from all ADX88178-treated animals approximately 65 minutes postadministration.
Plasma sample taken from all ADX88178-treated animals approximately 90 minutes postadministration.
Plasma sample taken from all ADX88178-treated animals approximately 66 minutes postadministration.
Plasma sample taken from all ADX88178-treated animals approximately 66 minutes postadministration.
Plasma sample taken from all ADX88178-treated animals approximately 120 minutes postadministration.
Plasma sample taken from all ADX88178-treated animals approximately 70 minutes postadministration.
EPM in mGlu4 KO and WT Mice.
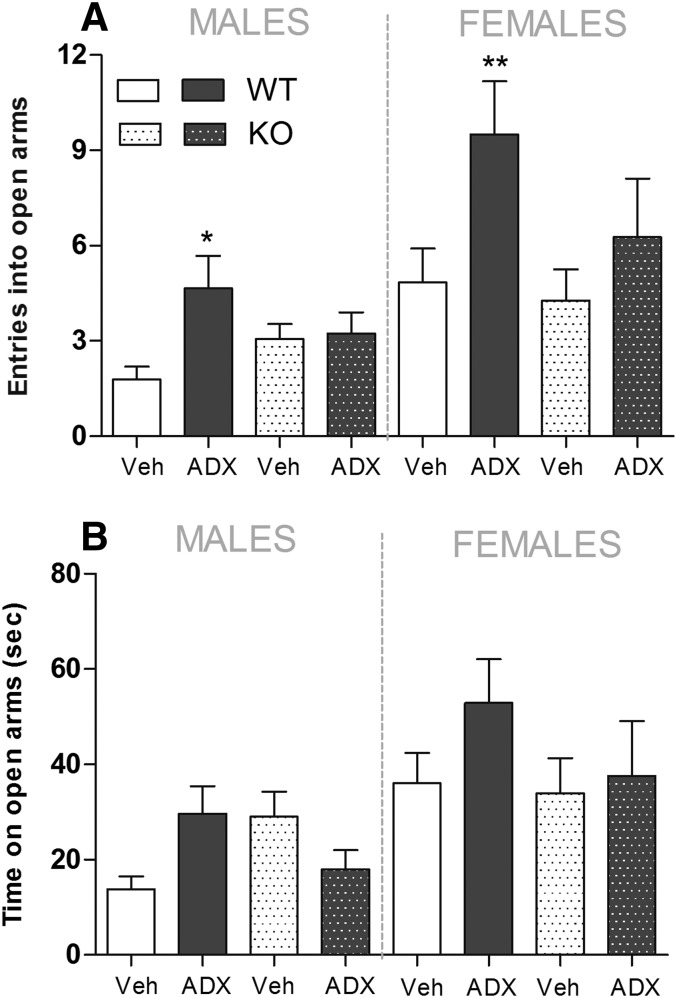
The analysis of open-arm entries revealed a significant overall effect of treatment [F(1,100) = 10.15; P < 0.01] and sex [F(1,100) = 16.49; P < 0.001], but not genotype [F(1,100) = 1.41; P = 0.24]. Although treatment × genotype interaction approached statistical significance [F(1,100) = 2.98; P = 0.09], no other interactions did so. Post-hoc analysis revealed that, among male WT mice, ADX88178-treated animals showed 2.5-fold increases (P < 0.05) in the number of open-arm entries compared with vehicle-treated controls (Fig. 3A). Similarly, among female WT mice, ADX88178-treated animals showed 2-fold increases (P < 0.01) in the number of open-arm entries compared with vehicle-treated controls (Fig. 3A). In male and female KO mice, effects of vehicle and ADX88178 on open-arm entries were similar (Fig. 3A).
Fig. 3.
Open-arm entries (A) and time spent in open arms (seconds) (B) in male and female WT and mGlu4 receptor KO mice in the EPM test. Before exposure to the maze, mice (n = 24–31/genotype) were treated p.o. with vehicle (Veh; 1% CMC) or ADX88178 (ADX; 10 mg/kg). Each point represents the observed mean (±S.E.M.). *P < 0.05; **P < 0.01 compared with the corresponding Veh group.
The analysis of time spent in open arms revealed a significant overall effect of sex [F(1,100) = 14.13; P < 0.001], but no effect of treatment [F(1,100) = 2.09; P = 0.15] or genotype [F(1,100) = 0.43; P = 0.51]. Although there was a significant treatment × genotype interaction [F(1,100) = 4.69; P < 0.05], no other interactions approached statistical significance. Post-hoc analysis revealed that, among male WT mice, ADX88178-treated animals tend to show 2-fold increases in the time spent in open arms (P = 0.07) compared with vehicle-treated controls (Fig. 3B). Similarly, among female WT mice, ADX88178-treated animals showed 1.5-fold increases in the time spent in open arms (P = 0.08) compared with vehicle-treated controls (Fig. 3B). In male and female KO mice, effects of vehicle and ADX88178 on open-arm entries were similar (Fig. 3B). There was no effect of treatment on closed-arm exploration in any of the groups studied (data not shown).
MB Test in Mice.
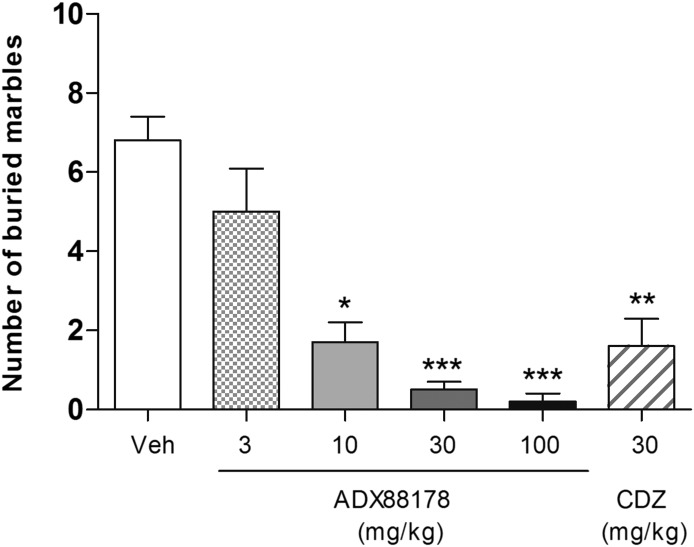
ADX88178 (3–100 mg/kg p.o.) caused dose-dependent reductions in the number of buried marbles [Kruskal-Wallis test, P < 0.001]. Specifically, mice treated at 10 mg/kg ADX88178 buried ∼75% (P < 0.05) fewer marbles than vehicle-treated controls, whereas those treated at higher doses (30 and 100 mg/kg) showed a full suppression of marble burying behavior (Fig. 4). Chlordiazepoxide-treated mice buried ∼75% (P < 0.01) fewer marbles compared with vehicle-treated controls (Fig. 4). The terminal concentrations of ADX88178 in plasma are present in Table 1. The minimum effective dose (MED) for ADX88178 in the MB test is 10 mg/kg, the ED50 is 4.5 mg/kg, in vivo EC50 in plasma is 152 ng/ml, equivalent to 560 nM, whereas the in vivo EC50 in cerebrospinal fluid (CSF; calculated from plasma protein binding) is 121 nM.
Fig. 4.
Number of buried marbles in male C57BL6/J mice (n = 10/group) following p.o. treatment with vehicle (Veh; 1% CMC), ADX88178 (3, 10, 30, 100 mg/kg), or chlordiazepoxide (CDZ; 30 mg/kg). Each point represents the observed mean (±S.E.M.). *P < 0.05; **P < 0.01; ***P < 0.001 compared with the Veh group.
Fear Conditioning in Mice.
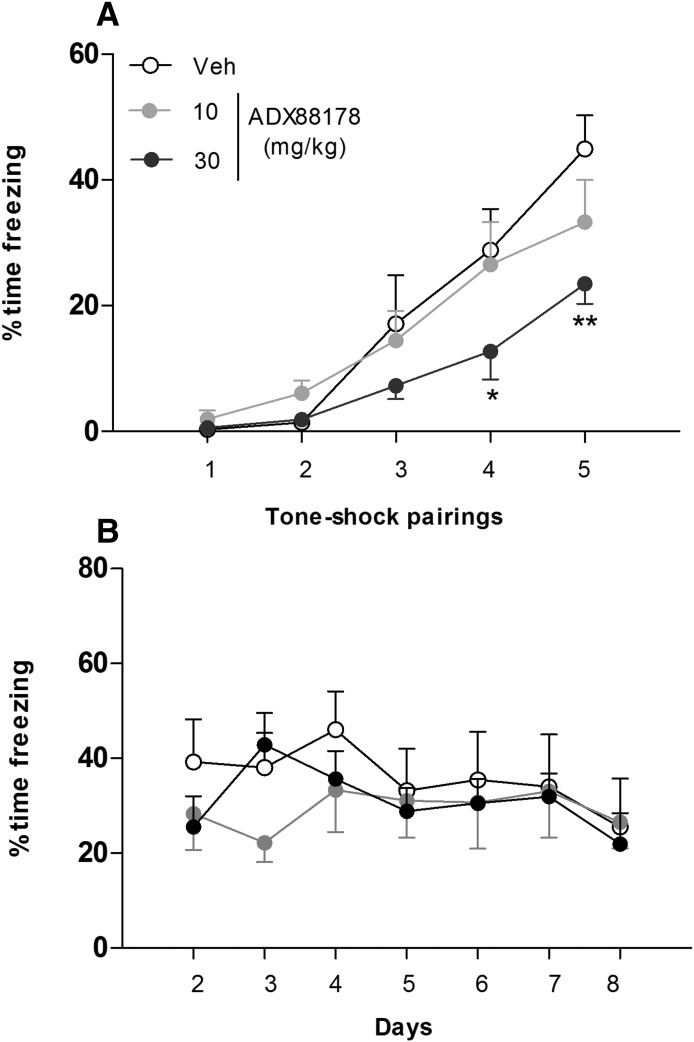
In the induction phase of fear conditioning, there was a significant effect of treatment [F(2,63) = 8.0; P < 0.01], trial [F(3,63) = 51.5; P < 0.001], and significant treatment × trial interaction [F(6,63) = 2.69; P < 0.05]. Post-hoc comparisons showed that mice treated with ADX88178 at 30 mg/kg, but not at 10 mg/kg, exhibited reduced acquisition of conditioned fear during the fourth (P < 0.05) and fifth trials (P < 0.01) as compared with vehicle-treated mice (Fig. 5A). This reduced acquisition was not caused by an effect of ADX88178 on baseline average motion or velocity during the shock (data not shown).
Fig. 5.
Percentage of C57BL6/J mice showing freezing responses in the induction phase of fear conditioning (A) and mean percentage of animals showing freezing across five exposures to tone on 7 consecutive days in the between-trial extinction phase (B). Animals were treated (p.o.) with vehicle (Veh; 1% CMC; n = 7), 10 mg/kg ADX88178 (n = 7), or 30 mg/kg ADX88178 (n = 10) 60 minutes before being introduced to the induction phase. Each point represents the observed mean (±S.E.M.). *P < 0.05; **P < 0.01 compared with the Veh group.
In the extinction phase, assessed across days 2–8 (Fig. 5B), there was a significant effect of days [F(6,120) = 2.34; P < 0.05], but no significant effect of treatment [F(2,120) = 0.31; P = 0.74] and no treatment × days interaction [F(12,120) = 0.97; P = 0.48].
FST in Mice.
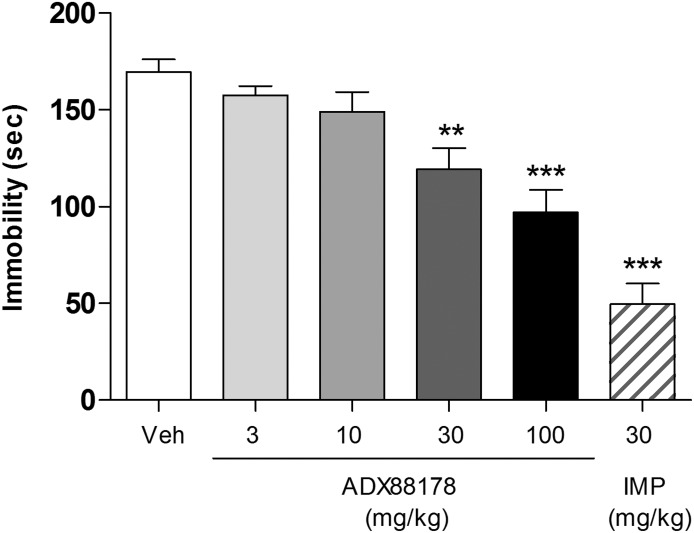
ADX88178 (3–100 mg/kg p.o.) dose-dependently reduced duration of immobility [F(4,45) = 10.42; P < 0.001] in the mouse FST (Fig. 6). Specifically, there were reductions in duration of immobility in mice treated with 30 and 100 mg/kg ADX88178 by ∼30% (P < 0.01) and 40% (P < 0.001) respectively, when compared with the vehicle-treated controls (Fig. 6). The terminal concentrations of ADX88178 in plasma are present in Table 1. Imipramine-treated mice exhibited ∼70% reduction (P < 0.001) in duration of immobility compared with vehicle-treated controls (Fig. 6).
Fig. 6.
Time (seconds) spent in immobility by C57BL6/J mice in the FST. Before exposure to water, animals (n = 10/group) were treated p.o. with vehicle (Veh; 1% CMC), ADX88178 (3, 10, 30, 100 mg/kg), or imipramine (IMP; 30 mg/kg). Each point represents the observed mean (±S.E.M.). **P < 0.01; ***P < 0.001 compared with the Veh group.
DOI-Induced Head Shakes in Mice.
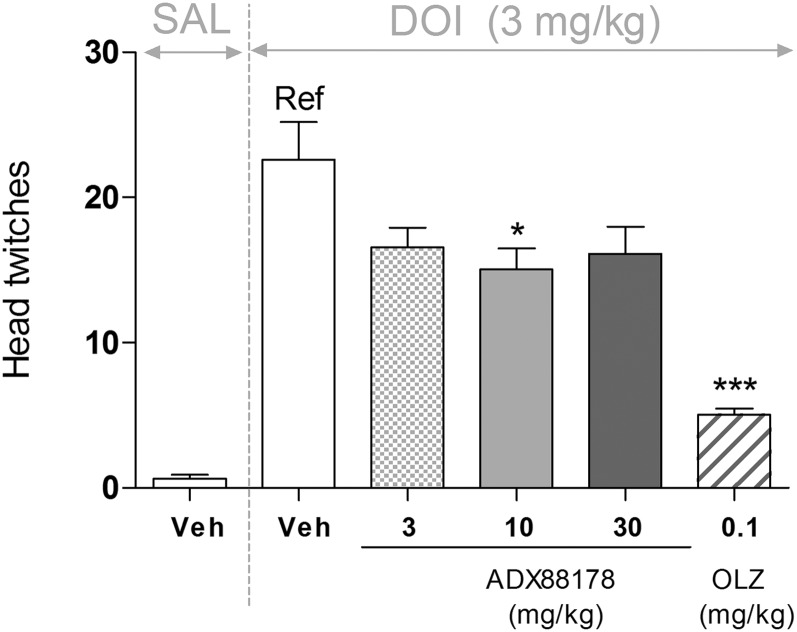
ADX88178 (3–30 mg/kg p.o.) had a significant effect on the number of DOI-induced head shakes [F(4,35) = 53.7; P < 0.001] in mice (Fig. 7). Specifically, at 3, 10, and 30 mg/kg ADX88178, there were overall similar (∼30%) reductions in the number of head shakes, which reached statistical significance (P < 0.05) at 10 mg/kg while missing it at 30 mg/kg (P = 0.056). The terminal concentrations of ADX88178 in plasma are present in Table 1. Olanzapine-treated animals showed 80% reductions (P < 0.001) in number of head shakes compared with the reference group (Fig. 7).
Fig. 7.
Number of DOI-induced head shakes in C57BL6/J mice (n = 10/group) that were pretreated (p.o.) with vehicle (Veh; 1% CMC), ADX88178 (3, 10, 30 mg/kg), or olanzapine (OLZ; 0.1 mg/kg) and then challenged (i.p.) with either saline (SAL) or DOI (3 mg/kg). Each point represents the observed mean (±S.E.M.). *P < 0.05; ***P < 0.001 compared with the reference (Ref) group.
MK-801–Induced Hyperactivity in Mice.
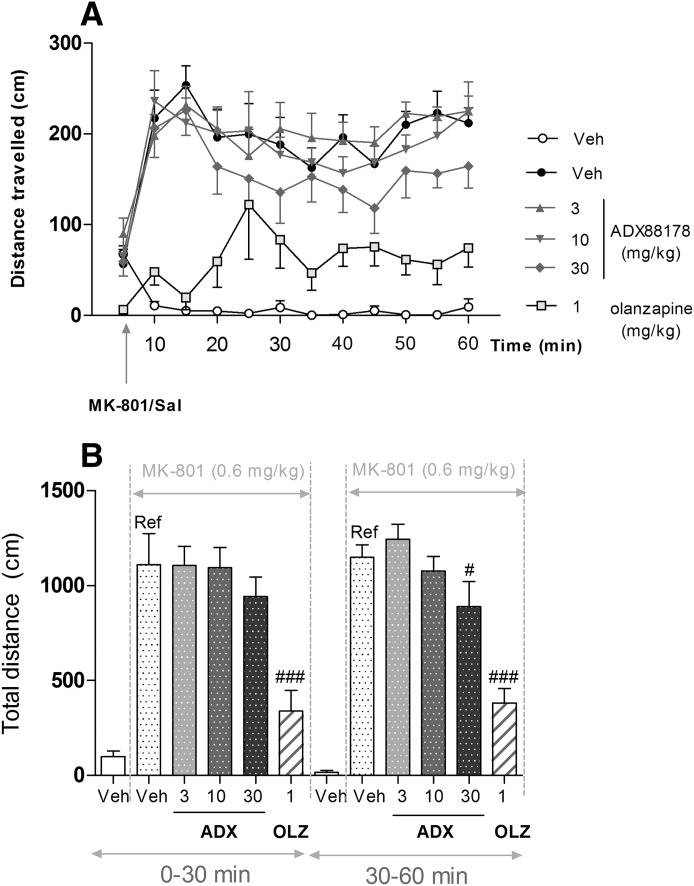
ADX88178 had no effect on the total distance traveled during the first 30-minute period, whereas during the second 30-minute period, the overall effect approached statistical significance [F(3,36) = 2.70; P = 0.059]. Planned comparisons revealed that during the latter period, animals treated with 30 mg/kg ADX88178 traveled approximately 25% less than the vehicle-treated controls (P < 0.05) compared with the reference group (Fig. 8, A and B). The terminal concentrations of ADX88178 in plasma are present in Table 1. Olanzapine-treated animals showed approximately a 70% reduction (P < 0.001) in total distance traveled compared with the reference group during the first and second 30-minute periods (Fig. 8, A and B).
Fig. 8.
Locomotor activity (distance traveled; centimeters) of C57BL6/J mice after being challenged with vehicle (saline; Sal) or MK-801 (0.6 mg/kg), expressed as a time course (A) or the total distance traveled (B) during 0–30 and 30–60 minutes. Animals (n = 8/group) were pretreated p.o. with vehicle (Veh; 1% CMC), ADX88178 (ADX; 1, 3, 10, 30 mg/kg), or olanzapine (OLZ; 3 mg/kg). Each point represents the observed mean. #P < 0.05; ###P < 0.001 compared with the reference (Ref) group.
CAR Test in Rats.
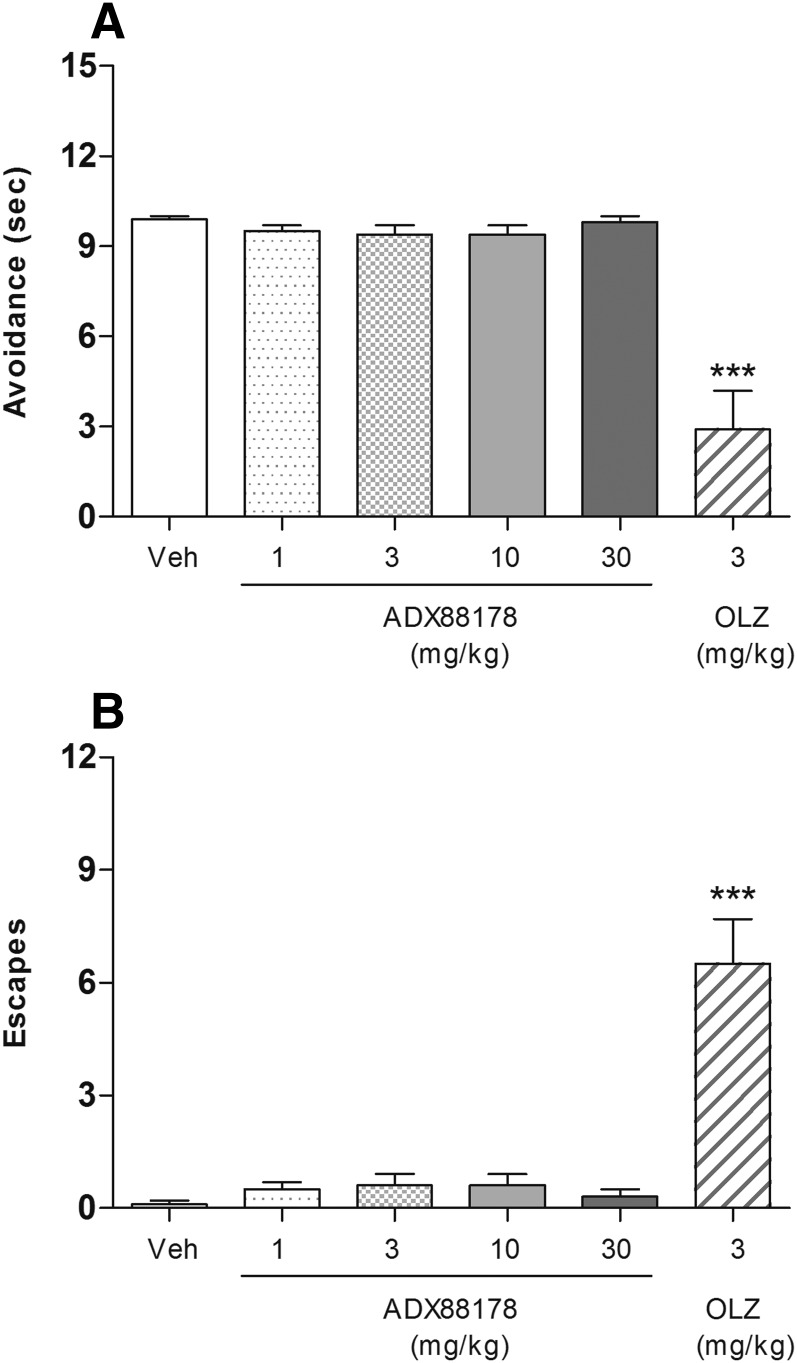
ADX88178 (1–30 mg/kg p.o.) had no effect in the rat CAR test, neither on the duration of avoidance response nor on the number of escapes (Fig. 9, A and B). The terminal concentrations of ADX88178 in plasma are present in Table 1. Olanzapine-treated animals showed a 70% reduction (P < 0.001) in the duration of avoidance response and a more than 60-fold increase (P < 0.001) in the number of escapes when compared with the vehicle-treated controls. None of the treated animals failed to escape the shock (data not shown).
Fig. 9.
Latency (seconds) to avoid electrical foot shock (A) and the number of escapes (B) exhibited by male Wistar rats during the CAR test (see text). Animals (n = 8/group) were pretreated p.o. with vehicle (Veh; 1% CMC), ADX88178 (1, 3, 10, 30 mg/kg), or olanzapine (OLZ; 3 mg/kg). Each point represents the observed mean. ***P < 0.001 compared with the Veh group.
sLMA Tests in Mice and Rats.
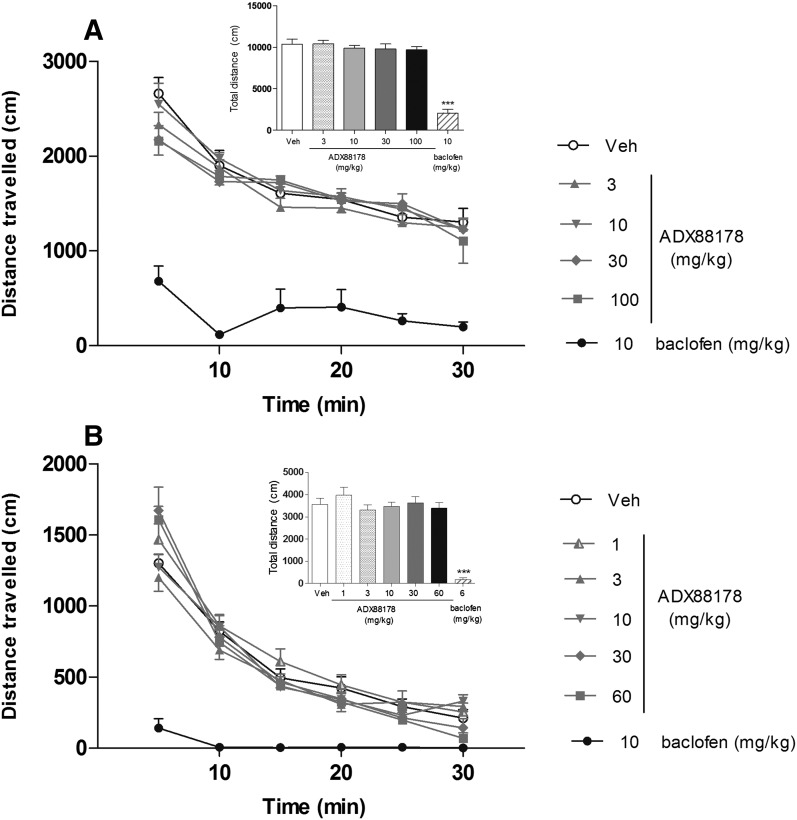
In mice, ADX88178 (3, 10, 30, 100 mg/kg p.o.) had no effect on sLMA, whereas baclofen (10 mg/kg) caused an 80% reduction of the total distance traveled during the test, when compared with the vehicle-treated controls (Fig. 10A). In rats, ADX88178 (1, 3, 10, 30, 60 mg/kg p.o.) had no effect on sLMA, whereas baclofen (10 mg/kg) caused a 95% (P < 0.001) reduction in the total distance traveled during the test, when compared with the vehicle-treated controls (Fig. 10B).
Fig. 10.
Spontaneous locomotor activity (distance traveled; centimeters) of C57BL6/J mice (A) and Sprague-Dawley rats (B) during 30 minutes, expressed as a time course and the total distance traveled (insets). Mice were pretreated (p.o.) with vehicle (Veh; 1% CMC), ADX88178 (3, 10, 30, and 100 mg/kg), or baclofen (10 mg/kg). Rats were pretreated (p.o.) with vehicle (1% CMC), ADX88178 (1, 3, 10, 30, and 60 mg/kg), or baclofen (10 mg/kg). Each point represents the observed mean (±S.E.M.). ***P < 0.001 compared with the Veh group.
Efficacy and Pharmacokinetic/Pharmacodynamic Interaction.
Evaluation of MEDs of ADX88178, associated plasma, and estimated CSF exposure across several experiments showed a good in vitro/in vivo correlation which was similar for several assays across therapeutic indications (Table 2). Specifically, following acute oral administration, the MED of ADX88178 in rats and mice, with the exception of the FST, was associated with mean plasma concentrations in the range of 220–625 ng/ml.
TABLE 2.
MEDs in several mouse and rat efficacy tests, measured plasma concentration, and estimated CSF concentration at the end of each experiment
| Test | Animal Models | Species | MED Dose | Pretreatment Time | Plasma Exposure | Plasma Exposure | CSF Exposurea | CSF Exposurea |
|---|---|---|---|---|---|---|---|---|
| mg/kg | min | ng/ml | nM | ng/ml | nM | |||
| Elevated plus maze | Anxiety | Mice | 3 | 60 | 229 | 842 | 49 | 181 |
| Elevated plus maze | Anxiety | Rats | 10b | 60 | 1129 | 4145 | 91 | 335 |
| Marble burying | Anxiety/OCD | Mice | 10 | 60 | 541 | 1987 | 41 | 149 |
| Forced swim test | Depression | Mice | 30 | 60 | 2994 | 10,997 | 225 | 825 |
| DOI-induced head twitches | Psychosis | Mice | 10 | 60 | 338 | 1243 | 25 | 93 |
| MK-801 hyperactivity | Psychosis | Mice | 30 | 60 | 624 | 2293 | 47 | 172 |
| Fear conditioning | Fear | Mice | 30 | 60 | na |
na, not available.
The theoretical CSF exposure is calculated from the plasma exposure using the unbound plasma protein binding values in mice (fu = 7.5%) and in rats (fu = 8.5%).
The lowest dose tested.
Discussion
Here, we evaluated the novel, potent, selective, and brain-penetrant mGlu4 receptor PAM ADX88178 in the context of in vivo models relevant to neuropsychiatric conditions. Previously, using intracellular calcium mobilization assays in human embryonic kidney 293 cells expressing the human and rat mGlu4 receptor, ADX88178 potentiated the response to glutamate with EC50 values of 3.5 and 9.1 nM, respectively (Le Poul et al., 2012).
In the EPM test in mice and rats, acute ADX88178 dose-dependently increased open-arm exploration with no changes in closed-arm activity, indicative of anxiolytic-like efficacy of the compound. This effect might be relevant to heightened anxiety, as in both tests vehicle-treated animals showed very low baseline activity in the open arms. In accord, ACPT-I increased open-arm exploration of Albino Swiss mice (Stachowicz et al., 2009), whereas a mGlu4 receptor preferred agonist LSP1-2111 (Wierońska et al., 2010) and a PAM VU0155041 (cis-2-[[(3,5-dichlorophenyl)amino]carbonyl]cyclohexanecarboxylic acid) (Duvoisin et al., 2011) did so in C57BL6/J mice. Importantly, the target specificity of the effect in the mouse EPM was confirmed in a follow-up experiment involving male and female mGlu4 receptor KO and WT mice. In a previously reported study, 12-month-old male mGlu4 receptor KO mice showed reduced open-arm exploration, indicative of increased anxiety-like reactivity, in comparison with their WT controls, whereas at the age of 6 months, no difference in open-arm exploration was detected between the genotypes (Davis et al., 2012). As the mGlu4 KO mice used here were approximately 7–8 months old, the lack of baseline differences in open-arm exploration was expected.
ADX88178 dose-dependently reduced the number of buried marbles in the mouse MB test. Whereas both EPM and MB tests are sensitive to typical anxiolytic drugs (Nicolas et al., 2006), the latter has also been linked to OCD (Thomas et al., 2009), which, according to the latest classification of mental disorders (Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition), now constitutes an independent disease. In accord, Lu AF21934 also showed an anti-OCD–like profile in the MB test in Albino Swiss mice (Sławińska et al., 2013a). Overall, these findings support a growing body of evidence for a broad, anxiolytic-like profile of mGlu4 receptor activators, and suggest that these compounds can be considered as a potential treatment of the spectrum of anxiety disorders, including elevated anxiety, which is comorbid with other diseases, such as Parkinson’s disease and multiple sclerosis.
A cued fear conditioning procedure has been used to model aspects of stress-related disorders in rodents (Grillon and Davis, 1997), and a freezing response has been used widely as an indicator of a conditioned fear response (Fanselow, 1994). It has also been reported that mGlu4 receptor KO mice show enhanced acquisition and delayed extinction of conditioned freezing responses compared with WT controls, mimicking some features of stress- and fear-related disorders (Davis et al., 2013). On the other hand, LSP1-2111 reduces conditioned freezing in mice, when given acutely prior to the start of fear conditioning training (i.e., acquisition) (Davis et al., 2013). The effect of LSP1-2111 on the acquisition of fear conditioning most likely involved the mGlu4 receptor, as it was absent in mGlu4 receptor KO mice (Davis et al., 2013). In a similar procedure used here, ADX88178 reduced acquisition of conditioned freezing in mice, when it was administered acutely prior to the start of the fear conditioning training. These findings support earlier evidence that activation of the mGlu4 receptor can reduce fear conditioning, whereas inhibition of the receptor appears to have the opposite effect. As seen with LSP1-2111, ADX88178 given to animals before the start of conditioning had no carryover effects on between-trial extinction. It remains to be investigated whether administration of ADX88178 (or other selective mGlu4 receptor activators) prior to the start of the extinction procedure can impact the rate of either within-session or between-session extinction. Also, experiments involving administration of ADX88178 immediately after the last conditioning session are needed to evaluate the role of the mGlu4 receptor in consolidation of fear memories.
There is growing evidence that abnormalities in the physiologic glutamate/GABA balance are linked to the pathophysiology of depression (Kendell et al., 2005). Increases in glutamate and reductions in GABA neurotransmission are detected in the cortex of depression patients, whereas therapeutic efficacy of an antidepressant following treatment is linked to a normalized glutamate/GABA equilibrium (Sanacora et al., 2003). Thus, it has been hypothesized that activation of mGlu4 receptors can restore the glutamate/GABA equilibrium and lead to antidepressant-like effects (Pilc et al., 2013). Thus far, the evidence linking activation of the mGlu4 receptor with an antidepressant-like effect in rodents has been inconclusive. It should be noted that i.c.v. administration of ACPT-I reduced immobility time in the rat FST (Tatarczyñska et al., 2002; Pałucha et al., 2004), the effect being blocked by a group III mGlu antagonist, CPPG [(RS)-α-cyclopropyl-4-phosphonophenylglycine]. Furthermore, enhanced depression-like immobility in the FST, as the outcome of the maternal separation procedure in the rat, has upregulated mGlu4 receptor protein levels in the hippocampus, whereas chronic treatment with the antidepressant drug, serotonin-norepinephrine reuptake inhibitor venlafaxine, normalized immobility times and mGlu4 receptor levels (Martisova et al., 2012). However, ACPT-I administered i.p. failed to show efficacy in the FST and TST in mice (Stachowicz et al., 2009), whereas LSP1-2111 and Lu AF21934 were inactive in TST (Wierońska et al., 2010; Sławińska et al., 2013a). The lack of effect of Lu AF21934 in TST, however, needs to be taken with caution, as the compound had a motor-suppressant effect which could have confounded any increases in mobility in the TST (Sławińska et al., 2013b). In the present study, ADX88178 dose-dependently reduced immobility times in the FST. However, the MED of ADX88178 in this test (30 mg/kg) and the concentration of the compound in plasma at the end of the test (1619 ng/ml) were higher compared with corresponding measurements linked to the activity of the compound in the mouse EPM and MB. Further studies involving disease-relevant models, such as Flinders sensitive line rats (Overstreet and Wegener, 2013) or a genetic line of “helpless” mice (El Yacoubi et al., 2003), as well as other domains of the disease such as anhedonia and social withdrawal can shed light on the role of the mGlu4 receptor in depression.
Even though the underlying pathophysiology of schizophrenia is still unknown, accumulating evidence suggests significant abnormalities in glutamatergic neurotransmission in schizophrenic patients (Javitt and Zukin, 1991; Javitt, 2010). For a decade, studies involving pharmacological manipulation of mGlu5 and mGlu2/3 receptors led the field of mGlu receptors as potential novel targets for schizophrenia (Conn et al., 2009), although recent preclinical evidence also implies the mGlu4 receptor could be a viable target for all domains of schizophrenia. For example, mGlu4 receptor activators were active in rodent models relevant for positive, negative, and cognitive symptoms of schizophrenia (Pałucha-Poniewiera et al., 2008; Wierońska et al., 2012, 2013; Sławińska et al., 2013b). It has been hypothesized that activation of mGlu4 receptors localized on glutamatergic terminals of the thalamocortical neurons (Corti et al., 2002) can counteract glutamatergic hyperactivity associated with schizophrenia. Here, ADX88178 resulted in reductions in DOI-mediated head twitches in mice that did not exceed 30%, showing a “flat” profile also seen with Lu AF21934 in this test. As with the latter, these data need to be taken with caution, as they lacked dose-dependency. Also, 30 mg/kg ADX88178 resulted in a modest (approximately 25%), albeit statistically significant reduction in MK-801–induced hyperactivity during the second 30 minutes of testing. In contrast to its effects in DOI and MK-801 hyperactivity tests, ADX88178 was inactive in the rat CAR test despite adequate plasma concentrations. We can only speculate on possible reasons why ADX88178 was inactive in this test. The CAR test may not be sensitive to mGlu4 receptor activators, as this was the first time an mGlu4 activator was tested in this assay. Previously, in addition to typical and atypical antipsychotic drugs, CAR tests showed sensitivity to a mGlu5 receptor PAM, ADX47273 [(4-fluorophenyl)-[(3S)-3-[3-(4-fluorophenyl)-1,2,4-oxadiazol-5-yl]piperidin-1-yl]methanone] (Liu et al., 2008). Alternatively, as seen with several GABAB receptor PAMs, the activity of mGlu4 PAMs may be brain-region dependent (Hensler et al., 2012). The fact that ADX88178 had no effect on sLMA in mice and rats, whereas Lu AF21934 significantly suppressed sLMA in mice at low doses (Sławińska et al., 2013a), partially supports this hypothesis. Overall, as with models of depression, more disease-relevant models of schizophrenia, such as those based on disruption of neurogenesis on gestational day 17 (Flagstad et al., 2005) or those involving 15q13.3 microdeletion (Fejgin et al., 2014), are needed to evaluate whether mGlu4 activators are potential novel pharmacotherapies for schizophrenia.
It needs to be emphasized that there was a good in vitro/in vivo correlation when evaluating MEDs of ADX88178, associated plasma, and estimated CSF exposure across several experiments (Table 2). Specifically, in all assays in rats and mice, with the exception of FST, the MED of ADX88178 was associated with mean plasma concentrations in the range of 220–625 ng/ml. These plasma concentrations are well aligned with the 241 ng/ml plasma concentration of ADX88178 associated with the MED of ADX88178 in the haloperidol-induced catalepsy test (3 mg/kg; Le Poul et al., 2012). However, in the MB test, the ED50 and CSF EC50 values (4.5 mg/kg and 121 nM, respectively) were higher than corresponding measures in the haloperidol-induced catalepsy test (1.1 mg/kg and 23 nM, respectively). The in vitro/in vivo correlations in other tests appear to follow the same trend. At this point, we can speculate that similar binding site occupancies by ADX88178 seem to lead to minimal anxiolytic and anti-Parkinsonian effects in several tests. However, markedly higher binding site occupancies may be required for the maximal anxiolytic effect, compared with those associated with the maximal anti-Parkinsonian effect. Further studies involving in vitro/in vivo correlations of ADX88178 in a range of assays can shed light on this question.
The effects of ADX88178 in models of anxiety, OCD, depression, fear-related disorders, and psychosis described in this paper were not confounded by any nonspecific changes in locomotor activity, as the compound had no effect in sLMA in mice and rats at up to 100 and 60 mg/kg, respectively. In contrast, Lu AF21934 resulted in robust and significant reductions in sLMA with a MED of 2 mg/kg (Sławińska et al., 2013a).
In conclusion, ADX88178 showed a robust anxiolytic- and anti-OCD–like profile likely to be mediated by the mGlu4 receptor. Thus, mGlu4 PAMs represent a potential novel treatment of anxiety disorders as well as heightened anxiety present as a comorbidity in Parkinson’s disease. Reduced acquisition of fear conditioning in response to ADX88178 provided other evidence suggesting that the mGlu4 receptor could be a novel target for treatment of post-traumatic stress disorder and other stress-related disorders. Although ADX88178 modestly reduced immobility in the mouse FST, additional studies are needed to evaluate mGlu4 as a target for treatment of depression. ADX88178 showed a modest reduction in DOI-induced head twitches (albeit with no dose-dependency), reduction in MK-801 hyperactivity in mice, but was inactive in the rat CAR test. Evaluation of the efficacy of ADX88178 in models relevant to negative symptoms and cognitive abnormalities as well as those involving disease-relevant models are needed for further evaluation of the mGlu4 receptor as a viable target for treatment of schizophrenia.
Acknowledgments
The authors thank Steven Vickers and Sharon Cheetham at RenaSci Ltd. for support in DOI-induced head twitch and conditioned avoidance response tests.
Abbreviations
- ACPT-I
(1S,3R,4S)-1-aminocyclopentane-1,3,4-tricarboxylic acid
- ADX47273
(4-fluorophenyl)-[(3S)-3-[3-(4-fluorophenyl)-1,2,4-oxadiazol-5-yl]piperidin-1-yl]methanone
- ADX88178
5-methyl-N-(4-methylpyrimidin-2-yl)-4-(1H-pyrazol-4-yl)thiazol-2-amine
- ANOVA
analysis of variance
- CAR
conditioned avoidance response
- CMC
carboxymethyl cellulose
- CPPG
(RS)-α-cyclopropyl-4-phosphonophenylglycine
- CS
conditioned stimulus
- CSF
cerebrospinal fluid
- DOI
2,5-dimethoxy-4-iodoamphetamine
- EPM
elevated plus maze
- FST
forced swim test
- KO
knockout
- LSP1-2111
(2S)-2-amino-4-[hydroxy[hydroxy(4-hydroxy-3-methoxy-5-nitro-phenyl)methyl]phosphoryl]butanoic acid
- Lu AF21934
(+)-(1S,2R)-N1-(3,4-dichlorophenyl)cyclohexane-1,2-dicarboxamide
- Lu AF32615
4-[(E)-2-phenylvinyl]-2-pyrimidinamine
- MB
marble burying
- MED
minimum effective dose
- mGlu
metabotropic glutamate receptor
- MK-801
(5S,10R)-(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine
- OCD
obsessive compulsive disorder
- PAM
positive allosteric modulator
- PHCCC
N-phenyl-7-(hydroxyimino)cyclopropa[b]chromen-1a-carboxamide
- QC
quality control
- sLMA
spontaneous locomotor activity
- TST
tail suspension test
- US
unconditioned stimulus
- VU0155041
cis-2-[[(3,5-dichlorophenyl)amino]carbonyl]cyclohexanecarboxylic acid
- WT
wild-type
Authorship Contributions
Participated in research design: Kalinichev, Le Poul, Boléa, Girard, Fonsi, Browne, Uslaner, Davis, Raber, Duvoisin, Reynolds, Poli, Celanire.
Conducted experiments: Girard, Fonsi, Royer-Urios, Davis.
Contributed new reagents or analytic tools: Fonsi, Royer-Urios.
Performed data analysis: Kalinichev, Girard, Campo, Fonsi, Uslaner, Davis, Raber, Duvoisin, Reynolds, Bate, Poli.
Wrote or contributed to the writing of the manuscript: Kalinichev, Le Poul, Boléa, Campo, Fonsi, Browne, Uslaner, Raber, Duvoisin, Bate, Reynolds, Poli, Celanire.
Footnotes
The fear conditioning experiments in mice performed at the Oregon Health & Science University were supported by the National Institutes of Health National Institute of Mental Health [Grant R01-MH77647 to J.R.].
References
- Bate ST, Clark RA. (2014) The Design and Statistical Analysis of Animal Experiments, Cambridge University Press, Cambridge [Google Scholar]
- Campo B, Kalinichev M, Lambeng N, El Yacoubi M, Royer-Urios I, Schneider M, Legrand C, Parron D, Girard F, Bessif A, et al. (2011) Characterization of an mGluR2/3 negative allosteric modulator in rodent models of depression. J Neurogenet 25:152–166 [DOI] [PubMed] [Google Scholar]
- Célanire S, Campo B. (2012) Recent advances in the drug discovery of metabotropic glutamate receptor 4 (mGluR4) activators for the treatment of CNS and non-CNS disorders. Expert Opin Drug Discov 7:261–280 [DOI] [PubMed] [Google Scholar]
- Conn PJ, Lindsley CW, Jones CK. (2009) Activation of metabotropic glutamate receptors as a novel approach for the treatment of schizophrenia. Trends Pharmacol Sci 30:25–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corti C, Aldegheri L, Somogyi P, Ferraguti F. (2002) Distribution and synaptic localisation of the metabotropic glutamate receptor 4 (mGluR4) in the rodent CNS. Neuroscience 110:403–420 [DOI] [PubMed] [Google Scholar]
- Davis MJ, Haley T, Duvoisin RM, Raber J. (2012) Measures of anxiety, sensorimotor function, and memory in male and female mGluR4⁻/⁻ mice. Behav Brain Res 229:21–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MJ, Iancu OD, Acher FC, Stewart BM, Eiwaz MA, Duvoisin RM, Raber J. (2013) Role of mGluR4 in acquisition of fear learning and memory. Neuropharmacology 66:365–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duty S. (2010) Therapeutic potential of targeting group III metabotropic glutamate receptors in the treatment of Parkinson’s disease. Br J Pharmacol 161:271–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvoisin RM, Villasana L, Davis MJ, Winder DG, Raber J. (2011) Opposing roles of mGluR8 in measures of anxiety involving non-social and social challenges. Behav Brain Res 221:50–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Yacoubi M, Bouali S, Popa D, Naudon L, Leroux-Nicollet I, Hamon M, Costentin J, Adrien J, Vaugeois JM. (2003) Behavioral, neurochemical, and electrophysiological characterization of a genetic mouse model of depression. Proc Natl Acad Sci USA 100:6227–6232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS. (1994) Neural organization of the defensive behavior system responsible for fear. Psychon Bull Rev 1:429–438 [DOI] [PubMed] [Google Scholar]
- Fejgin K, Nielsen J, Birknow MR, Bastlund JF, Nielsen V, Lauridsen JB, Stefansson H, Steinberg S, Sorensen HBD, Mortensen TE, et al. (2014) A mouse model that recapitulates cardinal features of the 15q13.3 microdeletion syndrome including schizophrenia- and epilepsy-related alterations. Biol Psychiatry 76:123–137 [DOI] [PubMed] [Google Scholar]
- Festing MFW, Overend P, Gaines RD, Borja MC, and Berdoy M (2002) The Design of Animal Experiments: Reducing the Use of Animals in Research Through Better Experimental Design, Ed. 1st. The Royal Society of Medicine Press Limited, London. [Google Scholar]
- Flagstad P, Glenthøj BY, Didriksen M. (2005) Cognitive deficits caused by late gestational disruption of neurogenesis in rats: a preclinical model of schizophrenia. Neuropsychopharmacology 30:250–260 [DOI] [PubMed] [Google Scholar]
- Grillon C, Davis M. (1997) Fear-potentiated startle conditioning in humans: explicit and contextual cue conditioning following paired versus unpaired training. Psychophysiology 34:451–458 [DOI] [PubMed] [Google Scholar]
- Hensler JG, Advani T, Burke TF, Cheng K, Rice KC, Koek W. (2012) GABAB receptor-positive modulators: brain region-dependent effects. J Pharmacol Exp Ther 340:19–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC. (2010) Glutamatergic theories of schizophrenia. Isr J Psychiatry Relat Sci 47:4–16 [PubMed] [Google Scholar]
- Javitt DC, Zukin SR. (1991) Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry 148:1301–1308 [DOI] [PubMed] [Google Scholar]
- Kalinichev M, Rouillier M, Girard F, Royer-Urios I, Bournique B, Finn T, Charvin D, Campo B, Le Poul E, Mutel V, et al. (2013) ADX71743, a potent and selective negative allosteric modulator of metabotropic glutamate receptor 7: in vitro and in vivo characterization. J Pharmacol Exp Ther 344:624–636 [DOI] [PubMed] [Google Scholar]
- Kendell SF, Krystal JH, Sanacora G. (2005) GABA and glutamate systems as therapeutic targets in depression and mood disorders. Expert Opin Ther Targets 9:153–168 [DOI] [PubMed] [Google Scholar]
- Le Poul E, Boléa C, Girard F, Poli S, Charvin D, Campo B, Bortoli J, Bessif A, Luo B, Koser AJ, et al. (2012) A potent and selective metabotropic glutamate receptor 4 positive allosteric modulator improves movement in rodent models of Parkinson’s disease. J Pharmacol Exp Ther 343:167–177 [DOI] [PubMed] [Google Scholar]
- Liu F, Grauer S, Kelley C, Navarra R, Graf R, Zhang G, Atkinson PJ, Popiolek M, Wantuch C, Khawaja X, et al. (2008) ADX47273 [S-(4-fluoro-phenyl)-3-[3-(4-fluoro-phenyl)-[1,2,4]-oxadiazol-5-yl]-piperidin-1-yl-methanone]: a novel metabotropic glutamate receptor 5-selective positive allosteric modulator with preclinical antipsychotic-like and procognitive activities. J Pharmacol Exp Ther 327:827–839 [DOI] [PubMed] [Google Scholar]
- Martisova E, Solas M, Horrillo I, Ortega JE, Meana JJ, Tordera RM, Ramírez MJ. (2012) Long lasting effects of early-life stress on glutamatergic/GABAergic circuitry in the rat hippocampus. Neuropharmacology 62:1944–1953 [DOI] [PubMed] [Google Scholar]
- Nicolas LB, Kolb Y, Prinssen EPM. (2006) A combined marble burying-locomotor activity test in mice: a practical screening test with sensitivity to different classes of anxiolytics and antidepressants. Eur J Pharmacol 547:106–115 [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Wegener G. (2013) The flinders sensitive line rat model of depression—25 years and still producing. Pharmacol Rev 65:143–155 [DOI] [PubMed] [Google Scholar]
- Pałucha A, Tatarczyńska E, Brański P, Szewczyk B, Wierońska JM, Kłak K, Chojnacka-Wójcik E, Nowak G, Pilc A. (2004) Group III mGlu receptor agonists produce anxiolytic- and antidepressant-like effects after central administration in rats. Neuropharmacology 46:151–159 [DOI] [PubMed] [Google Scholar]
- Pałucha-Poniewiera A, Kłodzińska A, Stachowicz K, Tokarski K, Hess G, Schann S, Frauli M, Neuville P, Pilc A. (2008) Peripheral administration of group III mGlu receptor agonist ACPT-I exerts potential antipsychotic effects in rodents. Neuropharmacology 55:517–524 [DOI] [PubMed] [Google Scholar]
- Pekhletski R, Gerlai R, Overstreet LS, Huang XP, Agopyan N, Slater NT, Abramow-Newerly W, Roder JC, Hampson DR. (1996) Impaired cerebellar synaptic plasticity and motor performance in mice lacking the mGluR4 subtype of metabotropic glutamate receptor. J Neurosci 16:6364–6373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilc A, Wierońska JM, Skolnick P. (2013) Glutamate-based antidepressants: preclinical psychopharmacology. Biol Psychiatry 73:1125–1132 [DOI] [PubMed] [Google Scholar]
- Sanacora G, Mason GF, Rothman DL, Hyder F, Ciarcia JJ, Ostroff RB, Berman RM, Krystal JH. (2003) Increased cortical GABA concentrations in depressed patients receiving ECT. Am J Psychiatry 160:577–579 [DOI] [PubMed] [Google Scholar]
- Sławińska A, Wierońska JM, Stachowicz K, Pałucha-Poniewiera A, Uberti MA, Bacolod MA, Doller D, Pilc A. (2013a) Anxiolytic- but not antidepressant-like activity of Lu AF21934, a novel, selective positive allosteric modulator of the mGlu₄ receptor. Neuropharmacology 66:225–235 [DOI] [PubMed] [Google Scholar]
- Sławińska A, Wierońska JM, Stachowicz K, Marciniak M, Lasoń-Tyburkiewicz M, Gruca P, Papp M, Kusek M, Tokarski K, Doller D, et al. (2013b) The antipsychotic-like effects of positive allosteric modulators of metabotropic glutamate mGlu4 receptors in rodents. Br J Pharmacol 169:1824–1839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachowicz K, Chojnacka-Wójcik E, Kłak K, Pilc A. (2006) Anxiolytic-like effects of group III mGlu receptor ligands in the hippocampus involve GABAA signaling. Pharmacol Rep 58:820–826 [PubMed] [Google Scholar]
- Stachowicz K, Kłak K, Kłodzińska A, Chojnacka-Wojcik E, Pilc A. (2004) Anxiolytic-like effects of PHCCC, an allosteric modulator of mGlu4 receptors, in rats. Eur J Pharmacol 498:153–156 [DOI] [PubMed] [Google Scholar]
- Stachowicz K, Kłodzińska A, Palucha-Poniewiera A, Schann S, Neuville P, Pilc A. (2009) The group III mGlu receptor agonist ACPT-I exerts anxiolytic-like but not antidepressant-like effects, mediated by the serotonergic and GABA-ergic systems. Neuropharmacology 57:227–234 [DOI] [PubMed] [Google Scholar]
- Tatarczyńska E, Pałucha A, Szewczyk B, Chojnacka-Wójcik E, Wierońska J, Pilc A. (2002) Anxiolytic- and antidepressant-like effects of group III metabotropic glutamate agonist (1S,3R,4S)-1-aminocyclopentane-1,3,4-tricarboxylic acid (ACPT-I) in rats. Pol J Pharmacol 54:707–710 [PubMed] [Google Scholar]
- Thomas A, Burant A, Bui N, Graham D, Yuva-Paylor LA, Paylor R. (2009) Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacology (Berl) 204:361–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierońska JM, Acher FC, Sławińska A, Gruca P, Lasoń-Tyburkiewicz M, Papp M, Pilc A. (2013) The antipsychotic-like effects of the mGlu group III orthosteric agonist, LSP1-2111, involves 5-HT₁A signalling. Psychopharmacology (Berl) 227:711–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierońska JM, Stachowicz K, Acher F, Lech T, Pilc A. (2012) Opposing efficacy of group III mGlu receptor activators, LSP1-2111 and AMN082, in animal models of positive symptoms of schizophrenia. Psychopharmacology (Berl) 220:481–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierońska JM, Stachowicz K, Pałucha-Poniewiera A, Acher F, Brański P, Pilc A. (2010) Metabotropic glutamate receptor 4 novel agonist LSP1-2111 with anxiolytic, but not antidepressant-like activity, mediated by serotonergic and GABAergic systems. Neuropharmacology 59:627–634 [DOI] [PubMed] [Google Scholar]